Abstract
BACKGROUND
HIV-1 infection is associated with intestinal inflammation, changes in the enteric microbiota (dysbiosis) and intestinal epithelial cell (IEC) damage. NKp44+ innate lymphoid cells (ILCs) play an important role in epithelial barrier maintenance via the production of IL-22, but also display functional plasticity and can produce inflammatory cytokines (e.g. IFNγ) in response to cytokine milieu and stimulatory signals. The objective of this pilot study was to enumerate frequencies of IL-22 and IFNγ-expressing colonic NKp44+ ILCs during untreated, chronic HIV-1 infection.
SETTING
A cross-sectional study was performed to compare numbers of cytokine-expressing ILCs in colonic biopsies of untreated, chronic HIV-1 infected (n=22) and uninfected (n=10) study participants. Associations between cytokine+ ILC and previously established measures of virological, immunological and microbiome indices were analyzed.
METHODS
Multi-color flow cytometry was used to measure the absolute number of colonic CD3−NKp44±CD56± ILCs expressing IL-22 or IFNγ following in vitro mitogenic stimulation.
RESULTS
Numbers of colonic NKp44+ ILCs that expressed IFNγ were significantly higher in HIV-1 infected versus uninfected persons and positively correlated with relative abundances of dysbiotic bacterial species in the Xanthomoadaceae and Prevotellaceae bacterial families and with colonic mDC and T cell activation.
CONCLUSION
Higher numbers of inflammatory colonic ILCs during untreated chronic HIV-1 infection that associated with dysbiosis and colonic mDC and T cell activation suggest that inflammatory ILCs may contribute to gut mucosal inflammation and epithelial barrier breakdown, important features of HIV-1 mucosal pathogenesis.
Keywords: HIV-1, innate lymphoid cells, dysbiosis, microbial translocation, inflammation
INTRODUCTION
Disruption of intestinal homeostasis, intestinal epithelial cell (IEC) damage and changes in the microbiota (dysbiosis)1–5 are associated with HIV-1 infection. IEC damage leads to translocation of dysbiotic microbes6 and microbial translocation is linked to tissue and systemic immune activation and predicts disease progression in untreated HIV-1 infected persons7–10. Understanding the mechanisms that drive IEC damage and microbial translocation will be critical to controlling both gut and systemic inflammation during HIV-1 infection.
Maintenance of intestinal barrier integrity and homeostasis relies in part on the presence of IL-2211,12. Depletion of gut T helper 22 (Th22) and Th17 cells, subsets of which co-produce IL-22, is considered to be a major contributor to HIV-1 pathogenesis1,13–15. A pivotal publication by Cella et.al., described non-T cells capable of producing IL-22 in human mucosa-associated lymphoid tissues16. These non-T cells identified by the expression of an NK cell activation receptor NKp4417and the classic NK cell marker CD56, were termed NK22 cells. NK22 cells are now considered members of the recently identified family of Group 3 innate lymphoid cells (ILC3s), which play important and diverse roles in mucosal immunity18–20. In untreated HIV-infected individuals, frequencies of colonic IL-22-expressing non-T-cells that included NKp44+ cells were increased early in infection13. Further, frequencies of NKp44+ IL-22-producing cells positively correlated with an intact intestinal barrier in long-term ART-treated HIV infected persons21. Thus, NKp44+ ILCs may also play a critical role in regulating epithelial barrier health during HIV infection.
Multiple studies have suggested that ILCs, including ILC3s, display a degree of functional plasticity and can produce inflammatory cytokines (e.g. TNFα, IFNγ) in response to the local cytokine milieu (e.g. IL-2, IL-23, IL-12p70, IL-1β, IL-18) and/or exposure to stimulatory signals (e.g. ligation of NKp44)22–25. We have previously demonstrated that colonic myeloid dendritic cells (mDCs) produce IL-23 and IL-1β in response to exogenous exposure to mucosa-associated colonic commensal bacteria that are increased in relative abundance in untreated HIV-1 infected persons26. Moreover, HIV-1 induces the expression of NKp44L on CD4 T cells both in vitro and in vivo27–29. These observations raise the possibility that a microenvironment exists in the gut of HIV-1-infected persons that is conducive to IL-22-producing ILCs switching into inflammatory ILCs. Indeed, colonic NKp44+ ILCs demonstrated an altered phenotype by producing IFNγ rather than IL-17/IL-22 during pathogenic SIV infection30,31. Therefore, we evaluated the frequencies of IL-22-, IL-17- and IFNγ-producing colonic NKp44-expressing ILCs in the setting of untreated chronic HIV-1 infection.
METHODS
Study participants
Untreated, chronically HIV-1-infected adults and HIV-1-seronegative (uninfected) controls were enrolled in this cross-sectional study at the University of Colorado Anschutz Medical Campus. Inclusion and exclusion criteria are extensively detailed in previous publications26,32,33. All study participants voluntarily gave written, informed consent. This study was approved by the Colorado Multiple Institutional Review Board (COMIRB).
Surface and intracellular flow cytometry staining assays, acquisition and analysis
The collection, processing and storage of colon biopsies are detailed elsewhere26,32,33. Colonic cells were cultured in RPMI (Invitrogen) + 10% human AB serum (Gemini Bioproducts) + 1% penicillin/streptomycin/L-glutamine (Invitrogen) and stimulated with PMA (250ng/ml; Sigma-Aldrich) and ionomycin (1μg/ml; Sigma-Aldrich) and 0.1% Brefeldin A (Golgi Plug, BD Biosciences) for 16hrs. Cells were collected and frequencies of NKp44+CD56−, NKp44+CD56+ and NKp44−CD56+ cells and cytokine-expressing NKp44+CD56−, NKp44+CD56+ and NKp44− CD56+ cells were determined using standard multi-color intracellular cytokine flow cytometry protocols26,32–36. Cells were stained with viability dye (Aqua, Invitrogen), CD45 (clone: 2D1; PerCp-Cy5.5, eBioscience), CD3 (UCHT1; PE Texas Red, Beckman Coulter), NKp44 (P44-8; APC, Biolegend), CD56 (B159; PE-Cy5, BD Biosciences), IL-22 (22URTI; PE, eBioscience), IL-17 (N49-653; V450, BD Biosciences) and IFNγ (B27; AF700, BD Biosciences). All flow cytometry data was acquired on an LSRII Flow Cytometer (BD Biosciences) and analyzed using BD FACSDiva software version 6.1.2 (BD Biosciences)36 (Figure S1 Supplemental Digital Content). Evaluation of cytokine-expressing ILCs was only performed when there were at least 25 NKp44+CD56−, NKp44+CD56+ and NKp44−CD56+ events.
Enumeration of immunological, virological, and microbial parameters
Measurements of plasma IL-6, C-reactive protein, TNFα, IFNγ, IL-10, soluble CD14, lipoteichoic acid, lipopolysaccharide and intestinal fatty acid binding protein, colonic mucosa levels of HIV-1 RNA, frequencies of colonic mDC, plasmacytoid DC (pDC), CD4 and CD8 T cells, measurements of mDC activation/maturation (CD40/CD83) and T cell activation (CD38+HLA-DR+) and frequencies of IFNγ, IL-22 and IL-17-expressing Th cells have been previously published26,32,33. Laboratory and analytic methods used to profile the intestinal microbiomes were as described26,32,33,35.
Statistical analysis
Non-parametric statistics were performed with no adjustments for multiple comparisons due to the exploratory nature of this study. Analyses were performed using GraphPad Prism Version 6 for Windows (GraphPad Software, San Diego, CA). A p-value <0.05 was considered significant.
RESULTS
Absolute numbers of colonic ILCs and cytokine-expressing colonic ILCs were determined in a subset of untreated HIV-1 infected (N=22) and uninfected (N=10) study participants from a previously detailed clinical study26,32,33. Study participant characteristics are detailed Table S1, Supplemental Digital Content.
Absolute numbers of colonic ILCs
There were no significant differences in the absolute number of colonic NKp44+CD56− and NKp44+CD56+ ILCs between uninfected and HIV-1-infected subjects (Figure S2 Supplemental Digital Content). Conversely, HIV-1 infected individuals had significantly fewer NKp44−CD56+ cells compared to uninfected controls.
Cytokine profiles of colonic ILCs in healthy individuals
We first compared absolute numbers of IL-22, IL-17, and IFNγ-expressing CD3−ILCs in healthy uninfected persons (Figure 1A). Greater numbers of NKp44+CD56− ILCs expressed IL-22 compared with IFNγ or IL-17, although this latter comparison did not reach statistical significance. Similar numbers of NKp44+CD56+ expressed IL-22 or IFNγ, whereas fewer were capable of producing IL-17. Very few NKp44+CD56+ ILCs co-expressed IL-22 and IFNγ (data not shown), suggesting distinct cytokine-producing populations. The number of NKp44−CD56+ cells that expressed IFNγ was significantly greater than IL-22+ or IL-17+ expressing cells.
Figure 1.
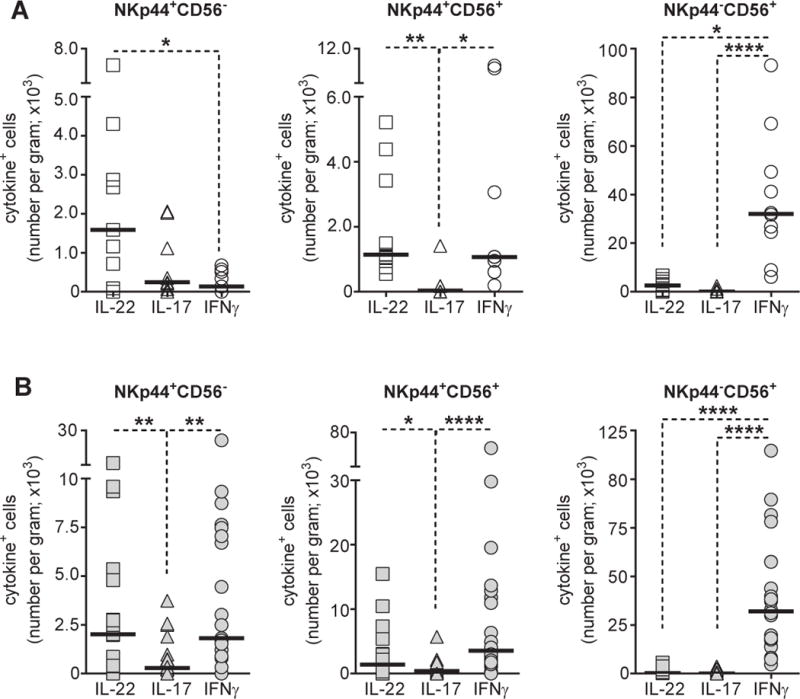
Cytokine profiles of colonic ILC subsets. A, Absolute numbers of cytokine+ NKp44+CD56− (N=9), NKp44+CD56+ (N=9) and NKp44−CD56+ (N=10) colonic ILCs in uninfected study participants. B, Absolute numbers of cytokine+ NKp44+CD56− (N=21), NKp44+CD56+ (N=22) and NKp44−CD56+ (N=22) colonic ILCs in HIV-1 infected study participants. Lines indicate the median value. Statistical analysis was performed using Dunn’s multiple comparisons test. *P<0.05; **P<0.01; ****P<0.0001.
Altered ILC cytokine profiles in HIV-infection
In HIV-1 infected individuals, comparable numbers of NKp44+CD56− ILCs expressed IL-22 or IFNγ (Figure 1B) with few NKp44+CD56− ILCs expressing both IL-22 and IFNγ (data not shown). Numbers of IL-22+ and IFNγ+ NKp44+CD56− ILCs were significantly greater than IL-17- expressing NKp44+CD56− ILCs. Cytokine profiles of the remaining ILC populations in HIV-1 infected persons were generally reflective of those observed in uninfected persons, with similar numbers NKp44+CD56+ ILCs producing either IL-22 or IFNγ and with NKp44−CD56+ ILCs primarily producing IFNγ (Figure 1B).
We next compared the absolute number of cytokine-producing ILCs between uninfected and HIV-1 infected persons. IFNγ-producing NKp44+CD56− and NKp44+CD56+ ILCs were significantly increased in number in HIV-1 infected relative to uninfected persons (Figures 2A, 2C). Absolute numbers of IFNγ+NKp44−CD56+ ILCs were not statistically different between the two cohorts (data not shown). Numbers of IL-22-expressing NKp44+CD56− or NKp44+CD56+ ILC populations were not significantly different between HIV-1 infected and uninfected study participants (Figure 2E). Although numbers of IL-22-expressing NKp44−CD56+ cells were low compared to IFNg+NKp44−CD56+ cells, significantly fewer NKp44−CD56+ cells expressing IL-22 were observed in HIV-1 infected persons (Figure 2E). No statistical differences between uninfected and HIV-1 infected individuals in the absolute numbers of IL-17-expressing ILC populations were noted (data not shown).
Figure 2.
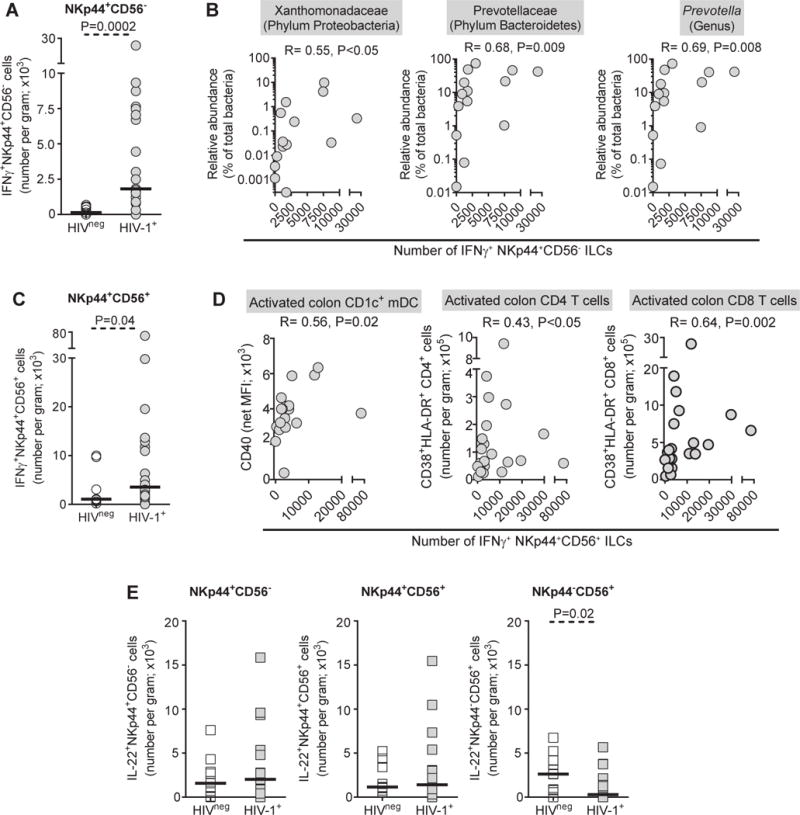
Absolute numbers of IFNγ-expressing NKp44+ ILCs are higher in HIV-1 infection and associate with microbial dysbiosis and colonic immune activation. A, Absolute numbers of IFNγ- expressing NKp44+CD56− ILCs in uninfected (HIVneg; N=9) and HIV-1-infected (HIV-1+; N=21) study participants expressed as the total number of IFNγ+ NKp44+CD56− ILC per gram of tissue. B, Correlations between the number per gram of IFNγ-expressing NKp44+CD56− ILCs and relative abundances of colonic mucosa-associated microbiota in HIV-1 infected study participants (N=14). C, Absolute number of IFNγ-expressing NKp44+CD56+ ILCs in HIVneg (N=9) and HIV-1+ (N=22) study participants expressed as the total number of IFNγ+ NKp44+CD56− ILC per gram of tissue. D, Correlations between the absolute number of IFNγ-expressing NKp44+CD56+ ILCs and CD40 expression levels on colonic CD1c+ mDC (N=18) and numbers of colonic CD38+HLA-DR+ CD4 and CD8 T cells (N=22). E, Absolute numbers of IL-22-expressing NKp44+CD56−, NKp44+CD56+, NKp44−CD56+ ILCs measured in uninfected (HIVneg; NKp44+CD56− N=9; NKp44+CD56+ N=9; NKp44−CD56+ N=10) and HIV-1-infected (HIV-1+; NKp44+CD56− N=21; NKp44+CD56+ N=22; NKp44−CD56+ N=22) study participants expressed as the total number of IL-22+ ILC subsets per gram of tissue. Lines represent median values. Statistical analysis to compare between independent groups were made using the Mann–Whitney test and correlations between variables were assessed using the Spearman test.
Associations of IFNγ-expressing colonic ILCs with dysbiotic microbes and markers of immune activation in HIV-1 infection
Given the finding of increased IFNγ+ ILCs in HIV-infected persons, we next addressed associations of these ILC populations with previously reported measures of clinical, virological, immunological and microbiome indices for the HIV-1 infected cohort26,32,33 (Table S2 Supplemental Digital Content). Absolute numbers of colonic IFNγ+NKp44+CD56− ILCs significantly correlated with the relative abundance of Xanthomonadaceae and Prevotellaceae families, with the Prevotella genus (Figure 2B), and with the individual species P. copri and P. stercorea (R=0.68, P=0.01; R=0.63, P=0.02 respectively; data not shown). Numbers of IFNγ+NKp44+CD56− ILCs inversely correlated with the percentages of CD83+CD1c+ mDC (R=− 0.51, P=0.03) and positively correlated with numbers of IFNγ-producing CD4 T cells (R=0.53, P=0.01) (data not shown). IFNγ+ NKp44+CD56+ ILC numbers were not significantly associated with dysbiotic microbes but instead positively correlated with colon CD1c+ mDC activation levels and with absolute numbers of activated colon CD4 and CD8 T cells in HIV-1 infected individuals (Figure 2D).
DISCUSSION
This study highlights that untreated, chronic HIV-1 infection is associated with higher numbers of colonic NKp44+ ILCs that express IFNγ. The difference in the number of these cells expressing IFNγ is unlikely related to an increase in the number of NKp44+ ILCs given that no significant difference in the overall number of each NKp44+ ILC subsets was observed between the two cohorts. IFNγ is an inflammatory cytokine that increases intestinal epithelial barrier permeability primarily via alterations in tight junction protein expression and thereby enhances bacterial transcytosis37–39. Accordingly, the presence of these inflammatory ILCs likely contributes to epithelial barrier breakdown and the resultant microbial translocation.
We, and others, have demonstrated that alterations in intestinal mucosa-associated bacterial communities during HIV-1 infection are associated with indicators of mucosal HIV-1 pathogenesis (reviewed in40). In our study, major findings from the microbiome analysis included higher relative abundance of mucosa-associated bacteria belonging to the Proteobacteria phylum (including Xanthomonadaceae) and of Prevotella spp. in HIV-1 infected persons26,33. Increased relative abundance of Prevotella spp. in HIV-1 infected persons associated with increased colonic mDC and T cell activation26,33. Other HIV-associated mucosal abnormalities included decreased percentages of colonic mDC expressing CD8326, a molecule reported to play a role in intestinal immune regulation41. In this current study, numbers of IFNγ-expressing NKp44+ ILCs positively correlated with relative abundances of bacterial species in the Xanthomoadaceae and Prevotellaceae families, with colonic mDC and T cell activation and inversely associated with the fraction of colonic mDCs expressing CD83. We have previously shown that enteric bacterial species found in high abundance in HIV-1 infected persons (e.g. Prevotella), are capable of inducing IL-23 and IL-1β from colonic mDC in vitro26, cytokines that contribute to the induction of inflammatory cytokines such as IFNγ and TNFα by ILCs25,42. Thus, we hypothesize that in the setting of HIV-1 infection, the shift in phenotype of primarily IL-22-producing ILCs to IFNγ- producing ILCs is linked to an intricate relationship between translocating bacteria, colonic mDC, and other signals within the inflammatory environment (e.g., NKp44L).
In contrast to the dramatic alterations in numbers of IFNγ-expressing NKp44+ ILCs associated with HIV-1 infection, absolute numbers of IL-22-expressing NKp44+ cells were similar in both HIV-1 infected and uninfected individuals, an observation in keeping with a number of other studies investigating gut ILCs during HIV-1 infection13,21,43,44. Conversely, one study which identified colonic tissue ILCs as CD3−IL-22+ using immunohistological techniques, found decreased numbers of these cells in untreated HIV-1 infected versus uninfected individuals45. In contrast to HIV-1 infection, both absolute frequencies of ILCs and frequencies of IL-22/IL-17- producing ILCs were significantly reduced during early and chronic SIV infection30,31,46–48. Of note, we observed a decrease in frequencies of NKp44−CD56+ cells that expressed IL-22, likely related to the overall decrease in absolute numbers of colonic NKp44−CD56+ cells as a whole. However, NKp44−CD56+ cells do not typically produce IL-22, so the impact of an overall decrease in this small population in untreated, chronic HIV-1 infected persons may be minor in the context of maintenance of IL-22+NKp44+ ILCs.
We acknowledge a number of limitations to this pilot study. The study was not powered to address alterations in numbers of colonic cytokine-producing ILCs in the setting of HIV-1 infection. The two study groups were not matched for sexual practice, which has recently been reported to impact the intestinal microbiome independent of HIV-1 infection49,50 and may drive mucosal immune cell activation and inflammation50 and therefore be a contributing factor to our current observations. Enumeration of the specific ILC subsets was based on criteria used to identify NK22 cells16. Since that time, the field of ILC biology has greatly expanded and a more rigorous and specific identification paradigm for the various ILC subsets (i.e., NK, ILC1s, ILC2, ILC3s) is being utilized19,20,42,51,52. Our ability to determine the composition of the specific ILC subsets (NK v ILC1 v ILC3) within the cytokine-producing populations based on this more recent nomenclature is limited here and future studies will be needed to incorporate these most recent definitions.
Despite these limitations, the observations that higher frequencies of IFNγ-producing ILCs, particularly in the NKp44+CD56− ILC population which typically do not produce IFNγ in the absence of HIV-1 infection, underscores the role these innate immune cells may play in HIV-1-associated mucosal inflammation and pathogenesis. Additional studies both in vivo and in vitro will be required to determine the extent to which the increased numbers are a result of a switch from IL-22 to IFNγ production versus expansion of the IFNγ-producing population versus an influx of inflammatory cells into the mucosal tissue. In summary, higher numbers of inflammatory colonic ILCs during untreated chronic HIV-1 infection associate with dysbiosis and gut mDC and T cell activation suggesting a critical interplay between gut ILCs, the microbiome and local immune responses that should be further explored.
Supplementary Material
Acknowledgments
We wish to express our sincere gratitude to all the study participants as well as the physicians and staff at the University of Colorado Infectious Disease Group Practice Clinic. We thank the staff at the Clinical and Translational Research Center (CTRC) and the University Hospital endoscopy clinic for their assistance with our clinical study and Zachary Dong and Daniel Hecht for assistance with study participant recruitment.
Source of Funding
This work was supported by National Institutes of Health (NIH) Grant R01 DK088663, RO1 AI118983 and, in part, by NIH/NCATS Colorado CTSI Grant Number UL1 TR000154.
Footnotes
This data was presented at the Conference on Retroviruses and Opportunistic Infections, Seattle, USA, February 12–14, 2017.
Conflicts of Interest: The authors have no conflict of interests to disclose
List of Supplemental Digital Content:
1. Supplemental Digital Content 1 Microsoft Word File.
References
- 1.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008;82:538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther. 2016;13:19. doi: 10.1186/s12981-016-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ullrich R, Zeitz M, Riecken EO. Enteric immunologic abnormalities in human immunodeficiency virus infection. Semin Liver Dis. 1992;12:167–174. doi: 10.1055/s-2007-1007388. [DOI] [PubMed] [Google Scholar]
- 6.Klase Z, Ortiz A, Deleage C, Mudd JC, Quinones M, Schwartzman E, et al. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunol. 2015;8:1009–1020. doi: 10.1038/mi.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 8.Marchetti G, Cozzi-Lepri A, Merlini E, Bellistri GM, Castagna A, Galli M, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 2011;25:1385–1394. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 9.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zevin AS, McKinnon L, Burgener A, Klatt NR. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS. 2016;11:182–190. doi: 10.1097/COH.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parks OB, Pociask DA, Hodzic Z, Kolls JK, Good M. Interleukin-22 Signaling in the Regulation of Intestinal Health and Disease. Front Cell Dev Biol. 2015;3:85. doi: 10.3389/fcell.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiber F, Arasteh JM, Lawley TD. Pathogen Resistance Mediated by IL-22 Signaling at the Epithelial-Microbiota Interface. J Mol Biol. 2015;427:3676–3682. doi: 10.1016/j.jmb.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012;5:670–680. doi: 10.1038/mi.2012.72. [DOI] [PubMed] [Google Scholar]
- 14.Page EE, Greathead L, Metcalf R, Clark SA, Hart M, Fuchs D, et al. Loss of Th22 Cells Is Associated With Increased Immune Activation and IDO-1 Activity in HIV-1 Infection. J Acquir Immune Defic Syndr. 2014;67:227–235. doi: 10.1097/QAI.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 15.Kim CJ, McKinnon LR, Kovacs C, Kandel G, Huibner S, Chege D, et al. Mucosal Th17 Cell Function Is Altered during HIV Infection and Is an Independent Predictor of Systemic Immune Activation. J Immunol. 2013;191:2164–2173. doi: 10.4049/jimmunol.1300829. [DOI] [PubMed] [Google Scholar]
- 16.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida FF, Belz GT. Innate lymphoid cells: models of plasticity for immune homeostasis and rapid responsiveness in protection. Mucosal Immunol. 2016;9:1103–1112. doi: 10.1038/mi.2016.64. [DOI] [PubMed] [Google Scholar]
- 19.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 20.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes SM, Pires AR, Ferreira C, Foxall RB, Rino J, Santos C, et al. Enteric mucosa integrity in the presence of a preserved innate interleukin 22 compartment in HIV type 1- treated individuals. J Infect Dis. 2014;210:630–640. doi: 10.1093/infdis/jiu126. [DOI] [PubMed] [Google Scholar]
- 22.Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, et al. Interleukin-12 and -23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity. 2015;43:146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 24.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL- 1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glatzer T, Killig M, Meisig J, Ommert I, Luetke-Eversloh M, Babic M, et al. RORgammat(+) innate lymphoid cells acquire a proinflammatory program upon engagement of the activating receptor NKp44. Immunity. 2013;38:1223–1235. doi: 10.1016/j.immuni.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Dillon SM, Lee EJ, Kotter CV, Austin GL, Gianella S, Siewe B, et al. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal Immunol. 2016;9:24–37. doi: 10.1038/mi.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fausther-Bovendo H, Sol-Foulon N, Candotti D, Agut H, Schwartz O, Debre P, et al. HIV escape from natural killer cytotoxicity: nef inhibits NKp44L expression on CD4+ T cells. AIDS. 2009;23:1077–1087. doi: 10.1097/QAD.0b013e32832cb26b. [DOI] [PubMed] [Google Scholar]
- 28.Sennepin A, Baychelier F, Guihot A, Nel I, Ho Tsong Fang R, Calin R, et al. NKp44L expression on CD4+ T cells is associated with impaired immunological recovery in HIV- infected patients under highly active antiretroviral therapy. AIDS. 2013;27:1857–1866. doi: 10.1097/qad.0b013e328361a3fe. [DOI] [PubMed] [Google Scholar]
- 29.Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, et al. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Richert-Spuhler LE, Evans TI, Gillis J, Connole M, Estes JD, et al. Hypercytotoxicity and rapid loss of NKp44+ innate lymphoid cells during acute SIV infection. PLoS Pathog. 2014;10:e1004551. doi: 10.1371/journal.ppat.1004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves RK, Rajakumar PA, Evans TI, Connole M, Gillis J, Wong FE, et al. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood. 2011;118:3321–3330. doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dillon SM, Kibbie J, Lee EJ, Guo K, Santiago ML, Austin GL, et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS. 2017;31:511–521. doi: 10.1097/QAD.0000000000001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983–994. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dillon SM, Lee EJ, Bramante JM, Barker E, Wilson CC. The natural killer cell interferon-gamma response to bacteria is diminished in untreated HIV-1 infection and defects persist despite viral suppression. J Acquir Immune Defic Syndr. 2014;65:259–267. doi: 10.1097/01.qai.0000435603.50598.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dillon SM, Lee EJ, Donovan AM, Guo K, Harper MS, Frank DN, et al. Enhancement of HIV-1 infection and intestinal CD4+ T cell depletion ex vivo by gut microbes altered during chronic HIV-1 infection. Retrovirology. 2016;13:5. doi: 10.1186/s12977-016-0237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dillon SM, Manuzak JA, Leone AK, Lee EJ, Rogers LM, McCarter MD, et al. HIV-1 infection of human intestinal lamina propria CD4+ T cells in vitro is enhanced by exposure to commensal Escherichia coli. J Immunol. 2012;189:885–896. doi: 10.4049/jimmunol.1200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci (Landmark Ed) 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beaurepaire C, Smyth D, McKay DM. Interferon-gamma regulation of intestinal epithelial permeability. J Interferon Cytokine Res. 2009;29:133–144. doi: 10.1089/jir.2008.0057. [DOI] [PubMed] [Google Scholar]
- 39.Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 2005;128:1258–1267. doi: 10.1053/j.gastro.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 40.Dillon SM, Frank DN, Wilson CC. The gut microbiome and HIV-1 pathogenesis: a two-way street. AIDS. 2016;30:2737–2751. doi: 10.1097/QAD.0000000000001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bates JM, Flanagan K, Mo L, Ota N, Ding J, Ho S, et al. Dendritic cell CD83 homotypic interactions regulate inflammation and promote mucosal homeostasis. Mucosal Immunol. 2015;8:414–428. doi: 10.1038/mi.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simoni Y, Fehlings M, Kloverpris HN, McGovern N, Koo SL, Loh CY, et al. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity. 2016;46:148–161. doi: 10.1016/j.immuni.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kloverpris HN, Kazer SW, Mjosberg J, Mabuka JM, Wellmann A, Ndhlovu Z, et al. Innate Lymphoid Cells Are Depleted Irreversibly during Acute HIV-1 Infection in the Absence of Viral Suppression. Immunity. 2016;44:391–405. doi: 10.1016/j.immuni.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kok A, Hocqueloux L, Hocini H, Carriere M, Lefrou L, Guguin A, et al. Early initiation of combined antiretroviral therapy preserves immune function in the gut of HIV-infected patients. Mucosal Immunol. 2015;8:127–140. doi: 10.1038/mi.2014.50. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Cheng L, Zhao J, Li G, Zhang L, Chen W, et al. Plasmacytoid dendritic cells promote HIV-1-induced group 3 innate lymphoid cell depletion. J Clin Invest. 2015;125:3692–3703. doi: 10.1172/JCI82124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012;5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu H, Wang X, Lackner AA, Veazey RS. Type 3 innate lymphoid cell depletion is mediated by TLRs in lymphoid tissues of simian immunodeficiency virus-infected macaques. FASEB J. 2015;29:5072–5080. doi: 10.1096/fj.15-276477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS. IL-17- producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV- infected macaques. Mucosal Immunol. 2012;5:658–669. doi: 10.1038/mi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noguera-Julian M, Rocafort M, Guillén Y, Rivera J, Casadellà M, Nowak P, et al. Gut Microbiota Linked to Sexual Preference and HIV Infection. EBioMedicine. 2016;5 doi: 10.1016/j.ebiom.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelley CF, Kraft CS, de Man TJ, Duphare C, Lee HW, Yang J, et al. The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: implications for HIV transmission and prevention. Mucosal Immunol. 2017;10:996–1007. doi: 10.1038/mi.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjorklund AK, Forkel M, Picelli S, Konya V, Theorell J, Friberg D, et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17:451–460. doi: 10.1038/ni.3368. [DOI] [PubMed] [Google Scholar]
- 52.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


