Abstract
Both sodium reduction and the DASH diet lower blood pressure (BP); however, the patterns of their effects on BP over time are unknown. In the DASH-Sodium trial, adults with pre-/stage 1 hypertension, not using antihypertensive medications, were randomly assigned to either a typical American diet (control) or DASH. Within their assigned diet, participants randomly ate each of three sodium levels (50, 100, and 150 mmol/d, at 2100 kcal) in 4-week periods. BP was measured weekly over 12wks; 412 participants enrolled (57% women, 57% black, mean age 48years, mean SBP/DBP 135/86 mmHg). For those assigned control, there was no change in SBP/DBP between weeks 1 and 4 on the high sodium diet (weekly change −0.04/0.06 mmHg/week) versus a progressive decline in BP on the low sodium diet (−0.94/−0.70 mmHg/week; P-interactions between time and sodium <0.001 for SBP and DBP). For those assigned DASH, SBP/DBP changed −0.60/−0.16 mmHg/week on the high versus −0.42/−0.54 mmHg/week on the low sodium diet (P-interactions between time and sodium=0.56 for SBP and 0.10 for DBP). When comparing DASH to control, DASH changed SBP/DBP by −4.36/−1.07 mmHg after 1wk, which accounted for most of the effect observed, with no significant difference in weekly rates of change for either SBP (P-interaction=0.97) or DBP (P-interaction=0.70). In the context of a typical American diet, a low sodium diet reduced BP without plateau, suggesting that the full effects of sodium reduction are not completely achieved by 4wks. In contrast, compared with control, DASH lowers BP within a week without further effect thereafter.
Keywords: sodium, diet, time course, trial, blood pressure, hypertension
Sodium intake is an important determinant of blood pressure (BP). The original DASH-Sodium feeding study demonstrated that decreasing sodium intake over a 4-week period lowered BP in adults with prehypertension or hypertension.1 This finding confirmed a role for dietary sodium reduction as a lifestyle intervention to lower BP. Furthermore, this result has since been replicated in numerous clinical trials.2,3 However, the time course of BP change is unknown. Given that many trials of sodium reduction last days or weeks, it is important to understand whether this effect increases over time.
The DASH diet has also been shown to be an important lifestyle approach to lower BP. Reports from the DASH trial have displayed BP change by week over 11 weeks in published figures suggesting a rapid reduction at 1–2 weeks with a plateau thereafter.4,5 However, no formal analyses of time course have been performed.
The purpose of this study was (1) to determine the time course of BP change from sodium reduction over 4 weeks, separately in the DASH and control diets, and (2) to determine the time course of BP change from the DASH diet compared to the control diet over the 12 weeks of feeding in the DASH-Sodium trial.
Methods
The original DASH-Sodium trial was an investigator-initiated, multicenter, randomized trial conducted between September 1997 through November 1999 with the support of the National Heart, Lung, and Blood Institute. Detailed methods have been published elsewhere.1 In brief, DASH-Sodium compared the effects of consuming three different sodium levels in the context of either the DASH diet or a control diet, typical of the US population. The study was conducted at four clinical centers within the United States (Baltimore, Maryland; Boston, Massachusetts; Durham, North Carolina; Portland, Oregon). Institutional Review Boards at each center approved the original study protocol including subsequent analyses. Written, informed consent was provided by all participants.
Participants
Adult men and women, aged 22 years and older, were recruited into the original DASH-Sodium trial at the four clinical centers. All participants had prehypertension or stage I hypertension based on an average systolic blood pressure (SBP) (measured at three screening visits) of 120 to 159 mm Hg and an average diastolic blood pressure (DBP) of 80 to 95 mm Hg. Excluded were persons with a prior diagnosis of heart disease, renal insufficiency, poorly controlled dyslipidemia, diabetes mellitus, or heart failure.1 The study also excluded persons using antihypertensive agents, insulin, or drinking more than 14 alcoholic drinks a week.
Dietary Interventions
Participants were fed either the DASH diet (intervention) or a control diet. The DASH diet (intervention), emphasized fruits, vegetables, and low-fat dairy foods with reduced intake of saturated fat, total fat, and cholesterol. In addition, the DASH diet emphasized whole grains, poultry, fish, and nuts with smaller amounts of red meat, sweets, and sugar-containing beverages. The control diet reflected foods common in a typical American diet.4
In addition, participants on either the DASH or control diets were fed each of three sodium levels: low, medium, and high, which at 2100 kcal provided a target of 50 mmol, 100 mmol, and 150 mmol of sodium per day, respectively. The high level was designed to reflect the sodium intake of a typical American. Further, sodium intake was adjusted to reflect the total energy requirements of each participant. As a result, larger or more active participants received more food and sodium than smaller or less active participants. A detailed composition of the diets may be found in Supplement Table S1.
Participants were provided all of their food, including snacks and meals. The main meal was consumed at the study centers while their remaining food was delivered to participants’ homes. Caloric intake was adjusted to keep participants’ weights constant throughout the trial. During a two-week run-in period, all participants ate the high sodium, control diet. They were subsequently randomized to either the DASH or control diet, following a parallel-arm design. These diets were consumed at each of the three sodium levels for 30 days in random order, according to a crossover design. Sodium levels were separated by, on average, 5-day washout periods during which participants could resume their usual diets. Over 98% of participants completed each of the intervention periods.1
Blood pressure and covariate measurements
The primary outcome of the original DASH-sodium study was BP, measured with random-zero sphygmomanometers while participants were seated. BP was measured twice during the run-in period, weekly during the first 3 weeks of each of the three 30-day intervention periods, and at five clinic visits during the last 9 days (at least two during the final 4 days) of each intervention period. A high BP at baseline was defined as having a mean SBP ≥140 mm Hg or DBP ≥90 mm Hg at baseline.
Additional covariates were ascertained via questionnaire, laboratory specimens, and physical examination. Body mass index (BMI) was derived from measured height and weight.
Statistical analysis
Study population characteristics were described using means (SD) and proportions. When comparing the effect of sodium over time, we determined the mean SBP and DBP at each week by sodium intake level (low, medium, or high) according to diet assignment (DASH versus control) per the study design. Individual means at each week were compared using t-tests with 95% confidence intervals calculated for the difference in measures at each week. Furthermore, a test for plateau of effects over time was performed using quadratic terms, which were evaluated with Wald tests.
Trends in SBP or DBP over time were determined via generalized estimating equation (GEE) regression models with a Huber and White robust variance estimator,6 which assumed an exchangeable working correlation matrix. Differences in weekly trends were examined with interaction terms between the sodium level and its weekly effect. Analyses were repeated in the subgroup of participants who had hypertension at baseline. In addition, we performed sensitivity analyses using autoregressive working correlation models for within-person correlations. Finally, to remove the possibility of carryover effects, we conducted a sensitivity analysis that used data from the first feeding period alone, following a parallel design.
Analyses were repeated comparing the weekly mean SBP and DBP between the DASH and control diets. As above, t-tests with 95% confidence intervals were used to compare weekly means and GEE was used in analyses of trends. Comparisons were repeated in a subgroup with hypertension at baseline.
All analyses were conducted with Stata version 14.0 (Stata Corporation, College Station, TX, USA). Missing data were minimal and evenly distributed throughout dietary interventions and time periods throughout the study (Supplement Tables S2–S3).
Results
Baseline characteristics of the 412 study participants are shown in Table 1. The mean age of participants was 48.2 (SD, 10.0) years, 56.8% were women, 57.9% were black, 38.8% were obese, and 40.8% had hypertension. The mean run-in SBP was 134.8 (9.5) mm Hg and the mean run-in DBP was 86 (4.5) mm Hg.
Table 1.
Baseline characteristics overall and by diet, mean (SD) or %
| Characteristic | Overall (N = 412) | Control Diet (N = 204) | DASH Diet (N = 208) |
|---|---|---|---|
| Age, yr | 48.2 (10.0) | 49.1 (10.4) | 47.4 (9.6) |
| Women, % | 56.8 | 54.4 | 59.1 |
| Black, % | 56.8 | 56.4 | 57.2 |
| High Blood Pressure*, % | 40.8 | 40.7 | 40.9 |
| Blood pressure, mm Hg | |||
| Systolic | 134.8 (9.5) | 135.4 (9.4) | 134.2 (9.6) |
| Diastolic | 85.7 (4.5) | 85.8 (4.1) | 85.6 (4.8) |
| Body mass index, kg/m2 | 29.2 (4.8) | 29.5 (5.0) | 28.8 (4.7) |
| Body mass index ≥30, % | 38.8 | 40.2 | 37.5 |
Defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg.
BP Time Course from Sodium Reduction
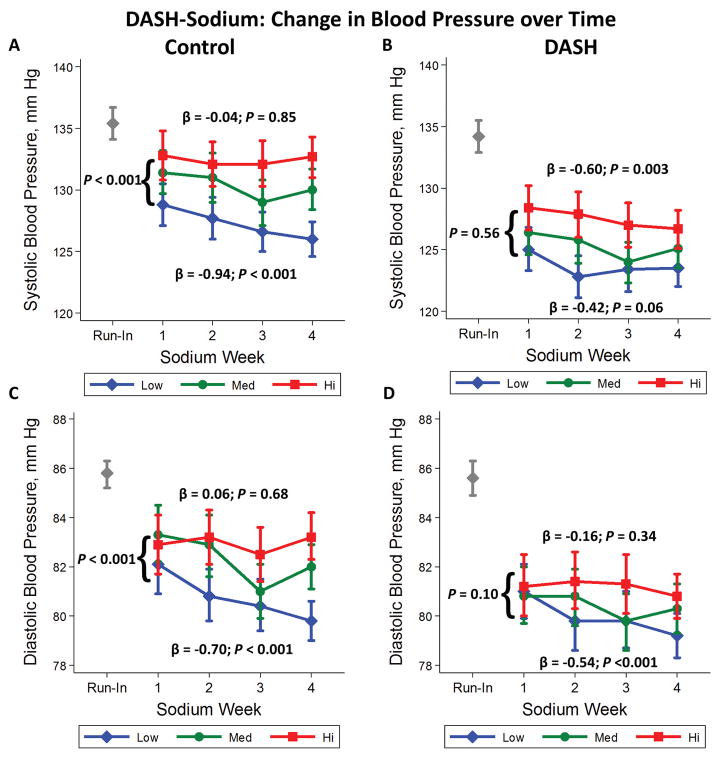
Among participants assigned the control diet, the difference in SBP between high and low sodium intake was −4.03 (−6.66, −1.39) mm Hg after week 1 and −6.66 (−8.84, −4.47) mm Hg after week 4 (Table 2; Figure 1A). The mean weekly change in SBP, from week 1 to week 4, on the high sodium diet was −0.04 mmHg/week versus −0.94 mmHg/week on the low sodium diet (P interaction for difference in trends <0.001). With regards to DBP (Table 2; Figure 1C), reducing sodium intake from high to low decreased DBP at week 1 (−0.80 mm Hg; 95% CI: −2.50, 0.89) and to a greater extent at week 4 (−3.43 mm Hg; 95% CI: −4.72, −2.13). The greater magnitude of the difference in DBP at week 4 compared to week 1, was consistent with an increasing trend in DBP reduction from reducing sodium over time (P-interaction = 0.004).
Table 2.
Difference in systolic and diastolic blood pressures between the high, medium, and low sodium diets according to DASH or control assignment
| Diet Control | Week
|
Mean Weekly Change (95% CI) | P-trend | P-interaction for difference in trends | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| SBP | |||||||
| High | 132.8 (14.3) | 132.1 (12.5) | 132.1 (13.1) | 132.7 (11.9) | −0.04 (−0.48, 0.39) | 0.85 | Ref |
| Medium | 131.4 (12.7) | 131.0 (14.2) | 129.0 (13.2) | 130.0 (11.7) | −0.56 (−0.92, −0.20) | 0.002 | 0.08 |
| Low | 128.8 (12.3) | 127.7 (12.4) | 126.6 (11.4) | 126.0 (10.0) | −0.94 (−1.34, −0.55) | <0.001 | <0.001 |
| High vs Med | −1.38 (−4.05, 1.29) | −1.08 (−3.75, 1.58) | −3.15 (−5.78, −0.51) | −2.66 (−5.00, −0.32) | |||
| P | 0.31 | 0.43 | 0.02 | 0.03 | |||
| High vs Low | −4.03 (−6.66, −1.39) | −4.41 (−6.88, −1.93) | −5.51 (−7.97, −3.05) | −6.66 (−8.84, −4.47) | |||
| P | 0.003 | 0.0005 | <0.001 | <0.001 | |||
| DBP | |||||||
| High | 82.9 (8.7) | 83.2 (7.8) | 82.5 (7.9) | 83.2 (6.9) | 0.06 (−0.23, 0.35) | 0.68 | Ref |
| Medium | 83.3 (8.2) | 82.9 (8.7) | 81.0 (7.9) | 82.0 (6.4) | −0.56 (−0.89, −0.23) | 0.001 | 0.004 |
| Low | 82.1 (8.4) | 80.8 (7.6) | 80.4 (7.6) | 79.8 (6.0) | −0.70 (−0.99, −0.40) | <0.001 | <0.001 |
| High vs Med | 0.43 (−1.24, 2.10) | −0.37 (−2.02, 1.27) | −1.47 (−3.06, 0.11) | −1.24 (−2.57, 0.09) | |||
| P | 0.61 | 0.65 | 0.07 | 0.07 | |||
| High vs Low | −0.80 (−2.50, 0.89) | −2.40 (−3.93, −0.86) | −2.07 (−3.63, −0.52) | −3.43 (−4.72, −2.13) | |||
| P | 0.35 | 0.002 | 0.009 | <0.001 | |||
| DASH | |||||||
| SBP | |||||||
| High | 128.4 (12.9) | 127.9 (13.2) | 127.0 (12.6) | 126.7 (11.2) | −0.60 (−1.00, −0.20) | 0.003 | Ref |
| Medium | 126.4 (13.0) | 125.8 (13.3) | 124.0 (11.9) | 125.1 (11.4) | −0.59 (−1.01, −0.17) | 0.006 | 0.92 |
| Low | 125.0 (12.8) | 122.8 (12.2) | 123.4 (12.8) | 123.5 (10.4) | −0.42 (−0.86, 0.02) | 0.06 | 0.56 |
| High vs Med | −1.94 (−4.50, 0.62) | −2.10 (−4.70, 0.51) | −3.02 (−5.44, −0.60) | −1.56 (−3.77, 0.65) | |||
| P | 0.14 | 0.11 | 0.01 | 0.17 | |||
| High vs Low | −3.35 (−5.88, −0.82) | −5.05 (−7.55, −2.54) | −3.61 (−6.13, −1.08) | −3.20 (−5.31, −1.09) | |||
| P | 0.01 | 0.0001 | 0.005 | 0.003 | |||
| DBP | |||||||
| High | 81.2 ( 9.2) | 81.4 ( 8.4) | 81.3 ( 8.5) | 80.8 ( 6.8) | −0.16 (−0.50, 0.17) | 0.34 | Ref |
| Medium | 80.8 (8.4) | 80.8 (8.2) | 79.8 (8.3) | 80.3 (7.2) | −0.30 (−0.59, −0.01) | 0.05 | 0.59 |
| Low | 81.0 ( 7.8) | 79.8 ( 8.3) | 79.8 ( 8.2) | 79.2 ( 6.3) | −0.54 (−0.82, −0.25) | <0.001 | 0.10 |
| High vs Med | −0.40 (−2.14, 1.35) | −0.67 (−2.30, 0.96) | −1.55 (−3.21, 0.10) | −0.54 (−1.91, 0.83) | |||
| P | 0.65 | 0.42 | 0.07 | 0.44 | |||
| High vs Low | −0.25 (−1.93, 1.42) | −1.65 (−3.29, −0.01) | −1.52 (−3.18, 0.14) | −1.60 (−2.88, −0.31) | |||
| P | 0.76 | 0.05 | 0.07 | 0.01 | |||
Figure 1.
Mean weekly systolic blood pressure (mm Hg) (A&B) and diastolic blood pressure (mm Hg) (C&D) while consuming high (squares), medium (circles), and low (diamonds) sodium levels. Means are presented according to assignment to the control (A&C) or DASH (B&D) diets. Beta’s represent weekly change in blood pressure. Vertical lines represent the 95% confidence intervals. The gray diamond represents the systolic or diastolic blood pressure measured after the run-in period at baseline.
In contrast, among participants assigned the DASH diet (Table 2; Figure 1B), the reduction in SBP from lowering sodium intake from high to low was virtually the same between weeks 1 and 4 (−3.35 versus −3.20 mm Hg; P-interaction = 0.56). Similarly, lowering sodium intake from high to low in the context of the DASH diet reduced DBP by week 2 (−1.65 mm Hg), but this effect did not vary over time (week 4 difference −1.60 mm Hg; P interaction = 0.10) (Table 2; Figure 1D). An examination of the medium sodium level in the groups above showed similar, albeit attenuated, trends among those assigned the control diet (Table 2).
In the subgroup of the participants with hypertension at baseline (Table 3), among those assigned the control diet, reducing sodium intake from high to low changed SBP −4.55 mm Hg at 1 week and −8.43 mm Hg at 4 weeks. The weekly trend on the high sodium diet was −0.07 mm Hg/week versus −1.45 mm Hg/week on the low sodium diet (P-interaction = 0.005). Similarly, among those assigned the control diet, reducing sodium intake from high to low changed DBP −1.29 mm Hg at 1 week and −4.57 mm Hg at 4 weeks (P-interaction = 0.001) with weekly trends of 0.24 mmHg/week (high) versus −0.85 mm Hg/week (low) (P-interaction < 0.001).
Table 3.
Difference in systolic and diastolic blood pressures between the high and low sodium diets overall and by diet in participants who were hypertensive at baseline
| Diet Control | Week
|
Mean Weekly Change (95% CI) | P-trend | P-interaction for difference in trends | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| SBP | |||||||
| High | 141.0 (13.8) | 139.9 (12.2) | 139.7 (14.0) | 140.9 (10.9) | −0.07 (−0.75, 0.61) | 0.84 | Ref |
| Medium | 139.0 (13.0) | 140.6 (14.4) | 137.5 (13.7) | 138.5 (12.0) | −0.36 (−0.96, 0.23) | 0.23 | 0.45 |
| Low | 136.5 (11.8) | 134.7 (13.1) | 132.3 (12.3) | 132.5 (10.8) | −1.45 (−2.13, −0.77) | <0.001 | 0.005 |
| High vs Med | −2.01 (−6.18, 2.16) | 0.65 (−3.52, 4.82) | −2.16 (−6.57, 2.26) | −2.40 (−6.01, 1.22) | |||
| P | 0.34 | 0.76 | 0.34 | 0.19 | |||
| High vs Low | −4.55 (−8.57, −0.52) | −5.24 (−9.22, −1.26) | −7.35 (−11.55, −3.15) | −8.43 (−11.85, −5.00) | |||
| P | 0.03 | 0.01 | 0.0007 | <0.001 | |||
| DBP | |||||||
| High | 85.5 ( 9.0) | 85.1 ( 8.7) | 85.5 ( 7.8) | 86.1 ( 6.8) | 0.24 (−0.25, 0.73) | 0.34 | Ref |
| Medium | 85.8 (8.2) | 86.1 (8.5) | 83.0 (8.5) | 84.5 (6.6) | −0.64 (−1.18, −0.10) | 0.02 | 0.007 |
| Low | 84.2 ( 8.9) | 82.4 ( 8.1) | 81.5 ( 8.5) | 81.6 ( 5.9) | −0.85 (−1.31, −0.38) | <0.001 | 0.001 |
| High vs Med | 0.27 (−2.42, 2.95) | 1.06 (−1.63, 3.75) | −2.47 (−5.07, 0.14) | −1.66 (−3.76, 0.45) | |||
| P | 0.84 | 0.44 | 0.06 | 0.12 | |||
| High vs Low | −1.29 (−4.09, 1.52) | −2.63 (−5.27, 0.02) | −4.00 (−6.59, −1.41) | −4.57 (−6.58, −2.56) | |||
| P | 0.37 | 0.05 | 0.003 | 0 | |||
| DASH | |||||||
| SBP | |||||||
| High | 135.3 (13.6) | 135.0 (13.3) | 133.0 (12.9) | 133.9 (10.6) | −0.61 (−1.36, 0.14) | 0.11 | Ref |
| Medium | 133.0 (14.1) | 133.9 (14.2) | 131.1 (11.9) | 131.9 (11.6) | −0.60 (−1.34, 0.13) | 0.11 | 0.94 |
| Low | 132.8 (13.6) | 128.8 (12.5) | 130.0 (13.4) | 128.7 (10.4) | −1.16 (−2.00, −0.33) | 0.006 | 0.37 |
| High vs Med | −2.32 (−6.67, 2.03) | −1.10 (−5.39, 3.20) | −1.90 (−5.80, 1.99) | −2.04 (−5.51, 1.43) | |||
| P | 0.2932 | 0.6154 | 0.3361 | 0.2482 | |||
| High vs Low | −2.53 (−6.75, 1.69) | −6.18 (−10.18, −2.18) | −3.00 (−7.10, 1.10) | −5.17 (−8.43, −1.91) | |||
| P | 0.24 | 0.0027 | 0.151 | 0.0021 | |||
| DBP | |||||||
| High | 84.0 ( 8.8) | 84.5 ( 7.6) | 84.6 ( 8.2) | 83.8 ( 6.5) | −0.10 (−0.64, 0.45) | 0.72 | Ref |
| Medium | 83.7 (8.5) | 83.8 (8.8) | 83.0 (8.6) | 83.4 (6.8) | −0.17 (−0.64, 0.31) | 0.49 | 0.92 |
| Low | 83.9 ( 7.9) | 82.6 ( 8.2) | 81.8 ( 8.4) | 81.4 ( 6.2) | −0.89 (−1.36, −0.42) | <0.001 | 0.03 |
| High vs Med | −0.31 (−3.02, 2.39) | −0.75 (−3.32, 1.82) | −1.58 (−4.22, 1.05) | −0.42 (−2.51, 1.67) | |||
| P | 0.82 | 0.56 | 0.24 | 0.69 | |||
| High vs Low | −0.12 (−2.70, 2.47) | −1.92 (−4.36, 0.53) | −2.81 (−5.41, −0.20) | −2.45 (−4.43, −0.47) | |||
| P | 0.93 | 0.12 | 0.03 | 0.02 | |||
In the subgroup of participants with hypertension at baseline assigned the DASH diet, reducing sodium intake from high to low changed SBP −2.53 mm Hg at 1 week and −5.17 mm Hg at 4 weeks. The weekly trend of change on the high sodium diet was −0.61 mm Hg/week versus −1.16 mm Hg/week on the low sodium diet, but these trends were not statistically significant (P-interaction = 0.37). Similarly, among those assigned the DASH diet, reducing sodium changed DBP −0.12 mm Hg (week 1) and −2.45 mm Hg (week 4). Weekly trends in DBP reduction (−0.1 mm Hg/week on high sodium versus −0.89 mm Hg/week on low sodium) were significantly different (P-interaction = 0.03).
BP Time Course of DASH versus Control Diet
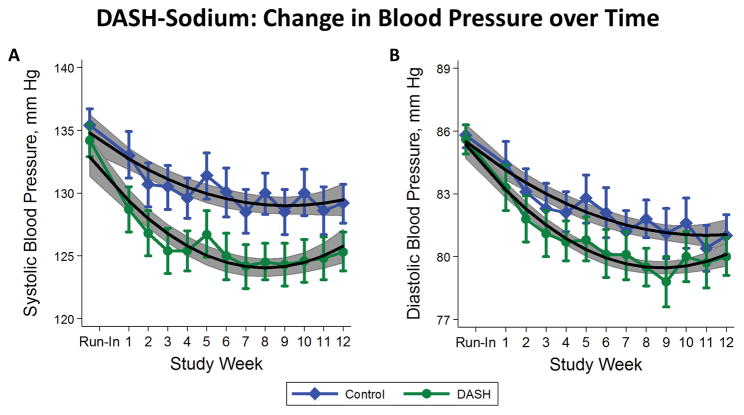
With regards to the DASH compared to the control diet, reduction in SBP occurred by week 1 (−4.36 mm Hg) and was maintained by week 12 (−3.82 mm Hg) (Supplemental Table S5; Figure 2A). Trends were curvilinear with an early reduction in SBP reflected by significant quadratic terms (P-values <0.001 for both control and DASH diets). Similarly, reduction in DBP at week 1 and 12 was −1.07 mm Hg and −0.99 mm Hg, respectively (Supplemental Table S4; Figure 2B). Trends were also curvilinear with P-values for quadratic terms being 0.01 for control and <0.001 for the DASH diet. A similar analysis comparing DASH to control over time in the subgroup with hypertension at baseline showed virtually the same results (Supplement Table S5). While the magnitudes of SBP and DBP reductions were greater among participants with hypertension, the majority of the BP reduction occurred in the first week of the 12-week intervention.
Figure 2.
Mean weekly systolic blood pressure (mm Hg) (A) and diastolic blood pressure (mm Hg) (B) while consuming the control diet (diamonds) or DASH diet (circles). Means are overlaid with quadratic linear fits to help visualize trends. Vertical lines represent the 95% confidence intervals for each mean. Gray shading represents the 95% confidence bands surrounding the quadratic models.
Sensitivity Analyses
Repeating sodium comparisons using an autoregressive type 1 correlation matrix (Supplement Table S6) slightly attenuated our findings. Similarly, limiting the sodium comparison to the first 4 weeks only, i.e. a parallel design, attenuated but did not change our findings. (Supplement Table S7).
Discussion
In the DASH-Sodium feeding study that enrolled a diverse population of individuals with pre- and stage 1 hypertension, we found that in the context of a typical American diet, a low sodium diet reduced BP at week 1 with no evidence of a plateau at 4 weeks, suggesting that the full effects of sodium reduction may not be entirely achieved within 4 weeks. In contrast, the DASH diet lowered BP compared with control within a week, and its effects appeared to plateau.
Consistent with a preponderance of evidence from other trials,2,3 the original DASH-Sodium trial demonstrated that lowering sodium lowered BP.1 However, prior trials of sodium reduction vary substantially with regards to the duration of the intervention, ranging from 4 days to 36 months.7,8 Furthermore, few studies reported BP measurements over time allowing for detailed, within-trial examination of the time course of BP change from sodium reduction. While this study does not identify the duration of intervention needed to achieve the full effect of sodium reduction, it does suggest that 4 weeks may not be sufficient.
Interestingly, the effect of sodium reduction on BP over time was not as pronounced in the DASH diet, and further there was no difference in trends between sodium levels over time while on the DASH diet. It is possible that this attenuation is related to the higher potassium content of the DASH diet,5 which might be responsible, at least in part, for its BP-lowering effects.9–11 Since both potassium and sodium are principal drivers of renin-angiotensin system, it may be that there is some redundancy in targeting this pathway by both reducing sodium and increasing potassium. Nevertheless, there was still a reduction in BP from reducing sodium intake in addition to eating more potassium, attesting to the value of combining both strategies to lower BP.
As for the time course of the DASH diet, we documented that the DASH diet lowered BP within 1 week of initiation, and effects were maintained throughout the duration of the study. These findings confirm the suggestion of data published in figures from the original DASH trial.
This study has a number of important implications for patients, physicians, and researchers. Data on time course are useful for physicians counseling their patients on the adoption of lifestyle interventions. While early effects can occur, full effects especially for sodium reduction likely do not occur within a month. Similarly, researchers studying the effects of sodium reduction and other interventions on BP might want to extend interventions beyond 4 weeks if a trial is to document the greatest possible effect. Still, reductions in BP before 4 weeks can be detected, but the effect size is smaller than what could occur with a longer intervention.
This study has limitations. First, each sodium period only lasted about 4 weeks. While our findings are highly relevant for other trials of sodium reduction, many of which were less than one month in duration, four weeks is too short for reliably inferring long-term trends. The ideal study, albeit impractical, would span years, if not decades. Hence, these analyses should be viewed as hypothesis generating and do not allow for conclusions about the time course of BP effects beyond one month. Similarly, we cannot speculate on the effects of the DASH diet beyond 12 weeks, the total duration of feeding in the DASH-Sodium trial. Second, the study may not be generalizable to people with conditions associated with salt retention, namely, patients with chronic kidney disease, medication-treated diabetes, and heart failure; these conditions were exclusion criteria in the DASH-Sodium trial. Last, our study did not have sufficient duration or size to observe clinical events such as cardiovascular disease or death.
Our study also has important strengths. It employed a randomized design with controlled feeding in a diverse population. Follow-up rates were extremely high. Furthermore, isocaloric feeding was utilized to minimize the effect of weight change on BP. Finally, BP measurements were assessed in triplicate with a standardized protocol nearly weekly. This afforded the opportunity to study the effect of these interventions over time.
Perspectives
In conclusion, in the context of a typical American diet, a low sodium diet reduced BP with no evidence of plateau at 4 weeks, suggesting that the full effects of sodium reduction may not have been fully achieved by 4 weeks. In contrast, the DASH diet lowers BP compared with control within a week, and its effects appear to plateau. The minimum length of time needed to observe the full effect of sodium reduction on BP should be the subject of future research.
Supplementary Material
Novelty and Significance.
What is New?
The DASH diet lowers BP within 1 week and its effects are sustained without evidence of further BP reduction.
Sodium reduction lowers BP through 4 weeks without signs of plateau.
What is Relevant?
Adults starting the DASH diet can expect BP effects soon, which is clinically useful for knowing when to reassess BP after starting this lifestyle intervention
Adults reducing sodium will on average experience greater reductions in BP over 4 weeks with possibly further BP reduction beyond 4 weeks.
Summary
While the effects of the DASH diet occur within 1 week, sodium reduction continues to lower BP up through 4 weeks of initiation.
Acknowledgments
We are indebted to the study participants for their sustained commitment to the DASH–Sodium Trial.
The authors thank William M Vollmer, PhD, senior investigator at Kaiser Permanente Center for Health Research, for reviewing the statistical section of this manuscript.
Source(s) of Funding
Supported by cooperative agreements and grants from the National Heart, Lung, and Blood Institute (U01-HL57173, to Brigham and Women’s Hospital; U01-HL57114, to Duke University; U01-HL57190, to Pennington Biomedical Research Institute; U01-HL57139 and K08 HL03857-01, to Johns Hopkins University; and U01-HL57156, to Kaiser Permanente Center for Health Research) and by the General Clinical Research Center Program of the National Center for Research Resources (M01-RR02635, to Brigham and Women’s Hospital, and M01-RR00722, to Johns Hopkins University).
SPJ is supported by a NIH/NIDDK T32DK007732-20 Renal Disease Epidemiology Training Grant and a K23HL135273-01 grant. MW is supported by a NHMRC Principal Research Fellowship.
Abbreviations used
- DASH
Dietary Approaches to Stop Hypertension
- GEE
generalized estimating equation
- CI
confidence interval
Footnotes
This trial is registered at clinicaltrials.gov, number: NCT00000608
Conflicts of interest
The authors have no conflicts of interest to report.
References
- 1.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, Karanja N, Lin PH DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 2.He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: meta-analysis of controlled trials. Hypertension. 2006;48:861–869. doi: 10.1161/01.HYP.0000245672.27270.4a. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 5.Conlin PR, Chow D, Miller ER, Svetkey LP, Lin PH, Harsha DW, Moore TJ, Sacks FM, Appel LJ. The effect of dietary patterns on blood pressure control in hypertensive patients: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Am J Hypertens. 2000;13:949–955. doi: 10.1016/s0895-7061(99)00284-8. [DOI] [PubMed] [Google Scholar]
- 6.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 7.He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013:CD004937. doi: 10.1002/14651858.CD004937.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017;4:CD004022. doi: 10.1002/14651858.CD004022.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm RH, Neaton JD, Elmer PJ, Svendsen KH, Levin J, Segal M, Holland L, Witte LJ, Clearman DR, Kofron P. The influence of oral potassium chloride on blood pressure in hypertensive men on a low-sodium diet. N Engl J Med. 1990;322:569–574. doi: 10.1056/NEJM199003013220901. [DOI] [PubMed] [Google Scholar]
- 10.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997;277:1624–1632. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 11.Krishna GG, Miller E, Kapoor S. Increased blood pressure during potassium depletion in normotensive men. N Engl J Med. 1989;320:1177–1182. doi: 10.1056/NEJM198905043201804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.