Abstract
BACKGROUND
Although early reperfusion is the most desirable intervention after ischemic myocardial insult, it may add to damage through oxidative stress.
OBJECTIVES
We investigated the cardioprotective effects of a single intravenous dose of heat shock protein (HSP72) coupled to a single-chain variable fragment (Fv) of monoclonal antibody 3E10 (3E10Fv) in a rabbit ischemia-reperfusion model. The Fv facilitates rapid transport of HSP72 into cells, even with intact membranes.
METHODS
A left coronary artery occlusion (40 min), reperfusion (3 h) model was employed in 31 rabbits. Of these, 12 rabbits received the fusion protein (Fv-HSP72) intravenously. The remaining 19 control rabbits received a molar equivalent of 3E10Fv alone (n = 6), HSP72 alone (n = 6), or phosphate buffered saline (n = 7). Serial echocardiographic examinations were performed to assess left ventricular (LV) function before and after reperfusion. Micro-single photon emission computed tomography imaging of 99mTc-labeled annexin-V was performed with micro-computed tomography to characterize apoptotic damage in vivo, followed by gamma counting of the excised myocardial specimens to quantify cell death. Histopathological characterization of the myocardial tissue, and sequential cardiac troponin I measurements were also undertaken.
RESULTS
Myocardial annexin-V uptake was 43% lower in the area at risk (p = 0.0003) in Fv-HSP72-treated rabbits compared to controls receiving HSP72 or 3E10Fv alone. During reperfusion, troponin I release was 42% lower and the echocardiographic LV ejection fraction 27% higher in the Fv-HSP72-treated group compared to controls. Histopathological analyses confirmed penetration of 3E10Fv-containing molecules into cardiomyocytes in vivo, and treatment with Fv-HSP72 showed fewer apoptotic nuclei compared to control rabbits.
CONCLUSIONS
A single-dose administration of Fv-HSP72 fusion protein at the time of reperfusion reduced myocardial apoptosis almost by half, and improved LV functional recovery following myocardial ischemia-reperfusion injury in rabbits. It might have a potential to serve as an adjunct to early reperfusion in the management of myocardial infarction.
Keywords: apoptosis, cytoprotection, heat shock proteins, myocardial infarction, necrosis
Introduction
The standard of care for acute myocardial infarction (AMI) is timely reperfusion, usually by percutaneous coronary intervention (PCI) or thrombolytic therapy. During coronary occlusion, ischemic myocytes endure hypoxic stress and further injury by oxidative stress during reperfusion. The ischemic and oxidative stresses result in irreversible myocardial damage. Pharmacological interventions have been proposed to reduce ischemia-reperfusion injury, but have not demonstrated significant efficacy in multicenter clinical trials (1–4). The use of targeted biologics that facilitate rapid entry of the therapeutic agent into myocytes might offer enhanced efficacy of reperfusion therapy.
Heat shock proteins (HSP) are a family of protective chaperone proteins that ensure correct folding and stabilization of newly synthesized critical intracellular proteins or corrective refolding of proteins damaged by oxidative and other cellular stressors. The cardioprotective effects of endogenously produced HSP72 have been previously demonstrated in both in vitro and in vivo studies (5–9). However, more than 3 h can elapse between endogenous HSP induction by stress stimuli (or even gene therapy) and production of a sufficient amount of intracellular protein for cardioprotection in vivo (10). Messenger ribonucleic acid transcription of HSP70 family members peaks between 2 and 4 h with significant protein accumulation at 24 h (7). This delay reduces the potential efficacy of strategies that promote endogenous levels of HSP72, such as in the setting of AMI, wherein prompt early intervention is essential.
We propose that carrier-mediated, direct intracellular delivery of HSP72 may facilitate effective cardioprotection in the immediate aftermath of acute myocardial ischemic injury (11). A single-chain variable fragment (Fv) of a monoclonal antibody 3E10 binds deoxyribonucleic acid (DNA) and penetrates viable cells through the energy independent channel equilibrative nucleoside transporter 2 (ENT2), located in the plasma membrane. To enter cells, 3E10 must bind nucleosides from DNA (12); hence, its specificity for tissues undergoing damage where extracellular DNA is accessible. Fusion of the 3E10-Fv to human HSP72 (referred to as Fv-Hsp70 in earlier publications) has been tested in a rat ischemic brain model (13). Recombinant Fv-HSP72 protein demonstrated a more than two-thirds reduction in cell death and provided neuroprotection to cortical motor function.
The goal of the present study was to determine if Fv-HSP72 administration could preserve ischemic myocardium in a rabbit model of left coronary artery ischemia-reperfusion injury. Radiolabeled annexin-V (an in vivo imaging marker of apoptosis) (14), cardiac troponin (cTn) I levels, and serial echocardiographic assessment of left ventricular ejection fraction (LVEF) were employed as objective indicators of myocardial damage.
Methods
In a rabbit ischemia-reperfusion model, we compared the cardioprotective effect of the Fv-HSP72 fusion protein against 3E10-Fv alone or HSP72 alone. The sample of 31 rabbits (12 in the experimental group and 19 controls) was determined to be sufficient to detect an effect size of Cohen’s D = 1.25 (a group difference in means equivalent to 1.25 standard deviations for the outcome variable) assuming 80% power and α = 0.017 (applying the Bonferroni correction to account for 3 simultaneous comparisons and a familywise error rate of 0.05). Animals were randomly assigned to groups. All animals were included in the analysis. Figure 1 summarizes the series of tests and samples collected from each rabbit in the study.
Figure 1. The Experimental Protocol.
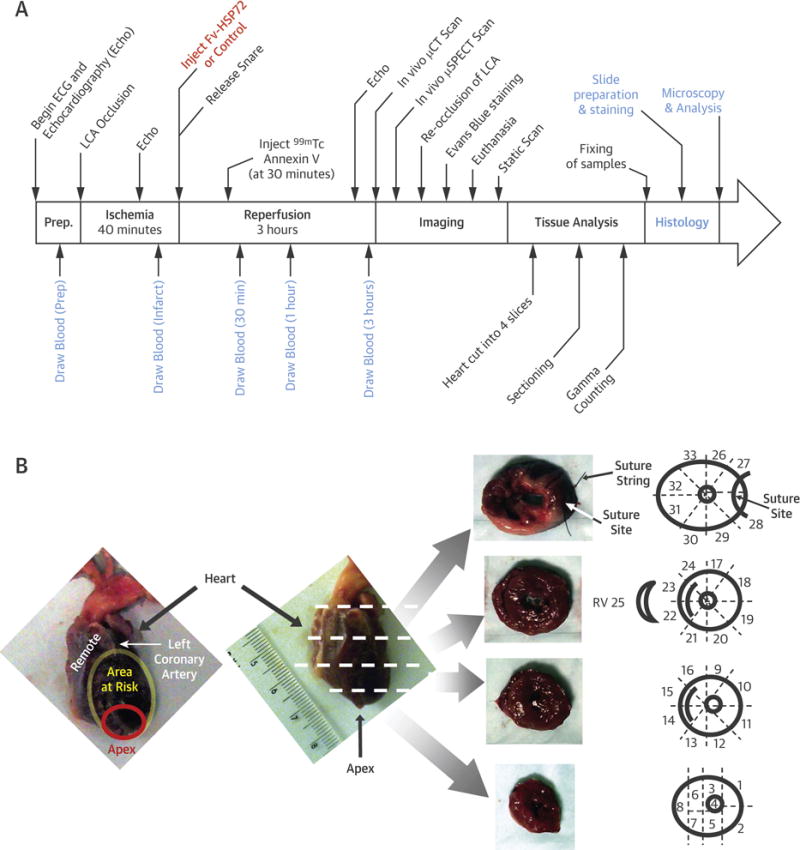
(A) Rabbits in each treatment group underwent the procedures in the timeline, with 40 min of ischemia followed by 3 h of reperfusion. (B) The territories of the heart investigated in this study were: 1) cells outside the left coronary artery (LCA) vascular bed were considered “remote” and acted as controls; 2) cells within the vascular bed of the LCA made up the area at risk; and 3) cells most vulnerable to damage during an LCA occlusion were located at the tip of the heart, a sub-region of the area at risk known as the apex. Sectioning of the heart into 4 slices followed by a systematic fragmentation (as illustrated) allowed for tracking of each piece through the gamma counting and histological procedures. μCT = micro-computed tomography; μSPECT = micro-single-photon emission computed tomographic; ECG = electrocardiogram; Fv-HSP72 = single-chain variable fragment 3E10-heat shock protein 72; RV = right ventricle.
Single-chain Fv was derived from monoclonal antibody 3E10. An Fv-HSP72 fusion protein was designed with the 3E10-Fv located at the N-terminal of the HSP72 protein. Complementary DNA for the Fv was ligated into the plasmid pPICZαA as previously described (15) and in the Online Appendix.
Recombinant human (rh)-annexin-V was obtained from a stock of wild-type annexin-V maintained at −80°C, produced by recombinant techniques under good laboratory practice conditions, as previously described. Annexin-V is a physiological protein that binds avidly to phosphatidylserine expressed on the outer leaflet of the cell membrane in apoptotic cells. Rh-annexin-V was derivatized with the nicotinic acid analog NHS-HYNIC (succinimidyl 6-hydrazinopyridine-3-carboxylate hydrochloride) at a 5.0:1.0 mol HYNIC-protein ratio which, after gentle mixing for 3 h at room temperature, resulted in a 0.9:1.0 mol HYNIC-protein product buffer using a previously described protocol (16). HYNIC is a bifunctional chelator with 1 moiety covalently binding the amino group on a lysine side chain on annexin-V and the other moiety conjugating to reduced 99mTc using tricine as a coligand, thereby producing a stable radiocomplex for molecular imaging.
To bind 99mTc to the HYNIC-annexin-V conjugate, 0.2 ml of a reduced tin (stannous ion)/tricine solution was added to a mixture of 20 to 30 mCi of 99mTc pertechnetate and 200 μg of HYNIC-annexin-V under anoxic conditions (16). Radiolabeling efficiency of 99mTc and annexin-V was consistently greater than 90% with a specific activity of 150 to 200 μCi/μg protein.
In Vitro Studies on Human Cardiomyocytes
Human primary cardiomyocytes (Cat. #6200, ScienCell, Carlsbad, California) were thawed and the 1 ml (1 × 106 cells/vial) suspended in 19 ml of phenol-red free cardiac myocyte media (includes 5% fetal bovine serum, 1% cardiac myocyte growth supplement, 1X penicillin/streptomycin; Cat. #6201-prf, ScienCell) prior to seeding into white, opaque bottom 96-well tissue culture plates at 5,000 cells per well (Cat. #3917, Corning Life Sciences, Tewksbury, Massachusetts). Cells were incubated overnight at 5% CO2 and 37° C before replacing the media in each well and further incubating for another day.
On the day of experiment, media was replaced with phenol-red free cardiac myocyte media containing 1X CellTox(TM) Green Dye (Cat. #G8741, Promega, Madison, WI). At time T = 0 h, media with or without hydrogen peroxide (H2O2) was added to each well and incubated for 30 min. The media in each well was further supplemented with 1 of the treatments in Figure 2 and cell death monitored on a 5-mode multidetection plate reader with fluorescence excitation at 490 nm, emission monitored at 525 nm, and a cut-off of 515 nm. Cells were kept at 5% CO2 and 37° C between readings. The percentage of cell death was determined by dividing the average fluorescent signal from each treatment with the average signal obtained after total lysis of cells not intoxicated with H2O2. Statistical significance in Figure 2 was calculated via Student t test.
Figure 2. In Vitro Studies With Fv-HSP72.
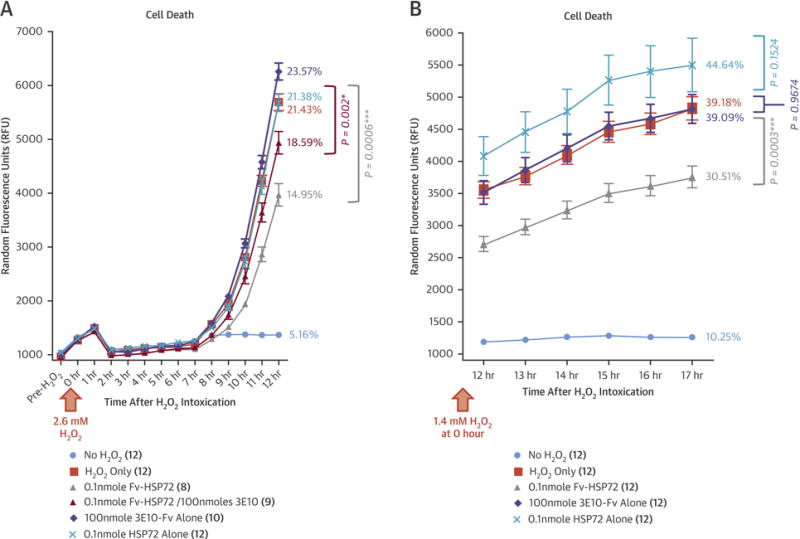
(A) Human primary cardiomyocytes were exposed to 2.6 mM hydrogen peroxide (H2O2) at time T = 0 h and throughout the course of the study. Treatment of cells 30 min after the start of intoxication with 3E10-Fv alone (
 ) or HSP72 alone (
) or HSP72 alone (
 ) did not affect the increase in cell death, while treatment with Fv-HSP72 (
) did not affect the increase in cell death, while treatment with Fv-HSP72 (
 ) significantly attenuated apoptosis (p = 0.0006). Addition of 3E10-Fv prior to Fv-HSP72 treatment (
) significantly attenuated apoptosis (p = 0.0006). Addition of 3E10-Fv prior to Fv-HSP72 treatment (
 ) inhibited Fv-HSP72 efficacy, but still significantly attenuated apoptosis at 12 h (p = 0.002). (B) To study cardiomyocyte exposure overnight, cells were intoxicated with 1.4 mM H2O2 at time T = 0 h and throughout the course of the study. Fv-HSP72 treatment at 30 min after the start of intoxication significantly reduced the percentage of cell death at 17 h compared to the H2O2-only control (p = 0.0003), while treatment with 3E10-Fv alone or HSP72 alone showed no statistically significant inhibition. Percentages reflect normalization of the average fluorescent signal at the last reading divided by the maximum signal obtained after total cell lysis in 3 of the No H2O2 control wells. Number of wells receiving each treatment cited in parentheses. *statistically significant; ***highly significant. Error bars represent SE of the mean. Abbreviations as in Figure 1.
) inhibited Fv-HSP72 efficacy, but still significantly attenuated apoptosis at 12 h (p = 0.002). (B) To study cardiomyocyte exposure overnight, cells were intoxicated with 1.4 mM H2O2 at time T = 0 h and throughout the course of the study. Fv-HSP72 treatment at 30 min after the start of intoxication significantly reduced the percentage of cell death at 17 h compared to the H2O2-only control (p = 0.0003), while treatment with 3E10-Fv alone or HSP72 alone showed no statistically significant inhibition. Percentages reflect normalization of the average fluorescent signal at the last reading divided by the maximum signal obtained after total cell lysis in 3 of the No H2O2 control wells. Number of wells receiving each treatment cited in parentheses. *statistically significant; ***highly significant. Error bars represent SE of the mean. Abbreviations as in Figure 1.
Induction of Ischemia and Reperfusion
Acute experimental myocardial ischemia (17) was produced in anesthetized New Zealand white male rabbits (weight: 2.5 to 3.0 kg). The heart was exposed through a parasternal thoracotomy and the pericardium was removed. A monofilament suture was placed on the lateral branch of the left coronary artery, which was occluded by tightening the snare created by passing the suture through polyethylene tubing and clamping the tube to prevent release of the snare. This protocol follows the Guidelines for the Care and Use of Laboratory Animals established by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee at the Mount Sinai School of Medicine.
At 40 min after occlusion, the snare was released for reperfusion. Rabbits received 1 of 5 treatments; 4 of which were administered via ear vein 1 min before reperfusion. The treatments were: 1) 20 mg (174 nmoles) of 3E10-Fv-HSP72 (Fv-HSP72 pre-reperfusion group; n = 6); 2) an equimolar amount of 3E10-Fv (174 nmoles; 3E10-Fv alone group; n = 6); 3) an equimolar amount of HSP72 (174 nmoles, HSP72 alone group; n = 6), and 4) phosphate buffered saline (PBS) (control group; n = 7). Furthermore, to determine whether there was any significant advantage of Fv-HSP72 administration prior to reperfusion, we undertook 1 more study group of 20 mg (174 nmoles) of 3E10Fv-HSP72 injected at 1 minute after reperfusion (Fv-HSP72 post-reperfusion group, n = 6). Investigators were blinded to the treatment.
Imaging
To detect apoptosis, ~7 mCi of 99mTc-labeled annexin-V was injected intravenously 30 min after the start of reperfusion. Rabbits were continuously monitored for another 2.5 h prior to micro-single-photon emission computed tomographic-micro-computed tomographic (μSPECT-μCT) imaging. Rabbits were imaged using a dual-head μSPECT gamma camera combined with μCT scanning.
After in vivo imaging, the left coronary artery was reoccluded at the original site of the snare. Evans blue dye was infused to document the area at risk (AAR) and the region outside the vascular bed of the occluded left coronary artery (the “remote” area) of the heart. The rabbits were euthanized with an anesthetic overdose of pentobarbital administered intravenously. This method was consistent with the recommendations of the American Veterinary Medical Association Guidelines on Euthanasia. The heart was removed for an ex vivo 1,800 second planar image of the myocardial distribution of 99mTc-annexin-V.
Quantitative Assessment
After the ex vivo planar scan, the heart was cut into 4 cross-sectional bread-loaf slices from apex to base (Figure 1). These 4 slices were further sectioned radially into 29 to 33 fragments which were weighed and counted in a gamma well counter. The percent injected dose (%ID) of 99mTc-annexin-V per gram of tissue was calculated in all myocardial fragments. A mean value of %ID/gram was calculated in 3 territories (Figure 1B); apex (the most vulnerable LV site in our model), AAR, and remote areas as published previously (17). The total weight of myocardial pieces belonging to AAR, or to the remote areas in each group, did not demonstrate any significant differences, indicating that the sizes of at-risk and unaffected areas were equal in all animals throughout the study.
Cardiac troponin I was measured using a commercially available enzyme-linked immunosorbent assay kit per manufacturer’s recommendations. Blood samples were collected immediately prior to coronary occlusion, during occlusion (just prior to reperfusion), and following reperfusion at 30 min, 1 h, and 3 h. Fresh serum was separated from red blood cells of each blood sample by centrifugation and stored frozen at −80°C for subsequent analysis.
Intraoperative echocardiography was performed by 2 experienced operators using a commercially available echo machine equipped with a 5 to 13 MHz linear probe. The ultrasound imaging was performed open chest by directly placing the probe on the heart.
To assess the sequential change of LV global systolic function during the protocol, echocardiography was performed at baseline, 30 min after occlusion, and 3 h after reperfusion per rabbit. In vivo echocardiograms were recorded in the Fv-HSP72 post-reperfusion, 3E10-Fv alone, and the HSP72 alone groups. LV 4-chamber and 2-chamber views were recorded to calculate LVEF using the modified Simpson’s method (18).
Histopathological Examination
Myocardial tissue specimens from the infarct zone, AAR, and the remote area were obtained for histological characterization. After paraformaldehyde fixation, the specimens were routinely processed and paraffin-embedded. Subsequently, 5 μm sections were prepared and stained with hematoxylin and eosin and Masson’s Trichrome. Tissue sections were also subjected to terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) to detect DNA fragmentation during apoptosis per manufacturer’s recommendations. TUNEL positive cells were counted in 5 high-power fields (400×) showing the maximum number of positive nuclei per section. Finally, sections were immunostained with an antibody for the c-myc tag embedded within the Fv-HSP72 on an automated immuno-staining system.
Statistical Analysis
Distributional properties and homogeneity of variance were examined for all study variables to ensure the assumptions for the selected analyses were supported. Student t tests were performed when comparing 2 groups. For multiple group comparisons, we employed 1-way analysis of variance (ANOVA) with post hoc Bonferroni-corrected independent sample t tests. P values < 0.05 were considered significant, with Bonferroni correction applied for multiple comparisons. Differential treatment effects by sample location (i.e., apex versus AAR sans apex versus remote region) were assessed with 2-way ANOVA testing the treatment times location interaction. All data were expressed as mean ± SD.
The Online Appendix provides additional information about the methods used.
Results
Because Fv-HSP72 has already been shown to penetrate COS-7 cells and non-dividing rat primary cortical neurons in vitro and protect them from H2O2-generated oxidative damage (15), we investigated its efficacy with human primary cardiomyocytes. Cells were seeded into opaque bottom 96-well tissue culture plates (5,000 cells/well) and allowed to adhere before replacement of the media with fresh CMM-prf media containing 1X CellTox™ Green, a cell-impermeant dye whose fluorescence is dependent on DNA binding. Exposure to H2O2 induces oxidative stress and subsequent apoptosis (19), leading to increasing exposure of DNA to the cell-impermeant green dye and concomitant increases in fluorescent signal (Figure 2). Wells receiving 0.1 nmoles Fv-HSP72 30 min after the start of H2O2 intoxication had significantly lower cell death than untreated controls. This reduction in cell death was compromised if the 3E10-Fv were added in molar excess as a competitive inhibitor prior to the Fv-HSP72 (Figure 2A). As expected, the same molar quantity of 3E10-Fv alone (100 nmoles) used in the competitive inhibition experiment did not affect apoptosis. Adding a molar equivalent of HSP72 alone (0.1 nmoles) also was not effective in reducing apoptosis compared to cells exposed to H2O2 only. Wells not intoxicated with H2O2 had very little cell death over the course of the experiments.
To determine the maximum signal achievable and calculate the percentage of cell death, some of these wells were exposed to lysis buffer and the values used to normalize the results. Whether cardiomyocyte intoxication included 12 h of H2O2 exposure (Figure 2A), overnight exposure (Figure 2B), or longer (in 1 study, up to 26 h; data not shown), Fv-HSP72 treatment inhibited cell death. Longer exposure of cells, either to a molar equivalent of HSP72 alone or a molar excess of 3E10-Fv alone, did not inhibit apoptosis (Figure 2B). These observations reflected the in vivo results subsequently obtained.
99MTC-Annexin-V Uptake
Figure 3 shows representative in vivo and ex vivo μSPECT-μCT images of each of the 5 subgroups of animals. The simultaneous CT images allowed precise localization of the radiotracer uptake in the cardiac silhouette. More intense and more extensive 99mTc-annexin-V uptake was seen in the 3 control groups compared with the 2 Fv-HSP72 treatment groups (quantification discussed later). Maximum 99mTc-annexin-V uptake was observed predominantly in the apical region of the heart. The ex vivo μSPECT images of 99mTc-annexin-V myocardial uptake confirmed substantially lower radiotracer uptake in the Fv-HSP72-treated animals compared to control animals. SPECT-CT images in the axial, coronal, and sagittal planes depicted the extent of 99mTc-annexin-V uptake in the myocardium (Online Figure 1).
Figure 3. Annexin-V Based Apoptosis Imaging in Rabbit Ischemia-Reperfusion Model Treated With Fv-HSP72 or Various Control Agents.
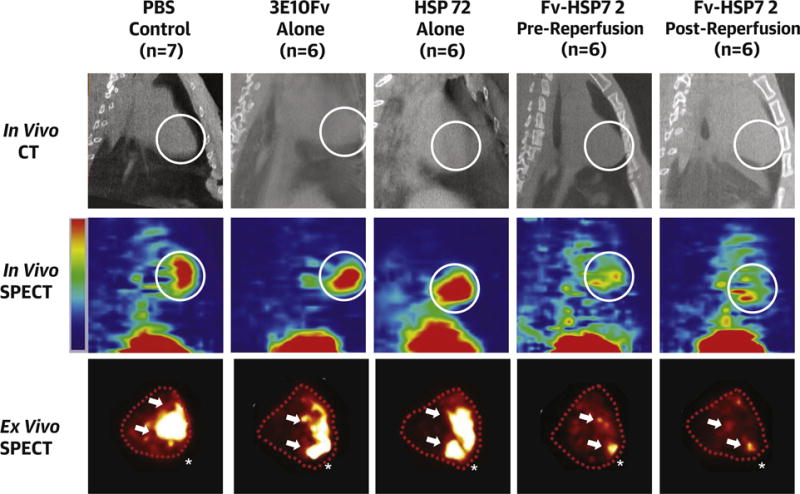
Higher and more extensive uptake of 99mTc-annexin-V is seen in control groups compared to the 2 therapy groups in in vivo sagittal slices of CT images and corresponding in vivo sagittal slices of SPECT images which were obtained 3 h after the injection of 99mTc-annexin-V (white circle = apex) and in ex vivo images of excised heart (traced with dotted lines). Arrows = high uptake area; * = apex. Abbreviations as in Figure 1.
Such uptake was quantitatively evaluated by gamma counting of the harvested myocardial sections and expressed as %ID/g tissue. Figure 4 shows %ID/g uptake in the apex, AAR, and the remote area. P values displayed in the figure were computed from 3 pairwise comparisons of samples exposed to a control treatment (PBS, 3E10-Fv alone, or HSP72 alone) versus samples exposed to the active treatment (Fv-HSP72 pre- or post-reperfusion) for each of the 3 locations using Bonferroni-corrected independent sample t tests (α = 0.01). Within each location, variance in %ID/g was similar between active and control treatment groups. Average %ID/g uptake was significantly lower in the Fv-HSP72 treated versus control groups (PBS, 3E10-Fv alone, HSP72 alone) for samples collected from the apex (0.37 ± 0.18 vs. 0.68 ± 0.20; p = 0.0001), and AAR (0.28 ± 0.12 vs. 0.49 ± 0.15; p = 0.0003), and marginally lower in the noninfarcted region (0.05 ± 0.01 vs. 0.06 ± 0.02; p = 0.016). Analysis of the AAR included %ID/g data from the apex, given its location as a subregion of the AAR. We also analyzed the %ID/g for the AAR as 2 separate regions comprising the apex and those fragments in the AAR outside the apex. Two-way ANOVA suggested significant differences in %ID/g tissue uptake among the 5 study groups (p < 0.0001), and between the 3 different locations within the heart (i.e., apex versus AAR sans apex versus remote; p < 0.0001). The size of the treatment effect differed across the sites (2-way ANOVA test of location × treatment interaction p = 0.042).
Figure 4. Gamma Counting for Quantitative 99mTc-Annexin-V Uptake in Treated and Control Animal Groups.
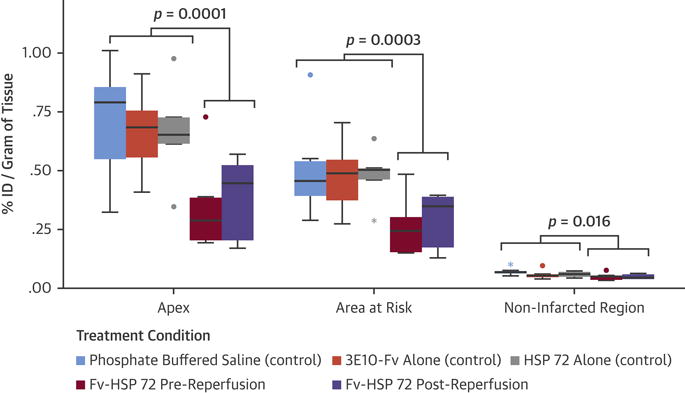
The percent injected dose (%ID)/g was significantly lower in the active versus control groups for samples collected from the apex (p = 0.0001), and area at risk (p = 0.0003), and were marginally lower in the noninfarcted region (p = 0.016). In these box plots, the midline is the median and the error bars are the minimum and maximum values that were not flagged as outliers. • = values >1.5 interquartile ranges (IQR) to <3 IQRs from either edge of box; * = values >3 IQRs. Abbreviations as in Figure 1.
Assessing Infarct Size
Figure 5 summarizes the cTn I concentration during the experiment. Independent sample t tests revealed significantly higher troponin I levels at 1 and 3 h post-reperfusion (data available for n = 25 animals; p = 0.043, and n = 21 animals; p = 0.041, respectively) for all 3 controls versus the rabbits that received Fv-HSP72 pre- or post-reperfusion. Echocardiography was performed in 3E10-Fv and HSP72 alone groups and the Fv-HSP72 post-reperfusion group. Figure 6 shows the sequential changes of LVEF in the control and active therapy groups. Repeated measures ANOVA reveals a significant time times treatment interaction for LVEF (p = 0.004). Paired comparisons with independent sample t-tests at each time point revealed no difference in LVEF at baseline or during occlusion; but, during the post-reperfusion period, LVEF was significantly higher for samples with Fv-HSP72 treatment (n = 3) compared to the control (n = 12) groups (47.9 ± 3.7% vs. 37.6 ± 3.6%; p = 0.022). Visually, one could see recovery of the wall motion abnormality to a level closer to baseline during reperfusion in Fv-HSP72-treated rabbits (Online Figure 2) compared to 3E10-Fv and HSP72-alone controls.
Figure 5. Troponin I Concentrations in Treated and Control Groups.
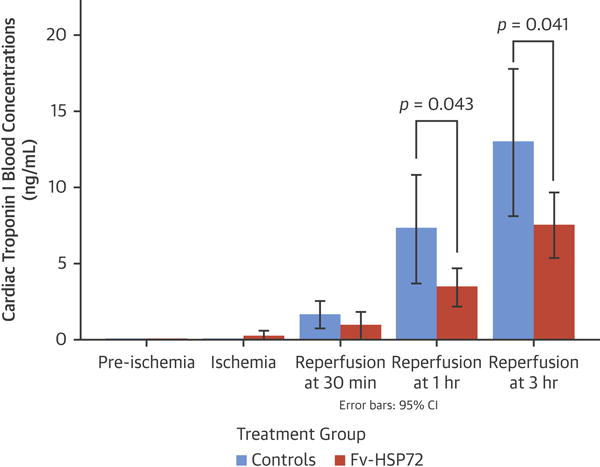
Cardiac troponin I levels were significantly lower at 1 and 3 h after reperfusion in therapy groups compared with the control groups. CI = confidence intervals; other abbreviations as in Figure 1.
Figure 6. LV Functional Assessment in Treated and Control Groups.
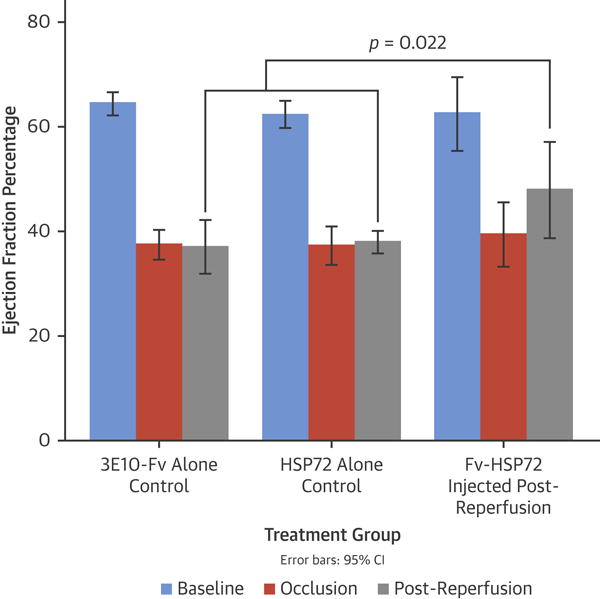
Left ventricular (LV) ejection fraction during the reperfusion period was significantly higher in the treatment group than control groups (p = 0.022). Other abbreviations as in Figures 1 and 5.
Histological Characterization
Myocardial tissue sections from the infarct zone, AAR, and remote myocardial region were examined from all 5 groups for histological, histochemical, and immunohistochemical characterization (Figure 7). Hematoxylin and eosin and Masson’s Trichrome staining revealed myocyte necrosis with contraction bands in the infarct zone. There appeared to be greater interstitial hemorrhage in the infarcted tissues of control rabbits compared to those treated with Fv-HSP72. TUNEL staining to detect DNA fragmentation during apoptosis was more numerous in the hearts of control rabbits compared to Fv-HSP72 treated animals. A linear correlation was seen between TUNEL positive nuclei and the %ID/g uptake of 99mTc-annexin-V in the heart fragments (p < 0.05; R2 = 0.5218). Finally, 3E10 localization with and without the HSP72 moiety was demonstrated by immunostaining for the c-myc tag with mouse monoclonal 9E10 seen as brown staining of the nucleus (arrows) in the cells. The HSP72 alone and PBS controls did not have a c-myc tag and, hence, no c-myc positive brown nuclei (data not shown).
Figure 7. Histopathological Characterization of Infarcted and Remote Myocardial Tissue in Fv-HSP72-Treated and Untreated Animals.
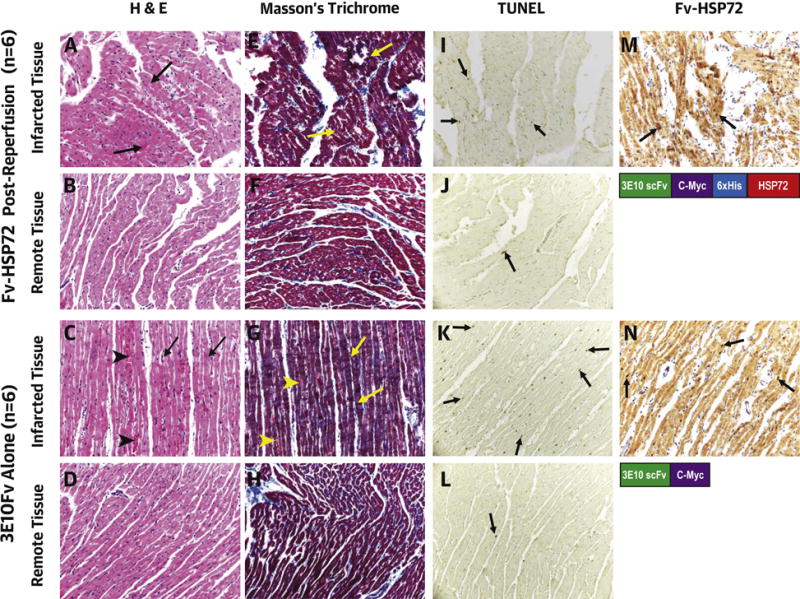
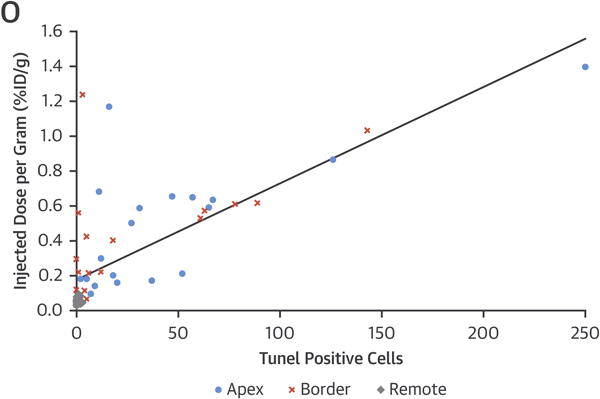
Hematoxylin and eosin (H&E) (A through D) and Masson’s Trichrome (E through H) staining show contraction band necrosis (arrows) in the infarcted area but not in the remote myocardial region in both groups. The 3E10-Fv control rabbits also show more interstitial hemorrhage in the infarcted area (arrowheads C and G). Necrotic myocytes appear grayish-blue on the Trichrome stain. TUNEL stain shows more apoptotic myocytes (arrows) in the infarct (I, K) compared with the remote myocardium (J, L). (M, N) 3E10 Fv (tagged with c-myc) penetration in the myocytes is highlighted by the positive nuclear staining with the anti-myc antibody (arrows). All images were taken at 200×. (O) Sections analyzed for TUNEL and %ID/g uptake of 99mTc-annexin-V (n = 57) showed a statistically significant linear correlation. Those fragments from the apex or border zone had greater TUNEL positive nuclei and annexin uptake than fragments in the remote regions unaffected by the LCA occlusion. TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling; other abbreviations as in Figures 1 and 4.
Discussion
The current study demonstrated that a single intravenous injection of Fv-HSP72 had a significant myocardial protective effect in the rabbit model of ischemia-reperfusion injury. The extent of annexin uptake in μSPECT images and gamma-counting of myocardial tissue specimens within the AAR was significantly lower in Fv-HSP72 treatment groups than 3 control groups, with a net reduction of 43% in myocardial damage. Also, the cTn I concentration was correspondingly lower with a net 42% reduction. The LVEF was 27% higher in Fv-HSP72 treatment groups compared to the control groups of animals.
Approaches Under Study
Although both necrosis and apoptosis play a significant role in cell death during ischemia and reperfusion, they constitute a continuum of myocardial injury. The ischemic injury is initiated by the energy-requiring process of apoptosis. However, because the ischemic cells are devoid of energy, the cells die of necrosis. Therefore, it is expected that an intervention targeted at any component of myocardial injury would reduce net cell death (20). Institution of reperfusion contributes to resolution of apoptosis within the AAR; ironically, it also imposes unintended adverse effects on myocardial cells (reperfusion injury) (21).
The ultimate treatment goal for patients with acute coronary syndrome is to achieve early reperfusion and resolution of ischemia by definitive intervention (such as PCI) or at least postpone cell death to allow subsequent definitive intervention if it is not available instantly (22). For the latter, thrombolytic therapy with pharmacological aids becomes important and is particularly important in low- and middle-income countries (23).
Multiple adjunctive pharmacological approaches have been proposed to reduce infarct size in animal models and clinical studies of ischemia-reperfusion injury, but they have demonstrated minimal success (3,4). A large randomized study showed only a 2.2% increase in ejection fraction, using continuous infusion of atrial natriuretic peptide in patients with AMI, and 29 of 277 patients (10.5%) in the atrial natriuretic peptide group had severe hypotension (24). Similarly, in another trial of 410 patients, a single intravenous cyclosporine A bolus just before primary PCI had no effect on ST-segment resolution or high-sensitivity cTnT and did not improve clinical outcomes or LV remodeling up to 6 months (25). Conversely, there is renewed interest in beta-1 selective blockers and multicenter studies are underway (26).
In patients with anterior Killip class ≤II ST-elevation myocardial infarction undergoing primary PCI, early intravenous metoprolol (delivered in the ambulance) before reperfusion resulted in higher long-term LVEF, reduced incidence of severe LV systolic dysfunction and implantable cardioverter-defibrillator indications, and fewer heart failure admissions. In addition to the pharmacological approaches, various biologics are being evaluated in experimental studies such as those targeting apoptotic cascades through caspase (22), and Ca(2+)-calmodulin-dependent protein kinase (27) pathways. Alternatively, fortification of protective biologics, such as Fv-HSP72, has demonstrated beneficial results in cerebral infarction (13).
More than 3 decades of research have demonstrated the cardioprotective effects of HSP72 in both in vitro and in vivo investigations. Expression of HSP72 is increased by elevated temperature or ischemic stimuli, gene transfection (28), and transgenic overexpression (29). HSP72 is a pleiotropic cytoprotectant, not only in the cardiovascular system but also in other organs, facilitating intracellular folding or unfolding, transportation, and chaperoning of proteins (30). HSP72 has also been implicated in the disruption of 3 apoptotic pathways: 1) ATP-dependent (the Apoptosome pathway) (31); 2) ATP-independent (apoptosis-inducing factor pathway) (32); and 3) the NF-κB pathway (33).
Heat shock proteins are largely intracellular proteins and their administration is not associated with any reported cytotoxicity. Since the cardioprotective effects of HSP72 have been demonstrated, even small molecules to induce transcription have also been employed (34). However, this approach has 2 limitations. First, both stimuli and gene therapy for HSP induction have a time lag of more than 3 h before reaching therapeutic levels; this time frame precludes their use as treatment for acute coronary syndromes. Second, the endogenous induction of HSP is reported to be less responsive with increasing age (35,36).
3E10-Fv-HSP72 Fusion Protein
The present study circumvents the HSP72 induction time lag by using an ENT2 transporter-mediated 3E10-Fv directed system to deliver therapeutic protein into living cells (Central Illustration). 3E10-Fv, a candidate antibody for protein delivery, is the single-chain fragment of a murine anti-DNA autoantibody 3E10 (37). It consists of the variable region of both the light and heavy chains of monoclonal antibody 3E10 fused with a short (GGGGS)3 linking sequence. 3E10-Fv penetrates cells through the energy-independent ENT2 channel, which is located in both the plasma and nuclear (38) membranes. The target selectivity of 3E10 in vivo is based on the simple concept that tissues undergoing significant cell injury possess a high concentration of extracellular DNA. Binding to DNA is necessary for 3E10 passage (12). Salvaging of nucleosides by surrounding cells, through the ENT2 channel, provides 3E10 the opportunity to enter those cells still hanging on to life. The toxicity of the Fv-HSP72 is negligible because it is produced by stable expression of Fv complementary DNA in Chinese hamster ovary cell lines (37); after localizing in the cell nucleus, 3E10-Fv is largely degraded within 4 h.
Central Illustration. Cardioprotective Effects of Fv-HSP72 on Ischemia-reperfusion Injury.
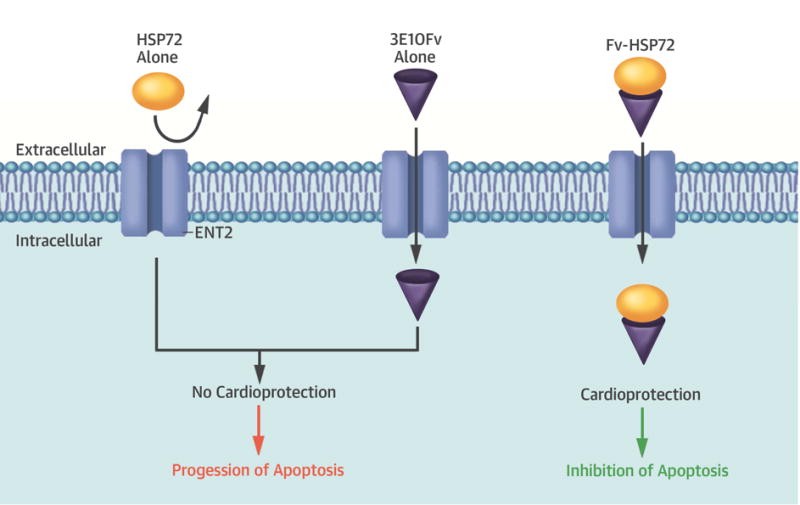
A single-chain variable fragment (Fv) of the 3E10 antibody transports heat shock protein (HSP)72 into cardiomyocytes through the equilibrative nucleoside transporter 2 (ENT2) channel, where the HSP inhibited apoptosis in a rabbit model of ischemia-reperfusion injury. Either moiety alone is not cardioprotective.
The antibody 3E10-Fv has been widely used to deliver numerous potentially therapeutic peptides or proteins into living cells, like p53 for cancer and micro-dystrophin for muscular dystrophy (39). In a previous study using a rat ischemic brain model, injection of Fv-HSP72 noticeably reduced infarct volume (68%) and improved sensorimotor function (13). In the current study, we demonstrated the cardioprotective effects of Fv-HSP72 in a myocardial ischemia-reperfusion model. Immunostaining for the c-myc tag incorporated into the Fv-HSP72 demonstrated penetration of the molecule into cardiomyocytes within the infarct zone, an important observation to counter the suggestion that the effects were due to induction of HSPs caused by the stress of ischemia and reperfusion. The administration of Hsp72 or 3E10-Fv alone did not reduce annexin-V myocardial uptake and had no effect on functional recovery. These data were consistent with the hypothesis that Fv-HSP72, not HSP72 alone, can penetrate the cell membrane and acts as a cardioprotective agent.
Based on messenger ribonucleic acid levels surveyed in the organs of mice and rats, ENT2 tissue distribution does vary, with heart and brain showing greater amounts than some other organs. ENT2 is also an energy independent channel through which molecules can readily pass down the concentration gradient between the extracellular and intracellular compartments. This means energy deficient but potentially viable ischemic cells, as seen in reperfusion injury, can still take up 3E10-Fv even when endocytosis and transporters cease to function. It can be further hypothesized that a minimal increase in the normal cells might render them more resistant to damage.
Molecular Imaging of Apoptotic Cells
Annexin-V, a human protein with molecular weight of 36 kDa, targets the expression of phosphatidylserine on the outer leaflet of the cell membrane in apoptotic cells (14,40). Phosphatidylserine is a constitutive plasma membrane anionic phospholipid that is actively recruited to the inner leaflet of the lipid bilayer. Phosphatidylserine is virtually absent from the outer surface of normal cells (41). After injecting 99mTc annexin-V, one can identify the site and extent of severely stressed and apoptotic cells, and gamma counting allows quantitative assessment of uptake within each cross section of the heart. Our data confirmed the cardioprotective effects of Fv-HSP72, correlating a substantially lower radiotracer uptake in μSPECT imaging and gamma counting of myocardial tissue segments compared to control groups. Histological analysis of the myocardium further illustrated the tissue protection afforded by the Fv-HSP72 treatment. TUNEL staining detects DNA fragmentation during apoptosis and those sections of infarcted tissue from Fv-HSP72 rabbits had fewer TUNEL-positive nuclei than control rabbits. Furthermore, there appeared to be less interstitial hemorrhage in Fv-HSP72-treated rabbits. Additionally, the echocardiographic data indicated functional salvage. These data demonstrated that the acute delivery of Fv-HSP72, but not Hsp72 alone, could at least partially resolve myocardial ischemia-reperfusion injury.
The cardiac troponin I measurement and LV functional assessment indicated that Fv-HSP72 reduced the infarct volume and improved LV functional recovery after an ischemia-reperfusion injury. Echocardiographic LV function and cardiac troponin I levels are not perfect measurements of infarct size; however, the modified trichrome staining with scintigraphy evidence offer significant insight into infarct size. Although triphenyl tetrazolium chloride staining of heart specimens is often used as a means of visualizing infarct size, conventional histopathological assessments are an acceptable alternative, especially for shorter reperfusion times (42). These assessments each provided data supporting the hypothesis that administration of Fv-HSP72 before or after reperfusion reduced myocyte cell death and infarct size.
Study Limitations
First, the effect of Fv-HSP72 was assessed only in the acute phase. Subacute and chronic effects of Fv-HSP72 need to be investigated with longer intervals of follow-up to determine the durability of this acute salvage. Second, dose dependency and frequency of Fv-HSP72 injection were not addressed in the current study. Future studies with a large animal would clarify them.
Conclusions
A single injection of Fv-HSP72 reduced myocardial apoptosis and improved LV function in an ischemia-reperfusion model of acute myocardial injury in rabbits. In contrast, injection of HSP72 or 3E10-Fv alone showed no evidence of cardioprotection, although 3E10-Fv is capable of transporting HSP72 into stressed, but potentially salvageable, cardiomyocyte. Fv-HSP72 needs further investigation in larger animals and in prospectively designed randomized clinical trials.
Supplementary Material
Online Figure 1: Power Point presentation showing 3-D reconstructions of four rabbit μSPECT images. In vivo images were captured for 2.5 s per view for 256 views in a 360° rotation allowing views of the 99mTc-annexin-V distribution in all 3 spatial axes (transverse, sagittal, and coronal). PBS control, 3E10-Fv alone, HSP72 alone, and Fv-HSP72 post-reperfusion rabbits are shown. Uptake of the 99mTc-annexin-V in the liver appears at the bottom of each image. Uptake in the heart is demarcated by the superimposed CT image of the spine and rib cage.
Online Figure 2: Power Point presentation showing echocardiography of rabbits. Slide 1: Wall motion analysis for an Fv-HSP72 treated rabbit calculated the ejection fraction to be 68.3% at baseline and 40.0% 30 minutes into the occlusion. The LVEF recovered to 50.0% 3 h after reperfusion. Slide 2: Wall motion analysis for a rabbit receiving 3E10Fv alone had the LVEF to be 61.5% at baseline, 38.9% during the occlusion, and it remained at 38.3% 3 h after reperfusion. Slide 3: Wall motion analysis for the HSP72 alone rabbit had the LVEF to be 63.4% at baseline, 36.7% during the occlusion, and it only increased to 37.9% 3 h after reperfusion.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE
Rapid delivery of HSP72 by using 3E10-Fv provided cardioprotective effects in a rabbit ischemia-reperfusion model. The biologics-based drugs are likely to play a greater role in disease management.
TRANSLATIONAL OUTLOOK
Rapid delivery of HSP72 using the 3E10-Fv carrier may be useful as an adjunct to reperfusion therapy for the treatment of acute myocardial infarction. Fv-HSP72 needs further investigation in larger animals and in prospectively designed randomized clinical trials.
Acknowledgments
Funding: This work was supported by a National Institutes of Health/National Heart, Lung, and Blood Institute grant (R43HL122012-01A1); T.T. was supported by Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad, T.N was supported by Uehara Memorial Foundation and SNMMI Wagner-Torizuka Fellowship.
ABBREVIATIONS AND ACRONYMS
- %ID/g
percent injected dose per gram
- AAR
area at risk
- CT
computed tomography
- ENT2
equilibrative nucleoside transporter 2
- Fv
single-chain fragment variable
- HSP
heat shock protein
- LVEF
left ventricular ejection fraction
- PCI
percutaneous coronary intervention
- SPECT
single-photon emission computed tomography
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors M.H.P., R.A.R. and G.T.R. declare they are owners of Rubicon Biotechnology, which is developing the Fv-HSP72 technology. J.B. does statistical consulting for Rubicon, however, he is not an employee nor does he have any ownership in the company. The authors R.N, R.H.W. and G.C. are employees of UCLA and the VA. They also have no ownership in Rubicon. All other authors have no conflicts of interest.
References
- 1.Ibanez B, Heusch G, Ovize M, Werf FV. Evolving Therapies for Myocardial Ischemia/Reperfusion Injury. J Am Coll Cardiol. 2015;14:1454–71. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur Heart J. 2017;38:774–84. doi: 10.1093/eurheartj/ehw224. [DOI] [PubMed] [Google Scholar]
- 3.Fokkema ML, Vlaar PJ, Vogelzang M, et al. Effect of high-dose intracoronary adenosine administration during primary percutaneous coronary intervention in acute myocardial infarction: a randomized controlled trial. Circ Cardiovasc Interv. 2009;2:323–9. doi: 10.1161/CIRCINTERVENTIONS.109.858977.109.858977. [DOI] [PubMed] [Google Scholar]
- 4.Cung TT, Morel O, Cayla G, et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N Engl J Med. 2015;373:1021–31. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 5.Hutter MM, Sievers RE, Barbosa V, Wolfe CL. Heat-shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein induced and the degree of myocardial protection. Circulation. 1994;89:355–60. doi: 10.1161/01.cir.89.1.355. [DOI] [PubMed] [Google Scholar]
- 6.Currie RW, Karmazyn M, Kloc M, Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988;63:543–9. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- 7.Knowlton AA, Brecher P, Apstein CS. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest. 1991;87:139–47. doi: 10.1172/JCI114963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly TJ, Sievers RE, Vissern FL, Welch WJ, Wolfe CL. Heat shock protein induction in rat hearts. A role for improved myocardial salvage after ischemia and reperfusion? Circulation. 1992;85:769–78. doi: 10.1161/01.cir.85.2.769. [DOI] [PubMed] [Google Scholar]
- 9.Plumier JC, Ross BM, Currie RW, et al. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95:1854–60. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guisasola MC, Desco MM, Gonzalez FS, et al. Heat shock proteins, end effectors of myocardium ischemic preconditioning? Cell Stress Chaperones. 2006;11:250–8. doi: 10.1379/CSC-181R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen JE, Tse CM, Chan G, Heinze ER, Nishimura RN, Weisbart RH. Intranuclear protein transduction through a nucleoside salvage pathway. J Biol Chem. 2007;282:20790–3. doi: 10.1074/jbc.C700090200. [DOI] [PubMed] [Google Scholar]
- 12.Weisbart RH, Chan G, Jordaan G, et al. DNA-dependent targeting of cell nuclei by a lupus autoantibody. Sci Rep. 2015;5:12022. doi: 10.1038/srep12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan X, Ander BP, Liao IH, et al. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke. 2010;41:538–43. doi: 10.1161/STROKEAHA.109.572537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narula J, Acio ER, Narula N, et al. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med. 2001;7:1347–52. doi: 10.1038/nm1201-1347. [DOI] [PubMed] [Google Scholar]
- 15.Hansen JE, Sohn W, Kim C, et al. Antibody-mediated Hsp70 protein therapy. Brain Res. 2006;1088:187–96. doi: 10.1016/j.brainres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Blankenberg FG, Vanderheyden JL, Strauss HW, Tait JF. Radiolabeling of HYNIC-annexin V with technetium-99m for in vivo imaging of apoptosis. Nat Protoc. 2006;1:108–10. doi: 10.1038/nprot.2006.17. [DOI] [PubMed] [Google Scholar]
- 17.Narula J, Petrov A, Pak KY, Lister BC, Khaw BA. Very early noninvasive detection of acute experimental nonreperfused myocardial infarction with 99mTc-labeled glucarate. Circulation. 1997;95:1577–84. doi: 10.1161/01.cir.95.6.1577. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metab Rev. 2000;32:307–26. doi: 10.1081/dmr-100102336. [DOI] [PubMed] [Google Scholar]
- 20.Kenis H, Zandbergen HR, Hofstra L, et al. Annexin A5 uptake in ischemic myocardium: demonstration of reversible phosphatidylserine externalization and feasibility of radionuclide imaging. J Nucl Med. 2010;51:259–67. doi: 10.2967/jnumed.109.068429. [DOI] [PubMed] [Google Scholar]
- 21.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 22.Dumont EA, Reutelingsperger CP, Smits JF, et al. Real-time imaging of apoptotic cell-membrane changes at the single-cell level in the beating murine heart. Nat Med. 2001;7:1352–5. doi: 10.1038/nm1201-1352. [DOI] [PubMed] [Google Scholar]
- 23.Alexander T, Mullasari AS, Narula J. Developing a STEMI system of care for low- and middle-income countries: the STEMI-India model. Glob Heart. 2014;9:419–23. doi: 10.1016/j.gheart.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Kitakaze M, Asakura M, Kim J, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–93. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 25.Ottani F, Latini R, Staszewsky L, et al. Cyclosporine A in Reperfused Myocardial Infarction: The Multicenter, Controlled, Open-Label CYCLE Trial. J Am Coll Cardiol. 2016;67:365–74. doi: 10.1016/j.jacc.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 26.Pizarro G, Fernandez-Friera L, Fuster V, et al. Long-term benefit of early pre-reperfusion metoprolol administration in patients with acute myocardial infarction: results from the METOCARD-CNIC trial (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction) J Am Coll Cardiol. 2014;63:2356–62. doi: 10.1016/j.jacc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, Zhang Y, Cui M, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22:175–82. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Murtuza B, Sammut IA, et al. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 2002;106:I270–6. [PubMed] [Google Scholar]
- 29.Hutter JJ, Mestril R, Tam EK, Sievers RE, Dillmann WH, Wolfe CL. Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation. 1996;94:1408–11. doi: 10.1161/01.cir.94.6.1408. [DOI] [PubMed] [Google Scholar]
- 30.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–84. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beere HM, Wolf BB, Cain K, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–75. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 32.Cande C, Cohen I, Daugas E, et al. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215–22. doi: 10.1016/s0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- 33.Chang CC, Chen SD, Lin TK, et al. Heat shock protein 70 protects against seizure-induced neuronal cell death in the hippocampus following experimental status epilepticus via inhibition of nuclear factor-kappaB activation-induced nitric oxide synthase II expression. Neurobiol Dis. 2014;62:241–9. doi: 10.1016/j.nbd.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Soti C, Nagy E, Giricz Z, Vigh L, Csermely P, Ferdinandy Heat shock proteins as emerging therapeutic targets. Br J Pharmacol. 2005;146:769–80. doi: 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fargnoli J, Kunisada T, Fornace AJ, Jr, Schneider EL, Holbrook NJ. Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci USA. 1990;87:846–50. doi: 10.1073/pnas.87.2.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locke M, Tanguay RM. Diminished heat shock response in the aged myocardium. Cell Stress Chaperones. 1996;1:251–60. doi: 10.1379/1466-1268(1996)001<0251:dhsrit>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisbart RH, Stempniak M, Harris S, Zack DJ, Ferreri K. An autoantibody is modified for use as a delivery system to target the cell nucleus: therapeutic implications. J Autoimmun. 1998;11:539–46. doi: 10.1006/jaut.1998.0212. [DOI] [PubMed] [Google Scholar]
- 38.Mani RS, Hammond JR, Marjan JM, et al. Demonstration of equilibrative nucleoside transporters (hENT1 and hENT2) in nuclear envelopes of cultured human choriocarcinoma (BeWo) cells by functional reconstitution in proteoliposomes. J Biol Chem. 1998;273:30818–25. doi: 10.1074/jbc.273.46.30818. [DOI] [PubMed] [Google Scholar]
- 39.Weisbart RH, Hansen JE, Nishimura RN, et al. An intracellular delivery vehicle for protein transduction of micro-dystrophin. J Drug Target. 2005;13:81–7. doi: 10.1080/10611860400029002. [DOI] [PubMed] [Google Scholar]
- 40.Boersma HH, Kietselaer BL, Stolk LM, et al. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med. 2005;46:2035–50. [PubMed] [Google Scholar]
- 41.Blankenberg FG, Strauss HW. Recent advances in the molecular imaging of programmed cell death: part I–pathophysiology and radiotracers. J Nucl Med. 2012;53:1659–62. doi: 10.2967/jnumed.112.108944. [DOI] [PubMed] [Google Scholar]
- 42.Redgfors B, Shao Y, Omervic E. Myocardial infarct size and area at risk assessment in mice. Exp Clin Cardiol. 2012;17:268–72. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Figure 1: Power Point presentation showing 3-D reconstructions of four rabbit μSPECT images. In vivo images were captured for 2.5 s per view for 256 views in a 360° rotation allowing views of the 99mTc-annexin-V distribution in all 3 spatial axes (transverse, sagittal, and coronal). PBS control, 3E10-Fv alone, HSP72 alone, and Fv-HSP72 post-reperfusion rabbits are shown. Uptake of the 99mTc-annexin-V in the liver appears at the bottom of each image. Uptake in the heart is demarcated by the superimposed CT image of the spine and rib cage.
Online Figure 2: Power Point presentation showing echocardiography of rabbits. Slide 1: Wall motion analysis for an Fv-HSP72 treated rabbit calculated the ejection fraction to be 68.3% at baseline and 40.0% 30 minutes into the occlusion. The LVEF recovered to 50.0% 3 h after reperfusion. Slide 2: Wall motion analysis for a rabbit receiving 3E10Fv alone had the LVEF to be 61.5% at baseline, 38.9% during the occlusion, and it remained at 38.3% 3 h after reperfusion. Slide 3: Wall motion analysis for the HSP72 alone rabbit had the LVEF to be 63.4% at baseline, 36.7% during the occlusion, and it only increased to 37.9% 3 h after reperfusion.


