Abstract
Background
Deep Brain Stimulation (DBS) is thought to improve the symptoms of selected neurological disorders by modulating activity within dysfunctional brain circuits. To date, there is no evidence that DBS counteracts progressive neurodegeneration in any particular disorder.
Objective/Hypothesis
We hypothesized that DBS applied to the fornix in patients with Alzheimer’s Disease (AD) could have an effect on brain structure.
Methods
In six AD patients receiving fornix DBS, we used structural MRI to assess one-year change in hippocampal, fornix, and mammillary body volume. We also used deformation-based morphometry to identify whole-brain structural changes. We correlated volumetric changes to hippocampal glucose metabolism. We also compared volumetric changes to those in an age-, sex-, and severity-matched group of AD patients (n = 25) not receiving DBS.
Results
We observed bilateral hippocampal volume increases in the two patients with the best clinical response to fornix DBS. In one patient, hippocampal volume was preserved three years after diagnosis. Overall, mean hippocampal atrophy was significantly slower in the DBS group compared to the matched AD group, and no matched AD patients demonstrated bilateral hippocampal enlargement. Across DBS patients, hippocampal volume change correlated strongly with hippocampal metabolism and with volume change in the fornix and mammillary bodies, suggesting a circuit-wide effect of stimulation. Deformation-based morphometry in DBS patients revealed local volume expansions in several regions typically atrophied in AD.
Conclusion
We present the first in-human evidence that, in addition to modulating neural circuit activity, DBS may influence the natural course of brain atrophy in a neurodegenerative disease.
Keywords: Deep brain stimulation, Alzheimer’s disease, Fornix, Hippocampus, MRI, Volume
Introduction
Chronic, high-frequency, electrical deep brain stimulation (DBS) is an effective treatment for movement disorders such as Parkinson’s disease, tremor, and dystonia [1]. More recently, DBS has been used on an experimental basis to treat patients with intractable psychiatric conditions including major depression [2,3], obsessive compulsive disorder [4,5], and anorexia nervosa [6]. The therapeutic mechanism of action underlying DBS in these various neuropsychiatric disorders remains uncertain, but likely involves modulation of activity within dysfunctional neural circuits [7]. As such, DBS is considered a symptomatic therapy, affecting neural circuit function, but is thought to be unable to influence progressive neurodegenerative processes acting on these circuits. Nevertheless, given the success of DBS in treating the symptoms of Parkinson’s disease, its use in other neurodegenerative disorders including Alzheimer’s disease (AD) is now also being considered.
AD is a neurodegenerative disease characterized by atrophy in several brain structures—notably the hippocampus—with amyloid and tau deposition, formation of neurofibrillary tangles, and cerebral hypometabolism most notably in posterior cortical regions [8]. These pathophysiological changes result in dysfunction in several neural circuits, including the default mode network [9] and the memory circuit of Papez [10]. In a recently published phase I clinical trial [11], we assessed the safety and possible benefits of DBS in six AD patients. Bilateral DBS applied to the fornix—the principal outflow tract from the hippocampus—was able to drive physiological activity within the memory circuit, and may have been associated with slowing in the expected rate of cognitive decline in some patients.
These promising results point to the potential effectiveness of fornix DBS as a symptomatic therapy in AD. However, additional findings from the trial, coupled with data from recent DBS studies in animal models, raise the possibility that fornix DBS might, additionally, induce plastic effects both within the memory circuit and across the entire brain. In particular, positron emission tomography (PET) data acquired serially from the AD patients enrolled in the trial showed a sustained increase in cortical glucose metabolism over one year in contrast to the cortical hypometabolism that is typically observed over time in AD [12]. Additionally, in rodents, DBS applied to several nodes of the memory circuit stimulates neurogenesis in the dentate gyrus of the hippocampus [13,14]; these new neurons are of normal morphology, integrate themselves into functional circuits, and appear to enhance memory [14,15].
Based on these observations, we hypothesized that the effects of fornix DBS may extend to changes in brain structure, and possibly to a slowing of progressive neurodegeneration in patients with AD. To test this hypothesis, we quantitatively analyzed serial structural magnetic resonance imaging (MRI) scans of the brain in AD patients treated in our phase I trial of fornix DBS. Specifically, we measured the volumes of the hippocampus, fornix, and mammillary bodies—critical components of the Papez circuit—in all patients at baseline and following one year of continuous fornix stimulation. We also looked for evidence of brain-wide structural changes, using a hypothesis-free, data-driven method known as deformation-based morphometry (DBM) [16]. Finally, we compared hippocampal volume changes over time in patients treated with DBS to those seen in an age-, sex-, and severity-matched group of untreated AD patients. Taken together, our results suggest that DBS may be accompanied by changes in brain structure, and in some cases prevent the progression of focal brain atrophy, in patients with a neurodegenerative disease.
Materials and methods
Participants
Patient selection, the DBS surgical implantation procedure, clinical evaluation, and follow-up have previously been addressed in detail in Laxton et al. [11]. Briefly, inclusion criteria were as follows: men or women, aged 40–80 years; satisfied the diagnostic criteria for probable AD; received the diagnosis of AD within the past 2 years; Clinical Dementia Rating (CDR) [17] score of 0.5 or 1.0; score between 18 and 28 on the Mini Mental State Examination (MMSE) [18]; on a stable dose of cholinesterase inhibitors for a minimum of 6 months prior to DBS implantation surgery. Exclusion criteria included: pre-existing structural brain abnormalities (such as tumor, infarction, or intracranial hematoma); other neurologic or psychiatric diagnoses; medical comorbidities that would preclude safe surgical implantation of a DBS system. The Research Ethics Boards of the University Health Network and the Centre for Addiction and Mental Health approved this study. Informed consent was obtained from either the patient or a surrogate after the nature and possible consequences of DBS surgery were explained. Patient demographics, medication use, and baseline and one-year Alzheimer’s Disease Assessment Scale-Cognitive Subscale-11 (ADAS-Cog) [19] scores are reported in Table 1. Additional neuropsychological data included: MMSE, verbal fluency as measured using animal fluency, intelligence quotient (IQ) measured using the Wechsler Abbreviated Scale of Intelligence [20], trail making test A and B [21], and face recognition using the Wechsler Memory Scale, 3rd edition [22] (Supplementary Tables 1 and 2). To provide a better indicator of hippocampus-mediated cognitive outcomes, we devised a composite measure comprised of the mean percent change over one year of fornix DBS across four memory-related neuropsychological measures: 1) the word recall component of the ADAS-Cog; 2) the word recognition component of the ADAS-Cog; 3) immediate face recognition; and 4) delayed face recognition.
Table 1.
Sex, age, baseline and one-year cognitive scores, and medication treatments for all six patients treated using fornix DBS. Note that higher ADAS-Cog scores represent worse performance.
| Patient | Sex | Baseline age (years) | Baseline ADAS-Cog | One year ADAS-Cog | Medication |
|---|---|---|---|---|---|
| 1 | Female | 51 | 18.67 | 20.66 | Donepezil |
| 2 | Female | 69 | 18.30 | 23.33 | Galantamine |
| 3 | Male | 58 | 28.67 | 42.67 | Galantamine |
| 4 | Male | 62 | 11.67 | 7.33 | Donepezil |
| 5 | Male | 60 | 24.00 | 30.67 | Donepezil, Memantine |
| 6 | Male | 64 | 13.33 | 15.33 | Rivastigmine |
ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale.
MRI volumetry of hippocampus, fornix, and mammillary bodies
Structural MRI scans were obtained at three time points: on the day of DBS implantation (i.e., pre-DBS), on the day immediately following initial DBS insertion (i.e., immediate post-DBS), and one year later (i.e., delayed post-DBS). Patient 4 was also re-scanned at three years. Immediate post-DBS scans were used as baseline scans in all assessments of volumetric change, in order to control for any image artifacts caused by—or potential volumetric influence due to—the presence of the DBS electrodes. All scans analyzed in this study, therefore, contained DBS electrodes at the fornix target. MRI scans were acquired with a 1.5 Tesla GE Signa EXCITE scanner (GE Healthcare, Waukesha, WI). A T1-weighted, three-dimensional spoiled gradient recalled (SPGR) volumetric sequence was obtained with the following parameters: repetition time (TR) = 11.9 ms, echo time (TE) = 5.0 ms, inversion time (TI) = 300 ms, flip angle = 20°, field-of-view = 25.9 cm × 25.9 cm, matrix = 256 × 256, slice thickness = 1.4 mm/0.7 mmoverlap yielding 248 slices. Effective voxel size was 0.5 × 0.5 × 1.4 mm = 0.35 mm3.
Using the software program Medical Image Processing and Visualization (MIPAV version 5.2.1, NIH, Bethesda, MD), all scans underwent N3 intensity non-uniformity correction [23] and realignment to the anterior-posterior commissure plane to correct for any minor variability due to head positioning [24]. Images were re-sampled into isotropic 1 mm3 voxels, as per the usual protocol in our laboratory. The use of isotropic voxels permits navigation through images in several different orientations at the same resolution, thereby providing a theoretical advantage in the reproducibility of structural boundary delineation [24].
Hippocampal, fornix, and mammillary body volume measurements were performed by manual segmentation on deidentified scans by two trained observers blinded to patient identity, scan date, and clinical outcome. Hippocampal measurements were performed using MIPAV according to the protocol of Watson et al. [25]. Fornix and mammillary body measurements were also performed using MIPAV according to the protocol of Copenhaver et al. [26]. Inter-rater reliability of volumetric measurements was assessed with the intraclass correlation coefficient (ICC). We report an absolute agreement standard for ICC, which for each structure represents the variance of the measurement, divided by the sum of the variance of the measurement and the variance over subjects [27]. Acceptable values for ICC are variable in the literature: some authors consider ICC > 0.7 to be adequate [27], while others argue for a more stringent threshold of ICC > 0.75 or 0.8 [28]. We chose to use the more stringent threshold of ICC > 0.8 for acceptable interrater reliability in our volumetric data.
Hippocampal glucose metabolism
As described in Laxton et al. [11], PET scans with the radiotracer [18F]-2-deoxy-2-fluoro-D-glucose ([18F]-FDG) to measure regional cerebral glucose metabolism were acquired preoperatively and in the on stimulation condition following 12 months of continuous DBS. PET scanning in patient #1 was acquired on a Siemens PET/CT scanner at Princess Margaret Hospital. PET scans in patients #2–6 were performed on a CPS/Siemens high-resolution research tomography scanner at the Centre for Addiction and Mental Health. [18F]-FDG was synthesized as described in Hamacher et al. [29]. During the radiotracer uptake period, subjects were maintained in a quiet, dimly lit room, with eyes open and ears unoccluded. Thirty minutes after a 5 mCi ± 10% radiotracer injection, patients were positioned in the scanner, and a 20-min emission scan was obtained, followed by a transmission scan. The last 10 min of the emission scan (40 min post-[18F]-FDG administration) were used for quantitative analysis.
Glucose metabolic rates were calculated (in mg/100 g/min) on a pixel-by-pixel basis by using a single venous blood sample (obtained 20 min after radiotracer injection) fit to a population curve [30]. This quantification method has been validated against arterial blood sampling and is sensitive to disease and medication effects in AD [31]. For the [18F]-FDG quantitative images, PET to PET registration was performed with statistical parametric mapping, version 5 (SPM5, Institute of Neurology, London, UK) using the normalized mutual information algorithm. The images were spatially normalized into standard 3-dimensional space relative to the anterior commissure using the Montreal Neurological Institute (MNI) ICBM 152 stereotactic template within SPM5. Voxel-wise, statistical analyses were performed with SPM5. The glucose metabolism images were smoothed with an isotropic Gaussian kernel (full width at half maximum, 4 mm). The glucose metabolic rates were normalized by scaling to a common mean value across all scans, after establishing that the global means did not differ significantly between groups and across conditions (P > 0.1).
MRI data were rigidly aligned (three translations and rotations) to PET data in order to get information regarding total glucose metabolism in the hippocampus [32]. Hippocampal labels obtained via manual segmentation were transformed into the image space of the PET data using nearest neighbor resampling so that total metabolism in the hippocampus could be estimated. A mask for the pons was drawn on the ICBM-152 model as per the approach of Ishii et al. [33] and was then nonlinearly transformed to fit each subject’s baseline neuroanatomy using a region-of-interest nonlinear registration approach [34]. The total glucose metabolism within the pons was then estimated in each patient and used as a normalization factor when reporting hippocampal values.
Deformation-based morphometry
Deformation-based morphometry (DBM) is an unbiased, data- driven method which generates a local measure of volume expansion or contraction over time at the voxel level [16]. DBM required two steps. First, immediate post-DBS implantation scans were nonlinearly registered to one-year scans for each patient [35]. The Jacobian of the determinant for each deformation field was then estimated, yielding a voxel-by-voxel measure of expansion and contraction. Second, MRI volumes were combined to create a population-specific template in order to align each of the Jacobian determinant maps in a common space [36,37]. Maps were blurred (6 mm Gaussian kernel) and voxel-wise statistics were conducted using the interval of time between the two scans in a linear model with the RMINC software package. The final average was warped to MNI-space. We report clusters with peak-values reaching P < 0.01 (uncorrected) and having minimum cluster size of 250 26-face-, edge-, and corner-connected voxels.
Comparison to untreated AD patients
In order to compare structural brain changes in our DBS patients to those expected in AD—specifically the rate of hippocampal atrophy—we used publicly available imaging data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://www.adni-info.org). We generated a set of AD patients from the ADNI database matched to our DBS patients in age, sex, and neurocognitive severity at diagnosis. Specifically, we identified all ADNI patients who met the following criteria: probable diagnosis of AD at baseline and 12 months, 1.5T T1-weighted MRI scans available at baseline and 12 month time points, age at baseline within range of ages for the DBS group, baseline MMSE score within the range of scores for the DBS group, and baseline ADAS-Cog score within the range of scores for the DBS group. In total, 26 ADNI subjects were identified who met these criteria. MRI scans for all patients were inspected for motion or other artifacts; in one patient, wrap-around artifact caused anterior-posterior commissure realignment to fail repeatedly, so this patient was excluded from further analysis, leaving a dataset of 25 matched patients in total. No additional N3 intensity non-uniformity correction was performed as this step had already been included in the ADNI image-processing pipeline.
We completed hippocampal volume measurements using manual segmentation as described above on both baseline and 12-month scans for all 25 matched AD subjects. Segmentations were performed on scans realigned to the anterior-posterior commissure plane. DBM was also performed on the group of matched AD subjects, using the same methodology described above.
Results
Hippocampus, fornix, and mammillary body volume changes in response to fornix DBS for AD
To test the hypothesis that fornix DBS could produce structural effects within the circuit of Papez, we measured the volume of the hippocampus on MRI scans of the brain at baseline and following one year of continuous stimulation in six patients with AD. In contrast to expected progressive atrophy of the hippocampus which characterizes AD, two of six patients instead showed a striking increase in right and left hippocampal volume following one year of continuous fornix DBS. Mean (i.e., average of right and left) hippocampal enlargement was 5.6% in patient 1, and 8.2% in patient 4 (Fig. 1A, Supplementary Table 5). Increasing our confidence in this finding was the fact that hippocampal volume measurements were highly concordant across observers: hippocampal volume measurements exhibited a strong intraclass correlation coefficient (ICC) of 0.93 (Supplementary Table 4). Hippocampal volume increases of this magnitude suggest the possibility, at least in certain patients, that fornix DBS may have a trophic effect.
Figure 1.
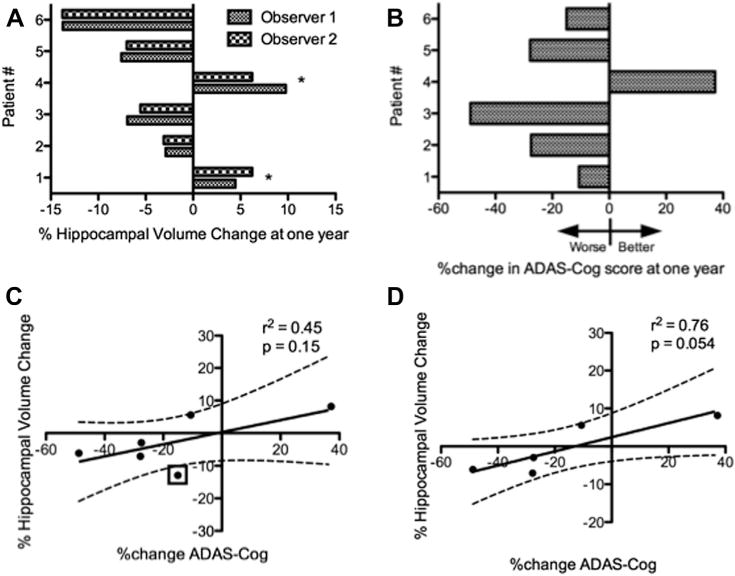
Hippocampal volume changes after one year of continuous fornix DBS and associated cognitive change. (A) Relative changes in mean (i.e., average of right and left) hippocampal volume for each patient after one year of fornix DBS. Unexpectedly, patients 1 and 4 demonstrated hippocampal enlargement (*). (B) ADAS-Cog score changes for each patient following one year of DBS. Patients 1 and 4, who showed evidence of hippocampal enlargement, also showed the best preservation of cognitive function at one year. (C) There was no significant correlation between change in ADAS-Cog score and hippocampal volume at the group-wide level, though patient #6 (marked with a box) appeared to be an outlier, showing relative preservation of ADAS-Cog score despite considerable hippocampal volume loss. (D) When patient 6 was excluded, there was a nearly significant correlation between change in ADAS-Cog score and hippocampal volume.
In light of the hippocampal volume changes we observed, we also looked for evidence of volumetric changes in other key structures within the Papez circuit, specifically the fornix and mammillary bodies. Unlike the hippocampus, neither structure showed enlargement in any subject (Fig. 2A and B). However, patients 1 and 4—in whom we observed stimulation-associated hippocampal volume increases—demonstrated the slowest atrophy rate in both the fornix and mammillary bodies. Furthermore, volume changes in the fornix and mammillary bodies were highly correlated with hippocampal volume change across the study population (Fig. 2C and D). Taken together, these results strongly suggest a circuit-wide structural effect of fornix DBS.
Figure 2.
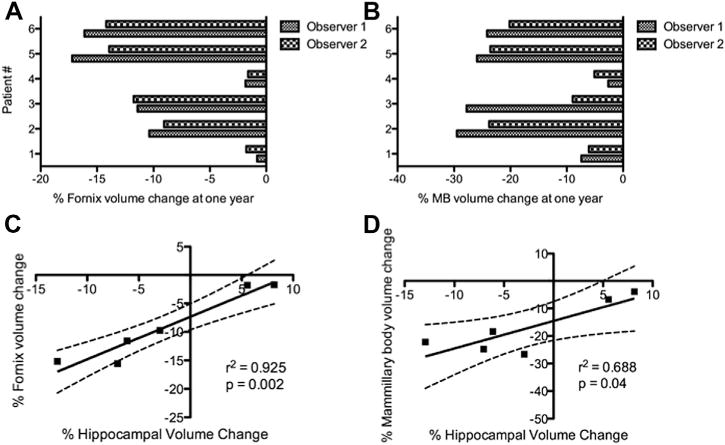
Relationship between hippocampal, fornix, and mammillary body volume change in response to fornix DBS. (A) Relative changes in mean (i.e., average of right and left) fornix volume for each patient after one year of fornix DBS. (B) Relative changes in mean (i.e., average of right and left) mammillary body (MB) volume for each patient after one year of fornix DBS. (C) Hippocampal volume change and fornix volume change are highly correlated. (D) Hippocampal volume change and mammillary body volume change are also highly correlated. Taken together, the results in (C) and (D) support the notion of a circuit-wide neuroprotective effect of DBS in AD.
The significant hippocampal volume increase, relative preservation of volume in the fornix and mammillary bodies, and improvement in ADAS-Cog score (see below) we observed in patient 4 suggested that this patient might represent a unique case of supranormal response to fornix DBS. Consequently, we re-imaged patient 4 three years after initial DBS implantation. Along with four other patients, patient 4 had chosen to continue receiving active fornix DBS beyond the conclusion of the one-year trial. We assessed hippocampal, fornix, and mammillary body volumes in patient 4 on three-year post-DBS implantation MRI scans obtained using the same scanner and scan acquisition parameters as at baseline and one-year time points. Remarkably, the volumes of these structures appeared to stabilize and showed little to no further atrophy over the three year time period (Fig. 3). Although limited to a single patient, these long-term observations are contrary to the expected progressive hippocampal atrophy observed in AD patients—and indeed in normal aging—and argue for a durable and sustained neuroprotective effect of fornix DBS across the Papez circuit.
Figure 3.
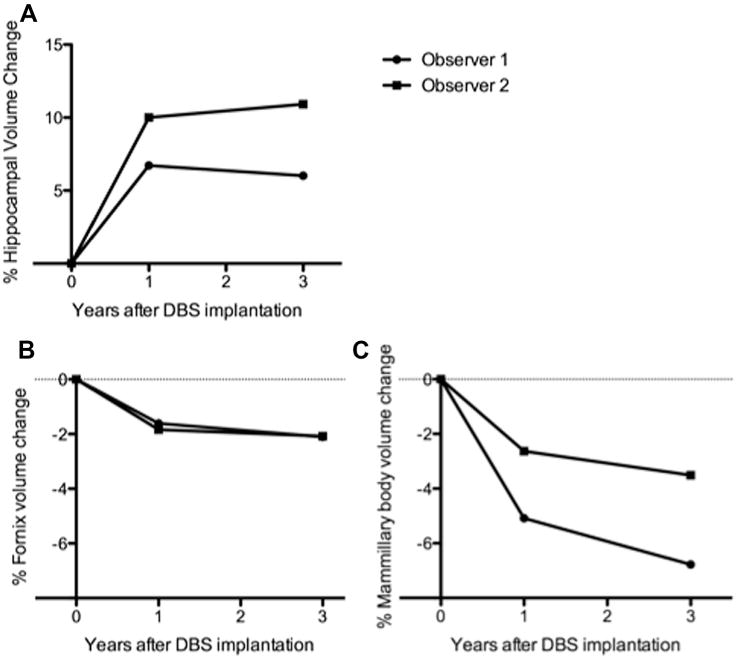
Volume changes in the hippocampus, fornix, and mammillary bodies in patient 4 after three years of continuous fornix DBS. (A) Hippocampal volume is well-preserved at three years in patient 4. (B) and (C) Volumes of the fornix and mammillary bodies are also well-preserved three years after initial DBS implantation in patient 4. These results may argue for a durable neuroprotective effect of fornix DBS in some patients with AD.
Association between hippocampal volume and cognitive outcome
Given that the primary outcome measure in this phase I trial was the ADAS-Cog score after one year of continuous fornix DBS, we looked for associations between hippocampal volume change and ADAS-Cog scores. The hippocampal volume increases we measured in patients 1 and 4 indeed appeared to correspond to cognitive outcome (Fig. 1B). Patient 4, who demonstrated a large increase in hippocampal volume of >8%, was the only patient in the trial whose score on the ADAS-Cog improved from baseline following one year of continuous fornix DBS, contrary to what is expected in AD. Patient 1, whose total hippocampal volume increased by >5%, did not show an improvement in ADAS-Cog score at one year but showed the least deterioration among the remaining patients. Based on these data, it appears that increasing hippocampal size with fornix DBS is not associated with any obvious deleterious effects and may instead be tied to cognitive benefit.
Note that, despite the relative long-term preservation of volume in the hippocampus (as well as the fornix and mammillary bodies) observed in patient #4, this patient’s cognitive function did ultimately decline: the patient’s baseline ADAS-Cog score of 11.67 improved to 7.33 at 12 months, but by 3 years it had worsened to 15.33. IQ, verbal fluency, and performance on the trail making test had also all declined at 3 years, though immediate and delayed face recognition remained preserved (Supplementary Table 2).
At the group-wide level, we did not initially identify a statistically significant correlation between one-year change in ADAS-Cog score and one-year change in hippocampal volume (Fig. 1C). However, a stronger, effectively statistically significant correlation (Pearson r2 = 0.76, P = 0.054, Fig. 1D) became apparent after reanalyzing the data following the exclusion of patient #6, who appeared to be an outlier with a well-preserved ADAS-Cog score (third-best clinical outcome after patients #1 and #4) despite a large decrease in hippocampal volume over 12 months. Since the ADAS-Cog assesses several domains, each of which is variably dependent on intact hippocampal function, we also examined whether changes in hippocampal volume were related to changes on specific components of the ADAS-Cog. We did not find any such relationships at the group-wide level, though patient #4’s improvement on the ADAS-Cog was largely driven by the word recognition component. Raw scores for each patient on each component of the ADAS-Cog at baseline and follow-up are included in Supplementary Table 3.
To look for any potential relationship between hippocampal structural change and predominantly hippocampus-mediated cognitive performance (i.e., that related to episodic memory), we created a composite memory measure as described in the Methods. We found that only patients #1 and #4—who had an increase in hippocampal volume—showed a net increase in this composite score over one year (Supplementary Fig. 1A). Furthermore, hippocampal volume change was significantly correlated with change in this composite score (Supplementary Fig. 1B).
Changes in hippocampal glucose metabolism in response to fornix DBS for AD
We hypothesized that a trophic effect underlying hippocampal enlargement in response to fornix DBS might be linked to DBS-induced changes in hippocampal metabolism. Indeed, we found that relative hippocampal glucose metabolism increased only in patients 1 and 4, the same patients who demonstrated increases in hippocampal volume (Fig. 4A). Across the entire patient cohort, changes in hippocampal glucose metabolism and hippocampal volume were well correlated (Fig. 4B). These results suggest that the structural and possible trophic effects of fornix DBS may depend on its ability to successfully enhance metabolism within the AD-affected hippocampus. Note that hippocampal metabolism was not correlated with cognitive outcome per se, neither at baseline (Pearson r2 = 0.07, P = 0.63) nor one-year follow-up (Pearson r2 = 0.0004, P = 0.97). It is possible that PET scans performed before and after a memory activation task might have been better suited to evaluate the change in functional—as opposed to structural—response of memory circuits following one year of fornix DBS, but we did not perform provocative scans of this nature in any of the six enrolled patients.
Figure 4.
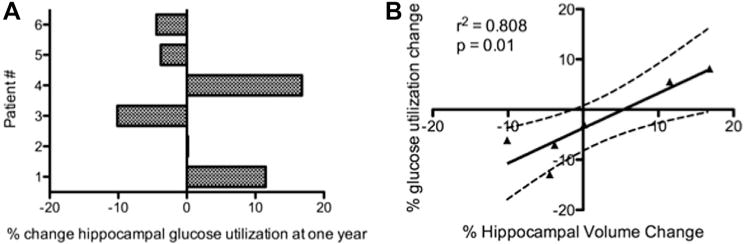
Change in hippocampal metabolism after one year of fornix DBS. (A) Hippocampal glucose metabolism (normalized by pontine glucose metabolism) increased following one year of fornix DBS in patients 1 and 4, in whom we also observed increases in hippocampal volume. (B) Across all patients, hippocampal glucose metabolism tracked hippocampal volume change over one year, implying that any favorable effects of fornix DBS against neurodegeneration may depend on its ability to successfully enhance metabolism within the AD-affected hippocampus.
Brain-wide structural changes in response to fornix DBS for AD
Exploratory DBM analysis revealed several clusters of volume expansion throughout the brain at the group level in response to one year of fornix DBS (Fig. 5, Table 2). Notably, several of these volume expansions were located in areas known to atrophy in AD, including the right and left parahippocampal gyrus, right superior temporal gyrus, left inferior parietal lobule, and bilateral precuneus. We also observed clusters of volume expansion in areas typically unaffected by AD, in particular in the thalamus and superior frontal gyrus, though given the close proximity of these areas to the implanted DBS electrodes, we cannot rule out the possibility that they merely represent mechanical re-expansion in areas deformed by the initial neurosurgical implantation procedure.
Figure 5.
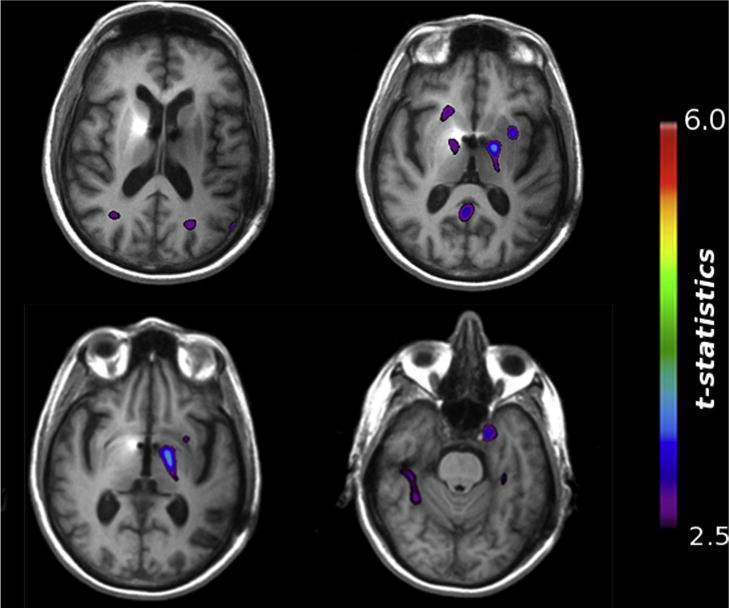
Brain-wide structural effects of fornix DBS in AD assessed using deformation-based morphometry (DBM). Representative axial brain slices showing representative clusters of volume increase across all patients following one year of fornix DBS. Many significant clusters were identified, particularly in several regions typically atrophied in AD (see Table 2).
Table 2.
Significant clusters of local volume increase over one year identified by DBM across all six patients treated using fornix DBS.
| t-value | MNI−x | MNI−y | MNI−z | Anatomical region | Cluster size (voxels) |
|---|---|---|---|---|---|
| 6 | −17 | 7.3 | 60 | Left posterior superior frontal gyrusa | 22,987 |
| 3.2 | 9 | 2 | 58 | Right posterior superior frontal gyrusa | 28,782 |
| 2.8 | 26 | −68 | 32 | Right parieto-occipital fissure | 19,202 |
| 3.2 | 16 | −15 | 23 | Right caudate tail | 3056 |
| 2.7 | −40 | −55 | 21 | Left inferior parietal lobule | 4225 |
| 3 | 85 | −62.2 | 15 | Left/right precuneus | 4856 |
| 2.8 | 27 | 10 | 7 | Right putamen | 2027 |
| 2.6 | −21 | 20 | 8 | Left caudate/putamen | 1695 |
| 2.6 | −14 | −7 | 5 | Left thalamusa | 977 |
| 3.5 | 14 | −4.4 | 4 | Right thalamusa | 7703 |
| 3.3 | −20 | −22 | −22 | Left parahippocampal gyrus | 1503 |
| 2.8 | 19 | −23 | −23 | Right parahippocampal gyrus | 2018 |
| 2.7 | 37 | 13 | −26 | Right antero-superior temporal gyrus | 295 |
| 3.4 | −19 | −40 | 33 | Left cerebellum | 4198 |
| 2.6 | 28 | −47 | 33 | Right cerebellum | 718 |
MNI = coordinate in MNI-space.
Regions which may be susceptible to electrode-related mechanical deformation.
Comparison of fornix DBS and matched AD patients
The 25 ADNI patients we analyzed were well matched to our 6 DBS patients: there were no between-group differences in sex, mean age, mean baseline MMSE score, or mean baseline ADAS-Cog score (Table 3). We also found no difference in mean baseline hippocampal volume between groups (Fig. 6A), suggesting a similar degree of neurodegeneration in both groups at baseline. However, we did find that the degree of volume loss over one year was significantly higher in the ADNI group compared to the DBS group (−9.5% ± 1.2% vs. −2.6% ± 3.3%; t = 2.338, df = 29, P = 0.027; Fig. 6B), suggesting that fornix DBS may be associated with an overall slowing of hippocampal atrophy when compared to a suitably matched group of AD patients.
Table 3.
Demographic and clinical characteristics of DBS patients compared to 25 matched AD subjects from the ADNI database.
| Characteristic | AD DBS (n = 6) | ADNI matched (n = 25) | P-value |
|---|---|---|---|
| Mean age (years) | 60.7 ± 6.1 | 63.9 ± 4.4 | 0.14 |
| Male:Female | 4:2 | 16:9 | 0.90 |
| Mean baseline MMSE | 22.3 ± 4.5 | 23.6 ± 1.8 | 0.27 |
| Mean baseline ADAS-Cog | 19.1 ± 6.4 | 18.7 ± 7.0 | 0.89 |
| Mean baseline hippocampal volume (mm3) | 2180.5 ± 399.0 | 2213.1 ± 476.4 | 0.88 |
| One year ADAS-Cog | 23.3 ± 12.3 | 23.8 ± 10.6 | 0.92 |
| One year MMSE | 21.5 ± 6.2 | 19.7 ± 4.8 | 0.44 |
MMSE: Mini-mental status examination; ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive Subscale. All values are mean ± standard deviation; P-values are based on an unpaired two-tailed Student’s t-test.
Figure 6.
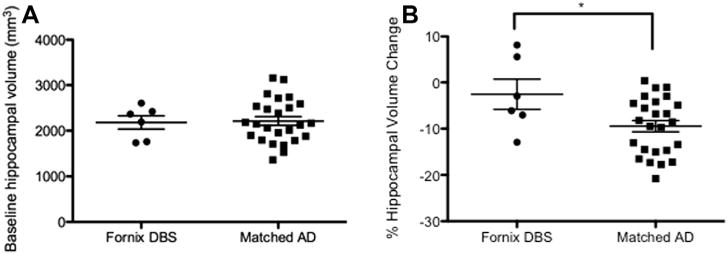
Comparison of baseline hippocampal volume and hippocampal atrophy rate between fornix DBS and matched AD patients. (A) Baseline hippocampal volume was no different between DBS and matched AD patients. (B) % mean hippocampal atrophy over one year was significantly greater in the matched AD group compared to the DBS group (*P < 0.05).
Given our finding of hippocampal enlargement in some DBS patients, we examined the matched ADNI group to see if any similar hippocampal volume increases occurred spontaneously. Across the entire set of 25 matched ADNI patients, no individual patient demonstrated bilateral increases in hippocampal volume. Mean hippocampal volume increased in only a single subject, by a magnitude of only 0.38%, driven by unilateral left hippocampal growth of 0.94%. This volume increase would be well within literature estimates for scan-rescan reliability, which are conservatively on the order of 2–4% [38]. Otherwise, hippocampal volume increased unilaterally in 4 additional patients, with a maximum measured unilateral increase of 2.2% in one patient. To compare the incidence of hippocampal enlargement between the DBS and matched AD groups, we used the binomial test. We found that the incidence of bilateral hippocampal enlargement was, in fact, significantly different between groups (i.e., 2/6 DBS vs. 1/25 matched AD, P = 0.020). In addition, the incidence of hippocampal enlargement expressed on a per hemisphere basis, was also significantly different between groups (4/12 hemispheres DBS vs. 5/50 hemispheres matched AD, P = 0.021).
At the whole-brain level, DBM applied to the matched ADNI group demonstrated several clusters of volume loss in regions typically affected by AD, including the right hippocampus, bilateral temporal poles, and bilateral precuneus (Fig. 7, Table 4). Unlike in the DBS group, there were no intra-parenchymal clusters of volume expansion. In keeping with expected brain-wide atrophy in AD, the matched ADNI group did exhibit ventricular enlargement with significant intra-ventricular clusters bilaterally (left: t = 7.1; MNI x = −9.0, y = −11.5, z = 22.9; right: t = 6.2; MNI x = 12.0, y = 5.0, z = 22.9).
Figure 7.
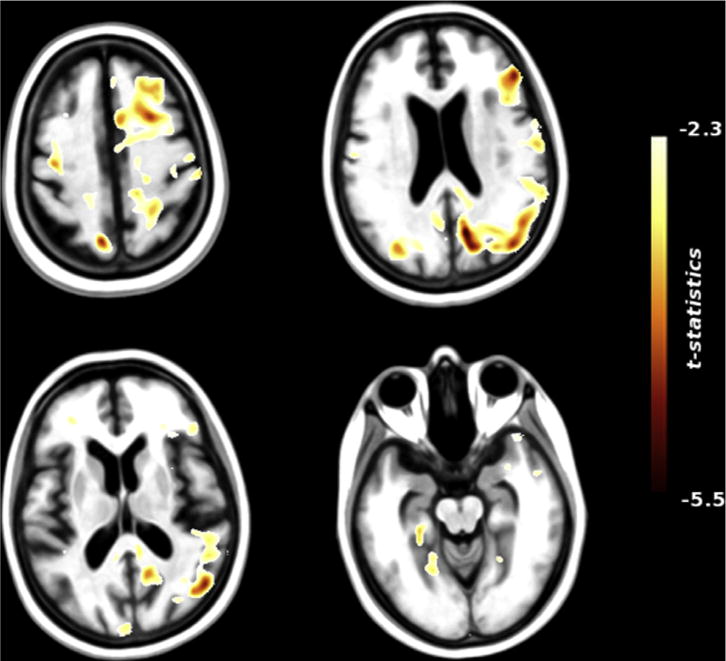
Brain-wide structural changes in matched AD patients assessed using deformation-based morphometry (DBM). Representative axial brain slices showing representative clusters of volume decrease over one year across a group of 25 AD patients from the ADNI database. Several significant clusters of volume loss were identified, in regions known to be atrophic in AD (see Table 4). Clusters of ventricular enlargement were observed bilaterally, consistent with brain-wide atrophy in AD (not shown). Unlike AD DBS patients, no intraparenchymal clusters of volume increase were seen.
Table 4.
Significant clusters of local volume decrease over one year identified by DBM across 25 matched AD patients from the ADNI database.
| t-Value | MNI−x | MNI−y | MNI−z | Anatomical region | Cluster size (voxels) |
|---|---|---|---|---|---|
| −3.5 | 42 | 54 | 23 | Right middle frontal gyrus | 105,228a |
| −3 | 33 | 63 | 23 | Right lateral super frontal gyrus | 105,228a |
| −2.9 | 56 | −6 | 16 | Right operculum | 105,228a |
| −3.5 | −3 | 24 | 11 | Genu of the corpus callosum | 1040 |
| −2.8 | 19 | −6 | 16 | Right caudate/anterior thalamus | 187 |
| −3.5 | 25 | −28 | 13 | Posterior thalamus | 3690 |
| −5.3 | −41 | 56 | 34 | Superior parietal lobule | 1992 |
| −3.2 | −45 | −81 | 11 | Parieto-occipital junction | 586 |
| −3.5 | −12 | −76 | −9 | Left precuneus | 963 |
| −3 | 24 | −71 | −4 | Right precuneus | 175 |
| −3.2 | 25 | −20 | −6.1 | Right hippocampus | 127 |
| −2.3 | 31 | 20 | −42 | Right temporal pole | 5383 |
| −2.7 | −41 | 0 | −37 | Left temporal pole | 251 |
MNI = coordinate in MNI-space.
Right middle frontal gyrus, lateral superior frontal gyrus, and operculum together make up one large cluster of volume loss.
Discussion
Using quantitative MRI volumetry, we found that the volume of the hippocampus increased over 12 months in response to chronic DBS applied to the fornix in some AD patients. These hippocampal volume increases were accompanied by increases in relative hippocampal glucose metabolism. Overall, volume change in the hippocampus was highly correlated with volume change in the fornix and mammillary bodies, suggesting a circuit-wide effect of fornix DBS. In a single patient, hippocampal enlargement was sustained three years after initial DBS implantation, potentially arguing for a durable structural effect of fornix stimulation. Importantly, we did not observe any convincing increases in hippocampal volume in a group of 25 AD patients matched by age, sex, and baseline cognitive function to our 6 DBS patients, and found that the mean rate of hippocampal atrophy was significantly slower in our DBS group compared to the matched AD group. Finally, we found preliminary evidence that fornix stimulation was associated with several clusters of local volume expansion in temporoparietal regions of the brain far from the Papez circuit, but known to be atrophic in AD. Taken together, these results provide—to our knowledge—the first in-human evidence that: 1) DBS may influence the structure of the brain; and, 2) hippocampal and other focal atrophy can potentially be slowed, and possibly reversed, by DBS in some patients with a neurodegenerative disorder.
The notion that DBS can produce plastic changes in the brain is gaining acceptance. Indeed, functional plasticity has been implicated in the clinical benefit observed in patients with Parkinson’s disease, dystonia, or depression treated by DBS [7], given the delay of weeks to months before maximal therapeutic effect is achieved. So far, evidence of structural neuroplasticity occurring in response to DBS has come entirely from pre-clinical studies of DBS in rodents. Toda et al. [13] showed in adult rats that DBS applied to the anterior nucleus of the thalamus—itself a critical node within the Papez circuit—produced a 2- to 3-fold increase in new cells within the dentate gyrus of the hippocampus, most of which assumed a neuronal fate. Hippocampal neurogenesis also follows DBS of the entorhinal cortex [14] and anterior nucleus of the thalamus [15] in mice. Outside the hippocampus, structural change in the form of increased proliferation of apical and basilar dendrites in neurons of the prefrontal cortex has been shown to accompany DBS of the nucleus accumbens [39]. In spite of these mounting animal data, there has been no analogous evidence of structural neuroplasticity accompanying DBS in humans. To our knowledge, there are no published studies examining neuroanatomical changes over time in patients receiving DBS for any indication.
The hippocampus possesses a particular capacity for structural neuroplasticity, and several quantitative human MRI studies have suggested that it can enlarge under certain training paradigms or on recovery from neurological disease states (for a review see Ref. [40]). That being said, hippocampal enlargement has, to our knowledge, never been reported in AD patients, who instead classically exhibit progressive hippocampal atrophy compared to age-matched healthy controls [41]. Significant hippocampal atrophy can be detected during the pre-AD period of amnestic mild cognitive impairment, and continues following conversion to AD. Shortly after diagnosis, the mean atrophy rate has been estimated to be on the order of 4–5% per year [41], though the reported literature averages are highly variable. Consequently, the 5–8% increases we observed in two patients with early AD are not only unexpected, but appear to be of a magnitude sufficient to bring hippocampal volumes back to pre-disease levels. That these two patients also had the best cognitive outcomes in response to fornix DBS further suggests that therapeutic efficacy of stimulation may be linked to structural effects on the hippocampus. More generally, our finding at the group-wide level that changes in hippocampal volume appear to track changes in memory-specific, largely hippocampus-mediated neuropsychological measures, further supports this possibility.
There are several limitations of this study, and caution needs to be exercised to avoid over-interpreting the data. Obviously, the sample size of six patients is small, and weakens attempts at identifying group-wide effects, particularly in the brain-wide exploratory analysis using DBM. Further, given the inherent variability of neuropsychological test scores—especially in a population with dementia—even statistically significant group-wide correlations between structural brain changes and cognitive outcome need to be interpreted with extreme caution. To some extent, these issues are partially overcome by the prospective, within-subject design of the study, and by carefully controlling for potential confounders (e.g., medication dosage was never changed in any patient, baseline MRI scans were obtained after electrode insertion to ensure that any electrode artifact was consistent between baseline and one-year follow-up). Another concern is that hippocampal enlargement was observed in only two patients, and could represent a chance finding. We believe this is unlikely for several reasons. First, we used a rigorous approach to hippocampal volumetry involving observers blinded to patient identity, clinical outcome, and scan date, and these observers segmented the hippocampus—as well as the fornix and mammillary bodies—with high inter-rater reliability. Second, the magnitude of volume change we observed appears too large to be accounted for merely by scan- rescan error [38]. Third, we did not observe any instances of bilateral hippocampal enlargement in a matched group of AD patients untreated with DBS. Finally, hippocampal volume changes were highly correlated with the volumes of other structures in the Papez circuit, and with changes in hippocampal glucose metabolism, arguing for a biological, circuit-wide DBS effect which resulted in hippocampal enlargement in some subjects.
Why only two patients demonstrated hippocampal enlargement in our study remains a matter of uncertainty. In the case of patient 4, who demonstrated remarkably preserved hippocampal, fornix, and mammillary body volumes three years after DBS implantation, one possible explanation is that the diagnosis of AD was erroneous. Admittedly, we do not have pathological confirmation of AD by autopsy in this patient, nor in any other patients. However, patient 4 met all study inclusion criteria, had evidence of clinically significant memory impairment on enrollment, and, importantly, did not have abnormally large hippocampi at baseline. In fact, baseline hippocampal volume was neither correlated with subsequent hippocampal volume change (Supplementary Fig. 2) nor clinical outcome in response to DBS across the entire patient group. It is also important to note that, despite preserved hippocampal, fornix, and mammillary body volume, patient 4 ultimately demonstrated progressive worsening of cognitive function by three years of follow-up. Interestingly, this patient still had preserved scores on the immediate and delayed face recognition tasks, which may be related to preserved hippocampal integrity. However, we speculate that while fornix DBS may have a durable trophic effect on the Papez circuit, it may be that as AD progresses, cognitive function eventually becomes decoupled from Papez circuit integrity as more profound, brain-wide neurodegeneration occurs.
The exact mechanisms underlying the structural effects of DBS we observed are still uncertain, and there are several possibilities. We speculate that, in addition to neurogenesis and increases in neuritic arborization which have been implicated in animal models of DBS, volume changes both within and outside the hippocampus could also be the result of axonal remodeling, synaptogenesis, gliogenesis, increase in neuronal size, increased microvascularization, increased extracellular fluid, or a combination of these factors [40,42].
Conclusions
Our findings provide preliminary evidence in support of the notion that DBS can change the structure of the human brain, and may potentially have restorative properties in neurodegenerative conditions such as AD. These data provide a significant impetus for future work aimed at uncovering similar evidence of structural neuroplasticity in other patient populations treated by DBS, using non-invasive, multi-modal imaging. Further study into the potential mechanisms underlying such plasticity will also be critically important, and may point the way to applications of DBS to harness plasticity for therapeutic ends.
Supplementary Material
Acknowledgments
Funding disclosures: T.S. is supported by a Canadian Institutes of Health Research (CIHR) fellowship award (#234784). A.W.L. received support from the Surgeon Scientist Program, Department of Surgery, University of Toronto, and the Neurosurgical Research and Education Foundation of the American Association of Neurological Surgeons. A.M.L. is a Canada Research Chair in Neuroscience and is supported by the R.R. Tasker Chair in Functional Neurosurgery. Additional support was provided by the Dana Foundation and Krembil Neuroscience Discovery Fund.
Footnotes
Conflicts of interest: A.M.L. is a consultant to Medtronic, St Jude, and Boston Scientific. A.M.L. serves on the scientific advisory board of Ceregene, Codman, Neurophage, Aleva and Alcyone Life Sciences. A.M.L. is co-founder of Functional Neuromodulation Inc. and holds intellectual property in the field of Deep Brain Stimulation. All other authors declare no relevant conflicts.
Authorship contributions: T.S., A.W.L., and A.M.L. conceived and designed the study. T.S. supervised all data collection and analysis, and drafted most of the manuscript. M.M.C. collected deformation-based morphometry data, performed neuroimaging data quality control, and wrote part of the Methods. A.B., M.L., and T. O. collected and analyzed MRI volumetric data. A.W.L., D.F.T-W. and M.P.M performed and analyzed clinical assessments. C.I.W. and G.S.S. collected and analyzed PET data and wrote part of the Methods. All authors contributed to critically revising the manuscript, and approved the final version before submission.
Supplementary data
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.brs.2014.11.020.
References
- 1.Pizzolato G, Mandat T. Deep brain stimulation for movement disorders. Front Integr Neurosci. 2012;6:2. doi: 10.3389/fnint.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Holtzheimer PE, Kelley ME, Gross RE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez-Ponce F, Velasco-Campos F, Castro-Farfan G, et al. Preliminary study in patients with obsessive-compulsive disorder treated with electrical stimulation in the inferior thalamic peduncle. Neurosurgery. 2009;65(6 Suppl):203–9. doi: 10.1227/01.NEU.0000345938.39199.90. discussion 209. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384–93. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 6.Lipsman N, Woodside DB, Giacobbe P, et al. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet. 2013;381(9875):1361–70. doi: 10.1016/S0140-6736(12)62188-6. [DOI] [PubMed] [Google Scholar]
- 7.Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77(3):406–24. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 9.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101(13):4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12(1):27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laxton AW, Tang-Wai DF, McAndrews MP, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68(4):521–34. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 12.Smith GS, Laxton AW, Tang-Wai DF, et al. Increased cerebral metabolism after 1 year of deep brain stimulation in Alzheimer disease. Arch Neurol. 2012;69(9):1141–8. doi: 10.1001/archneurol.2012.590. [DOI] [PubMed] [Google Scholar]
- 13.Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg. 2008;108(1):132–8. doi: 10.3171/JNS/2008/108/01/0132. [DOI] [PubMed] [Google Scholar]
- 14.Stone SS, Teixeira CM, Devito LM, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31(38):13469–84. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamani C, Stone SS, Garten A, Lozano AM, Winocur G. Memory rescue and enhanced neurogenesis following electrical stimulation of the anterior thalamus in rats treated with corticosterone. Exp Neurol. 2011;232(1):100–4. doi: 10.1016/j.expneurol.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Chung MK, Worsley KJ, Paus T, et al. A unified statistical approach to deformation-based morphometry. Neuroimage. 2001;14(3):595–606. doi: 10.1006/nimg.2001.0862. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. The Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 21.Reitan RM. Trail making test: manual for administration, scoring and interpretation. Indianapolis, IN: Indiana University Medical Center; 1958. [Google Scholar]
- 22.Wechsler D. Wechsler Memory Scale – third edition manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 23.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 24.Boccardi M, Bocchetta M, Apostolova LG, et al. Establishing magnetic resonance images orientation for the EADC-ADNI manual hippocampal segmentation protocol. J Neuroimaging. 2014;24(5):509–14. doi: 10.1111/jon.12065. [DOI] [PubMed] [Google Scholar]
- 25.Watson C, Cendes F, Fuerst D, et al. Specificity of volumetric magnetic resonance imaging in detecting hippocampal sclerosis. Arch Neurol. 1997;54(1):67–73. doi: 10.1001/archneur.1997.00550130049015. [DOI] [PubMed] [Google Scholar]
- 26.Copenhaver BR, Rabin LA, Saykin AJ, et al. The fornix and mammillary bodies in older adults with Alzheimer’s disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry Res. 2006;147(2–3):93–103. doi: 10.1016/j.pscychresns.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Nugent AC, Luckenbaugh DA, Wood SE, Bogers W, Zarate CA, Jr, Drevets WC. Automated subcortical segmentation using FIRST: test-retest reliability, interscanner reliability, and comparison to manual segmentation. Hum Brain Mapp. 2013 Sep;34(9):2313–29. doi: 10.1002/hbm.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 29.Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of nocarrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med. 1986;27(2):235–8. [PubMed] [Google Scholar]
- 30.Takikawa S, Dhawan V, Spetsieris P, et al. Noninvasive quantitative fluorodeoxyglucose PET studies with an estimated input function derived from a population-based arterial blood curve. Radiology. 1993;188(1):131–6. doi: 10.1148/radiology.188.1.8511286. [DOI] [PubMed] [Google Scholar]
- 31.Smith GS, Kramer E, Ma Y, et al. Cholinergic modulation of the cerebral metabolic response to citalopram in Alzheimer’s disease. Brain. 2009;132(Pt 2):392–401. doi: 10.1093/brain/awn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- 33.Ishii K, Soma T, Kono AK, et al. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with lewy bodies and those with mild Alzheimer’s disease. J Nucl Med. 2007;48(5):704–11. doi: 10.2967/jnumed.106.035691. [DOI] [PubMed] [Google Scholar]
- 34.Chakravarty MM, Sadikot AF, Germann J, Bertrand G, Collins DL. Towards a validation of atlas warping techniques. Med Image Anal. 2008;12(6):713–26. doi: 10.1016/j.media.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Robbins S, Evans AC, Collins DL, Whitesides S. Tuning and comparing spatial normalization methods. Med Image Anal. 2004;8(3):311–23. doi: 10.1016/j.media.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Borghammer P, Ostergaard K, Cumming P, et al. A deformation-based morphometry study of patients with early-stage Parkinson’s disease. Eur J Neurol. 2010;17(2):314–20. doi: 10.1111/j.1468-1331.2009.02807.x. [DOI] [PubMed] [Google Scholar]
- 37.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv. 2006;9(Pt 2):58–66. doi: 10.1007/11866763_8. [DOI] [PubMed] [Google Scholar]
- 38.Morey RA, Selgrade ES, Wagner HR, 2nd, Huettel SA, Wang L, McCarthy G. Scan-rescan reliability of subcortical brain volumes derived from automated segmentation. Hum Brain Mapp. 2010;31(11):1751–62. doi: 10.1002/hbm.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falowski SM, Sharan A, Reyes BA, Sikkema C, Szot P, Van Bockstaele EJ. An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery. 2011;69(6):1281–90. doi: 10.1227/NEU.0b013e3182237346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8(4):189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 41.Barnes J, Bartlett JW, van de Pol LA, et al. A meta-analysis of hippocampal atrophy rates in Alzheimer’s disease. Neurobiol Aging. 2009;30(11):1711–23. doi: 10.1016/j.neurobiolaging.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn Sci. 2011;15(10):475–82. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


