Abstract
Background and study aims
Endoscopic treatment of malignant colorectal polyps is often challenging, especially for early rectal cancer (ERC) localized close to the dentate line. Conversely, the surgical approach may result in temporary or definitive stoma and in frequent post-surgical complications. The Full-Thickness Resection Device (FTRD ® ) System (Ovesco Endoscopy, Tübingen, Germany) is a novel system that, besides having other indications, appears to be promising for wall-thickness excision of intestinal T1 carcinoma following incomplete endoscopic resection. However, follow-up data on patients treated with this device are scarce, particularly for ERC.
Patients and methods
Six consecutive patients with incomplete endoscopic resection of T1-ERC were treated with the FTRD and their long-term outcomes were evaluated based on a detailed clinical and instrumental assessment.
Results
The endoscopic en bloc full-thickness resection was technically feasible in all patients. The histopathologic analysis showed a complete endoscopic resection in all cases, and a full-thickness excision in four. Neither complications, nor disease recurrence were observed during the 1-year follow-up period.
Conclusions
The FTRD System is a promising tool for treating ERC featuring a residual risk of disease recurrence after incomplete endoscopic mucosal resection in patients unfit for surgery or refusing a surgical approach.
Introduction
Thanks to the widespread implementation of screening protocols, colorectal malignant lesions are an increasingly detected pathology, being reported in up to 12 % of resected polyps 1 .
Complete endoscopic resection of rectal lesions can be achieved with snare-polypectomy, endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). However, subsequent histopathological examination of the resected specimen may reveal signs of incomplete resection raising the need for additional treatments ( Table 1 ) 2 .
Table 1. Histopathological criteria for high risk malignant polyps according to Ueno et al. 2 .
| Low tumor differentiation grade (G3) |
| Haggitt’s levels (pedunculated polyps): 3 – 4 |
| Kikuchi's levels (sessile polyps): sm3 |
| Width of submucosal invasion: ≥ 4000 µm |
| Depth of submucosal invasion: ≥ 2000 µm |
| Positive tumor budding |
| Distance from the excision margin < 1 mm |
| Presence of vascular invasion |
Limited by post-polypectomy submucosal fibrosis, a rescue endoscopic therapy is often challenging, especially for rectal lesions localized close to the dentate line 3 . On the other hand, major rectal surgery often results in temporary or definitive stoma with a remarkable impact on patients reported outcomes. In addition, post-surgical complications, such as disturbed defecation, sexual and urinary dysfunctions or anastomotic dehiscence, may arise from rectal surgery even in tertiary referral centers 4 5 .
To overcome these limitations, a novel endoscopic therapeutic tool, called the Full-Thickness Resection Device (FTRD ® ) System (Ovesco Endoscopy, Tübingen, Germany) has recently been introduced. This endoscopic approach is technically successful for intestinal T1 carcinoma following incomplete resection, non-lifting adenoma or adenoma arising in difficult positions (i. e. the neck of the diverticulum, appendix, and dentate line). In selected cases, the FTRD proved effective for endoscopic treatment of small submucosal tumors and for diagnostic purposes 6 7 8 9 10 11 12 . The main features of this technique lie in generating a pseudopolyp involving the intestinal wall-thickness within an endoscopic cap (diameter 13 mm, length 23 mm) followed by an en bloc resection using a hot snare technique (monofilament, 14-mm polypectomy snare preloaded in the tip of the cap), after the deployment of a modified over-the-scope clip (diameter 14 mm) to seal the likely underlying transmural defect 6 7 8 9 10 11 12 . The FTRD can be quickly pre-loaded on the tip of an endoscope with a tip diameter of 11.2 – 13.2 mm. Initial experiences using the FTRD have shown promising results, especially in high risk patients and in lesions located in peculiar anatomic sites where standard endoscopic or surgical approaches would carry considerable risks and require aggressive strategies 6 7 8 9 10 11 12 .
The aim of this pilot study was to assess for the first time the feasibility and long-term clinical impact of endoscopic treatment with the new FTRD in selected patients with T1-early rectal cancer (ERC) following an incomplete endoscopic mucosal excision.
Patients and methods
Consecutive patients diagnosed with T1-ERC following incomplete endoscopic resection or showing submucosal involvement (R1) at histological examination were evaluated for endoscopic full-thickness resection (EFTR) after a complete oncologic work-up. The inclusion criteria were: (i) residual rectal lesion or rectal scar < 20 mm; (ii) increased probability of disease recurrence as defined by the histopathological evidence of > 1 criteria according to Ueno et al. 2 ; (iii) no lymphatic or metastatic disease at computed tomography (CT) scan and rectal endoscopic ultrasound (EUS); (iv) patients defined as “unfit for surgery” according to their underlying general condition, or who had refused the surgical option despite having received exhaustive information about the natural history of the disease and the presence of a valid surgical option. The exclusion criteria were patients < 18 years old, pregnancy, severe uncontrolled coagulopathy, and inability to provide an informed consent. These patients were offered and accepted to undergo the EFTR procedure, providing their informed consent. This study was carried out in accordance with the Declaration of Helsinki adopted in 1964 incorporating all later amendments.
Antibiotic prophylaxis with iv cefalosporin was administered to all patients. Before EFTR, the target lesion was identified under white-light endoscopic imaging and marked using the FTRD marking probe. EFTR was performed in a standard technique 6 7 8 9 10 11 12 using a preloaded FTRD including a tissue anchor, a modified 14-mm over-the-scope clip and a monofilament, 14-mm polypectomy snare connected to a standard electrosurgical device (VIO; ERBE Elektromedizin GmbH, Tübingen, Germany; ENDO CUT ® Q*, effect 3, cutting duration/interval 4/1). All procedures were conducted by expert endoscopists with initial experience in the use of the FTRD (less than 10 procedures) using high-resolution endoscopes with a tip diameter of 11.2 – 13.2 mm and digitally recorded. After endoscopic treatment, patient outcomes were strictly monitored performing a tailored oncologic work-up including endoscopy, CT scan, and rectal EUS. Two expert gastrointestinal pathologists performed the histopathological analysis on the en bloc specimens obtained by EFTR.
Results
From June 2015 to February 2016, six patients were consecutively treated at the IRCCS Policlinico San Donato, San Donato Milanese, Italy (5 men, mean age 63 years, range 51 – 78 years). All patients had previously received a rectal EMR within the previous 1 – 3 months, thereby receiving the histopathological diagnosis of high risk malignant polyp 2 ( Table 2 ). Each patient had been treated with EFTR at hospital admission and discharged home within the following 24 hours. The endoscopic full-thickness resection was technically feasible in all cases within 8 – 15 minutes (15 – 30 minutes including lesion detection, demarcation, and FTRD assembly). No immediate or late complications occurred. No patient reported any symptom related to the endoscopic procedure. High resolution endoscopic images displaying the original rectal lesions, the EFTR procedures, and the follow-up are reported in Fig. 1 . A full-length demonstrative video was also recorded ( Video 1 ).
Table 2. T1 early rectal cancer features before endoscopic mucosal resection, indications for endoscopic full-thickness resection, and follow-up.
| Patient # | Rectal site | Endoscopic features before EMR | Level of invasion of each ERC according to the EMR specimen | Positive Ueno’s criteria based on the en bloc EMR specimen | Indication for EFTR | Pre-EFTR staging based on Endoscopy, EUS, CT | Histology based on the en bloc EFTR specimens | Oncologic follow-up |
| 1 | Distal | 30 mm, I s, Kudo V, negative lifting sign | sm3 | Tumor budding, excision margin, Kikuchi’s level, width of submucosal invasion | Incomplete endoscopic excision of T1-ERC unfit for surgery (ASA IV) | T0, N0, M0 | R0, full-thickness resection; histology negative for residual disease | Endoscopy, EUS, and CT negative at 3 and 12 months; Endoscopy and EUS negative at 18 months |
| 2 | Distal | 20 mm, I sp, Kudo IIIL, negative lifting sign | sm3 | Tumor budding, Haggitt’s level, excision margin, depth and width of submucosal invasion | Incomplete endoscopic excision of T1-ERC refusing surgery (ASA II) | T0, N0, M0 | R0, full-thickness resection; histology negative for residual disease | Endoscopy, EUS and CT negative at 6 and 12 months |
| 3 | Distal | 18 mm, I sp, Kudo IIIL, negative lifting sign | sm3 | Haggitt’s level, excision margin, depth and width of submucosal invasion | Incomplete endoscopic excision of T1-ERC refusing surgery (ASA III) | T0, N0, M0 | R0, complete submucosal resection but no muscularis propria layer in the specimen; histology negative for residual disease | Endoscopy, EUS and CT negative at 6 and 12 months |
| 4 | Proximal | 6 mm, I s, Kudo V, negative lifting sign | sm3 | Haggitt’s level, excision margin | Incomplete endoscopic excision of T1-ERC unfit for surgery (ASA IV) | T1, N0, M0 | R0, full-thickness resection; histology positive for adenocarcinoma (sm2) | Endoscopy, EUS and CT negative at 6 months. Patient died from severe cardiac disease at the 8th follow-up month |
| 5 | Distal | 7 mm, I s, Kudo IV, negative lifting sign | sm1 | Low tumor differentiation grade, excision margin | Incomplete endoscopic excision of T1-ERC unfit for surgery (ASA IV) | T0, N0, M0 | R0, full-thickness resection; histology negative for residual disease | Endoscopy, EUS and CT negative at 6 and 12 months |
| 6 | Distal | 18 mm, I s, Kudo IIIL, negative lifting sign | sm1 | Tumor budding, excision margin, width of submucosal invasion | Incomplete endoscopic excision of T1-ERC refusing surgery (ASA III) | T0, N0 | R0, complete submucosal resection but no muscularis propria layer in the specimen; histology negative for residual disease | Endoscopy, EUS and CT negative at 6 and 12 months |
ASA, physical status classification system adopted by the American Society of Anesthesiologists; EFTR, endoscopic full-thickness resection performed with the Full-Thickness Resection Device (FTRD ® ) System; EMR, endoscopic mucosal resection; ERC, early rectal cancer; EUS, endoscopic ultrasound.
Fig. 1.
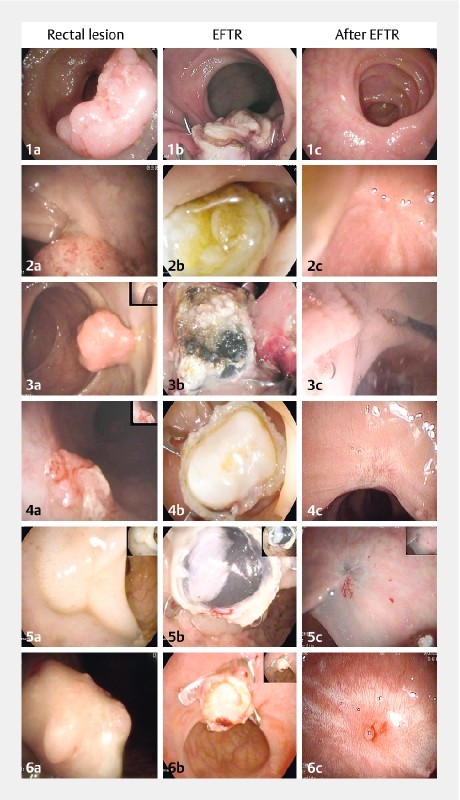
High-resolution endoscopic images. a T1-early rectal lesions in the six patients (1 – 6) before endoscopic mucosal resection. b Images taken following the endoscopic full-thickness resection procedures using the Full-Thickness Resection Device (FTRD ® ) System. c Images taken at the 6-month follow-up.
The histopathologic analysis performed on the en bloc resected specimen demonstrated a complete endoscopic resection in all patients, with the achievement of a full-thickness excision in four patients. The resected specimen included the submucosal layer with an estimated depth ≥ 1000 µm (1250 – 3050 µm) but not the muscularis propria in the two remaining patients ( Table 2 ). By revising these two endoscopic charts, no evident technical issue affecting the endoscopic procedures has been identified.
During follow-up, all patients underwent an oncologic work-up with endoscopy, CT scan, and rectal EUS every 6 months ( Table 2 ). All patients were in oncologic remission after a median follow-up of 12 months (range 12 – 18 months) without any radio- or chemotherapy. One patient died from cardiac failure at the 8th month of follow-up after showing no sign of disease recurrence at the 6-month oncological work-up.
Discussion
Our series confirms that EFTR with the newly introduced FTRD System is feasible and safe in T1-ERC. This study also shows that EFTR is a valid option for intestinal tumor excision following incomplete endoscopic resection in patients without evidence of metastatic or lymphatic disease when the standard surgical option is contraindicated, refused by the patient or the patient is at high risk.
The proper management of high risk malignant polyps relies on a multidisciplinary decision-making process currently based on the estimated risk of residual disease, as well as on several patient-specific features such as age, general global assessment, underlying morbidities, long-term prognosis, and the patient’s wishes 13 . The proper management of T1 ERC is remarkably influenced by the risk of lymph node micrometastasis. Therefore, following the endoscopic removal of colorectal malignant polyps, the specific risk of disease recurrence depends on the histopathological identification of standardized microscopic criteria and the nodal status 2 . The precise impact of each single microscopic criterion and their interrelationship is still unclear. Nonetheless, when several adverse risk factors are present and the risk of residual disease is substantial (> 20 %), the decision-making process to undergo further surgical treatment is usually straightforward 13 . Exceptions include those patients whose comorbidities outweigh the risk of surgery and those refusing major rectal surgery to avoid the risk of definitive stoma. Conversely, routine follow-up without further treatment is the best option after endoscopic removal of very low risk (< 3 %) malignant polyps 13 .
Notably, margin positivity on its own does not appear to be an independent risk factor for lymph node metastasis, with the risk of nodal metastasis being similar in patients with and without margin involvement 14 . Previous studies have clearly shown that the risk of disease recurrence is often overestimated by histopathological assessment of involved margins within the resected specimens 15 16 . Indeed, radical surgery for incomplete endoscopic resection of early colon-rectal cancers provides tumor-free specimens in up to 76 % of cases, thereby failing to improve the 2-year survival rates 17 while imposing significant risks of immediate morbidity and long-term complications on the patient.
Consequently, we performed a full thickness endoscopic resection following supposedly incomplete (R1) endoscopic excision with endoscopic mucosa resection of T1-ERC in those patients either refusing or unfit for the standard surgical options. In the present series, all but one of the specimens obtained by EFTR showed fibrotic submucosal tissue with no dysplastic residue. Within these cases, no residual or recurrent dysplastic tissue was observed when performing EFTR. In the remaining patient with incomplete endoscopic excision of T1-ERC, cancer recurrence was already evident at endoscopy.
As compared to established surgical curative treatments for rectal cancer (i. e. lower anterior resection with total mesorectal excision and abdominoperineal resection), less invasive trans-anal full-thickness excision techniques (e. g., conventional trans-anal excision, trans-anal endoscopic microsurgery (TEM), or trans-anal minimally invasive surgery (TAMIS)) have comparable 5- and 10-year survival rates 18 19 but clear advantages in limiting either surgery-related mortality or morbidity, and the need for a permanent stoma 19 . However, by reducing both the resected specimens and the mesorectal lymph node assessment, these treatments hamper the exact disease staging, thereby implying an increased risk of local recurrence and missed micrometastasis 20 . In addition, following either TEM or TAMIS, major complications have been reported in 1.5 – 7 % of patients and conversion to laparotomy with or without total mesorectal excision or temporary stoma is sometimes necessary 21 22 23 .
Within this context, our initial results indicate that the endoscopic approach with the FTRD is a valid alternative to trans-anal full-thickness excision techniques for non-pedunculated T1-ERC smaller than 20 mm (i. e. estimated maximal size referring to the luminal diameter of the rectal naïve lesion or to the scar following previous EMR or polypectomy), resulting in low comorbidity, fast operating time, and anesthesiology-free procedures. These results are consistent with other recent series showing positive outcomes when the FTRD has been used for the treatment of adenomatous and early colonic cancer, including both right- and left-sided lesions 6 7 8 9 10 11 12 . According to these reports, the FTRD is technically feasible for lower gastrointestinal mucosal lesions up to 30 mm. However, we believe that lesions ≤ 20 mm represent an ideal target for stiff tissues (e. g., incomplete resections with tissue fibrotic retraction, nonlifting naïve lesions) and angulated positions (e. g., distal rectum, recto-sigmoid junction, colonic flexure).
When compared to ESD or EMR, EFTR has the potential to allow for en bloc radical endoscopic excision of T1-ERC involving all submucosal layers, with reduced risk of bleeding, perforation, and post-polypectomy syndrome, which appear considerable even in referral ESD centers 24 25 . Furthermore, ESD is technically difficult, especially in fibrotic tissue due to previous excisions, it is time-consuming, and requires a prolonged learning curve for inexperienced endoscopists 24 . In fact, ESD outcomes from Western studies are substantially worse compared with Eastern studies, thereby limiting generalizability of the results 20 . However, ESD represents the only reasonable endoscopic approach for superficial (sm1) T1-ERC with a diameter exceeding 30 mm (i. e. large non-pedunculated colorectal polyps), since the use of the FTRD would not be feasible for technical reasons (cap diameter/length 13 /23 mm) 6 7 8 , while EMR often leads to piecemeal resection, challenging histopathological assessment of R0 resection, and increased risk of incomplete excision 24 .
Our positive experience with EFTR has some inherent limitations. Despite being prospectively designed, the main drawback of this pilot study is the relatively short follow-up, which includes patients with a full negative oncologic work-up at 6 to 18 months since the time of EFTR. Secondly, six patients are not enough to rule out the risk of potential EFTR-related complications, such as the entrapment of other pelvic structures close to the rectal wall; to date, such complications have never been documented in the literature 6 7 8 9 10 11 12 . Finally, a complete full-thickness rectal excision was not feasible in one-third of our patients, where the deepest submucosal layer but not the muscularis propria was included in the resected specimens. A post-hoc revision of these endoscopic procedures was not able to identify any technical feature clearly affecting the successful full-thickness resections in those two patients. Post-polypectomy fibrotic changes following previous EMR can increase the stiffness of the rectal wall and thus impair the endoscopic suction of the deepest layers within the snare housing. In any case, the deepest layer of the suctioned tissue remains within the over-the-scope clip, being thereby bound to ischemic injuries and fibrotic remodeling, thus decreasing the risk of local recurrence.
In conclusion, this study provides initial evidence in favor of EFTR with the newly introduced FTRD System for rectal malignant polyps featuring a medium risk of disease recurrence after endoscopic mucosal resection in patients either unfit for surgery or refusing the standard surgical approach.
Acknowledgements
This independent research has received no funding. Preliminary data from this work was presented at the following meeting: FISMAD 2017 (Bologna, Italy – oral presentation).
Footnotes
Competing interests None
References
- 1.Wasif N, Etzioni D, Maggard M A et al. Trends, patterns, and outcomes in the management of malignant colonic polyps in the general population of the United States. Cancer. 2011;117:931–937. doi: 10.1002/cncr.25657. [DOI] [PubMed] [Google Scholar]
- 2.Ueno H, Mochizuki H, Hashiguchi Y et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–394. doi: 10.1053/j.gastro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K, Koide Y, Katsuno H. When is local excision appropriate for “early” rectal cancer? Surg Today. 2014;44:2000–2014. doi: 10.1007/s00595-013-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu S W, Ma C C, Yang Y. Role of protective stoma in low anterior resection for rectal cancer: a meta-analysis. World J Gastroenterol. 2014;20:18031–18037. doi: 10.3748/wjg.v20.i47.18031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glimelius B, Tiret E, Cervantes A et al. Rectal cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2013;24:vi81–vi88. doi: 10.1093/annonc/mdt240. [DOI] [PubMed] [Google Scholar]
- 6.Schimdt A, Damm M, Caca K et al. Endoscopic full-thickness resection using a novel over-the-scope device. Gastroenterology. 2014;147:740–742. doi: 10.1053/j.gastro.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 7.Richter-Schrag H J, Walker C, Thimme R et al. Full thickness resection device (FTRD): experience and outcome for benign neoplasms of the rectum and colon. Chirurg. 2016;87:316–325. doi: 10.1007/s00104-015-0091-z. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A, Bauerfeind P, Gubler C et al. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47:719–725. doi: 10.1055/s-0034-1391781. [DOI] [PubMed] [Google Scholar]
- 9.Sarker S, Gutierrez J P, Council L et al. Over-the-scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy. 2014;46:758–761. doi: 10.1055/s-0034-1365513. [DOI] [PubMed] [Google Scholar]
- 10.Fähndrich M, Sandmann M. Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy. 2015;47:76–79. doi: 10.1055/s-0034-1377975. [DOI] [PubMed] [Google Scholar]
- 11.Lagoussis P, Soriani P, Tontini G E et al. Over-the-scope clip-assisted full thickness resection after incomplete resection of rectal adenocarcinoma. Endoscopy. 2016;48:E59–E60. doi: 10.1055/s-0042-100197. [DOI] [PubMed] [Google Scholar]
- 12.Andrisani G, Pizzicannella M, Martino M et al. Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: a single-centre study. Dig Liver Dis. 2017;49:1009–1013. doi: 10.1016/j.dld.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Williams J G, Pullan R D, Hill J et al. Management of the malignant colorectal polyps: ACPGBI position statement. Colorectal Dis. 2013;15:1–38. doi: 10.1111/codi.12262. [DOI] [PubMed] [Google Scholar]
- 14.Hassan C, Zullo A, Risio M et al. Histologic risk factors and clinical outcome in colorectal polyp: a pooled-data analysis. Dis Colon Rectum. 2005;48:1588–1596. doi: 10.1007/s10350-005-0063-3. [DOI] [PubMed] [Google Scholar]
- 15.Morson B C, Bussey H JR, Samoorian S. Policy of local excision for early cancer of the colon rectum. Gut. 1977;18:1045–1050. doi: 10.1136/gut.18.12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith K JE, Jones P F, Burke D A et al. Lymphatic vessels distribution in the mucosa and submucosa and potential implications for T1 colorectal tumors. Dis Colon Rectum. 2011;54:35–40. doi: 10.1007/DCR.0b013e3181fb0e7a. [DOI] [PubMed] [Google Scholar]
- 17.Gill M D, Rutter M D, Holtham S J et al. Management and short-term outcome of malignant colorectal polyps in the north of England. Colorectal Dis. 2013;15:169–176. doi: 10.1111/j.1463-1318.2012.03130.x. [DOI] [PubMed] [Google Scholar]
- 18.Sajid M S, Farag S, Leung P et al. Systematic review and meta-analysis of published trials comparing the effectiveness of transanal endoscopic microsurgery and radical resection in the management of early rectal cancer. Colorectal Dis. 2014;16:2–14. doi: 10.1111/codi.12474. [DOI] [PubMed] [Google Scholar]
- 19.Kidane B, Chadi S A, Kanters S et al. Local resection compared with radical resection in the treatment of T1N0M0 rectal adenocarcinoma: a systematic review and meta-analysis. Dis Colon Rectum. 2015;58:122–140. doi: 10.1097/DCR.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 20.Bartel M J, Brahmbhatt B S, Wallace M B. Management of colorectal T1 carcinoma treated by endoscopic resection from the Western perspective. Dig Endosc. 2016;28:330–341. doi: 10.1111/den.12598. [DOI] [PubMed] [Google Scholar]
- 21.Guerrieri M, Baldarelli M, de Sanctis A et al. Treatment of rectal adenomas by transanal endoscopic microsurgery: 15 years’ experience. Surg Endosc. 2010;24:445–449. doi: 10.1007/s00464-009-0585-1. [DOI] [PubMed] [Google Scholar]
- 22.Palma P, Freudenberg S, Samel S et al. Transanal endoscopic microsurgery: indications and results after 100 cases. Colorectal Dis. 2004;6:350–355. doi: 10.1111/j.1463-1318.2004.00671.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee L, Burke J P, deBeche-Adams Tet al. Transanal minimally invasive surgery for local excision of benign and malignant rectal neoplasia: outcomes from 200 consecutive cases with midterm follow up Ann Surg 2017 10.1097/SLA.0000000000002190[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Probst A, Ebigbo A, Märkl B et al. Endoscopic submucosal dissection for early rectal neoplasia: experience from a European center. Endoscopy. 2017;49:222–232. doi: 10.1055/s-0042-118449. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguti F S, Nahas C S, Marques C F et al. Endoscopic submucosal dissection versus transanal endoscopic microsurgery for the treatment of early rectal cancer. Surg Endosc. 2014;28:1173–1179. doi: 10.1007/s00464-013-3302-z. [DOI] [PubMed] [Google Scholar]


