Abstract
Background
Venous thromboembolism (VTE) is a common complication of hematologic malignancies. Prolonged periods of thrombocytopenia are experienced universally by patients undergoing treatment for these diseases, yet data to guide management of anticoagulation in this setting are lacking.
Objectives
To obtain data on the management and outcomes of VTE in patients with thrombocytopenia related to the treatment of hematologic malignancies.
Methods
This was an observational cohort study of patients experiencing VTE during periods of treatment-related thrombocytopenia over a five-year period at the Fred Hutchinson Cancer Research Center. Medical records were reviewed for diagnostic, treatment and outcomes data, including bleeding events (categorized by WHO criteria) and progression or recurrence of VTE.
Results
Eighty-two patients meeting inclusion criteria were identified. Forty-eight percent were male and the median age was 55. Sixty-seven patients received anticoagulation, 88% of these were managed with transfusion support for a platelet goal of 50×109/L. Thirty-one patients experienced bleeding events, 22 of which were grade 2 and 9 of which were grade 3/4. The median platelet count at the time of bleeding event was 54×109/L. Seven patients experienced progression of thrombosis and/or recurrence. Eleven patients experienced transfusion reactions and 30 experienced volume overload requiring diuretics or dialysis.
Conclusions
While bleeding events were not uncommon, the majority of events were non-major/non-clinically relevant. Most bleeding events occurred while the platelet count was within the ‘goal’ range of ≥ 50×109/L, and many patients experienced transfusion related adverse events. Prospective studies are urgently needed to identify the optimal transfusion strategy for these patients.
Keywords: Thromboembolism, Venous, Thromboembolism, Pulmonary, Hematologic Neoplasms, Platelet Transfusion Hemorrhage
Introduction
Patients undergoing curative intent therapy and hematopoietic stem cell transplant (HSCT) for hematologic malignancies suffer a number of treatment- and disease-related adverse effects, including prolonged cytopenias and increased risk of both thrombosis and bleeding. While prolonged periods of severe thrombocytopenia (platelets <10×109/L) clearly increase risk of bleeding (as high as 79% in allogeneic transplant patients), [1] low platelet counts do not appear to reduce the risk of venous thromboembolism (VTE). These patients have a number of prothrombotic risk factors, including active malignancy, hospitalization, infection and central venous access. VTE rates as high as 5% in the 180 days post-HSCT have been noted.[2]
While cancer-associated thrombosis[3–5] has been well-studied and a number of guidelines, including those from the National Comprehensive Cancer Network (NCCN) and the Anticoagulation Forum, address management, [6, 7] few address management in the setting of platelet counts <50×109/L. Those that do base recommendations solely on expert opinion due to the absence of well-controlled studies.[7, 8] While concern about bleeding is noteworthy, risk of recurrent thrombosis, which is particularly elevated in the first 30 days, [9] must be minimized. Practice patterns and opinions on the subject vary widely[10] and patients with severe thrombocytopenia are usually excluded from randomized clinical trials of anticoagulants[3].
Options for the management of VTE in this population include holding or dose-reducing anticoagulation and administering platelet transfusions to achieve a pre-determined goal platelet count while delivering therapeutic anticoagulation. While transfusions toward an increased platelet goal (approximately 50×109/L) are recommended in the acute setting, [8] there are currently no published studies addressing adverse effects associated with such an increased goal or comparing this goal to any other. Here we report our experience with management of thrombosis in the setting of treatment-related thrombocytopenia, including outcomes associated with an increased platelet transfusion threshold, at the Fred Hutchinson Cancer Research Center.
Methods
The Hutch Integrated Data Repository and Archive (HIDRA) was used to identify patients requiring chemotherapy and/or HSCT for hematologic malignancies who also had diagnosis codes for upper or lower extremity VTE and/or pulmonary embolism (PE) between 2009 and 2014. Patients were considered consecutively and exclusion criteria were minimized to reduce bias. Medical records were then reviewed for inclusion criteria, which included age ≥18 years, thrombocytopenia related to the treatment of a hematologic disorder with curative-intent chemotherapy and/or HSCT and imaging-confirmed diagnosis of a venous thrombotic event. Thrombocytopenia was defined as a platelet count <50×109 in the absence of platelet transfusion support for a minimum of three days or a requirement for platelet transfusions in order to maintain a platelet count ≥50×109. Patients were included only if treatment-induced thrombocytopenia overlapped with the 30-day period following diagnosis of VTE. Patients were excluded if records during this time period were incomplete due to transfer of care elsewhere.
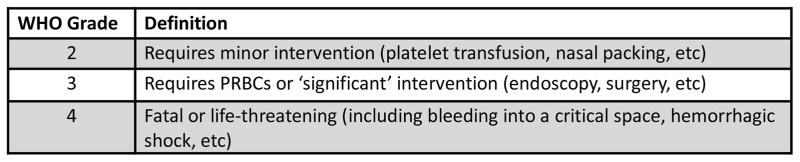
Medical records were reviewed for demographic data, hematologic diagnosis and treatment, VTE event including location and diagnostic imaging, VTE treatment including anticoagulant agent and total days of therapy, number of platelet transfusions, transfusion reactions and outcomes including thrombosis progression, new PE and bleeding events during the 30 days following diagnosis of VTE. Bleeding was graded by WHO criteria (Figure 1).[1, 11] Mortality was assessed at 90 days.
Figure 1.
WHO Bleeding Criteria
Due to the retrospective nature of this study, including inconsistency in timing of platelet counts, lack of post-transfusion platelet counts and limited documentation of the details of transfusion administration ‘at-risk’ time (hours or days during which the patient was anticoagulated and had a platelet count <50×109/L) could not be accurately calculated. As a surrogate, number of anticoagulated days with thrombocytopenia (days on which the patient received anticoagulation and experienced a platelet count <50×109/L at some point regardless of transfusions received later in the day) were calculated.
Demographic and treatment data were assessed and reported with standard descriptive statistics. The student’s t-test was used to compare platelet count at time of bleeding event between patients with grade 2 vs grade 3/4 bleeding. One-way ANOVA analysis was used to compare remaining metrics between the three groups (non-bleeding subjects vs subjects with grade 2 bleeding vs subjects with grade 3/4 bleeding). These metrics included mean platelet count, number of anticoagulated days with thrombocytopenia and total days of anticoagulation.
Results
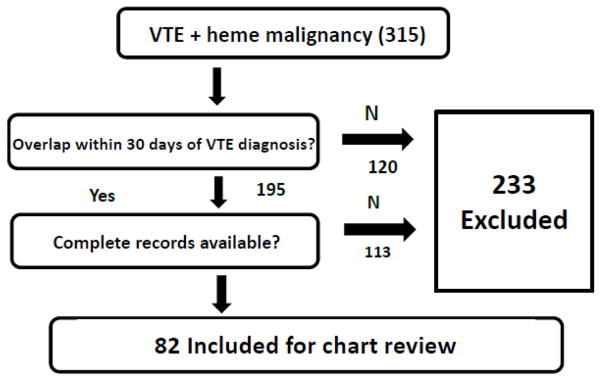
We identified 316 VTE events occurring in 315 patients undergoing treatment for a hematologic disorder between January 1, 2009 and December 21, 2014. A total of 233 subjects were excluded due to lack of overlap between thrombocytopenia and the 30-day period following the thrombotic event or incomplete access to records during the same period due to follow-up care received in another location (Figure 2). Characteristics of the remaining 82 patients and 83 events are reported in Table 1.
Figure 2.
Flow Diagram of Selection Criteria
Table 1.
Characteristics of Subjects
| Characteristic | Value |
|---|---|
| No. of patients | 82 |
| Age, range y (median) | 19–84 (55) |
| Sex, male/female, no (%) | 39 (48)/43 (52) |
| Primary Disease | |
| Acute myeloid leukemia, no (%) | 61 (74) |
| Lymphoma, no (%) | 12 (15) |
| MDS/MPN, no (%) | 7 (9) |
| Other, no (%) | 2 (2) |
| Treatment | |
| Chemotherapy, no (%) | 48 (59) |
| HSCT, no (%) | 31 (38) |
| Autologous, no (%) | 9 (11) |
| Allogeneic/cord blood, no (%) | 22 (27) |
| Other, no (%) | 3 (4) |
| Thrombosis | |
| Line associated, no (%) | 63 (77) |
| DVT, not line associated, no (%) | 11 (13) |
| Distal, no (%) | 5 (6) |
| Proximal, no (%) | 6 (7) |
| Pulmonary embolism, no (%) | 8 (10) |
| Initial Treatment | |
| Anticoagulation, no (%) | 65 (79) |
| Unfractionated heparin, no (%) | 30 (37) |
| LMWH, no (%) | 33 (40) |
| Other, no (%) | 2 (2) |
| Transfusion Threshold | |
| ≥ 50×109/L, no (%) | 55 (85) |
| < 50×109/L, no (%) | 3 (5) |
| Not stated, no (%) | 7 (11) |
| Treatment at 30 Days | |
| Anticoagulation, no (%) | 67 (82) |
| Unfractionated heparin, no (%) | 16 (20) |
| LMWH, no (%) | 48 (89) |
| Other, no (%) | 3 (4) |
| Transfusion Threshold | |
| ≥ 50×109/L, no (%) | 59 (88) |
| < 50×109/L, no (%) | 2 (3) |
| Not stated, no (%) | 6 (9) |
| Days of anticoagulation, range d (median) | 1 – 30 (26.5) |
| Days anticoagulation held, range (median) | 0 – 22 (0) |
Details regarding thrombosis diagnosis and location are reported in Table 1. Fourteen patients with VTE, including 7/8 patients with PE, 5/6 patients with proximal thrombosis and 2/5 patients with distal thrombosis were treated with anticoagulation at the time of diagnosis. Fifty-one of 63 patients with catheter-associated thrombosis were treated with anticoagulation upon diagnosis, 27 catheters were removed on diagnosis, and in 3 cases the catheter had been removed prior to diagnosis. Twenty-four of the 27 patients also received anticoagulation.
Initial treatment included parenteral treatment with unfractionated heparin (UFH) or low molecular weight heparin (LMWH) (46% vs 51% respectively). Two patients were treated with bivalirudin. Oral anticoagulation was not used in initial treatment. Anticoagulation was started later (within the first 30 days) in 2 patients, and discontinued within 30 days in 21 of 51 patients with catheter associated thrombosis and 3/14patients with DVT/PE. Nearly all patients who were anticoagulated either had anticoagulation held while the platelet count was <50×109 or had an increased transfusion threshold of 50×109/L. Patients received a median of six platelet transfusions (range 0–29) in the first thirty days following diagnosis. Eleven (13%) reported transfusion reactions (6 cases of hives, one unspecified rash, two chills without documented fever, 2 febrile, non-hemolytic transfusion reactions). Thirty (37%) patients experienced volume overload requiring diuretic therapy or dialysis.
Bleeding events occurred in 31 patients and 22 (71%) were WHO grade 2 (Table 3). Four events (all grade 2) occurred in the absence of anticoagulation, two while anticoagulation was held after initiation and two in patients in whom anticoagulation was never initiated. Three bleeding events, all grade 2, were noted among patients in whom anticoagulation was avoided during periods of thrombocytopenia (morning platelet count <50×109). Among the 58 patients who received anticoagulation during periods of thrombocytopenia, 19 experienced WHO grade 2 bleeding and 9 experienced WHO grade 3/4 bleeding. Patients with no bleeding, WHO grade 2 bleeding and WHO grade 3/4 bleeding received anticoagulation on a median of 4.5, 8 and 4 days (p = 0.020) with a platelet count <50×109, respectively. Patients among all three groups received a similar number of days of anticoagulation (median 26.5 vs 27 vs 20, p = 0.545).
Median platelet count at the time of bleeding events was 54 (range 8–518). This did not differ significantly between WHO grade 2 vs grade 3/4 bleeding (52.5 vs 54, p = 0.516). Thrombosis progression or possible/probable PE occurred in 7 patients (9%), all of whom had been selected to receive anticoagulation. Two of these patients had catheter-associated thrombosis, in one the catheter had been removed three days prior to imaging diagnosis of progression (five days after the initial VTE diagnosis), in another the catheter remained in place. Seventeen patients (21%) died within 90 days; 4 of these deaths appeared to be bleeding related. In all 4 cases of fatal bleeding, patients were on anticoagulation with platelet count >50×109 at the time of the bleed.
Discussion
Management of thrombosis in patients with prolonged, treatment-related thrombocytopenia carries many challenges. Here we describe management of 82 patients over a five-year period at the Fred Hutchinson Cancer Research Center/University of Washington. The majority of cases were catheter-associated, in keeping with previously reported findings [2], and the majority of these were managed with anticoagulation with or without catheter removal. Despite high rates of anticoagulation (>80%), the rate of thrombus extension and PE was impressive (9% at 30 days). This is consistent with previous findings demonstrating highest risk of recurrence in the first 30 days following diagnosis[9] and highlights the importance of attempting to administer anticoagulation during this period in the absence of absolute contraindications.
While direct comparisons cannot be made, it is noteworthy that bleeding rates among severely thrombocytopenic patients not receiving anticoagulants are as high as 79%, [1] with WHO grade 3 and 4 events occurring in as many as 15% of HSCT patients by day 180.[2] Bleeding rates among our patient population were near or below both of these thresholds and the majority of events occurred during a time the platelet count was >50×109/L, further suggesting that the relationship between bleeding, platelet count and anticoagulation is complex and nonlinear. Further adding to the complexity is the observation that the majority of bleeding events occurring in anticoagulated patients were WHO Grade 2. In virtually all cases these bleeds would not be considered to be clinically significant (mucocutaneous bleeding including menorrhagia, epistaxis, oral or hemorrhoidal bleeding). However, progression of thrombosis was symptomatic in nearly all cases and included at least one possible/probable PE.
While the bleeding and thrombosis rates in our study are consistent with those previously reported, the high rate of adverse effects associated with a platelet transfusion threshold of 50×109/L is, to our knowledge, a new observation. It is likely that our estimates underrepresent true rates of the complications of platelet transfusion, because our study is retrospective and we were unable to accurately evaluate for additional adverse outcomes such as alloimmunization. Furthermore, we were unable to quantify such burdens as financial cost, depletion of resources and inconvenience to patients who required prolonged hospitalizations and/or frequent trips to the infusion center to maintain such a high threshold. Perhaps the greatest ‘adverse effect’ of these high platelet thresholds, given high rates of VTE progression/recurrence, is the fact that they resulted in frequent discontinuation of anticoagulation during this high-risk period (36% within 30 days). These facts strongly support the need for prospective studies comparing a transfusion threshold of 50×109/L to other, lower, thresholds that might enable continued anticoagulation while reducing transfusion related adverse effects and costs.
Our study has a number of limitations primarily due to its retrospective nature. The etiology of certain adverse outcomes, such as volume overload and death, is difficult to assess on chart review and may, in some cases, have been incorrectly attributed. Furthermore, it is probable that a number of adverse events were not reported in the record and were therefore not reported in this study. In the specific case of volume overload, a treatment approach which requires therapy with diuretics or dialysis should, however, also incorporate care to minimize and eliminate any avoidable contribution, such as unnecessary transfusions. In the case of hemorrhagic adverse effects, WHO grade 3 and 4 events generally require interventions which would be noted in the medical record and are less likely to be underreported. Future studies would benefit from close monitoring of bleeding symptoms and transfusion-related outcomes.
Another limitation of our study is our inability to determine ‘at risk’ time, such as anticoagulated time below a certain platelet count, due to variable timing of transfusions and frequencies of platelet checks between patients. While we are able to report the most recent platelet count prior to the time of each bleed, we are unable to identify subsets of a population which may have spent a greater amount of time anticoagulated at a lower count and therefore ‘at risk.’ The relative uniformity of management at our institution prevented any comparison of bleeding risk between patients transfused to different thresholds.
Our data suggest that patients who experience thrombosis in close proximity to a period of treatment-related thrombocytopenia (within 30 days) stand to benefit from anticoagulation. Anticoagulated patients who were transfused to maintain a platelet count of 50×109 had a risk of bleeding similar to those reported in unanticoagulated patients with lower transfusion thresholds, but also suffered many transfusion-related consequences. Our observations underscore the need for future, prospective studies designed to identify a platelet count at which need for transfusions and transfusion-related complications may be minimized without increasing the rate of clinically significant bleeding.
Table 2.
Outcomes Among Patients Receiving Anticoagulation for VTE
| Characteristic | Values | p-value |
|---|---|---|
| Thrombosis progression | No (%) of patients | |
| Catheter-associated thrombosis progression | 2 (3) | |
| DVT/PE progression | 2 (11) | |
| Pulmonary embolism | 3 (4) | |
| Mortality | No (%) of patients | |
| Death within 90 days | 17 (21) | |
| Bleeding Outcomes | No (%) of patients | |
| Events (total) | 31 (37) | |
| Grade 2 | 22 (71) | |
| Grade 3 | 5 (16) | |
| Grade 4 | 4 (13) | |
| Events (in the absence of anticoagulation) | 4 (5) | |
| Grade 2 | 4 (100) | |
| Grade 3 | 0 | |
| Grade 4 | 0 | |
| Degree of thrombocytopenia | Platelets x109/L, range (median) | |
| At time of bleed | 8–518 (54) | |
| Grade 2 | 25–518 (52.5) | |
| Grade 3/4 | 8–403 (54) | p = 0.516* |
| Mean 30 day platelet count, range | ||
| No bleeding | 14–283 (73) | |
| Grade 2 | 32–124 (57) | |
| Grade 3/4 | 48–275 (80) | p = 0.074* |
| Duration of Thrombocytopenia | Days, range (median) | |
| Days with morning platelet count <50, range (median) | ||
| No bleeding, range (median) | 0–24 (4.5) | |
| Grade 2, range (median) | 2–30 (8) | |
| Grade 3/4, range (median) | 1–12 (4) | p = 0.020* |
All p-values are the result of ANOVA comparisons between the no-bleeding, grade 2 bleeding and grade ¾ bleeding groups.
Acknowledgments
Funding: This research was supported (in part) by the NHLBI under award number T32HL007093.
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: The authors have no relevant conflicts of interest to report.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: A waiver of informed consent was granted by the IRB (based upon lack of direct patient contact, minimal risk and inability to contact many subjects due to death or other circumstances).
References
- 1.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, Gernsheimer TB, Ness PM, Brecher ME, Josephson CD, Konkle BA, Woodson RD, Ortel TL, Hillyer CD, Skerrett DL, McCrae KR, Sloan SR, Uhl L, George JN, Aquino VM, Manno CS, McFarland JG, Hess JR, Leissinger C, Granger S. Dose of prophylactic platelet transfusions and prevention of hemorrhage. The New England journal of medicine. 2010;362:600–13. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerber DE, Segal JB, Levy MY, Kane J, Jones RJ, Streiff MB. The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention. Blood. 2008;112:504–10. doi: 10.1182/blood-2007-10-117051. [DOI] [PubMed] [Google Scholar]
- 3.Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M. Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer I. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. The New England journal of medicine. 2003;349:146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 4.Lee AY, Bauersachs R, Janas MS, Jarner MF, Kamphuisen PW, Meyer G, Khorana AA Investigators C. CATCH: a randomised clinical trial comparing long-term tinzaparin versus warfarin for treatment of acute venous thromboembolism in cancer patients. BMC cancer. 2013;13:284. doi: 10.1186/1471-2407-13-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee AY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, Khorana AA Investigators C. Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients With Active Cancer: A Randomized Clinical Trial. JAMA. 2015;314:677–86. doi: 10.1001/jama.2015.9243. [DOI] [PubMed] [Google Scholar]
- 6.Khorana AA, Carrier M, Garcia DA, Lee AY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. Journal of thrombosis and thrombolysis. 2016;41:81–91. doi: 10.1007/s11239-015-1313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streiff MB. The National Comprehensive Cancer Center Network (NCCN) guidelines on the management of venous thromboembolism in cancer patients. Thrombosis Research. 2010;125:S128–S33. doi: 10.1016/S0049-3848(10)70030-X. [DOI] [PubMed] [Google Scholar]
- 8.Carrier M, Khorana AA, Zwicker J, Noble S, Lee AY Subcommittee on H, Malignancy for the SSCotI. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH. J Thromb Haemost. 2013;11:1760–5. doi: 10.1111/jth.12338. [DOI] [PubMed] [Google Scholar]
- 9.Houghton DE, Key NS, Zakai NA, Laux JP, Shea TC, Moll S. Analysis of anticoagulation strategies for venous thromboembolism during severe thrombocytopenia in patients with hematologic malignancies: a retrospective cohort. Leukemia & lymphoma. 2017:1–9. doi: 10.1080/10428194.2017.1306644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuelson BT, Gernsheimer T, Estey E, Garcia DA. Variability in management of hematologic malignancy patients with venous thromboembolism and chemotherapy-induced thrombocytopenia. Thromb Res. 2016;141:104–5. doi: 10.1016/j.thromres.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]