Abstract
Background
Although inflammatory bowel disease (IBD) is a failure in maintaining tolerance to the intestinal microbiota, few studies have investigated the use of immunologic tolerance as a treatment approach for IBD. We hypothesized that induction of immune tolerance at a distal site could suppress intestinal inflammation through a process of bystander regulation.
Methods
Epicutaneous tolerance was induced by topical application of OVA using a Viaskin® patch for 48 h. In some experiments, a single feed of OVA was used to drive epicutaneous tolerance-induced Tregs to the intestine. The mechanism of tolerance induction was tested using neutralizing antibodies against TGF-β, IL-10, and Treg depletion using Foxp3-DTR mice. The capacity of skin-draining Tregs, or epicutaneous tolerance, to prevent or treat experimental IBD was tested using T cell transfer colitis, DSS colitis, and ileitis in SAMP-YITFc mice. Weight loss, colonic inflammatory cytokines and histology were assessed.
Results
Epicutaneous exposure to OVA induced systemic immune tolerance by a TGF-β-dependent, but IL-10 and iFoxp3+ Treg-independent mechanism. Skin draining Tregs suppressed the development of colitis. Epicutaneous tolerance to a model antigen prevented intestinal inflammation in the DSS and SAMP-YITFc models, and importantly could halt disease in mice already experiencing weight loss in the T cell transfer model of colitis. This was accompanied by a significant accumulation of LAP+ and Foxp3+ Tregs in the colon.
Conclusions
This is the first demonstration that epicutaneous tolerance to a model antigen can lead to bystander suppression of inflammation and prevention of disease progression in preclinical models of IBD.
Keywords: Epicutaneous tolerance, bystander suppression, Inflammatory bowel disease, regulatory T cells
Introduction
Inflammatory bowel disease (IBD) is believed to be the result of a failure to develop tolerance to normal gut bacteria in genetically predisposed individuals.1,2 Despite the central role of immune regulatory pathways in the pathogenesis of IBD, relatively few studies have examined the potential use of immune tolerance-inducing strategies for the treatment of IBD. Some studies utilizing oral tolerance have shown success in ameliorating disease using foreign antigens and intrinsic antigens like murine cecal antigen-1 and colonic extracted protein.3–7 Another rodent study failed to show induction of oral tolerance with antigen alone and showed that nasal tolerance induction was more efficacious for treating colitis.15 In addition, a drawback of the oral tolerance approach for the treatment of IBD is that patients with Crohn’s disease (CD) have suppressed capacity to generate immune tolerance via the oral route.8,9 We hypothesized that delivering antigen by alternative routes could be more effective for eventual use in human subjects.
The skin is a highly active immune organ capable of inducing effector cells,10 as well as protective immunity and immune tolerance.11–15 Epicutaneous immunotherapy delivering antigen with Viaskin® patches suppresses food allergy and eosinophilic esophagitis in mice by Treg-dependent mechanisms.16,17 Epicutaneous-induced tolerance (ET) to haptens can also suppress colitis induced by the same hapten.18,19 We investigated whether ET to a model dietary protein antigen could suppress colitis in an antigen-nonspecific or bystander manner, which is essential for application of ET as a therapeutic strategy in IBD where inciting antigens are multiple or unknown.
We demonstrate that ET can be achieved by epicutaneous antigen exposure to the same extent as that which is induced orally in a TGF-β-dependent manner. Importantly we show that ET can abrogate intestinal inflammation in murine models of IBD via bystander suppression.
Materials and Methods
Mice
BALB/c, C57BL/6, and CD45.1 mice were obtained from Jackson Laboratories (Bar Harbor, ME). OTII/RAG1−/−, RAG1−/−, and Foxp3-GFP-DTR mice were obtained from existing colonies at The Icahn School of Medicine at Mount Sinai. For all mice, bedding was exchanged between experimental groups and where possible littermates were used in experiments to control for possible differences in colonic flora. Experiments were carried out using age and gender matched groups. All mice were maintained under specific pathogen-free conditions and all procedures were approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee.
Oral and Epicutaneous Exposure
To induce tolerance, mice were exposed orally by gavage feeding with OVA 1mg daily for 5 consecutive days. For epicutaneous exposure, mice were anesthetized and dorsal fur removed with depilatory cream (Veet, Reckitt Benckiser, Parsippany, NJ), followed the day after by application of 100 µg of OVA or PBS imbedded in an antigen delivery device, Viaskin® (DBV Technologies, France) for 48 hours.13 Viaskin consists of a central transparent plastic membrane charged with electrostatic forces and therefore is able to retain dry antigen in the central part. When applied to the skin, it creates an occlusive chamber, which generates moisture and allows the antigen to be absorbed by intact skin.16,20 Antigen passes intracellularly and is take up by dendritic cells that induce Tregs.16,21,22
Immunization of mice and measurement of antigen specific cytokines
Three days after oral or epicutaneous exposure, mice were immunized bilaterally in the hock with 1mg OVA plus complete Freund’s adjuvant (Sigma, St. Louis, MO) using glass syringes and 27G needles. On day 15, mice were boosted with 1mg OVA plus incomplete Freund’s adjuvant. Mice were sacrificed on day 22. Hock draining lymph nodes were harvested and cells put into culture with 100µg/mL of OVA for 72 hours. Supernatants were harvested and IFN-γ, IL-10, IL-5 and IL-13 levels measured by ELISA per the manufacturer’s instructions (BD Biosciences, San Jose, CA).
Ablation of induced Foxp3+ Tregs
Foxp3-GFP-DTR mice on a C57BL/6 background23 were exposed on the skin as described above. Three days later the experimental group was injected with 75 ng per gram body weight of DT (Sigma) to deplete any OVA-specific Foxp3+ Tregs that were induced by the application of OVA-Viaskin. Control mice were not given DT. Depletion of greater than 90% of Foxp3+ cells was confirmed by flow cytometry using antibodies against CD4 (clone GK1.5) and CD25 (clone PC61) (Affymetrix, Waltham, MA) and examining CD4+CD25+ cells for GFP positivity the following day. The total Treg compartment was allowed to rebound prior to immunization. Thus, induced Tregs, but not total Tregs were targeted using this approach as previously described.24
IL-10R and TGF-β Neutralization
ET was induced as described above. Mice were given either anti-IL-10R (200µg) or anti-TGF-β (1mg) antibodies or an isotype control (BioXcell, West Lebanon, NH) via IP injection six hours prior to hock immunizations as described above.
Co-transfer of CD25+ T cells from the SLN or MLN
Cells were obtained from spleens, MLN and SLN of C57BL/6 mice and CD4+ T cells were enriched by negative selection using a MACS micro bead system (Miltenyi, San Diego, CA). The resulting CD4+ enriched population from the spleens was labeled with APC-conjugated anti-CD4 (clone L3T4), PE-conjugated anti-CD62L (clone MEL-14) and FITC-conjugated anti-CD45RB antibodies (clone C363.16A) (Affymetrix). Subpopulations of CD4+ cells from spleens were sorted by flow cytometry to obtain naïve T cells. Splenic CD4+CD45RBhi T cells (3.5 × 105) were adoptively transferred by intraperitoneal injection into recipient littermate RAG1−/− mice. CD25+ cells were isolated from the MLN or SLN using the EasySep Mouse CD25 Regulatory T cell Positive Selection Kit according to the manufacturer’s instructions (Stemcell Technologies, Vancouver, Canada). CD25+ cells (3.5 × 105) from either MLN or SLN were co-transferred or not with splenic CD4+CD45RBhi T cells. Transfer was confirmed by flow cytometry of peripheral blood in all mice after 2 weeks. Weights were recorded semi-weekly. All mice were sacrificed once any mouse lost 20% of their initial weight. Colonic histology was scored and colonic tissue was cultured overnight. Cytokine secretion was measured in the supernatants (IL-4, IL-6, IL-10, IL-12, TNF-α, IFN-γ, and IL-17) by cytometric bead array per the manufacturer’s instructions (BD Biosciences).
DSS model of colitis
Six-week-old, littermate male C57BL/6 mice were exposed or not exposed on the skin to Viaskin-OVA once as described above and fed 1mg OVA to draw Tregs to the gastrointestinal tract. Two percent DSS (MP Biomedicals, Solon, OH) was given in the drinking water for 5 days and then normal drinking water was given back for another 5 days. Mice were weighed daily. Colonic histology was scored. Colonic tissue was cultured overnight and cytokine secretion was measured in the supernatants as described above. Colonic tissue was analyzed by rtPCR as described below.
SAMP/YITFc Model
Co-housed SAMP/YITFc mice were exposed or not exposed weekly for 5 weeks to Viaskin-OVA as described above starting at 4 weeks of age when they are known to develop ileal inflammation in our facility. This was followed by a single gavage feeding of 1mg of OVA to all mice to increase gut homing and activate Tregs. Mice were sacrificed and ileal tissue was analyzed for cytokines (IFN-γ, TNF-α, IL-10) by rtPCR and for histological inflammation.
CD45RbHI-OTII Transfer model of colitis
CD4+CD45RBhi T cells (3.5 × 105) were isolated from spleens of C57BL/6 mice as described above and were adoptively transferred by intraperitoneal injection into recipient 6–8-week-old, littermate RAG1−/− mice. Weights were monitored weekly. At week six after ensuring mice lost weight, CD4+ T cells were isolated from spleens of OTII/RAG1−/− mice, which do not have any mature T cells (or Tregs), and 3.5 × 105 cells were transferred by intraperitoneal injection into recipient RAG1−/− mice. T cells with OVA-specific TCRs were added since the initial small number of transferred cells was unlikely to contain any cells with an OVA-specific TCR. The mice were then exposed or not to Viaskin-OVA once a week for 3 weeks. This was followed 3 days later by gavage feeding all mice 1mg of OVA once to increase gut homing and activate Tregs. Weights were monitored, and at week 14 mice were sacrificed. Colonic histology was scored as described below. Colonic tissue was cultured overnight. Cytokine secretion was measured in the supernatants as described above. Lamina propria lymphocytes were isolated as described below and stained to look at T cell phenotype by flow cytometry. Colonic samples were also examined by rtPCR as described below.
Treg transfer into the CD45RbHI Transfer model of colitis
To expand the population of OVA-specific cells, C57BL/6 mice were immunized to OVA by IP injection of 200µl of 1mg/mL OVA mixed 1:1 with Alum IP once a week for two weeks followed by gavage feeding with 1mg OVA with 10µg cholera toxin once a week for two weeks.25 Following immunization, mice were tolerized with Viaskin-OVA weekly for 8 weeks to induce OVA-specific Tregs. CD25+CD4+ cells were isolated from the spleens using EasySep Mouse CD25 Regulatory T cell Positive Selection Kit (Stemcell Technologies). Total CD25+CD4+ Tregs from immunized and tolerized mice were then transferred into mice with established colitis as demonstrated by weight loss. As controls, CD25+CD4+ Tregs from mice that had not been tolerized were used. Tregs were co-transferred into RAG−/− mice receiving CD4+CD45RBhi T cells (3.5 × 105) to induced colitis. The day following Treg transfer, mice were gavage fed with 1mg of OVA. Weights were monitored and 3 weeks later mice were sacrificed.
Isolation and assessment of lamina propria lymphocytes
For producing a single cell suspension from the lamina propria, colons were flushed with HBSS medium, cut longitudinally and washed three times in 15ml ice-cold HBSS, 5% FBS and vortexed for 15–20 seconds. Tissues were incubated on a rocker for 20 min at 37°C in 25ml RPMI containing 5% FBS, 1mM dithiothreitol and 3mM Ethylenediaminetetraacetic acid. The remaining tissue was rinsed twice in RPMI, 5% FBS, and digested for 1 hr at 37°C with RPMI, 5% FBS, Collagenase D (1.6 mg/ml; Roche) and DNase I (0.5mg/ml; Roche). The isolated cells were homogenized using a 20g syringe and filtered through a 70µm cell strainer. Cells were washed twice in RPMI, 5% FBS. Cell pellets were resuspended in 8ml 44% iso-osmotic Percoll (GE Healthcare) in RPMI and transferred to FBS-coated 15 ml polystyrene tubes. Five ml of 66% iso-osmotic Percoll/RPMI was carefully layered below the cell layer using pasteur pipets. Tubes were then centrifuged for 20 min at 2,800 rpm, 4°C, with brakes off. Using a plastic collection pipet, interface cells were collected. Purified cells were washed and labeled with antibodies against CD45.1(clone A20), CD4(clone GK1.5), CD25(clone PC61), Foxp3(clone FJK-16s), and LAP(clone TW7-16B4) (Affymetrix or BioLegend, San Diego, CA). Samples were acquired on a BD LSRII Cell Analyzer (BD Biosciences), and data analyses were conducted with FlowJo software (FlowJo, Ashland, OR).
Quantitative real-time (RT) PCR
RNA was extracted from colonic specimens using Trizol (Life Technologies) followed by isopropanol precipitation. RNA was then reverse-transcribed to complementary DNA (cDNA) by M-MLV reverse transcriptase and random hexamers per the manufacturer’s protocol (Live Technologies). RT-PCR was performed using qPCR SYBR Green mix (Quiagen, Germantown, MD) and a VIIA7 real-time PCR system. Fold induction was calculated over control cells using the ΔΔCt method. Results were multiplied by one thousand for better visual representation. Primer sequences are listed in Supplementary Table 1.
Histological Scoring of Intestinal Inflammation
Histological scoring was done on one complete cross-section of a Swiss roll26 of colon or ileum for each mouse. The severity of colitis and ileitis was scored by two independent pathologists blinded to the treatment groups by examining the involvement of the epithelium, mucosa, submucosa, and muscularis. A score was also added to indicate the presence and extent of ulcerations. A total score was calculated by adding the individual scores for a maximal score of 20.27
Statistics
Differences between groups were analyzed by Mann-Whitney T-test when two groups were present or by ANOVA when comparing multiple groups. This was followed by either non-parametric Mann-Whitney U-test or Bonferroni analysis when appropriate. Data analysis was done using Prism software (GraphPad, San Diego, CA). A value of p<0.05 was considered statistically significant. P values are indicated by *p<0.05, **p<0.01, ***p<0.001.
Results
Systemic immune tolerance is induced by epicutaneous antigen exposure
To determine if epicutaneous exposure to OVA could induce systemic immune tolerance, mice were exposed to OVA by Viaskin® patch or OVA orally by gavage and subsequently immunized to OVA (Fig 1A). OVA-specific IFN-γ production was examined in the draining lymph node and was significantly suppressed by prior oral or epicutaneous exposure to OVA (Fig 1B). Prior epicutaneous exposure to OVA also caused a significant increase in OVA-specific IL-10 production (Fig 1B). The Th2 cytokines IL-5 and IL-13 were not altered (data not shown). Thus, the epicutaneous route effectively induces systemic immune tolerance to a model antigen.
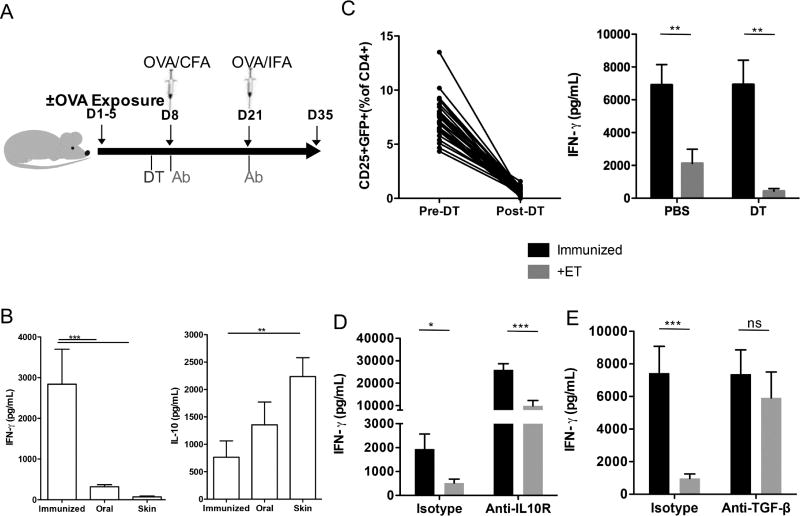
Figure 1. Epicutaneous exposure to OVA induced systemic tolerance in a TGF-β dependent manner.
A. Experimental protocol: mice were exposed to OVA on days 1–5 and subsequently immunized on days 8 and 21. To determine the mechanism, mice were given neutralizing antibodies 6 hours prior to immunization or Foxp3-GFP-DTR mice were given DT the day prior to immunization. B. OVA-specific IFN-γ and IL-10 measured by ELISA in mice not exposed (immunized) or exposed orally (oral) or on the skin to OVA (Viaskin) prior to immunization with OVA (n=3 experiments of 8–10 mice/group); C. Depletion of Foxp3+ T cells after administration of DT as measured by flow cytometry in the peripheral blood and OVA-specific IFN-γ measured by ELISA in mice not exposed (immunized) or exposed on the skin to OVA (+Viaskin) with depletion (DT) or without (PBS) depletion of induced Foxp3+ Tregs (n=3 pooled experiments of 4–5 mice/group); D. OVA-specific IFN-γ measured by ELISA in mice not exposed (immunized) or exposed on the skin to OVA (+Viaskin) with (Anti-IL10R) or without (Isotype) an IL-10R antibody (n=2 pooled experiments of 5–6 mice/group). E. OVA-specific IFN-γ measured by ELISA in mice not exposed (immunized) or exposed on the skin to OVA (+Viaskin) with (Anti-TGF-β) or without (Isotype) a TGF-β antibody (n=2 pooled experiments of 5 mice/group). (*p≤0.05, **p≤0.01, ***p≤0.001)
ET is mediated by TGF-β-dependent but IL-10 and Foxp3+ iTreg –independent mechanisms
To determine the role of Tregs and Treg-associated molecules in ET, we used neutralizing anti-IL-10 or TGF-β antibodies or Foxp3-DTR mice (Fig 1A). Foxp3-DTR mice were given DT after OVA exposure to ablate all Foxp3+ Tregs including those induced by OVA (Fig 1C), but the total Foxp3+ Treg population was allowed to recover prior to immunization. This depletion approach, which effectively suppresses oral tolerance,24 had no effect on ET (Fig 1C). Similarly, neutralization of IL-10 had no impact on ET (Fig 1D). In contrast, neutralization of TGF-β completely abrogated the suppressive effect of ET on OVA-specific IFN-γ production (Fig 1E).
This indicates that TGF-β, but not IL-10 or induced Foxp3+ Tregs, is required for ET.
Skin-derived Tregs prevent colitis
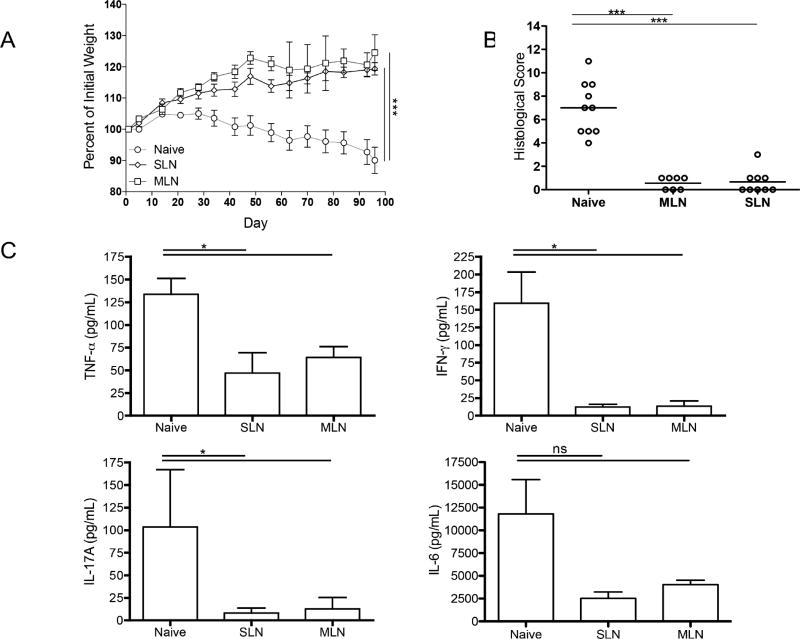
We next sought to determine if ET could suppress colitis. However, we first examined the capacity of Tregs isolated from the skin-draining LNs to suppress colitis. We compared the capacity of skin-derived or intestinal-derived CD25+ Tregs to suppress the development of colitis when co-transferred with CD45RBhi T cells into Rag1−/− mice. Mice receiving CD25+ cells from either SLN or MLN maintained growth curves, while mice receiving CD45RBhi T cells without Tregs lost weight (Fig 2A). Treg transfer from either site resulted in reduced histologic inflammation in the colon compared to mice receiving no Tregs (Fig 2B). This correlated with suppressed secretion of inflammatory cytokines (TNF-α, IFN-γ and IL-17) by the colon by skin-derived Tregs (Fig 2C).
Figure 2. Skin-derived Tregs prevent colitis.
A. Weight curves of RAG1−/− mice given CD4+CD45+RBhi T cells isolated from C57BL/6 mice without (Naïve) or with CD4+CD25+ T cells isolated from either skin-draining LNs (SLN) or mesenteric LNs (MLN) B. Histological scores of colonic specimens from RAG1−/− mice given CD4+CD45+RBhi T cells isolated from C57BL/6 mice without (Naïve) or with CD4+CD25+ T cells isolated from either SLNs or MLNs C. Cytokine production measured by ELISAs from the supernatant of colonic specimens cultured overnight from RAG1−/− mice given CD4+CD45+RBhi T cells isolated from C57BL/6 mice without (Naïve) or with CD4+CD25+ T cells isolated from either SLNs or MLNs. (n=2 pooled experiments of 3–5 mice/group) (*p≤0.05, **p≤0.01, ***p≤0.001)
Thus, skin-derived Tregs can effectively suppress the development of colitis.
Prevention and treatment of colitis and ileitis by ET
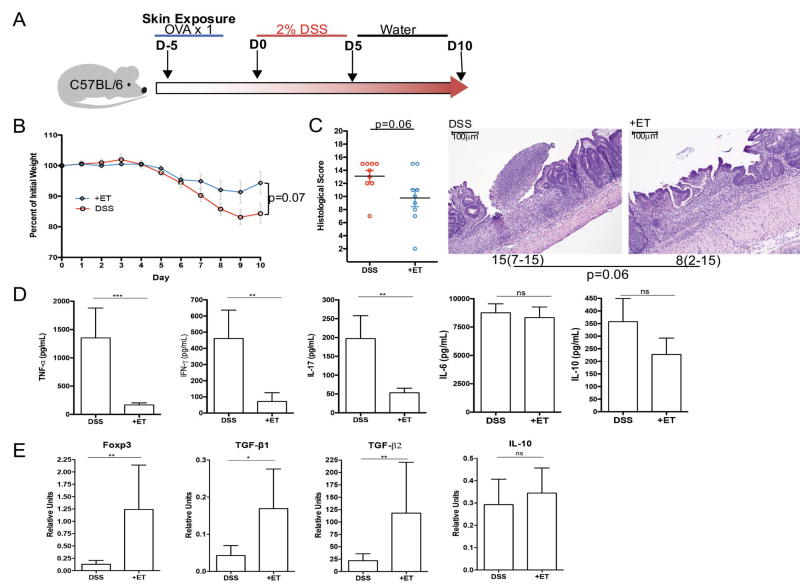
We applied ET to three pre-clinical models to test the potential of ET for prevention or treatment of IBD. We began by utilizing an epithelial injury model of colitis caused by dextran sodium sulfate (DSS) in the drinking water (Fig 3A). Mice treated with ET showed a significant reduction in the production of TNF-α, IFN-γ, and IL-17A by the inflamed colon (Fig 3D). ET-treated mice had increased mRNA expression of Foxp3, TGFβ1, and TGFβ2 but not IL-10 in the colon compared to untreated mice receiving DSS (Fig 3E). Despite this immunomodulation, histological damage and weight loss were not significantly affected in this acute injury model (Fig 3B and 3C).
Figure 3. Epicutaneous tolerance abrogates colitis in the DSS model.
A. Experimental protocol: C57BL/6 mice were exposed once to OVA on the skin and then given 5 days of 2% DSS in the drinking water followed by 5 days of normal water. B. Weight curves for mice exposed to OVA (+ET) or not (DSS) prior to DSS treatment. C. Histological scores for colons from mice exposed to OVA (+ET) or not (DSS) prior to DSS treatment and median histological scoring of colon specimens with ranges in parenthesis from mice exposed to OVA (+ET) or not (DSS) prior to DSS treatment shown under a representative H&E sections of colon demonstrating less severe inflammation and ulceration in mice exposed to OVA (scale bar = 100µm). D. Cytokine production as measured by ELISA from supernatants of colon cultures from mice exposed to OVA (+ET) or not (DSS) prior to DSS treatment. E. Colonic expression of regulatory markers as measured by rtPCR from mice exposed to OVA (+ET) or not (DSS) prior to DSS treatment. (n=2 independent experiments of 4–5 mice/group) (*p≤0.05, **p≤0.01, ***p≤0.001)
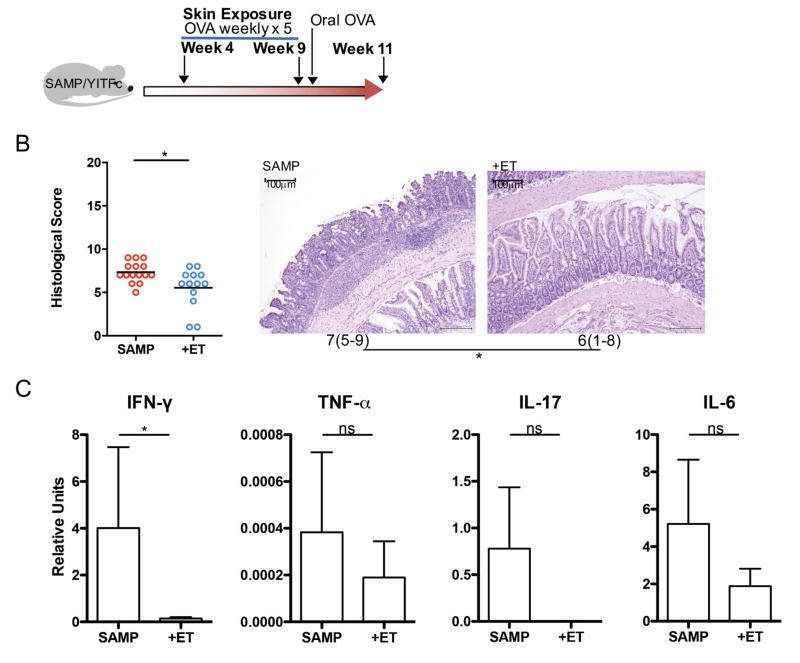
We also tested the impact of ET in a spontaneous model of ileitis, the SAMP/YITFc model (Fig 4A). These mice develop modest inflammation in the ileum at 4 weeks of age but do not lose weight in our facility (data not shown). SAMP-YITFc mice treated with ET had significantly decreased histological inflammation (Fig 4B) and expression of the predominant inflammatory cytokine in the intestine of this model, IFN-γ (Fig 4C) as compared to untreated littermate controls.
Figure 4. Epicutaneous tolerance treats ileitis in SAMP/YITFc mice.
A: Experimental protocol: SAMP/YITFc mice were exposed or not to OVA on the skin weekly for 5 weeks starting at 4 weeks old. This was followed by a one-time oral exposure to OVA in all mice. B: Histological scores for ileums from SAMP/YITFc mice exposed (+ET) or not (SAMP) to OVA and median histological scoring of ileum specimens with ranges in parenthesis from SAMP/YITFc mice exposed (+ET) or not (SAMP) to OVA shown under a representative H&E section of ileum demonstrating less severe inflammation and villus blunting in those mice exposed to OVA (scale bar = 100µm). C: Inflammatory cytokines IFN-γ, TNF-α, IL-17 and IL-6 as measured by rtPCR in mice not exposed (SAMP) or exposed to OVA (+ET). (n= 2 pooled experiments of 5–6 mice/group) (*p≤0.05)
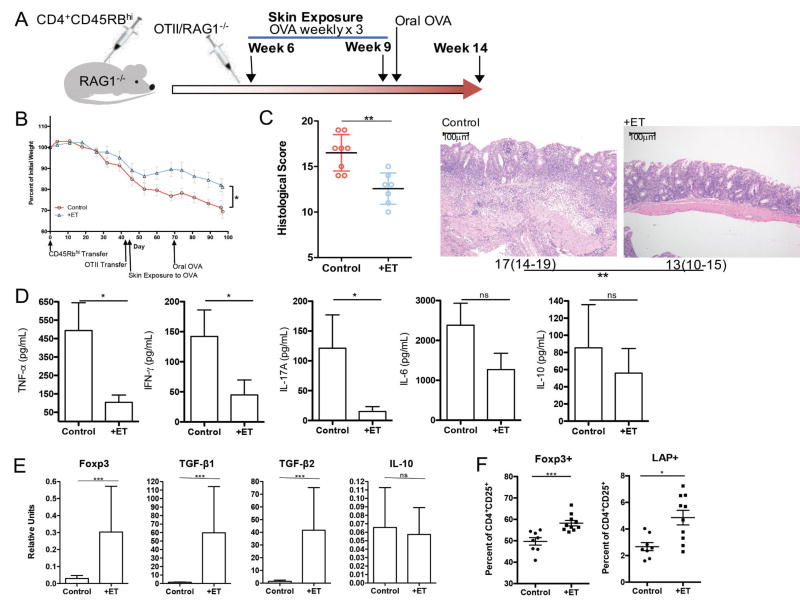
We subsequently utilized a CD45RBhi T cell transfer model to determine if ET could effectively halt the progression of disease. Rag1−/− mice that had received CD45RBhi CD4+ T cells and had begun to lose weight were treated with ET or left untreated, followed by a single gavage with OVA to induce homing of Tregs to the gastrointestinal tract (Fig 5A). ET-treated mice were significantly protected from further weight loss as compared to untreated controls (Fig 5B). Histological inflammation was also significantly decreased in mice treated with ET (Fig 5C). The colonic production of TNF-α, IFN-γ and IL-17 was significantly reduced compared to untreated mice (Fig 5D). Consistent with previous data using Viaskin-OVA for food allergy,22 ET was associated with a significant increase in mRNA expression of Foxp3, TGF-β1, and TGFβ2 in the colonic tissue, but no change in IL-10 (Fig 5E). By flow cytometry, we also identified a significant increase in CD4+CD25+Foxp3+ as well as CD4+CD25+LAP+ Tregs within the colonic lamina propria of ET-treated mice (Fig 4F).
Figure 5. Epicutaneous tolerance treats colitis in the T cell Transfer Model.
A. Experimental protocol: RAG1−/− mice were injected with CD4+CD45+RBhi T cells isolated from C57BL/6 mice and OTII/RAG1−/− mice followed by skin exposure weekly for 3 weeks to OVA. This was followed by a one-time oral exposure to OVA in all mice. B. Weight curves of mice exposed on the skin exposure to OVA (+ET) or not (Control). C. Histological score for colons from mice exposed on the skin exposure to OVA (+ET) or not (Control) and median histological scoring of colon specimens with ranges in parenthesis from mice exposed on the skin exposure to OVA (+ET) or not (Control) shown under a representative H&E section of colon demonstrating less severe inflammatory infiltrate in treated mice (scale bar = 100µm). D. Cytokine production as measured by ELISA from supernatants of colon cultures from mice exposed to OVA (+ET) or not (Control). E. Colonic regulatory marker expression as measured by rtPCR from mice exposed to OVA (+ET) or not (Control). F. Colon lamina propria Treg percentages assessed by flow cytometry in mice exposed to OVA (+ET) or not (control). (n=2 pooled experiments of 4–5 mice/group) (*p≤0.05, **p≤0.01, ***p≤0.001)
These data demonstrate that ET effectively prevents and halts the progression of disease via bystander suppression in three unique pre-clinical models of IBD.
OVA-specific Tregs mediate the reduction in inflammation in the transfer model of colitis
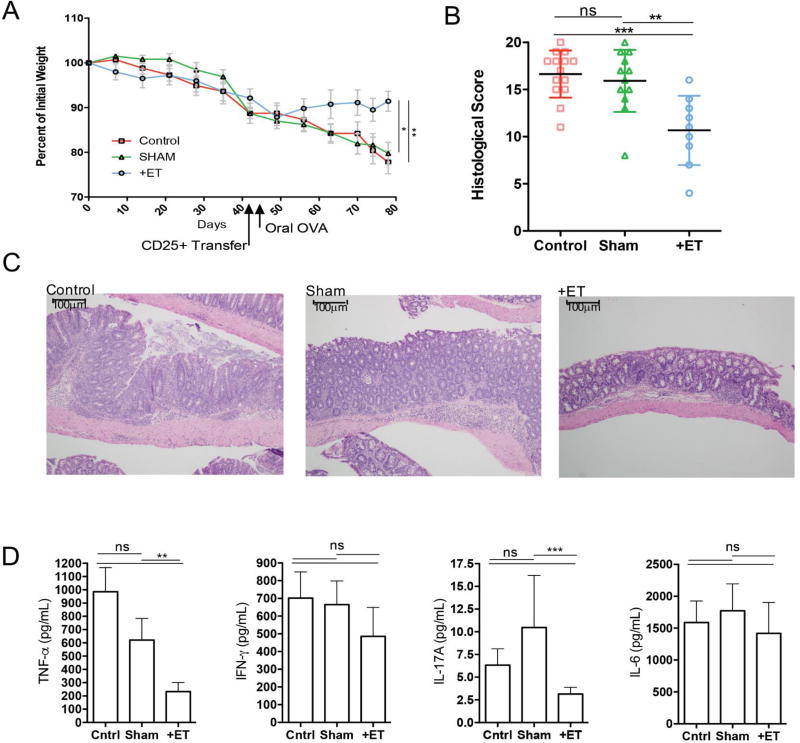
To determine the role of antigen experienced Tregs in the suppression of colitis by ET, we transferred CD25+CD4+ Tregs derived either from mice that had been treated with ET or left untreated into mice beginning to lose weight following CD45RBhi T cell transfer. Treg transfer into mice that are already symptomatic is not as efficient in suppressing disease as co-transfer is at preventing disease,28 which was confirmed by our transfer of Tregs from sham-treated mice. However, administration of Tregs derived from mice treated with ET significantly reduced weight loss (Fig 6A), histological inflammation (Fig 6B and 6C) and colonic TNF-α and IL-17A production (Fig 6D) when compared to controls receiving no transfer. These data demonstrate that ET induces an enhanced suppressive capacity in Tregs that can terminate disease progression in T cell-mediated colitis.
Figure 6. OVA-specific Tregs mediate the reduction in inflammation in the transfer model of colitis.
A. Weight curves of mice with colitis and given CD25+ T cells from immunized and OVA (+ET) exposed mice, mice not exposed to OVA (SHAM) or mice not given CD25+ T cells (control). B. Histological scores for colon specimens from immunized and OVA (+ET) exposed mice, mice not exposed to OVA (SHAM) or mice not given CD25+ T cells (control). C. Median histological scoring of colon specimens with ranges in parenthesis from immunized and OVA (+ET) exposed mice, mice not exposed to OVA (SHAM) or mice not given CD25+ T cells (control) shown under a representative H&E section of colon demonstrating less severe inflammation in the ETI group as compared to control and Sham groups (scale bar = 100µm). D. Cytokine production as measured by ELISA from supernatants of colon cultures from immunized and OVA (+ET) exposed mice, mice not exposed to OVA (SHAM) or mice not given CD25+ T cells (control). (n=2 pooled experiments of 4–10 mice/group) (*p≤0.05, **p≤0.01, ***p≤0.001)
Discussion
We show for the first time that epicutaneous administration of a model protein antigen can prevent or halt disease in three distinct pre-clinical models of IBD. Importantly, the antigen used for ET was not involved in colitis pathogenesis, indicating that this was a process of bystander suppression. This is critical for the translation of our findings to human IBD, where antigens are poorly defined and it is likely that multiple antigens trigger inappropriate immune reactivity.
The use of immune tolerance induction to treat human IBD has been attempted through several approaches. Direct administration of the regulatory cytokine IL-10 to humans was not effective for the treatment of CD.29 Other drugs like anti-TNF-α therapies may indirectly increase Treg numbers and that this increase may correlate with clinical response.30–33 Desreumaux et al. showed that administration of in vitro-generated OVA-specific Tregs to patients with refractory CD was well tolerated and had dose-related efficacy. The OVA-specific immune response correlated with clinical response.34 Thus generation of active regulatory responses may be more effective than administration of regulatory cytokines in the treatment of CD. The generation of Tregs by administration of antigen to patients in vivo is likely to be safer and less costly than the generation of Tregs in vitro and reinfusion back to patients. However, induction of oral tolerance in patients with CD is significantly impaired.8,9 Therefore, alternative routes of tolerance induction may be required and our data support the epicutaneous route as a safe and effective route of induction of Tregs to suppress inflammation in IBD.
The epicutaneous route of tolerance induction has been extensively studied for the treatment of food allergy.17,25 There are also studies showing that ET can suppress colitis when haptens are used to induce tolerance and colitis.18,19 In both of these examples, there is antigen-specific immune suppression. However, there is evidence that Tregs can suppress immune responses to bystander antigens. For example, induction of ET to one dietary antigen can suppress the development of allergy to new dietary allergens.25 We observed that the induction of ET to OVA could suppress inflammation in three distinct pre-clinical models of IBD whose pathogenesis is unrelated to OVA. The DSS model is an acute injury model of colitis that models aspects of the innate inflammation observed in IBD. The SAMP/YITFc mice develop spontaneous ileitis and cecal inflammation mediated by monocytes and Th1 cells and is similar to human Crohn’s disease both clinically and histologically.35–37 The T cell transfer model recapitulates more aspects of human disease: a chronic, progressive disease with diarrhea and weight loss, heavily inflamed colon, and a Th1/Th17 dominated cytokine profile. In all three models, ET effectively suppressed inflammation, and importantly was able to halt the progression of disease in the T cell transfer model. Thus, for the first time we show that ET is feasible to prevent and treat pre-clinical models through a mechanism of bystander suppression.
Tolerance to microbial antigens requires IL-10, as illustrated by the spontaneous inflammation in IL-10-deficient mice. Tolerance to dietary antigens, shown in oral tolerance experiments, requires TGF-β. ET appears to be more similar to oral tolerance than microbial tolerance mechanistically. Interestingly, induced Foxp3+ T cells were not required. This is in contrast to oral tolerance, that is dependent on Foxp3+ Tregs. The source of TGF-β may be LAP+ Tregs, that contribute to oral tolerance through TGF-β dependent mechanisms38–41 and that we found to be increased in the colon of mice treated with ET. LAP+ Tregs have also been shown to contribute to suppression of food allergy in ET-treated mice.22 Additional studies are needed to determine the duration of the protective effect, as well as the mechanism of bystander suppression by Tregs of ET treated mice.
Within the clinical setting, the induction of Tregs via epicutaneous exposure to antigens may have a role as an adjuvant therapy to prevent the escalation of treatments or the need for surgical intervention. It also would be useful to help maintain remission. Interestingly, evidence demonstrates that antibodies to bacterial antigens are elevated up to 3 years before the development of CD.42,43 Thus with use of select antigens, we may be able to alter this aberrant immune response and prevent disease in at risk populations.
In conclusion, we show for the first time that epicutaneous tolerance to a model antigen can prevent and halt the progression of disease in three distinct pre-clinical models of IBD. We propose that this novel and safe approach to generate Tregs has the potential to be developed into an effective treatment for IBD.
Supplementary Material
Acknowledgments
In memory of Dr. Lloyd Mayer whose mentorship played a vital role in this project and who continues to inspire us. We also acknowledge the Center for Comparative Medicine and Surgery at the Icahn School of Medicine at Mount Sinai. Dr. Yeretssian is no longer affiliate with Mount Sinai and is currently employed by The Leona M. and Harry B. Helmsley Charitable Trust, but the article in no way represents the work, views or opinions of Helmsley.
Grant support: NIH 1 K08 DK102978-01A1, collaborative project support from DBV Technologies
Abbreviations
- ET
epicutanous tolerance
- MLN
mesenteric lymph node
- OVA
ovalbumin
- SLN
skin-draining lymph node
- Tregs
Regulatory T cells
Footnotes
Disclosures: DD, MCB, GY, LT, AI, TL VG, and JFC have no disclosures; LM, PHB and HAS are employed partially or fully by DBV Technologies
Author contributions: Conceived and designed the study: DD; Consulted on study design: MCB, LM, GY HAS; Performed the experiments: DD, ST, LT, AI, TL, VG; Analyzed and interpreted the data: DD, ST; Contributed to the writing of the manuscript: DD, CB, LM, ST, GY, LT, AI, TL, VG, JFC, PHB, HAS
Contributor Information
David Dunkin, Division of Pediatric Gastroenterology and The Mindich Child Health and Development Institute (MCHDI), The Icahn School of Medicine at Mount Sinai (ISMMS), NY, NY, USA.
M. Cecilia Berin, Division of Pediatric Allergy and Immunology, The Immunology Institute and MCHDI, ISMMS, NY, NY, USA.
Lucie Mondoulet, DBV Technologies, Bagneux, France.
Steven Tobar, Division of Pediatric Gastroenterology and MCHDI, ISMMS, NY, NY, USA.
Garabet Yeretssian, The Icahn School of Medicine at Mount Sinai, Immunology Institute and Tisch Cancer Institute, New York, NY, USA.
Leticia Tordesillas, Division of Pediatric Allergy and Immunology, The Immunology Institute and MCHDI, ISMMS, NY, NY, USA.
Alina Iuga, Department of Pathology, Columbia University Medical School, NY, NY.
Thibaut Larcher, National Veterinary School, Nantes, France.
Virginia Guillespie, Department of Comparative Pathology, ISMMS, NY, NY.
Pierre-Henri Benhamou, DBV Technologies, Bagneux, France.
Jean-Frederic Colombel, Division of Gastroenterology and Co-Director of the IBD Center, ISMMS, NY, NY, USA.
Hugh A. Sampson, Division of Pediatric Allergy and Immunology, The Immunology Institute and MCHDI, ISMMS, NY, NY and CSO, DBV Technologies, Bagneux, France.
Reference List
- 1.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128(7):1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 2.Torres J, Mehandru S, Colombel JF, et al. Crohn's disease. Lancet. 2016 doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 3.Mori K, Yamanishi H, Ikeda Y, et al. Oral administration of carbonic anhydrase I ameliorates murine experimental colitis induced by Foxp3-CD4+CD25− T cells. J Leukoc Biol. 2013;93(6):963–72. doi: 10.1189/jlb.1212612. [DOI] [PubMed] [Google Scholar]
- 4.Shan-Shan Z, Yu-Lan L. Therapeutic effects of mucosal tolerance on experimental colitis in rats. Eur J Gastroenterol Hepatol. 2009;21(10):1145–52. doi: 10.1097/MEG.0b013e32830edb29. [DOI] [PubMed] [Google Scholar]
- 5.Yue M, Shen Z, Yu CH, et al. The therapeutic role of oral tolerance in dextran sulfate sodium-induced colitis via Th1–Th2 balance and gammadelta T cells. J Dig Dis. 2013;14(10):543–51. doi: 10.1111/1751-2980.12068. [DOI] [PubMed] [Google Scholar]
- 6.Zhou P, Borojevic R, Streutker C, et al. Expression of dual TCR on DO11.10 T cells allows for ovalbumin-induced oral tolerance to prevent T cell-mediated colitis directed against unrelated enteric bacterial antigens. J Immunol. 2004;172(3):1515–23. doi: 10.4049/jimmunol.172.3.1515. [DOI] [PubMed] [Google Scholar]
- 7.Ben Ya'acov A, Lichtenstein Y, Zolotarov L, et al. The gut microbiome as a target for regulatory T cell-based immunotherapy: induction of regulatory lymphocytes by oral administration of anti-LPS enriched colostrum alleviates immune mediated colitis. BMC Gastroenterol. 2015;15:154. doi: 10.1186/s12876-015-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraus TA, Cheifetz A, Toy L, et al. Evidence for a genetic defect in oral tolerance induction in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(2):82–8. doi: 10.1097/01.MIB.0000200343.61707.52. discussion 81. 10.1097/01.MIB.0000200343.61707.52. [DOI] [PubMed] [Google Scholar]
- 9.Kraus TA, Toy L, Chan L, et al. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126(7):1771–8. doi: 10.1053/j.gastro.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 10.Dunkin D, Berin MC, Mayer L. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. J Allergy Clin Immunol. 2011;128(6):1251–58. e2. doi: 10.1016/j.jaci.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azukizawa H, Dohler A, Kanazawa N, et al. Steady state migratory RelB+ langerin+ dermal dendritic cells mediate peripheral induction of antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells. Eur J Immunol. 2011;41(5):1420–34. doi: 10.1002/eji.201040930. [DOI] [PubMed] [Google Scholar]
- 12.Majewska-Szczepanik M, Yamamoto N, Askenase PW, et al. Epicutaneous immunization with phosphorylcholine conjugated to bovine serum albumin (PC-BSA) and TLR9 ligand CpG alleviates pneumococcal pneumonia in mice. Pharmacol Rep. 2014;66(4):570–5. doi: 10.1016/j.pharep.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Mondoulet L, Dioszeghy V, Ligouis M, et al. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy. 2010;40(4):659–67. doi: 10.1111/j.1365-2222.2009.03430.x. [DOI] [PubMed] [Google Scholar]
- 14.Mondoulet L, Dioszeghy V, Vanoirbeek JA, et al. Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int Arch Allergy Immunol. 2011;154(4):299–309. doi: 10.1159/000321822. [DOI] [PubMed] [Google Scholar]
- 15.Van Kampen KR, Shi Z, Gao P, et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005;23(8):1029–36. doi: 10.1016/j.vaccine.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 16.Dioszeghy V, Mondoulet L, Dhelft V, et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol. 2011;186(10):5629–37. doi: 10.4049/jimmunol.1003134. [DOI] [PubMed] [Google Scholar]
- 17.Mondoulet L, Dioszeghy V, Larcher T, et al. Epicutaneous immunotherapy (EPIT) blocks the allergic esophago-gastro-enteropathy induced by sustained oral exposure to peanuts in sensitized mice. PLoS One. 2012;7(2):e31967. doi: 10.1371/journal.pone.0031967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt T, Lorenz N, Raker V, et al. Epicutaneous and Oral Low-Zone Tolerance Protects from Colitis in Mice. J Invest Dermatol. 2016;136(9):1831–9. doi: 10.1016/j.jid.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 19.Majewska-Szczepanik M, Goralska M, Marcinska K, et al. Epicutaneous immunization with protein antigen TNP-Ig alleviates TNBS-induced colitis in mice. Pharmacol Rep. 2012;64(6):1497–504. doi: 10.1016/s1734-1140(12)70947-7. [DOI] [PubMed] [Google Scholar]
- 20.Dupont C, Kalach N, Soulaines P, et al. Cow's milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. 2010;125(5):1165–7. doi: 10.1016/j.jaci.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 21.Mondoulet L, Dioszeghy V, Puteaux E, et al. Intact skin and not stripped skin is crucial for the safety and efficacy of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl Allergy. 2012;2(1):22. doi: 10.1186/2045-7022-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tordesillas L, Mondoulet L, Blazquez AB, et al. Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 24.Hadis U, Wahl B, Schulz O, et al. Intestinal Tolerance Requires Gut Homing and Expansion of FoxP3(+) Regulatory T Cells in the Lamina Propria. Immunity. 2011;34(2):237–46. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Mondoulet L, Dioszeghy V, Puteaux E, et al. Specific epicutaneous immunotherapy prevents sensitization to new allergens in a murine model. J Allergy Clin Immunol. 2015;135(6):1546–57. e4. doi: 10.1016/j.jaci.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 26.Moolenbeek C, Ruitenberg EJ. The "Swiss roll": a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15(1):57–9. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 27.Castaneda FE, Walia B, Vijay-Kumar M, et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129(6):1991–2008. doi: 10.1053/j.gastro.2005.09.017. doi: S0016-5085(05)01801-9 [pii] 10.1053/j.gastro.2005.09.017 [published Online First: 2005/12/14] [DOI] [PubMed] [Google Scholar]
- 28.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170(8):3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 29.Buruiana FE, Sola I, Alonso-Coello P. Recombinant human interleukin 10 for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2010(11) doi: 10.1002/14651858.CD005109.pub3. CD005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armuzzi A, Pugliese D, Danese S, et al. Infliximab in steroid-dependent ulcerative colitis: effectiveness and predictors of clinical and endoscopic remission. Inflamm Bowel Dis. 2013;19(5):1065–72. doi: 10.1097/MIB.0b013e3182802909. [DOI] [PubMed] [Google Scholar]
- 31.Boschetti G, Nancey S, Sardi F, et al. Therapy with anti-TNFalpha antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):160–70. doi: 10.1002/ibd.21308. [DOI] [PubMed] [Google Scholar]
- 32.Di Sabatino A, Biancheri P, Piconese S, et al. Peripheral regulatory T cells and serum transforming growth factor-beta: relationship with clinical response to infliximab in Crohn's disease. Inflamm Bowel Dis. 2010;16(11):1891–7. doi: 10.1002/ibd.21271. [DOI] [PubMed] [Google Scholar]
- 33.Kato K, Fukunaga K, Kamikozuru K, et al. Infliximab therapy impacts the peripheral immune system of immunomodulator and corticosteroid naive patients with Crohn's disease. Gut Liver. 2011;5(1):37–45. doi: 10.5009/gnl.2011.5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desreumaux P, Foussat A, Allez M, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn's disease. Gastroenterology. 2012;143(5):1207–17. e1–2. doi: 10.1053/j.gastro.2012.07.116. [DOI] [PubMed] [Google Scholar]
- 35.Corridoni D, Kodani T, Rodriguez-Palacios A, et al. Dysregulated NOD2 predisposes SAMP1/YitFc mice to chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2013;110(42):16999–7004. doi: 10.1073/pnas.1311657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosiewicz MM, Nast CC, Krishnan A, et al. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease. J Clin Invest. 2001;107(6):695–702. doi: 10.1172/JCI10956. [published Online First: 2001/03/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pizarro TT, Pastorelli L, Bamias G, et al. SAMP1/YitFc mouse strain: a spontaneous model of Crohn's disease-like ileitis. Inflamm Bowel Dis. 2011;17(12):2566–84. doi: 10.1002/ibd.21638. [published Online First: 2011/05/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oida T, Zhang X, Goto M, et al. CD4+CD25− T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170(5):2516–22. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Kuchroo VK, Inobe J, et al. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265(5176):1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 40.Miller A, Lider O, Roberts AB, et al. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992;89(1):421–5. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faria AM, Weiner HL. Oral tolerance and TGF-beta-producing cells. Inflamm Allergy Drug Targets. 2006;5(3):179–90. doi: 10.2174/187152806778256034. [DOI] [PubMed] [Google Scholar]
- 42.Israeli E, Grotto I, Gilburd B, et al. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut. 2005;54(9):1232–6. doi: 10.1136/gut.2004.060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choung RS, Princen F, Stockfisch TP, et al. Serologic microbial associated markers can predict Crohn's disease behaviour years before disease diagnosis. Aliment Pharmacol Ther. 2016;43(12):1300–10. doi: 10.1111/apt.13641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.