Abstract
Lipidomics is a rapidly developing field of study that focuses on the identification and quantitation of various lipid species in the lipidome. Lipidomics has now emerged in the forefront of scientific research due to the importance of lipids in metabolism, cancer, and disease. Using both targeted and untargeted mass spectrometry as a tool for analysis, progress in the field has rapidly progressed in the last decade. Having the ability to assess these small molecules in vivo has led to better understanding of several lipid-driven mechanisms and the identification of lipid-based biomarkers in neurodegenerative disease, cancer, sepsis, wound healing, and pre-eclampsia. Biomarker identification and mechanistic understanding of specific lipid pathways linked to a disease pathologies can form the foundation in the development of novel therapeutics in hopes of curing human disease.
Keywords: lipidomics, translational research, biomarkers
Introduction
Although Gorter and Grendel discovered the lipid bilayer in 1925, the scientific community generally viewed lipids as uninteresting for many years. Since this time, scientific studies have mainly focused on the accepted dogma of biology, DNA -> RNA -> Protein. Indeed, this dogma ignored the most prolific therapeutic in history, aspirin (acetylsalicyclic acid), which targets enzymes (i.e., cyclooxygenase 1 and 2 (COX-1 and- 2)) in the anabolic pathway of the biosynthesis of a lipid class known as eicosanoids. In the last several decades, more specific COX-2 inhibitors have also emerged to treat inflammatory disorders such as arthritis although cardiovascular concerns emerged limiting their current clinical use. Further, the main biomarker for cardiac ischemic diseases for decades has been the plasma levels of the lipid cholesterol, which is used in the clinical setting to this day for determining a patient’s risk and treatment with statins: therapeutics targeting another lipid biosynthetic pathway (i.e., the initial rate limiting step of the cholesterol biosynthetic pathway, HMG-CoA reductase). Although lipids were initially overlooked with exception of these few noted examples, a maturation of the lipid field has occurred over the last two decades leading to the rapid development of lipidomics. The term lipidomics refers to the large scale and quantitative analysis of both specific lipid classes as well as individual lipid species of various chain lengths within a specific lipid class usually involving mass spectrometric technologies and methodologies. This ability to quantitatively and qualitatively analyze multiple lipid classes and species with high sensitivity has led to an explosion in the lipid research field over this time period.
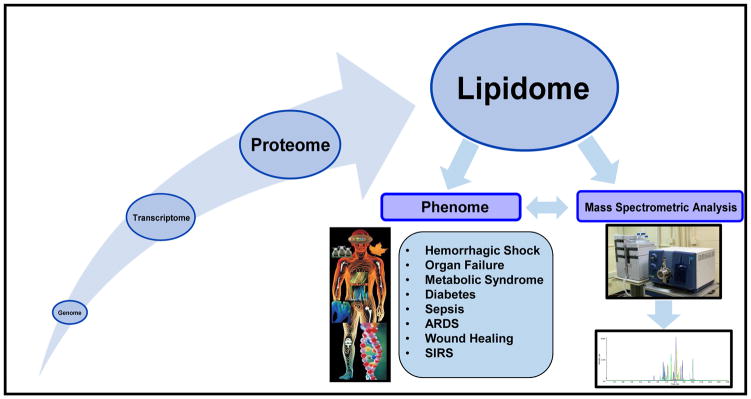
Why lipids in translational and clinical settings? Lipid molecules are a subset of the metabolome and serve as ubiquitous and multifunctional metabolites that are integral and essential to many diverse functions on both a cellular and organismal level (1–4). Primary functions of the lipidome include: (1) compartmentalization of cells and organelles via the formation of membranes; (2) storage of excess calories in order to provide a reservoir of energy when needed or to act as the primary energy source for high energy demand organs (eg, heart); (3) regulation of biochemical reactions via influencing the activity of transient and permanent membrane protein interaction; and (4) function as a reservoir of bioactive lipids that can be utilized in signaling following agonist induced hydrolysis or enzymatic/non-enzymatic covalent modifications (1–4). Acting as the boundaries of cells and organelles, lipids are directly exposed to biochemical changes in the intra- and extracellular milieu and as a result undergo chemical and structural modifications themselves. As such, changes to the lipidome will reflect the greater biochemical changes of the whole system. In this regard, the investigation of the lipidome is expected to provide a window to the biochemistry of the system of interest and will help identify biochemical anomalies arising from perturbations to the normal homeostatic and early biochemical processes. Lipids have also been shown to interact with proteins to regulate cell functions, for instance eicosanoids, which bind specific cellular receptors, play a very important role in both the inflammatory and pro-resolving responses in the immune system (5). Other lipids have been linked to disease states including cancer, neurological disease, sepsis, and metabolic syndromes, which has changed the initial dogma to DNA -> RNA -> Protein -> Lipids -> Phenome (Fig. 1). Hence, this new dogma showcases lipids as the biomolecules most amenable as biomarkers for clinical manifestations due to their relative proximity to the phenome. Thus, the continued growth and study of lipidomics is important to fully understand, diagnose, and treat numerous human diseases. In this review, we will briefly summarize the various classes of lipids typically analyzed in clinical research studies followed by discussion as to the roles of these lipids in various diseases pathophysiologies, and the role that lipidomics analyses are currently playing in translational research.
Figure 1. The new scientific dogma of biological systems.
The new scientific dogma for biological systems is DNA -> RNA -> Protein -> Lipids -> Phenome demonstrating that lipids are the most amenable biomolecule for biomarkers of human disease. UPLC-MS/MS is more commonly being used to determine novel lipid biomarkers in human disease and illness by observing lipid variance through the progression of disease states.
The roles of various lipid classes in disease pathophysiologies
Eicosanoids and Specialized Lipid Mediators
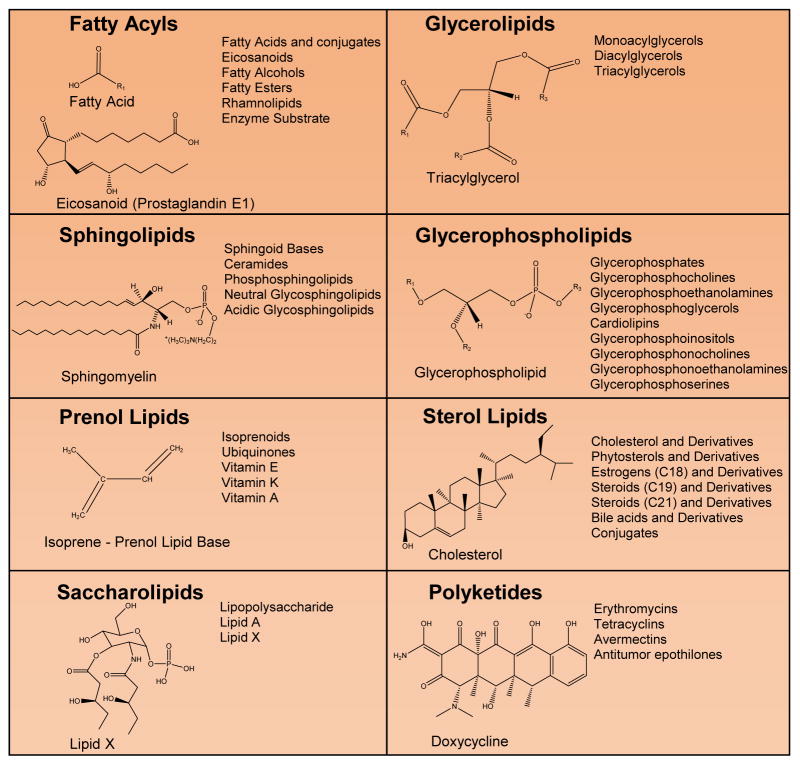
Due to the structural diversity of lipids, these biomolecules are commonly divided into eight classes; fatty acyls, glycerophospholipids, glycerolipids, sphingolipids, sterol lipids, prenol lipids, saccharolipids, and polyketides (Fig. 2). The class, fatty acyls, structurally contain a double bonded oxygen to a fatty acid alkyl group. Eicosanoids, fatty acids and conjugates, octadecanoids, docosanoids, fatty alcohols, fatty esters, and fatty aldehydes are all included in this group (6). In metabolism, fatty acyls enter the TCA cycle as acetyl-CoA, after undergoing beta oxidation (7). Eicosanoids, which are part of the oxylipin subclass of fatty acyls, has been one of the most long standing targets for therapeutics. The omega 3 and 6 polyunsaturated fatty acids (PUFA) such as arachidonic acid (AA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) are the precursor fatty acyls for the biosynthesis of this lipid class. Through the anabolic enzymes, cyclooxygenase (COX) enzymes (COX-1, COX-2), lipoxygenase (LOX) enzymes, and cytochrome P450, these precursor fatty acids are converted into bioactive lipid mediators, which are either pro-resolving or pro-inflammatory signaling molecules. Specifically the various classes of eicosanoids include prostaglandins, prostacyclins, thromboxanes, and leukotrienes. 3-PUFA-derived lipid mediators, also designated as specialized pro-resolution lipid mediators (SPMs), include the two main classes of E-resolvins and D-resolvins as well as Protectins and Maresins (8) (Fig. 3). Indeed, these bioactive lipids are well-established regulators of inflammatory responses with major roles in immunology and the pathogenesis of numerous disease states (1–4, 9–23). For example, aspirin, which inhibits and modulates the function of COX enzymes, is a therapeutic utilized at a low dose (81mg/day) for individuals at risk for heart disease demonstrating the causality of eicosanoids in the development of cardiovascular pathophysiologies.
Figure 2. The Eight Classes of Lipids.
Structural examples from the eight classes of lipids (fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, prenol lipids, sterol lipids, saccharolipids, and polyketides). Subclasses and examples are displayed to the right of each structure.
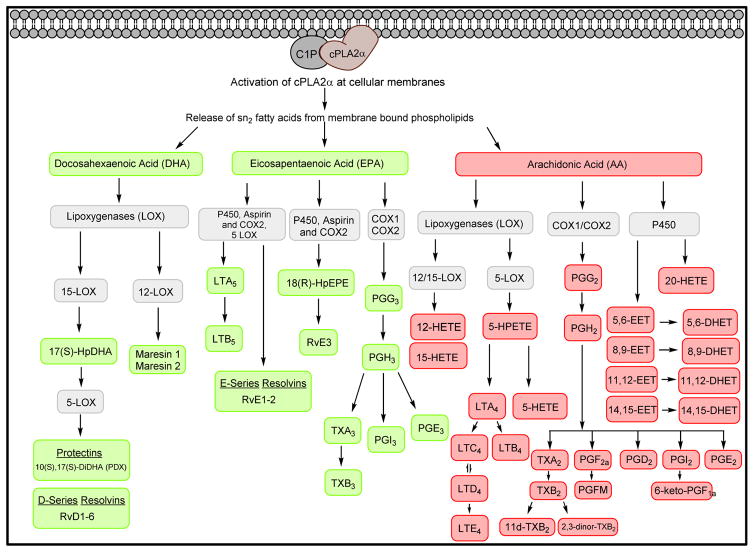
Figure 3. The Biosynthetic Pathways for Eicosanoids and Specialized Pro-Resolution Lipid Mediators (SPM).
The C1P/cPLA2α interaction at cellular membranes releases sn2 bound unsaturated fatty acids (AA, EPA, DHA) from membrane-bound phospholipids. Through interactions with cyclooxygenases, cytochrome P450, or lipoxygenases; AA, DHA, and EPA are converted into pro-inflammatory (red) or anti-inflammatory (green) lipid mediators.
Historically, the use of lipidomics to analyze oxylipins in a translational sense was highlighted by Klockenbusch and co-workers who showed that it is possible to diagnose pregnancy-induced hypertension (PIH), one of the hallmarks pre-eclampsia, using the ratio of two eicosanoids, 6-oxo-PGF1α/TXB2 ratio (TXB2 is a stable metabolite for measuring the levels of the active, but labile TXA2). The urine of women who developed PIH contained increased levels TxB2 and lower levels of 6-oxo-PGF1α, whereas the opposite was found in control patients (24). Shortly after, Walsh and co-workers published a study showing that the placental thromboxane produced in patients with pre-eclampsia, a disease state during pregnancy that results in high blood pressure and often kidney failure, is localized to the trophoblast cells in placenta driving a reduced ratio of prostacyclin/TXB2 (25). Although it is not known, the current hypothesis is that the increase of TXB2 and decrease of prostacyclin in pre-eclampsia are associated with oxidative stress, which is inferred to drive this change in the prostacyclin/TXB2 ratio due to lipid peroxidation (26) and the decreased levels of antioxidants found in pre-eclamptic patients (27–28). A later study showed similar results by identifying increased levels of 11-dehydro-TXB2 (TXM), another stable metabolite of TXA2, in the urine of pre-eclamptic women, compared to controls (i.e., women with normal pregnancies and no underlying physiologic conditions (e.g., gestational diabetes, hypertension)) (29). Whereas these studies highlighted the use of lipidomics as a diagnostic, an additional strength of these studies was the subsequent clinical trials that spawned from these early lipidomic findings, which investigated the use of low dose aspirin as a preventive therapy in pregnant women with an increased risk of developing pre-eclampsia. Aspirin proved to be >50% effective in reducing the development of pre-eclampsia for compliant patients, which was well-tolerated with minimal side-effects. More recently, The American College of Obstetricians and Gynecologists (ACOG), has directed that all pregnant women at risk for pre-eclampsia (e.g., obese, having a previous pregnancy with the diagnosis of pre-eclampsia) receive low dose aspirin further implicating the major role of eicosanoids in cardiovascular health as well as highlighting the strength of lipidomics research translating to clinical practice (30).
A more recent example of a disease state impacted by eicosanoids and SPMs is sepsis, a severe critical care disease with high mortality rates. The disorder is linked to a prolonged hyperactive immune response followed by an immunosuppressive stage (1,2,9). In preclinical animal studies, pro-inflammatory eicosanoids such as the COX product, TxA2/TxB2, and the 5-LO product, LTB4, are increased in models of endotoxic/septic shock (31–35). Blockade of their production has shown efficacy in ameliorating septic shock in animal models (31–35). In contrast, anti-inflammatory eicosanoids such as PGE1, prostacyclin, and 15-deoxy-delta (12,14)-PGJ2 are beneficial in septic shock models (31–35). Furthermore, Serhan and co-workers demonstrated that resolvin D2 (RvD2) preserved host immune function to facilitate the resolution of sepsis (34). Indeed, treatment of mice subjected to cecal ligation and puncture (CLP)-induced sepsis with RvD2 demonstrated a 40% increase in survival (36). The E-resolvins have also been shown to attenuate peritonitis in mouse models by reducing neutrophil infiltration (37). Overall, the literature strongly supports the paradigm of a critical balance between pro- and anti-inflammatory lipid mediators that can directly affect the resolution of underlying inflammatory and over-stimulated immune responses in various human disease states.
The above preclinical animal findings led to a recent clinical study on oxylipin levels in sepsis patients. Specifically, sepsis was highlighted as another example of how oxylipins/fatty acyls serve as potential biomarkers for certain diseases and even prognostic markers. Specifically, Serhan and co-workers were able to identify pro-resolving lipid mediators in 22 septic patients admitted to the ICU within 48 hours. In opposition to the hypothesis that pro-resolving lipid mediators would correlate with better outcome and survival, the protectin D1 isomer, 10S,17S-diHDHA, was shown to have a strong correlation with the development acute respiratory distress syndrome in septic patients. Furthermore, increases in the levels of prostaglandin F2a, resolvin E1, resolvin D5, 17-epi-resolvin D1, PD1, and 17-epi-PD1 were also observed in septic patients that did not survive >30 days. The current premise of this observation is that these pro-resolving mediators were unable to regulate inflammatory response in sepsis because the cognate receptors of these pro-resolving mediators were possibly downregulated(38). Hence, translational studies in lipidomics also have the potential to “bring to light” new and unexpected possibilities to identify key pathophysiologies. In on-going and follow-up studies, other laboratories are expanding upon the Serhan study examining whether lipid-based biomarkers can determine a sepsis patient’s responsiveness to intravenous anti-oxidant therapy, and the preservation of systemic lipid “pools” by these therapies may be a key mechanism of action.
There is also a large body of evidence that certain lipid species, in addition to their diagnostic/prognostic roles, also play causative roles in cancer. For example, eicosanoids and SPMs have been linked to inflammation associated with cancer development and progression, as well as with tumor growth and maintenance for colon, breast, and lung cancer (1,9, 12–14, 17, 31–34, 39–50). Indeed, Nemenoff and co-workers demonstrated over the last decade that various pro-inflammatory eicosanoids such as 5-LOX-derived leukotrienes and phospholipase A2 (PLA2)-derived eicosanoids regulate tumor formation, growth, and progression of lung cancer. Further supporting these findings, more aggressive cancer cells have been shown by Yamashita and co-workers to exhibit a decreased level of phosphatidylcholines and increased lysophosphatidylcholine content. These lipid changes are linked with the activity of the phospholipase (PLA2), the initial rate-limiting step in eicosanoid biosynthesis via hydrolysis at the sn2 fatty acyl ester bound of glycerophospholipids, which are overviewed later in this review. The overexpression of PLA2 is also associated with the malignant potential in human breast cancer (51). Importantly, aspirin reduced the risk of colorectal cancer incidence and mortality after an induction and latency period of approximately 10 years (52), and these lipid-findings aid to validate the accepted premise of the link between inflammation and cancer progression.
Another subset of fatty acyls are isoprostanes, which are generated from the peroxidation of fatty acids. For example, 8-iso-PGF2α, is a common isoprostane generated via lipid peroxidation of AA, which serves as a biomarker of oxidative stress in disease states like prediabetes and aneurysmal subarachnoid hemorrhage (aSAH) (53,54). Oxidative stress is also a hallmark in pre-eclampsia diagnosis, leading to the quantitation of isoprostanes in the urine of pregnant women. High urinary levels of isoprostanes have been associated with a 5-fold increased risk of pre-eclampsia, whereas increased anti-oxidant production is associated with a 3-fold decrease in risk of pre-eclampsia. This isoprostane imbalance is thought to be the direct result of maternal diet, but is still used as a biomarker for the early diagnoses of pre-eclampsia prior to clinical manifestation (55).
Another fatty acyl lipid sub-type are nitro-lipids, which are also linked to oxidative stress like isoprostanes, but are known to have anti-inflammatory properties (11, 19, 20, 56). Nitro fatty acids are generated via reaction of reactive nitrogen species (RNS) with susceptible unsaturated fatty acids (11), and their production is often observed in conjunction with ROS production in biological systems under stress (11, 57). Although these lipids are understudied, there are reported links to the production of these lipids to colon cancer progression (58).
A new application in using lipidomics to analyze oxylipins is in the development and quality control of lipid-based biological therapies. This novel application in lipid-based therapy development was highlighted in a recent study by our laboratory in which the lipid content of platelet-rich plasma (PRP), a biological therapy utilized in some clinical settings to heal chronic wounds, was analyzed by lipidomics coupled to experiments assaying the biological efficacy. In this regard, the lipid fraction of PRP was found to be the biologically active component in wound healing phenotypes. As for which specific bioactive lipids associated with wound healing phenotypes, increased levels of TXB2, PGF2α, LTB4, LXA4, 5-HETE, 12-HETE, and 15-HETE were observed in the lipid fraction of PRP in comparison to both platelet-poor-plasma and whole blood (59). Recapitulation studies showed a major role for the prostaglandin subspecies of eicosanoids in mediating the enhanced wound healing phenotype. With this knowledge, studies can begin to assess whether a synthetic-blend of lipid mediators can be developed and utilized to enhance wound healing in the clinic.
The above lipidomic findings can also be applied to quality control measures for the current biological PRP regime. Indeed, PRPs are developed from the same patient that the therapy will be applied. Unfortunately, PRP is only affective in 50% of cases, and thus, an underlying failure of the therapy is hypothesized to be linked to a quality control issue as to the bioactive lipid content. For example, a patient on low dose aspirin or with an underlying condition (e.g., diabetes) may have a different lipid content in their PRP versus other patients. This variability in the content of particular bioactive lipids in a PRP preparation may explain the 50% failure rate. Lipidomics in this translational context could be utilized to explore this hypothesis in depth, and validation of this hypothesis could lead to rapid implementation of a quality control measure prior to the application of PRP. A patient may simply need to change their diet or reduce a medication for a short period of time to allow for the production of a superior PRP preparation with appropriate bioactive lipid content.
Another example where the use of lipidomics may be applied for the quality control is in biological therapies related to stored blood products. In a recent collaborative study, the effect of using low (0.3mmol/L) and high doses (3mmol/L) of vitamin C on stored platelets was examined with the hypothesis that vitamin C would preserve platelet function over time (60). Whereas vitamin C did show positive effects on platelet function, high doses of vitamin C also induced the time-dependent generation of 11-HETE, 12-HETE, 15-HETE, PGE2 and TXB2, which would likely elicit a potent inflammatory response if delivered into a patient. Thus, the use of high doses of vitamin C as a preservative >8 days could have adverse effects on patient outcomes and platelet function. Therefore, lipidomics may be a key technology to provide an assay to identify these quality control issues and avoid negative therapeutic side-effects (61–64).
Glycerolipids and Glycerophospholipids
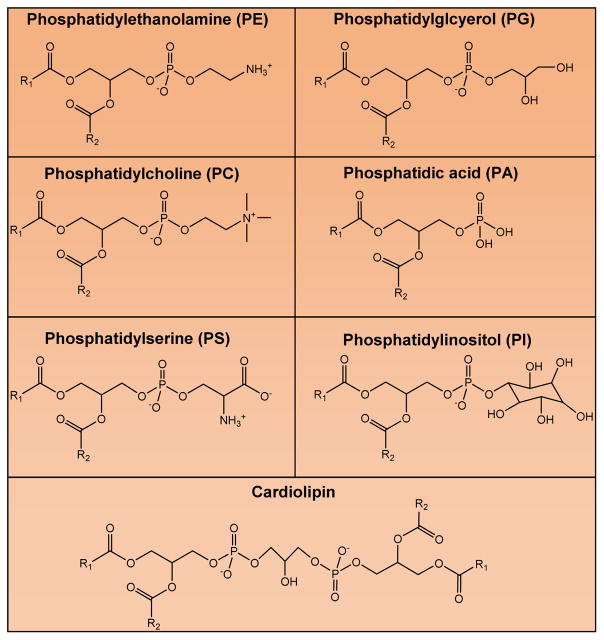
Glycerolipids are made up of a glycerol backbone, which is generally esterified with one to three fatty acid chains. Glycerolipids are categorized into either monoradylglycerols, diradylglycerols, triradylglycerols, glycosylmonoradylgycerols, or glycosyldiradylgycerols. Typically, fatty acids are esterified at all three hydroxyl positions of the glycerol backbone making glycerolipids, like triglycerides, which are very effective as functioning as energy stores. Glycerophospholipids contain a glycerol base with a phosphate group in the sn3 position, and typically two fatty acids in the sn1 and sn2 positions. Glycerophospholipids are the main components of membrane structures in cells, and these lipids play important roles in fatty acid storage and cellular signaling as well as serve as reservoirs for bioactive lipids. Examples of glycerophospholipids include phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidylinositides, phosphatidylglycerols (PG), cardiolipins (CL), and phosphatidic acid (PA) (Fig. 4). Of note, PA is a product of glucose metabolism that can be used to synthesize phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol (65,66).
Figure 4. Subclasses and Structures of Glycerophospholipids.
Structures are depicted for the glycerophospholipids; phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidylglycerols (PG), cardiolipins (CL), and phosphatidic acid (PA).
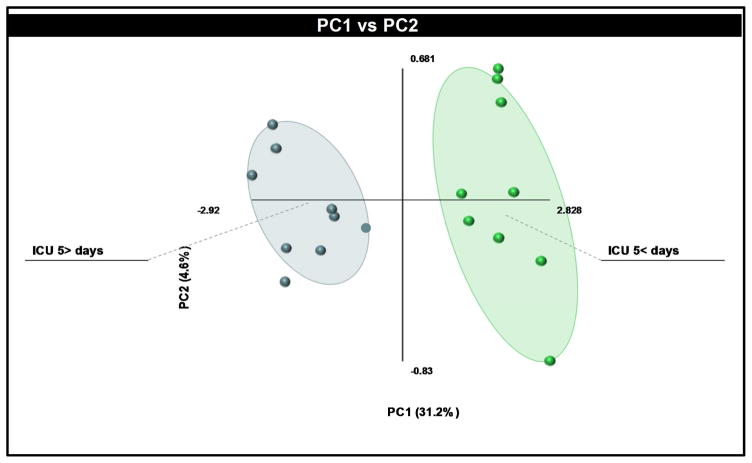
Glycerophospholipid metabolism is strongly linked to the biosynthesis of oxylipins due to their biological roles as reservoirs for bioactive lipids. For example, many PE, PS, and PC species contain PUFAs such as either EPA, DHA or AA in their sn2 position. When a cell is injured or exposed to inflammatory cytokines or bacterial wall components, the translocation to cellular membranes of a phospholipase A2 is induced, in many cases group IVA cytosolic phospholipase A2 (cPLA2α). At the membrane, other bioactive lipids such as the sphingolipid, ceramide-1-phosphate (C1P), or the glycerophospholipid, phosphatidylinositol 4,5-bisphosphate (PIP2), serve to anchor cPLA2α, and the enzyme proceeds to cleave PUFAs from the sn2 position of glycerophospholipids to drive the biosynthesis of oxylipins and either promote an inflammatory response or help resolve inflammation (5,19–23,64,67–68). In this regard, not only can oxylipins serve as translational biomarkers as previously discussed in this review, but also lysophosphatidylcholine (LPC), another product of the action of PLA2 enzymes. Indeed, LPC has recently been identified as a cervical cancer biomarker in uterine fibroids by Yin and co-workers (65). Furthermore, a lack of glycerophospholipid catabolism is also “telltale” in certain pathophysiologies as irregular PC metabolism has been implicated in breast and ovarian tumor progression (69). For example, the PC species, PC 36:4, was also reported as an early lipid biomarker for the development of pre-eclampsia, which suggested that a lack of PC hydrolysis and subsequent generation of eicosanoids is an indicator of an inability of the body to resolve certain inflammatory disorders (70). Unpublished and preliminary findings by our laboratory also support this premise and implicate both increased levels of PC with arachidonate in the sn2 position and decreased levels of LPC as early biomarkers of a poorer outcome for sepsis patients (Fig. 5).
Figure 5. Sepsis patients differ significantly in their lipid profiles depending on outcome.
In a small preliminary study, lipids were extracted from the plasma of 12 sepsis patients transferred to pulmonary ICU. This lipid extract was interrogated by targeted lipidomic analysis for bioactive lipids followed by Partial Least Squares Discriminant Analysis (PLS-DA). The resultant scores plot demonstrated clear separation between groups (shaded area = 95% confidence interval); groups were either < 5 days in the ICU or > 5 days in the ICU. The corresponding loadings plot identified the top lipids that contributed to the separation based on time the patient spent in the ICU. Of note, increased levels of phosphatidylcholine (20:4 fatty acyl in the sn2 position) and decreased levels of lysophosphatidylcholine in the >5 days in the ICU group were the main contributing lipids.
Whereas glycerolipids serve as biomarkers and possibly therapeutics, they also have been shown to be causative in disease pathophysiologies like oxylipins. For example, Fang and co-workers have shown that the glycerophospholipid, lysophosphatidic acid (LPA), drives the migration and metastasis of ovarian cancer cells (71). Furthermore, LPA is found in ascites fluid from patients with ovarian cancer and may serve as a prognostic factor (71). Also of note, the Fang laboratory showed that the elevated levels of LPA found in ovarian cancer enhances lactate efflux and glycolytic rate in the cancer cells. This work was the first to suggest that LPA signaling promotes glucose metabolism in cancer cells making the biosynthesis of LPA a therapeutic target (71).
Glycerophospholipids have been shown to play a role in Alzheimer’s disease (AD) as well. AD occurs through the aggregation of plaques in the brain that consist of amyloid-beta (Aβ). One of the earliest studies in lipids and AD was reported in 1987 in which Stokes and Hawthorne compared phosphatidylinositol (PI) levels in the brain anterior temporal cortex of 17 patients with AD and 18 control patients (72). The study found that PI in the cortex of patients with AD was approximately 50% less than cortex from control patients. This study led to the hypothesis that reduced amounts of certain glycerophospholipids could be facilitating Aβ aggregation. A more recent study in 2014 highlighted this possible role for glycerophospholipids. Specifically, a study by Bana and co-workers focused on preventing Aβ aggregation in AD through use of liposomes bi-functionalized with phosphatidic acid and with a modified ApoE-derived peptide (mApoE-PA-LIP) (73). This study concluded that while mono-functionalized (PA-LIP or mApoE-LIP) is not able to destabilize preformed Aβ aggregates, bi-functionalized mApoE-PA-LIP possessed potent efficacy in this regard. These findings were suggestive of a synergistic relationship between PA and MaPoE, and thus, one can now hypothesize that these lipid-based therapeutics could be combined with the current drug therapy for AD; specifically, cholinesterase inhibitors. For example, acetylcholinesterase induces Aβ formation in AD. Cholinesterase inhibitors, in particular, help to reduce Aβ aggregation and slow the process of neurodegeneration (74–75). Based on the findings in the Bana study, the clinical effectiveness of cholinesterase inhibitors may be enhanced or prolonged by co-administration of bi-functionalized mApoE-PA-LIP.
In another example, Federoff and co-workers published a seminal study on glycerophospholipids in AD that highlighted the role that lipidomics can play in translational research as well as the clinical setting. In particular, this study identified several glycerophospholipids in the peripheral blood that could serve as biomarkers for the later on-set of AD; in particular, the phenoconversion of older adults to either amnestic mild cognitive impairment or AD within a 2–3 year time-frame. Importantly, the predictive accuracy was 90% based on significant decreases in specific species of PCs containing long diacyl chains (e.g., 36:6, 38:0). Thus, this study highlights how the use of lipidomics can be used to identify blood-based biomarkers, which is an attractive, simple, and minimally-invasive option to determine the later on-set of disease states with high sensitivity, specificity, and accuracy. (76)
Sphingolipids
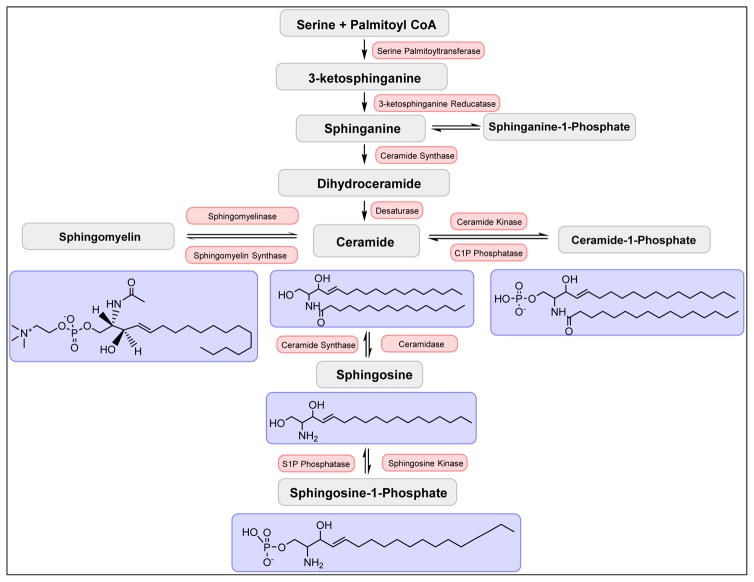
Sphingolipids are a large class of lipids that differ from glycerol lipids in containing a sphingosine backbone in lieu of a glycerol backbone (Fig. 6). Ceramide is a central molecule in the sphingolipid biosynthetic pathway in mammalian cells derived from either a de novo, sphingomyelin hydrolysis, or salvage-reacylation pathway (77). Ceramide can then be converted to additional bioactive sphingolipids such as sphingomyelin (SM), ceramide-1-phosphate (C1P), and sphingosine (and subsequently sphingosine-1-phosphate (S1P) via sphingosine kinases) (77) (Fig. 6). Some sphingolipids such as C1P play important roles in regulating immune response through regulation of eicosanoid biosynthesis (5,19–23,58,60–62), while others, namely S1P, have been implicated in cancer, atherosclerosis, diabetes and osteoporosis (78). Indeed, two decades of research in the lipid field have shown distinct bioactive roles for both S1P and ceramide. For example, numerous seminal works by the Spiegel laboratory has shown major roles for S1P in several cancer phenotypes (67,78–82), which have led to the initial design of phase1/2 clinical trials in colorectal and breast cancer for the therapeutic, Fingolimod, an S1P receptor antagonist (83,110). Our laboratory, in a recent lipidomic and translational study, showed elevated levels of S1P in the plasma of patients with end stage renal disease. This elevation predicted patients who clinically manifested with clotting complications while undergoing dialysis (84). As sphingolipids can act as regulators of thrombin production (85), these findings led to the hypothesis that S1P mediates a feed-forward, amplification mechanism. Specifically, increased levels of S1P are induced during chronic inflammation, which decreases anticoagulants, but induces thrombin. Thrombin increases coagulation and induces the additional release of S1P from platelets, which “drives” an amplifying cycle of hypercoagulopathy. Furthermore, thrombin and S1P are implicated in acting synergistically to induce inflammation, which in turn continues to drive clotting complications via this amplification mechanism. (84).
Figure 6. The sphingolipid biosynthetic pathway.
An overview of the sphingolipid biosynthetic pathway is depicted. In the de novo biosynthesis of ceramide, serine and palmitoyl-CoA are initially condensed by serine-palmitoyl transferase followed by three additional enzymatic steps involving a reductase, synthase, and desaturase respectively. Ceramide, thus produced, can be further metabolized to ceramide-1-phosphate via ceramide kinase, sphingomyelin via sphingomyelin synthases, and sphingosine via ceramidases. Sphingosine can be further converted to sphingosine-1-phosphate by the action of sphingosine kinases.
For several decades, ceramides have also been implicated in modulating apoptosis as a result of the pioneering work of the Hannun and Obeid laboratories (86–87). Under stress induction, increased levels of ceramides are derived from one of the three main biosynthetic pathways discussed in the above section. In the case of SM hydrolysis, activation of enzymes known as sphingomyelinases (SMases) convert a main component of neuronal cells, SM, to ceramide (87). This knowledge led to current research on the role of sphingolipids in neurodegenerative diseases, and links between ceramide formation and neurological diseases have now been reported analogous to those studies previously discussed in regard to glycerophospholipids (88). For example, Filippov and co-workers quantified the levels of various species of ceramides in AD using lipidomics (88). When compared to control patients without neurological disease, they found statistically significant increases of C16:0, C18:0, and C20:0 ceramide levels in the brain tissues of patients with AD and other neuropathological diseases. They also found that the range in ceramide levels of brains with neural disease are much higher than those of healthy controls leading to the conclusion that regulation of ceramide biosynthesis is highly dysfunctional when individuals experience neurodegeneration.
Additional translational studies using lipidomic approaches demonstrated increased levels of sphingomyelin in cerebrospinal fluid (CSF) of AD patients (89). Specifically, Kosicek and co-workers observed an increase of 50.4 ± 11.2% of total sphingomyelins in the CSF of patients with prodromal AD compared to cognitively normal controls (89). Interestingly, no significant changes of total sphingomyelins were found in the CSF of patients with mild or moderate AD. They postulated that this could be due ceramide turnover in early AD. More recently, the Spiegel laboratory reported that S1P, and the anabolic enzyme for S1P production, sphingosine kinase 1, regulated endocytic trafficking, which plays significant roles in AD, cancer and diseases associated with lipid storage (79).
Due to the diverse function of sphingolipids, they have not only been implicated in cancer and neurological disorders, but the process of wound healing and tissue regeneration as well. For example, C1P-derived from the sphingolipid anabolic enzyme, ceramide kinase (CERK), has been implicated to play an important role in fibroblast migration, which was linked to effects on the biosynthesis of eicosanoids and the role of this bioactive sphingolipid in anchoring cPLA2α. More specifically, lipidomic analyses showed that C1P levels were increased in the early stages of wound healing in order to promote release of AA from membrane-bound glycerophospholipids by cPLA2α and initiate eicosanoid biosynthesis. Specific increases of 5-HETE, 12-HETE, 15-HETE, and PGE2 were observed as CERK-dependent using lipidomics, all of which have reported roles in fibroblast migration and wound closure rates (63). Recapitulation studies validated the requirement of PGE2 biosynthesis for proper migration and polarity of fibroblasts in response to mechanical trauma. These studies highlight how lipidomic approaches identified a novel interplay between sphingolipids, glycerolipids, and oxylipins in biological mechanisms, which led to the examination of PRP efficacy via lipidomics previously discussed in this review.
Sterols
Sterol lipids contain a polar hydroxyl group located at the 3-position of the A-ring of a four-ring steroid (90). Cholesterol is the most predominant sterol lipid in mammalians and is synthesized from Acetyl-CoA in the cytosol to Hydroxymethylglutaryl-CoA (HMG-CoA) in the endoplasmic reticulum (ER) via Hydroxymethylglutaryl-CoA synthase (HMGCS). There, it is ultimately converted into cholesterol via four additional biosynthetic steps (91). Cholesterol plays an important role in cell membrane fluidity by being able to both cause membrane stability and by decreasing flexibility of nearby unsaturated acyl chains in membranes. Cholesterol also helps to maintain fluidity through inhibition of acyl-chain packing of cellular membranes (92).
High cholesterol is one of the primary risk factors of atherosclerosis, a disease marked by fat deposition on arterial walls. Therapeutic treatments for atherosclerosis are currently focused on the reduction of the accumulation of cholesterol esters. One potential treatment to reduce cholesterol esters involves delivering cholesterol ester hydrolase (CEH) to hepatocytes via galactose-functionalized polyamidoamine (PAMAM) dendrimer generation 5 (Gal-G5). Upregulation of CEH induces an increase in the hydrolysis of cholesterol esters to free cholesterol, which is then secreted as bile acids. This pre-clinical work by Hongliang He and coworkers demonstrated a promising treatment for alleviating the accumulation of cholesterol esters in patients suffering from atherosclerosis. (92)
Although much of the work associated with sterols is in relation to cardiovascular disease, recent studies have demonstrated significant correlations between certain steroid hormone profiles in pre-eclampsia and cancer risk (59–68). Using lipidomic approaches, Konieczna and co-workers identified higher concentrations of cortisone and cortisol in both males and females with urogenital tract cancer (bladder, kidney, prostate and testis cancer). Thus, the utilization of lipidomics to discover biomarkers helps to further our understanding as well as elucidate the causes and mechanisms of steroid-related cancers (93). In another study using lipidomic approaches, Pertegal and co-workers tied low plasma levels of 2-methoxyestradiol to pre-eclampsia (94). The onset of pre-eclampsia generally begins around 20 weeks, and currently the only cure is for the mother to deliver. However, having biomarkers to detect either early on-set pre-eclampsia before clinical manifestation or patients at risk of pre-eclampsia, offers healthcare professionals the ability to properly accommodate, and if necessary, provide pre-eclamptic mothers with additional clinical care such as low-dose aspirin.
Prenols, Saccharolipids, and Polyketides
Three additional lipid classes are prenols, saccharolipids, and polyketides, which are currently understudied in regard to lipidomic technologies at this time. Prenol lipids are derived from the 5-carbon precursors, dimethylallyl diphosphate and isopentenyl diphosphate (90). Several examples of prenol lipids include vitamins A, E, and their isoprenoid precursors, carotenoids. Deficiencies in these vitamins tend to have serious effects. For instance, Vitamin A deficiency has been shown to weaken the immune system, resulting in higher mortality among vitamin A-deficient humans (95). Vitamin E deficiencies can cause both peripheral neuropathy and ataxia (96–97). Lipidomic approaches have been employed in the study of prenol lipids to elucidate the effect of vitamin E deficiency on brain phospholipid concentrations. For example, Choi and co-workers found that low vitamin E concentrations are correlated with a ~60% depletion of 4 DHA-containing phospholipids and 19 lyso phospholipids in zebra fish brains. Due to the decrease of DHA-containing phospholipids in Vitamin E-deficient zebra fish, vitamin E is postulated to play a role in the homeostatic balance of anti-inflammatory and inflammatory lipids in the brain (98).
Saccharolipids are lipids that contain a monosaccharide backbone attached to fatty acyl chains, as opposed to the glycerol backbone in glycerophospholipids and glycerolipids linked to fatty acyl chains (90). Lipopolysaccharide is highly acylated and found on the outer membrane of gram-negative bacteria, such as Escherichia coli and Salmonella. It plays an important role in maintaining hydrophobicity in the membrane to prevent passive diffusion of antibiotics (99).
Polyketides are composed of at least two carbonyl groups connected by a carbon. They are synthesized by fungi and bacteria and have antibiotic, immunosuppressant, anti-tumor, anti-fungal, and anti-parasitic properties. Two clinical examples include erythromycin and lovastatin (100). Erythromycin is currently being studied as a potential treatment in gastrointestinal disorders and inflammatory diseases (101). Lovastatin is a HMG-CoA reductase inhibitor commonly used to help lower low-density lipoprotein (LDL) and triglycerides, while increasing high-density lipoprotein (HDL) in the blood. It is commonly prescribed for patients who have high levels of plasma cholesterol (102). Lipidomics approaches can be utilized to study polyketides and bacterial virulence. Using fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS), Jain and co-workers discovered two polyketides, phthiocerol dimycocerosate (PDIM) and sulfolipid-1 (SL-1), are required for Mycobacterium tuberculosis virulence (103).
Lipidomics: Mass spectrometry as a tool to analyze lipids in the laboratory, translational, and clinical setting
Mass spectrometric analysis is a relatively new tool used to study lipids both quantitatively and qualitatively. Prior to the adaptation of mass spectrometric approaches to the analysis of lipids, initial difficulties were apparent in detecting and quantifying lipids due to their small molecular weights, instability, and limits of detection. Earlier studies with lipidomics involved radiolabeling, which comes with safety and detection limitations, or by enzyme-linked immunosorbent assay (ELISA), which although very sensitive, has accuracy limits due to antibody specificity within a particular lipid class. Furthermore, some lipids can also be difficult to study in-vivo due to short half-lives or fast metabolism (TXA2, 15-Deoxy PGJ2). Fortunately, some eicosanoids do have stable metabolites. For instance, TXB2 is the stable metabolite of TXA2 and is fairly easy to detect with liquid chromatography mass spectrometry (LC/MS-MS) or by enzyme-linked immunosorbent assay (ELISA). With the advent of mass spectrometry, many, although not all, of the early limitations to the study of lipidomics have, in theory, been overcome. For example, the invention of electrospray ionization (ESI) in 1984 by John Fenn forever revolutionized lipidomics, though ESI was not fully appreciated for another decade. Indeed, this mass spectrometric technique allowed researchers to quantitatively study femtomole levels of small molecules such as lipids, even those as labile as eicosanoids in biological samples.
As the study of lipids has evolved and more is understood about these complex molecules, and the analytical techniques to study lipids have rapidly advanced over the last decade. As detailed through-out this review, the role of lipidomic analyses is undeniable in the perpetuation of both analyzing the potential role of these lipid mediators in underlying the pathophysiology of certain diseases and in identifying lipid based-biomarkers to provide clinical insight. Although a number of biological samples can be utilized for lipidomics analyses, the focus in the translational and clinical setting is minimally-invasive or non-invasive. As such, many initial lipidomic studies in a translational/clinical research setting utilize human plasma and urine as the two main biological samples. Additionally, other biological fluids such as breath condensates, saliva, and wound fluid are also being utilized for the same non-invasive philosophy. Various extraction techniques are utilized by researchers depending on the lipid class being examined and biological sample. A general-purpose extraction technique for lipidomics is the Bligh-Dyer extraction (104), which is often used to when extracting lipids from plasma, tissues and cell pellets such as sphingolipids. Other lipid classes require a solid phase extraction to purify and concentrate, such as the examination of eicosanoids and SPMs from tissue culture media, urine, and plasma (38,62).
Two main techniques are employed for lipidomic analysis by mass spectrometric techniques, these include targeted and untargeted (i.e., systems-level) lipidomics. In the case of targeted lipidomics, usually a pre-separation using ultra-high pressure liquid chromatography/high pressure liquid chromatography (UPLC/HPLC) method for one particular lipid class is developed coupled to heavy isotope or synthetic lipid standards with Triple Quadrupole Mass Spectrometry (QQQ). QQQ is a mass spectrometric technique, often couple with the ESI developed by John Fenn, where the first and third quadrupoles are mass filters for precursor and product ions, and the second quadrupole causes fragmentation from the precursor ion to the product ion through either argon or nitrogen gas. QQQ is used for targeted/quantitative measurements of specific lipids due to a greater sensitivity in targeted Multiple Reaction Monitoring (MRM) analysis, which allows for rapid analysis of multiple precursor/product ion pairs in a single run. Newer QQQ models have ability to rapidly switch between positive and negative mode in the same analytical run, reducing the total instrument run time by 50%. The analysis and quantitation of a specific lipid species in this type of targeted method is verified via three measures of confidence: the known UPLC/HPLC retention time of the analyte of interest, the precursor ion mass/charge ratio (m/z), and its “structurally specific” product ion. This technique is often utilized in the research setting when a specific lipid class is being examined based on a pre-existing hypothesis or specialization of the researcher in question.
Untargeted or Systems-Level Lipidomics is utilized in discovery-based research paradigms where targeted lipidomics is insufficient. Quehenberger and co-workers highlighted the limitation of targeted lipidomics, which usually focus on one or a few specific lipid classes, as this laboratory group identified only 600 lipid species in human plasma with combined targeted/quantitative and untargeted/qualitative approaches (105). Indeed, the lipidome is a major subset of the entire metabolome, which comprises over 180,000 lipids. Furthermore, a majority of the lipid changes occurring during the course of a human disease cannot be postulated a priori, and thus, a targeted approach may not provide data on numerous lipid classes associated with a disease pathophysiology. As such, a discovery-based/systems level analysis is required in order to identify lipids of importance that are not included within a targeted lipidomic platform. In untargeted lipidomics, Quadrupole Time-of-Flight (QTOF) mass spectrometry is often employed. This is a mass spectrometric technique that measures an ionized compounds mass based on ion flight time, which is often coupled to a limited, but high-throughput analysis of >100 samples per day. QTOF analysis offers high resolution mass spectra across a broader mass than QQQ. Although this technique offers a broader mass range and theoretically could examine the entire lipidome, untargeted lipidomics has significantly lower sensitivity than a QQQ approach, therefore only high abundance lipids are usually assessed. Furthermore, the accuracy of quantitation is limited in systems level analyses. Indeed, relative values for lipid species contained in a compound library in possession of an individual laboratory are obtained in an analytical run with a relative ion intensity provided for an identified lipid species. Currently, a number of mass spectrometry companies provide lipid-centric compound libraries for these types of analyses and research endeavors (106). Of note, mass spectrometry-based technologies are advancing rapidly, and new hybrid instruments combining QTOF and QQQ technologies are beginning to foster both high sensitivity and accurate lipid analyses on a systems level.
In a translational or clinical research setting, the combination of systems level/discovery-based lipidomics with targeted methods is a powerful tool to determine new paradigms in disease pathophysiologies, as well as responses of patients to therapy and overall outcomes. Indeed, the two methods serve to cross-validate findings for high abundance lipids, and thereby, enhance the scientific rigor of a particular study or analysis. The addition of multiple targeted methods allows for addition data to be obtained on low abundance lipids not detected via untargeted analysis. The Federoff study on AD highlights the strength of a combination approach (76). In this example, both targeted and untargeted lipidomics was employed on 124 plasma samples of two groups of patients, one control group with normal function, and a mixed group that reflected the earliest clinical signs of AD. Untargeted lipidomics yielded 2700 positive mode analytes and 1700 negative mode analytes for a total of 4400 lipid species. Targeted lipidomics was then used verify and quantitate changes between groups due to the limitation in accuracy related to the relative quantitation provided by systems level analyses. Using these combined approaches, decreases of (PC diacyl (aa) C36:6, PC aa C38:0, PC aa C38:6, PC aa C40:1, PC aa C40:2, PC aa C40:6, PC acyl-alkyl (ae) C40:6), lysophophatidylcholine (lysoPC a C18:2), and acylcarnitines (ACs) (Propionyl AC (C3) and C16:1-OH) were found in the memory impairment group vs the controls. This study was the first to collect large amounts of lipid data from patients, which then determined new lipid biomarkers for early onset AD. This study also serves as an example for the powerful ability of a combined untargeted and targeted lipidomics to diagnose human diseases prior to clinical manifestation at the biochemical level using minimally invasive techniques.
Limitations of a Mass Spectrometric Approach in Lipidomics
Although mass spectrometry is a useful tool driving the lipidomics field, there are still a number of limitations to be addressed. For example, the separation of multiple isomers using the same elution profile can be difficult. Indeed, a major limitation in the lipid signaling field has been the inability to specifically analyze the generation of specific phosphoinositides such as PI(4,5)P from PI(3,4)P. Currently differential mobility sources are beginning to separate lipid isomers, which seems to be a promising solution in the future as to soon overcoming this limitation.
Another limitation in utilizing mass spectrometric approaches in certain situations is cost. The strength of lipidomics is the ability to examining 30 to 100 lipids in a targeted and quantitative fashion. Thus, a large amount of data is provided to a researcher for relatively low cost. Unfortunately, if sample is limited and only one or two lipid species a desired for analysis (e.g., only PGE2 is required versus 40 additional eicosanoids), it would likely be more prudent and cost-effective to utilize an ELISA for PGE2 due to the high sensitivity and reduced cost. Other major drawbacks to using mass spectrometry include the significant “up-front” cost of the mass spectrometers, the limited portability, and the required technical expertise needed to develop protocols, extract biological samples, run samples, and maintain the instruments. Hence, institutional support for equipment, experienced personnel at the doctoral level, and service contracts are usually required for this type of research.
Lipidomics utilizing mass spectrometric techniques also suffers from a limited ability to perform high throughput screening. Although 96 plate formats and platforms coupled to robotics systems are becoming available, certain lipid classes require >10 minute HPLC/UPLC pre-separations. In these cases, it is feasible to run approximately 100–200 samples/day. Thus, High-throughput screening (HTS) examining between 10,000 to 100,000 samples per day is not usually feasible at this time in regard to lipidomic analyses (107). Another major limitation is due to the variability of techniques and competitiveness among research laboratories and lipidomics cores/facilities. In regard to variability, not all research laboratories undertaking translational and clinical research in lipidomics have the same standard operating procedures for sample processing, extraction, allowed storage time and storage guidelines, and lipid analysis protocols. In many cases, different types of mass spectrometers are utilized with different analytical software. Whereas genomics data and databases are becoming standardized with open-access to the scientific community, the lipidomics field is far behind this type of standardization. Standardization of techniques and standard operating procedures are clearly a need and required for lipidomics as translational research continues on lipid-based biomarkers of human diseases. Fortunately, a number of laboratories are moving forward to address this need and these concerns, specifically those laboratories originally associated with the LIPID MAPS Consortium via annual scientific conferences (108–109).
Regardless of the noted limitations with current technologies associated with lipidomic analysis, the recent strides in field of lipidomics coupled to increased sensitivity in mass spectrometric analysis over the last decade show great promise for translational research such as the identification of lipid-based biomarkers in human disease. Large numbers of clinical samples can now be rapidly analyzed for numerous lipid classes using a powerful combination of targeted and untargeted lipidomics. Although sphingolipids, glycolipids, eicosanoids, and glycerophospholipids are implicated in many cancers and neurological diseases as well as serving as biomarkers in immunology and wound healing, much is still unknown about the biological roles and metabolism of lipids. With the help of new analytical techniques as well as additional enhancement of mass spectrometric technologies, we will have a better understanding of these biomolecules that will hopefully lead to the development of treatments and cures for currently enigmatic diseases.
Acknowledgments
This work was supported by research grants from the Veteran’s Administration (VA Merit Review, I BX001792 (CEC) and a Research Career Scientist Award, 13F-RCS-002 (CEC)); from the National Institutes of Health via HL125353 (CEC), HD087198 (CEC), RR031535 (CEC), and NH1C06-RR17393 (to Virginia Commonwealth University for renovation). Services and products in support of the research project were generated by the VCU Massey Cancer Center Shared supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
All authors have read and agree with the Authorship Statement as laid out by Translational Research. All authors also hereby confirm they have read the policy on disclosure and potential conflicts of interest, and all authors have disclosed any such potential conflicts as laid out by the guidelines in Translational Research. In this regard, none of the authors have no potential conflicts to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Seam N, Suffredini AF. Mechanisms of sepsis and insights from clinical trials. Drug Discov Today Dis Mech. 2007;4(2):83–93. doi: 10.1016/j.ddmec.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011 Jun;(62):1–8. [PubMed] [Google Scholar]
- 3.Ott J, Hiesgen C, Mayer K. Lipids in critical care medicine. Prostaglandins Leukot Essent Fatty Acids. 2011 Nov;85(5):267–73. doi: 10.1016/j.plefa.2011.04.011. Epub 2011 May 4. Review. [DOI] [PubMed] [Google Scholar]
- 4.Fetterman JW, Jr, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm. 2009 Jul 1;66(13):1169–79. doi: 10.2146/ajhp080411. Review. [DOI] [PubMed] [Google Scholar]
- 5.Stahelin RV, Subramanian P, Vora P, Cho W, Chalfant CE. Ceramide-1-phosphate binds group IVA cytosolic phospholipase A2 via a novel site in the C2 domain. J Biol Chem. 2007;282:20467–74. doi: 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- 6.Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov. 2005;4(7):594–610. doi: 10.1038/nrd1776. https://doi.org/10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- 7.Berg JM, Tymoczko JL, Stryer L. Biochemistry. Biochemistry textbook. (5) 2006:1120. https://doi.org/10.1007/s13398-014-0173-7.2.
- 8.Nagase T, Uozumi N, Ishii S, Kume K, Izumi T, Ouchi Y, Shimizu T. Acute lung injury by sepsis and acid aspiration: a key role for cytosolic phospholipase A2. Nat Immunol. 2000 Jul;1(1):42–6. doi: 10.1038/76897. [DOI] [PubMed] [Google Scholar]
- 9.Wenzel RP. Treating sepsis. N Engl J Med. 2002;347(13):966–967. doi: 10.1056/NEJMp020096. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre JS, Marleau S, Milot V, Lévesque T, Picard S, Flamand N, Borgeat P. Toll-like receptor ligands induce polymorphonuclear leukocyte migration: key roles for leukotriene B4 and platelet-activating factor. FASEB J. 2010 Feb;24(2):637–47. doi: 10.1096/fj.09-135624. Epub 2009 Oct 20. [DOI] [PubMed] [Google Scholar]
- 11.Lefebvre JS, Lévesque T, Picard S, Paré G, Gravel A, Flamand L, Borgeat P. Extra domain A of fibronectin primes leukotriene biosynthesis and stimulates neutrophil migration through activation of Toll-like receptor 4. Arthritis Rheum. 2011 Jun;63(6):1527–33. doi: 10.1002/art.30308. [DOI] [PubMed] [Google Scholar]
- 12.Zardi EM, Zardi DM, Dobrina A, Afeltra A. Prostacyclin in sepsis: a systematic review. Prostaglandins Other Lipid Mediat. 2007 Feb;83(1–2):1–24. doi: 10.1016/j.prostaglandins.2006.12.004. Epub 2006 Dec 29. Review. [DOI] [PubMed] [Google Scholar]
- 13.Cook JA. Eicosanoids. Crit Care Med. 2005 Dec;33(12 Suppl):S488–91. doi: 10.1097/01.ccm.0000196028.19746.42. Review. [DOI] [PubMed] [Google Scholar]
- 14.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009 Oct 29;461(7268):1287–91. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamurthy VR, Dougherty A, Haller CA, Chaikof EL. Total synthesis and bioactivity of 18(R)-hydroxyeicosapentaenoic acid. J Org Chem. 2011 Jul 1;76(13):5433–7. doi: 10.1021/jo2002243. Epub 2011 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65(6):1043–51. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 17.Clark JD, Schievella AR, Nalefski EA, Lin LL. Cytosolic phospholipase A2. J Lipid Mediat Cell Signal. 1995;12(2–3):83–117. doi: 10.1016/0929-7855(95)00012-f. [DOI] [PubMed] [Google Scholar]
- 18.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 19.Pettus BJ, Kitatani K, Chalfant CE, Taha TA, Kawamori T, Bielawski J, Obeid LM, Hannun YA. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol. 2005;68(2):330–5. doi: 10.1124/mol.104.008722. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian P, Stahelin RV, Szulc Z, Bielawska A, Cho W, Chalfant CE. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. J Biol Chem. 2005;280(18):17601–7. doi: 10.1074/jbc.M414173200. [DOI] [PubMed] [Google Scholar]
- 21.Pettus BJ, Chalfant CE, Hannun YA. Sphingolipids in inflammation: roles and implications. Curr Mol Med. 2004;4(4):405–18. doi: 10.2174/1566524043360573. [DOI] [PubMed] [Google Scholar]
- 22.Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, Evans JH, Freiberg J, Roddy P, Hannun YA, Chalfant CE. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem. 2004;279(12):11320–6. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- 23.Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE. Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J Biol Chem. 2003;278(40):38206–13. doi: 10.1074/jbc.M304816200. [DOI] [PubMed] [Google Scholar]
- 24.Klockenbusch W, Somville T, Hafner D, Strobach H, Schr??r K. Excretion of prostacyclin and thromboxane metabolites before, during, and after pregnancy-induced hypertension. European Journal of Obstetrics and Gynecology and Reproductive Biology. 1994;57(1):47–50. doi: 10.1016/0028-2243(94)90110-4. https://doi.org/10.1016/0028-2243(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 25.Walsh SW, Wang Y. Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. Journal of Clinical Endocrinology and Metabolism. 1995;80(6):1888–1893. doi: 10.1210/jcem.80.6.7775637. https://doi.org/10.1210/jcem.80.6.7775637. [DOI] [PubMed] [Google Scholar]
- 26.Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, McLaughlin MK. Lipid peroxidation in pregnancy: New perspectives on preeclampsia. American Journal of Obstetrics and Gynecology. 1989;161(4):1025–1034. doi: 10.1016/0002-9378(89)90778-3. https://doi.org/10.1016/0002-9378(89)90778-3. [DOI] [PubMed] [Google Scholar]
- 27.Hubel Ca. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222(3):222–235. doi: 10.1177/153537029922200305. https://doi.org/pse22216 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Siddiqui Ia, Jaleel A, Tamimi W, Al Kadri HMF. Role of oxidative stress in the pathogenesis of preeclampsia. Archives of Gynecology and Obstetrics. 2010;282(5):469–74. doi: 10.1007/s00404-010-1538-6. https://doi.org/10.1007/s00404-010-1538-6. [DOI] [PubMed] [Google Scholar]
- 29.Perneby C, Vahter M, Åkesson A, Bremme K, Hjemdahl P. Thromboxane metabolite excretion during pregnancy - Influence of preeclampsia and aspirin treatment. Thrombosis Research. 2011;127(6):605–606. doi: 10.1016/j.thromres.2011.01.005. https://doi.org/10.1016/j.thromres.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Roberts JM, Druzin M, August PA, Gaiser RR, Bakris G, Granger JP, … Sibai BM. ACOG Guidelines: Hypertension in pregnancy. American College of Obstetricians and Gynecologists. 2012 https://doi.org/doi:10.1097/01.AOG.0000437382.03963.88.
- 31.Lamour NF, Wijesinghe DS, Mietla JA, Ward KE, Stahelin RV, Chalfant CE. Ceramide kinase regulates the production of tumor necrosis factor α (TNFα) via inhibition of TNFα-converting enzyme. J Biol Chem. 2011 Dec 16;286(50):42808–17. doi: 10.1074/jbc.M111.310169. Epub 2011 Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinkovska-Galcheva VT, Boxer LA, Mansfield PJ, Harsh D, Blackwood A, Shayman JA. The formation of ceramide-1-phosphate during neutrophil phagocytosis and its role in liposome fusion. J Biol Chem. 1998;273(50):33203–9. doi: 10.1074/jbc.273.50.33203. [DOI] [PubMed] [Google Scholar]
- 33.Lennartz MR. Phospholipases and phagocytosis: the role of phospholipid-derived second messengers in phagocytosis. Int J Biochem Cell Biol. 1999 Mar-Apr;31(3–4):415–30. doi: 10.1016/s1357-2725(98)00108-3. Review. [DOI] [PubMed] [Google Scholar]
- 34.Rubin BB, Downey GP, Koh A, Degousee N, Ghomashchi F, Nallan L, Stefanski E, Harkin DW, Sun C, Smart BP, Lindsay TF, Cherepanov V, Vachon E, Kelvin D, Sadilek M, Brown GE, Yaffe MB, Plumb J, Grinstein S, Glogauer M, Gelb MH. Cytosolic phospholipase A2-alpha is necessary for platelet-activating factor biosynthesis, efficient neutrophil-mediated bacterial killing, and the innate immune response to pulmonary infection: cPLA2-alpha does not regulate neutrophil NADPH oxidase activity. J Biol Chem. 2005;280(9):7519–29. doi: 10.1074/jbc.M407438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menten-Dedoyart C, Faccinetto C, Golovchenko M, Dupiereux I, Van Lerberghe PB, Dubois S, Desmet C, Elmoualij B, Baron F, Rudenko N, Oury C, Heinen E, Couvreur B. Neutrophil extracellular traps entrap and kill Borrelia burgdorferi sensu stricto spirochetes and are not affected by Ixodes ricinus tick saliva. J Immunol. 2012 Dec 1;189(11):5393–401. doi: 10.4049/jimmunol.1103771. Epub 2012 Oct 29. [DOI] [PubMed] [Google Scholar]
- 36.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009 Oct;119(10):2868–78. doi: 10.1172/JCI39421. Epub 2009 Oct 1. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balgoma D, Montero O, Balboa MA, Balsinde J. Lipidomic approaches to the study of phospholipase A2-regulated phospholipid fatty acid incorporation and remodeling. Biochimie. 2010 Jun;92(6):645–50. doi: 10.1016/j.biochi.2009.11.010. Epub 2009 Dec 5. [DOI] [PubMed] [Google Scholar]
- 38.Dalli J, Colas RA, Quintana C, Barragan-Bradford D, Hurwitz S, Levy BD, … Baron RM. Human Sepsis Eicosanoid and Proresolving Lipid Mediator Temporal Profiles. Critical Care Medicine. 2016;45(1):1. doi: 10.1097/CCM.0000000000002014. https://doi.org/10.1097/CCM.0000000000002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rittirsch Daniel, Huber-Lang Markus S, Flierl Michael A, Ward Peter A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nature Protocols. 2009:4, 31, 36. doi: 10.1038/nprot.2008.214. Published online: 11 December 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011 Apr;19(4):198–208. doi: 10.1016/j.tim.2011.01.001. Review. [DOI] [PubMed] [Google Scholar]
- 41.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG the Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013 Feb 11; doi: 10.1073/pnas.1222878110. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajuebor MN, Das AM, Virág L, Flower RJ, Szabó C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999 Feb 1;162(3):1685–91. [PubMed] [Google Scholar]
- 43.Suram S, Silveira LJ, Mahaffey S, Brown GD, Bonventre JV, Williams DL, Gow NA, Bratton DL, Murphy RC, Leslie CC. Cytosolic phospholipase A(2)α and eicosanoids regulate expression of genes in macrophages involved in host defense and inflammation. PLoS One. 2013 Jul 25;8(7):e69002. doi: 10.1371/journal.pone.0069002. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, Hotchkiss RS, Buchman TG, Coopersmith CM. Effects of aging on the immunopathologic response to sepsis. Crit Care Med. 2009 Mar;37(3):1018–23. doi: 10.1097/CCM.0b013e3181968f3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010 Apr;78(4):1582–92. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Puri P, Massey HD, Idowu MO, Brophy DF, Voelkel NF, Fowler AA, 3rd, Natarajan R. Attenuation of Sepsis-induced Organ Injury in Mice by Vitamin C. JPEN J Parenter Enteral Nutr. 2013 Aug 5; doi: 10.1177/0148607113497760. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Fowler AA, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin EJ, Brophy DF, Gupta S, Fisher BJ, Natarajan R. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014 doi: 10.1186/1479-5876-12-32. Manuscript in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Ward KR, Voelkel NF, Fowler AA, 3rd, Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. 2012 Jul 1;303(1):L20–32. doi: 10.1152/ajplung.00300.2011. Epub 2012 Apr 20. [DOI] [PubMed] [Google Scholar]
- 49.Swartz DE, Seely AJ, Ferri L, Giannias B, Christou NV. Decreased systemic polymorphonuclear neutrophil (PMN) rolling without increased PMN adhesion in peritonitis at remote sites. Arch Surg. 2000 Aug;135(8):959–66. doi: 10.1001/archsurg.135.8.959. [DOI] [PubMed] [Google Scholar]
- 50.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008 May;8(5):349–61. doi: 10.1038/nri2294. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen C. COX-2’s new role in inflammation. (2010) Nature Chem Biol. 2010;6:401–402. doi: 10.1038/nchembio.375. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Albeniz X, Chan a. Aspirin for the prevention of colorectal cancer. Best Practice & Research Clinical Gastroenterology. 2012;25:461–472. doi: 10.1016/j.bpg.2011.10.015. https://doi.org/10.1016/j.bpg.2011.10.015.Aspirin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan DS, Yan M, Hassan M, Fang ZB, Chen MT. Plasma 8-iso-Prostaglandin F2α, a possible prognostic marker in aneurysmal subarachnoid hemorrhage. Clinica Chimica Acta. 2017;469(April):166–170. doi: 10.1016/j.cca.2017.04.005. https://doi.org/10.1016/j.cca.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Kant M, Akıs M, Calan M, Arkan T, Bayraktar F, Dizdaroglu M, Islekel H. Elevated urinary levels of 8-oxo-2′-deoxyguanosine, (5′R)- and (5′;S)-8,5′-cyclo-2′-deoxyadenosines, and 8-iso-prostaglandin F2a as potential biomarkers of oxidative stress in patients with prediabetes. DNA Repair. 2016;48:1–7. doi: 10.1016/j.dnarep.2016.09.004. https://doi.org/10.1016/j.dnarep.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scholl TO, Leskiw M, Chen X, Sims M, Stein TP. Oxidative stress, diet, and the etiology of preeclampsia. American Journal of Clinical Nutrition. 2005;81(6):1390–1396. doi: 10.1093/ajcn/81.6.1390. https://doi.org/81/6/1390 [pii] [DOI] [PubMed] [Google Scholar]
- 56.Smith WL, Urade Y, Jakobsson P-J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev. 2011;111:5821–5865. doi: 10.1021/cr2002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haeggström JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev. 2011;111:5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 58.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nature Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoeferlin LA, Huynh QK, Mietla JA, Sell SA, Tucker J, Chalfant CE, Wijesinghe DS. The Lipid Portion of Activated Platelet-Rich Plasma Significantly Contributes to Its Wound Healing Properties. Advances in Wound Care. 2015;4(2):100–109. doi: 10.1089/wound.2014.0589. https://doi.org/10.1089/wound.2014.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohammed BM, Sanford KW, Fisher BJ, Martin EJ, Contaifer D, Jr, Warncke UO, … Natarajan R. Impact of high dose vitamin C on platelet function. World Journal of Critical Care Medicine. 2017;6(1):37. doi: 10.5492/wjccm.v6.i1.37. https://doi.org/10.5492/wjccm.v6.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamour NF, Stahelin RV, Wijesinghe DS, Maceyka M, Wang E, Allegood JC, Chalfant CE. Ceramide kinase uses ceramide provided by ceramide transport protein: localization to organelles of eicosanoid synthesis. J Lipid Res. 2007;48(6):1293–1304. doi: 10.1194/jlr.M700083-JLR200. https://doi.org/10.1194/jlr.M700083-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Simanshu DK, Kamlekar R-K, Wijesinghe DS, Zou X, Zhai X, Mishra SK, Molotkovsky JG, Malinina L, Hinchcliffe EH, Chalfant CE, Brown RE, Patel DJ. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature. 2013;500:463–467. doi: 10.1038/nature12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wijesinghe DS, Brentnall M, Mietla Ja, Hoeferlin La, Diegelmann RF, Boise LH, Chalfant CE. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. The Journal of Lipid Research. 2014;55(7):1298–1309. doi: 10.1194/jlr.M048207. https://doi.org/10.1194/jlr.M048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nature Reviews Immunology. 2015;15(8):511–23. doi: 10.1038/nri3859. https://doi.org/10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin M zhu, Tan S, Li X, Hou Y, Cao G, Li K, … Lou G. Identification of phosphatidylcholine and lysophosphatidylcholine as novel biomarkers for cervical cancers in a prospective cohort study. Tumor Biology. 2016;37(4):5485–5492. doi: 10.1007/s13277-015-4164-x. https://doi.org/10.1007/s13277-015-4164-x. [DOI] [PubMed] [Google Scholar]
- 66.Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, Shimizu T, … Dennis EA. Update of the LIPID MAPS comprehensive classification system for lipids. J Lipid Res. 2009;50(Suppl):S9–14. doi: 10.1194/jlr.R800095-JLR200. https://doi.org/R800095-JLR200 [pii]\r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50:S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Six DA, Dennis EA. Essential Ca2+-independent role of the Group IVA cytosolic phospholipase A2 C2 domain for interfacial activity. J Biol Chem. 2003;278:23842–23860. doi: 10.1074/jbc.M301386200. [DOI] [PubMed] [Google Scholar]
- 69.Podo F, Sardanelli F, Iorio E, Canese R, Carpinelli G, Fausto A, Canevari S. Abnormal Choline Phospholipid Metabolism in Breast and Ovary Cancer:Molecular Bases for Noninvasive Imaging Approaches. Current Medical Imaging Reviews. 2007;3(2):123–137. https://doi.org/10.2174/157340507780619160. [Google Scholar]
- 70.Anand S, Young S, Esplin MS, Peaden B, Tolley HD, Porter TF, … Graves SW. Detection and confirmation of serum lipid biomarkers for preeclampsia using direct infusion mass spectrometry. Journal of Lipid Research. 2016;57(4):687–96. doi: 10.1194/jlr.P064451. https://doi.org/10.1194/jlr.P064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukherjee A, Ma Y, Yuan F, Gong Y, Fang Z, Mohamed EM, … Fang X. Lysophosphatidic acid up-regulates hexokinase II and glycolysis to promote proliferation of ovarian cancer cells. Neoplasia. 2015;17(9):723–734. doi: 10.1016/j.neo.2015.09.003. https://doi.org/10.1016/j.neo.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stokes CE, Hawthorne JN. Reduced Phosphoinositide Concentrations in Anterior Temporal Cortex of Alzheimer-Diseased Brains. Journal of Neurochemistry. 1987;48(4):1018–1021. doi: 10.1111/j.1471-4159.1987.tb05619.x. https://doi.org/10.1111/j.1471-4159.1987.tb05619.x. [DOI] [PubMed] [Google Scholar]
- 73.Bana L, Minniti S, Salvati E, Sesana S, Zambelli V, Cagnotto A, … Re F. Liposomes bi-functionalized with phosphatidic acid and an ApoE-derived peptide affect Aβ aggregation features and cross the blood-brain-barrier: Implications for therapy of Alzheimer disease. Nanomedicine: Nanotechnology, Biology, and Medicine. 2014;10(7):1583–1590. doi: 10.1016/j.nano.2013.12.001. https://doi.org/10.1016/j.nano.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Inestrosa NC, Sagal JP, Colombres M. Acetylcholinesterase interaction with Alzheimer amyloid beta. Sub-Cellular Biochemistry. 2005;38:299–317. doi: 10.1007/0-387-23226-5_15. https://doi.org/10.1007/b100942. [DOI] [PubMed] [Google Scholar]
- 75.Lan JS, Zhang T, Liu Y, Yang J, Xie SS, Liu J, … Ding Y. Design, synthesis and biological activity of novel donepezil derivatives bearing N -benzyl pyridinium moiety as potent and dual binding site acetylcholinesterase inhibitors. European Journal of Medicinal Chemistry. 2017;133:184–196. doi: 10.1016/j.ejmech.2017.02.045. https://doi.org/10.1016/j.ejmech.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 76.Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Federoff HJ. Plasma phospholipids identify antecedent memory impairment in older adults. Nature Medicine, advance on. 2014;(4):415–418. doi: 10.1038/nm.3466. https://doi.org/10.1038/nm.3466. [DOI] [PMC free article] [PubMed]
- 77.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cellular Signalling. 2008 doi: 10.1016/j.cellsig.2007.12.006. https://doi.org/10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed]
- 78.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends in Cell Biology. 2012;22(1):50–60. doi: 10.1016/j.tcb.2011.09.003. https://doi.org/10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lima S, Milstien S, Spiegel S. Sphingosine and Sphingosine Kinase 1 Involvement in Endocytic Membrane Trafficking. Journal of Biological Chemistry. 2017;292(8) doi: 10.1074/jbc.M116.762377. jbc.M116.762377. https://doi.org/10.1074/jbc.M116.762377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagahashi M, Hait NC, Maceyka M, Avni D, Takabe K, Milstien S, Spiegel S. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Advances in Biological Regulation. 2012;29(6):997–1003. doi: 10.1016/j.jbior.2013.10.001. https://doi.org/10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liang J, Nagahashi M, Kim EY, Harikumar KB, Huang W, Hait NC, … Spiegel S. NIH Public Access. 2014;23(1):107–120. https://doi.org/10.1016/j.ccr.2012.11.013.Sphingosine-1-Phosphate. [Google Scholar]
- 82.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, … Spiegel S. Sphingosine-1-Phosphate Links Persistent STAT3 Activation, Chronic Intestinal Inflammation, and Development of Colitis-Associated Cancer. Cancer Cell. 2013;23(1):107–120. doi: 10.1016/j.ccr.2012.11.013. https://doi.org/10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hait NC, Avni D, Yamada a, Nagahashi M, Aoyagi T, Aoki H, … Spiegel S. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERα expression and enhances hormonal therapy for breast cancer. Oncogenesis. 2015;4(6):e156. doi: 10.1038/oncsis.2015.16. https://doi.org/10.1038/oncsis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Contaifer D, Carl DE, Warncke UO, Martin EJ, Mohammed BM, Van Tassell B, … Wijesinghe DS. Unsupervised analysis of combined lipid and coagulation data reveal coagulopathy subtypes among dialysis patients. Journal of Lipid Research. 2016:804–828. doi: 10.1194/jlr.P068833. https://doi.org/10.1194/jlr.P068833. [DOI] [PMC free article] [PubMed]
- 85.Deguchi H, Yegneswaran S, Griffin JH. Sphingolipids as Bioactive Regulators of Thrombin Generation. Journal of Biological Chemistry. 2004;279(13):12036–12042. doi: 10.1074/jbc.M302531200. https://doi.org/10.1074/jbc.M302531200. [DOI] [PubMed] [Google Scholar]
- 86.Ariga T, Jarvis WD, Yu RK. Role of sphingolipid-mediated cell death in neurodegenerative diseases. Journal of Lipid Research. 1998;39(1):1–16. [PubMed] [Google Scholar]
- 87.Arboleda G, Morales LC, Benítez B, Arboleda H. Regulation of ceramide-induced neuronal death: Cell metabolism meets neurodegeneration. Brain Research Reviews. 2009;59(2):333–346. doi: 10.1016/j.brainresrev.2008.10.001. https://doi.org/10.1016/j.brainresrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 88.Filippov V, Song MA, Zhang K, Vinters HV, Tung S, Kirsch WM, … Duerksen-Hughes PJ. Increased ceramide in brains with alzheimer’s and other neurodegenerative diseases. Journal of Alzheimer’s Disease. 2012;29(3):537–547. doi: 10.3233/JAD-2011-111202. https://doi.org/10.3233/JAD-2011-111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kosicek M, Zetterberg H, Andreasen N, Peter-Katalinic J, Hecimovic S. Elevated cerebrospinal fluid sphingomyelin levels in prodromal Alzheimer’s disease. Neuroscience Letters. 2012;516(2):302–305. doi: 10.1016/j.neulet.2012.04.019. https://doi.org/10.1016/j.neulet.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 90.Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Murphy RC, … Dennis Ea. A comprehensive classification system for lipids. Journal of Lipid Research. 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. https://doi.org/10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 91.Holthuis JCM, Menon AK. Lipid landscapes and pipelines in membrane homeostasis. Nature. 2014;510(7503):48–57. doi: 10.1038/nature13474. https://doi.org/10.1038/nature13474. [DOI] [PubMed] [Google Scholar]
- 92.He H, Lancina MG, Wang J, Korzun WJ, Yang H, Ghosh S. Bolstering cholesteryl ester hydrolysis in liver: A hepatocyte-targeting gene delivery strategy for potential alleviation of atherosclerosis. Biomaterials. 2017;130:1–13. doi: 10.1016/j.biomaterials.2017.03.024. https://doi.org/10.1016/j.biomaterials.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Konieczna L, Baczek T, Belka M, Fel A, Markuszewski M, Struck W, … Kaliszan R. Steroid profiles as potential biomarkers in patients with urogenital tract cancer for diagnostic investigations analyzed by liquid chromatography coupled to mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2013;73:108–115. doi: 10.1016/j.jpba.2012.03.016. https://doi.org/10.1016/j.jpba.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 94.Pertegal M, Fenoy FJ, Bonacasa B, Mendiola J, Delgado JL, Hernández M, … Hernández I. 2-Methoxyestradiol Plasma Levels Are Associated With Clinical Severity Indices and Biomarkers of Preeclampsia. Reproductive Sciences (Thousand Oaks, Calif) 2015;22(2):198–206. doi: 10.1177/1933719114537716. https://doi.org/10.1177/1933719114537716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esidence PER, Alendar WORKC. THE AUSTRALIAN MULTI - CENTRE STUDY OF ENVIRONMENTAL FACTORS AND IMMUNE FUNCTION Gosford, NSW Dog Birds N/A Hornsby, NSW Cat yes Tertiary. Group. 2001:1–7. [Google Scholar]
- 96.Kowdley KV, Mason JB, Meydani SN, Cornwall S, Grand RJ. Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption. Gastroenterology. 1992;102(6):2139–2142. doi: 10.1016/0016-5085(92)90344-x. https://doi.org/10.5555/uri:pii:001650859290344X. [DOI] [PubMed] [Google Scholar]
- 97.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radical Biology and Medicine. 2007;43(1):4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. https://doi.org/10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi J, Leonard SW, Kasper K, McDougall M, Stevens JF, Tanguay RL, TM Novel function of vitamin E in regulation of zebrafish (Danio rerio) brain lysophospholipids discovered using lipidomics. J Lipid Res. 2015;56(6):1182–90. doi: 10.1194/jlr.M058941. https://doi.org/10.1194/jlr.M058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Badalà F, Nouri-mahdavi K, Raoof DA. NIH Public Access. Computer. 2008;144(5):724–732. doi: 10.1016/j.ajo.2007.07.010. https://doi.org/10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cane DE. Programming of erythromycin biosynthesis by a modular polyketide synthase. Journal of Biological Chemistry. 2010;285(36):27517–27523. doi: 10.1074/jbc.R110.144618. https://doi.org/10.1074/jbc.R110.144618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McDaniel R, Thamchaipenet A, Gustafsson C, Fu H, Betlach M, Ashley G. Multiple genetic modifications of the erythromycin polyketide synthase to produce a library of novel “unnatural” natural products. Proceedings of the National Academy of Sciences. 1999;96(5):1846–1851. doi: 10.1073/pnas.96.5.1846. https://doi.org/10.1073/pnas.96.5.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeller FP, Uvodich KC. Lovastatin for hypercholesterolemia. Drug Intelligence and Clinical Pharmacy. 1988;22(7–8):542–545. doi: 10.1177/106002808802200703. Retrieved from http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L18203412. [DOI] [PubMed] [Google Scholar]
- 103.Jain M, Petzold CJ, Schelle MW, Leavell MD, Mougous JD, Bertozzi CR, Cox JS. Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(12):5133–5138. doi: 10.1073/pnas.0610634104. https://doi.org/10.1073/pnas.0610634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bligh EG, Dyer WJ. Canadian Journal of. Canadian Journal of Biochemistry and Physiology. 1959;37(8):911–917. doi: 10.1139/o59-099. https://doi.org/dx.doi.org/10,1139/cjm2014-0700. [DOI] [PubMed] [Google Scholar]
- 105.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, … Dennis Ea. Lipidomics reveals a remarkable diversity of lipids in human plasma. Journal of Lipid Research. 2010;51(11):3299–3305. doi: 10.1194/jlr.M009449. https://doi.org/10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lam C, Law C. Untargeted Mass Spectrometry-Based Metabolomic Profiling of Pleural Effusions: Fatty Acids as Novel Cancer Biomarkers for Malignant Pleural Effusions. Journal of Proteome Research. 2014;13:4040–4046. doi: 10.1021/pr5003774. https://doi.org/10.1021/pr5003774. [DOI] [PubMed] [Google Scholar]
- 107.Szymański P, Markowicz M, Mikiciuk-Olasik E. Adaptation of high-throughput screening in drug discovery-toxicological screening tests. International Journal of Molecular Sciences. 2012;13(1):427–452. doi: 10.3390/ijms13010427. https://doi.org/10.3390/ijms13010427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Southeastern Regional Lipid Conference 2016. Avanti Polar Lipids. 2016 Web. < https://www.serlc.us/>.
- 109.Lipidomics and Bioactive Lipids in Metabolism and Disease (B6) Abide Therapeutics Inc; 2017. Web. http://www.keystonesymposia.org/17B6. [Google Scholar]
- 110.Hait NC, Avni D, Yamada A, Nagahashi M, Aoyagi T, Aoki H, Dumur CI, Zelenko Z, Gallagher EJ, Leroith D, Milstien S, Takabe K, Spiegel S. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERα expression and enhances hormonal therapy for breast cancer. Oncogenesis. 2015 Jun 8;4:e156. doi: 10.1038/oncsis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]