Abstract
Many tissues in our body have a tubular shape and are constantly exposed to various stresses. Luminal pressure imposes tension on the epithelial and myoepithelial or smooth muscle cells surrounding the lumen of the tubes. Contractile forces generated by actomyosin assemblies within these cells oppose the luminal pressure and must be calibrated to maintain tube diameter homeostasis and tissue integrity. In this review, we discuss mechanotransduction pathways that can lead from sensation of cell stretch to activation of actomyosin contractility, providing rapid mechanochemical feedback for proper tubular tissue function.
Keywords: Stretch, epithelial tubes, smooth muscle, mechanotransduction, contractility
Introduction
Tubular structures, such as blood vessels, exocrine glands, and airway passages, are a common feature of the animal body plan. In the simplest form, tubes have a central lumen that is enveloped by a layer of epithelial or endothelial cells. Physiological tubes often consist of multiple layers of cells, including myoepithelial or smooth muscle cells. Though each tubular structure in the body is anatomically distinct, they all experience external forces that influence their structure and function. Cells in tubes become stretched as a result of intraluminal pressure, either in a constant regime or cyclically, for example following inhalation and exhalation of air from the lungs. Cells also experience shear stress when fluid or air flows through the tube. The response of epithelial and endothelial cells to shear stress has been extensively reviewed elsewhere [1–3]. Here, we focus on the response of epithelial tubes to stretch, and in particular on signaling pathways that are initiated by stretch and lead directly to activation of actomyosin contractility.
Increasing luminal pressure acts to stretch tubular structures, which are elastic at short time scales [4], just as pumping air into the inner tube of a bicycle tire will inflate it. In contrast to a tire tube, whose passive response to stretching depends solely on its material properties, cells can actively respond by modulating their cytoskeleton (see Figure 1). Mounting a cellular response to stretch requires conversion of physical force into intracellular biochemical signals, a process named mechanotransduction [5,6]. Mechanosensing proteins directly perceive mechanical cues and are activated or inactivated in response. Signals from mechanosensors, located at sites of cell-matrix and cell-cell adhesion, the cytoskeleton and the plasma membrane, are then relayed to secondary messengers, which function through various pathways to allow cells to respond to the stress. This response could be a rapid response, such as an increase in intracellular tension or an increase in intracellular calcium concentration. Additionally, there could be a delayed response, such as signaling to the nucleus, culminating in changes in gene expression [7,8] or reorientation of the cytoskeleton and cells at an angle to the direction of stress [9–12].
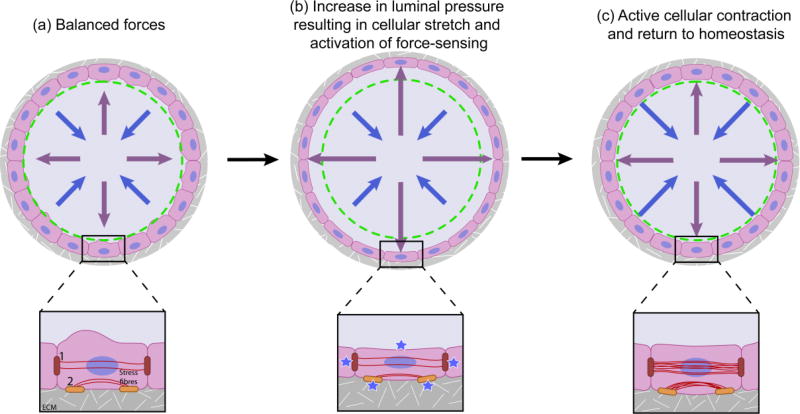
Figure 1. A balance between luminal pressue and cellular contractility determines the diameter of epithelial tubes.
Schematic depiction of the force balance within epithelial tubes (top panel) and within individual cells of tubes (bottom panel). (a) In a state of homeostasis, the outward luminal pressure (purple arrows) is balanced by the mechanical properties of the cells (blue arrows). Cells are attached to their neighbors through cell-cell junctions (marked as 1) and to the ECM through integrin adhesions (marked as 2). (b) Upon an increase in intraluminal pressure, as upon inhalation of air in lung alveoi, the cells around the tube stretch and force-sensing occurs at the membrane, integrin adhesions and cell-cell junctions (blue stars). (c) In response to force-sensing at integrin adhesions and cell-cell junctions, the cells increase their intracellular tension by active contraction of the actin cytoskeleton (red lines), culminating in return to a state of homeostasis within the tube.
The most direct response to cell stretching is an increase in intracellular tension to balance the applied force. The actomyosin contractile apparatus builds up this tension, which is essential to maintain the integrity of the cells and also of the tubular structure as a whole. Luminal pressure is countered by passive mechanical resistance and contractility of the cells surrounding the lumen, and these forces must be balanced to maintain a stable tube diameter (Figure 1). Further, a reduction in the intraluminal pressure would ultimately induce a reversal of the contracted state of the cells, resulting in homoeostasis in the relaxed state. Epithelial tube contractility is also externally regulated by hormones and neurotransmitters [13–15]. However, we would like to suggest here that mechanotransduction pathways leading directly from stretch-sensing to activation of the contractile apparatus are the most effective way to respond to external forces, and are probably employed by tubular structures in vivo.
The contractile apparatus in smooth muscle and in non-muscle cells is composed of arrays of actin and myosin II filaments [16]. As in skeletal muscle, ATP hydrolysis powers the inward sliding of actin filaments towards bipolar filaments of myosin motors [17]. However, in contrast with the highly organized and stable sarcomere structure of skeletal muscle, actomyosin in smooth muscle and in non-muscle cells is more loosely organized into sarcomere-like stress fibers that can assemble and disassemble within minutes. Actin polymerization from its free monomeric form (G-actin) to filamentous actin (F-actin) is catalyzed by actin nucleation and elongation factors, such as formins and the Arp2/3 complex [18,19]. In vitro, as airway smooth muscle cells are stretched, an increase in the ratio of F-actin:G-actin is observed [20]. Further remodeling of actin filaments by actin bundling and crosslinking proteins gives rise to a network of filamentous actin. In vivo, smooth muscle actin isoform ACTA2−/− mice show impaired mammary gland contraction and milk ejection, highlighting the role of actin in tube contractility [21]. Myosin II consists of two heavy chains, two regulatory light chains and two essential light chains. Phosphorylation of two residues on the regulatory myosin light chain, MLC, is required for the activity of myosin [22]. Myosin light chain kinase, MLCK, phosphorylates MLC, promoting contractility. Conversely, myosin light chain phosphatase, MLCP, inhibits contractility by dephosphorylation of MLC [23]. Thus, contractility can be controlled at two main levels: regulation of F-actin polymerization/remodeling and regulation of MLC phosphorylation.
In the following sections, we highlight three major signaling pathways that can be activated by cell stretching and lead to actin polymerization and/or myosin phosphorylation and hence to actomyosin contractility. A schematic representation of these pathways is shown in Figure 2.
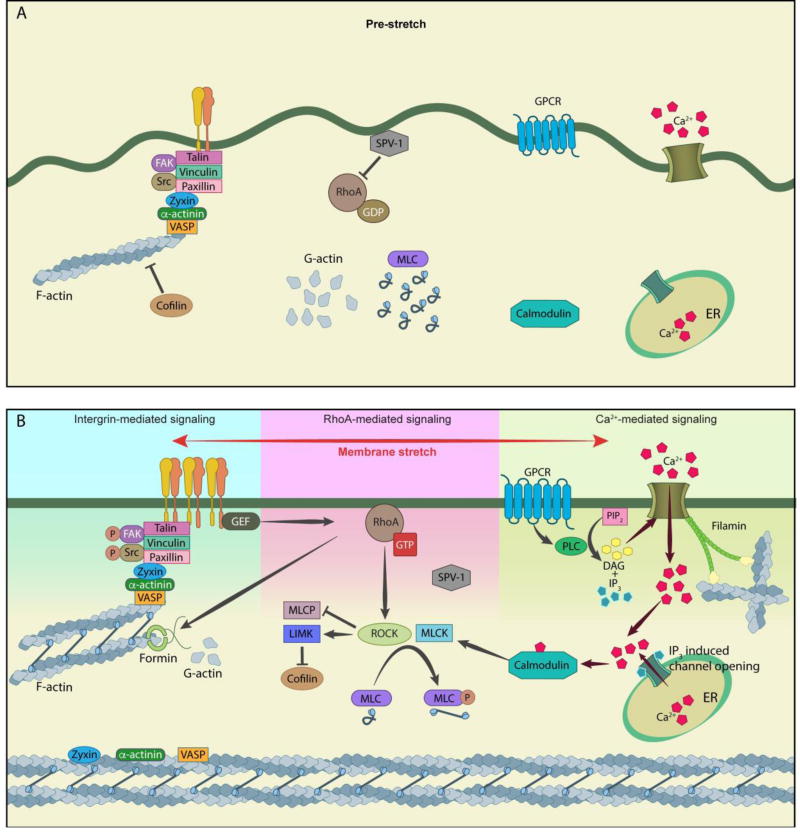
Figure 2. Stretch-induced mechanotransduction pathways leading to actomyosin contractility.
(A) Schematic depicting the interiors of a cell in a pre-stretch condition. The RhoGAP SPV-1 is attached to the membrane via its F-BAR domain and actively inhibits RhoA, calcium levels in the cells are low, myosin II is primarily inactive and the level of F-actin is low because of little polymerization and severing by cofilin. (B) Schematic depicting activation of the integrin-mediated pathway, RhoA-mediated pathway and calcium-mediated pathway, in response to stretch. Activation of integrins results in assembly of focal adhesions, Src and FAK phosphorylation, redistribution of zyxin, VASP and alpha-actinin to reinforce stress fibers and recruitment of RhoGEFs. Activation of RhoA activates downstream pathways resulting in phosphorylation and activation of myosin. In parallel, actin polymerization is enhanced by RhoA-mediated activation of formins and inhibition of the actin-severing activity of cofilin. Activation of calcium(Ca2+)-mediated signaling induces calcium-dependent steps ultimately resulting in myosin activation through MLCK. Filamin functions as a link between calcium influx and actin. All three pathways converge at activation of the actomyosin contractile machinery and consequently increase intracellular tension.
Integrin-mediated signaling
Integrins are heterodimeric transmembrane receptors [24]. They bind extracellular matrix (ECM) ligands with their extracellular head domains and bind to multiple intracellular proteins through their short cytoplasmic tails. It is through integrins that the ECM is connected to the intracellular contractile machinery. Cyclically stretched endothelial cells fail to reorient their cytoskeleton upon inhibition of integrin function [25], highlighting the importance of integrins in transducing mechanical cues. In addition, cyclically stretched endothelial cells show an upregulation of β3-integrin [26]. In vivo, knockout of β1-integrin in mice urothelium affects mechanosensing of urinary bladder filling (stretch), resulting in incontinence [27]. Additionally, integrins may also have a role in completion of the contraction/relaxation cycle in vivo, as a conditional mutant of integrin α3β1 in the mammary myoepithelial cells results in hypercontractility of the alveoli in the mammary gland [28].
Integrins can exist in an inactive or in an active state and their activation can be brought about by internal or external factors [29]. Inside-out signaling refers to the activation of integrins following the binding of talin or kindlin to the cytoplasmic tail [30,31]. On the other hand, outside-in integrin signaling occurs when integrins are activated by binding of a ligand within the ECM. Activation of integrins induces a conformational change in their structure, leading to clustering and a change from a low affinity state to a high affinity state, which forms focal adhesion sites [32]. In the high affinity activated state, clustering of integrins promotes focal adhesion kinase (FAK) activity, which in turn recruits Src-family kinase to adhesion sites [33]. As seen in umbilical vein endothelial cells, stretch promotes phosphorylation of focal adhesion kinase (FAK) [34], which in turn interacts with focal adhesion proteins, such as paxillin [35]. Additionally, cyclic stretching promotes increased expression and enhanced recruitment of focal adhesion components, such as paxillin and vinculin, at focal adhesion sites, as is seen in stretched smooth muscle cells [36,37]. In parallel, force applied to cells induces conformational changes in proteins, such as that of talin. This change in conformation exposes cryptic binding sites for other signaling/adapter proteins, such as that of vinculin on stretched talin [38].
Integrin-mediated signaling functions to reinforce the cytoskeletal machinery. One way it does this is through the LIM domain protein zyxin. Zyxin localizes to focal adhesion sites [39] and is known to bind to both the actin crosslinking protein, α-actinin and the actin polymerization protein, Ena/VASP [40–42]. Upon cellular stretch, zyxin, along with VASP (in a zyxin dependent manner), mobilizes from focal adhesion sites to stress fibers and thus contributes to actin remodeling [43,44]. Interestingly, zyxin is highly expressed in cells of the vasculature, lungs and urinary bladder, which experience stretch forces in vivo [45]. In vivo, zyxin is not essential for mechanotransduction, as zyxin null mice are viable [46]. However, vascular smooth muscle cells from zyxin null mice show reduced contractility with a poorly organized actin network [47].
Another way integrin-mediated signaling reinforces the actin cytoskeleton is through recruitment and activation of Rho guanine nucleotide exchange factors (GEFs), which stimulate RhoA signaling (discussed below).
Calcium-mediated signaling
Calcium plays a central role in cell contractility, in non-muscle cells, primarily through the calcium-calmodulin dependent activation of myosin light chain kinase, MLCK [48]. MLCK phosphorylates the myosin regulatory light chains, resulting in myosin activation, bipolar filament formation, and contraction of the actomyosin cytoskeleton [49].
One important source of calcium is through ion channels at the plasma membrane, which allow for influx of calcium into the cell from the extracellular environment. Mechanical stimulation, such as stretch, may activate ion channels. Influx of calcium through ion channels is essential for a mechanoresponse to stretch. For example, inhibition of stretch-activated calcium channels with gadolinium chloride almost completely abolishes calcium influx and subsequent cytoskeletal remodeling in cultured endothelial cells [50]. Possible mechanisms include direct mechanosensing (change in conformation of the channel under tension), activation through a tethered cytoskeletal element or activation by an upstream protein in the pathway. Ion channels expressed at the endothelial cell membrane have been studied extensively and among them, the transient receptor potential vanilloid (TRPV) superfamily of channels play an important role in mechanosignaling [25]. Inhibition of TRPV4 function in capillary endothelial cells, either by using an inhibitor molecule or through specific siRNA treatment, inhibited influx of calcium and subsequent reorientation of cells to stretch [25]. Similarly, TRPV1 and TRPV4, expressed in the urothelium, have been proposed to sense bladder distension and are required for normal bladder function [51–53]. Other studies have shown a crosstalk between integrins and TRPV channels with a mutual dependency for activation [25,54]. Further, TRPV4 associates with α-catenin at adherens junctions [55], F-actin [56], and non-muscle myosin IIa [57] in different cell types, consistent with a role in mechanotransduction.
Another major source of calcium is calcium stored in the endoplasmic reticulum. Calcium entry from both intracellular and extracellular sources can be activated by G-protein coupled receptors (GPCRs). GPCRs are integral membrane proteins, expressed at the cell surface, that can be activated by the action of agonists, such as activation of the GPCR oxytocin receptor upon binding of oxytocin [15]. Importantly, it has been suggested that the GPCR angiotensin II type 1, AT1 receptor in cardiac myocytes is directly mechanosensitive [58,59], but identification of other mechanosensitive GPCRs is still elusive.
Among other effectors, GPCR activates phospholipase C (PLC) leading to the release of calcium from internal stores. PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to produce the second messengers, diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 diffuses into the cytoplasm and binds to IP3 receptors at the endoplasmic reticulum, promoting the release of the intracellular stored calcium pool. Additionally, DAG can activate ion channels at the plasma membrane and allow for influx of extracellular calcium [60,61].
In connected cells, propagation of intercellular calcium waves through gap junctions is important for coordinated tissue responses to mechanical input. For example, gap junctions are required for the propagation of calcium waves through human retinal pigment epithelium in response to mechanical stimulation [62], and the gap junction protein innexin-12 is required for propagation of calcium waves in response to the stretch of oocyte entry in the C. elegans spermatheca [63], a contractile myoepthelial tissue in the nematode’s reproductive system. Although little is known about the response of gap junctions to mechanical perturbation in epithelial tissues, evidence from cardiomyocytes and bone cells suggests that the composition and localization of gap junctions is responsive to mechanical stimulation [64–67]. If a similar mechanism is operational in epithelial cells, gap junctions could, in addition to transmitting the signal between cells, contribute to the spatio-temporal regulation of mechanically triggered calcium signaling.
Filamin, an actin-binding and mechanically-sensitive scaffold protein, also contributes to contractility through crosslinking the actin cytoskeleton and by initiating calcium influx [68]. For example, a reduction of function mutant of filamin in the C. elegans spermatheca has a significantly disrupted actin cytoskeleton and fails to initiate calcium waves, resulting in impaired spermathecal contractile activity [63,69]. Similarly, a deletion mutant of filamin-A in smooth muscle cells in mice results in disruption of calcium influx upon increase in intraluminal pressure and consequently impairs myogenic behaviour by lowering of basal arterial pressure [70]. Filamin anchors many transmembrane proteins, including the CaR extracellular calcium receptor, to the actin cytoskeleton [71]. While it has not been directly demonstrated, filamin tethering of stretch-gated ion channels to the cytoskeleton might enable them to be activated by actomyosin contraction. Although these interactions are stabilizing or activating, the interaction between filamin and channels, such as the polycystin TRP-type channel in human renal epithelial cells, can also be inhibitory [72].
RhoA-mediated signaling
The small G protein RhoA is a key regulator of contractility [73]. RhoA switches between an active GTP-bound state and an inactive GDP-bound state. In its inactive state, RhoA is retained in the cytosol, associated with a GDP dissociation inhibitor (GDI). GTPase activating proteins (GAPs) inactivate RhoA by promoting hydrolysis of GTP (bound to RhoA) to GDP. Activation of RhoA is mediated by guanine nucleotide exchange factors (GEFs) [74]. Once activated and localized to the membrane, RhoA can signal to downstream pathways to respond to stretch. The pivotal role of RhoA in mechanosignaling is highlighted in cultured airway smooth muscle cells expressing a dominant negative mutant of RhoA wherein the cells failed to reorient in response to stretch [20].
We recently identified a RhoGAP protein, SPV-1, that regulates contractility of the C. elegans spermatheca [75]. Contraction of the spermatheca is driven by calcium-calmodulin activation of myosin light chain kinase and RHO-1 activation of Rho kinase (ROCK), which leads to the phosphorylation and activation of non-muscle myosin II [63,76]. In addition to its GAP domain, SPV-1 also posseses an F-BAR domain, which binds curved membranes. In a relaxed membrane state, SPV-1 binds to the membrane through its F-BAR domain and effectively prevents spermatheca contractility by inhibiting RhoA activity through its GAP domain. Upon membrane stretch (oocyte entry into spermatheca), SPV-1 dissociates from the membrane, with subsequent recruitment of active RhoA that induces contractility of the spermatheca [75].
GEFs are often recruited and/or activated by upstream effectors, such as integral membrane receptors, including integrins and Gq-coupled GPCRs, and kinases. For example, upon activation of integrins by applying force on fibroblast cells with magnetic beads, the GEFs GEF-H1 (ARHGEF2) and LARG (ARHGEF12) are activated and recruited to focal adhesion sites [77]. These GEFs activate RhoA, also present at focal adhesion sites, and thus promote contractility. Likewise, cyclic stretch of HUVECs showed enrichment of the GEF Solo (ARHGEF40) at adherens junctions [78]. Similar to GEF-H1 and LARG, Solo activates RhoA, though at cell-cell contact sites, promoting contracility.
RhoA promotes contractility by regulating both actin remodeling and myosin phosphorylation status. Binding of RhoA induces a conformational change in Rho kinase (ROCK) [79,80]. This conformational change of ROCK results in its autophosphorylation and activation. ROCK activation is important for contractile response, as upon inhibition of ROCK activity with the ROCK inhibitor Y27632, the basal tone of cyclically stretched isolated human bronchi is significantly affected [81]. Once activated, ROCK phosphorylates MLC [82], enabling myosin binding to actin and contraction [83]. Also, ROCK phosphorylates the myosin binding subunit of MLCP, relieving its inhibitory effect on MLC [84]. As a consequence, MLC is now maintained in a phosphorylated active status, enabling contraction. Activated ROCK also affects actin stability by stimulation of LIM kinase which phosphorylates and inhibits the actin-severing protein, cofilin [85]. In parallel, activated RhoA also activates the formin protein, mDia1 [86], driving polymerization of F-actin that can incorporate into stress fibers. As a result of formin activation and cofilin inhibition, filamentous actin structures are stabilized and together with the activity of myosin can increase cellular tension/contractility.
It has also been suggested that actin filaments themselves can sense tension [87]: tensed actin filaments show an enhanced affinity for myosin [88] while hindering cofilin binding [89]. Both cell culture [90–92] and in vivo work [93–96], for example, in the epithelial tubes of Drosophila trachea and C. elegans spermatheca, have shown that cell stretch and contraction influences actin organization, suggesting reorganization of the actin cytoskeleton is an important cellular mechanism for adaptation to the mechanical microenvironment. Stress fibers, contractile actomyosin bundles, are common among cells exposed to physical stress and have been well-studied within the context of cell migration [97–102]. However, much less is known about regulation of contractile stress fiber-like structures in epithelial tubes.
Conclusions and future directions
In this review, we have highlighted pathways through which cell contractility can be directly activated in response to stretching forces. Cells in vivo, particularly those in tubular structures, commonly experience stretching forces. However, very few studies have addressed the cellular responses to stretching in vivo. This is partly because of technical challenges and a shortage of amenable in vivo models. Additionally, the fact that multiple mechanical cues influence cells in vivo and that multiple biochemical pathways also regulate contractility, further complicates the issues at hand. In the future, advanced imaging techniques, novel biosensors and use of conditional mutants should further enhance our understanding of cellular contractile responses to stretch within tubular tissues in vivo. Further studies are also required to better understand the assembly and organization of the contractile machinery within in vivo tubes. Basic questions include how actin bundles are aligned within cells and what determines their dominant orientation, which proteins contribute to the development and maintenance of stress fiber-like structures and how forces might contribute to these processes.
We expect that tube homeostasis is achieved through a combination of both short term and long term regulation of the actomyosin cytoskeleton and that mechanotransduction processes play a role in both, in combination with biochemical signals such as hormones and neuropeptides. Understanding the implications of stretch on cells is of clinical importance, because errors in the mechanotransduction process may impact various disease states. For example, one of the underlying causes of asthma is excessive hypercontractility of the bronchial airways. As another example, an increase in vascular stiffness, such as by deposition of collagen, results in increased cellular tension and consequently high blood pressure. Thus, knowledge of the mechanotransduction pathways involved in regulating tubular contractility might suggest novel therapeutic targets.
Acknowledgments
We thank Chun Xi Wong (MBI communications) for help with the illustrations. This work was supported by a grant from the National Institutes of Health NIGMS (GM110268) to E.J.C and by a Ministry of Education Tier2 grant (MOE2015-T2-1-045) to R.Z.B.
Abbreviations
- ATP
adenosine triphosphate
- DAG
diacylglycerol
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- GAP
GTPase activating protein
- GDI
guanine dissociation factor
- GEF
guanine nucleotide exchange factor
- GPCR
G protein-coupled receptors
- HUVEC
Human Umbilical Vein Endothelial Cells
- IP3
inositol 1,4,5-trisphosphate
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PLC
phospholipase C
- ROCK
Rho-associated protein kinase
- TRPV
transient receptor potential vanilloid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Invest. 2016;126:821–828. doi: 10.1172/JCI83083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeyens N, Schwartz MA. Biomechanics of vascular mechanosensation and remodeling. Mol. Biol. Cell. 2016;27:7–11. doi: 10.1091/mbc.E14-11-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am. J. Physiol. Renal Physiol. 2010;299:F1220–F1236. doi: 10.1152/ajprenal.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamm RD. Airway wall mechanics. Annu. Rev. Biomed. Eng. 1999;1:47–72. doi: 10.1146/annurev.bioeng.1.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev. Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Paluch EK, Nelson CM, Biais N, Fabry B, Moeller J, Pruitt BL, Wollnik C, Kudryasheva G, Rehfeldt F, Federle W. Mechanotransduction: use the force(s) BMC Biol. 2015;13:47. doi: 10.1186/s12915-015-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho S, Irianto J, Discher DE. Mechanosensing by the nucleus: From pathways to scaling relationships. J. Cell Biol. 2017 doi: 10.1083/jcb.201610042. jcb.201610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 9.Moretti M, Prina-Mello A, Reid AJ, Barron V, Prendergast PJ. Endothelial cell alignment on cyclically-stretched silicone surfaces. J. Mater. Sci. Mater. Med. 2004;15:1159–1164. doi: 10.1023/B:JMSM.0000046400.18607.72. [DOI] [PubMed] [Google Scholar]
- 10.Ives CL, Eskin SG, McIntire LV. Mechanical effects on endothelial cell morphology: in vitro assessment. In Vitro Cell. Dev. Biol. 1986;22:500–7. doi: 10.1007/BF02621134. [DOI] [PubMed] [Google Scholar]
- 11.Kanda K, Matsuda T, Oka T. Two-dimensional orientational response of smooth muscle cells to cyclic stretching. Asaio J. 1992;38:M382–5. doi: 10.1097/00002480-199207000-00060. [DOI] [PubMed] [Google Scholar]
- 12.Dartsch PC, Hämmerle H, Betz E. Orientation of cultured arterial smooth muscle cells growing on cyclically stretched substrates. Acta Anat. (Basel) 1986;125:108–13. doi: 10.1159/000146146. [DOI] [PubMed] [Google Scholar]
- 13.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J. Physiol. 2008;586:5047–61. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson K-EE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol. Rev. 2004;84:935. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 15.Reversi A, Cassoni P, Chini B. Oxytocin receptor signaling in myoepithelial and cancer cells. J. Mammary Gland Biol. Neoplasia. 2005;10:221–229. doi: 10.1007/s10911-005-9583-7. [DOI] [PubMed] [Google Scholar]
- 16.Murrell M, Oakes PW, Lenz M, Gardel ML. Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 2015;16:486–498. doi: 10.1038/nrm4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper G. Cell A Mol. Approach. 2. Sinauer Associates; Sunderland (MA): 2000. Actin, Myosin, and Cell Movement; pp. 1–7. [Google Scholar]
- 18.Zigmond SH. Formin-induced nucleation of actin filaments. Curr. Opin. Cell Biol. 2004;16:99–105. doi: 10.1016/j.ceb.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am. J. Physiol. Cell Physiol. 2005;288:C1145–C1160. doi: 10.1152/ajpcell.00387.2004. [DOI] [PubMed] [Google Scholar]
- 20.Smith PG, Roy C, Zhang YN, Chauduri S. Mechanical Stress Increases RhoA Activation in Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2003;28:436–442. doi: 10.1165/rcmb.4754. [DOI] [PubMed] [Google Scholar]
- 21.Haaksma CJ, Schwartz RJ, Tomasek JJ. Myoepithelial cell contraction and milk ejection are impaired in mammary glands of mice lacking smooth muscle alpha-actin. Biol. Reprod. 2011;85:13–21. doi: 10.1095/biolreprod.110.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikebe M, Hartshorne DJ. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J. Biol. Chem. 1985;260:10027–31. [PubMed] [Google Scholar]
- 23.Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: Structure, regulation and function. Mol. Cell. Biochem. 2004;259:197–209. doi: 10.1023/B:MCBI.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z, Guo SS, Fässler R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016;215:445–456. doi: 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 Channels Mediate Cyclic Strain-Induced Endothelial Cell Reorientation Through Integrin-to-Integrin Signaling. Circ. Res. 2009;104:1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki M, Naruse K, Asano Y, Okamoto T, Nishikimi N, Sakurai T, Nimura Y, Sokabe M. Up-regulation of integrin beta 3 expression by cyclic stretch in human umbilical endothelial cells. Biochem. Biophys Commun. 1997;239:372–6. doi: 10.1006/bbrc.1997.7364. [DOI] [PubMed] [Google Scholar]
- 27.Kanasaki K, Yu W, von Bodungen M, Larigakis JD, Kanasaki M, Ayala de la Pena F, Kalluri R, Hill WG. Loss of 1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype. FASEB J. 2013;27:1950–1961. doi: 10.1096/fj.12-223404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raymond K, Cagnet S, Kreft M, Janssen H, Sonnenberg A, Glukhova MA. Control of mammary myoepithelial cell contractile function by α3β1 integrin signalling. EMBO J. 2011;30:1896–906. doi: 10.1038/emboj.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calderwood D, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013;14:503–17. doi: 10.1038/nrm3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bledzka K, Bialkowska K, Sossey-Alaoui K, Vaynberg J, Pluskota E, Qin J, Plow EF. Kindlin-2 directly binds actin and regulates integrin outside-in signaling. J. Cell Biol. 2016;213:97–108. doi: 10.1083/jcb.201501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 2011;3:1–21. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitra SK, a Hanson D, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 34.Naruse K, Yamada T, Sai XR, Hamaguchi M, Sokabe M. Pp125FAK is required for stretch dependent morphological response of endothelial cells. Oncogene. 1998;17:455–463. doi: 10.1038/sj.onc.1201950. [DOI] [PubMed] [Google Scholar]
- 35.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J. Cell Sci. 2010;123:1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 36.Na S, Trache A, Trzeciakowski J, Sun Z, Meininger GA, Humphrey JD. Time-dependent changes in smooth muscle cell stiffness and focal adhesion area in response to cyclic equibiaxial stretch. Ann. Biomed. Eng. 2008;36:369–380. doi: 10.1007/s10439-008-9438-7. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham JJ, Linderman JJ, Mooney DJ. Externally applied cyclic strain regulates localization of focal contact components in cultured smooth muscle cells. Ann. Biomed. Eng. 2002;30:927–935. doi: 10.1114/1.1500408. [DOI] [PubMed] [Google Scholar]
- 38.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–41. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J. Cell Sci. 2008;121:2795–804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- 40.Drees B, Friederich E, Fradelizi J, Louvard D, Beckerle MC, Golsteyn RM. Characterization of the interaction between zyxin and Ena/VASP family of proteins. J. Biol. Chem. 2000;275:22503–22511. doi: 10.1074/jbc.M001698200. [DOI] [PubMed] [Google Scholar]
- 41.Reinhard M, Jouvenal K, Tripier D, Walter U. Identification, purification, and characterization of a zyxin-related protein that binds the focal adhesion and microfilament protein VASP (vasodilator-stimulated phosphoprotein) Proc Natl Acad Sci U S A. 1995;92:7956–7960. doi: 10.1073/pnas.92.17.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B, Trueb B. Analysis of the alpha-actinin/zyxin interaction. J. Biol. Chem. 2001;276:33328–35. doi: 10.1074/jbc.M100789200. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman LM, Jensen CC, Chaturvedi A, Yoshigi M, Beckerle MC. Stretch-induced actin remodeling requires targeting of zyxin to stress fibers and recruitment of actin regulators. Mol. Biol. Cell. 2012;23:1846–1859. doi: 10.1091/mbc.E11-12-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macalma T, Otte J, Hensler ME, Bockholt SM, Louis HA, Kalff-Suske M, Grzeschik KH, von der Ahe D, Beckerle MC. Molecular characterization of human zyxin. J. Biol. Chem. 1996;271:31470–8. doi: 10.1074/jbc.271.49.31470. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman LM, Nix DA, Benson B, Boot-Hanford R, Gustafsson E, Jamora C, Menzies AS, Goh KL, Jensen CC, Gertler FB, Fuchs E, Fässler R, Beckerle MC. Targeted Disruption of the Murine zyxin Gene. Mol. Cell. Biol. 2003;23:70–79. doi: 10.1128/MCB.23.1.70-79.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh S, Kollar B, Nahar T, Suresh Babu S, Wojtowicz A, Sticht C, Gretz N, Wagner AH, Korff T, Hecker M. Loss of the mechanotransducer zyxin promotes a synthetic phenotype of vascular smooth muscle cells. J. Am. Heart Assoc. 2015;4:e001712. doi: 10.1161/JAHA.114.001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh MP. Calmodulin and the regulation of smooth muscle contraction. Mol. Cell. Biochem. 1994;135:21–41. doi: 10.1007/BF00925958. [DOI] [PubMed] [Google Scholar]
- 49.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am. J. Physiol. 1998;274:H1532–H1538. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- 51.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J. Biol. Chem. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J. Clin. Invest. 2007;117:3453–3462. doi: 10.1172/JCI31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 54.Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol. 2010;2:435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen DAW, Hoenderop JG, Jansen KCFJ, Kemp AW, Heesakkers JPFA, Schalken JA. The mechanoreceptor TRPV4 is localized in adherence junctions of the human bladder urothelium: a morphological study. J. Urol. 2011;186:1121–7. doi: 10.1016/j.juro.2011.04.107. [DOI] [PubMed] [Google Scholar]
- 56.Becker D, Bereiter-Hahn J, Jendrach M. Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur. J. Cell Biol. 2009;88:141–152. doi: 10.1016/j.ejcb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Masuyama R, Mizuno A, Komori H, Kajiya H, Uekawa A, Kitaura H, Okabe K, Ohyama K, Komori T. Calcium/calmodulin-signaling supports TRPV4 activation in osteoclasts and regulates bone mass. J. Bone Miner. Res. 2012;27:1708–1721. doi: 10.1002/jbmr.1629. [DOI] [PubMed] [Google Scholar]
- 58.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat. Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 59.Schnitzler MMy, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helliwell RM, Large WA. Alpha 1-adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1,2-diacyl-sn-glycerol. J. Physiol. 1997;499(Pt 2):417–28. doi: 10.1113/jphysiol.1997.sp021938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–63. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 62.Abu Khamidakh AE, Juuti-Uusitalo K, Larsson K, Skottman H, Hyttinen J. Intercellular Ca2+ wave propagation in human retinal pigment epithelium cells induced by mechanical stimulation. Exp. Eye Res. 2013;108:129–139. doi: 10.1016/j.exer.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Kovacevic I, Orozco JM, Cram EJ. Filamin and Phospholipase C-ε Are Required for Calcium Signaling in the Caenorhabditis elegans Spermatheca. PLoS Genet. 2013;9:e1003510. doi: 10.1371/journal.pgen.1003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salameh A, Dhein S. Effects of mechanical forces and stretch on intercellular gap junction coupling. Biochim. Biophys. Acta - Biomembr. 2013;1828:147–156. doi: 10.1016/j.bbamem.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 65.Pimentel RC, Yamada KA, Kléber AG, Saffitz JE. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ. Res. 2002;90:671–677. doi: 10.1161/01.RES.0000014823.75393.4D. [DOI] [PubMed] [Google Scholar]
- 66.Salameh A, Wustmann A, Karl S, Blanke K, Apel D, Rojas-Gomez D, Franke H, Mohr FW, Janousek J, Dhein S. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circ. Res. 2010;106:1592–602. doi: 10.1161/CIRCRESAHA.109.214429. [DOI] [PubMed] [Google Scholar]
- 67.Matsuda T, Fujio Y, Nariai T, Ito T, Yamane M, Takatani T, Takahashi K, Azuma J. N-cadherin signals through Rac1 determine the localization of connexin 43 in cardiac myocytes. J. Mol. Cell. Cardiol. 2006;40:495–502. doi: 10.1016/j.yjmcc.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura F, Stossel TP, Hartwig JH. The filamins: Organizers of cell structure and function. Cell Adhes. Migr. 2011;5:160–169. doi: 10.4161/cam.5.2.14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kovacevic I, Cram EJ. FLN-1/Filamin is required for maintenance of actin and exit of fertilized oocytes from the spermatheca in C. elegans. Dev. Biol. 2010;347:247–257. doi: 10.1016/j.ydbio.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Retailleau K, Arhatte M, Demolombe S, Peyronnet R, Baudrie V, Jodar M, Bourreau J, Henrion D, Offermanns S, Nakamura F, Feng Y, Patel A, Duprat F, Honoré E. Arterial Myogenic Activation through Smooth Muscle Filamin A. Cell Rep. 2016;14:2050–8. doi: 10.1016/j.celrep.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 71.Awata H, Huang C, Handlogten ME, Miller RT. Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein. J. Biol. Chem. 2001;276:34871–9. doi: 10.1074/jbc.M100775200. [DOI] [PubMed] [Google Scholar]
- 72.Wang Q, Dai XQ, Li Q, Wang Z, del R M, Cantero, Li S, Shen J, Tu JC, Cantiello H, Chen XZ. Structural interaction and functional regulation of polycystin-2 by filamin. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lessey EC, Guilluy C, Burridge K. From mechanical force to RhoA activation. Biochemistry. 2012;51:7420–7432. doi: 10.1021/bi300758e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rossman KL, Der CJ, Sondek J. GEF means Go: turning on Rho GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 75.Tan PY, Zaidel-Bar R. Transient membrane localization of SPV-1 drives cyclical actomyosin contractions in the C elegans spermatheca. Curr. Biol. 2015;25:141–151. doi: 10.1016/j.cub.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 76.Wissmann A, Ingles J, Mains PE. The Caenorhabditis elegans mel-11 Myosin Phosphatase Regulatory Subunit Affects Tissue Contraction in the Somatic Gonad and the Embryonic Epidermis and Genetically Interacts with the Rac Signaling Pathway. Dev. Biol. 1999;209:111–127. doi: 10.1006/dbio.1999.9242. [DOI] [PubMed] [Google Scholar]
- 77.Guilluy C, Swaminathan V, Garcia-Mata R, Timothy O’Brien E, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 2011;13:724–729. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abiko H, Fujiwara S, Ohashi K, Hiatari R, Mashiko T, Sakamoto N, Sato M, Mizuno K. Rho guanine nucleotide exchange factors involved in cyclic-stretch-induced reorientation of vascular endothelial cells. J. Cell Sci. 2015;128:1683–1695. doi: 10.1242/jcs.157503. [DOI] [PubMed] [Google Scholar]
- 79.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 80.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le Guen M, Grassin-Delyle S, Naline E, Buenestado A, Brollo M, Longchampt E, Kleinmann P, Devillier P, Faisy C. The impact of low-frequency, low-force cyclic stretching of human bronchi on airway responsiveness. Respir. Res. 2016;17:151. doi: 10.1186/s12931-016-0464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 83.Craig R, Smith R, Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. Nature. 1983;302:436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- 84.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 85.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 87.Galkin VE, Orlova A, Egelman EH. Actin filaments as tension sensors. Curr. Biol. 2012;22 doi: 10.1016/j.cub.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uyeda TQP, Iwadate Y, Umeki N, Nagasaki A, Yumura S. Stretching actin filaments within cells enhances their affinity for the myosin ii motor domain. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayakawa K, Tatsumi H, Sokabe M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J. Cell Biol. 2011;195:721–727. doi: 10.1083/jcb.201102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A. 2012;18:910–9. doi: 10.1089/ten.TEA.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skwarek-Maruszewska A, Hotulainen P, Mattila PK, Lappalainen P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J. Cell Sci. 2009;122:2119–2126. doi: 10.1242/jcs.046805. [DOI] [PubMed] [Google Scholar]
- 92.Greiner AM, Chen H, Spatz JP, Kemkemer R. Cyclic Tensile Strain Controls Cell Shape and Directs Actin Stress Fiber Formation and Focal Adhesion Alignment in Spreading Cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hannezo E, Dong B, Recho P, Joanny J-F, Hayashi S. Cortical instability drives periodic supracellular actin pattern formation in epithelial tubes. Proc. Natl. Acad. Sci. 2015;112 doi: 10.1073/pnas.1504762112. 201504762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mason FM, Tworoger M, Martin AC. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat. Cell Biol. 2013;15:926–36. doi: 10.1038/ncb2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hosono C, Matsuda R, Adryan B, Samakovlis C. Transient junction anisotropies orient annular cell polarization in the Drosophila airway tubes. Nat. Cell Biol. 2015;17:1569–76. doi: 10.1038/ncb3267. [DOI] [PubMed] [Google Scholar]
- 96.Wirshing ACE, Cram EJ. Myosin activity drives actomyosin bundle formation and organization in contractile cells of the C. elegans spermatheca. Mol. Biol. Cell. 2017 doi: 10.1091/mbc.E17-01-0029. mbc.E17-01-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tojkander S, Gateva G, Lappalainen P. Actin stress fibers - assembly, dynamics and biological roles. J. Cell Sci. 2012;125:1855–1864. doi: 10.1242/jcs.098087. [DOI] [PubMed] [Google Scholar]
- 98.Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J. Microsc. 2008:446–454. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 99.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–12. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000;29:545–76. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- 101.Burridge K, Wittchen ES. The tension mounts: Stress fibers as force-generating mechanotransducers. J. Cell Biol. 2013;200:9–19. doi: 10.1083/jcb.201210090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pellegrin S, Mellor H. Actin stress fibres. J. Cell Sci. 2007;120:3491–9. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]