Abstract
Recent studies suggest that dual-frequency intravascular ultrasound (IVUS) transducers allow detection of superharmonic bubble signatures, enabling acoustic angiography for microvascular and molecular imaging. In this paper, a dual-frequency IVUS cylindrical array transducer was developed for real-time superharmonic imaging. A reduced form-factor lateral mode transmitter (2.25 MHz) was used to excite microbubbles effectively at 782 kPa with single-cycle excitation while still maintaining the small size and low profile (5 Fr) (3 Fr = 1 mm) for intravascular imaging applications. Superharmonic microbubble responses generated in simulated microvessels were captured by the high frequency receiver (30 MHz). The axial and lateral full-width half-maximum of microbubbles in a 200-µm-diameter cellulose tube were measured to be 162 µm and 1039 µm, respectively, with a contrast-to-noise ratio (CNR) of 16.6 dB. Compared to our previously reported single-element IVUS transducers, this IVUS array design achieves a higher CNR (16.6 dB vs 11 dB) and improved axial resolution (162 µm vs 616 µm). The results show that this dual-frequency IVUS array transducer with a lateral-mode transmitter can fulfill the native design requirement (~3–5 Fr) for acoustic angiography by generating nonlinear microbubble responses as well as detecting their superharmonic responses in a 5 Fr form factor.
Keywords: Dual-frequency ultrasound, IVUS array, Lateral mode transducer, Superharmonic, Acoustic angiography
1. Introduction
Vulnerable plaques that develop from atherosclerosis are a challenge to identify and treat, yet remain a leading cause of death [1]. Since the vasa vasorum, a network of microvasculature which supports large arteries (e.g. coronary artery) and can infiltrate vulnerable plaques, has been suggested as a biomarker of plaque vulnerability [2], it has been hypothesized that the capability for imaging this microvascular network using ultrasound contrast agents (i.e. microbubbles) and contrast enhanced ultrasound might enable clinicians to distinguish vulnerable atherosclerotic plaques from stable ones [3–5].
Studies have shown that low frequency (~1–5 MHz) acoustic waves excite microbubbles effectively since these low frequency waves are close to the resonance frequency of the microbubble contrast agents. On the other hand, high-frequency reception (15–30 MHz) can be used to collect the broadband superharmonic responses of microbubbles. This broadband response of excited microbubbles had encouraged various approaches for superharmonic imaging to detect microbubbles and separate their response from more bandlimited low frequency tissue background. Kruse et al. demonstrated that wideband microbubble response beyond 15 MHz was present with 2.25 MHz excitation utilizing confocally aligned commercial piston transducers [6]. Gessner et al. reported 3-D contrast imaging with a mechanically scanned 2.5 MHz / 30 MHz transducer [7]. Lindsey et al. showed the balance of CTR and resolution as a function of transmit/receive frequencies and pressure, while transmit at lower frequency (1.5 MHz vs 3.5 MHz) yields a higher CTR (22 dB vs 18 dB) under same pressure (1 MPa) and at same receive frequency (15 MHz) [8]. Wang et al. reported preliminary superharmonic imaging results with an array transmitter (2.25 MHz) and commercial piston receivers (15 MHz, 20 MHz and 25 MHz) [9]. Kim et al. presented phantom evaluation of a 2 MHz / 14 MHz 1–3 composite transducer [10]. Li et al. showed the superharmonic imaging of a 3 MHz / 18 MHz co-linear array [11].
The application of superharmonic imaging with a high frequency receiver (beyond second harmonic), implemented in 3-D is termed acoustic angiography [12, 13]. This approach can yield a high contrast-to-tissue ratio (CTR) (>10 dB) and high resolution (on the order of 100 µm), to provide visualization of contrast flowing in microvessels, i.e., volumetric microvascular images with little or no tissue background, which can resemble x-ray or computed tomography (CT) angiography [14]. However, this approach requires an extremely wideband system to cover a frequency range over an order of magnitude, and is thus is best implemented by dual-frequency transducers [15].
Acoustic angiography transducer technology has been successfully translated into intravascular ultrasound (IVUS) devices, where the catheter size must be limited to allow navigation within peripheral and coronary vessels. Ma et al. reported a single-element dual-frequency rotational intravascular ultrasound transducer (6.5 MHz / 30 MHz) for superharmonic imaging [16]. Wang et al. continued this work to achieve a lower center-frequency transmitter in a single-element, dual-frequency rotational IVUS transducer (2.25 MHz / 30 MHz) using a reduced form factor lateral mode transmitter [17]. Lowering the frequency on transmission increased contrast-to-noise ratio (CNR) (13 dB vs 11 dB), and reduced pulse lengths (0.1 µs vs 0.8 µs) compared to using a non-optimal transmission center frequency. These advances have demonstrated encouraging progress on the ability to perform acoustic angiography from an intravascular form factor, however, rotational dual element devices involve challenges of multi-wire slip rings and other technical difficulties associated with the moving device. Thus, our intent has been to move towards dual-frequency array transducers for superharmonic intravascular imaging.
IVUS array transducers have several desirable features over single-element IVUS transducers. For one, array catheters are stationary, which eliminates non-uniform rotational displacements (NURDs) that can be generated when rotational transducers are revolved slightly off-axis [18–20]. Arrays also use beamforming during transmission and reception—a feature that optimizes image quality, to obtain higher frame rates and decreased point spread functions [21]. Despite their many advantages, compared to the conventional, single-frequency IVUS arrays, dual-frequency superharmonic IVUS arrays present several challenges in both design and fabrication that need to be addressed to determine their feasibility for IVUS. The additional low-frequency transmission element of the dual-frequency superharmonic IVUS arrays conflicts with the catheter size, which must be small (< 6 Fr) (3 Fr = 1 mm) for intravascular ultrasound to be utilized in coronary arteries [22]. Lateral mode transmitter, with a compact size and high output efficiency, is thus an attractive option for developing small-size dual-frequency IVUS arrays for superharmonic imaging [23].
In this work, we report a dual-frequency IVUS array with a lateral mode low frequency transmitter (2.25 MHz) for acoustic angiography. The purpose of this study is to assess the feasibility of this design for real-time superharmonic imaging. In order to excite microbubbles effectively while still maintaining a small size requirement, a lateral mode transmitter with a center frequency of 2.25 MHz was selected. The center frequency of the high-frequency receiver was chosen to be 30 MHz to obtain higher resolution for both B-mode and contrast-specific dual-frequency imaging. This dual-frequency IVUS array was designed, fabricated and characterized, followed by evaluation via real-time superharmonic imaging using the Verasonics system.
2. Methods
2.1 IVUS Array Design and Fabrication
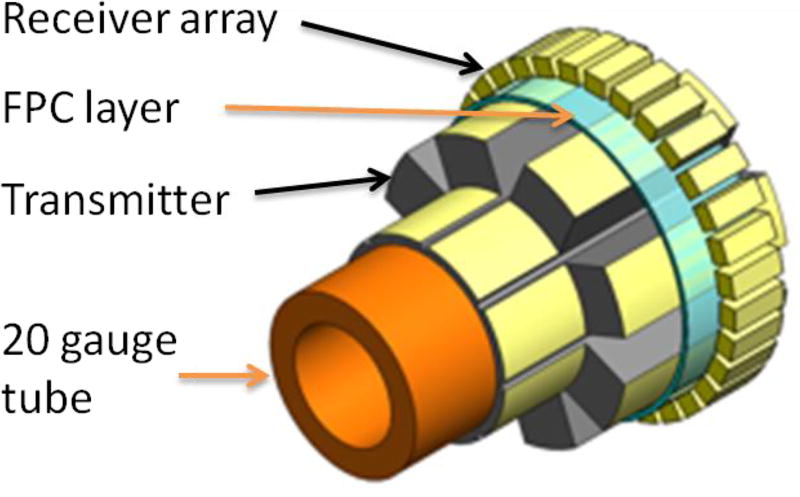
The dual-frequency IVUS array consists of 8 low-frequency lateral mode transmission sub-elements (2.25 MHz) and 32 high-frequency receiving elements (30 MHz), with an acoustic isolation layer built in-between two active layers. The dual-frequency IVUS array was built onto a 20-gauge (0.9-mm-diameter) stainless steel tube with a total outer diameter of 1.7 mm (5 Fr) (Figure 1). The size of this prototyped IVUS array is slightly larger than a single-frequency Volcano Eagle Eye® IVUS array (~3.5 Fr).
Figure 1.
Structure of the dual-frequency IVUS array (not in scale).
Pb(Mg1/3Nb2/3)O3] −x[PbTiO3] (PMN-PT) single crystal was selected as the piezoelectric material for both the low-frequency transmitter and the high-frequency receiver, due to its high coupling coefficients (kt = 0.62, k31 = 0.51), high dielectric constant (εr = 8266) and low transverse frequency coefficient (N31 = 721) [23]. The dimensions of the low-frequency element are 6 mm × 0.35 mm × 0.30 mm in order to achieve a lateral mode transmission at 2.25 MHz (determined by the element width), with a pitch of 650 microns, which is smaller than one wavelength at 2.25 MHz (680 microns). The high-frequency element was designed with dimensions of 1 mm × 0.13 mm × 0.06 mm to obtain a center frequency of 30 MHz (thickness mode). The pitch of high-frequency elements was designed to be 160 microns, which is larger than three wavelengths at 30 MHz (51 microns). This pitch size is a compromise of the desired aspect ratio (> 2) and crystal volume fraction (80 %) based on our fabrication capability. A polyimide (PI) film-based, single-layer flexible printed circuit (FPC) with a thickness of 12.5 µm (Tech-Etch, Plymouth, MA), used for both electrical connectivity and acoustic isolation, was placed between the two piezoelectric layers. The thickness of the isolation layer, the material selection, and the pulse-echo responses of high-frequency elements, were designed and simulated using the Krinholtz, Leedom, and Matthaei (KLM) transmission line model [24]. For the low-frequency lateral mode transmitter, the electric impedance spectrum was simulated using COMSOL (COMSOL, Inc., Burlington, MA). This IVUS array has similar design parameters as previously reported flat arrays, and the detailed method of designing the transducer can be found elsewhere [23,25]. Primary design parameters are listed in Table I.
Table I.
Design parameters of a dual-frequency IVUS array.
| Parameters | Transmission Layer |
Receiving layer |
|---|---|---|
| Center Frequency | 2.25 MHz | 30 MHz |
| Material | PMN-PT | PMN-PT |
| Impedance (MRayl) | 32 | 32 |
| Width (mm) | 0.35 | 0.13 |
| Length (mm) | 6 | 1 |
| Thickness (µm) | 300 | 65 |
| # of element | 8 sub-elements | 32 elements |
| Pitch (µm) | 650 | 160 |
| Width (µm) | 350 | 130 |
| Isolation layer Impedance (MRayl) | - | 3.5 |
| Isolation layer Thickness (µm) | - | 12.5 |
2.2 Array Fabrication
A PMN-PT single crystal chip (1 mm × 5.2 mm × 0.3 mm) and a PMN-PT single crystal sheet (6 mm × 5.2 mm × 0.3 mm) with Cr/Au electrodes on both top and bottom surfaces were prepared first. The PMN-PT single crystal sheet was then diced with a pitch of 650 µm and a kerf of 300 µm to form the 8 low-frequency elements.
The high-frequency elements were first sub-diced to a depth of 100 µm from one side, with a 160 µm pitch and a 30 µm kerf. After sub-dicing, the chip was aligned with the customized flexible printed circuit and bonded with Epo-tek 301 (Epoxy Technology Inc, Billerica, MA). Extra Epo-tek 301 was poured around the chip to cover the area of 3 mm × 5 mm to be the matching layer of the low-frequency transmitter. The integrated two layers were lapped to 65 µm after curing. Then, the top electrode (100Å Ti, 1000Å Au) of the high-frequency element was deposited using an electron beam evaporation process.
The high-frequency array was then bonded onto the low-frequency transmitter with Epo-tek 301. The dual-frequency IVUS array was wrapped onto a 20-gauge tube (Figure 2) by applying additional sub-dices into the high-frequency elements. Common grounds of both the high-frequency receiver and low-frequency transmitter were connected with coaxial cables (#40232, Hitachi cable, Manchester, NH) and silver epoxy (8331-14G, M.G. Chemicals, Surrey, B.C., Canada).
Figure 2.
Photograph image of the dual-frequency IVUS array.
The other end of the FPC was bonded to a 50-pin, 0.5 mm pitch printed circuit board (PCB). Three coaxial cables (MCX 40232, Hitachi Cable America Inc., Purchase, NY) were used to connect the electrodes to the PCB. A Parylene C layer with a thickness of 20 µm was coated using the SCS Labcoter®2 vacuum deposition system (PDS 2010, Specialty Coating Systems, Inc., Indianapolis, IN) to serve as both the passivation and matching layer for the high-frequency elements. Finally, the PCB was connected to the Verasonics (Verasonics, Redmond, VA) system through a 2-meter multi-core coaxial cable.
2.3 IVUS Array Characterization
The IVUS array was characterized by measuring the complex electrical impedances of both high-frequency and low-frequency elements, any pulse-echo responses or crosstalk from the high-frequency elements, and the acoustic pressure of the low-frequency transmitter.
The electrical impedance and phase were measured with an impedance analyzer (Agilent 4294A, Agilent Technologies Inc, Santa Clara, CA). The center frequency and fractional bandwidth of the high-frequency elements were derived from the pulse-echo measurement, which was obtained in a water tank using a pulser/receiver (5900 PR, Olympus Corp, Waltham, MA) and an oscilloscope (Agilent DSO7014B, Agilent Technologies Inc, Santa Clara, CA). During the pulse-echo test, the energy of the applied pulse was set to 1 µJ. An aluminum bar (50 mm × 30 mm × 5 mm) was placed in front of the high-frequency element as the reflection target at a distance of 2 mm away from the aperture. The crosstalk was measured by exciting each high-frequency element with a 2-cycle sinusoidal burst generated by an arbitrary function generator (AFG3101, Tektronix Inc., Beaverton, OR) at the element’s resonance with an amplitude of 10 Vpp. The response of adjacent elements was measured and compared to the excitation voltage.
The acoustic pressure measurement of the low-frequency transmitter was conducted with a hydrophone (HNA-0400, ONDA Co, Sunnyvale, CA). A 1-cycle sinusoidal burst at 2.7 MHz was generated by an arbitrary function generator (AFG3101, Tektronix Inc., Beaverton, OR) and amplified to 70 V with a radio-frequency amplifier (Model 3200L, Electronic Navigation Industries Inc., Rochester, NY). Pressure output of the low-frequency transducer at 8 mm from the aperture was recorded using an in-house LabVIEW (National Instruments Co., Austin, TX) data acquisition system.
2.4 Real-time Superharmonic Imaging of IVUS Array
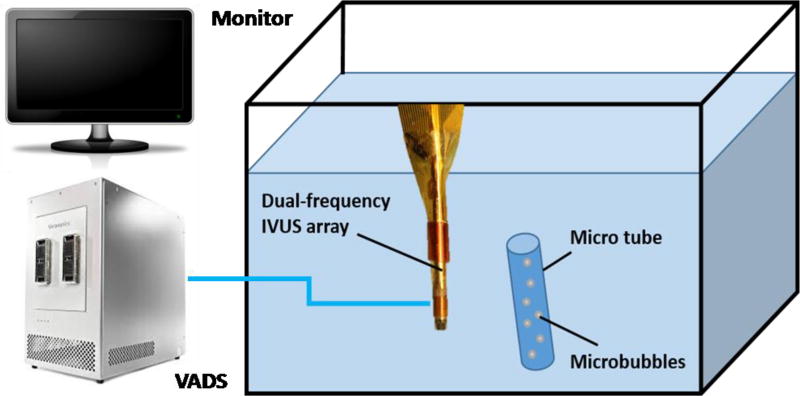
The real-time superharmonic imaging of the dual-frequency IVUS array was evaluated in a water tank (filled with room temperature degassed water) using a Verasonics system (Verasonics Vantage, Redmond, WA) to drive the transducer (Figure 3). Contrast tests were conducted using in-house poly-dispersed (1–5 µm diameter) lipid-coated microbubbles [26] at diluted concentrations (1 × 108 MBs/mL). Microbubbles were pumped through a cellulose micro-tube with a 200 µm diameter made of acoustically transparent material. The flow rate was set to be 10 mL/hr (8.8 cm/s).
Figure 3.
Experimental setup of the contrast test (not in scale).
The Verasonics sampling frequency (quadruple of the base frequency) was set to be 62.5 MHz (15.6 MHz as base frequency). The superharmonic images were rendered using a proprietary Verasonics curved linear beamforming algorithm and a built-in bandpass filter (19–30 MHz). Because of the convex shape and large pitch of the high-frequency elements, this IVUS array would be susceptible to grating lobes. To avoid this, the maximum number of receiver elements in each acquisition was set to 5 during superharmonic imaging with this dual-frequency IVUS array. This reduced receiving aperture size (0.8 mm) established a smaller signal acquisition field and a more limited steering angle, which suppressed the grating lobe artifact. On the other hand, reducing the size of the receiving aperture also decreased the lateral resolution as well. A constant f-number on the receiver was set to be 1.26.
The Verasonics curved linear beamforming algorithm supports forming an image with up to 180-degrees for real-time imaging. The 360-degree scan was obtained in offline processing by reconstructing two 180-degree data sets, with each of them averaged at 8 acquisitions. The dynamic range was set to −17 dB. The axial and lateral superharmonic image resolutions were calculated quantitatively from the axial and lateral pulse profiles of the microbubble responses recorded from the 200 µm tube. The CNR of the array transducer was measured as the ratio between the maximum of microbubble responses and the mean of the noise in a focused area of 3 mm × 2 mm.
| (1) |
A 1-cycle burst plane wave generated from the low-frequency transmitter excited the microbubble contrast agents in the micro-tube (5 mm away from the transducer). Then, higher-order, non-linear microbubble responses were collected with the high-frequency elements. The superharmonic backscatter from the microbubbles was digitized and reconstructed with a dynamic receive focusing of filtered signals. Due to the output limitation of the Verasonics system at the low frequency (maximum 55V at 2.25 MHz), the excitation frequency increased slightly to 2.7 MHz under the excitation voltage of 70 V and achieved 782 kPa peak negative pressure.
3. Experimental RESULTS
3.1 IVUS Array Characterization
The resonance frequencies of both the high-frequency receiving elements and the low-frequency transmitter were characterized first. The measured electrical impedance spectrums of the high and low-frequency elements are shown in Figures 4a and Figure 4b, respectively. The resonance of high frequency elements is around 30 MHz, and that of the low-frequency transmitter is around 2.5 MHz. Both measurements correspond with our design.
Figure 4.
Measured electric impedance (blue) and phase (red). a) High frequency element. b) Low frequency element.
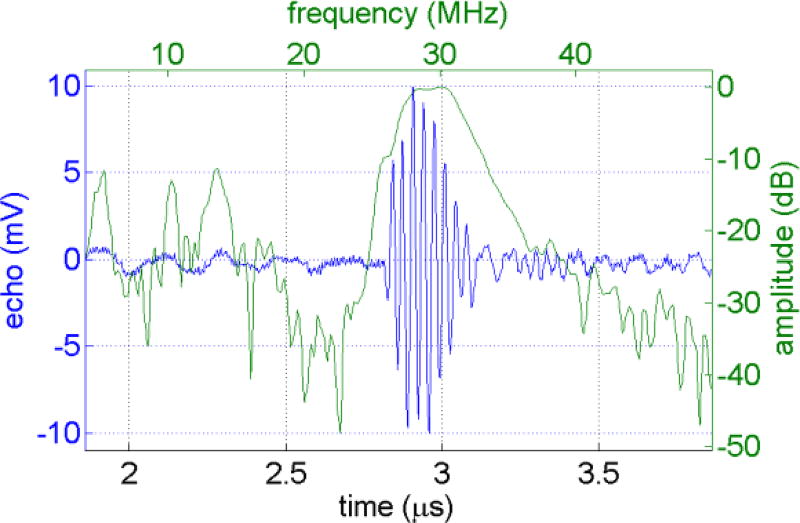
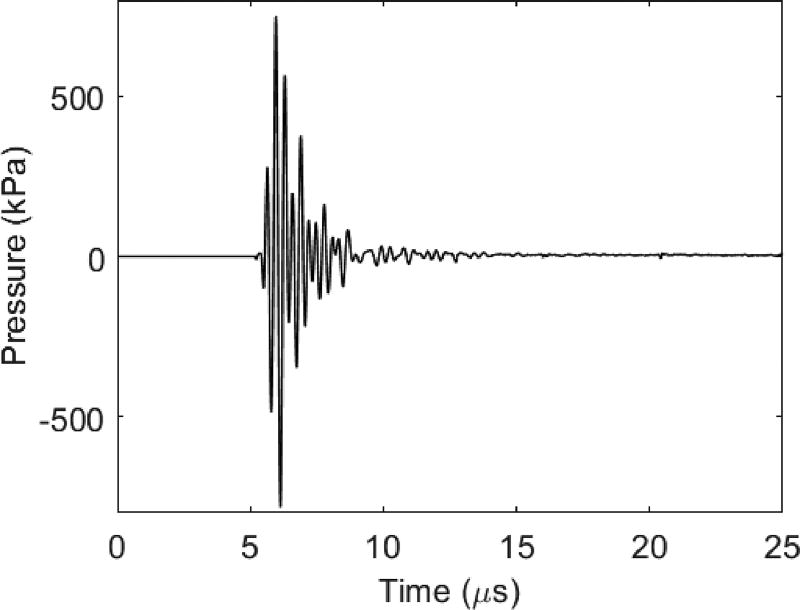
Both the pulse-echo response of receiving elements and the acoustic pressure of the transmitter were tested to evaluate the performance of the dual-frequency IVUS array. The pulse-echo result of a typical high-frequency element show a center frequency of 29.6 MHz with a bandwidth of 18% (Figure 5). The crosstalk of adjacent high-frequency elements is −29.7 dB. The peak negative pressure of the low-frequency transmitter, placed at 8 mm axially from the transducer surface, under 1-cycle of a 70 V excitation at 2.7 MHz, is 782 kPa (Figure 6). This is high enough to excite the microbubbles for superharmonic imaging purposes.
Figure 5.
Pulse-echo response of a typical high frequency element.
Figure 6.
Acoustic pressure of the low frequency transmitter under 1-cycle 70 V excitation at 2.7 MHz.
3.2 Superharmonic Imaging
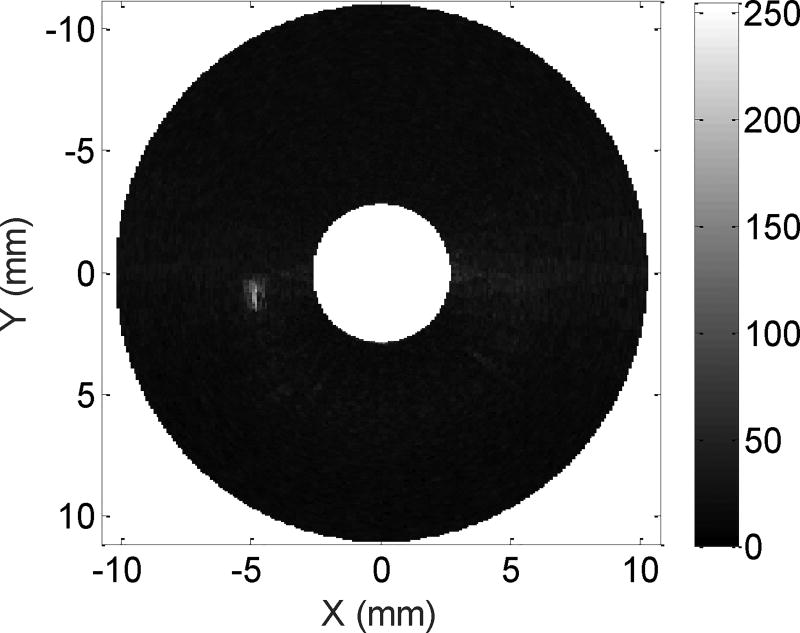
By reconstructing two 180-degree data sets offline, the superharmonic images of a 200 µm micro-tube with the dual-frequency IVUS array were obtained (Figure 7). The axial and lateral estimates of the image resolution were calculated to be 162 µm and 1039 µm, respectively, by measuring the full-width half-maximum (FWHM) of the microbubble responses (Figure 8). As expected, the lateral resolution of this IVUS array transducer is poor (1039 µm vs 200 µm) compared to the previously reported flat dual-frequency array [23] due to a much smaller aperture size (0.8 mm vs 5 mm). The CNR of the superharmonic image is 16.6 dB.
Figure 7.
Superharmonic imaging of IVUS array.
Figure 8.
Microbubble responses of the micro-tube. a) Axial; b) Lateral.
Compared to our initial 6.5 MHz / 30 MHz single-element IVUS transducer design [16], this dual-frequency IVUS array transducer with a lateral mode transmitter (2.25 MHz / 30 MHz) has better CNR (16.6 dB vs 11 dB) and higher axial resolution (162 µm vs 616 µm). It also requires a lower input voltage (70 V vs 98 V) and a shorter transmission pulse (1-cycle vs 5-cycle). This result also shows a higher CNR (16.6 dB vs 13 dB) compared to that of the single-element lateral mode dual-frequency IVUS reported in [17], by taking advantage of the dynamic focusing of the array receiver and higher peak negative pressure (782 kPa vs 575 kPa) from the array transmitter.
4. Discussion
It must be noted that the calculated FWHM was not the observed resolution of the IVUS array. The FWHM is based on microbubble responses in a micro-tube, which has a significant size difference compared to the wavelength of the receiver (200 µm vs 51µm). The limitations of the Verasonics system in both range of frequency (< 31.25 MHz) and scan angle (< 180 degree) limited the performance of the imaging system.
Additionally, due to the relatively large pitch size of the high-frequency elements (160 µm vs 51µm), the maximum number of elements in each acquisition was set to 5 to eliminate the grating lobe artifact, which decreased the lateral resolution greatly (1039 µm). The pitch size of the high-frequency elements cannot be optimized with the current thickness mode receiver design, which requires an aspect ratio greater than 2.
Moreover, based on our fabrication capability, a coated Parylene layer was used as the matching layer of the high-frequency elements. The acoustic impedance of Parylene (2.6 MRayl) made it less than an ideal matching material for the PMN-PT single crystal (32 MRayl), and thus the performance of high-frequency receiver in both bandwidth and sensitivity was impacted. The performance could be improved with a better matching material (~7 MRayl), or a dual matching layer design, or an alternate piezoelectric material with lower acoustic impedance (e.g. 1–3 composite material) (~18 MRayl).
In future work, the pitch size of the high-frequency elements (30 MHz) may be improved (< 50 µm) by implementing a finer flexible circuit and a high-frequency 1–3 composite material working in 3-3 mode. The 1–3 composite material also have favorable merits including high electromechanical coupling coefficients (~0.9) for broad bandwidth (> 70 %) and low acoustic impedance (~18 MRayl) for better acoustic matching, which would result in a better imaging quality. Furthermore, a better imaging platform with a broader bandwidth (up to 60 MHz) and an advanced beamforming algorithm should be developed to achieve better real-time 360-degree superharmonic imaging for IVUS application.
5. Conclusion
In this paper, the design, fabrication, characterization and phantom-imaging of a dual-frequency PMN-PT IVUS array (2.25 MHz / 30 MHz) with a lateral mode transmitter were presented and followed by superharmonic imaging. This IVUS array transducer is small enough (5 Fr) to use in IVUS catheter sizes that are typically used for coronary interventions (~3–5 Fr). Dual-frequency array transducers are challenging to fabricate in form factors applicable for intravascular imaging and thus studies for their unique applications are limited. To our knowledge, this is the first 360-degree dual-frequency superharmonic IVUS array reported.
Under a 70 V 1-cycle burst excitation, superharmonic imaging with the prototype dual-frequency IVUS array was obtained, with offline reconstruction, using the Verasonics system. The axial and lateral FWHM of the micro-tube were measured to be 162 µm and 1039 µm, respectively. The CNR was calculated as 16.6 dB. This IVUS array transducer shows improved imaging metrics results compared to our initial single-element IVUS transducer design (CNR: 16.6 dB vs 11 dB, axial resolution: 162 µm vs 616 µm). These results suggest promising results for the future expansion of this technology for use outside of the lab.
=A dual-frequency IVUS array transducer is developed for acoustic angiography.
The small size is obtained with a reduced form-factor lateral mode transmitter.
The superharmonic imaging is rendered using the Verasonics system.
High CNR and good spatial resolution are achieved with 1-cycle burst excitation.
Acknowledgments
This work was supported by the National Institutes of Health [R01EB015508] and the Department of Defense [W81XWH-12-1-0303]. The authors would sincerely thank the editor and the reviewers for their valuable comments that improved this paper substantially.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108(14):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005;25(10):2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 3.Maresca D, Skachkov I, Renaud G, Jansen K, van Soest G, de Jong N, van der Steen AFW. Imaging microvasculature with contrast-enhanced ultraharmonic ultrasound. Ultrasound in Med. & Biol. 2014;40(6):1318–1328. doi: 10.1016/j.ultrasmedbio.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Staub D, Schinkel AFL, Coll B, Coli S, van der Steen AFW, Reed JD, Krueger C, Thomenius KE, Adam D, Sijbrands EJ, Folkert J. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging. 2010;3(7):761–771. doi: 10.1016/j.jcmg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Staub D, Patel MB, Tibrewala A, Ludden D, Johnson M, Espinosa P, Coll B, Jaeger KA, Feinstein SB. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke. 2010;41(1):41–47. doi: 10.1161/STROKEAHA.109.560342. [DOI] [PubMed] [Google Scholar]
- 6.Kruse DE, Ferrara KW. A new imaging strategy using wideband transient response of ultrasound contrast agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2005;52(8):1320–1329. doi: 10.1109/tuffc.2005.1509790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gessner RC, Lukacs M, Lee M, cherin E, Foster FS, Dayton PA. High-resolution, high-contrast ultrasound imaging using a prototype dual-frequency transducer: In vitro and in vivo studies. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010;57(8):1772–1781. doi: 10.1109/TUFFC.2010.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindsey BD, Rojas JD, Martin KH, Shelton SE, Dayton PA. Acoustic characterization of contrast-to-tissue ratio and axial resolution for dual-frequency contrast-specific acoustic angiography imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2014;61(10):1668–1687. doi: 10.1109/TUFFC.2014.006466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Ma J, Jiang X, Martin KH, Dayton PA. An array transmitter for dual-frequency contrast enhanced intravascular ultrasound imaging. IEEE Int. Ultrason. Symp. 2014:2104–2107. [Google Scholar]
- 10.Kim J, Li S, Kasoji S, Dayton PA, Jiang X. Phantom evaluation of stacked-type dual-frequency 1–3 composite transducers: A feasibility study on intracavitary acoustic angiography. Ultrasonics. 2015;63:7–15. doi: 10.1016/j.ultras.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Kim J, Wang Z, Jiang X, Kasoji S, Lindsey B, Dayton PA. A 3 MHz/18 MHz dual-layer co-linear array for transrectal acoustic angiography. IEEE Int. Ultrason. Symp. 2015 [Google Scholar]
- 12.Gessner RC, Aylward SR, Dayton PA. Mapping microvasculature with acoustic angiography yields quantifiable differences between healthy and tumor-bearing tissue volumes in a rodent model. Radiology. 2012;264(3):733–740. doi: 10.1148/radiol.12112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin KH, Dayton PA. Current status and prospects for microbubbles in ultrasound theranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013;5(4):329–345. doi: 10.1002/wnan.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gessner RC, Frederick CB, Foster FS, Dayton PA. Acoustic angiography: A new imaging modality for assessing microvasculature architecture. Int. J. Biomed. Imaging. 2013;2013 doi: 10.1155/2013/936593. art. no. 936593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin KH, Lindsey BD, Ma J, Lee M, Li S, Foster FS, Jiang X, Dayton PA. Dual-frequency piezoelectric transducers for contrast enhanced ultrasound imaging. Sensors. 2014;14(11):20825–20842. doi: 10.3390/s141120825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Martin KH, Dayton PA, Jiang X. A preliminary engineering design of intravascular dual-frequency transducers for contrast-enhanced acoustic angiography and molecular imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2014;61(5):870–880. doi: 10.1109/TUFFC.2014.6805699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Martin KH, Dayton PA, Jiang X. A dual frequency IVUS transducer with a lateral mode transmitter for contrast enhanced intravascular ultrasound imaging. ASME Int. Mech. Eng. Congress. 2015:V003T03A019–V003T03A019. [Google Scholar]
- 18.Ciompi F, Gatta C, Pujol O, R-Leor O, Ferre JM, Radeva P. Recent advances in biomedical signal processing. Bentham Science Publishers; 2011. Reconstruction and Analysis of Intravascular Ultrasound Sequences; pp. 231–250. [Google Scholar]
- 19.Neglén P, Stenting V. Noninvasive vascular diagnosis: a practical guide to therapy. 2. Springer; 2007. Using Intravascular Ultrasound; pp. 406–413. [Google Scholar]
- 20.Back MR, Kopchok GE, White RA. Peripheral Endovascular Interventions. 2. Springer; New York: 1999. Intravascular Ultrasound Imaging; pp. 195–216. [Google Scholar]
- 21.Macovski A. Ultrasonic imaging using arrays. Proc. IEEE. 1979;67(4):484–495. [Google Scholar]
- 22.Szabo TL, Lewin PA. Ultrasound transducer selection in clinical imaging practice. Journal of Ultrasound in Medicine. 2013;32(4):573–582. doi: 10.7863/jum.2013.32.4.573. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Martin KH, Huang W, Dayton PA, Jiang Xiaoning. Contrast Enhanced Superharmonic Imaging for Acoustic Angiography Using Reduced Form-factor Lateral Mode Transmitters for Intravascular and Intracavity Applications. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016;64(2):311–319. doi: 10.1109/TUFFC.2016.2619687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krimholtz R, Leedom DA, Mattaei GL. New equivalent circuits for elementary piezoelectric transducers. Electronics Letters. 1970;41:398–399. [Google Scholar]
- 25.Wang Z, Huang W, Jiang X, Martin KH, Dayton PA. Dual-frequency IVUS array for contrast enhanced intravascular ultrasound imaging. IEEE Int. Ultrason. Symp. 2015:1–4. [Google Scholar]
- 26.Borden MA, Sarantos MR, Stieger SM, Simon SI, Ferrara KW, Dayton PA. Ultrasound radiation force modulates ligand availability on targeted contrast agents. Mol. Imaging. 2006;5:139–147. [PMC free article] [PubMed] [Google Scholar]