Abstract
In the present work, a new protocol for fast separation and quantification of JH III from biological samples using liquid chromatography coupled to electrospray tandem mass spectrometry is described. In particular, the proposed protocol improves existing methodologies by combining a limited number of sample preparation steps with fast LC-MS/MS detection, providing lower limits of detection and demonstrated matrix effect control, together with high inter and intraday reproducibility. A limit of detection of 8 pg/mL (0.32 pg on column) was achieved, representing a 15-fold gain in sensitivity with respect to previous LC-MS based protocols. The performance of the LC-MS/MS protocol is comparable to previously described JH III quantitation protocol based on fluorescence detection, with the added advantage that quantification is independent of the availability of fluorescent tags that are often unavailable or show quite diverse responses on a batch-to-batch basis. Additionally, a detailed description of the JH III fragmentation pathway is provided for the first time, based on isolation of the molecular ion and their intermediate fragments using in-source MS/MS, MS/MSn and FT-ICR MS/MS measurements. The JH III workflow was evaluated as a function of developmental changes, sugar feeding and farnesoic acid stimulation in mosquitoes and can be applied to the detection of other juvenile hormones.
Keywords: Juvenile hormone III, Liquid chromatography, MRM, quantification, extraction
TOC image
INTRODUCTION
Juvenile hormones (JHs) are synthesized by the corpora allata (CA) and play key roles in many processes in insect development and reproduction, including inhibition of metamorphosis, caste determination and differentiation, stimulation of flight and migration, stimulation of reproduction, regulation of diapause, stress resistance, and aging(1–6). Several JHs have been identified and characterized in insects, with JH III being the most widespread(6–9). A common structural feature for all JHs is the presence of an epoxide group near one end and a methyl ester on the other. JH titers in small insects are often in the femtomole to picomole range, which makes it challenging to detect by most typical analytical techniques(10–14). Mosquito-borne diseases are major public health problems in developing countries. The discovery of mosquito-specific control agents depends on basic research on the biology of mosquitoes. Juvenile hormone (JH) is a major hormonal regulator that controls development and reproduction in mosquitoes.
The most widely used analytical methods for identification and detection of JHs include nuclear magnetic resonance (NMR), infrared spectroscopy (IR), and gas chromatography (GC)–mass spectrometry (MS) (14–19). During GC-MS, fragment ions from the electron or chemical ionization process are typically used to identify the JH molecules; however, this analysis typically requires lengthy preparation steps(19–23). More recently, several studies have shown the advantages of a number of additional techniques for the identification and quantification of JHs, such as direct analysis in real time-MS (DART-MS), high performance liquid chromatography-MS/MS (HPLC- MS/MS), HPLC with fluorescence detection (HPLC-FD) and ultra-performance liquid chromatography-MS (UPLC-MS)(18, 24–32). A variety of ionization sources have been utilized prior to the MS analysis, such as electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), atmospheric pressure photoionization (APPI) and atmospheric pressure thermospray ionization (APTSI); allowing the detection of the JH III molecular ion in the protonated and sodiated forms(33). While some studies showed the advantages of using the sodiated species for quantification in single ion monitoring mode (SIM), only the protonated species allowed for multiple reaction monitoring (MRM)(25).
In the present work, we describe an extraction protocol followed by an HPLC-ESI-MS/MS analysis that increased sensitivity and reproducibility, while reducing the analysis time for JH III detection in mosquito samples. The features of the method proposed are compared with previously established MS- and FD-based methods. Additionally, a detailed description of the fragmentation behavior of JH III [M+H]+ molecular ion is described for the first time.
MATERIALS AND METHODS
Materials and reagents
Certified standard solutions for JH III and its deuterated analog (JH III-D3) were obtained from Toronto Research Chemicals (Toronto, Canada). Sodium chloride, potassium chloride, hydrochloric acid, sodium hydroxide, ammonium acetate, ammonium formate and ammonium hydroxide salts were analytical grade or better (Fisher Scientific, Pittsburgh, PA). Water, methanol, hexane and acetonitrile were all Optima grade or better (Fisher Scientific). Chromatographic mobile phases (0.1% formic acid in water, and 0.1% formic acid in acetonitrile) of Optima LC-MS grade were also purchased from Fisher Scientific, and used as received. Tissue culture media Gibco M-199, silanized LC vials and silanized LC vials with fused 250 μL inserts were also purchased from Fisher Scientific. The tuning mix calibration standard (G24221A) was obtained from Agilent Technologies (Santa Clara, CA).
Sample preparation and storage
Biological samples were prepared following the protocol described in supplemental Figure S9. Briefly, preparations were of intact corpora allata-corpora cardiaca (CA-CC) complexes connected to the brain and head capsule, and are denoted as BR-CA-CC complexes, as described in reference (22). The aorta-CA-CC was left connected to the intact head capsule to facilitate the visualization and transfer of the glands. BR-CA-CC were dissected in a drop of mosquito saline-buffer containing 138 mM NaCl, 8.4 mM KCl, 4 mM CaCl2, 12 mM NaH2PO4 and 42.5 mM sucrose(34). After dissection, the BR-CA-CC complexes were incubated in 150 μL of tissue culture media M-199, containing 2% Ficoll 400 and 50 μM methionine. Incubations of BR-CA-CC complexes were carried out in a humid chamber in silanized 2 mL vials for 4 h in the dark at 32°C, and under continuous gentle agitation. After incubation, 10 μL of 6.25 ppb JH III-D3 in acetonitrile were added to each sample, followed by 600 μL of Hexane. Samples were vortexed for 1 minute, and spun for 5 minutes at 4°C and 2000 g. The organic phase was transferred to a new silanized vial and dried under nitrogen flow. Dried extracts were re-suspended in 100 μl of acetonitrile, vortexed 1 minute, transferred to a new silanized vial with a fused 250 μL insert and stored at −20°C.
Mass Spectrometry Analysis
The mass spectrometry analyses were carried out in-house using HPLC-MS/MS. Briefly, sample injections (40 μL) and LC separations were performed by a Prominence LC-20AD Ultra-Fast Liquid Chromatograph (Shimadzu, Kyoto, Japan), equipped with a Dionex Acclaim 120 C18 Column (250×2.1 mm, 5 μm) obtained from Thermo Scientific (Sunnyvale, CA). Column temperature was kept at 40°C. A 15 minutes binary gradient program between 0.1% formic acid dissolved in water (mobile phase A) and 0.1% formic acid dissolved in acetonitrile (mobile phase B) was run according to the following timetable: hold 5% B for 0.5 min; ramp to 98% B in 7.5 min; hold 98% B for 3 min; return to 5% B in 0.5 min; hold 5% B for 3.5 min. Flow rate was constant at 0.8 mL/min. Detection was performed by a QTRAP® 5500 triple quadrupole mass spectrometer (AB Sciex, Ontario, Canada) equipped with a Turbo V™ ion source. The mass spectrometer was operated under ESI positive mode ionization with multiple reaction monitoring (MRM) of two transitions per compound. MRM detection and electrospray source parameters were optimized by infusing 1 mg/L solutions of each compound in 0.1% formic acid in acetonitrile. Source parameters were: curtain gas = 10 psi; spray Voltage = 5000 V; temperature 400°C; ion source gas 1= 40 psi; ion source gas 2= 50 psi; entrance potential = 7.0 V. MRM transitions parameters are listed in Table 1.
Table 1.
Common MRM parameters utilized for JH III and JH III-D3 detection on the QTRAP® 5500 triple quadrupole mass spectrometer.
| Compound | DP1 (V) | Q1 (Da) | CE2 (V) | Q3 (Da) | CXP3 (V) |
|---|---|---|---|---|---|
| JH III | 46 | 267 | 11 | 235 | 30 |
| 19 | 147 | 16 | |||
| JH III-D3 | 46 | 270 | 11 | 235 | 28 |
| 17 | 147 | 6 |
Declustering Potential;
Collision Energy;
Collision cell exit potential.
JH III and JH III-D3 chemical structures and purities were confirmed by MS/MS and accurate mass measurements using Fourier transform ion cyclotron resonance-MS (nanoESI-FT-ICR MS). In-source fragmentation and MS/MSn capabilities in the QTRAP® 5500 triple quadrupole mass spectrometer (AB Sciex, Ontario, Canada) were used to determine the fragmentation pathways of JH III and JH III-D3 (see Table 1 and 2). nanoESI FT-ICR MS/MS experiments were performed in positive ion mode in a 7T Solarix FT-ICR MS spectrometer (Bruker Daltonics, Inc., Billerica, MA). Ion transmission was optimized for high sensitivity for JH III and its fragments in the m/z 100−300 range. Ions were accumulated in the collision cell (2 MHz, 1000 Vpp) for 4s. FT-ICR MS spectra were acquired over 200-time domain acquisitions at 2 Mword (1 s transient). FT-ICR signals were processed using a half-sine apodization followed by Fast-Fourier transform and broadband phase correction (absorption spectra using absorption mode processing, AMP), resulting in a 2-fold increase in mass resolution.
Table 2.
Typical fragment ion and dissociation pathways observed using in-source MS/MS, MS/MSn and FT-ICR MS/MS for JH III [M+H]+ molecular ion.
| Parent m/z | Fragment m/z | |
|---|---|---|
| In-source MS/MS | 267 | 249, 235, 217, 189, 147 |
| 249 | 217, 189, 147 | |
| 235 | 217, 189, 147 | |
| 217 | 189, 147 | |
| 189 | 147 | |
| MS/MSn | 267 -> 235 | 217, 207, 189 |
| 267 -> 249 | 217, 189, 147 | |
| 267 -> 217 | 189, 147 | |
| 267 -> 189 | 147 | |
| FT-ICR MS/MS | 267.195461 C16H27O3 |
249.18492 C16H25O2 |
| 235.169298 C15H23O2 |
||
| 217.15878 C15H21O |
||
| 207.174417 C14H23O |
||
| 189.163858 C14H21 |
||
| 147.116827 C11H15 |
RESULTS AND DISCUSSION
The main aim of this study was to develop a protocol for fast and accurate measurement of JH III from biological samples using mass spectrometry. In particular, our efforts were focused on: 1) minimizing the number of steps during sample preparation prior to the MS analysis, 2) developing a fast LC-MS/MS protocol, 3) improving sample storage protocols, 4) evaluate inter and intraday analysis variation, 5) refining the interpretation of the JH III MS/MS fragmentation pathway, 6) enhancing limits of detection while reducing matrix effects, and 7) comparing the performance with a previously utilized JH III quantitation protocol based on fluorescence detection(30).
Mass Spectrometry Analysis
JH III can be typically detected in the protonated and sodiated forms (e.g., m/z = 267 [M+H]+ and m/z= 289 [M+Na]+), depending on the spraying and solvent conditions(24). Since the fragmentation of the sodiated molecular ion [M+Na]+ does not produce diagnostic fragment ions, previous studies have preferred LC-MS for JH identification and quantitation, with the caveat that quantification accuracy may be compromised by potential interferences due to the complex nature of the biological sample(28, 29). Alternatively, the fragmentation of the protonated form m/z = 267 [M+H]+ provides a variety of signature fragmentation pathways that can be used to identify the JH III structure (Figure 1 top). The [M+H]+ molecular ion can undergo fragmentation via collision induced activation using in-source MS/MS and the MS/MSn linear trap region of the LC-QQQ (see Table 2). Inspection of the in-source and MS/MSn data permitted the construction of the JH III [M+H]+ fragmentation pathway (Figure 2). Upon collision induced activation, the JH III [M+H]+ main fragmentation pathways lead to the observation of m/z = 235 [M+H-CH3OH]+ and m/z= 249 [M+H-H2O]+ ions, corresponding to the loss of one methanol group and one water molecule, respectively. Further activation of m/z= 235 and 249 generates m/z = 217 [M+H-CH3OH-H2O]+ and m/z = 207 [M+H-CH3OH-CO]+, corresponding to the combined loss of one methanol group and water, and to the loss of a CO group, respectively. In contrast to previous reports(25), our data support the generation of m/z = 217 as a product of 267->235->217 and 267->249->217. The activation of m/z= 217 produces m/z= 189 [M+H-CH3OH-H2O-CO]+, which further fragments into m/z = 147 [M+H-CH3OH-H2O-CO-CH3CH=CH2]+. Complementary analysis using nESI-FT-ICR MS/MS supports the above proposed mechanism. The nESI-FT-ICR MS/MS showed the fragmentation of [M+H]+ (m/z= 267.195461, C16H27O3) into [M+H-H2O]+ (m/z= 249.18492, C16H25O2), [M+H-CH3OH]+ (m/z= 235.16929, C16H23O2), [M+H-CH3OH-H2O]+ (m/z= 217.158786, C15H21O), [M+H-CH3OH-CO]+ (m/z= 207.174417, C14H23O), [M+H-CH3OH-H2O-CO]+ (m/z= 189.163858, C14H21) and [M+H-CH3OH-H2O-CO-CH3CH=CH2]+ (m/z= 147.116827, C11H15) with sub-ppm mass accuracy (see Table 2). Previous studies utilizing chemical ionization ion trap mass spectrometry, performed the quantification based on the summed intensities of six diagnostic ions (m/z= 235, 217, 189, 147, 125, and 111)(19). Although this approach is also feasible in the case of LC-QQQ instruments, using a lower number of MRM transitions allows the monitoring of a higher number of points across the LC peak relative to the aforementioned MRM strategies and therefore provides higher sensitivity. The selection of the transitions to perform the JH III quantification was based on their relative abundance. That is, the most abundant fragmentation transitions utilized were 267->235 (primary) and 267-> 147 (secondary). Previous reports have also utilized 267-> 235 as a primary and 267-> 217 as secondary MRM transitions for LC-MS/MS analysis(25, 27).
Figure 1.
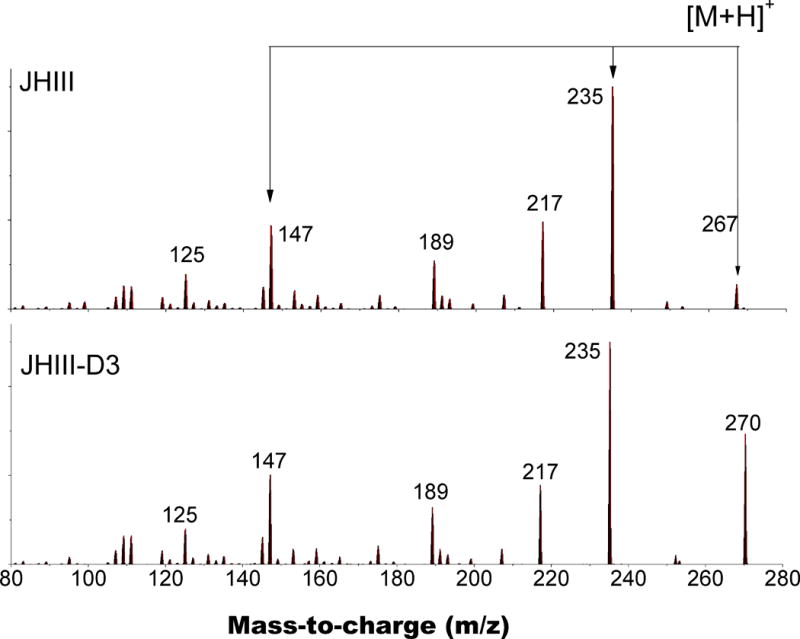
Typical MS/MS fragmentation spectra for JH III [M+H]+ and JH III-D3 [D+H]+.
Figure 2.
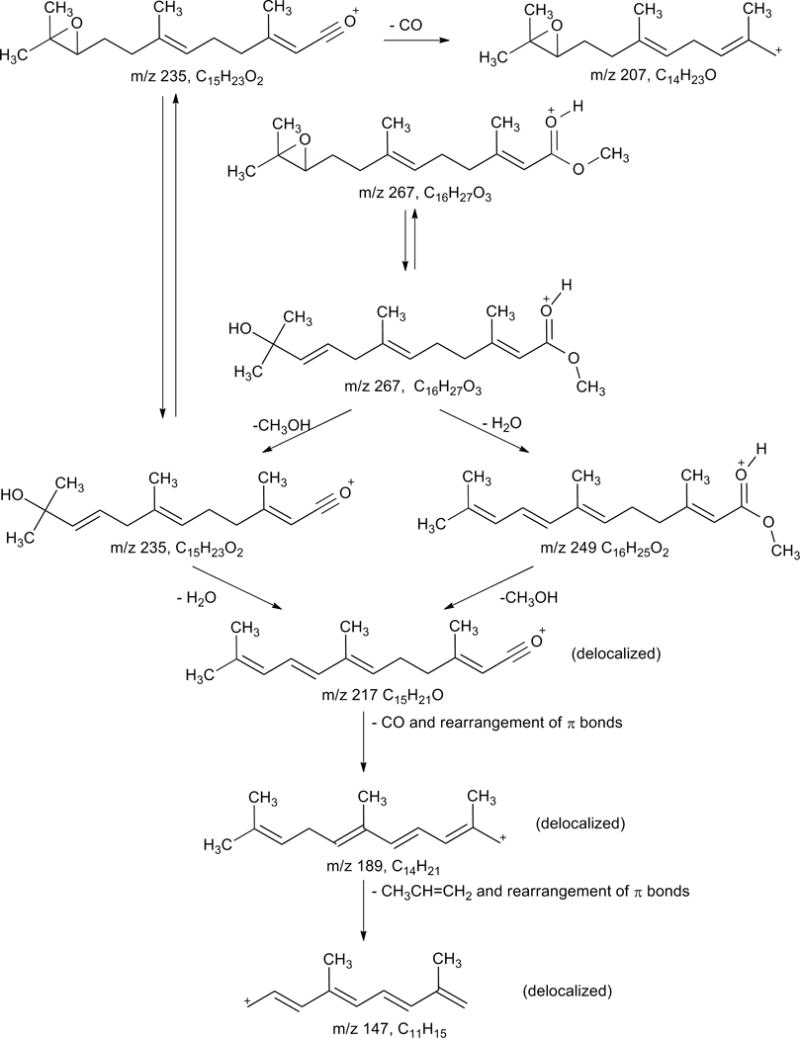
Fragmentation scheme for JH III [M+H]+ and JH III-D3 [D+H]+ proposed based on isolation of the molecular ion and fragment ions using in- source MS/MS, MS/MSn and FT-ICR MS/MS.
The heavy isotopomer JH III-D3 was utilized as an internal standard to normalize all sample preparation, extraction and analysis steps in order to accurately quantify the amount of JH III hormone produced by the BR-CA-CC complexes. The JH III-D3 molecular ion was observed in the protonated form [D+H]+, and the stoichiometry was confirmed utilizing nESI-FT-ICR MS (m/z = 270.21420) with sub-ppm accuracy. The fragmentation of the JH III-D3 [D+H]+ molecular ion showed multiple similarities to JH III [M+H]+ (Figure 1). That is, the loss of the deuterated methanol group led to the observation of m/z= 235 [M+H-CD3OH]+; further activation showed the same fragmentation pattern as the JH III [M+H]+ molecular ion.
A plausible mechanistic sequence for the generation of JH III fragment ions is shown in Figure 2 and in the supplementary materials. Briefly, it is proposed that opening of the oxirane to form a tertiary alcohol and an alkene would facilitate the elimination of water (perhaps, via a 6-electron cyclic transition state) leading to the formation of m/z = 249 [M+H-H2O]+ from m/z = 267 [M+H]+ and m/z = 217 [M+H-CH3OH-CO]+ from m/z = 235 [M+H-CH3OH ]+. Similarly, elimination of methanol accounts for the formation of both m/z = 235 [M+H-CH3OH]+ from m/z = 267 [M+H]+ and m/z = 217 [M+H-CH3OH-CO]+ from m/z = 249 [M+H-H2O]+. The loss of methanol is confirmed by the aforementioned isotopic labeling study with JH III-D3. In each case, the exclusion of the methanol generates a molecular ion in which the positive charge will reside on the C≡O oxygen. The subsequent loss of CO from m/z = 217 [M+H-CH3OH-CO]+ to form m/z = 189 observed by the nESI-FT-ICR MS/MS analysis is unlikely to occur on the basis of direct cleavage from an sp2carbon. Thus, it is proposed that a rearrangement of the C-2 double bond occurs, allowing the CO to leave with the generation of a resonance-stabilized allylic carbocation that has the potential to undergo a Wagner-Meerwein hydride shift to form an even more stable allylic carbocation in which the positive charge is delocalized over an entire 7-carbon conjugated triene array. Whether the rearrangement and elimination are separate steps or a concerted process is not clear. Further rearrangement of this system allows the elimination of propene as a neutral fragment generating m/z = 147 [M+H-CH3OH-H2O-CO-CH3CH=CH2]+ as an even more delocalized allylic carbocation in which the positive charge can be delocalized over the entire 9-carbon tetraene unit. The loss of CO from m/z = 235 [M+H-CH3OH]+ to generate m/z = 207 [M+H- CH3OH-CO]+ is proposed to occur via a similar initial rearrangement of the C-2 double bond. The resultant allylic carbocation will also be resonance stabilized. It is unclear whether the loss of CO occurs on the oxirane or the rearranged oxirane or both.
HPLC-MS/MS method validation
The HPLC-MS/MS workflow was developed using standards and biological samples, with the extraction protocol described in supplemental Figure S9. The HPLC consisted of a short 15 minutes run with JH III eluting at around 8.2 minutes (Figure 3). As expected, JH III and its heavy analog JH III-D3 co-eluted, and their detection was based on monitoring the 267->235 and 270->235 as primary and 267->147 and 270->147 as secondary transitions. Notice that the primary MS/MS channel is 4–5 fold more abundant that the secondary channel. Comparison between the standard and the biological samples showed no changes on retention time, as well as on the ratio between transitions, allowing the use of these parameters for analyte confirmation purposes.
Figure 3.
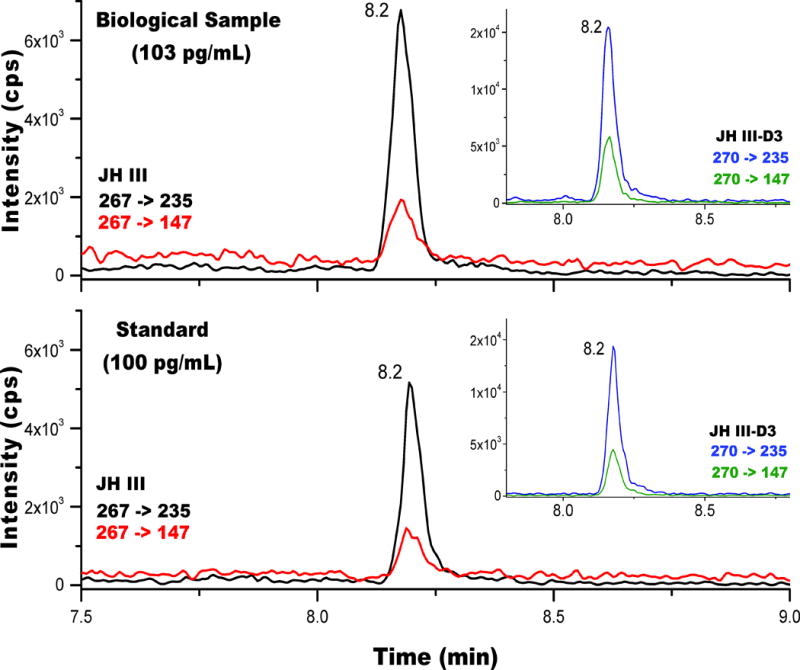
Typical ion extracted chromatogram for JH III from a biological sample (top) and from a standard solution (bottom) using the 267-> 235 (primary) and the 267->147 (confirmation) MRM transitions. In the inset, the corresponding chromatograms for JH III-D3 are shown.
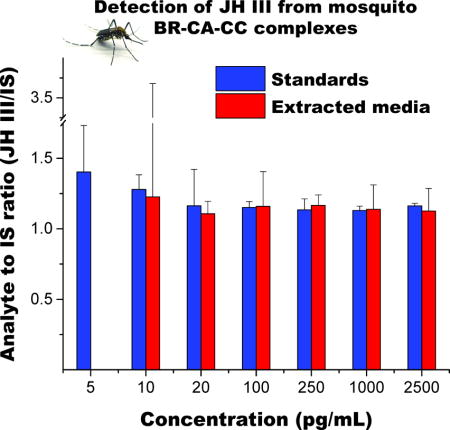
The effect of analyte extraction was evaluated by comparing the instrument response of analyte to internal standard ratio (JH III/JH III-D3), for pure- and media-extracted standards over a wide concentration range (Figure 4a). An extraction recovery of near 55% was routinely observed regardless of the analyte concentration. No improvements were observed by performing a double extraction with hexane (Figure 4b). The reduced sensitivity caused by the extraction efficiency is evidenced in the reduced slope on the linear response of the instrument as a function of analyte concentration (Figure 4c), justifying the use of JH III-D3 to normalize this step.
Figure 4.
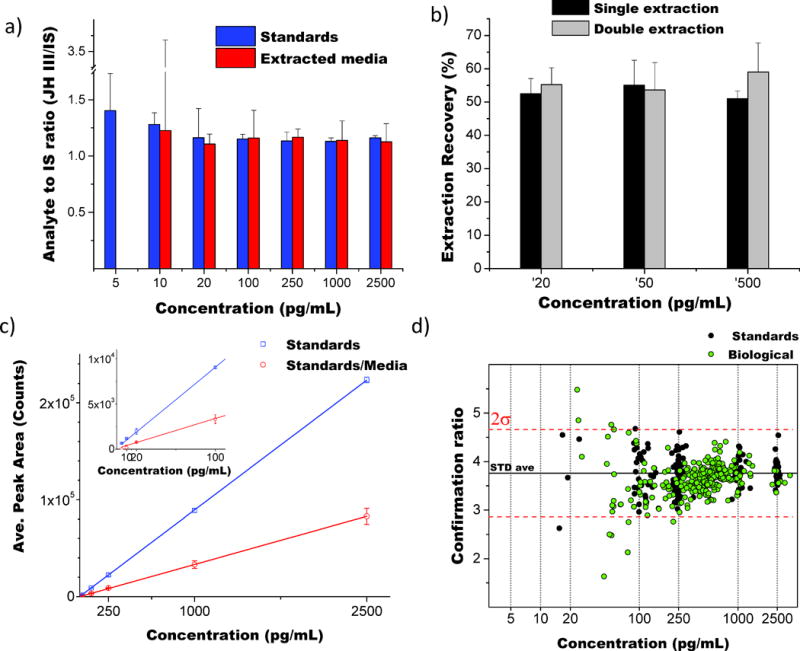
Response curves as a function of the JH III concentration in the sample. A) Analyte to internal standard ratio (JH III/JH III-D3) for non-extracted and extracted samples; b) extraction recovery for single and double extraction; c) method response for non-extracted and extracted samples; and d) confirmation ratio for internal standards and biological samples.
The HPLC-MS/MS method limit of detection (LOD), defined as 3 times the background analytical response, was determined experimentally to be 8 pg/mL (0.32 pg on column) by spiking increasing quantities of JH III in ACN (at 5, 10, 20, 100, 250 and 1000 pg/mL in triplicates, n=18), and calculating the uncertainty on the intercept of the obtained curve. A LOD of 19 pg/mL (0.76 pg on column) was obtained by spiking the analyte into M-199 medium and submitting the prepared solutions to the complete extraction protocol; this higher LOD is a consequence of a reduced signal after extraction. However, the obtained protocol LOD is approximately 10 times lower than previous LC-MS based methodologies for the detection of JH III in hemolymph (LOD = of 4–5 pg on column(27, 29, 31)).
Inter and intra-day reproducibility was evaluated by extracting and analyzing spiked M-199 media with 500 pg/mL of JH III. The protocol was halted at the drying step (see supplemental Figure S9) and the extracted standards were stored dry for 0 (reference, n=32), 1, 4, 5 and 7 days (n=5 each). There were no significant differences between the measurements in terms of peak areas or recovery (supplemental Tables S1 and S2), which suggested that the analyte extracted from spiked media could be stored dry for at least 7 days. Most intra-day variability occurred between one standard deviation from the pooled mean of all measurements. A relative standard deviation (RSD) of 10% or lower was obtained for each analysis day, while an RSD of 16% was obtained in the complete dataset.
Analysis of JH III from biological samples
In order to evaluate the applicability of this analysis protocol, JH III synthesized in vitro by mosquito BR-CA-CC complexes under different developmental and physiological conditions were analyzed. Samples were prepared following the protocol previously described (see supplemental Figure S9). Calibration curves were obtained by plotting the peak area ratio of JH III to JH III-D3 as a function of the analyte concentration. Linearity was observed over a wide concentration range (R2 > 0.999; 5 to 2500 pg/mL, 0.2 pg to 100 pg on column). The concentration of JH III-D3 was constant in biological samples and calibration solutions (625 pg/mL). Calibration standards were analyzed in duplicate at the beginning and at the end of each analytical sequence, with calibration verification performed every 12 runs by injecting 250 pg/mL of JH III in acetonitrile buffer, allowing a 15% deviation. A control blank (non-extracted) was analyzed with every batch, consistently displaying the absence of JH III signal. The analysis sequence also included carryover tests by verifying the absence of a JH III signal upon injecting the control blank after the highest calibration standard, as well as after every calibration verification standard.
In each LC-QQQ analysis batch, duplicates of a negative control and two concentrations of positive controls (50 pg/mL and 500 pg/mL) were extracted according to supplemental Figure S9 and measured, with deviations of 15% or lower consistently observed. JH III compound identifications were considered positive when a signal-to-noise ratio larger than 3 were present in both the quantification and confirmation MRM transitions, with a difference of less than 0.05 min in retention time relative to that of the JH III-D3. The results of the analysis of over 1500 samples revealed that the confirmation ratios for internal standards and biological samples were constantly within two standard deviations (Figure 4d).
In order to investigate the effect of sample storage on analyte signal, 10 biological samples were split after hexane extraction and the resulting extract aliquots were dried under nitrogen. One of the dried aliquots was stored at 4°C and the second was reconstituted in acetonitrile for immediate analysis. Five of the stored aliquots were reconstituted and analyzed after 5 days, while the remaining samples were analyzed after 30 days of storage. There were no significant differences in terms of recovery relative to the initial measured concentration (see supplemental Tables S1 and S2) as a function of storage time, suggesting that dried biological samples can be correctly quantified after a month of dry storage. Previous work has shown that JH III is stable for up to one month and up to six 6 months in methanol if stored at 4°C and −18°C, respectively(25). Similar stabilities were detected when JH III was stored in hexane and acetonitrile. However, the possibility to store dry samples largely facilitates the handling and shipping for inter-laboratory comparisons and complementary measurements.
Further validation of the JH III quantitation protocol was attained by analyzing JH III synthesis by BR-CA-CC adult female mosquito preparations in vitro as a function of developmental changes, sugar feeding and farnesoic acid stimulation. The results of these experiments were compared with previous studies using HPLC-FD(35–37). The comparison of the MS and the FD results showed good agreement for all the cases, considered reinforcing the value of the new developed protocol to assess JH III levels in mosquitoes (Figure 5). The new LC-MS/MS protocol showed similar high sensitivity, accuracy and reproducibility compared to the previously reported HPLC-FD(30), with the advantage of having selective MS detection that enables absolute quantification by using a heavy analog as an internal standard. The HPLC-FD protocol, while also sensitive, depends on commercially made tags that are often unavailable, require derivatization steps and show quite diverse responses on a batch-to-batch basis. On the contrary, this LC-MS/MS protocol includes a straightforward extraction protocol followed by a sensitive and reproducible HPLC-ESI-MS/MS analysis for absolute JH III detection in mosquito samples.
Figure 5.
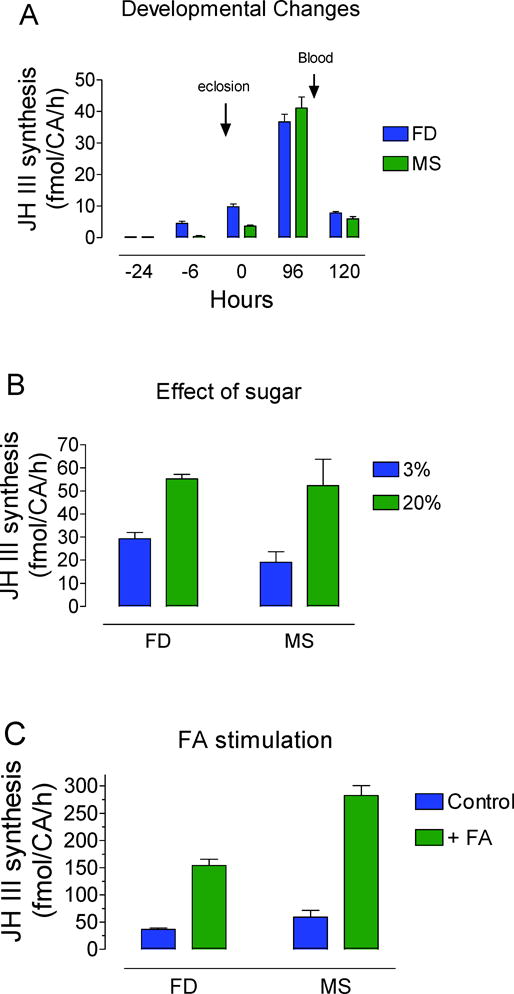
Comparison of FD and MS methods to quantify JH III synthesis as a function of: A) Developmental changes. Synthesis was evaluated from BR-CA-CC preparations dissected from pupae and adult female mosquitoes at different hours before and after adult eclosion. FD values are from reference(35). B) Effect of sugar feeding. MS: Newly eclosed adult females were fed for 4 days on 3% or 20% sucrose. FD values are from reference(37). C) MS: BR-CA-CC preparations were dissected from 4 day-old adult female mosquitoes and stimulated with 40μM farnesoic acid (FA). FD values are from reference(36).
CONCLUSIONS
An analytical workflow for fast, ultra-trace quantitation of JH III from biological samples was developed. The fragmentation pathway of JH III [M+H]+ molecular ion was studied, and a mechanistic model is proposed. A HPLC-MS/MS workflow based on MRM using 267-> 235 and 267 -> 147 was optimized for quantitative analysis, with higher sensitivity and a reduced number of sample preparation steps. A better analytical performance of the proposed protocol was demonstrated in terms of reproducibility, sensitivity and routine applicability, with higher sensitivity than previous LC-MS applications for the detection of JH III and with similar sensitivity to spectrofluorometric methods without the need of lengthy derivatization steps. Results showed that the storage protocol allows for quantification of samples with at least one month of dry storage. Further developments of the proposed workflow can be used to the analysis of other juvenile hormones.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (Grant No. 2R01AI045545-15 to FGN and R00GM106414 to FFL) and the Advanced Mass Spectrometry Facility of Florida International University.
Footnotes
Notes
The authors declare no competing financial interest.
SUPPORTING INFORMATION
The proposed mechanisms of fragmentations are outlined in the supplementary material. Figure S1. Rearrangement of m/z= 267. Figure S2. Formation of m/z= 249 from rearranged m/z= 267. Figure S3. Formation of m/z= 235 (works the same way from rearranged m/z= 267. Figure S4. Formation of m/z= 217 from rearranged m/z= 235. Figure S5. Formation of m/z= 217 from m/z= 249. Figure S6. Formation of m/z= 207 from m/z= 235. Figure S7. Formation of m/z= 189 from m/z= 217. Figure S8. Formation of m/z= 147 from m/z= 189. Figure S9. Protocol for extraction and analysis of JH III synthesized in vitro by mosquito BR-CA-CC preparations. BR-CA-CC: brain corpora allata-corpora cardiaca. ACN: acetonitrile. Figure S10. Typical ESI-MS spectra of for JH III [M+H]+ and [M+Na]+ and JH III-D3 [D+H]+ and [D+Na]+. Table S1. Effects of storage on analyte signals. Comparisons of peak areas and percent recoveries (based on response factors, RF) of JH III and JH III-D3 after standards were dried and stored at 4°C. Table S2. Effects of storage on analyte signals. Comparison of analyte recovery (expressed as %) as a function of storage time. Biological samples were extracted, split, dried and stored at 4°C for 5 and 30 days (n= 5 and n=4, respectively).
References
- 1.Goodman WG, Cusson M. The Juvenile Hormones. In: Gilbert LI, editor. Insect Endocrinology. San Diego: Academic Press; 2012. pp. 310–65. [Google Scholar]
- 2.Gilbert LI, Granger NA, Roe RM. The juvenile hormones: historical facts and speculations on future research directions. Insect Biochemistry and Molecular Biology. 2000;30(8–9):617–44. doi: 10.1016/s0965-1748(00)00034-5. [DOI] [PubMed] [Google Scholar]
- 3.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annual review of entomology. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt G, Davey K. Cellular and Molecular Actions of Juvenile Hormone. ll. Roles of Juvenile Hormone in Adult Insects. Advances in insect physiology. 1996;26(1) [Google Scholar]
- 5.Watanabe D, Maekawa K. Relationships between frontal-gland formation and mandibular modification during JH III-induced presoldier differentiation in the termite Reticulitermes speratus (Isoptera: Rhinotermitidae) Entomological Science. 2012;15(1):56–62. [Google Scholar]
- 6.Engel KC, Stokl J, Schweizer R, Vogel H, Ayasse M, Ruther J, et al. A hormone-related female anti-aphrodisiac signals temporary infertility and causes sexual abstinence to synchronize parental care. Nat Commun. 2016;7 doi: 10.1038/ncomms11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noriega FG. Juvenile hormone biosynthesis in insects: What is new, what do we know, and what questions remain? International Scholarly Research Notices. 2014;2014 doi: 10.1155/2014/967361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riddiford LM. Cellular and Molecular Actions of Juvenile Hormone I. General Considerations and Premetamorphic Actions. In: Evans PD, editor. Advances in Insect Physiology. Vol. 24. Academic Press; 1994. pp. 213–74. [Google Scholar]
- 9.Schooley DA, Judy KJ, Bergot BJ, Hall MS, Siddall JB. Biosynthesis of the juvenile hormones of Manduca sexta: labeling pattern from mevalonate, propionate, and acetate. Proceedings of the National Academy of Sciences. 1973;70(10):2921–5. doi: 10.1073/pnas.70.10.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kort Cd, Granger N. Regulation of the juvenile hormone titer. Annual review of entomology. 1981;26(1):1–28. [Google Scholar]
- 11.Mayoral JG, Nouzova M, Yoshiyama M, Shinoda T, Hernandez-Martinez S, Dolghih E, et al. Molecular and functional characterization of a juvenile hormone acid methyltransferase expressed in the corpora allata of mosquitoes. Insect biochemistry and molecular biology. 2009;39(1):31–7. doi: 10.1016/j.ibmb.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trumbo ST, Robinson GE. Social and nonsocial stimuli and juvenile hormone titer in a male burying beetle, Nicrophorus orbicollis. Journal of insect physiology. 2008;54(3):630–5. doi: 10.1016/j.jinsphys.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Girard J, Madhavan K, McMorris T, De Loof A, Chong J, Arunachalam V, et al. Identification of a juvenile hormone from Musca domestica. Insect Biochemistry. 1976;6(4):347–50. [Google Scholar]
- 14.Borovsky D, Carlson D, Hancock R, Rembold H, Van Handel E. De novo biosynthesis of juvenile hormone III and I by the accessory glands of the male mosquito. Insect biochemistry and molecular biology. 1994;24(5):437–44. doi: 10.1016/0965-1748(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 15.Röller H, Dahm KH, Sweely CC, Trost BM. The Structure of the Juvenile Hormone. Angewandte Chemie International Edition in English. 1967;6(2):179–80. [Google Scholar]
- 16.Bergot BJ, Ratcliff M, Schooley DA. Method for quantitative determination of the four known juvenile hormones in insect tissue using gas chromatography—mass spectroscopy. Journal of Chromatography A. 1981;204:231–44. [Google Scholar]
- 17.Yin C-M, Zou B-X, Jiang M, Li M-F, Qin W, Potter TL, et al. Identification of juvenile hormone III bisepoxide (JHB3), juvenile hormone III and methyl farnesoate secreted by the corpus allatum of Phormia regina (Meigen), in vitro and function of JHB3 either applied alone or as a part of a juvenoid blend. Journal of Insect Physiology. 1995;41(6):473–9. [Google Scholar]
- 18.Ichikawa A, Ono H, Furuta K, Shiotsuki T, Shinoda T. Enantioselective separation of racemic juvenile hormone III by normal-phase high-performance liquid chromatography and preparation of [2H3]juvenile hormone III as an internal standard for liquid chromatography–mass spectrometry quantification. Journal of Chromatography A. 2007;1161(1–2):252–60. doi: 10.1016/j.chroma.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Teal P, Proveaux A, Heath R. Analysis and quantitation of insect juvenile hormones using chemical ionization ion-trap mass spectrometry. Analytical Biochemistry. 2000;277(2):206–13. doi: 10.1006/abio.1999.4377. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Simuta PETY. Juvenile hormone: action in regulation of sexual maturity in Caribbean fruit flies and potential use in improving efficacy of sterile insect control technique for tephritid fruit flies. IOBC wprs Bulletin. 2002:25. [Google Scholar]
- 21.Rembold H, Llackner B. Convenient method for the determination of picomole amounts of juvenile hormone. Journal of Chromatography A. 1985;323(2):355–61. [Google Scholar]
- 22.Li Y, Unnithan GC, Veenstra JA, Feyereisen R, Noriega FG. Stimulation of JH biosynthesis by the corpora allata of adult female Aedes aegypti in vitro: effect of farnesoic acid and Aedes allatotropin. Journal of Experimental Biology. 2003;206(11):1825–32. doi: 10.1242/jeb.00371. [DOI] [PubMed] [Google Scholar]
- 23.Sperling S, Kühbandner S, Engel KC, Steiger S, Stökl J, Ruther J. Size Exclusion High Performance Liquid Chromatography: Re-Discovery of a Rapid and Versatile Method for Clean-Up and Fractionation in Chemical Ecology. Journal of Chemical Ecology. 2015;41(6):574–83. doi: 10.1007/s10886-015-0584-8. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Linse KD, Taub-Montemayor TE, Rankin MA. Comparison of radioimmunoassay and liquid chromatography tandem mass spectrometry for determination of juvenile hormone titers. Insect biochemistry and molecular biology. 2007;37(8):799–807. doi: 10.1016/j.ibmb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Qi Y, Hou Y, Zhao J, Li Y, Xue X, et al. Quantitative determination of juvenile hormone III and 20-hydroxyecdysone in queen larvae and drone pupae of Apis mellifera by ultrasonic-assisted extraction and liquid chromatography with electrospray ionization tandem mass spectrometry. Journal of Chromatography B. 2011;879(25):2533–41. doi: 10.1016/j.jchromb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Navare AT, Mayoral JG, Nouzova M, Noriega FG, Fernández FM. Rapid direct analysis in real time (DART) mass spectrometric detection of juvenile hormone III and its terpene precursors. Analytical and bioanalytical chemistry. 2010;398(7–8):3005–13. doi: 10.1007/s00216-010-4269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ares A, Nozal M, Bernal J, Martín-Hernández R, Higes M, Bernal J. Liquid chromatography coupled to ion trap-tandem mass spectrometry to evaluate juvenile hormone III levels in bee hemolymph from Nosema spp. infected colonies. Journal of Chromatography B. 2012;899:146–53. doi: 10.1016/j.jchromb.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Westerlund SA, Hoffmann KH. Rapid quantification of juvenile hormones and their metabolites in insect haemolymph by liquid chromatography–mass spectrometry (LC-MS) Analytical and bioanalytical chemistry. 2004;379(3):540–3. doi: 10.1007/s00216-004-2598-x. [DOI] [PubMed] [Google Scholar]
- 29.Furuta K, Ichikawa A, Murata M, Kuwano E, Shinoda T, Shiotsuki T. Determination by LC-MS of juvenile hormone titers in hemolymph of the silkworm, Bombyx mori. Bioscience, biotechnology, and biochemistry. 2013;77(5):988–91. doi: 10.1271/bbb.120883. [DOI] [PubMed] [Google Scholar]
- 30.Rivera-Perez C, Nouzova M, Noriega FG. A quantitative assay for the juvenile hormones and their precursors using fluorescent tags. Plos One. 2012;7(8):e43784. doi: 10.1371/journal.pone.0043784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazaki M, Mao L, Henderson G, Laine RA. Liquid chromatography–electrospray ionization-mass spectrometric quantitation of juvenile hormone III in whole body extracts of the Formosan subterranean termite. Journal of Chromatography B. 2009;877(27):3175–80. doi: 10.1016/j.jchromb.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe D, Gotoh H, Miura T, Maekawa K. Soldier presence suppresses presoldier differentiation through a rapid decrease of JH in the termite Reticulitermes speratus. Journal of Insect Physiology. 2011;57(6):791–5. doi: 10.1016/j.jinsphys.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Vilaró F, Pérez-Hedo M, Eras J, Canela R, Eizaguirre M. UHPLC–MS Analysis of Juvenile Hormone II in Mediterranean Corn Borer (Sesamia nonagrioides) Hemolymph Using Various Ionization Techniques. Journal of agricultural and food chemistry. 2012;60(12):3020–5. doi: 10.1021/jf204621h. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Hernandez-Martinez S, Unnithan GC, Feyereisen R, Noriega FG. Activity of the corpora allata of adult female Aedes aegypti: effects of mating and feeding. Insect biochemistry and molecular biology. 2003;33(12):1307–15. doi: 10.1016/j.ibmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Rivera-Perez C, Nouzova M, Lamboglia I, Noriega FG. Metabolic analysis reveals changes in the mevalonate and juvenile hormone synthesis pathways linked to the mosquito reproductive physiology. Insect biochemistry and molecular biology. 2014;51:1–9. doi: 10.1016/j.ibmb.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nouzova M, Rivera-Perez C, Noriega FG. Allatostatin-C reversibly blocks the transport of citrate out of the mitochondria and inhibits juvenile hormone synthesis in mosquitoes. Insect biochemistry and molecular biology. 2015;57:20–6. doi: 10.1016/j.ibmb.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernández-Martínez S, Rivera-Perez C, Nouzova M, Noriega FG. Coordinated changes in JH biosynthesis and JH hemolymph titers in Aedes aegypti mosquitoes. Journal of insect physiology. 2015;72:22–7. doi: 10.1016/j.jinsphys.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


