Abstract
Objectives
To characterize clinical and imaging features in patients with pathologically confirmed demyelinating lesions.
Methods
In this retrospective chart review, we analyzed clinical-radiological-pathological correlations in patients >15 years old who underwent brain biopsy at our institution between 2000–2015 and had inflammatory demyelination on neuropathology.
Results
Of 31 patients, the mean age was 42 years (range 16 to 69 years) and 55% were female. All but one of the biopsied lesions were considered tumefactive demyelinating lesions (TDLs) by imaging criteria, measuring >2 cm on contrast-enhanced brain MRI. On clinical follow-up, the final diagnosis was a CNS malignancy in 2 patients (6.5%). In patients without malignant tumor, the TDL was solitary in 12 (41%) and multifocal in 17 (59%), with contrast enhancement in all but one case, primarily in an incomplete rim enhancement pattern (75.9%). Of 16 patients with at least 12 months of clinical follow-up, 7 (43.8%) had a clinical relapse. Of patients without a prior neurologic history, relapse occurred in 2/7 (29%) in solitary TDL and 2/6 (33%) in multifocal lesions at initial presentation. Recurrent TDLs occurred in 3 patients, all with initially solitary TDLs. Stratifying by CSF analysis, 4 of 6 patients (67%) with either an elevated IgG Index or >2 oligoclonal bands suffered a clinical relapse compared to 2/8 (25%) with non-inflammatory CSF.
Conclusions
Pathologically confirmed TDLs call for careful clinical correlation, clinical follow-up and imaging surveillance. Although sometimes clinically monophasic, tumefactive demyelinating lesions carried nearly a 45% risk of near-term clinical relapse in our study, even when presenting initially as a solitary mass lesion.
Keywords: Multiple Sclerosis, Tumefactive, Demyelination, MRI, Neuropathology, Brain biopsy
1. Introduction
Tumefactive demyelinating lesions (TDLs) are defined radiologically as tumor- or mass-like white matter dominant lesions exceeding 2 cm in size [1]. On MRI, TDLs can be solitary or multifocal and often exhibit peri-lesional edema, a rim of reduced diffusion, and rim or irregular peripheral enhancement following the administration of intravenous gadolinium contrast [1,2]. TDLs can be challenging to diagnose on radiological grounds alone owing to their imaging similarity to malignant brain tumor, abscess and autoimmune or vasculitic processes.
The prevalence of TDLs has been reported to be 1–2 per 1000 cases of multiple sclerosis (MS), making it an uncommon presentation of demyelinating disease [1]. TDLs have also been associated with atypical presentations of neuromyelitis optica (NMO) and acute disseminated encephalomyelitis (ADEM) [1,3–5]. However, many patients with TDLs never completely fulfill diagnostic criteria for these conditions, and the tumefactive demyelinating event may be a standalone diagnosis [1,5–8].
One of the earliest published case series suggested that TDLs, unlike typical MS lesions, carry only about a 10% risk for relapse or conversion to clinically-definite MS [6]. However, a subsequent report, based on 168 patients, reported a risk of relapse or conversion to MS of 70% [1]. In other published case series, the rate of relapse and conversion to clinically-definite MS varies substantially, making it challenging for clinicians to counsel patients with TDLs about short or longer-term prognosis [3,5,7–12]. Limited data exist regarding factors associated with risk of relapse following TDL presentation. Potential risk factors suggested by other authors include age at onset, enhancement pattern, and the presence of other typical MS lesions [7,8]. However, study design has varied considerably between publications, especially as some studies included patients with an existing diagnosis of multiple sclerosis prior to development of a TDL, which can influence or bias analysis of relapse risk [1,3,7–10]. Many studies also incorporate data from patients diagnosed radiologically as TDL without pathologic confirmation, some of whom may in fact have alternate diagnoses [3,6,9,12].
Clinicians are also sometimes faced with the conundrum of evaluating a patient with previously unsuspected inflammatory demyelination on brain biopsy, such as following biopsy of a mass lesion initially may have been suspected to be a high-grade glioma. Counseling brain biopsy-confirmed TDL patients about diagnosis, prognosis and preventive immunomodulatory or immunosuppressive therapy can be challenging given the minimal and variable evidence base, especially when there is no other evidence of MS or another autoimmune or demyelinating condition.
The primary aim of our study was to identify patients with pathologically confirmed demyelinating lesions, and evaluate clinical and radiographic factors associated with risk of clinical relapse. We hypothesized that: (1) solitary TDLs would carry a lower risk of relapse than multifocal TDL presentations; and (2) inflammatory CSF profile would be associated with a higher risk of relapse. We also evaluated for potential contributory comorbidities, because numerous TDL case reports highlight potential associations and risk factors ranging from various cancers, autoimmune diseases, human immunodeficiency virus (HIV), and exposure to certain medications [6,13–22].
2. Methods
2.1 Study Cohort
In this retrospective chart review, we identified patients older than 15 years of age who underwent brain biopsy at UCSF between January 2000 and December 2015 and received a final neuropathological diagnosis of inflammatory demyelination. Subjects under age 15 were excluded from this analysis, as TDLs in younger patients may have different clinical characteristics and epidemiology [23]. We elected to include a single case of inflammatory demyelination in which the radiologic criterion for a TDL of 2 cm was not met, as the starting point of the analysis was all patients with pathologically confirmed demyelination. The UCSF Committee on Human Research approved the study protocol for retrospective chart review.
2.2 Clinical Characteristics
Demographic and clinical variables were abstracted from the electronic health record by a MS trained neurologist (MT) into a case review form. The following details were recorded: pre-morbid medical history, age at biopsy, sex, race, clinical or imaging relapse, CSF immunoglobulin index, presence of oligoclonal bands, and serum aquaporin 4 IgG testing. Clinical relapse was defined as a new clinical event consistent with a typical inflammatory demyelinating disease or multiple sclerosis relapse lasting at least 24 hours and occurring at least 30 days from the index demyelinating event.
2.3 Neuroimaging
MR images were analyzed by a neuroradiologist (SC) and neuroradiology fellow (JEV), and the following variables were recorded: lesion number, lesion location, presence of edema, enhancement pattern, lesion appearance (solid, cystic, or mixed) and interval appearance of lesions following biopsy. Follow-up imaging was also reviewed. Radiological progression was defined as the appearance of enlarging or new T2/FLAIR or gadolinium enhancing lesions at least 30 days after biopsy.
2.4 Pathological Evaluation
All cases originally reported as compatible with a demyelinating lesion were identified through a pathology database search, and pathology materials were subjected to a central review by one of the authors (TT). Routine sections, special stains and immunohistochemical studies performed as a part of the original pathology work-up was re-evaluated and the cases were classified as having inflammatory demyelination or “other.” Additional special stains or immunohistochemical studies were also performed when necessary for inclusion/exclusion to the study. Cases without sufficient pathology material and with a pathological diagnosis other than inflammatory demyelination were excluded. In cases where there was subsequent material, this material was also reviewed and recorded. We excluded cases in which the brain biopsy was performed and/or read-out at another institution, even if the patient was subsequently evaluated clinically at UCSF. We also excluded autopsy-only cases.
2.5 Statistical Analysis
Summary statistics were calculated. Comparisons of categorical variables were performed utilizing the Fisher exact test. STATA 12 (StataCorp LP, College Station, TX) was used for these analyses.
3. Results
We identified 31 patients with brain biopsy-confirmed inflammatory demyelinating lesions. On clinical follow-up, 2 (6.5%) patients went on to have confirmed neoplastic diagnoses based on clinical follow-up and additional diagnostics: 1 with diffuse astrocytoma with a gliomatosis pattern of spread (discovered at autopsy 1 year after the biopsy that showed demyelination) and another with primary CNS lymphoma (confirmed by a second brain biopsy 12 weeks later). Review of initial conventional MRI imaging was unable to discriminate between demyelination and neoplastic process in either of these subjects. However, follow-up imaging in both cases demonstrated elevated cerebral blood volume on dynamic susceptibility contrast perfusion sequences that subsequently favored neoplasm rather than demyelination. Both of these patients were excluded from the following analysis of relapse risk given these alternative diagnoses.
Of the remaining 29 patients, ages ranged from 16 to 69 years, with a mean age of 41.6 years; 51.7% were female (Table 1). All but one patient had at least one TDL on imaging review, with one biopsied patient lacking a lesion >2 cm. The demyelinating lesion diagnosed on brain biopsy was the first neurological event for 18 of the patients, while 3 carried a history of a prior demyelinating event, including 1 with prior optic neuritis and 2 already carrying a diagnosis of multiple sclerosis. Serum anti-aquaporin4 (AQP4) IgG results were available for 13/29 patients in our demyelinating cohort, all of whom tested negative for the autoantibody. Serum testing for antibodies against myelin oligodendrocyte glycoprotein (anti-MOG) was not available for any of the subjects in our cohort. While the importance of anti-MOG testing in NMO spectrum disorders and other demyelinating disease phenotypes including TDLs has since been highlighted in emerging literature,[24] MOG testing was not performed in any of these patients and was not clinically available during the epoch under study. Glucocorticoids were administered in 69% of patients (70% after biopsy, 20.7% at various time points before biopsy). Plasma exchange was administered to 48% of patients, typically due to an inadequate or incomplete response to corticosteroids. Immunomodulation with MS-specific therapies or other immunosuppressive therapies was initiated in 71% of patients either at discharge or during follow-up. Clinical relapses occurred in 40% of patients, with a median follow-up duration of 22 months. Mean expanded disability status scale (EDSS) was 3.5 at last follow-up.
Table 1.
Non-malignant brain biopsy-confirmed inflammatory-demyelination: Study Cohort
| Demographics | (n=29) |
|
| |
| Age in years, mean (Range) | 41.4 (16–69) |
|
| |
| Sex (%female) | 51.7% |
|
| |
| Race/Ethnicity | |
|
| |
| Caucasian | 13 (44.8%) |
|
| |
| Asian | 6 (20.7%) |
|
| |
| Black | 2 (6.9%) |
|
| |
| Other/Unknown | 8 (27.6%) |
|
| |
| Clinical History | |
|
| |
| Prior Demyelination Events | 3 (10.3%) |
|
| |
| Prior Neurologic History* | 8 (27.6%) |
|
| |
| Radiographic Features | |
|
| |
| Lesion Number | |
|
| |
| Solitary | 12 (41.4%) |
|
| |
| Multifocal | 17 (58.6%) |
|
| |
| Edema | 23 (79.3%) |
|
| |
| Enhancement | 28 (96.6%) |
|
| |
| Incomplete Rim | 22 (75.9%) |
|
| |
| Nodular | 4 (13.8%) |
|
| |
| Patchy | 2 (6.9%) |
|
| |
| Radiating/Linear | 2 (6.9%) |
|
| |
| Complete Rim | 1 (3.4%) |
|
| |
| Spinal Lesions (n=24) | 6 (25%) |
| Laboratory Features | |
| Serum anti-AQP4 IgG (n=13) | 0 (0%) |
|
| |
| Treatment | |
|
| |
| Glucocorticoids | 20 (69.0%) |
|
| |
| Plasma Exchange | 14 (48.1%) |
|
| |
| Immunosuppressive therapy (n=21) | 15 (71.4%) |
|
| |
| Prognosis | |
|
| |
| Follow-up duration, months (median) | 22 |
|
| |
| Clinical Relapse | |
|
| |
| All Subjects# | 8 (40%) |
|
| |
| ≥12 months follow-up (n=16) | 7 (43.8%) |
|
| |
| MRI Progression (n=22) | |
|
| |
| All Subjects | 9 (40.9%) |
|
| |
| ≥12 months follow-up (n=16) | 6 (37.5%) |
|
| |
| Recurrent TDL | 3 (13.6%) |
|
| |
| Last EDSS, mean (SD) | 3.5 (2.4) |
Prior Neurological History included suspected stroke (n=1), seizure disorder (n=3), autoimmune encephalitis (n=1), facial palsy (n=1), paraparesis (n=1), optic neuritis (n=1).
20 patients with available follow-up.
On review of baseline brain MR imaging, there was a solitary TDL in 12 (41%) and multifocal lesions in 17 (59%) (Table 1). Edema surrounded the lesions in 23 cases (79%). Enhancement following the administration of gadolinium was observed in all but one of the 29 cases reviewed. Patterns of enhancement were classified as follows from most to least prevalent: incomplete rim (75.9%), nodular (13.8%), patchy (6.9%), radiating/linear (6.9%), and complete rim enhancement (3.4%) (Figure 1). Spinal lesions were less common, with only 6 of 24 (25%) patients with spinal imaging demonstrating abnormalities (1 with a longitudinally extensive lesion, 4 with enhancing lesions typical of demyelinating disease and 1 with a patchy T2 hyperintensity). Imaging progression, defined as development of new lesions over time, was observed in 41% of patients with follow-up imaging available (n = 22). Recurrent TDLs were observed in 3 patients (13.6%), all of whom presented initially with a solitary TDL. A recurrent solitary TDL occurred in one patient with smoldering multiple myeloma that had recently transformed to active myeloma, and in another patient with recurrent Balo’s concentric sclerosis-like lesions. The third patient had concurrent myelitis at initial presentation, but multifocal recurrence, and is suspected to have a seronegative NMO spectrum disorder.
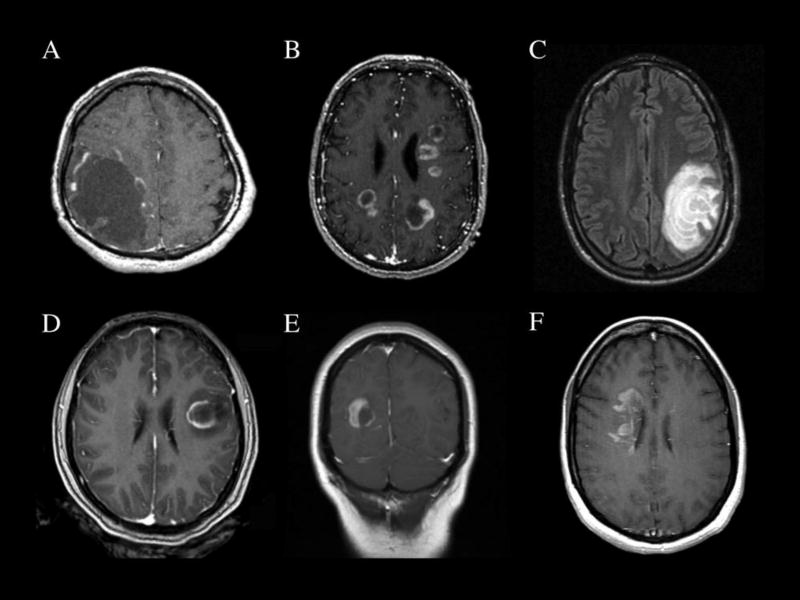
Figure 1.
Imaging Patterns of Brain-biopsy Confirmed Tumefactive Demyelinating Lesions: A) Solitary TDL with cystic appearance (T1 post-gadolinium); B) multifocal demyelinating lesions (T1 post-gadolinium); C) Balo-like tumefactive demyelinating lesion showing concentric rings of demyelination (FLAIR). Enhancement patterns can include: D) Incomplete rim enhancement; E) nodular enhancement; F) and patchy enhancement (D–F, T1 post-gadolinium). Each scan is from a different individual, none of whom were later diagnosed with a malignancy on follow-up.
In order to test the hypothesis that multifocal TDL presentations carry a higher risk of relapse, patients diagnosed with TDL as a first neurological event (n = 18) were grouped as solitary or multifocal based on their baseline imaging presentation (Table 2). The rate of relapse was similar between the groups, with 2 (29%) of 7 solitary and 2 (33.3%) of 6 multifocal TDL patients suffering a clinical relapse within the follow-up period. Imaging progression was seen in 40% and 43%, respectively. The only parameters for which a difference was seen between patients with solitary versus multifocal presentations were CSF protein and rate of treatment with intravenous corticosteroids and immunosuppression, neither of which is useful in clinical practice as a prognostic variable. However, we observed that subjects with solitary TDLs were predominantly male and trended toward an older age of onset.
Table 2.
Solitary versus Multifocal Brain Biopsy-Confirmed TDL presenting as a first lifetime neurological event.
| Solitary TDL (n=10) |
Multifocal Lesions (n=8) |
||
|---|---|---|---|
| Demographics | |||
| Age in years, mean (SD) | 45.9 (18.1) | 36.8 (13.9) | ns |
| Sex (%female) | 30% | 75% | P=0.15 |
| Radiographic Features | |||
| Enhancement n(%) | 10 (100%) | 8 (100%) | |
| Edema n(%) | 10 (100%) | 5 (62.5%) | P=0.07 |
| Spinal Lesions n(%) | 1 (12.5%) | 2 (28.6%) | ns |
| Laboratory Features | |||
| CSF Studies | |||
| Elevated IgG/OCB n(%) | 0 (0%) | 4 (66.7%) | P=0.06 |
| WBC (cells/µL) mean (SD) | 11.2 (21.7) | 10 (11.7) | ns |
| Protein (mg/dL) mean (SD) | 102 (75.6) | 39.1 (13.0) | P=0.04* |
| Glucose (mg/dL) mean (SD) | 70 (32.6) | 62.5 (18.9) | ns |
| Treatment n (%) | |||
| IV Methylprednisolone | 3 (30%) | 7 (87.5%) | P=0.02* |
| Plasma Exchange | 2 (20%) | 5 (62.5%) | ns |
| MS Disease Modifying Therapy | 2 (28.6%) | 7 (87.5%) | P=0.04* |
| Prognosis | |||
| Median follow-up duration in months | 20 | 24 | ns |
| Clinical Relapse n(%) | 2 (28.6%) | 2 (33.3%) | ns |
| MRI Progression n(%) | 4 (40%) | 3 (42.9%) | ns |
| Last EDSS, mean (SD) | 2.8 (2.3) | 3.6 (2.7) | ns |
A CSF exam with at least 2 unique oligoclonal bands or elevated IgG Index was associated with a trend toward higher relapse rate when compared to patients without evidence of intrathecal immunoglobulin synthesis but did not reach statistical significance (Table 3). Of patients without at least 2 oligoclonal bands or an elevated IgG index, 25% later relapsed, and 33% developed new lesions on follow-up imaging.
Table 3.
Inflammatory versus Non-Inflammatory CSF profile and risk of relapse following a brain biopsy-confirmed TDL. Inflammatory CSF profile was defined as having elevated IgG Index or ≥2 oligoclonal bands. Non-inflammatory CSF was defined by normal IgG Index and <2 oligoclonal bands.
| No inflammation on CSF Exam (n=11) |
Intrathecal inflammation on CSF Exam (n=10) |
||
|---|---|---|---|
| Demographics | |||
| Age in years, mean (SD) | 42.5 (15.8) | 33.8 (12.0) | |
| Sex (%female) | 36.4% | 70% | ns |
| Radiographic Features | |||
| Lesion Number | |||
| Solitary n(%) | 5 (45.5%) | 1 (10%) | P=0.15 |
| Multifocal n(%) | 6 (54.5%) | 9 (90%) | |
| Spinal Lesions n(%) | 2 (22.2%) | 3 (30%) | ns |
| Treatment | |||
| DMT n(%) | 6 (66.7%) | 8 (100%) | ns |
| Prognosis | |||
| Median follow-up in months | 19.5 | 47 | P=0.08 |
| Clinical Relapse n(%)# | 2/8 (25%) | 4/6 (66.7%) | ns |
| MRI Progression n(%) | 3/9 (33.3%) | 4/6 (66.7%) | ns |
| Last EDSS, mean (SD) | 2.8 (2.3) | 4 (2.6) | ns |
Patients with available follow-up.
4. Discussion
Of all brain biopsy confirmed demyelinating lesions diagnosed at our institution over a 15-year period in patients age 15 and older, 6.5% went on to have a neoplastic diagnosis on clinical follow-up. Consistent with our results, multiple other case reports have described confirmed tumefactive demyelination on brain biopsy in patients that turned out to have a final diagnosis of glioma or primary CNS lymphoma [25–29]. Notably, the patient in our study subsequently diagnosed with primary CNS lymphoma had >5 unique oligoclonal bands on CSF evaluation, a finding common in MS. Clinical-pathological correlation and close clinical and imaging follow-up, including dedicated imaging protocols optimized to distinguish demyelination from malignancy when clinically indicated, is recommended when the neuropathological diagnosis is inflammatory demyelination, especially when the diagnosis of a demyelinating disease is not otherwise suspected [2].
The results from our study suggest that the rate of clinical relapse and imaging progression following tumefactive demyelinating lesions is considerably higher than reported in the initial study by Kepes et al [6] – and higher than what we had hypothesized going into this analysis – but similar to many other reports in the published literature [1,3,6–12]. In our series, patients with solitary TDLs were at a similar risk of relapse or imaging progression to those with multiple lesions at the time of biopsy. Intrathecal immunoglobulin synthesis was observed much more frequently in patients with multifocal MRI presentations, and together this was associated with a trend toward increased risk of relapse but was not statistically significant. Importantly, none of the patients in our series with solitary TDLs that relapsed on clinical follow-up had inflammatory CSF, suggesting that the absence of oligoclonal bands or other markers of intrathecal inflammation is not necessarily reassuring in the context of a solitary TDL. Taken together, the results of our study suggest that the risk of relapse following the initial diagnosis or TDL is comparable to other typical clinically isolated syndrome (CIS) presentations, and close follow-up and individualized consideration of a preventive disease modifying therapy is advisable [30]. However, as with CIS generally, some patients with TDL end up having a monophasic course on longer-term follow-up.
In our analysis, we reviewed past medical histories and comorbidities for possible associations with TDLs owing to anecdotal observations within our center as well as published case reports of TDLs occurring in the setting of various cancers [6,13,15,25,26,28]. In our series, we identified two patients with a known history of monoclonal gammopathy, one with monoclonal gammopathy of unknown significance (MGUS) who did not relapse with 18 months of follow-up, and another with smoldering multiple myeloma whose TDL occurred temporally in association with the conversion to active myeloma and who went on to have another TDL relapse. Additional co-morbidities of note included a patient with a prior history of optic neuritis who developed a tumefactive relapse during the postpartum period (leading to a diagnosis of relapsing-remitting MS and who clinically stabilized with Rituximab), one patient who developed a TDL 2 years after suffering from anti-NMDAR encephalitis, and another patient with a TDL that occurred immediately after waking up from general anesthesia for an elective procedure and did not relapse or have radiological disease progression over more than 2 years of follow-up. Other comorbidities reported in the existing literature include autoimmune diseases (systemic lupus erythematosus, Sjogren’s syndrome) and HIV,[16,17,20–22] none of which was observed in our series during the period under study although diagnostic protocols were not standardized in this retrospective real-world analysis. In some instances, TDLs may represent a rebound phenomenon or very severe relapse manifestation of MS, as with postpartum presentations and reports during transition off certain disease modifying therapies such as natalizumab and fingolimod [31–33], though such cases do not typically make it to brain biopsy given the clinical context and therefore were not captured in our analysis. Despite reports of NMOSD with AQP4 antibodies presenting as a TDL [3,34], none of the patients in our cohort tested positive for aquaporin-4 antibodies, although some of the cases occurred during a time period before such testing was available or commonplace.
Limitations of our study include the relatively small sample size (although comparable with many other published series on this topic), limited follow-up duration and some missing data given that this was a retrospective chart review. While a strength of our study is inclusion of only pathologically-proven cases, our results may not be generalizable to suspected TDL cases that do not proceed to biopsy.
5. Conclusions
Our study supports the recommendation that patients with TDLs confirmed by brain biopsy receive close follow-up, because clinical relapse is fairly common and misdiagnosis can occur even after pathologically confirmation, probably due to sampling bias of tissue biopsied. Inflammatory CSF is associated with a risk of relapse approaching 67% in our study, but relapses can occur in patients with non-inflammatory CSF, particularly if there is a solitary TDL. Given numerous reports of malignancy in association with TDL presentation, evaluation for malignancy and possibly monoclonal gammopathy in patients presenting with TDL at an atypical age for demyelinating disease should be considered. While no seropositive NMO spectrum disorders were identified in our series, testing for anti-aquaporin-4 antibodies is advisable and MOG-antibodies may also be considered. Longer-term follow-up and prospective studies are needed to clarify risk of relapse, overall prognosis and the potential role for preventive immunosuppression in varied clinical presentations of TDLs.
Highlights.
-
○
Tumefactive demyelinating lesions (TDLs) carry a substantial risk of relapse, regardless of lesion number or size at presentation
-
○
Some patients with solitary TDLs relapse again with TDLs
-
○
Pathologically confirmed tumefactive demyelination can sometimes be associated with a final malignant diagnosis
-
○
Pathologically confirmed inflammatory demyelinating lesions call for careful follow-up
Acknowledgments
Funding: Dr. Tremblay was supported by a National Multiple Sclerosis Society Institutional Clinician Training award. Javier E. Villanueva-Meyer was supported by NIH grant T32 EB001631-12.
Dr. Gelfand reports research support to UCSF from Genentech, MedDay and Quest Diagnostics; prior compensation for consulting for Genentech; and compensation for medical legal consulting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Tremblay reports no disclosures. Dr. Villanueva-Meyer reports no disclosures. Dr. Cha reports no disclosures. Dr. Tihan reports no disclosures.
References
- 1.Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, Thomsen K, Mandrekar J, Altintas A, Erickson BJ, König F, Giannini C, Lassmann H, Linbo L, Pittock SJ, Brück W. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131:1759–1775. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mabray MC, Cohen BA, Villanueva-Meyer JE, Valles FE, Barajas RF, Rubenstein JL, Cha S. Performance of apparent diffusion coefficient values and conventional MRI features in differentiating tumefactive demyelinating lesions from primary brain neoplasms. Am. J. Roentgenol. 2015;205:1075–1085. doi: 10.2214/AJR.14.13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong IH, Kim SH, Hyun JW, Joung AR, Cho HJ, Kim HJ. Tumefactive demyelinating lesions as a first clinical event: Clinical, imaging, and follow-up observations. J. Neurol. Sci. 2015;358:118–124. doi: 10.1016/j.jns.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Hardy TA, Tobin WO, Lucchinetti CF. Exploring the overlap between multiple sclerosis, tumefactive demyelination and Balos concentric sclerosis. Mult. Scler. J. 2016:1–7. doi: 10.1177/1352458516641776. [DOI] [PubMed] [Google Scholar]
- 5.Wattamwar PR, Baheti NN, Kesavadas C, Nair M, Radhakrishnan A. Evolution and long term outcome in patients presenting with large demyelinating lesions as their first clinical event. J. Neurol. Sci. 2010;297:29–35. doi: 10.1016/j.jns.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Kepes JJ. Large focal tumor-like demyelinating lesions of the brain: Intermediate entity between multiple sclerosis and acute disseminated encephalomyelitis? A study of 31 patients. Ann. Neurol. 1993;33:18–27. doi: 10.1002/ana.410330105. [DOI] [PubMed] [Google Scholar]
- 7.Kilic AK, Kurne AT, Oguz KK, Soylemezoglu F, Karabudak R. Mass lesions in the brain: tumor or multiple sclerosis? Clinical and imaging characteristics and course from a single reference center. Turk. Neurosurg. 2013;23:728–35. doi: 10.5137/1019-5149.JTN.7690-12.3. [DOI] [PubMed] [Google Scholar]
- 8.Wallner-Blazek M, Rovira A, Fillipp M, Rocca MA, Miller DH, Schmierer K, Frederiksen J, Gass A, Gama H, Tilbery CP, Rocha AJ, Flores J, Barkhof F, Seewann A, Palace J, Yousry T, Montalban X, Enzinger C, Fazekas F. Atypical idiopathic inflammatory demyelinating lesions: Prognostic implications and relation to multiple sclerosis. J. Neurol. 2013;260:2016–2022. doi: 10.1007/s00415-013-6918-y. [DOI] [PubMed] [Google Scholar]
- 9.Altintas a, Petek B, Isik N, Terzi M, Bolukbasi F, Tavsanli M, Saip S, Boz C, Aydin T, Arici-Duz O, Ozer F, Siva a. Clinical and radiological characteristics of tumefactive demyelinating lesions: follow-up study. Mult. Scler. J. 2012;18:1448–1453. doi: 10.1177/1352458512438237. [DOI] [PubMed] [Google Scholar]
- 10.Nagappa M, Taly AB, Sinha S, Bharath RD, Mahadevan A, Bindu PS, Saini JS, Prasad C, Shankar SK. Tumefactive demyelination: Clinical, imaging and follow-up observations in thirty-nine patients. Acta Neurol. Scand. 2013;128:39–47. doi: 10.1111/ane.12071. [DOI] [PubMed] [Google Scholar]
- 11.Kuan YC, Wang KC, Yuan WH, Tsai CP. Tumefactive Multiple Sclerosis in Taiwan. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siri A, Carra-Dalliere C, Ayrignac X, Pelletier J, Audoin B, Pittion-Vouyovitch S, Debouverie M, Lionnet C, Viala F, Sablot D, Brassat D, Ouallet JC, Ruet A, Brochet B, Taillandier L, Bauchet L, Derache N, Defer G, Cabre P, de Seze J, Lebrun Frenay C, Cohen M, Labauge P. Isolated tumefactive demyelinating lesions: diagnosis and long-term evolution of 16 patients in a multicentric study. J. Neurol. 2015;262:1637–1645. doi: 10.1007/s00415-015-7758-8. [DOI] [PubMed] [Google Scholar]
- 13.Broadfoot JR, Archer HA, Coulthard E, Appelman APA, Sutak J, Braybrooke JP, Love S. Paraneoplastic tumefactive demyelination with underlying combined germ cell cancer. Pract. Neurol. 2015;15:451–455. doi: 10.1136/practneurol-2015. [DOI] [PubMed] [Google Scholar]
- 14.de Medeiros FC, de Albuquerque LAF, Pittella JEH, de Souza RB, Gomes Neto AP, Christo PP. Open-Ring Enhancement in Pseudotumoral Multiple Sclerosis: Important Radiological Aspect. Case Rep. Neurol. Med. 2014;2014:1–5. doi: 10.1155/2014/951690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahta A, Qu Y, Nastic D, Sundstrom M, Kim RY, Saria M, Santagata S, Kesari S. Relapsing tumefactive lesion in an adult with medulloblastoma previously treated with chemoradiotherapy and stem cell transplant. Pathol. Oncol. Res. 2012;18:539–543. doi: 10.1007/s12253-011-9464-x. [DOI] [PubMed] [Google Scholar]
- 16.Solomon IH, Perrin RJ, Clifford DB, Ances BM. Tumefactive demyelination in a patient with human immunodeficiency virus. J. Neurovirol. 2013;19:265–269. doi: 10.1007/s13365-013-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uriel A, Stow R, Johnson L, Varma A, du Plessis D, Gray F, Herwadkar A, Holland J, Lewthwaite P, Wilkins E. Tumefactive demyelination-an unusual neurological presentation of HIV. Clin. Infect. Dis. 2010;51:1217–1220. doi: 10.1086/656812. [DOI] [PubMed] [Google Scholar]
- 18.Cereda CW, Zecca C, Mazzucchelli L, Valci L, Staedler C, Bassetti CL, Gobbi C. Tumefactive demyelinating lesions during etanercept treatment requiring decompressive hemicraniectomy. Mult. Scler. J. 2013;19:820–823. doi: 10.1177/1352458512461969. [DOI] [PubMed] [Google Scholar]
- 19.Vaknin-Dembinsky A, Bdolah Y, Karussis D, Rosenthal G, Petrou P, Fellig Y, Abramsky O, Lossos A. Tumefactive demyelination following in vitro fertilization (IVF) J. Neurol. Sci. 2015;348:256–258. doi: 10.1016/j.jns.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Ben Sassi S, Nabli F, Boubaker A, Ben Ghorbel I, Neji S, Hentati F. Pseudotumoral brain lesion as the presenting feature of primary Sjögren’s syndrome. J. Neurol. Sci. 2014;339:214–216. doi: 10.1016/j.jns.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Verma R, Arya K. Tumefactive demyelination associated with systemic lupus erythematosus. Case Reports. 2012;2012:bcr0220125743–bcr0220125743. doi: 10.1136/bcr.02.2012.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindzen E, Jewells V, Bouldin T, Speer D, Royal W, Markovic-Plese S. Progressive tumefactive inflammatory central nervous system demyelinating disease in an acquired immunodeficiency syndrome patient treated with highly active antiretroviral therapy. J. Neurovirol. 2008:1–5. doi: 10.1080/13550280802304753. [DOI] [PubMed] [Google Scholar]
- 23.Hanumanthe SB, Francisco C, Hart J, Graves J, Waubant E. Biopsy-Supported Tumefactive Demyelination of the Central Nervous System in Children. J. Child Neurol. 2016;31:1528–1533. doi: 10.1177/0883073816664667. [DOI] [PubMed] [Google Scholar]
- 24.Jurynczyk M, Geraldes R, Probert F, Woodhall MR, Waters P, Tackley G, DeLuca G, Chandratre S, Leite MI, Vincent A, Palace J. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain. 2017;140:617–627. doi: 10.1093/brain/aww350. [DOI] [PubMed] [Google Scholar]
- 25.Golombievski EE, McCoyd MA, Lee JM, Schneck MJ. Biopsy Proven Tumefactive Multiple Sclerosis with Concomitant Glioma: Case Report and Review of the Literature. Front. Neurol. 2015;6:150. doi: 10.3389/fneur.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalus S, Di Muzio B, Gaillard F. Demyelination preceding a diagnosis of central nervous system lymphoma. J. Clin. Neurosci. 2016;24:146–148. doi: 10.1016/j.jocn.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto J, Shimajiri S, Nakano Y, Nishizawa S. Primary central nervous system lymphoma with preceding spontaneous pseudotumoral demyelination in an immunocompetent adult patient: A case report and literature review. Oncol. Lett. 2014;7:1835–1838. doi: 10.3892/ol.2014.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohe Y, Hayashi T, Mishima K, Nishikawa R, Sasaki A, Matsuda H, Uchino A, Tanahashi N. Central nervous system lymphoma initially diagnosed as tumefactive multiple sclerosis after brain biopsy. Intern. Med. 2013;52:483–8. doi: 10.2169/internalmedicine.52.8531. [DOI] [PubMed] [Google Scholar]
- 29.Roemer SF, Scheithauer BW, Varnavas GG, Lucchinetti CF. Tumefactive demyelination and glioblastoma: a rare collision lesion. Clin Neuropathol. 2011;30:186–191. doi: 10.5414/NP300201. [DOI] [PubMed] [Google Scholar]
- 30.JM G. Risk of multiple sclerosis after a clinically isolated syndrome: From magnetic resonance imaging to oligoclonal bands to activated t cells. JAMA Neurol. 2017;74:262–263. doi: 10.1001/jamaneurol.2016.5143. http://dx.doi.org/10.1001/jamaneurol.2016.5143. [DOI] [PubMed] [Google Scholar]
- 31.Faissner S, Hoepner R, Lukas C, Chan A, Gold R, Ellrichmann G. Tumefactive multiple sclerosis lesions in two patients after cessation of fingolimod treatment. Ther. Adv. Neurol. Disord. 2015;8:233–8. doi: 10.1177/1756285615594575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jander S, Turowski B, Kieseier BC, Hartung HP. Emerging tumefactive multiple sclerosis after switching therapy from natalizumab to fingolimod. Mult Scler. 2012;18:1650–1652. doi: 10.1177/1352458512463768. [DOI] [PubMed] [Google Scholar]
- 33.Verhaeghe A, Deryck OM, Vanopdenbosch LJ. Pseudotumoral rebound of multiple sclerosis in a pregnant patient after stopping natalizumab. Mult. Scler. Relat. Disord. 2014;3:279–281. doi: 10.1016/j.msard.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Popescu BFG, Guo Y, Jentoft ME, Parisi JE, Lennon VA, Pittock SJ, Weinshenker BG, Wingerchuk DM, Giannini C, Metz I, Brück W, Shuster EA, Carter J, Boyd CD, Clardy SL, Cohen BA, Lucchinetti CF. Diagnostic utility of aquaporin-4 in the analysis of active demyelinating lesions. Neurology. 2015;84:148–58. doi: 10.1212/WNL.0000000000001126. [DOI] [PMC free article] [PubMed] [Google Scholar]