Abstract
Sortilin 1(Sort1) is a vesicle trafficking receptor that mediates protein sorting in the endocytic and exocytic pathways. Sort1 is a component of the GLUT4 storage vesicles in adipocytes and is also involved in the regulation of adipogenesis. Sort1 protein is reduced in adipose of obese mice and humans, but the underlying cause is not fully understood. Here we report that insulin/PI3K/AKT signaling cascade critically regulates adipose Sort1 protein abundance. Administration of a PI3K inhibitor rapidly decreased Sort1 protein but not mRNA in adipose of chow-fed mice. In 3T3-L1 adipocytes, serum-starvation or inhibition of the PI3K/AKT signaling also decreased Sort1 protein without affecting Sort1 mRNA expression. Sort1 protein downregulation upon PI3K inhibition was blocked by pretreatment of MG132 but not Bafilomycin A1, suggesting that PI3K inhibition caused Sort1 degradation via the proteasome pathway. Using a phospho-specific Sort1 antibody, we showed that endogenous Sort1 was phosphorylated at S825 adjacent to the DXXLL sorting motif on the cytoplasmic tail. We demonstrated that mutagenesis that abolished Sort1 S825 phosphorylation decreased insulin-stimulated Sort1 localization on the plasma membrane and Sort1 protein stability in 3T3-L1 adipocytes. However, endogenous Sort1 phosphorylation at S825 was not affected by insulin stimulation or by inhibition of PI3K. In conclusion, this study revealed an important role of insulin signaling in regulating adipose Sort1 protein stability, and further suggests that impaired insulin signaling may underlie reduced adipose Sort1 in obesity. The cellular events downstream of insulin/PI3K/AKT signaling that mediates insulin regulation of Sort1 stability requires further investigation.
Keywords: insulin resistance, diabetes, obesity, glucose metabolism, protein trafficking
1. Introduction
Sortilin 1 (Sort1) belongs to the family of vacuolar protein sorting 10 protein (VPS10P)-domain receptors (1–3). Sort1 is a single-pass transmembrane protein that mediates intracellular vesicle trafficking in the endocytic or exocytic pathways (3). In trafficking vesicles, the N-terminal luminal domain of Sort1 interacts with its protein ligands while the cytoplasmic domain of Sort1 facilitates the recruitment of adaptor proteins that are involved in vesicular trafficking. Sortilin 1 is expressed in many tissues including metabolically active tissues such as liver, muscle and adipose, immune cells such as macrophages and lymphocytes, and the central nerve system (2, 4). Sort1 has been shown to mediate the intracellular trafficking of proteins that are involved in distinct cellular pathways in various tissues and cell types (4–8). Recent studies revealed a role of Sort1 in the regulation of lipoprotein metabolism and both genetic variations and pathological changes of Sort1 function may be linked to cardiovascular disease risk (9–14).
Obesity and diabetes are associated with impaired glucose uptake and metabolism in adipose tissue, which may be partially caused by insulin resistance and reduced GLUT4 expression (15). In 3T3-L1 adipocytes, Sort1 has been identified as a component of the GLUT4 storage vesicle (GSV) involved in insulin-dependent glucose uptake (4, 5). It has been shown that both the formation of GSVs and their insulin responsiveness require the presence of Sort1 in 3T3-L1 adipocytes (16–18). Insulin-stimulated glucose uptake was significant reduced in Sort1-deficient 3T3-L1 adipocytes (16). Mice lacking Sort1 maintained overall glucose homeostasis but showed reduced basal glycolytic activity in adipose tissue (19). More recently, it was reported that increased Sort1 expression repressed adipogenesis by regulating the trafficking and function of delta-like 1 homologue (DLK1), an inhibitor of adipocyte differentiation (20, 21). Whole body Sort1 knockout mice were not obese (19, 22). Studies carried out in tissue specific Sort1 knockout mice are still needed to better define the role of Sort1 in regulating adipose biology and function. In addition, it has been reported that adipose Sort1 protein abundance was reduced in obese mice and obese humans, but the underlying mechanisms require further investigation (23). This study identified that insulin signaling through the PI3K/AKT cascade played an important role in regulating Sort1 protein stability in adipose tissue of mice and 3T3-L1 adipocytes, which suggests that impaired insulin signaling may be a possible cause of decreased adipose Sort1 in obesity.
2. Materials and Methods
Reagents
Antibodies against Sort1 (ab16640; Lot: GR64653-1), GLUT4 (ab48547; Lot: Ab654) and α-Tubulin (ab7291; Lot: GR122217-1) were from Abcam (Cambridge, MA). Antibodies against phospho-AKT (Ser-473; 4060; Lot: 19), AKT (4691; Lot: 17), ubiquitin (3933), LC3B (3868, Lot: 9), and Histone 3 (9717; Lot: 8), TNFα and wortmannin were purchased from Cell Signaling Technology (Danvers, MA). Antibody against GGA2 (H-175, sc-30103; Lot: K0305) was purchased from Santa Cruz Biotechnology (Dallas, Texas). Antibodies against FLAG (M2) (F1804; Lot: SLBJ4607V) and Actin (A5441; Lot: 063M4808), Anti-FLAG (M2) magnetic beads, AKT1/2 inhibitor VIII, cycloheximide, chloroquine, Bafilomycin A1, MG132, and TBCA were purchased from Sigma (St. Louis, MO). The antibody against phospho-Sort1 (S825) was developed by Epitomics (Burlingame, CA). Insulin was purchased from Eli Lilly and Company (Indianapolis, IN). PX866 was purchased from Cayman Chemical (Ann Arbor, MI). Alexa Fluor 488 IgG (A-21202; Lot: 1572559) and Alexa Fluor 594 (A-21207; Lot: 1558726) were purchased from Life Technologies (Grand Island, NY).
Mice
Male 8 weeks old C57BL/6J mice and Ob/Ob mice were purchased from the Jackson Lab (Bar Harbor, ME). Mice were housed under a normal light-dark cycle (light on from 6 am – 7 pm) with free access to food and water. Ob/Ob mice were sacrificed at 12 weeks of age after overnight (5 pm – 9 am) fasting. C57BL/6J mice at 10 weeks of age were fed a Western diet (TD.88137) purchased from Envigo (Indianapolis, IN), and sacrificed after overnight (5 pm – 9 am) fasting. Px866 was dissolved in sterile 1× PBS with 5% ethanol. At around 9 am, Px866 was administered to 10-week old non-fasted C57BL/6J mice through intraperitoneal injection at 8 mg/kg in a 100-µl volume (24). Control mice were injected with vehicle. Mice were then fasted for 8 h. A drop of blood was collected from the tail and used for measurement of blood glucose with a glucometer. Mice were then sacrificed. All animal protocols were approved by the Institutional Animal Care and Use Committee.
Cell culture
Mouse 3T3-L1 preadipocytes were a gift from Dr. Gökhan Hotamisligil (Harvard University School of Public Health). Differentiation was induced by culturing preadipocytes in DMEM supplemented with 10% bovine calf serum, 0.5 mM isobutyl methyl xanthine, 10 µM dexamathasone, 5 µg/ml insulin and 1 µM thiazolidinedione for 3 days, after which cells were cultured in maintenance medium (DMEM with 10% FBS, 1% P/S and 5 µg/ml insulin). Lenti-WT-Sort1-FLAG and Lenti-S825A-Sort1-FLAG (C-terminal-tagged) were generated by Capital Biosciences Inc. (Gaithersburg, MD). Heterogeneous pools of stable 3T3-L1 cells were generated by selecting infected cells with 2.5 µg/ml puromycin. Stable cells were maintained in 2 µg/ml puromycin-containing DMEM in further experiments.
Western blotting
Cell lysates or tissue homogenates were prepared in 1X RIPA buffer containing 1% SDS and protease inhibitor cocktail Sigma (St. Louis, MO). Lysates were incubated on ice for 30 min followed by brief sonication. After centrifugation, supernatant was transferred to a new tube and protein concentrations were determined by a BCA assay kit (ThermoFisher Scientific, Waltham, MA). Lysates containing equal amount of protein were used for SDS-PAGE and Western blotting. Densitometry was performed with ImageJ software and normalized to loading controls unless noted otherwise.
Co-immunoprecipitation assay
Differentiated 3T3-L1 cells with stable expression of WT or S825A Sort1-FLAG were lysed in modified RIPA buffer containing protease inhibitor cocktail and phosphatase inhibitor. Sort1-FLAG was precipitated with Anti-FLAG (M2) magnetic beads. Differentiated 3T3-L1 cells that did not express Sort1-FLAG were used in the immunoprecipitation as negative controls. Precipitated Sort1-FLAG, GGA2 and GLUT4 were detected by Western blotting.
Real-time PCR
Total RNA was isolated with Tri-reagent (ThermoFisher Scientific, Waltham, MA). Real-time PCR were performed with SYBR master mix (Bio-Rad Laboratories Inc., Hercules, CA). Amplification of 18S was used as an internal control. Relative mRNA expression was quantified using the comparative CT (Ct) method and expressed as 2−ΔΔCt.
Immunofluorescent confocal microscopy
Cells were fixed in 4% paraformaldehyde, permeabilized in 0.1% tween-20 and 0.3 M glycine. Primary antibodies and Alexa Fluor-conjugated secondary antibodies were used for staining. Images were taken with a Leica DM550Q confocal microscope and acquired with LAS AF software (Leica Microsystems Inc., Buffalo Grove, IL).
Surface biotinylation assay
Biotinylation assay was performed with Pierce Cell Surface Protein Isolation Kit (Cat#: 89881) purchased from ThermoFisher Scientific (Waltham, MA) per manufacturer’s instruction. Briefly, cells were immediately washed with ice-cold 1x PBS after treatments and incubated in Sulfo-NHS-SS-Biotin solution at 4 °C for 30 min. Biotinylated proteins were purified from cell lysates with NeutrAvidin Agarose-containing columns. Biotinylated protein and flow-through were used for Western blotting.
Statistical analysis
Results were expressed as mean ± SE or mean ± S.D. as noted in figure legend. Statistical analysis was performed by Student’s t-test. A p < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Blocking the PI3K/AKT pathway causes Sort1 downregulation in mouse adipose tissue
Consistent with previous reports (19, 23), we found that adipose Sort1 protein was significantly decreased in genetic obese Ob/Ob mice and in Western diet-induced obese mice (Fig 1A, 1C). The Sort1 mRNA tended to be lower in obese mice but the reduction was not statistically significant (Fig 1B, 1D). Similarly, a previous study suggested that elevated tumor necrosis factor α (TNFα) played a role in mediating the transcriptional inhibition of adipose Sort1 in obese mice, but Sort1 protein appeared to be reduced to a much larger extent than Sort1 mRNA in obese mice (23). Feeding mice a Western diet for 1 week decreased adipose Sort1 protein but not mRNA (Fig 1E, 1F), suggesting that adipose Sort1 started to decrease at the early stage of diet-induced obesity development. A previous study demonstrated that challenging mice a high fat diet for 3 days was sufficient to cause early onset insulin resistance in adipose due to acute lipid overload (25). Currently, the cause of adipose Sort1 downregulation in obesity is not clear. To test if adipose Sort1 downregulation could potentially be due to decreased insulin/PI3K/AKT signaling in obese mice, we injected chow-fed C57BL/6J mice with a PI3K inhibitor Px866 or vehicle and mice were sacrificed 8 h post injection. This treatment significantly decreased the phosphorylation levels of AKT in the white adipose of mice (Fig 2A), confirming effective inhibition of the PI3K/AKT signaling. Px866 significantly decreased Sort1 protein (Fig 2A) without altering Sort1 mRNA expression (Fig 2B). As positive controls, GLUT4 protein was decreased and plasma glucose was increased upon Px866 treatment (Fig 2A, 2C). These results suggest that chronically attenuated PI3K/AKT signaling as a result of insulin resistance may be a potential mechanism underlying lower adipose Sort1 protein abundance in obese mice.
FIGURE 1. Sort1 protein was decreased in white adipose of obese mice.
A, B. Sort1 protein and mRNA levels in white adipose of C57BL/6J mice fed a Western diet for 8 weeks. A representative bot was shown. Densitometry values are expressed as mean ± SE (n=5–6). C, D. Sort1 protein and mRNA levels in white adipose of WT C57BL/6J and Ob/Ob mice. Densitometry values are expressed as mean ± SE (n=3). E, F. Sort1 protein and mRNA levels in white adipose of C57BL/6J mice fed a Western diet for 1 wk. Densitometry values are expressed as mean ± SEM (n=3). For all mRNA measurement, results are expressed as mean ± SE (n=4–5). “*”, p< 0.05 vs. chow-fed controls. NS, not significant.
FIGURE 2. Blocking PI3K/AKT signaling caused downregulation of Sort 1 in mice.
A. Sort1 protein in white adipose of mice 8 h post injection of Px866 or vehicle. Relative Sort1 band intensity values (mean ± SE) are shown (n=3). B. Sort1 mRNA in white adipose of mice 8 h post injection of Px866 or vehicle. n=4–5. C. Plasma glucose of mice injected with PX866. n=4–5. Results are expressed as mean ± SE. “*”, p< 0.05, vs. vehicle group. NS, not significant.
3.2. Inhibition of PI3K or AKT decreases Sort1 protein abundance in 3T3-L1 adipocytes
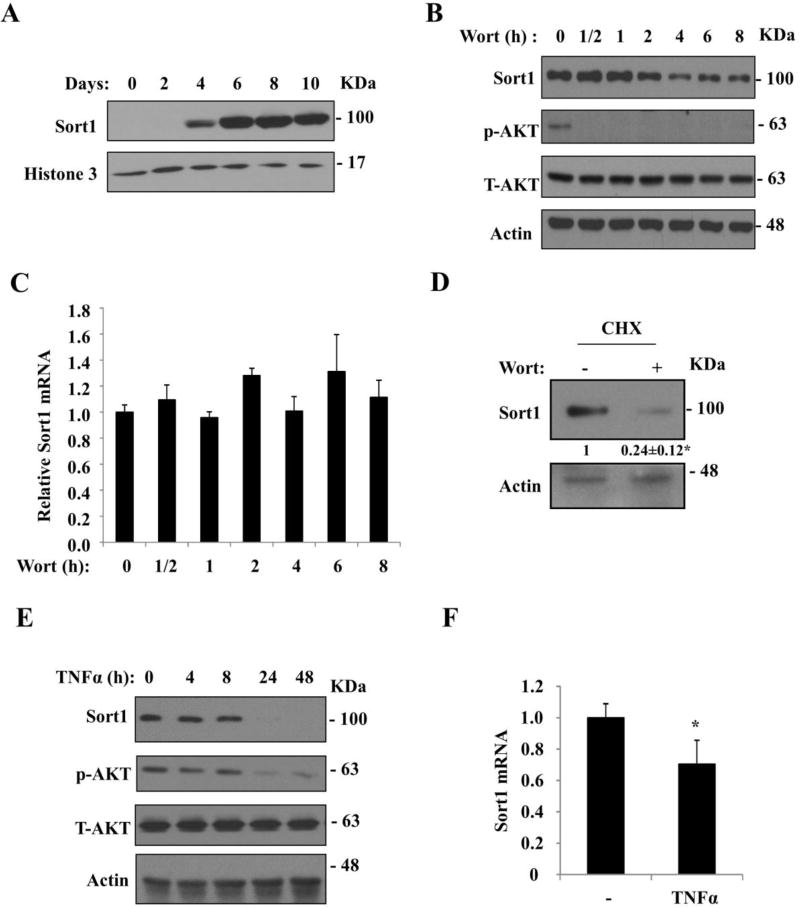
We next elaborated on this finding in differentiated 3T3-L1 adipocytes in vitro. It has been reported that undifferentiated 3T3-L1 fibroblast cells do not express Sort1, while Sort1 mRNA and protein are highly expressed when these cells differentiated into adipocytes (4, 26). Consistently, we found that the expression of Sort1 reached relatively constant levels after 6 days post induction of differentiation (Fig 3A). Cells between 8–11 days post induction of differentiation were used for the following experiments. Consistent with our in vivo observations, we found that treating differentiated 3T3-L1 adipocytes with the PI3K inhibitor wortmannin rapidly decreased Sort1 protein without affecting Sort1 mRNA (Fig 3B, 3C). Wortmainnin also decreased Sort1 protein in cells pretreated with cycloheximide which blocked protein synthesis (Fig 3D), suggesting a posttranslational mechanism of Sort1 downregulation. Increased cytokines are known to play an important role in inhibiting adipose insulin signaling in obesity (27). We also found that Sort1 protein was decreased in 3T3-L1 adipocytes only after 24 h or longer TNFα treatment, which correlated with decreased AKT phosphorylation (Fig 3E). Consistent with a previous report (23), TNFα also decreased Sort1 mRNA expression, but to a less extent than Sort1 protein (Fig 3F). Culturing differentiated adipocytes in serum-free medium overnight decreased Sort1 protein (Fig 4A), while addition of insulin into the culture medium increased Sort1 protein, but not Sort1 mRNA, in overnight serum-starved cells (Fig 4B, 4C). Insulin induction of Sort1 protein was blocked by either wortmannin or an AKT inhibitor (Fig 4D, 4E). Wortmannin-induced Sort1 downregulation was prevented by the proteasome inhibitor MG132, but not by the lysosome inhibitor Bafilomycin A1 (Fig 4F, 4G). Ubiquitinated proteins and LC3B were measured as controls in these experiments. These results suggest that inhibition of cellular PI3K signaling probably promoted proteasome-dependent Sort1 degradation.
FIGURE 3. PI3K inhibitor and TNFα decreased Sort1 protein in 3T3-L1 adipocytes.
A. Western blotting measurement of Sort1 protein expression during 3T3-L1 differentiation into adipocytes. B, C. Sort1 protein and mRNA in differentiated 3T3-L1 adipocytes cultured in insulin-containing maintenance medium and treated with 1 µM wortmannin (Wort) in time course. Results of a representative real-time PCR assay was shown. Real-time PCR was done in triplicates and results are expressed as mean ± SD. D. Differentiated 3T3-L1 adipocytes were cultured in maintenance medium and pre-treated with 100 µg/ml cycloheximide (CHX) for 1 h, and then treated with 1 µM wortmannin for 6 h. Sort1 protein was detected by Western blotting. A representative blot was shown. The average densitometry value of 3 independent experiments was shown under the blot. E, F. Differentiated 3T3-L1 adipocytes were treated with 20 ng/ml mouse TNFα for time indicated. Culture medium containing TNFα was replaced every 24 h in cells treated for 48 h. E. Sort1 protein was detected by Western blotting. F. Sort1 mRNA was modestly reduced after 24 h TNFα treatment. Results are expressed as mean± SD. “*”, p< 0.05, vs. vehicle-treated controls. In A, B, D and E, anti-Sort1 antibody was used to detect Sort1 protein.
FIGURE 4. Insulin increased Sort1 protein in serum-starved 3T3-L1 adipocytes.
A. Western blotting detection of Sort1 protein with anti-Sort1 antibody in differentiated 3T3-L1 adipocytes cultured in 10% FBS containing DMEM or FBS-free DMEM for 16 h. B, C. Detection of Sort1 protein or mRNA in differentiated 3T3-L1 adipocytes cultured in FBS-free DMEM for 16 h, and treated with 100 nM insulin in time course. Real-time PCR results of a representative assay were performed in triplicates and expressed as mean ± SD. No significant changes were detected. D, E. Differentiated 3T3-L1 adipocytes were cultured in serum-free DMEM for 16 h, and then pre-treated with 1 µM wortmannin (Wort) (D) or 10 µM AKT inhibitor (AKT-I) (E) for 1 h before 100 nM insulin treatment for 6 h. Sort1 protein was detected by Western blotting with anti-Sort1 antibody. Vehicle: DMSO. Relative Sort1 band intensity of three independent experiments was expressed as bar graphs below the blot. “*”, vs. vehicle alone. F, G. Differentiated 3T3-L1 adipocytes were cultured in maintenance medium and were pre-treated with 10 µM MG132 (F) or 10 nM Bafilomycin A1 (BafA1) (G) for 1 h before 1 µM wortmannin (Wort) treatment for 2 h. Sort1 protein was detected by Western blotting with anti-Sort1 antibody. Relative Sort1 band intensity of three independent experiments was expressed as bar graphs below the blot. Ub. Ubiquitin antibody. “*”, vs. vehicle alone. “#”, vs. BafA1 alone.
3.3. S825 phosphorylation of Sort1 was not affected by insulin or PI3K/AKT inhibition
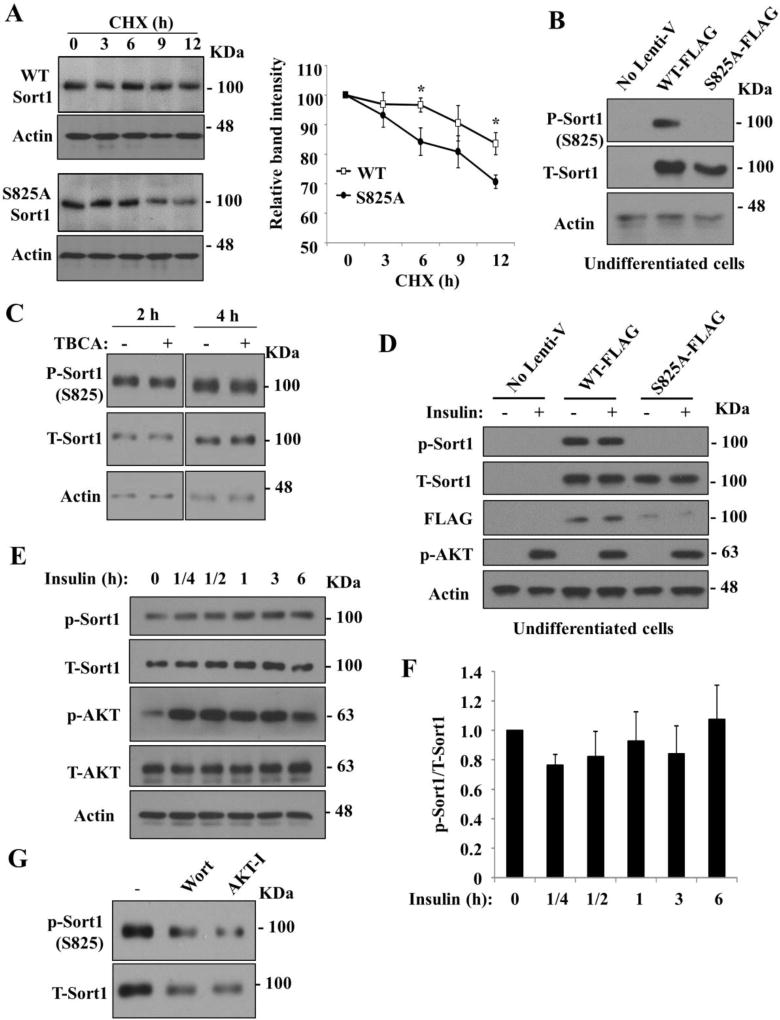
The mechanism underlying the rapid posttranslational downregulation of adipose Sort1 caused by blocking PI3K/AKT signaling is still not clear. Recently, we and others have identified a putative Sort1 phosphorylation site serine-825 (S825) via proteomics approaches (24, 28, 29). S825 is located on the cytoplasmic tail of Sort1 immediately adjacent to a conserved DXXLL motif (S825DEDLL in Sort1) that mediates Sort1 interaction with the trafficking adaptor Golgi–associated, γ-adaptin ear–containing, ARF-binding protein-2 (GGA2) (28, 30, 31). Abolishing S825 phosphorylation by S-A mutagenesis appeared to alter Sort1 cellular localization in vascular smooth muscle cells (29) and decrease protein stability in liver cells (24). In differentiated 3T3-L1 adipocytes stably expressing WT Sort1-FLAG or S825A Sort1-FLAG, we found that WT Sort1-FLAG showed minimal reduction in protein abundance after protein synthesis was blocked by cycloheximide over a 12-h period, suggesting that Sort1 is a relatively stable protein in 3T3-L1 adipocytes (Fig 5A). In contrast, S825A Sort1-FLAG protein showed a faster degradation rate after cycloheximide treatment (Fig 5A). S825 is a consensus casein kinase target and was shown to be phosphorylated by casein kinase 2 (CK2) and the Golgi-localized casein kinase the Family with sequence similarity 20, member C (Fam20C) in in vitro kinase assays (28, 29). Although CK2 was generally thought to be constitutively active in various cell types (32), some studies also suggested that CK2 was rapidly activated by insulin, insulin-like growth factor-I and epidermal growth factor in 3T3-L1 adipocytes (33, 34). Activated CK2 may in turn promote cell proliferation in response to growth factor signaling (35, 36). These previous findings raise the question whether S825 phosphorylation is involved in insulin/PI3K/AKT regulation of Sort1 in adipocytes.
FIGURE 5. Phospho-Sort1 to total Sort1 ratio was not altered by insulin or PI3K inhibitor in 3T3-L1 adipocytes.
A. Differentiated 3T3-L1 adipocytes stably expressing WT-Sort1-FLAG or S825A-Sort1-FLAG were cultured in maintenance medium and treated with 100 µg/ml cycloheximide (CHX) for time indicated. Western blot was performed with anti-FLAG antibody. Right panel: Average Sort1 band intensity (not normalized to Actin band) of three independent experiments with 0 h set as 100. “*”, vs. WT at the same time point. B. Detection of phospho-Sort1 and total Sort1 with anti-Sort1 antibody in undifferentiated 3T3-L1 cells (no-Lenti-V) and undifferentiated 3T3-L1 cells stably expressing WT-Sort1-FLAG or S825A-Sort1-FLAG. These cells were cultured in serum-containing medium. C. Detection of endogenous phospho-Sort1 and total Sort1 in differentiated 3T3-L1 adipocytes cultured in maintenance medium and and treated with 10 µM TBCA. Controls were treated with DMSO. D. Detection of phospho-Sort1 and total Sort1 in undifferentiated 3T3-L1 cells (no-Lenti-V) and undifferentiated 3T3-L1 cells stably expressing WT-Sort1-FLAG or S825A-Sort1-FLAG. Cells were serum-starved overnight. Some cells were then treated with 100 nM insulin for 1 h. Note: endogenous Sort1 protein was not detected in undifferentiated 3T3-L1 cells by anti-Sort1 antibody. E, F. Differentiated 3T3-L1 adipocytes were cultured in serum/insulin-free medium overnight, and then treated with 100 nM insulin in time course. Phospho-Sort1 and total Sort1 was measured by Western blotting. In F, the band intensity of phospho-Sort1 and total Sort1 was determined by ImageJ software. Results of 3 independent experiments were plotted as mean ± SD. No significant changes of the p-Sort1/T-Sort1 ratio were detected. G. Phospho-Sort1 and total Sort1 protein was measured by Western blotting in differentiated 3T3-L1 adipocytes cultured in maintenance medium and treated with vehicle (DMSO), 1 µM wortmannin (Wort) or 10 µM AKT inhibitor (AKT-I) for 2 h.
To better address these questions, we developed antibodies specifically detecting S825 phosphorylated Sort1. Fig 5B shows that in undifferentiated 3T3-L1 cells that did not express endogenous Sort1, phospho-Sort1 antibodies only detected WT Sort1-FLAG, but not S825A Sort1-FLAG. These result validated the specificity of the phospho-Sort1 antibodies. We next treated differentiated 3T3-L1 adipocytes with a CK2 inhibitor Tetrabromocinnamic acid (TBCA) and measured endogenous Sort1 phosphorylation at S825 (37). However, TBCA treatment for 2 h and 4 h did not affect S825 phosphorylation (Fig 5C). One possible explanation may be the existence of enzyme redundancy so that kinases other than CK2 may also phosphorylate S825 in 3T3-L1 adipocytes. We next investigated if S825 phosphorylation was affected by insulin/PI3K signaling. In undifferentiated 3T3-L1 cells that did not express endogenous Sort1, insulin treatment did not further increase the S825 phosphorylation of WT Sort1-FLAG (Fig 5D). In differentiated 3T3-L1 adipocytes, phosphorylated endogenous Sort1 was readily detectable in overnight serum-starved cells (Fig 5E). However, insulin increased the phospho-Sort1 and total Sort1 to similar extent, and the ratio of phospho-Sort1 to total Sort1 was not altered by insulin treatment over the course of 6 h (Fig 5E, 5F). Furthermore, treating differentiated 3T3-L1 adipocytes with wortmannin or AKT inhibitor for 2 h rapidly decreased both phosphorylated Sort1 and total Sort1 to similar extent (Fig 5G). In summary, our results confirmed that endogenous Sort1 was phosphorylated at S825 in 3T3-L1 adipocytes. The S825 phosphorylation was readily detectable in several experimental conditions, which left an impression that a relatively high percentage of endogenous Sort1 may be phosphorylated at S825 in 3T3-L1 adipocytes. It may be speculated that the constitutive activity of CK2 and other unidentified kinases phosphorylate Sort1 at S825 at high baseline levels to maintain Sort1 protein stability and constitutive trafficking function in cells (32, 35, 36).
3.4. Abolishing phosphorylation of S825 prevented insulin-stimulated Sort1 plasma membrane localization
It was suggested that phosphorylation of the serine residue immediately adjacent to the DXXLL di-leucine motif in many trafficking receptors regulates di-leucine motif interaction with the trafficking adaptor GGA2 (28, 30, 31). We next investigated if S825 phosphorylation is involved in insulin-regulated Sort1 plasma membrane localization. In differentiated 3T3-L1 adipocytes expressing WT Sort1-FALG or S825A Sort1-FLAG, both WT Sort1 and S825A Sort1 were primarily visualized as perinuclear puncta that substantially co-localized with GLUT4 (Fig 6A). Some Sort1 puncta also dispersed throughout the cells and showed co-localization with GLUT4 (Fig 6A). By using surface biotinylation assays, we were able to detect rapid plasma membrane enrichment of endogenous Sort1 in insulin-stimulated 3T3-L1 adipocytes (Fig 6C), which was consistent with a previous report (5). This experimental approach was used to further investigate the plasma translocation of WT and S825A Sort1-FLAG in response to insulin. Similar to endogenous Sort1, plasma membrane localization of WT Sort1-FLAG was stimulated by insulin (Fig 6D). In contrast, S825A Sort1-FLAG membrane localization was not enriched after insulin stimulation (Fig 6E). Longer insulin stimulation for 15 min decreased S825A Sort1-FLAG amount on the cell surface (Fig 6E). It is not known if this could be a combined result of S825A Sort1-FLAG endocytosis without stimulated membrane enrichment. Consistent with the lack of insulin-stimulated membrane enrichment of S825A Sort1-FLAG, the amount of GGA2 that co-precipitated with S825A Sort1-FLAG was also less than that co-precipitated with WT-Sort1-FLAG in a co-immunoprecipitation assay (Fig 6F). These results showed that abolishing S825 phosphorylation reduced Sort1 interaction with trafficking adaptor GGA2 and insulin-dependent Sort1 trafficking to the plasma membrane.
FIGURE 6. Abolishing S825 phosphorylation prevented insulin-regulated Sort1 membrane localization in 3T3-L1 adipocytes.
A. Confocal microscope of differentiated 3T3-L1 adipocytes stably expressing WT-Sort1-FLAG or S825A-Sort1-FLAG. Cells were cultured in FBS-free medium for 16 h. Sort1-FLAG was stained with anti-FLAG antibody. Blue: DAPI staining of nuclei. B. Confocal microscope of differentiated 3T3-L1 adipocytes stably expressing WT-Sort1-FLAG or S825A-Sort1-FLAG. Cells were cultured in FBS-free medium for 16 h and then treated with 100 nM insulin for 15 min. Sort1 (green) was stained with anti-FLAG antibody. Nuclei were stained with DAPI (Blue). C. Differentiated 3T3-L1 adipocytes were cultured in serum-free medium for 16 h, and treated with 100 nM insulin for 2 min. Surface biotinylation assays were performed. Endogenous biotinylated Sort1 and unbound Sort1 in the flow-through were detected with Sort1 antibody. D, E. Differentiated 3T3-L1 adipocytes expressing WT Sort1-FLAG or S825A Sort1-FLAG were cultured in serum-free medium for 16 h, then treated with 100 nM insulin for 2 and 15 min. Biotinylated Sort1-FLAG and unbound Sort1-FLAG in the flow-through were detected with anti-FLAG antibody. Actin in flow-through was also blotted. F. Differentiated 3T3-L1 adipocytes stably expressing WT-Sort1-FLAG or S825A-Sort1-FLAG were subjected to co-immunoprecipitation with anti-FLAG magnetic beads. Co-precipitated GGA2 and GLUT4 were measured by Western blotting. Differentiated adipocytes without stable Sort1-FLAG expression was subjected to immunoprecipitation as negative controls.
Despite being a relatively stable protein (Fig 5A), we have provided several lines of in vitro and in vivo evidence to show that Sort1 downregulation in response to inhibition of PI3K/AKT signaling was strong and rapid, which suggests stimulated Sort1 protein degradation under this condition. The downstream mechanism that caused such rapid Sort1 destabilization remains to be determined. The parallel changes of phosphorylated and total Sort1 suggest that alteration of S825 phosphorylation did not appear to be an event prior to total Sort1 changes caused by insulin treatment or by PI3K/AKT inhibition. Therefore, although protein phosphorylation is considered as a common mechanism by which cellular signaling regulates protein stability and degradation, our results do not support a potential mechanism by which insulin/PI3K/AKT signaling regulates Sort1 protein stability via altering Sort1 S825 phosphorylation. However, by studying the functional link of S825 phosphorylation to Sort1 stability and trafficking, we learned that abolishing S825 phosphorylation decreased Sort1 trafficking to the plasma membrane upon insulin stimulation, which correlated with reduced Sort1 protein stability. This observation may indirectly imply a possibility that cellular events that alter Sort1 trafficking process may be linked to Sort1 degradation. This is also supported by a recent study showing that altered AP-1-dependent Sort1 trafficking also affected cellular Sort1 protein degradation and Sort1 protein abundance without changes in Sort1 mRNA expression in adipocytes and in adipose tissue of mice (20). In addition, it is known that functional GSV formation depends on the key protein components to form a stable complex, and some experimental conditions that reduced GSV formation, such as by reducing Sort1 expression, also destabilized GLUT4 and caused GLUT4 degradation in adipocytes (16, 38). Whether Sort1 protein is also stabilized after it is incorporated into protein complex requires further investigation. As many protein components in GSV are insulin-regulated proteins (38), further studies are needed to investigate if inhibition of insulin/PI3K/AKT signaling somehow impairs processes such as Sort1-containg protein complex formation and/or vesicular trafficking process, which leads to Sort1 destabilization.
4. Conclusions
The major finding of this study is that the insulin/PI3K/AKT signaling cascade plays an important role in regulating adipose Sort1 at posttranslational levels, which suggests that impaired insulin signaling may be an underlying mechanism that results in lower adipose Sort1 protein in obese mice and obese humans (23). Adipose Sort1 downregulation occurred at early stage of diet-induced obesity, which was consistent with previous findings that challenging mice a high fat diet for 3 days was sufficient to cause early onset insulin resistance in adipose due to acute lipid overload (25). Sort1 is a component of GSV in adipocytes, and Sort1 loss-of-function was shown to decrease insulin-dependent glucose uptake into the adipocytes (16). Therefore, down-regulation of adipose Sort1 may be implicated in altered adipose glucose metabolism under obesity and insulin resistant conditions. However, the in vivo role of Sort1 in adipose glucose metabolism seems to be more complex as mice lacking Sort1 globally showed reduced basal adipose glycolytic activity but normal insulin-stimulated adipose glucose uptake (19). Sort1 has also been shown to inhibit adipogenesis by regulating the intracellular trafficking of DLK1, a receptor that inhibits adipogenesis (20, 21), and increased adipose Sort1 expression decreases adipogenesis and adipose mass. This finding raises another question whether downregulation of adipose Sort1 in obesity helps promote adipose differentiation in response to higher dietary lipid uptake. We recently reported that Sort1 knockout mice gained similar body weight as their WT counterparts when placed on a high fat diet (19). However, study of the genotype-dependent effects in high fat diet feeding model is limited due to the rapid and strong downregulation of Sort1 in the adipose tissue of WT control mice. Whether adipose-specific Sort1 transgenic mice have reduced adipogenesis and lower fat mass remains to be determined. Furthermore, it should be noted that Sort1 has also been implicated in various other cellular pathways in adipocytes such as mediating the endocytic targeting of lipoprotein lipase (7) and adiponectin (39) and neurotensin signaling (40). However, the functional roles of Sort1 in regulating these pathways are less well characterized in adipocytes and in vivo. Because findings from this study suggest that adipose Sort1 is highly sensitive to downregulation under insulin resistant conditions, the potential pathophysiological significance of decreased Sort1 in other aspects of adipose function in obesity and diabetes needs to be further defined by future studies.
Highlights.
Adipose Sortilin 1 protein is strongly downregulation in obesity.
Insulin/PI3K/AKT signaling critically controls Sort1 protein abundance in adipocytes
Sortilin 1 S825 phosphorylation regulates Sort1 trafficking function in adipocytes
Acknowledgments
This work was supported in part by an American Diabetes Association Junior Faculty Award (T.L.), National Institutes of Health grant 1R01DK102487-01 (T.L) and the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) of the National Institutes of Health.
Abbreviations
- Sort1
Sortilin1
- VPS10P
vacuolar protein sorting 10 protein
- GSV
GLUT4 storage vesicle
- DLK1
delta-like 1 homologue
- TNFα
tumor necrosis factor α
- GGA2
Golgi–associated, γ-adaptin ear–containing, ARF-binding protein-2
- CK2
casein kinase
- TBCA
Tetrabromocinnamic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have nothing to disclose
Author contributions
J.L, C.C, Y.L. D.M. and Y.W. designed and performed the experiments and analyzed the data. W.X.D. and T.L. supervised the study, designed and analyzed the data. T.L. wrote the manuscript.
References
- 1.Zsurger N, Mazella J, Vincent JP. Solubilization and purification of a high affinity neurotensin receptor from newborn human brain. Brain research. 1994;639:245–252. doi: 10.1016/0006-8993(94)91737-x. [DOI] [PubMed] [Google Scholar]
- 2.Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272:3599–3605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- 3.Hermey G. The Vps10p-domain receptor family. Cell Mol Life Sci. 2009;66:2677–2689. doi: 10.1007/s00018-009-0043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin BZ, Pilch PF, Kandror KV. Sortilin is a major protein component of Glut4-containing vesicles. J Biol Chem. 1997;272:24145–24147. doi: 10.1074/jbc.272.39.24145. [DOI] [PubMed] [Google Scholar]
- 5.Morris NJ, Ross SA, Lane WS, Moestrup SK, Petersen CM, Keller SR, Lienhard GE. Sortilin is the major 110-kDa protein in GLUT4 vesicles from adipocytes. J Biol Chem. 1998;273:3582–3587. doi: 10.1074/jbc.273.6.3582. [DOI] [PubMed] [Google Scholar]
- 6.Mazella J, Zsurger N, Navarro V, Chabry J, Kaghad M, Caput D, Ferrara P, Vita N, Gully D, Maffrand JP, Vincent JP. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273:26273–26276. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen MS, Jacobsen C, Olivecrona G, Gliemann J, Petersen CM. Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem. 1999;274:8832–8836. doi: 10.1074/jbc.274.13.8832. [DOI] [PubMed] [Google Scholar]
- 8.Ni X, Morales CR. The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic. 2006;7:889–902. doi: 10.1111/j.1600-0854.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 9.Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, Wahlstrand B, Hedner T, Corella D, Tai ES, Ordovas JM, Berglund G, Vartiainen E, Jousilahti P, Hedblad B, Taskinen MR, Newton-Cheh C, Salomaa V, Peltonen L, Groop L, Altshuler DM, Orho-Melander M. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myocardial Infarction Genetics, C. Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC, Siscovick DS, O'Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Melander O, Altshuler D, Ardissino D, Merlini PA, Berzuini C, Bernardinelli L, Peyvandi F, Tubaro M, Celli P, Ferrario M, Fetiveau R, Marziliano N, Casari G, Galli M, Ribichini F, Rossi M, Bernardi F, Zonzin P, Piazza A, Mannucci PM, Schwartz SM, Siscovick DS, Yee J, Friedlander Y, Elosua R, Marrugat J, Lucas G, Subirana I, Sala J, Ramos R, Kathiresan S, Meigs JB, Williams G, Nathan DM, MacRae CA, O'Donnell CJ, Salomaa V, Havulinna AS, Peltonen L, Melander O, Berglund G, Voight BF, Kathiresan S, Hirschhorn JN, Asselta R, Duga S, Spreafico M, Musunuru K, Daly MJ, Purcell S, Voight BF, Purcell S, Nemesh J, Korn JM, McCarroll SA, Schwartz SM, Yee J, Kathiresan S, Lucas G, Subirana I, Elosua R, Surti A, Guiducci C, Gianniny L, Mirel D, Parkin M, Burtt N, Gabriel SB, Samani NJ, Thompson JR, Braund PS, Wright BJ, Balmforth AJ, Ball SG, Hall A, Wellcome Trust Case Control, C. Schunkert H, Erdmann J, Linsel-Nitschke P, Lieb W, Ziegler A, Konig I, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Schunkert H, Samani NJ, Erdmann J, Ouwehand W, Hengstenberg C, Deloukas P, Scholz M, Cambien F, Reilly MP, Li M, Chen Z, Wilensky R, Matthai W, Qasim A, Hakonarson HH, Devaney J, Burnett MS, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Knouff CW, Waterworth DM, Walker MC, Mooser V, Epstein SE, Rader DJ, Scheffold T, Berger K, Stoll M, Huge A, Girelli D, Martinelli N, Olivieri O, Corrocher R, Morgan T, Spertus JA, McKeown P, Patterson CC, Schunkert H, Erdmann E, Linsel-Nitschke P, Lieb W, Ziegler A, Konig IR, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Holm H, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Engert JC, Do R, Xie C, Anand S, Kathiresan S, Ardissino D, Mannucci PM, Siscovick D, O'Donnell CJ, Samani NJ, Melander O, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Altshuler D. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjolby M, Andersen OM, Breiderhoff T, Fjorback AW, Pedersen KM, Madsen P, Jansen P, Heeren J, Willnow TE, Nykjaer A. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell metabolism. 2010;12:213–223. doi: 10.1016/j.cmet.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, Pirruccello JP, Muchmore B, Prokunina-Olsson L, Hall JL, Schadt EE, Morales CR, Lund-Katz S, Phillips MC, Wong J, Cantley W, Racie T, Ejebe KG, Orho-Melander M, Melander O, Koteliansky V, Fitzgerald K, Krauss RM, Cowan CA, Kathiresan S, Rader DJ. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bi L, Chiang JY, Ding WX, Dunn W, Roberts B, Li T. Saturated fatty acids activate ERK signaling to downregulate hepatic sortilin 1 in obese and diabetic mice. Journal of lipid research. 2013;54:2754–2762. doi: 10.1194/jlr.M039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Wang Y, Matye DJ, Chavan H, Krishnamurthy P, Li F, Li T. Sortilin 1 Modulates Hepatic Cholesterol Lipotoxicity in Mice via Functional Interaction with Liver Carboxylesterase 1. The Journal of biological chemistry. 2017;292:146–160. doi: 10.1074/jbc.M116.762005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson AL, Knight JB. Regulation of GLUT4 expression in vivo and in vitro. Frontiers in bioscience : a journal and virtual library. 2003;8:s401–409. doi: 10.2741/1072. [DOI] [PubMed] [Google Scholar]
- 16.Shi J, Kandror KV. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev Cell. 2005;9:99–108. doi: 10.1016/j.devcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Kandror KV. The luminal Vps10p domain of sortilin plays the predominant role in targeting to insulin-responsive Glut4-containing vesicles. The Journal of biological chemistry. 2007;282:9008–9016. doi: 10.1074/jbc.M608971200. [DOI] [PubMed] [Google Scholar]
- 18.Huang G, Buckler-Pena D, Nauta T, Singh M, Asmar A, Shi J, Kim JY, Kandror KV. Insulin responsiveness of glucose transporter 4 in 3T3-L1 cells depends on the presence of sortilin. Mol Biol Cell. 2013;24:3115–3122. doi: 10.1091/mbc.E12-10-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Matye DJ, Wang Y, Li T. Sortilin 1 knockout alters basal adipose glucose metabolism but not diet-induced obesity in mice. FEBS letters. 2017 doi: 10.1002/1873-3468.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baltes J, Larsen JV, Radhakrishnan K, Geumann C, Kratzke M, Petersen CM, Schu P. sigma1B adaptin regulates adipogenesis by mediating the sorting of sortilin in adipose tissue. Journal of cell science. 2014;127:3477–3487. doi: 10.1242/jcs.146886. [DOI] [PubMed] [Google Scholar]
- 21.Moon YS, Smas CM, Lee K, Villena JA, Kim KH, Yun EJ, Sul HS. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Molecular and cellular biology. 2002;22:5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabinowich L, Fishman S, Hubel E, Thurm T, Park WJ, Pewzner-Jung Y, Saroha A, Erez N, Halpern Z, Futerman AH, Zvibel I. Sortilin deficiency improves the metabolic phenotype and reduces hepatic steatosis of mice subjected to diet-induced obesity. Journal of hepatology. 2015;62:175–181. doi: 10.1016/j.jhep.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Kaddai V, Jager J, Gonzalez T, Najem-Lendom R, Bonnafous S, Tran A, Le Marchand-Brustel Y, Gual P, Tanti JF, Cormont M. Involvement of TNF-alpha in abnormal adipocyte and muscle sortilin expression in obese mice and humans. Diabetologia. 2009;52:932–940. doi: 10.1007/s00125-009-1273-3. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Matye DJ, Li T. Insulin resistance induces posttranslational hepatic sortilin 1 degradation in mice. J Biol Chem. 2015;290:11526–11536. doi: 10.1074/jbc.M115.641225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60:2474–2483. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hivelin C, Mazella J, Coppola T. Sortilin derived propeptide regulation during adipocyte differentiation and inflammation. Biochemical and biophysical research communications. 2017;482:87–92. doi: 10.1016/j.bbrc.2016.10.139. [DOI] [PubMed] [Google Scholar]
- 27.Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1771–1776. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001;20:2180–2190. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goettsch C, Hutcheson JD, Aikawa M, Iwata H, Pham T, Nykjaer A, Kjolby M, Rogers M, Michel T, Shibasaki M, Hagita S, Kramann R, Rader DJ, Libby P, Singh SA, Aikawa E. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. The Journal of clinical investigation. 2016;126:1323–1336. doi: 10.1172/JCI80851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y, Doray B, Poussu A, Lehto VP, Kornfeld S. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science. 2001;292:1716–1718. doi: 10.1126/science.1060896. [DOI] [PubMed] [Google Scholar]
- 31.Kato Y, Misra S, Puertollano R, Hurley JH, Bonifacino JS. Phosphoregulation of sorting signal-VHS domain interactions by a direct electrostatic mechanism. Nature structural biology. 2002;9:532–536. doi: 10.1038/nsb807. [DOI] [PubMed] [Google Scholar]
- 32.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. The Biochemical journal. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sommercorn J, Mulligan JA, Lozeman FJ, Krebs EG. Activation of casein kinase II in response to insulin and to epidermal growth factor. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:8834–8838. doi: 10.1073/pnas.84.24.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klarlund JK, Czech MP. Insulin-like growth factor I and insulin rapidly increase casein kinase II activity in BALB/c 3T3 fibroblasts. J Biol Chem. 1988;263:15872–15875. [PubMed] [Google Scholar]
- 35.Trembley JH, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2 in health and disease: CK2: a key player in cancer biology. Cell Mol Life Sci. 2009;66:1858–1867. doi: 10.1007/s00018-009-9154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filhol-Cochet O, Loue-Mackenbach P, Cochet C, Chambaz EM. Casein kinase 2 and the cell response to growth factors. Cellular & molecular biology research. 1994;40:529–537. [PubMed] [Google Scholar]
- 37.Pagano MA, Poletto G, Di Maira G, Cozza G, Ruzzene M, Sarno S, Bain J, Elliott M, Moro S, Zagotto G, Meggio F, Pinna LA. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem : a European journal of chemical biology. 2007;8:129–139. doi: 10.1002/cbic.200600293. [DOI] [PubMed] [Google Scholar]
- 38.Pilch PF. The mass action hypothesis: formation of Glut4 storage vesicles, a tissue-specific, regulated exocytic compartment. Acta Physiol (Oxf) 2008;192:89–101. doi: 10.1111/j.1748-1716.2007.01788.x. [DOI] [PubMed] [Google Scholar]
- 39.Karki S, Chakrabarti P, Huang G, Wang H, Farmer SR, Kandror KV. The multi-level action of fatty acids on adiponectin production by fat cells. PloS one. 2011;6:e28146. doi: 10.1371/journal.pone.0028146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, Starr ME, Kim JT, Harris JW, Yiannikouris FB, Katz WS, Nilsson PM, Orho-Melander M, Chen J, Zhu H, Fahrenholz T, Higashi RM, Gao T, Morris AJ, Cassis LA, Fan TW, Weiss HL, Dobner PR, Melander O, Jia J, Evers BM. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533:411–415. doi: 10.1038/nature17662. [DOI] [PMC free article] [PubMed] [Google Scholar]