SUMMARY
Retinoblastomas develop due to the loss of the Rb protein, yet the cell type in which Rb suppresses retinoblastoma, and the cellular circuitry that underlies the need for Rb are undefined. Here, we show that retinoblastoma cells express markers of post-mitotic cone precursors, but not markers of other retinal cell types. We also demonstrate that human cone precursors prominently express MDM2 and N-Myc, that retinoblastoma cells require both of these proteins for proliferation and survival, and that MDM2 is specifically needed to suppress ARF-induced apoptosis in cultured retinoblastoma cells. Interestingly, retinoblastoma cell MDM2 expression was regulated by the cone-specific RXRγ transcription factor and a human-specific RXRγ consensus binding site, and proliferation required RXRγ as well as the cone-specific thyroid hormone receptor-β2. These findings provide support for a cone precursor origin of retinoblastoma and suggest that human cone-specific signaling circuitry sensitizes to the oncogenic effects of RB1 mutations.
INTRODUCTION
Retinoblastoma is a childhood retinal tumor that has provided numerous insights into human cancer biology. For instance, retinoblastoma was one of the first malignancies to be recognized as having hereditary features, and it engendered the characterization of one of the first tumor suppressors genes to be cloned, RB1 (Weller, 1937; Friend et al., 1986). In turn, the retinoblastoma protein, Rb, was found to have crucial roles in cell cycle control and differentiation, and to govern a signaling pathway that is inactivated in most if not all human cancers (Weinberg, 1995; Cobrinik, 2005; Skapek et al., 2006). However, despite the general significance of Rb in cancer biology, the basis for its crucial role in retinoblastoma pathogenesis has not been defined.
Retinoblastomas are thought to develop due to the inactivation of RB1, either with somatic inactivation of both RB1 alleles, or with a germline RB1 mutation and somatic inactivation of the second allele in hereditary cases. Biallelic RB1 mutations may initially result in the production of benign retinomas, with subsequent genetic changes mediating malignant transformation (Dimaras et al., 2008). Germline RB1 mutations predispose to an average of five retinoblastomas, usually bilaterally, and with most forming in the first year (Abramson and Gombos, 1996), but predispose to only a ~0.5% chance of developing other cancers per year (Kleinerman et al., 2005). The exceptionally high rate of retinoblastoma arising from the minuscule retinal cell population implies that the tumors derive from a cell type that is unusually sensitive to the loss of Rb function.
One way that a cell may be sensitized to the loss of Rb is by having signaling circuitry that fails to respond to Rb loss with appropriate countermeasures. Indeed, at least two cellular responses to Rb loss impede tumorigenesis in other settings, but evidently fail to do so in the cells from which retinoblastomas arise. First, in diverse settings including explanted mouse retinas, Rb loss is compensated by increased expression of the Rb-related p107 (Donovan et al., 2006). Second, loss of Rb can deregulate E2F transcription factors and elicit E2F-dependent apoptosis (Chen et al., 2007). Among other effects, deregulated E2Fs induce expression of a CDKN2A isoform that encodes ARF, which inhibits MDM2 and promotes p53-mediated apoptosis (Iaquinta and Lees, 2007). As Rb-deficient retinas and pre-malignant retinomas have greatly increased CDKN2AARF expression (Laurie et al., 2006; Chen et al., 2007; Dimaras et al., 2008), it seems that the ARF-induced apoptotic response may be impaired in early stages of retinoblastoma tumorigenesis.
To gain insight into the circuitry that sensitizes to Rb loss, we sought to relate features of retinoblastoma to human retinal development. As retinoblastomas form as early as 21 weeks of gestation (Maat-Kievit et al., 1993), we initially sought clues to this circuitry by evaluating Rb’s developmental expression pattern. We found that Rb is expressed in a cell cycle-dependent manner in retinal progenitor cells, but was not detected in the early post-mitotic precursors of the different retinal neurons. However, Rb was detected in older, maturing retinal precursors, and at exceptionally high levels in maturing cone precursor cells (Lee et al., 2006).
Rb’s prominent expression in cone precursors was intriguing, as cultured retinoblastoma cells generally express cone markers (Bogenmann et al., 1988), but not glial markers as was once believed (Virtanen et al., 1988), and the tumors have cone but not rod phototransduction activities (Hurwitz et al., 1990). In addition, retinoblastomas are topographically distributed across the retina in a pattern that mimics that of L/M cones (Munier et al., 1994). Nevertheless, the significance of the cone circuitry has been unclear, as cone features were not consistently detected in retinoblastomas in situ, and cells with properties of other retinal cell types are also present in the tumors (Gonzalez-Fernandez et al., 1992; Nork et al., 1995). Moreover, mice with targeted loss of Rb and Rb-related proteins produced tumors with amacrine or horizontal cell, but not cone cell, features (Robanus-Maandag et al., 1998; Chen et al., 2004; Dannenberg et al., 2004; MacPherson et al., 2004; Ajioka et al., 2007). Thus, in the current study, we examined the relevance of cone-specific signaling circuitry to retinoblastoma tumorigenesis.
RESULTS
Widespread expression of L/M cone photoreceptor markers in retinoblastoma tumors
To assess the cellular phenotypes of retinoblastoma cells, we stained a panel of tumors with retinal cell type-specific markers. The panel included tumors displaying a range of differentiation states, with and without prior chemotherapy, and from bilaterally and unilaterally affected patients (Table S1).
As mature photoreceptor features were previously detected in only a subset of retinoblastomas, we initially examined whether the tumors more generally express proteins that are characteristic of immature photoreceptor precursors. These included CRX, which is specific to cones, rods, and bipolar cells (Bibb et al., 2001); RXRγ, which is specific to cones and ganglion cells (Mori et al., 2001); TRβ2, which is specific to cones (Ng et al., 2001); and NRL, which is specific to rods (Swain et al., 2001) (Figures 1A–D and S1).
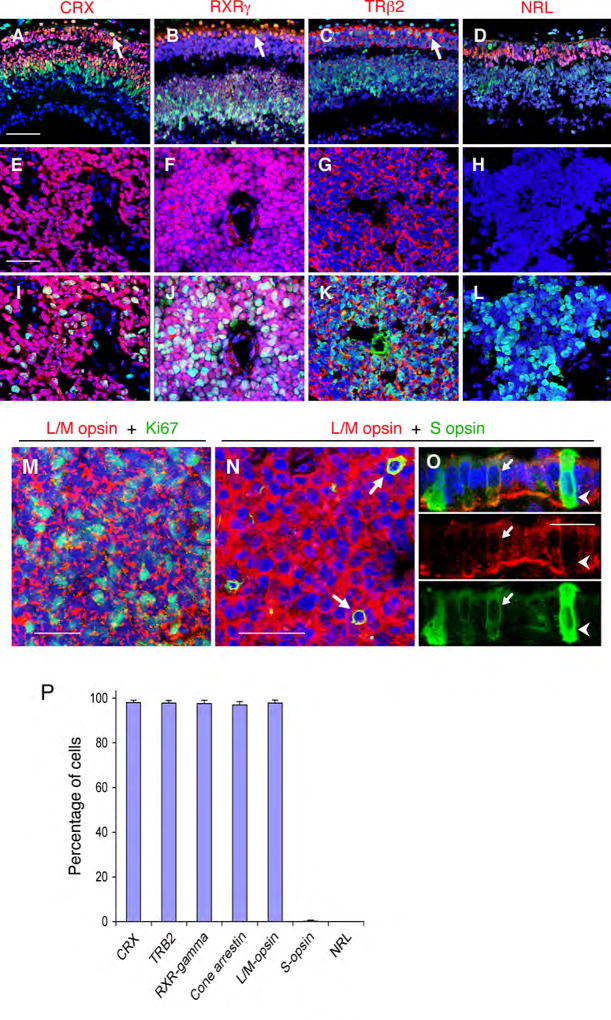
Figure 1. Cone precursor markers in developing retina and retinoblastoma tumors.
(A–D) Photoreceptor precursor markers (red) and Rb (green) in gestational wk 16 human retina. Note co-expression of Rb with cone markers CRX, RXRγ, and TRβ2 but not with the rod marker NRL (arrows, see also Figure S1). (E–L) Cone but not rod precursor markers (red) in retinoblastomas without (E–H) or with (I–L) Ki67 co-staining. The specificity of TRβ2 staining was confirmed by knockdown analyses (Figure S1). (M) Co-expression of L/M opsin (red) and Ki67 (green) in retinoblastoma. (N–O) Co-expression of L/M opsin and S opsin (arrows) in retinoblastoma (N) and in wk 18 perifoveal L/M cone precursors, but not in S cone precursors (O). Scale bars, 50 µm in A-N; 20 µm in O. (P) Mean percentage of cells expressing each marker in representative sections of 10 tumors (see Table S1). Error bars indicate standard deviation.
CRX, RXRγ, and TRβ2 were detected in the majority of cells in each of 40 tumors, including >95% of cells in 10 quantitatively evaluated samples, whereas NRL generally was not detected (Figure 1E–H, 1P, and Table S2). CRX, RXRγ, and TRβ2 were expressed in all tumor regions, and in morphologically differentiated cells as well as in proliferating cells that express Ki67 or phosphorylated histone H3 (Figure 1I–L, and data not shown). In keeping with their neoplastic status, CRX+, RXRγ+, and TRβ2+ tumor cells lacked detectable Rb (Figure 2A, B), whereas CRX+, RXRγ+, and TRβ2+ cone precursors in the central retina had prominent Rb expression (Figures 1A–D and S1). In addition, the vast majority of cells in each of 22 retinoblastomas expressed cone-specific arrestin (Figures 1P and Figure S2).
Figure 2. Retinoblastoma cells express cone but not glial markers.
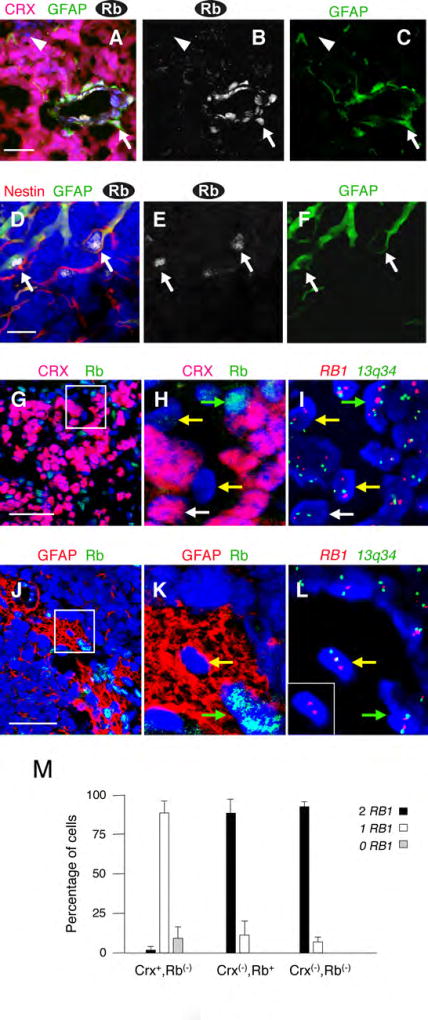
(A–F) Rb expression in retinoblastoma glia. (A–C) Rb (white) in endothelial cells and GFAP+ perivascular glia (green, arrow), but not in CRX+ tumor cells (red). Rare cells lack Rb, CRX, and GFAP (arrowhead). (D–F) Rb (white) in non-perivascular cells that co-express GFAP (green) and Nestin (red, arrows). (G–M) RB1 FISH of CRX−,Rb− and GFAP+,Rb−cells. Sections were co-stained for Rb and either CRX (G–H) or GFAP (J–K), and then probed by FISH (I, L), and nuclei with two control 13q34 signals (green) examined for RB1 (red). Boxed regions in G and J are magnified in H-I and K-L, and the inset in L shows the RB1 FISH for the cell in the center of the image. Arrows in G-L indicate CRX+Rb− tumor cells (white), non-neoplastic Rb+ cells (green), and CRX−,Rb− or GFAP+,Rb− cells (yellow). (M) The percentage of cells with the indicated CRX and Rb staining that have 2, 1, or 0 RB1 signals. Error bars indicate standard deviation for six sections from two tumors. Scale bars: 50 µm in A, G, J; 20 µm in D.
As co-expression of CRX, RXRγ, TRβ2, and cone arrestin was indicative of a cone phenotype, we examined whether retinoblastoma cells resemble short wavelength-sensitive S cones, or the developmentally distinct long and medium wavelength-sensitive L/M cones, by staining 22 tumors for S and L/M opsins. L/M opsin was detected in >95% of cells in each of the tumors, including proliferating Ki67+ cells and each of ~4,000 CRX+ cells evaluated in a co-staining analysis (Figures 1M, 1P, and S3). In contrast, S opsin was detected in only 0.1 – 2% of cells in 16 (73%) of the samples, and always in L/M opsin+ cells (Figure 1N, P). As S and L/M opsins are transiently co-expressed in developing L/M but not S cones (Cornish et al., 2004) (Figure 1O), these findings indicate that retinoblastomas largely consist of L/M cone precursor-like cells.
Cells within retinoblastomas that lack cone markers express Rb and/or retain wild type RB1 alleles
We next examined whether retinoblastoma cells also express markers of retinal neurons other than cones. In these analyses, samples were co-stained for cell type-specific markers and for Rb, in order to identify non-neoplastic Rb+ cells. We detected no tumor cells expressing the amacrine and horizontal cell-specific syntaxin, the ganglion cell-specific Brn-3b, or the horizontal, amacrine, bipolar, and progenitor cell-specific Prox1 (Table S2). Occasional tumors had clusters of cells that expressed the retinal progenitor cell- and bipolar cell-specific Chx10 or the progenitor, ganglion, horizontal, amacrine, or pigment epithelium cell-specific Pax6. However, all Chx10+ and Pax6+ cells co-expressed Rb (Figure S4) and were concluded to derive from the normal retina. Similarly, a cluster of cells expressing the rod-specific rhodopsin was detected in one tumor, yet these cells also co-expressed Rb (Figure S4). One sample had a highly differentiated retinoma-like region in which the majority of cells expressed cone markers and <1% expressed rhodopsin (Figure S5). However, these cells also seemed to be non-neoplastic, as no rhodopsin+ cells co-stained for Ki67. Thus, in this series, neoplastic retinoblastoma cells expressed markers of cones but not other retinal neurons.
We also examined retinoblastomas for expression of glial and progenitor cell markers. Each of 20 tumors had cells that co-expressed glial fibrillary acidic protein (GFAP) and Nestin (Table S2). The vast majority of these cells co-expressed Rb (Figure 2A–F), consistent with their being non-neoplastic astrocytes or Müller glia. However, Rb was not detected in rare GFAP+ cells (Figure 2K), or in rare cells that lacked both GFAP and the cone marker CRX (Figure 2A–C, arrowhead).
To assess whether the rare CRX−,Rb− or GFAP+,Rb− cells derive from RB1 mutated tumor cells, we examined whether they retained wild type RB1 alleles. We first identified tumors in which one of the RB1 alleles was deleted, then immunostained and recorded the positions of CRX−,Rb− and GFAP+,Rb− cells, and then defined their RB1 status using two-color fluorescence in situ hybridization (FISH) for RB1 (at 13q14) and for a 13q34 locus that served as a FISH positive control.
As expected, nearly all (89% +/− 7.6%) CRX+,Rb− retinoblastoma cells had one RB1 hybridization signal, and nearly all (88% +/− 8.9%) non-neoplastic CRX−,Rb+ cells had two RB1 signals (Figure 2G–I and 2M). The lack of RB1 signal in ~10% of CRX+,Rb− cells, and the presence of only one RB1 signal in ~10% of CRX−,Rb+ cells (Figure 2M) reflects the limited sensitivity of the FISH assay. Importantly, nearly all (93 +/− 2.2%) CRX−,Rb− cells in both tumors also had two RB1 signals (Figure 2M). The similar proportion of CRX−,Rb+ and CRX−,Rb− cells with two RB1 signals implied that most, and potentially all, of the CRX− cells retained two RB1 alleles. Many of these may be microglia, as all tumors had cells that expressed the CD68 marker, and ~ one-half of these lacked detectable Rb (Table S2 and Figure S6). Similarly, GFAP+,Rb− cells generally had two RB1 signals (Figure 2J–L).
In summary, Rb− cells that lacked the cone-related CRX, or that expressed the glial marker GFAP, generally had two RB1 alleles and were non-neoplastic, whereas Rb− cells that expressed CRX retained one RB1 allele and were the neoplastic component.
Neoplastic cone precursor-like cells propagate retinoblastoma tumors
While the above studies showed that retinoblastoma cells generally express cone markers, it remained possible that rare cells that lack a cone phenotype might propagate the tumors. To address this possibility, we serially engrafted primary retinoblastomas to the subretinal space of nude mice (Figure 3A), and examined the propagation of cone-like and non-cone-like cells, using human nuclear antigen (HuNu) as a human cell marker.
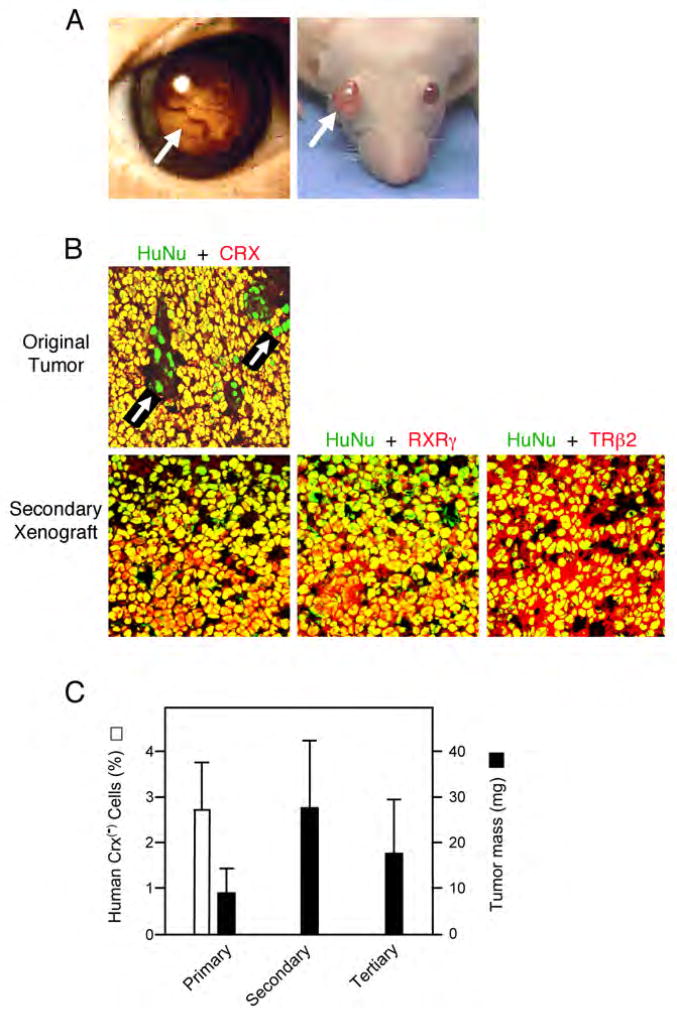
Figure 3. Cone precursor-like cells propagate retinoblastoma.
(A) Human retinoblastoma and mouse subretinal xenografts (arrows). (B) Original retinoblastoma (top) and secondary xenograft (bottom) co-stained with human nuclear antigen (HuNu, green) and either CRX, RXRγ, or TRβ2 (red). Most cells in the original tumor co-stained with HuNu and CRX (yellow). Occasional green nuclei (arrows) represent HuNu+,CRX− cells. Green HuNu+ human cells lacking CRX, RXRγ, or TRβ2 were not detected in xenografts. (C) Percentage of human cells that lack CRX in samples used in primary, secondary and tertiary xenografts (□) and average mass of primary, secondary, and tertiary xenografts (■). Error bars indicate standard deviation.
As expected, virtually all cells in the original human tumors were HuNu+. ~2.7% of the cells lacked CRX (Figure 3B–C), of which the majority were likely to be glia and other non-neoplastic cells as described above. Engrafting this population resulted in tumors that had human HuNu+,CRX+ tumor cells and mouse HuNu−,CRX− cells, but no HuNu+,CRX− cells among an estimated more than 8,000 cells examined (Figure 3C). Secondary grafts produced tumors of similar size, in which each of at least 8,000 HuNu+ cells expressed CRX, TRβ2, RXRγ, and L/M opsin (Figure 3B–C and data not shown), implying that cone-specific proteins were expressed in each of at least 32,000 engrafted cells. Likewise, cells from secondary grafts produced similar-sized tumors in tertiary grafts, and cells from tertiary grafts produced tumors in a fourth round. Although cone markers were detected in each of ~32,000 engrafted cells, re-engraftment of as few as 100 cells generated tumors in three out of four eyes.
Tumors also developed after engrafting two retinoblastomas that consisted entirely of cone-like L/M opsin+ cells following two months in culture. In these cultures, CRX, TRβ2, RXRγ, and L/M opsin were detected in each of at least 8,000 HuNu+ cells, and tumors developed after engrafting 1,000 cells (the least attempted) in each of 4 eyes (Figure S7). Thus, two approaches with three tumors demonstrated that cone precursor-like cells propagate retinoblastoma in an orthologous site.
The cone phenotype of human but not mouse retinal tumors correlates with robust Rb expression in human but not mouse cone precursors
While the above studies showed that cone markers were prominent in human retinoblastomas, amacrine and horizontal cell markers were prominent in mouse retinal tumors that resulted from loss of Rb and Rb-related proteins (Robanus-Maandag et al., 1998; Chen et al., 2004; MacPherson et al., 2004; Dannenberg et al., 2004; Ajioka et al., 2007). To address this discrepancy, we compared retinal cell marker expression in tumors from the two species. In this analysis, the cone markers CRX and TRβ2 were highly expressed in human retinoblastomas but not detected in Rb/p130-deficient mouse tumors, whereas the horizontal and amacrine markers Pax6 and syntaxin were prominent in the mouse tumors but not detected in human retinoblastomas (Figures 4A–B and Figure S8).
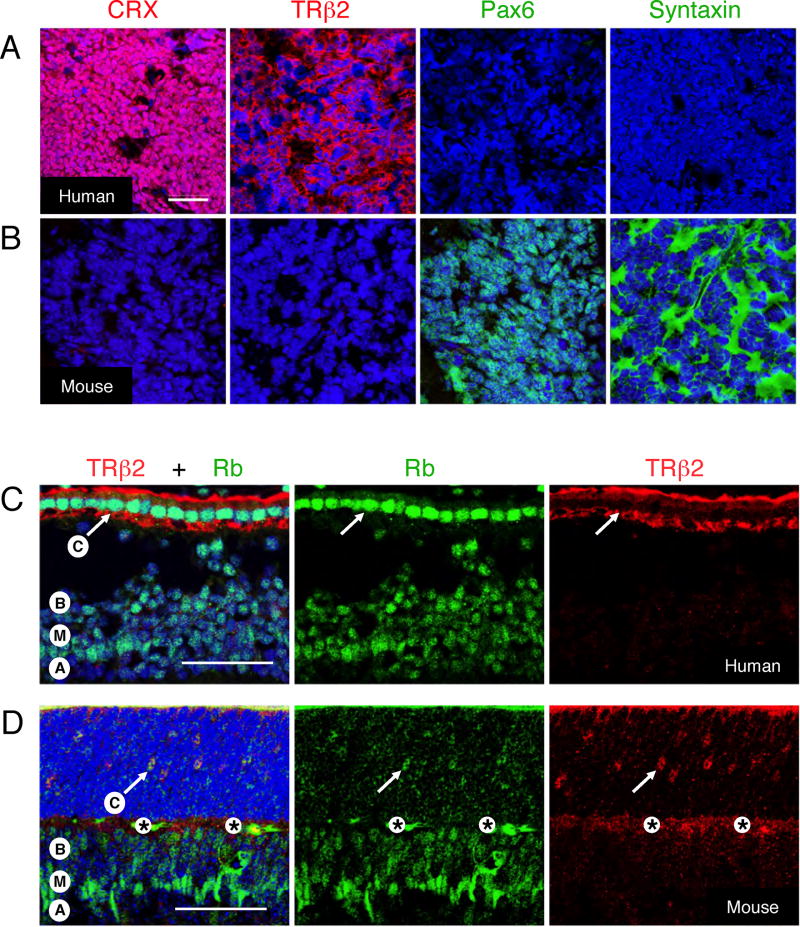
Figure 4. Species-specific retinal tumor phenotypes and Rb expression patterns.
(A–B) Human retinoblastoma (A) and a Rb1−/−,p130−/− retinal tumor (B) stained for cone markers CRX and TRβ2 (red), or for amacrine and horizontal cell markers syntaxin and Pax6 (green), and with DAPI (blue), using antibodies that stain the appropriate mouse and human retinal cells (Figure S8). (C–D) Prominent Rb (green) in TRβ2+ cone precursors (arrows) in human wk 18 fovea (C), but not in mouse P12 central retina (D). Labels indicate positions of cone (C), bipolar (B), Müller (M), and amacrine (A) cells, and an antibody-independent signal (*). Note that the P12 cone precursors in (D) are appropriately positioned in the outer nuclear layer (Rich et al., 1997; Ng et al., 2009). Scale bars, 50 µm.
The cone phenotype of human but not mouse retinal tumors was notable given that Rb was found to be highly expressed in maturing human cone precursors (Lee et al., 2006) but was not detected in immature mouse cone precursors from E14 to P5, nor in mature mouse cones (Spencer et al., 2005 and XLX, data not shown). Thus, we determined whether Rb was induced during human but not mouse cone maturation, by evaluating human foveae at fetal wk 18 (which follows the onset of L/M opsin expression at wk 14.5 but precedes emergence of inner and outer segments (Hendrickson and Provis, 2006)) and mouse retinas at P8, P12, and P16 (which brackets the onset of M opsin expression and emergence of cone outer segments at P11 (Szel et al., 1993)). In a side-by-side comparison, there was an intense Rb signal in human TRβ2+ cone precursors but only a faint signal in mouse cone precursors, whereas Rb signals were similar in human and mouse Müller and bipolar cells (Figures 4C–D and Figure S9). Thus, Rb appeared to be robustly expressed during the post-mitotic maturation of human but not mouse cones, correlating with the L/M cone phenotype of human but not mouse retinal tumors.
We also detected Rb in mouse Prox1+ amacrine and horizontal cells at P12 and P42, and in horizontal but not amacrine cells at P5, yet in neither cell type at P0. However, the Rb signal in these cells never exceeded that of Müller cells (Figure S10 and data not shown). Thus, in contrast to the situation in humans, mouse retinal tumors are jointly suppressed by Rb and Rb-related proteins, and resemble retinal cell types that have relatively modest Rb expression.
MDM2 and N-Myc are highly expressed in maturing human cone precursors
The retinoblastoma cone precursor phenotype and the Rb expression pattern suggested that Rb might have an anti-proliferative role in cone precursor cells. We reasoned that if Rb was required to prevent proliferation, then cone precursors might have features that impede the E2F- and ARF-mediated apoptotic responses that often accompany Rb loss. To address this possibility, we examined human retinas for expression of MDM2, which can abrogate E2F- and ARF-mediated responses by inhibiting p53 (Kowalik et al., 1998; Lomazzi et al., 2002), using an antibody (SMP14) that is specific to p53-binding MDM2 isoforms.
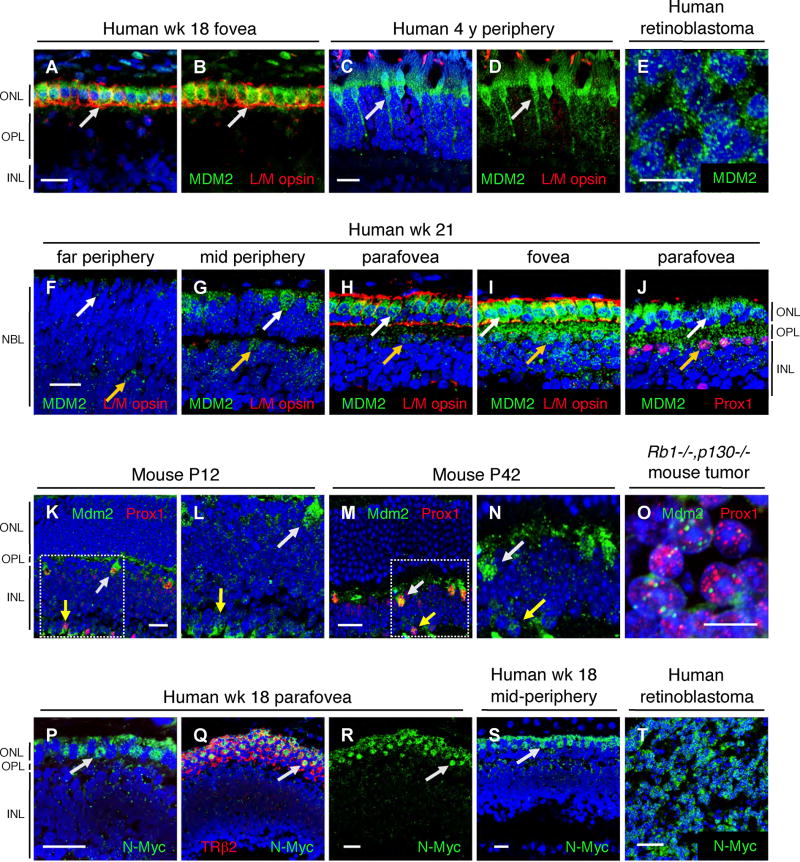
Remarkably, MDM2 was highly expressed in foveal cone precursors at gestational wks 16, 18, and 21 (Figure 5A, B, I, and data not shown). The MDM2 signal diminished in progressively more peripheral and less mature cone precursors (Figure 5F–I), suggesting that MDM2 levels increase during cone cell maturation. Concordantly, MDM2 was prominent in mature cones in tumor-associated retinas from retinoblastoma patients, including cones in the far periphery (Figure 5C–D and Figure S11). MDM2 was detected at lower levels in horizontal cells but generally was not detected in other retinal cell types (Figure 5J and data not shown). MDM2 was also highly expressed in each of 8 retinoblastomas (Figures 5E), despite that each of the tumors had a near diploid MDM2 copy number (Figure S12).
Figure 5. MDM2 and N-Myc expression in human cone precursors.
(A–J) MDM2 (green) in human gestational wk 18 (A, B) or wk 21 (F–J) retina, an uninvolved retina from a 4-year old retinoblastoma patient (C–D), or a retinoblastoma tumor (E); and co-stained for L/M opsin (A–D and F–I, red) or Prox1 (J, red). The same section was imaged with fixed parameters in F–I. White and yellow arrows indicate MDM2+ cones and horizontal cells, respectively. (K–O) Mdm2 (green) and Prox1 (red) in mouse P12 (K–L) or P42 (M–N) retina, or in a Rb1−/−,p130−/− mouse retinal tumor (O). Boxed regions in K and M are shown at higher magnification in L and N. White and yellow arrows indicate Mdm2+ horizontal and amacrine cells, respectively. (P–T) N-Myc (green) in wk 18 parafovea (P–R) or mid-periphery (S), or in human retinoblastoma (T), with TRβ2 co-staining (Q, red). Arrows indicate N-Myc+ cones. Scale bars, 20 µm for all panels except 10 µm in E and O.
We next examined MDM2 expression in mouse retinas, wherein combined loss of Rb1 and related genes appears to elicit amacrine or horizontal cell tumors. Little or no MDM2 was detected in immature cones from E14 to P5, in maturing cone precursors at P8, P12, and P16, or in mature cones at P42 (Figure 5K, M, and data not shown). While we cannot rule out that the lack of Mdm2 signal in mouse cones results from epitope masking, this seems unlikely as Mdm2 was readily detected in Prox1+ horizontal cells and at lower levels in Prox1+ amacrine cells at P14 and P42; as well as in the Prox1+ tumor cells in Rb/p130-deficient mice (Figure 5K–O). However, Mdm2 was not detected in immature amacrine or horizontal cells at E14 or P0 (data not shown). Thus, MDM2 was highly expressed in the human and mouse cells that resemble the retinal tumors in the two species, and particularly during their post-mitotic maturation.
As tumorigenesis requires proliferative as well as survival signaling, we also examined whether post-mitotic cone precursors express proliferation-related proteins. While human cone precursors lacked detectable E2F1 and cyclins D1, A2, and B, they highly expressed N-Myc. N-Myc was prominent in perifoveal cone precursors at wks 16 and 18, and in more peripheral positions at wks 18 and 21 (Figure 5P–S and data not shown), but was not detected in mature cones of the post-natal human retina nor in mouse cone precursors at P0, P5, or P12 (data not shown). The N-Myc staining appeared to be specific, as two N-Myc antibodies stained cone precursor nuclei in similar retinal positions (data not shown). N-Myc was also prominent in each of 8 retinoblastomas that had a near diploid MYCN copy number (Figures 5T and Figure S12).
MDM2 and N-Myc are required for retinoblastoma cell proliferation and survival
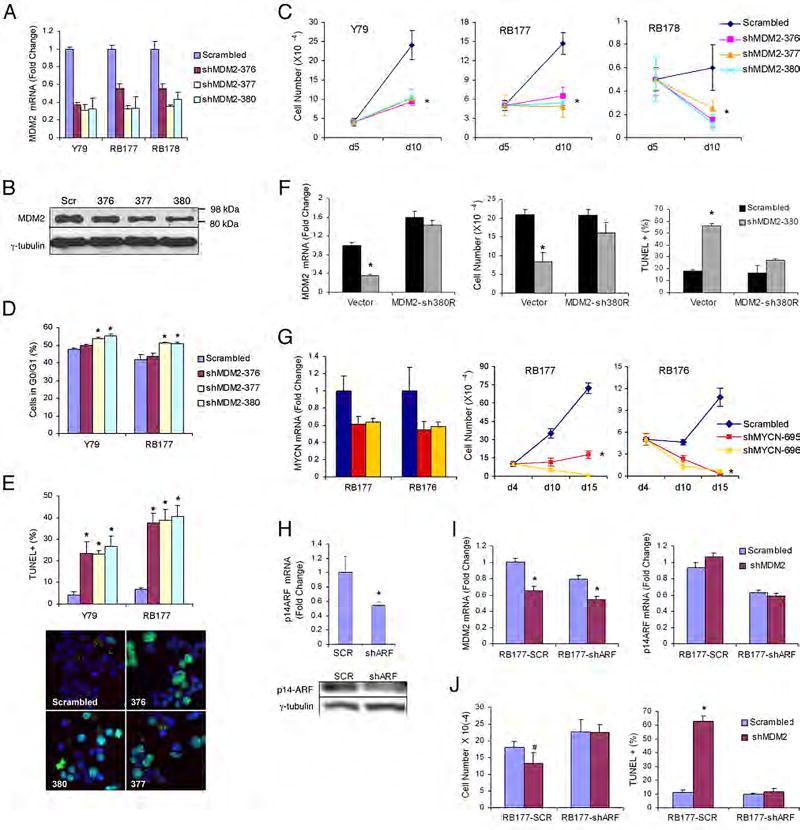
The prominent expression of MDM2 and N-Myc in cone precursors and retinoblastoma cells suggested that these proteins might be relevant to retinoblastoma development and propagation. To evaluate this possibility, we used lentiviral shRNA vectors to decrease MDM2 or N-Myc expression in Y79 and early passage RB176, RB177 or RB178 cells, which lack MDM2 and MYCN amplification (Figure S12).
Three shRNAs were found to decrease MDM2 mRNA and protein by ~60–70%, compared to a scrambled shRNA control, at five days after transduction (Figure 6A, B). Over the next five days, each MDM2 shRNA impaired cell proliferation and induced apoptosis, and two shRNAs increased the proportion of G0/G1 cells (Figure 6C–E), whereas expression of an shRNA-resistant MDM2 cDNA abrogated these effects (Figure 6F). These findings suggest that constitutively high MDM2 expression is crucial for retinoblastoma cell proliferation and survival.
Figure 6. Roles for MDM2 and N-Myc in retinoblastoma cells.
(A) qRT-PCR of MDM2 RNA, 5 days after infection of Y79, RB177, or RB178 cells with MDM2 shRNAs or a scrambled control. (B) Immunoblot analysis of MDM2 and γ-tubulin (as a control) in Y79 cells on day 5 after infection. (C) Growth of Y79, RB177, and RB178 cells after puromycin selection, plating on day 5, and counting on days 5 and 10 after infection. (D) The proportion of G0/G1 cells in Y79 and RB177 cultures, 9 days after infection. (E) The percentage of TUNEL+ Y79 and RB177 cells, 8 days after infection (top) and representative TUNEL staining (bottom). (F) RB177 cells transduced with sh380 resistant MDM2 cDNA and re-infected with pLKO-shMDM2-380 or a scrambled shRNA control. Left, qRT-PCR analysis of MDM2 RNA, 4 days after re-infection. Middle, cell numbers after plating on day 4 and counting on day 10 after re-infection. Right, percentage of TUNEL+ cells 11 days after re-infection. (G) qRT-PCR analysis of MYCN RNA on day 5, and cell growth after plating on day 4 and counting on days 4, 10, and 15 after transduction of RB177 or RB176 with MYCN shRNAs. (H) RB177 cells transduced with pLKO-shARF-331 or a scrambled control, and analyzed for CDKN2AARF RNA (top) and ARF protein (bottom). (I) qRT-PCR analysis of MDM2 RNA and CDKN2AARF RNA, 4 days after re-infection of RB177-shARF and control cells with pLKO-shMDM2-377 or control shRNA. (J) Cell numbers and TUNEL staining 5 days after re-infection with MDM2 or control shRNA vectors. Error bars indicate standard deviation, and (*) or (#) indicate P < 0.01 or P = 0.016, respectively.
Similarly, shRNAs that diminished MYCN expression by ~ 40–50% impaired proliferation and promoted cell death in retinoblastoma cultures (Figure 6G).
MDM2 suppresses ARF-induced apoptosis in retinoblastoma cells
As MDM2 is a major target of the ARF-mediated oncogenic stress response, we hypothesized that MDM2 may impede ARF-induced apoptosis in retinoblastoma cells. To test this idea, we determined whether a reduction in ARF could diminish the need for MDM2. We first produced RB177 derivatives that stably expressed either of two CDKN2AARF-specific shRNAs or a scrambled shRNA control (Figure 6H and data not shown). The cells were then transduced with an shRNA against MDM2 (Figure 6I), and examined for proliferation and TUNEL staining. Interestingly, the ARF-directed shRNAs increased RB177 proliferation and abrogated the anti-proliferative and pro-apoptotic effects of the MDM2 shRNA (Figure 6J), indicating that MDM2 did indeed suppress ARF-induced apoptosis in retinoblastoma cells.
A human-specific RXR element and the cone-specific RXRγ regulate retinoblastoma cell MDM2 expression
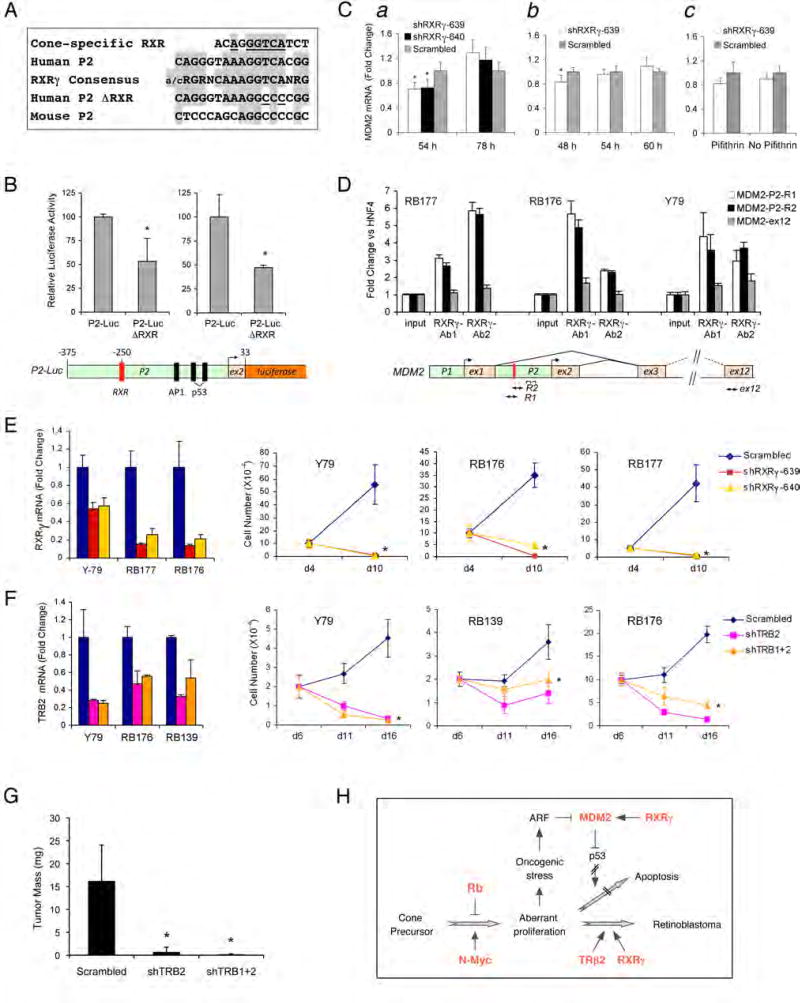
The expression of MDM2 in human cones and retinoblastoma cells suggested that cone-specific circuitry might direct MDM2 expression during retinoblastoma tumorigenesis. To address this possibility, we searched the MDM2 P1 and P2 promoters for cone-specific control elements that were identified in an unbiased bioinformatics analysis (Danko et al., 2007). This revealed an element in the human but not mouse P2 promoter that matched a cone-specific RXR-like element (Danko et al., 2007) at each of six invariant positions, and matched the consensus RXRγ homodimer binding site (Dowhan et al., 1994) at 14 of 15 positions (Figure 7A). We then examined whether this human-specific element promotes MDM2 expression, using an MDM2-P2-Luc reporter gene and a mutant ΔRXR version that had two human-to-mouse nucleotide substitutions. Notably, the two substitutions significantly diminished MDM2-P2 promoter activity in retinoblastoma cells (Fig. 7A–B).
Figure 7. Roles for cone-specific transcription factors in retinoblastoma cellMDM2expression, proliferation, and survival.
(A) A human MDM2 P2 promoter element (second row) with identity to a cone-specific RXR element (Danko et al., 2007) at each of six invariant positions (top row, underlined), and identity to the consensus RXRγ homodimer binding site at 14 of 15 positions (third row, shaded, from Table 1 of Dowhan et al., 1994), but differing from murine sequences (fifth row, aligned as in Figure S13). Human-to-mouse substitutions in P2-Luc-ΔRXR (fourth row) are underlined. (B) Top, luciferase activities after transfection of P2-Luc and P2-Luc-ΔRXR in two experiments. Bottom, P2-Luc structure. (C) qRT-PCR analysis of MDM2 RNA in RB177 cells at 54 and 78 h after transduction with RXRγ shRNAs (a); at 48, 54, and 60 h after transduction (b); and at 30 h after transduction in the presence or absence of 60 nM pifithrin-α. p-nitro, cyclic (EMD Biosciences) for the last 6h (c). (D) Top, ChIP analysis of RB177, RB176, and Y79 assayed by qRT-PCR directly (input) or after immunoprecipitation with two RXRγ antibodies. Values are the ratio of the MDM2 PCR products R1, R2, or ex12 to an HNF4α product. Bottom, the human MDM2 locus, showing positions of the RXR site and PCR products. (E) qRT-PCR analysis of RXRγ RNA on day 4, and cell growth after plating on day 4 after transduction of Y79, RB176, or RB177 with RXRγ shRNAs. (F) qRT-PCR analysis of TRβ2 RNA at day 5, and cell growth after plating on day 5 and counting on days 6, 11, and 16 after transduction of Y79, RB176, or RB139 cells with the indicated TRβ shRNAs. (G) Mean tumor mass 50 days after engrafting Y79 cells transduced with shTRβ2, shTRβ1+2, or scrambled shRNAs. (H) Potential role of cone precursor signaling proteins in retinoblastoma tumorigenesis. Proteins in Red are highly expressed during human cone precursor maturation. Error bars indicate standard deviation, and asterisks indicate P < 0.05.
We next examined whether RXRγ is important for MDM2 expression. We found that each of two shRNAs that diminished RXRγ expression also reduced expression of MDM2, yet the effect was transient and MDM2 RNA subsequently increased (Figure 7Ca,b). The initial effect of RXRγ knockdown was enhanced by the p53 inhibitor, pifithrin-α. p-nitro, cyclic (Figure 7Cc), and by p53 knockdown (data not shown), suggesting that p53 opposed the reduction in MDM2 expression. Moreover, in chromatin immunoprecipitation (ChIP) experiments, two RXRγ antibodies enriched for MDM2 sequences that surround the P2 RXR site, but not for sequences that were in exon 12 (Figure 7D). We conclude that a human-specific MDM2 promoter element and the cognate cone-specific RXRγ transcription factor contribute to retinoblastoma cell MDM2 expression.
The cone-specific transcription factors RXRγ and TRβ2 are required for retinoblastoma cell proliferation and survival
As RXRγ was found to promote MDM2 expression and is implicated in numerous aspects of cone biology, we examined whether this factor could contribute to retinoblastoma tumorigenesis. Concordantly, RXRγ knockdown dramatically impaired the proliferation and survival of Y79, RB176, and RB177 cells (Figure 7E). Moreover, RXRγ had effects beyond regulating MDM2, as the shRNA-induced decline in MDM2 was followed by increased expression of MDM2 (Figure 7C) as well as other p53 target genes, including CDKN1A and 14-3-3σ (data not shown).
We also examined the role of the cone-specific TRβ2 (Ng et al., 2001), using shRNAs directed against sequences that are specific to TRβ2 or that are shared with TRβ1. These shRNAs impaired proliferation of Y79, RB139, and RB176 cells, and suppressed tumorigenesis when shRNA-transduced Y79 cells were engrafted to the mouse subretinal space (Figure 7F–G). We conclude that the cone-specific TRβ2 and RXRγ contribute to retinoblastoma cell proliferation and survival.
DISCUSSION
Germline RB1 mutations have long been known to predispose to retinoblastoma, yet the basis for the selective predilection to retinoblastoma, as opposed to other tumors, has not been defined. In this study, we evaluated whether the underlying cellular circuitry of retinoblastoma cells might sensitize to the oncogenic effects of RB1 mutations.
Retinoblastoma cells resemble L/M cone photoreceptor precursors
Several lines of evidence indicated that retinoblastoma cells resemble neoplastic L/M cone precursors.
First, the vast majority of cells in all tumors expressed cone-specific proteins, as well as proteins that are concurrently expressed only in cones, such as CRX and RXRγ. These proteins are not merely associated with the cone phenotype, but include transcription factors such as CRX, RXRγ, and TRβ2, that are among the earliest cone proteins to be expressed and that dictate numerous cone cell features (Furukawa et al., 1999; Ng et al., 2001; Roberts et al., 2005). While cone-specific mRNAs and photo-transduction activities were earlier detected in cultured retinoblastomas (Bogenmann et al., 1988; Hurwitz et al., 1990), we know of only two studies that evaluated cone-specific protein expression in retinoblastomas in situ. One study reported widespread expression of cone transducin-α (Rodrigues et al., 1992), whereas a second showed that antibodies against chicken opsins detected cells in ~ one-half of retinoblastoma samples (Gonzalez-Fernandez et al., 1992). Our results with an anti-human L/M opsin antibody, on the contrary, demonstrate that retinoblastomas consistently exhibit features of maturing L/M cone precursors.
Second, rare cells that expressed markers of retinal neurons other than cones were found to co-express Rb, and were concluded to derive from the surrounding normal retina. Similarly, cells that expressed glial and progenitor markers generally co-expressed Rb, and rare GFAP+,Rb− and CRX−,Rb− cells generally retained two RB1 alleles. These findings imply that the vast majority and potentially all tumor cells that lack cone markers are non-neoplastic.
Finally, cone precursor-like cells propagated retinoblastoma tumors, with engraftment of as few as 100 cells sufficient to initiate tumorigenesis. Thus, cone-like cells appear to propagate the tumors in the absence of a distinct stem cell population.
We also confirmed that the human retinoblastoma phenotype differed from that of mouse tumors that develop due to the combined loss of Rb and Rb-related proteins. The distinction between the mouse and human tumors appeared to be absolute, as we found no evidence of human retinoblastoma cells that lacked cone markers, and no evidence of mouse tumor cells that expressed cone markers.
A role for cone precursor MDM2 expression in retinoblastoma tumorigenesis
To address whether cone precursor circuitry might contribute to retinoblastoma, we examined whether cones have distinctive circuitry that could impede an ARF-mediated apoptotic response to Rb loss. We found that the ARF target, MDM2, was expressed at exceptionally high levels in human cone precursors, and suppressed ARF-induced apoptosis in retinoblastoma cells. The MDM2 expression and resistance to ARF signaling seem to ensue at least in part from cone precursor circuitry, as human MDM2 promoter activity depended upon an RXR promoter element and on the cognate RXRγ protein.
Importantly, cone precursor MDM2 expression provides a rationale for the lack of classical p53 pathway mutations in retinoblastoma tumors. In most cancers, the p53 pathway is inactivated by mutation of TP53 or CDKN2AARF, or by amplification of MDM2. Moreover, these changes appear to be necessary for the tumors to avert ARF-mediated responses to oncogenic stress, rather than to avert the DNA damage response (Christophorou et al., 2006; Efeyan et al., 2006). Thus, it has been puzzling that CDKN2A and TP53 mutations have not been detected in retinoblastoma, and that MDM2 amplification is rare (Kato et al., 1996; Schlamp et al., 1997; Laurie et al., 2006). Our findings suggest that the ARF-mediated oncogenic stress response is inactivated in retinoblastomas due to the robust expression of MDM2, in the absence of MDM2 amplification. Nevertheless, retinoblastoma cells retain an effective p53-mediated DNA damage response (Kondo et al., 1997), and are sensitive to the MDM2 antagonist Nutlin-3a (Elison et al., 2006), as expected for cells that rely on robust MDM2 expression.
It has also been proposed that the p53 pathway is impaired in retinoblastoma due to copy number gains of the MDM2-related gene, MDM4 (also called MDMX) on chromosome 1q32.1 (Laurie et al., 2006). However, while MDM4 was shown to impede the p53-mediated radiation response, it was not shown to promote retinoblastoma cell survival in the absence of radiation, or to oppose ARF-mediated apoptotic signals. Moreover, a recent study suggested that a gene other than MDM4 may be a more general target of chromosome 1q gains (Dimaras et al., 2008), and MDM4 amplification was rare in our samples (Figure S12). Thus, while MDM4 copy number gains may contribute to some retinoblastomas, our findings suggest that robust expression of the unamplified MDM2 has a more general role in averting the ARF-mediated oncogenic stress response, and could act during early stages of tumorigenesis before Rb-deficient cells acquire additional genetic aberrations.
Notably, MDM2 was prominent in human but not mouse cones, yet in the horizontal cells of both species and in Prox-1+ amacrine cells in mice. The high MDM2 levels in mouse horizontal and amacrine cells is intriguing in light of evidence that mouse retinal tumors that arise due to combined Rb1 family mutations derive from apoptosis-resistant cells (Chen et al., 2004); specifically from horizontal cells in some genetic backgrounds (Ajioka et al., 2007) and potentially from amacrine cells in others (Robanus-Maandag et al., 1998; Chen et al., 2004). Thus, MDM2 appears to be well situated to contribute to mouse horizontal or amacrine cell tumors upon combined loss of Rb and Rb-related proteins, and to contribute to human cone precursor-like retinoblastomas upon loss of Rb. However, MDM2 evidently does not predispose to horizontal or amacrine cell tumors upon loss of only Rb, possibly due to the redundant actions of p107 and p130 in these cell types.
Roles for additional cone-specific circuitry in retinoblastoma
We also found that human cone precursors prominently expressed N-Myc and that retinoblastoma cells require N-Myc for proliferation and survival. The apoptotic response to N-Myc knockdown is consistent with the need for persistent Myc expression in numerous mouse tumor models, and may reflect the altered expression of diverse Myc target genes, yet continued CDKN2AARF expression, when Myc is incompletely suppressed (Shachaf et al., 2008). Interestingly, MYCN was amplified in a subset of mouse retinal tumors, but generally is not amplified in human retinoblastomas (Corson and Gallie, 2007; MacPherson et al., 2007). Thus, while further study is required, these observations are consistent with a role for cone precursor-related N-Myc expression in retinoblastoma tumorigenesis.
This study also revealed roles for the cone cell transcription factors RXRγ and TRβ2 in retinoblastoma cells. As these factors regulate opsin expression in cone precursors, but are not expressed in proliferating retinal cells (Ng et al., 2001; Roberts et al., 2005), their post-mitotic roles appear to be co-opted for proliferative purposes during retinoblastoma tumorigenesis. Moreover, our data indicate that RXRγ promotes MDM2 expression in retinoblastoma cells, and suggest that RXRγ could likewise contribute to MDM2 expression in human cone precursors via an RXRγ recognition element in the human MDM2 promoter. As the RXRγ element was present in the human but not mouse promoter, this element could contribute to the uniquely human proclivity to develop cone-like retinoblastoma tumors. Likewise, our data suggest that horizontal and amacrine cell circuitry might promote Mdm2 expression and tumorigenesis in mouse retinoblastoma models.
Implications for the retinoblastoma cell of origin
As cone precursor circuitry was crucial to retinoblastoma cell proliferation, it is of interest to consider whether this circuitry is adopted during the course of tumorigenesis, or is continuously present due to the tumor’s origin from cone precursor cells.
A cone-related origin of retinoblastoma was previously suggested by the L/M cone-like distribution of the tumors over the surface of the retina (Munier et al., 1994). This distribution is difficult to reconcile with an origin from cells that are unrelated to cones, but is consistent with an origin either from cone precursors or from cone-directed retinal progenitor cells. Our results provide support for a cone precursor but not progenitor origin, as we did not detect neoplastic cells that have progenitor features in 40 retinoblastoma tumors.
It was also suggested that retinoblastomas might arise from cells that reside in the retinal inner nuclear layer (INL), rather than from outer nuclear layer (ONL) cells such as cones, based on a potentially nascent tumor that was adjacent to the INL and had INL-like nuclear morphology (Gallie et al., 1999). However, as the tumor was almost entirely within the ONL, and its nuclear morphology has uncertain significance, this sample is also consistent with a cone precursor origin.
A cone precursor origin is also supported by the robust expression of Rb, MDM2, and N-Myc – and the abrupt decline in p27 (Lee et al., 2006) – during cone precursor maturation, and with Rb’s ability to reassert growth control when restored to cone precursor-like retinoblastoma cells (Cobrinik et al., 2006). Thus, our findings support a model in which a lack of Rb permits the aberrant proliferation of N-Myc+ cone precursors, robust MDM2 expression impedes an ARF-mediated apoptotic response, and cone factors such as RXRγ and TRβ2 further promote cell proliferation and survival. Moreover, as Rb loss can elicit mitotic instability (Hernando et al., 2004), the aberrantly proliferating cells could rapidly acquire additional cytogenetic changes that lead to malignancy (Figure 7H). As Rb, MDM2, and N-Myc are prominent in maturing but not nascent cone precursors, this model implies that contextual features that are specific to human cone maturation could impose the requirement for Rb tumor suppressor function.
Finally, a cone precursor origin accords with the prediction that human and mouse retinal tumors derive from different intrinsically death-resistant retinal precursors (Pacal and Bremner, 2006), and suggests that cell type-specific MDM2 expression may underlie the death-resistant phenotypes. Thus, while this study does not experimentally demonstrate that retinoblastomas derive from cone precursors within the developing human retina, the ability to reconcile the distinct features of the human and mouse retinal tumors further supports the cone precursor model.
In summary, this study shows that retinoblastoma cells express numerous components of the cone precursor signaling circuitry and rely upon this circuitry for their proliferation and survival. The findings appear consistent with a cone precursor origin of retinoblastoma, and suggest that elements of the cone precursor program may provide novel targets for retinoblastoma therapy. More generally, the findings suggest that cell type-specific as well as species-specific signaling circuitry sensitizes specific cells to specific oncogenic mutations.
EXPERIMENTAL PROCEDURES
Immunostaining and FISH
Retinas and retinoblastoma samples were prepared, sectioned, and immunostained as previously described (Lee et al., 2006) and as detailed in Supplemental Data. Some sections were imaged by confocal microscopy, probed for RB1 and a 13q34 hybridization control, and cells with immunostaining profiles of interest and two 13q34 signals examined for hybridization to RB1, as described in Supplemental Data.
Xenografts
2 × 105 retinoblastoma cells were injected into the subretinal space of nude mice 1 day after explant, and examined 60 days after primary and secondary grafts, 48 days after tertiary grafts, or 4–6 months after grafts of 104, 103, or 102 cells. Sections were stained for HuNu with a FITC-conjugated second antibody and for cone markers with a Cy3-conjugated second antibody. More than 8,000 HuNu+ cells were examined per marker.
shRNA knockdowns
Cells infected with pLKO lentiviral shRNA vectors were selected for 48–72 h starting 48 h after infection, plated at 2 × 104 cells per well for counting, attached to cover slips for immunostaining or TUNEL, or analyzed by FACS, qRT-PCR, or immunoblotting. RB177 cells transduced with pLKO shCDKN2AARF constructs or controls were selected, re-infected with pLKO-shMDM2-377 or a control, counted on days 1 and 5, and analyzed by qRT-PCR on day 4 and by TUNEL on day 5 after infection. RB177 cells were transduced with UINZ-MDM2-sh380R or the UGINZ vector, selected in G418, and transduced with pLKO-shMDM2-380 as in Supplemental Data.
Luciferase Assays
Cells were transfected with pRL-TK (Promega), P2-Luc, and P2-Luc-ΔRXR (as described in Supplemental Data) and the firefly:Ranilla luciferase ratio determined at 72 hours.
Chromatin Precipitation
(ChIP) assays were performed with RXRγ antibodies H-105 (Ab1) and Y-20 (Ab2) (Santa Cruz) and qRT-PCR as in Supplemental Data.
Supplementary Material
Acknowledgments
We thank Drs. J. Nathans and A. Swaroop for antibodies, H. te Riele for mouse tumors, J. Blaydes for plasmids, and M. Pappas for compiling Table S1. This work was supported by the Starr Foundation Tri-Institutional Stem Cell Initiative, Research to Prevent Blindness, and the Fund for Ophthalmic Knowledge.
References
- Abramson DH, Gombos DS. The topography of bilateral retinoblastoma lesions. Retina. 1996;16:232–239. doi: 10.1097/00006982-199616030-00009. [DOI] [PubMed] [Google Scholar]
- Ajioka I, Martins RA, Bayazitov IT, Donovan S, Johnson DA, Frase S, Cicero SA, Boyd K, Zakharenko SS, Dyer MA. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131:378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb LC, Holt JK, Tarttelin EE, Hodges MD, Gregory-Evans K, Rutherford A, Lucas RJ, Sowden JC, Gregory-Evans CY. Temporal and spatial expression patterns of the CRX transcription factor and its downstream targets. Critical differences during human and mouse eye development. Hum Mol Genet. 2001;10:1571–1579. doi: 10.1093/hmg/10.15.1571. [DOI] [PubMed] [Google Scholar]
- Bogenmann E, Lochrie MA, Simon MI. Cone cell-specific genes expressed in retinoblastoma. Science. 1988;240:76–78. doi: 10.1126/science.2451289. [DOI] [PubMed] [Google Scholar]
- Chen D, Livne-bar I, Vanderluit JL, Slack RS, Agochiya M, Bremner R. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell. 2004;5:539–551. doi: 10.1016/j.ccr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Chen D, Opavsky R, Pacal M, Tanimoto N, Wenzel P, Seeliger MW, Leone G, Bremner R. Rb-Mediated Neuronal Differentiation through Cell-Cycle-Independent Regulation of E2f3a. PLoS Biol. 2007;5:e179. doi: 10.1371/journal.pbio.0050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- Cobrinik D, Francis RO, Abramson DH, Lee TC. Rb induces a proliferative arrest and curtails Brn-2 expression in retinoblastoma cells. Mol Cancer. 2006;5:72. doi: 10.1186/1476-4598-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish EE, Xiao M, Yang Z, Provis JM, Hendrickson AE. The role of opsin expression and apoptosis in determination of cone types in human retina. Exp Eye Res. 2004;78:1143–1154. doi: 10.1016/j.exer.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Corson TW, Gallie BL. One hit, two hits, three hits, more? Genomic changes in the development of retinoblastoma. Genes Chromosomes Cancer. 2007;46:617–634. doi: 10.1002/gcc.20457. [DOI] [PubMed] [Google Scholar]
- Danko CG, McIlvain VA, Qin M, Knox BE, Pertsov AM. Bioinformatic identification of novel putative photoreceptor specific cis-elements. BMC Bioinformatics. 2007;8:407. doi: 10.1186/1471-2105-8-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg JH, Schuijff L, Dekker M, van der Valk M, te Riele H. Tissue-specific tumor suppressor activity of retinoblastoma gene homologs p107 and p130. Genes Dev. 2004;18:2952–2962. doi: 10.1101/gad.322004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimaras H, Khetan V, Halliday W, Orlic M, Prigoda NL, Piovesan B, Marrano P, Corson TW, Eagle RC, Jr, Squire JA, et al. Loss of RB1 induces non-proliferative retinoma; increasing genomic instability correlates with progression to retinoblastoma. Hum Mol Genet. 2008 doi: 10.1093/hmg/ddn024. [DOI] [PubMed] [Google Scholar]
- Donovan SL, Schweers B, Martins R, Johnson D, Dyer MA. Compensation by tumor suppressor genes during retinal development in mice and humans. BMC Biol. 2006;4:14. doi: 10.1186/1741-7007-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowhan DH, Downes M, Sturm RA, Muscat GE. Identification of deoxyribonucleic acid sequences that bind retinoid-X receptor-gamma with high affinity. Endocrinology. 1994;135:2595–2607. doi: 10.1210/endo.135.6.7988448. [DOI] [PubMed] [Google Scholar]
- Efeyan A, Garcia-Cao I, Herranz D, Velasco-Miguel S, Serrano M. Tumour biology: Policing of oncogene activity by p53. Nature. 2006;443:159. doi: 10.1038/443159a. [DOI] [PubMed] [Google Scholar]
- Elison JR, Cobrinik D, Claros N, Abramson DH, Lee TC. Small molecule inhibition of HDM2 leads to p53-mediated cell death in retinoblastoma cells. Arch Ophthalmol. 2006;124:1269–1275. doi: 10.1001/archopht.124.9.1269. [DOI] [PubMed] [Google Scholar]
- Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- Gallie BL, Campbell C, Devlin H, Duckett A, Squire JA. Developmental basis of retinal-specific induction of cancer by RB mutation. Cancer Res. 1999;59:1731s–1735s. [PubMed] [Google Scholar]
- Gonzalez-Fernandez F, Lopes MB, Garcia-Fernandez JM, Foster RG, De Grip WJ, Rosemberg S, Newman SA, VandenBerg SR. Expression of developmentally defined retinal phenotypes in the histogenesis of retinoblastoma. Am J Pathol. 1992;141:363–375. [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A, Provis J. Comparison of development of the primate fovea centralis with peripheral retina. In: Sernagor E, Eglen S, Harris B, Wong R, editors. Retinal Development. Cambridge: Cambridge University Press; 2006. pp. 126–149. [Google Scholar]
- Hernando E, Nahle Z, Juan G, Diaz-Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald W, Benezra R, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- Hurwitz RL, Bogenmann E, Font RL, Holcombe V, Clark D. Expression of the functional cone phototransduction cascade in retinoblastoma. J Clin Invest. 1990;85:1872–1878. doi: 10.1172/JCI114648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaquinta PJ, Lees JA. Life and death decisions by the E2F transcription factors. Curr Opin Cell Biol. 2007;19:649–657. doi: 10.1016/j.ceb.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato MV, Shimizu T, Ishizaki K, Kaneko A, Yandell DW, Toguchida J, Sasaki MS. Loss of heterozygosity on chromosome 17 and mutation of the p53 gene in retinoblastoma. Cancer Lett. 1996;106:75–82. doi: 10.1016/0304-3835(96)04305-4. [DOI] [PubMed] [Google Scholar]
- Kleinerman RA, Tucker MA, Tarone RE, Abramson DH, Seddon JM, Stovall M, Li FP, Fraumeni JF., Jr Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23:2272–2279. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Kondo S, Liu J, Haqqi T, Barnett GH, Barna BP. Involvement of p53 and WAF1/CIP1 in gamma-irradiation-induced apoptosis of retinoblastoma cells. Exp Cell Res. 1997;236:51–56. doi: 10.1006/excr.1997.3693. [DOI] [PubMed] [Google Scholar]
- Kowalik TF, DeGregori J, Leone G, Jakoi L, Nevins JR. E2F1-specific induction of apoptosis and p53 accumulation, which is blocked by Mdm2. Cell Growth Differ. 1998;9:113–118. [PubMed] [Google Scholar]
- Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C, Teunisse A, Lam S, Ramos Y, Mohan A, et al. Inactivation of the p53 pathway in retinoblastoma. Nature. 2006;444:61–66. doi: 10.1038/nature05194. [DOI] [PubMed] [Google Scholar]
- Lee TC, Almeida D, Claros N, Abramson DH, Cobrinik D. Cell cycle-specific and cell type-specific expression of Rb in the developing human retina. Invest Ophthalmol Vis Sci. 2006;47:5590–5598. doi: 10.1167/iovs.06-0063. [DOI] [PubMed] [Google Scholar]
- Lomazzi M, Moroni MC, Jensen MR, Frittoli E, Helin K. Suppression of the p53- or pRB-mediated G1 checkpoint is required for E2F-induced S-phase entry. Nat Genet. 2002;31:190–194. doi: 10.1038/ng891. [DOI] [PubMed] [Google Scholar]
- Maat-Kievit JA, Oepkes D, Hartwig NG, Vermeij-Keers C, van Kamp IL, van de Kamp JJ. A large retinoblastoma detected in a fetus at 21 weeks of gestation. Prenat Diagn. 1993;13:377–384. doi: 10.1002/pd.1970130510. [DOI] [PubMed] [Google Scholar]
- MacPherson D, Conkrite K, Tam M, Mukai S, Mu D, Jacks T. Murine bilateral retinoblastoma exhibiting rapid-onset, metastatic progression and N-myc gene amplification. Embo J. 2007;26:784–794. doi: 10.1038/sj.emboj.7601515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson D, Sage J, Kim T, Ho D, McLaughlin ME, Jacks T. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 2004;18:1681–1694. doi: 10.1101/gad.1203304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Ghyselinck NB, Chambon P, Mark M. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest Ophthalmol Vis Sci. 2001;42:1312–1318. [PubMed] [Google Scholar]
- Munier FL, Balmer A, van Melle G, Gailloud C. Radial asymmetry in the topography of retinoblastoma. Clues to the cell of origin. Ophthalmic Genet. 1994;15:101–106. doi: 10.3109/13816819409057835. [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Ng L, Ma M, Curran T, Forrest D. Developmental expression of thyroid hormone receptor beta2 protein in cone photoreceptors in the mouse. Neuroreport. 2009 doi: 10.1097/WNR.0b013e32832a2c63. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nork TM, Schwartz TL, Doshi HM, Millecchia LL. Retinoblastoma. Cell of origin. Arch Ophthalmol. 1995;113:791–802. doi: 10.1001/archopht.1995.01100060117046. [DOI] [PubMed] [Google Scholar]
- Pacal M, Bremner R. Insights from animal models on the origins and progression of retinoblastoma. Curr Mol Med. 2006;6:759–781. doi: 10.2174/1566524010606070759. [DOI] [PubMed] [Google Scholar]
- Rich KA, Zhan Y, Blanks JC. Migration and synaptogenesis of cone photoreceptors in the developing mouse retina. J Comp Neurol. 1997;388:47–63. [PubMed] [Google Scholar]
- Robanus-Maandag E, Dekker M, van der Valk M, Carrozza ML, Jeanny JC, Dannenberg JH, Berns A, te Riele H. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev. 1998;12:1599–1609. doi: 10.1101/gad.12.11.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor gamma is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest Ophthalmol Vis Sci. 2005;46:2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- Rodrigues MM, Rajagopalan S, Lee L, Nair CN, Advani SH, Donoso L, Chader GJ, Wiggert B. Retinoblastoma: messenger RNA for interphotoreceptor retinoid binding protein. Curr Eye Res. 1992;11:425–433. doi: 10.3109/02713689209001796. [DOI] [PubMed] [Google Scholar]
- Schlamp CL, Poulsen GL, Nork TM, Nickells RW. Nuclear exclusion of wild-type p53 in immortalized human retinoblastoma cells. J Natl Cancer Inst. 1997;89:1530–1536. doi: 10.1093/jnci/89.20.1530. [DOI] [PubMed] [Google Scholar]
- Shachaf CM, Gentles AJ, Elchuri S, Sahoo D, Soen Y, Sharpe O, Perez OD, Chang M, Mitchel D, Robinson WH, et al. Genomic and proteomic analysis reveals a threshold level of MYC required for tumor maintenance. Cancer Res. 2008;68:5132–5142. doi: 10.1158/0008-5472.CAN-07-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapek SX, Pan YR, Lee EY. Regulation of cell lineage specification by the retinoblastoma tumor suppressor. Oncogene. 2006;25:5268–5276. doi: 10.1038/sj.onc.1209710. [DOI] [PubMed] [Google Scholar]
- Spencer C, Pajovic S, Devlin H, Dinh QD, Corson TW, Gallie BL. Distinct patterns of expression of the RB gene family in mouse and human retina. Gene Expr Patterns. 2005;5:687–694. doi: 10.1016/j.modgep.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Swain PK, Hicks D, Mears AJ, Apel IJ, Smith JE, John SK, Hendrickson A, Milam AH, Swaroop A. Multiple phosphorylated isoforms of NRL are expressed in rod photoreceptors. J Biol Chem. 2001;276:36824–36830. doi: 10.1074/jbc.M105855200. [DOI] [PubMed] [Google Scholar]
- Szel A, Rohlich P, Mieziewska K, Aguirre G, van Veen T. Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: a developmental study. J Comp Neurol. 1993;331:564–577. doi: 10.1002/cne.903310411. [DOI] [PubMed] [Google Scholar]
- Virtanen I, Kivela T, Bugnoli M, Mencarelli C, Pallini V, Albert DM, Tarkkanen A. Expression of intermediate filaments and synaptophysin show neuronal properties and lack of glial characteristics in Y79 retinoblastoma cells. Lab Invest. 1988;59:649–656. [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Weller CV. Intrinsic factors in the etiology of neoplasms. Am J Cancer. 1937;30:39–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.