Abstract
Malignant tumors of the central nervous system (CNS) cause substantial morbidity and mortality, yet efforts to optimize chemo- and radiotherapy have largely failed to improve dismal prognoses. Over the past decade, RNA-sequencing (RNA-seq) has emerged as a powerful tool to comprehensively characterize the transcriptome of CNS tumor cells in one high-throughput step, leading to improved understanding of CNS tumor biology and suggesting new routes for targeted therapies. RNA-seq has been instrumental in improving diagnostic classification of brain tumors, characterizing oncogenic fusion genes, and shedding light on intra-tumor heterogeneity. Currently, RNA-seq is beginning to be incorporated into regular neuro-oncology practice in the form of precision neuro-oncology programs, which use information from tumor sequencing to guide implementation of personalized targeted therapies. These programs show great promise in improving patient outcomes for tumors where single agent trials have been ineffective. As RNA-seq is a relatively new technique, many further applications yielding new advances in CNS tumor research and management are expected in the coming years.
Keywords: RNA sequencing, Brain tumor, Neuro-Oncology, Precision Medicine, Microarray, Nanostring
INTRODUCTION
Cancer is characterized by global changes to the RNA transcriptome, causing aberrations of metabolism, immune signaling, cell growth, motility, and genome integrity.(1) While profiling of alterations in tumor DNA has certainly improved our understanding of tumorigenesis, assessment of RNA changes (the “transcriptome”) offers additional and non-redundant information on tumor biology. Many human cancers harboring the same histology and recurrent DNA alterations can be further characterized by widely distinct patterns of global gene expression.(2, 3) Transcriptional signatures now augment or surpass information on tumor biology and clinical prognosis provided by histology alone.(4) From a therapeutic perspective, changes in the sequence and quantity of RNA transcripts often translates into changes in their encoded proteins, resulting in cancer-specific druggable targets and immunogenic molecules.(5) In addition, many RNA molecules exert direct regulatory control over a variety of cellular processes, including transcription, translation, and protein function, and thus individual RNA species themselves can be valuable biomarkers or therapeutic targets.(6) For tumors of the CNS, transcriptional profiling has led to more precise molecular categorization of tumors and has identified novel tumor-specific transcripts that drive oncogenesis.(3, 7–10) Thus, the transcriptome represents a comprehensive description of the current state of the CNS tumor cell that is diagnostically and therapeutically relevant.
The emergence of RNA-seq over the past ten years represents a new era in which cancer transcriptomes can be comprehensively characterized in a high-throughput, unbiased manner. This technology is revolutionizing cancer research and clinical oncology and will be a key component of precision medicine protocols that aim to improve outcomes for aggressive cancers by using therapies tailored for individual patients. In this review, we discuss how RNA-seq is driving dramatic change in our understanding and treatment of CNS tumors.
RNA testing in clinical oncology and the power of RNA-seq
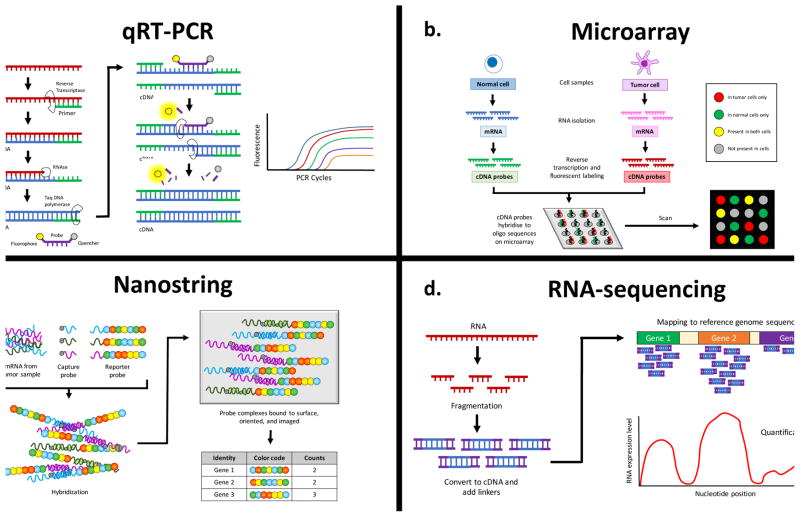
Measurement of individual RNA species has been used in clinical medicine for decades. Quantitative reverse transcription PCR (qRT-PCR) is considered the gold-standard technique due to high reproducibility and accuracy and is often used to confirm results obtained with newer methods (Figure 2A).(11) Beginning in the 1990s, DNA microarrays (ordered collections of DNA probes which hybridize to fluorescently labeled and reverse-transcribed RNA samples) allowed researchers to measure expression levels of thousands of transcripts simultaneously (Figure 2B).(12) Microarray studies demonstrated that RNA expression profiles could be used to categorize types of cancers and predict response to chemotherapy.(13) Hierarchical clustering of gene expression profiles can be used to sort tumors based on their transcriptional signatures (unsupervised analysis). Alternatively, it is possible to identify groups of genes that correlate with a particular tumor histology or type (supervised analysis), which is useful for highlighting genes involved in different tumor phenotypes.(13)
Figure 2.
Techniques for characterizing RNA transcripts. (A) qRT-PCR (TaqMan®). Sample RNA is converted into cDNA and amplified by PCR in the presence of a target-specific oligonucleotide bound to a fluorescent probe and fluorescence quencher. As DNA polymerase synthesizes the new DNA strand, it cleaves the fluorescent probe off the oligonucleotide, freeing it to fluoresce. The fluorescence grows stronger with each PCR cycle as more fluorescent probes are freed. (B) Microarray. RNA is extracted from normal and tumor cells, reverse transcribed, and labeled with fluorescent probes (green for normal cDNA, red for tumor cDNA). The cDNAs are applied to a microarray chip, where they bind to complementary sequences from annotated genes. The relative amount of green versus red fluorescence corresponds to the relative expression of genes in normal versus tumor cells. (C) Nanostring. Extracted RNA is hybridized with target-specific capture probes and reporter probes. The reporter probes contain precisely ordered fluorescent barcodes to identify each target. The probe-target complexes are then aligned by an electric current, and the fluorescent barcodes are counted to digitally quantitate each target. (D) RNA-seq. Extracted RNA is fragmented, reverse-transcribed, and modified with linkers to aid sequencing. The cDNA is sequenced with NGS technology, and the resulting sequences are aligned against a reference genome to reveal the expression levels of various genes in the sample.
A number of multi-gene expression panels have been approved for use in clinical oncology.(14, 15) However, microarrays have been limited in the clinical setting by problems with lab-to-lab variability and low signal-to-noise ratios.(11) The Nanostring nCounter system was developed to quantitate the expression levels of hundreds of genes in poor-quality clinical samples with improved sensitivity, dynamic range, and reproducibility.(16) It uses a capture probe and a reporter probe with a fluorescent bar code to digitally count transcripts and is capable of detecting lower abundance mRNAs than microarrays (Figure 2C).(17) Prosigna is a Nanostring-based clinical assay that was FDA-cleared in 2013 and measures the expression of 50 genes to estimate the risk of distant recurrence in hormone-positive breast cancer.(18) Since then, it has become the most widely used Nanostring-based clinical test in oncology, and has driven some clinical labs to obtain a Nanostring device.(18, 19)
One of the most prominent drawbacks of hybridization-based approaches is the inability to detect transcripts with previously unknown significance for a given cancer.(20) RNA-seq solves this problem because it uses next generation sequencing (NGS) technology to comprehensively characterize the transcriptome to single-base resolution in one high-throughput step, without the need for transcript-specific probes (Figure 2D). It has a wider dynamic range than microarrays, is highly accurate and reproducible for both technical and biological replicates, and requires less RNA sample than microarrays.(20) Importantly, RNA-seq can characterize expressed fusion genes, to which cancers often exhibit extreme oncogene addiction due to their unique functions that cannot be recapitulated by overexpressing either partner protein alone.(21–23) In contrast to DNA sequencing, RNA-seq uniquely allows detection of tumor-specific alternative splicing in addition to RNA editing events, which play a critical role in glioblastoma (GBM) and other CNS tumors.(24–26) Furthermore, RNA-seq is valuable for interpreting the significance of genetic variants found at the DNA level. For example, RNA-seq can determine whether heterozygous single nucleotide polymorphisms (SNPs) cause differences in allele-specific expression in cancer cells compared to normal cells,(27) and it can be used to determine the extent to which truncating mutations cause nonsense-mediated transcript decay.(28) From a diagnostics perspective, RNA-seq can characterize extracellular RNAs (exRNAs) in blood, CSF, or other body fluids that may be more accessible than tissues.(11) Finally, RNA-seq can be used to characterize long non-coding RNAs (lncRNAs), microRNAs (miRNAs), PIWI-interacting RNAs, and tRNAs that are emerging as important mediators of oncogenesis.(11)
RNA-seq has been approved for clinical use in a small number of cases,(29) but several challenges have thus far limited the more widespread adoption of RNA-seq in the clinic (reviewed in (11)). One major challenge is that most pathology samples are usually formalin-fixed and paraffin-embedded, which causes partial degradation of RNA. To overcome this, some sequencing programs, such as the MiOncoSeq platform at the University of Michigan, have moved to an exon-targeting RNA probe capture library, which can more readily detect splice junctions, fusions, and variants in degraded samples than a traditional polyA-enrichment protocol.(30) Another barrier to clinical implementation is the complexity and nonstandardization of existing RNA-seq data acquisition and analysis protocols, raising questions about how RNA-seq tests will be regulated and certified. Recently, full-packaged pipelines have emerged with the intent of being used for clinical applications,(31, 32) and costs of RNA-seq have fallen substantially due to improvements in construction of cDNA libraries and reductions in costs of NGS.(33, 34) Given the potential for RNA-seq to transform clinical testing, there is widespread optimism that remaining obstacles can be overcome to demonstrate analytic validity and clinical utility.(11)
RNA-SEQ ILLUMINATES KEY FEATURES OF BRAIN TUMORS
Almost five thousand children will be diagnosed with brain tumors in the United States in 2017, making brain cancer the most common solid pediatric malignancy and the most deadly cancer in children and adolescents.(35, 36) In addition, the survivors of pediatric brain cancer frequently experience long-term neurological or neuroendocrine sequelae.(37) Recent RNA-seq of large cohorts of pediatric brain tumors have revealed exciting new insights, such as the fact that many of these tumors are defined by fusions and rearrangements, as seen in extracranial solid tumors and leukemias.(9, 38, 39) Although the routine application of RNA-seq in clinical laboratories is in its infancy, and for the most part limited to research studies, there are some key observations in large retrospective cohorts profiled by RNA-seq that illustrate the power of RNA-seq in routine care.(9, 38, 39) RNA-seq as a diagnostic tool allows for improved classification and identification of actionable lesions and will be a key component of the practice of neuro-oncology moving forward.
One excellent example of RNA-seq helping to redefine the classification of a tumor type is the entity formerly classified as primitive neuroectodermal tumors of the CNS (CNS-PNET). Historically, CNS-PNET have been considered a very heterogeneous entity, with widely variable outcome and histologic findings. A recent study of 323 institutionally diagnosed CNS-PNET profiled by genome-wide methylation arrays and transcriptional profiling revealed that over 60% of cases could be re-classified as another entity when clustered with a large reference set of well-defined brain tumors.(9) Interestingly, of the remainder, four distinct clusters emerge with distinct genetic lesions identified using a combined approach of DNA and RNA sequencing. These new entities termed “CNS neuroblastoma with FOXR2 activation” (CNS NB-FOXR2), “CNS Ewing sarcoma family tumor with CIC alteration” (CNS EFT-CIC), “CNS high-grade neuroepithelial tumor with MN1 alteration” (CNS HGNET-MN1), and “CNS high-grade neuroepithelial tumor with BCOR alteration” (CNS HGNET-BCOR), are defined by distinct recurrent gene fusions. The CNS NB-FOXR2 sub-group has multiple recurrent fusions involving FOXR2 with JMJD1C or various other fusion partners, resulting in activation of FOXR2 expression. In other cases, amplifications and deletions bring enhancers into close proximity, likewise resulting in activation of FOXR2 expression. Many of these events, including FOXR2 fusions and activation of FOXR2, are detectable using routine RNA-seq. CNS EFT-CIC tumors are characterized by CIC rearrangements detectable by break-part FISH, including a recurrent CIC-NUTM1 gene fusion, as well as upregulation of the ETS transcription factor family, similar to peripheral Ewing sarcoma. CNS HGNET-MN1 tumors are characterized by fusions detectable by break-apart FISH involving the MN1 gene, which has previously been found to be activated in leukemias. Interestingly, astroblastomas, a tumor type that was previously very poorly defined, have similar DNA methylation profiles to the CNS HGNET-MN1 group. Finally, CNS HGNET-BCOR tumors harbor in-frame internal tandem duplications of BCOR which can be identified either by RNA or DNA sequencing.(9)
The clinical implications of these four new entities are still being established, but their classification nevertheless provides a powerful diagnostic tool to distinguish them from other morphological mimics. The same study revealed that entities such as pediatric supratentorial ependymoma and glioblastoma were frequently misdiagnosed as CNS-PNET.(9) Altogether, the study demonstrates that RNA-seq can improve upon the use of FISH probes by providing fusion analysis of multiple targets at once, allowing for both an unbiased and more cost-effective evaluation of diagnostic fusions. Given that recurrent oncogenic gene fusions occur in multiple CNS tumor types, RNA-seq will be an invaluable tool for their diagnosis and classification.
Molecular profiling has also improved understanding of other embryonal tumors such as atypical teratoid rhabdoid tumors (ATRT). As early as the 1980s, neuropathologists noted some small round blue cell tumors of a teratoid rhabdoid appearance had monosomy 22 and behaved distinctly from medulloblastoma in terms or poorer prognosis and younger age distribution.(40) DNA microarray gene expression data later revealed that ATRT, PNET, and medulloblastoma are, in fact, molecularly distinct tumors.(41) Cytogenetic screening showed that ATRT harbor homozygous inactivation of SMARCB1 by deletion or mutation in chromosome 22q11.2,(42) and exome sequencing confirmed that loss of SMARCB1 is the only recurrent coding alteration.(43) Nevertheless, DNA methylation profiling, whole-exome DNA sequencing, and RNA-seq have established three distinct molecular subgroups: ATRT-TYR, ATRT-MYC, ATRT-SHH, which have distinct gene expression profiles.(44, 45) ATRT-TYR is defined by an over-expression of the melanosomal markers DCT, MITF, or TYR and is frequently found infratentorially in children under 1 year of age.(44) ATRT-MYC tumors overexpress MYC and are most often supratentorial. A minority of ATRT cases retain SMARCB1 expression, harbor SMARCA4 mutations, and cluster in the ATRT-SHH subgroup of tumors, which overexpress MYCN and GLI2.(44)
Medulloblastoma, a small round blue cell tumor of the cerebellum, historically had been stratified only by clinical staging. However, a series of profoundly impactful studies have distinguished four biologically distinct molecular subgroups of medulloblastoma: WNT, SHH, Group 3, and Group 4.(46–52) The WNT group, characterized by CTNNB1 mutations, was the first clinically described subgroup and affected patients experience very good outcomes with greater than 90% overall survival (OS).(46, 52, 53) WNT medulloblastomas are characterized by activation of the WNT pathway, as evidenced by expression of many downstream WNT targets.(51) Several mutations associated with the hedgehog pathway, including SUFU and SMO, were already known to be associated with medulloblastoma, but RNA expression analysis helped distinguish the SHH subgroup that included frequent PTCH1 mutations and a higher incidence of affected infants (Figure 1).(47, 54) Group 3 medulloblastomas are characterized by pronounced overexpression of MYC, the greatest incidence of metastatic disease, and the worst outcomes with long-term survival less than 50%.(47, 50) RNA-seq revealed group 3 medulloblastomas frequently harbor PVT1-MYC fusions, resulting in high levels of MYC expression, or PVT1-NDRG1 fusions.(39) In multiple cancer types, inhibition of BET bromodomains suppresses the MYC-associated transcriptional signature, and studies suggest that bromodomain inhibitors may be a viable therapeutic strategy in medulloblastoma with MYC overexpression (Figure 1).(55)
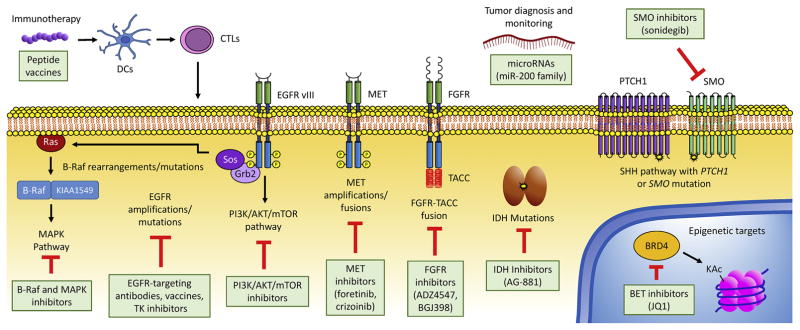
Figure 1.
Key molecular alterations in CNS tumors. Many of the alterations identified in this schematic are targetable through approved or investigational drugs. RNA-seq is especially valuable for characterizing expressed fusion genes, but future applications may include the development of personalized immunotherapies and detection of extracellular RNAs for tumor diagnosis and monitoring. DC: dendritic cell; CTL: cytotoxic lymphocyte; TK: tyrosine kinase; KAc: acetylated lysine.
In contrast, recurrent gene fusions have not been identified in group 4 medulloblastoma, although tandem duplication of the Parkinson’s disease-associated gene SNCAIP occurs in 10% of cases.(39) A comprehensive analysis of the enhancer landscape in group 4 tumors using ChIP-seq, RNA-seq, and DNA methylation profiling led to the identification of LMX1A as a master transcriptional regulator for this sub-group, and upper rhombic lip cells, in which LMX1A is a critical regulator, were proposed as the likely cells-of-origin for group 4 tumors.(56) As the subgroups of medulloblastoma have distinct epidemiology, response to various therapies and prognosis, it is critical to routinely subgroup these tumors through comprehensive, validated approaches such as limited gene expression panels and genome-wide methylation arrays.(57) NanoString platforms, such as one developed at The University of Toronto/Sick Kids Hospital to perform subgroup analysis of medulloblastoma using expression data for just 22 targets, are currently quicker and more effective than RNA-seq for identifying subgroup alone.(57)
Pilocytic astrocytomas represent a minority of adult gliomas but are the most common primary brain tumor of childhood, accounting for nearly 20% of all pediatric CNS tumors.(58) Most of these tumors exhibit BRAF aberrations, especially tandem duplications resulting in KIAA1549-BRAF fusion genes and less frequently BRAF V600E mutations (Figure 1).(59, 60) These mutations are important beyond low-grade gliomas, as a subset of histologically diagnosed GBM display pleomorphic xanthoastrocytoma-like methylation profiles and BRAF V600E mutations, and these tumors are associated with a prognosis intermediate between classic GBM and low-grade glioma.(61) Interestingly, gene expression profiles may be able to distinguish between BRAF-KIAA-duplicated and BRAF V600E-mutated, and can potentially differentiate pilocytic astrocytomas and diffuse gliomas.(62, 63) In the subset of pilocytic astrocytoma cases in which KIAA1549-BRAF fusions are not present, RNA-seq was critical in the identification of additional fusion genes involving BRAF or RAF1, including RNF130-BRAF, NFIA-RAF1, and others (64–66). Multiple clinical trials investigating BRAF inhibitors and other inhibitors of the MAPK pathway for the treatment of low-grade pediatric gliomas are currently ongoing.(67)
Ependymomas have long been known to undergo divergent clinical courses and survival outcomes.(68, 69) Recent molecular analyses have clarified the clinical diversity of this histopathologic diagnosis.(70) Ependymomas are clinically and molecularly subdivided into two groups: supratentorial and infratentorial (posterior fossa). Whole genome and RNA sequencing isolated a novel fusion, C11orf95-RELA, in a majority of supratentorial ependymomas, while the remaining tumors frequently harbor C11orf95-YAP1.(38) The C11orf95-RELA fusion is both a prognostic and diagnostic marker in supratentorial ependymoma, and is now included as a diagnostic tool in the revised 2016 WHO classification.(38, 71) The C11orf95 fusion partner is also clinically significant as patients with RELA-fusion ependymomas have a median age of 8 years and 57% of these patients will experience recurrence, while YAP1-fusion ependymomas most commonly occur under 2 years of age and may be less likely to progress or recur.(71)
Posterior fossa ependymomas, which are distinct from supratentorial ependymoma by methylation and RNA expression profiling, can be further classified into two sub-groups, PF-EPN-A and PF-EPN-B.(71, 72) PF-EPN-A ependymomas display a promotor CpG island methylator phenotype (CIMP+), occur at a median age of 3 years, and frequently recur.(71, 72) PF-EPN-B ependymomas have a CIMP− phenotype, are more common in adults with a median age of 30 years, and are associated with a better prognosis.(71, 72)
Over 70,000 American adults will be diagnosed with a primary brain tumor in 2017, leading to extensive morbidity and mortality, with especially dismal prognoses for the approximately 1/3 of brain tumors that are malignant.(35) GBM (grade IV glioma) is the most common and most aggressive type of CNS tumor in adults, with a median survival of 14.6 months after treatment with surgery, radiation, and chemotherapy.(73) Molecular profiling of gliomas led to identification of five groups with distinct prognoses: tumors harboring mutations in the TERT promoter, mutations in IDH, and codeletion of chromosome arms 1p and 19q (oligodendrogliomas); tumors with TERT and IDH mutations (grade II and III astrocytomas); tumors with IDH mutations only (grade II and III astrocytomas); triple-negative tumors (majority are grade IV gliomas); and tumors with TERT mutations only (majority are grade IV gliomas, worst prognosis).(74) Comprehensive multi-platform studies that include RNA-seq have recently succeeded in further sub-classifying gliomas according to gene expression and DNA methylation, and have identified factors driving progression from low grade glioma to full-blown GBM, such as hypomethylation and overexpression of cell cycle genes.(7, 75) Another important contribution of RNA-seq to the molecular characterization of GBM was its use in identifying fusion transcripts and other altered transcripts in GBM, such as FGFR-TACC fusions, EGFR-SEPT14 fusions, EGFRvIII, and PTPRZ1-MET fusions in secondary GBM.(10, 76–78)
RNA-seq-based studies have also been instrumental in characterizing intra-tumoral heterogeneity within GBM tumors, and revealing that GBM contains a mixture of cells with varying genomic and transcriptomic profiles.(3) Interestingly, gene copy number changes are generally conserved in nearly all cells within an individual GBM, but expression profiles are much more heterogeneous. Recent sequencing efforts of individual GBM cells demonstrate large transcriptional diversity among cells from the same tumor and no clear boundaries separating the transcriptional signatures of different tumors.(79) In addition, individual tumors contain a wide range of cells with varying amounts of cell cycle, hypoxia, and differentiation (“stemness”) gene expression signatures. Increased tumor heterogeneity is associated with decreased survival, underscoring the contribution of heterogeneity to tumor aggressiveness.(79)
From a therapeutics perspective, intra-tumoral heterogeneity increases the chances that a subset of cells are resistant to a given small molecule inhibitor. To illustrate this point, amplifications and/or mutations in EGFR are oncogenic drivers in approximately 50% of GBM and are a leading molecular target for therapeutic intervention (Figure 1).(80, 81) However, small molecule inhibitors of EGFR have had disappointing clinical results in GBM patients.(82) One explanation for the ineffectiveness of EGFR inhibition is that GBM contains distinct subpopulations of cells with amplification of different receptor tyrosine kinases (RTK).(83, 84) While one subpopulation may be sensitive to EFGR inhibition, another subpopulation with amplification of PDGFRA or MET would be resistant.(83, 84) Furthermore, different subpopulations within a GBM tumor harbor different molecular alterations in the EGFR gene, and different EGFR variants could have varying sensitivities to any single small molecule EGFR inhibitor.(79, 80, 85) Thus, successful treatment of GBM could potentially require a cocktail of inhibitors targeting multiple RTKs and multiple RTK variants.(85) In the future clinical setting, RNA-seq of bulk tumor and single cells could be used to characterize the ensemble of RTK alterations and aid selection of particular targeted therapies.
RNA-SEQ AS A COMPONENT OF PRECISION NEURO-ONCOLOGY
For aggressive brain cancers, attempts to optimize cytotoxic chemotherapy and radiation regimens have failed to improve dismal prognoses. Precision neuro-oncology programs have begun using NGS technologies to identify oncogenic molecular alterations that can be targeted with rationally designed inhibitors. A unique advantage of RNA-seq in the context of precision medicine is its ability to detect expressed fusion genes that are often oncogenic drivers in brain cancer.(22) For example, RNA-seq was used to identify the FGFR3-TACC3 fusion gene in 3.1% of adult GBM (Figure 1). FGFR3-TACC3 gains constitutive kinase activity that causes invasive, rapidly growing high-grade glioma.(76) Small molecule FGFR3 inhibitors prolong survival of mice harboring intracranial gliomas driven by FGFR3-TACC3.(76) Compassionate use of an FGFR inhibitor in two GBM patients showed promising results, supporting further clinical investigation of such drugs in FGFR-TACC-positive patients.(86)
As another example, RNA-seq has been valuable in characterizing gene fusions involving the MET oncogene, which are found in 10% of pediatric GBM as well as 15% of adult secondary GBM, where they are associated with a poor prognosis (Figure 1).(10, 87) MET inhibitors like crizotinib are promising therapies for the treatment of GBMs harboring MET fusions or amplifications.(87) As discussed above, RNA-seq can also be used to identify C11orf95–RELA fusions that drive oncogenic NF-κB signaling in more than two-thirds of supratentorial ependymoma,(38) PVT1-MYC fusions in medulloblastoma,(39) and TTYH1-C19MC fusions in embryonal tumors with multilayered rosettes,(88) all of which carry important diagnostic and prognostic information.
Clinical studies suggest that precision oncology approaches have the potential to dramatically improve outcomes for cancer patients. A meta-analysis of 570 Phase II studies containing 32,149 cancer patients showed that those receiving a precision treatment strategy had a significantly higher median response rate (31% versus 10.5%) and median progression-free survival (5.9 versus 2.7 months) compared with standard treatment.(89) This study defined precision therapy very conservatively, including treatments based on a single biomarker.(89) The incorporation of comprehensive sequencing data into precision medicine programs has the potential to further improve outcomes.
Pioneering precision medicine programs, such as INFORM (Germany) and MiOncoSeq (University of Michigan), have used RNA-seq to screen for fusions, structural variants, and gene expression changes that have diagnostic and therapeutic implications.(22, 90, 91) In a pilot INFORM study, a set of pediatric tumors was analyzed with whole-exome, low-coverage whole-genome, and RNA sequencing together with methylation and expression microarray. 26/52 (50%) patients harbored a potentially targetable alteration, and in ten patients the physician decided to administer a matched targeted therapy, with responses observed in some tumors that did not respond to traditional treatments.(90) Our group recently applied paired germline and tumor DNA and RNA sequencing to 50 children and young adults with high-risk brain tumors through the MiOncoSeq program, and found high feasibility and actionability, especially in glial brain tumors (manuscript in preparation).
These groundbreaking precision oncology studies frequently identified multiple targets per patient, suggesting that combined therapies may optimize response in future studies.(22, 90) Their results also suggested that clinical sequencing can be used not only to identify treatment targets but also to characterize genetic variants that would affect absorption, distribution, metabolism and excretion (ADME) properties of candidate drugs.(90) Finally, these studies revealed areas for future improvement, such as longer-than-expected sequencing times that delayed clinical action and a lack of FDA-approved agents for pediatric patients.(22)
One consideration regarding a precision oncology-based approach for malignant brain tumors is their intra-tumoral heterogeneity, which raises concern that one biopsy sample may not capture molecular alterations found in other regions of the tumor.(79) In addition to heterogeneity within a GBM tumor, recurrent gliomas exhibit substantial genetic divergence from the initial tumor that cannot be accounted for by heterogeneity in the original tumor.(92) Therefore, careful biopsy planning, with re-biopsy and re-profiling of recurrent malignant brain tumors, will be necessary for optimal responses.(92)
INVESTIGATIONAL APPLICATIONS OF RNA-SEQ TO NEURO-ONCOLOGY
Identifying significant alterations and pathways
RNA-seq can be a powerful adjunct in the evaluation of expression of mutant alleles derived from DNA-panel sequencing. As next generation panel DNA sequencing is becoming more prevalent in routine clinical use, previously unreported variants are frequently being observed where it is unclear if these variants are activating or damaging. In many instances relevant immunohistochemistry is not available, but the expression of the mutant transcript can help in determining the nature of the mutation. Similarly, rearrangements identified by either SNP arrays or whole genome sequencing can be evaluated by RNA-seq to determine if coding exons are fused in frame, which can be extremely helpful in the workup of novel aberrations. Thus, RNA-seq data can provide an additional layer of evidence in determining if an experimental therapy is warranted when actionable targets are identified using either DNA sequencing or copy-number arrays.
Another area of tremendous clinical potential is the evaluation of actionable signaling pathways using RNA-seq. This is particularly exciting in conditions such as GBM where actionable fusions are rare, and recurrent somatic nucleotide variants are not actionable. Pathway analysis can be performed using a variety of tools including Gene Set Enrichment Analysis (GSEA) and g:Profiler, and has the potential to identify pathways that are aberrant rather than single lesions.(93, 94) Single sample pathway analysis is becoming more refined as methods such as single-sample GSEA (ssGSEA) and Individual Gene Sets Analysis (IGSA) have the potential to allow single patient samples to be analyzed for pathway activation.(95–97) As network analysis becomes more sophisticated, single runs of RNA-seq can be analyzed for pathway over-activation in a reproducible, robust and reliable manner. This can be of special importance in samples where hundreds of somatic nucleotide variants are observed, or alternatively in the evaluation of a somatic nucleotide variant of unknown significance. The demonstration that downstream targets of an activated and actionable mutation or fusion are overexpressed provides additional evidence that a driver event has been uncovered, and provides further rationale for targeted therapies. Although in its infancy, pathway analysis using RNA-seq has the potential to open novel therapeutic avenues in neuro-oncology.
Development of new diagnostics and therapeutics
RNA-seq is an ongoing source of progress in many other aspects of neuro-oncology research, diagnosis, and treatment. In the lab, transcriptome analysis will continue to be a valuable method for investigating brain tumor biology. For example, RNA-seq was recently used to characterize the molecular mechanism of an inherited non-coding single nucleotide polymorphism, rs55705857, which is strongly associated with IDH-mutant glioma development (Figure 1).(98) RNA-seq and proteomic analyses of grade II gliomas demonstrated that the risk allele is associated with increased MYC transcriptional network activity. Notably, RNA-seq identified expression of enhancer-associated RNA, a feature of active enhancers, from the genetic region encompassing rs55705857, which helped to implicate the region as an enhancer of MYC expression.(98) The identification of massive over-expression of oncogenes being driven by non-coding mutations in super-enhancer regions will likely only become more frequent as this field is refined, in part using RNA-seq.
RNA-seq can be used to characterize not only mRNAs but also miRNAs and lncRNAs. Measurement of miRNAs in CSF using RNA-seq is a potential source of new diagnostic and prognostic biomarkers for CNS tumors (Figure 1). For example, the levels of miRNAs miR-200a and miR-125b distinguish GBM from brain metastases with 95% accuracy.(99) MiRNAs could also be used to monitor tumor status after treatment, as miR-10b and miR-200 family members are not detectable in CSF of GBM and brain metastases patients in remission.(99) Additionally, extracellular miRNA-containing vesicles released from GBM could theoretically be isolated from CSF and sequenced for monitoring brain tumor status.(100, 101) RNA-seq can likewise be used to characterize lncRNAs, which have an emerging role in oncogenesis.(102) In brain tumors, lncRNA expression profiles have been used to distinguish different histologic subtypes of glioma and to differentiate primary from recurrent glioma.(103, 104) The lncRNAs HOTAIR and Pnky have been proposed as therapeutic targets in glioma based on their overexpression in tumor cells and role in inhibiting differentiation.(105, 106)
Single cell RNA-seq (scRNA-seq) is an especially exciting tool that will be invaluable in the study of heterogeneous transcriptional profiles within brain tumors. The power of this technique was demonstrated in the characterization of transcriptomes of individual cells from normal human brain samples, with all major cell types of the brain identified.(107) For heterogeneous brain tumors, scRNA-seq has likewise become an important method for molecularly characterizing the range of cells within a tumor. For instance, scRNA-seq revealed that IDH-mutant gliomas contain three subpopulations of cells: two non-proliferating subpopulations with astrocyte-like and oligodendrocyte-like expression programs, and a third subpopulation of undifferentiated stem-like cells with increased proliferation potential.(108, 109) The identification of cancer stem cells (CSCs) in glioma raises the possibility that therapies designed to induce differentiation of CSCs could halt cancer growth.(109) Additionally, the heterogeneity of brain tumors may be fueled by epigenetic reprogramming of differentiated cancer cells back to CSCs, which is augmented in the presence of chemotherapy agents and microenvironmental factors like hypoxia.(110, 111) Thus, treatments that target the reprogramming process could reduce tumor heterogeneity and tumor aggressiveness (Figure 1).(79, 110)
RNA-seq will also be an invaluable tool in the development of anticancer immunotherapies, as it can characterize RNA species that may be translated into tumor-specific antigens with unique amino acid sequences not present in normal cells (Figure 1). One common mechanism for generating tumor-specific transcripts is alternative splicing, by which exons and introns are variously skipped or included.(112) For example, in B-cell lymphoma an alternative splicing event of CD20 creates a tumor-specific splice junction which could be leveraged as an immunotherapy, as a peptide that spans the splice junction is capable of activating CD8 and CD4 T-cell responses in transgenic mice.(113) Interestingly, CNS tumors are enriched in tissue-specific splicing events, suggesting that CNS tumors may be particularly amenable to alternative transcript-directed immunotherapies.(114) Notably, immunotherapy is already a promising treatment strategy for some CNS tumors, including mutated IDH1 peptides for glioma.(115, 116)
In the future, comprehensive genome and transcriptome analysis by DNA and RNA sequencing could be used to develop precision cancer vaccines based on the somatic mutations and variants found in individual brain tumors.(117, 118) In particular, RNA-seq could identify a pool of immunogenic mutations for each patient that could be targeted in combination to create multivalent T-cell targeting of the tumor.(118) Such efforts would need to overcome a number of obstacles, including the fact that not all cancer-specific mutations are immunogenic, and many tumors are surrounded by a highly immunosuppressive environment.(117, 118)
CONCLUSIONS
The ability to comprehensively characterize cancer transcriptomes is rapidly altering the practice of neuro-oncology. RNA-seq has clearly surpassed previous tools for expression analysis. Its use in clinical neuro-oncology will continue to expand with the current steady pace of improvements in terms of efficiency and reduction of its cost. RNA-seq has already provided important insights into brain cancer biology, including improving brain tumor diagnosis and the identification of prognostic subgroups. Furthermore, RNA-seq is beginning to be used in clinical precision oncology programs to identify targetable genetic alterations for individual patients. We look forward to and support the routine integration of RNA-seq into the diagnostic approach for all high-grade brain tumors. It is now clear that RNA-seq will play a role in providing much-needed improved outcomes for patients with malignant brain cancers.
Acknowledgments
C.K. is supported by NIH/NINDS Grant K08-NS099427-01. D.S.R. acknowledges training grant support from the University of Michigan Chemistry-Biology Interface (CBI) training program (NIH Grant 5T32GM008597) and from the University of Michigan Medical Scientist Training Program (NIH Grant 5T32GM007863). N.A.V. is supported by the McKenna Claire Foundation for Pediatric Brain Cancer. V.R. is supported by grants from Meagan’s Walk, the Garron Family Cancer Center and the Collaborative Ependymoma Research Network. The authors have read the journal’s statement on potential conflicts of interest and have no financial or personal relationships with organizations that could potentially be perceived as influencing the described research. All authors have read the journal’s authorship statement, and the manuscript has been reviewed by and approved by all named authors. No editorial support was used in the writing of this manuscript.
Abbreviations
- CNS
central nervous system
- RNA-seq
RNA sequencing
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- NGS
next generation sequencing
- GBM
glioblastoma
- SNPs
single nucleotide polymorphisms
- exRNAs
extracellular RNAs
- lncRNAs
long non-coding RNAs
- miRNAs
microRNAs
- CNS-PNET
primitive neuroectodermal tumors of the CNS
- CNS NB-FOXR2
CNS neuroblastoma with FOXR2 activation
- CNS EFT-CIC
CNS Ewing sarcoma family tumor with CIC alteration
- CNS HGNET-MN1
CNS high-grade neuroepithelial tumor with MN1 alteration
- CNS HGNET-BCOR
CNS high-grade neuroepithelial tumor with BCOR alteration
- ATRT
atypical teratoid rhabdoid tumors
- CIMP
CpG island methylator phenotype
- RTK
receptor tyrosine kinase
- ADME
absorption, distribution, metabolism and excretion
- GSEA
Gene Set Enrichment Analysis
- ssGSEA
single-sample Gene Set Enrichment Analysis
- IGSA
Individual Gene Sets Analysis
- CSC
cancer stem cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Torchia J, Golbourn B, Feng S, et al. Integrated (epi)-Genomic Analyses Identify Subgroup-Specific Therapeutic Targets in CNS Rhabdoid Tumors. Cancer Cell. 30(6):891–908. doi: 10.1016/j.ccell.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhaak RGW, Hoadley KA, Purdom E, et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sager M, Yeat NC, Pajaro-Van der Stadt S, Lin C, Ren Q, Lin J. Transcriptomics in cancer diagnostics: developments in technology, clinical research and commercialization. Expert Review of Molecular Diagnostics. 2015;15(12):1589–603. doi: 10.1586/14737159.2015.1105133. [DOI] [PubMed] [Google Scholar]
- 5.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16(4):219–33. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 6.Arun G, Diermeier S, Akerman M, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes & Development. 2016;30(1):34–51. doi: 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceccarelli M, Barthel Floris P, Malta Tathiane M, et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell. 2016;164(3):550–63. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comprehensive Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. New England Journal of Medicine. 2015;372(26):2481–98. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturm D, Orr Brent A, Toprak Umut H, et al. New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. Cell. 2016;164(5):1060–72. doi: 10.1016/j.cell.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao ZS, Chen HM, Yang MY, et al. RNA-seq of 272 gliomas revealed a novel, recurrent PTPRZ1-MET fusion transcript in secondary glioblastomas. Genome Res. 2014;24(11):1765–73. doi: 10.1101/gr.165126.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byron SA, Van Keuren-Jensen KR, Engelthaler DM, Carpten JD, Craig DW. Translating RNA sequencing into clinical diagnostics: opportunities and challenges. Nat Rev Genet. 2016;17(5):257–71. doi: 10.1038/nrg.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo G, Zegar C, Giordano A. Advantages and limitations of microarray technology in human cancer. Oncogene. 2003;22(42):6497–507. doi: 10.1038/sj.onc.1206865. [DOI] [PubMed] [Google Scholar]
- 13.Mischel PS, Cloughesy TF, Nelson SF. DNA-microarray analysis of brain cancer: molecular classification for therapy. Nat Rev Neurosci. 2004;5(10):782–92. doi: 10.1038/nrn1518. [DOI] [PubMed] [Google Scholar]
- 14.Slodkowska EA, Ross JS. MammaPrint™ 70-gene signature: another milestone in personalized medical care for breast cancer patients. Expert Review of Molecular Diagnostics. 2009;9(5):417–22. doi: 10.1586/erm.09.32. [DOI] [PubMed] [Google Scholar]
- 15.Cronin M, Sangli C, Liu M-L, et al. Analytical Validation of the Oncotype DX Genomic Diagnostic Test for Recurrence Prognosis and Therapeutic Response Prediction in Node-Negative, Estrogen Receptor–Positive Breast Cancer. Clinical Chemistry. 2007;53(6):1084. doi: 10.1373/clinchem.2006.076497. [DOI] [PubMed] [Google Scholar]
- 16.Veldman-Jones MH, Brant R, Rooney C, et al. Evaluating Robustness and Sensitivity of the NanoString Technologies nCounter Platform to Enable Multiplexed Gene Expression Analysis of Clinical Samples. Cancer Research. 2015;75(13):2587. doi: 10.1158/0008-5472.CAN-15-0262. [DOI] [PubMed] [Google Scholar]
- 17.Geiss GK, Bumgarner RE, Birditt B, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotech. 2008;26(3):317–25. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 18.Wallden B, Storhoff J, Nielsen T, et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med Genomics. 2015;8:54. doi: 10.1186/s12920-015-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NanoString Technologies, Inc. Nanostring Technologies. 2017 [Available from: http://www.nanostring.com/company/corp_overview.
- 20.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertens F, Johansson B, Fioretos T, Mitelman F. The emerging complexity of gene fusions in cancer. Nat Rev Cancer. 2015;15(6):371–81. doi: 10.1038/nrc3947. [DOI] [PubMed] [Google Scholar]
- 22.Mody RJ, Wu YM, Lonigro RJ, et al. Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA. 2015;314(9):913–25. doi: 10.1001/jama.2015.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Xia J, Jia P, Pao W, Zhao Z. Application of next generation sequencing to human gene fusion detection: computational tools, features and perspectives. Briefings in Bioinformatics. 2012 doi: 10.1093/bib/bbs044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickrell JK, Marioni JC, Pai AA, et al. Understanding mechanisms underlying human gene expression variation with RNA sequencing. Nature. 2010;464(7289):768–72. doi: 10.1038/nature08872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han L, Diao L, Yu S, et al. The Genomic Landscape and Clinical Relevance of A-to-I RNA Editing in Human Cancers. Cancer Cell. 28(4):515–28. doi: 10.1016/j.ccell.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galeano F, Rossetti C, Tomaselli S, et al. ADAR2-editing activity inhibits glioblastoma growth through the modulation of the CDC14B/Skp2/p21/p27 axis. Oncogene. 2013;32(8):998–1009. doi: 10.1038/onc.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuch BB, Laborde RR, Xu X, et al. Tumor Transcriptome Sequencing Reveals Allelic Expression Imbalances Associated with Copy Number Alterations. PLOS ONE. 2010;5(2):e9317. doi: 10.1371/journal.pone.0009317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivas MA, Pirinen M, Conrad DF, et al. Effect of predicted protein-truncating genetic variants on the human transcriptome. Science. 2015;348(6235):666. doi: 10.1126/science.1261877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He J, Abdel-Wahab O, Nahas MK, et al. Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood. 2016 doi: 10.1182/blood-2015-08-664649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cieslik M, Chugh R, Wu YM, et al. The use of exome capture RNA-seq for highly degraded RNA with application to clinical cancer sequencing. Genome Res. 2015;25(9):1372–81. doi: 10.1101/gr.189621.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalari KR, Nair AA, Bhavsar JD, et al. MAP-RSeq: Mayo Analysis Pipeline for RNA sequencing. BMC Bioinformatics. 2014;15(1):224. doi: 10.1186/1471-2105-15-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasser S, Kurdoglu AA, Izatt T, et al. Biocomputing 2015. World Scientific; 2014. An Integrated Framework for Reporting Clinically Relevant Biomarkers From Paired Tumor/Normal Genomic and Transcriptomic Sequencing Data in Support of Clinical Trials in Personalized Medicine; pp. 56–67. [PubMed] [Google Scholar]
- 33.Hou Z, Jiang P, Swanson SA, et al. A cost-effective RNA sequencing protocol for large-scale gene expression studies. Sci Rep. 2015;5:9570. doi: 10.1038/srep09570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Dijk EL, Auger H, Jaszczyszyn Y, Thermes C. Ten years of next-generation sequencing technology. Trends in Genetics. 2014;30(9):418–26. doi: 10.1016/j.tig.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 35.2016 CBTRUS Fact Sheet: Central Brain Tumor Registry of the United States. 2016 [Available from: http://www.cbtrus.org/factsheet/factsheet.html.
- 36.Curtin SCMA, Anderson RN. Declines in cancer death rates among children and adolescents in the United States, 1999–2014. Hyattsville, MD: National Center for Health Statistics; 2016. NCHS data brief, no 257. [Google Scholar]
- 37.Armstrong GT, Conklin HM, Huang S, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro-Oncology. 2011;13(2):223–34. doi: 10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker M, Mohankumar KM, Punchihewa C, et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506(7489):451–5. doi: 10.1038/nature13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Northcott PA, Shih DJH, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biegel JA, Rorke LB, Emanuel BS. Monosomy 22 in rhabdoid or atypical teratoid tumors of the brain. The New England journal of medicine. 1989;321(13):906. doi: 10.1056/nejm198909283211317. [DOI] [PubMed] [Google Scholar]
- 41.Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415(6870):436–42. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 42.Judkins AR, Mauger J, Ht A, Rorke LB, Biegel JA. Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. The American journal of surgical pathology. 2004;28(5):644–50. doi: 10.1097/00000478-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Lee RS, Stewart C, Carter SL, et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. The Journal of Clinical Investigation. 2012;122(8):2983–8. doi: 10.1172/JCI64400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johann PD, Erkek S, Zapatka M, et al. Atypical Teratoid/Rhabdoid Tumors Are Comprised of Three Epigenetic Subgroups with Distinct Enhancer Landscapes. Cancer cell. 2016;29(3):379–93. doi: 10.1016/j.ccell.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Torchia J, Golbourn B, Feng S, et al. Integrated (epi)-Genomic Analyses Identify Subgroup-Specific Therapeutic Targets in CNS Rhabdoid Tumors. Cancer Cell. 2016;30(6):891–908. doi: 10.1016/j.ccell.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho YJ, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–30. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kool M, Koster J, Bunt J, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PloS one. 2008;3(8):e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Remke M, Hielscher T, Northcott PA, et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29(19):2717–23. doi: 10.1200/JCO.2011.34.9373. [DOI] [PubMed] [Google Scholar]
- 49.Pugh TJ, Weeraratne SD, Archer TC, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488(7409):106–10. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(11):1408–14. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta neuropathologica. 2012;123(4):465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clifford SC, Lusher ME, Lindsey JC, et al. Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle. 2006;5(22):2666–70. doi: 10.4161/cc.5.22.3446. [DOI] [PubMed] [Google Scholar]
- 53.Ellison DW, Onilude OE, Lindsey JC, et al. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children’s Cancer Study Group Brain Tumour Committee. J Clin Oncol. 2005;23(31):7951–7. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- 54.Taylor MD, Liu L, Raffel C, et al. Mutations in SUFU predispose to medulloblastoma. Nature genetics. 2002;31(3):306–10. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 55.Bandopadhayay P, Bergthold G, Nguyen B, et al. BET-bromodomain inhibition of MYC-amplified medulloblastoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(4):912–25. doi: 10.1158/1078-0432.CCR-13-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin CY, Erkek S, Tong Y, et al. Active medulloblastoma enhancers reveal subgroup-specific cellular origins. Nature. 2016;530(7588):57–62. doi: 10.1038/nature16546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Northcott PA, Shih DJ, Remke M, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615–26. doi: 10.1007/s00401-011-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-oncology. 2014;16(Suppl 4):iv1–63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67(9):878–87. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 60.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. The Journal of clinical investigation. 2008;118(5):1739–49. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korshunov A, Ryzhova M, Hovestadt V, et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta neuropathologica. 2015;129(5):669–78. doi: 10.1007/s00401-015-1405-4. [DOI] [PubMed] [Google Scholar]
- 62.Bergthold G, Bandopadhayay P, Hoshida Y, et al. Expression profiles of 151 pediatric low-grade gliomas reveal molecular differences associated with location and histological subtype. Neuro-oncology. 2015;17(11):1486–96. doi: 10.1093/neuonc/nov045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kleinschmidt-DeMasters BK, Donson AM, Vogel H, Foreman NK. Pilomyxoid Astrocytoma (PMA) Shows Significant Differences in Gene Expression vs. Pilocytic Astrocytoma (PA) and Variable Tendency Toward Maturation to PA. Brain pathology. 2015;25(4):429–40. doi: 10.1111/bpa.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones DTW, Hutter B, Jager N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–32. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–12. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yde CW, Sehested A, Mateu-Regué À, et al. A new NFIA:RAF1 fusion activating the MAPK pathway in pilocytic astrocytoma. Cancer Genetics. 2016;209(10):440–4. doi: 10.1016/j.cancergen.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Olow A, Mueller S, Yang X, et al. BRAF Status in Personalizing Treatment Approaches for Pediatric Gliomas. Clinical Cancer Research. 2016;22(21):5312. doi: 10.1158/1078-0432.CCR-15-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fisher ER, Hazard JB, Gardner WJ. Intracranial ependymoma; clinicopathologic observations. Cleveland Clinic quarterly. 1951;18(4):260–9. doi: 10.3949/ccjm.18.4.260. [DOI] [PubMed] [Google Scholar]
- 69.Foreman NK, Love S, Thorne R. Intracranial ependymomas: analysis of prognostic factors in a population-based series. Pediatric neurosurgery. 1996;24(3):119–25. doi: 10.1159/000121027. [DOI] [PubMed] [Google Scholar]
- 70.Pajtler KW, Mack SC, Ramaswamy V, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta neuropathologica. 2016 doi: 10.1007/s00401-016-1643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pajtler KW, Witt H, Sill M, et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer cell. 2015;27(5):728–43. doi: 10.1016/j.ccell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mack SC, Witt H, Piro RM, et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506(7489):445–50. doi: 10.1038/nature13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New England Journal of Medicine. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 74.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. New England Journal of Medicine. 2015;372(26):2499–508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mazor T, Pankov A, Johnson Brett E, et al. DNA Methylation and Somatic Mutations Converge on the Cell Cycle and Define Similar Evolutionary Histories in Brain Tumors. Cancer Cell. 2015;28(3):307–17. doi: 10.1016/j.ccell.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh D, Chan JM, Zoppoli P, et al. Transforming Fusions of FGFR and TACC Genes in Human Glioblastoma. Science. 2012;337(6099):1231. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frattini V, Trifonov V, Chan JM, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013;45(10):1141–9. doi: 10.1038/ng.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–77. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vivanco I, Robins HI, Rohle D, et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov. 2012;2(5):458–71. doi: 10.1158/2159-8290.CD-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu F, Hon Gary C, Villa Genaro R, et al. EGFR Mutation Promotes Glioblastoma through Epigenome and Transcription Factor Network Remodeling. Molecular Cell. 60(2):307–18. doi: 10.1016/j.molcel.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brandes AA, Franceschi E, Tosoni A, Hegi ME, Stupp R. Epidermal Growth Factor Receptor Inhibitors in Neuro-oncology: Hopes and Disappointments. Clinical Cancer Research. 2008;14(4):957. doi: 10.1158/1078-0432.CCR-07-1810. [DOI] [PubMed] [Google Scholar]
- 83.Szerlip NJ, Pedraza A, Chakravarty D, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):3041–6. doi: 10.1073/pnas.1114033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Snuderl M, Fazlollahi L, Le Long P, et al. Mosaic Amplification of Multiple Receptor Tyrosine Kinase Genes in Glioblastoma. Cancer Cell. 2011;20(6):810–7. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 85.Francis JM, Zhang C-Z, Maire CL, et al. EGFR Variant Heterogeneity in Glioblastoma Resolved through Single-Nucleus Sequencing. Cancer Discovery. 2014;4(8):956. doi: 10.1158/2159-8290.CD-13-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di Stefano AL, Fucci A, Frattini V, et al. Detection, Characterization, and Inhibition of FGFR–TACC Fusions in IDH Wild-type Glioma. Clinical Cancer Research. 2015;21(14):3307. doi: 10.1158/1078-0432.CCR-14-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.International Cancer Genome Consortium PedBrain Tumor P. Recurrent MET fusion genes represent a drug target in pediatric glioblastoma. Nat Med. 2016;22(11):1314–20. doi: 10.1038/nm.4204. [DOI] [PubMed] [Google Scholar]
- 88.Kleinman CL, Gerges N, Papillon-Cavanagh S, et al. Fusion of TTYH1 with the C19MC microRNA cluster drives expression of a brain-specific DNMT3B isoform in the embryonal brain tumor ETMR. Nat Genet. 2014;46(1):39–44. doi: 10.1038/ng.2849. [DOI] [PubMed] [Google Scholar]
- 89.Schwaederle M, Zhao M, Lee JJ, et al. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. Journal of Clinical Oncology. 2015;33(32):3817–25. doi: 10.1200/JCO.2015.61.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Worst BC, van Tilburg CM, Balasubramanian GP, et al. Next-generation personalised medicine for high-risk paediatric cancer patients - The INFORM pilot study. European Journal of Cancer. 65:91–101. doi: 10.1016/j.ejca.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 91.Roychowdhury S, Iyer MK, Robinson DR, et al. Personalized Oncology Through Integrative High-Throughput Sequencing: A Pilot Study. Science Translational Medicine. 2011;3(111):111ra21. doi: 10.1126/scitranslmed.3003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim J, Lee I-H, Cho Hee J, et al. Spatiotemporal Evolution of the Primary Glioblastoma Genome. Cancer Cell. 28(3):318–28. doi: 10.1016/j.ccell.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 93.Reimand J, Arak T, Adler P, et al. g:Profiler—a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Research. 2016;44(W1):W83–W9. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences. 2005;102(43):15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tarca AL, Bhatti G, Romero R. A Comparison of Gene Set Analysis Methods in Terms of Sensitivity, Prioritization and Specificity. PLOS ONE. 2013;8(11):e79217. doi: 10.1371/journal.pone.0079217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barbie DA, Tamayo P, Boehm JS, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462(7269):108–12. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu L, Chen X, Zhang D, et al. IGSA: Individual Gene Sets Analysis, including Enrichment and Clustering. PLOS ONE. 2016;11(10):e0164542. doi: 10.1371/journal.pone.0164542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oktay Y, Ülgen E, Can Ö, et al. IDH-mutant glioma specific association of rs55705857 located at 8q24.21 involves MYC deregulation. Scientific Reports. 2016;6:27569. doi: 10.1038/srep27569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Teplyuk NM, Mollenhauer B, Gabriely G, et al. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro-Oncology. 2012;14(6):689–700. doi: 10.1093/neuonc/nos074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Vos KE, Abels ER, Zhang X, et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro-Oncology. 2016;18(1):58–69. doi: 10.1093/neuonc/nov244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Santiago-Dieppa DR, Steinberg J, Gonda D, Cheung VJ, Carter BS, Chen CC. Extracellular vesicles as a platform for ‘liquid biopsy’ in glioblastoma patients. Expert review of molecular diagnostics. 2014;14(7):819–25. doi: 10.1586/14737159.2014.943193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haemmerle M, Gutschner T. Long Non-Coding RNAs in Cancer and Development: Where Do We Go from Here? International Journal of Molecular Sciences. 2015;16(1) doi: 10.3390/ijms16011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Y, Wu J-J, Lin X-B, et al. Differential lncRNA expression profiles in recurrent gliomas compared with primary gliomas identified by microarray analysis. International Journal of Clinical and Experimental Medicine. 2015;8(4):5033–43. [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X, Sun S, Pu JKS, et al. Long non-coding RNA expression profiles predict clinical phenotypes in glioma. Neurobiology of Disease. 2012;48(1):1–8. doi: 10.1016/j.nbd.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Pastori C, Kapranov P, Penas C, et al. The Bromodomain protein BRD4 controls HOTAIR, a long noncoding RNA essential for glioblastoma proliferation. Proceedings of the National Academy of Sciences. 2015;112(27):8326–31. doi: 10.1073/pnas.1424220112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramos AD, Attenello FJ, Lim DA. Uncovering the roles of long noncoding RNAs in neural development and glioma progression. Neuroscience Letters. 2016;625:70–9. doi: 10.1016/j.neulet.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Darmanis S, Sloan SA, Zhang Y, et al. A survey of human brain transcriptome diversity at the single cell level. Proceedings of the National Academy of Sciences. 2015;112(23):7285–90. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tirosh I, Venteicher AS, Hebert C, et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature. 2016;539(7628):309–13. doi: 10.1038/nature20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Venteicher AS, Tirosh I, Hebert C, et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017;355(6332) doi: 10.1126/science.aai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Safa AR, Saadatzadeh MR, Cohen-Gadol AA, Pollok KE, Bijangi-Vishehsaraei K. Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs. Genes & Diseases. 2015;2(2):152–63. doi: 10.1016/j.gendis.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suvà ML, Riggi N, Bernstein BE. Epigenetic Reprogramming in Cancer. Science. 2013;339(6127):1567. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dvinge H, Bradley RK. Widespread intron retention diversifies most cancer transcriptomes. Genome Medicine. 2015;7(1):45. doi: 10.1186/s13073-015-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vauchy C, Gamonet C, Ferrand C, et al. CD20 alternative splicing isoform generates immunogenic CD4 helper T epitopes. International Journal of Cancer. 2015;137(1):116–26. doi: 10.1002/ijc.29366. [DOI] [PubMed] [Google Scholar]
- 114.Sveen A, Kilpinen S, Ruusulehto A, Lothe RA, Skotheim RI. Aberrant RNA splicing in cancer; expression changes and driver mutations of splicing factor genes. Oncogene. 2016;35(19):2413–27. doi: 10.1038/onc.2015.318. [DOI] [PubMed] [Google Scholar]
- 115.Dunn-Pirio AM, Vlahovic G. Immunotherapy approaches in the treatment of malignant brain tumors. Cancer. 2017;123(5):734–50. doi: 10.1002/cncr.30371. [DOI] [PubMed] [Google Scholar]
- 116.Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–7. doi: 10.1038/nature13387. [DOI] [PubMed] [Google Scholar]
- 117.Dutoit V, MD, Walker PR, Dietrich P-Y. Immunotherapy of Brain Tumors. In: OMGC, editor. Immuno-Oncology: Prog Tumor Res. Basel: Karger; 2015. [DOI] [PubMed] [Google Scholar]
- 118.Vonderheide RH, Nathanson KL. Immunotherapy at Large: The road to personalized cancer vaccines. Nat Med. 2013;19(9):1098–100. doi: 10.1038/nm.3317. [DOI] [PubMed] [Google Scholar]