Abstract
Stem cells are particularly ‘plastic’ cell types that are induced by various cues to become specialized, tissuefunctional lineages by switching on the expression of specific gene programs. Matrix stiffness is among the cues that multiple stem cell types can sense and respond to. This seminar-style review focuses on mechanosensing of matrix elasticity in the differentiation or early maturation of a few illustrative stem cell types, with an intended audience of biologists and physical scientists. Contractile forces applied by a cell’s acto-myosin cytoskeleton are often resisted by the extracellular matrix and transduced through adhesions and the cytoskeleton ultimately into the nucleus to modulate gene expression. Complexity is added by matrix heterogeneity, and careful scrutiny of the evident stiffness heterogeneity in some model systems resolves some controversies concerning matrix mechanosensing. Importantly, local stiffness tends to dominate, and ‘durotaxis’ of stem cells toward stiff matrix reveals a dependence of persistent migration on myosin-II force generation and also rigid microtubules that confer directionality. Stem and progenitor cell migration in 3D can be further affected by matrix porosity as well as stiffness, with nuclear size and rigidity influencing niche retention and fate choices. Cell squeezing through rigid pores can even cause DNA damage and genomic changes that contribute to de-differentiation toward stem cell-like states. Contraction of acto-myosin is the essential function of striated muscle, which also exhibit mechanosensitive differentiation and maturation as illustrated in vivo by beating heart cells and by the regenerative mobilization of skeletal muscle stem cells.
Keywords: matrix elasticity; matrix stiffness; nucleus; lamin-A,C; myosin-II
Introduction
In response to various inductive cues, embryonic stem cells give rise to the roughly 200 different specialized cell types in human tissues. Within many of these tissues, one or more resident adult stem cell types helps to mature, maintain, and repair the tissue throughout life. Physically, these tissues vary widely but consistently across species, where a tissue such as marrow is easily deformed by small forces whereas the surrounding bone is rigid. Quantifiable differences in tissue stiffness (Fig. 1A) are increasingly understood to influence lineage specification and maturation of stem cells. As is typical of other cell types, stem cells adhere and tug on their environment at the micron scale, sensing the resistance or stiffness as a micro-elasticity rather than a bulk tissue elasticity. The micro-elasticity of tissue across all species tested by atomic force microscopy (AFM) is in the kilo-Pascal (kPa) range (Fig. 1A,B), which is many orders of magnitude softer than rigid glass coverslips and tissue culture plastic in wide use for reductionist approaches to stem cell biology. Hydrogels, such as polyacrylamide (PA, or PAAm) and crosslink-modified hyaluronic acid (HA), can be tuned to mimic in vivo stiffness of tissues in vitro (Fig. 1B,C). For example, an adult stem cell referred to as a mesenchymal stem cell (MSC, also known as mesenchymal stromal cell) differentiates toward osteogenic cells in vitro in response to substrates at least as stiff as pre-calcified bone (>20 kPa) rather than substrates that are soft like fat or marrow (<3 kPa) [1]. Such conclusions have spurred some controversy, however, with a few in vitro studies of – for example – MSC osteogenesis on/in novel material systems seeming to contradict stiffness-directed differentiation. A more consistent view of the field based on some key principles of polymer physics is one aim of this seminar-style review of mechanosensing by stem cells.
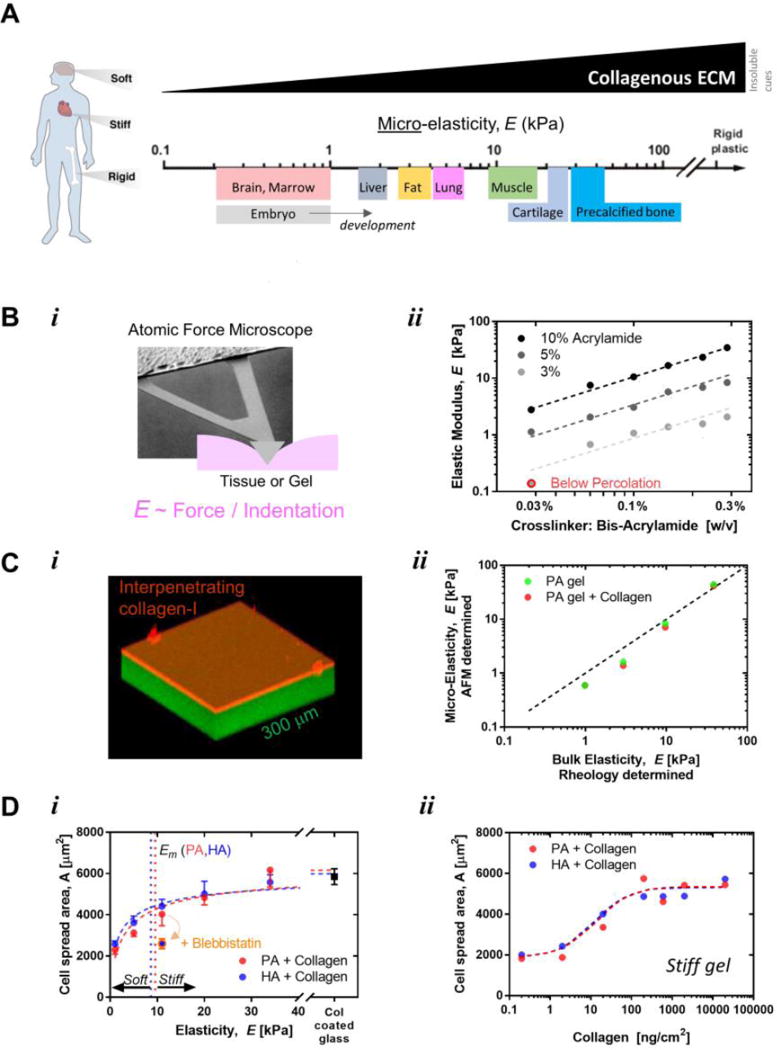
Figure 1. Universal scale of micro-stiffness for tissues.
A. The body is composed of tissues that vary over log scales of stiffness. Atomic force microscopy can be used to probe the stiffness of tissues at a micro-scale similar to that probed by cells. Soft tissues have orders of magnitude lower stiffness than glass or tissue culture plastic. Adapted from [89].
B. Gels can be engineered to mimic the stiffness of tissues found in vivo. AFM indentations on gel or tissue yield the elastic properties and fit well with models when probed at 1 mm/sec that is relevant to cell mechanics. The predictable scaling over log scales of gel elasticity with polymer density or crosslinking in gels verifies the mechanical properties of in vitro gel systems. Adapted from [90].
C. Polyacrylamide gels are covalently attached to glass and then coated with a thin layer of covalently attached collagen-I to functionalize the gel which becomes inert and stable [91]. Gel systems can be characterized microscopically and mechanically, which demonstrates homogeneity by the high agreement between micro-elasticity measured by AFM and bulk elasticity measured with rheology, unaltered by collagen coating.
D. Projected area of MSCs versus matrix elastic modulus of both hylaronic acid (HA) and polyacrylamide (PA) gels coated with collagen I. The projected area can be fit with the a Hill equation: A = AmaxB/(Em+ E). Where A is the calculated projected area of the cell, Amax is the maximum area of the cell on a rigid substrate, B is a constant where B = Em(Amax— Amin), and Em is the matrix elasticity where the area is the mid-point between the maximum and minimum (and the delineation between soft and stiff gels). On the right is MSC projected area vs surface concentration of collagen l, with the focus of this review on the saturated portion of the curve. Adapted from [38].
Throughout this review we will typically assume that the density of an adhesive ligand (collagen, fibronectin, etc.) that is attached to a gel (Fig. 1C,D) is abundant rather than limiting, with the earliest studies of mechanosensing by lineage-committed fibroblast and smooth muscle cell lines showing that gel mechanics matters most after such ligands are attached to a gel [2, 3]. Ligand heterogeneity can also be important to stem cells and can promote haptotaxis, but haptotaxis does not strictly require a cell to mechanosense its microenvironment. Furthermore, although micropatterns of a ligand on a rigid substrate can control the shapes of stem cells that spread on rigid substrates [4], a large pattern of ligand on top of a soft gel simply cannot activate the acto-myosin machinery sufficiently to promote cell spreading from the rounded morphology observed on soft matrix. For any homogeneously stiff surface, however, it is widely observed that inhibition of myosin-II contractility is sufficient to eliminate spreading of any cell, including an MSC (Fig. 1D). Matrix elasticity is thus upstream of cell spreading. Spreading of MSCs indeed depends on the stiffness of a collagen-functionalized gel, which is supported by the fact that spreading occurs on gels of very different materials, ranging from purely synthetic and neutral polyacrylamide (PA) to naturallyderived and anionic but modified crosslinkable hyaluronic acid (HA) (Fig. 1D). A hyperbolic fit of the spread area of almost any isolated cell versus matrix E typically has a characteristic half-max Em ∼ 5–10 kPa, and so a matrix is “stiff” for E > Em whereas a matrix is “soft” for E < Em. Such terminology is completely analogous to biochemical regimes of “dilute” or “concentrated” for ligand concentrations that are respectively less than or greater than the affinity of a ligand for its receptor. Thus the cell-scale micro-stiffness and any heterogeneous elasticity of material systems for cell culture are crucial to understand, which means that micro-mechanical measurements require as much attention as mapping any other physicochemical property when investigating stem cell niche properties.
Length scales for cell sensing are also important to consider. Cells are microns in size but might in principle sense at the atomic scales that are resolved by protein crystallography. Considering that life is carbon-based, i.e. organic, the stiffness of a covalent bond between two carbon atoms is conceivably relevant, but the elastic modulus of C-C is readily estimated from diamond to be ∼1000 GPa, which is far stiffer than tissues (Fig. 1A). Tissues are of course highly hydrated, and all molecules fluctuate in space and time as they interact non-covalently at 300 Kelvin; all of this motion collectively softens tissues, cells, and matrix by orders of magnitude when considering micron-scale properties. The distribution of material thus affects stiffness. As an additional important and relevant example, collagen helices pack very densely as they exclude water, and collagen fibers can be measured to have an elastic modulus of ∼10 GPa [5]. However, when such a small, rigid molecule is attached to a soft gel, the latter dominates when pulled upon. In other words, in a collection of springs in series, the softest spring extends the most. Cells in tissues likewise adhere to and deform the network of extracellular matrix molecules, not necessarily individual molecules, and thus matrix stiffness is a convolution of the intrinsic properties of matrix molecules, their connectivity, and the overall geometry of the matrix. Such properties can vary substantially within a tissue or culture system, and so a separate section of this review focuses on various forms of mechanical heterogeneity and their impacts on stem cells including their motility.
The use of stem cells in mechanosensing studies has both advantages and disadvantages relative to the use of lineage-committed cells. One key advantage is that relationships can be uncovered between microenvironment cues and the key fate choice of differentiation, whereas a disadvantage is that many transcript and protein levels eventually change in differentiation and can complicate more general conclusions about cell biology. On the other hand, lineage-committed cells on substrates of different stiffness are often assumed to maintain constant transcript and protein levels, even though some limited plasticity in expression is likely. Indeed, most cell types including stem cells spread more on stiff substrates than on soft substrates with more prominent adhesions and acto-myosin cytoskeletal assembly on stiff substrates. It has also long been observed that a cell’s nucleus spreads in proportion to cell spreading [6], with more recent evidence indicating that one of the main nuclear structural proteins, lamin-A,C, increases with matrix stiffness and contributes to lineage maturation (probably through the proteins and genes that lamin-A,C regulates) [7]. More generally, given the central role of the nucleus in gene expression changes that are key to any differentiation process, we summarize some of the growing evidence that nuclear factors also mechanosenses matrix stiffness.
Mechanosensitivity of MSC differentiation
Stem cells are of course capable of differentiating into various cell types of the body but are also often defined as having some capacity to reproduce more stem cells via self-renewal. Both processes potentially depend on matrix mechanics although such stem cell fate decisions have traditionally been controlled with soluble growth factors and small molecules (such as retinoic acid) that regulate signaling pathways [8]. Human bone marrow-derived MSCs had been known to be inducible toward bone, cartilage, and fat among other lineages, and they were the first stem cell type used to illustrate matrix mechanosensing. MSCs cultured atop collagen-I-functionalized PA gels (Fig. 1B–D) that mimic the elasticity of soft neural tissue, stiff muscle tissue, or stiffer developing bone provided evidence that matrix elasticity can direct stem cell fate [1]. Not only did MSCs express markers for neural, muscle, or bone cells when grown on gels of the corresponding tissue stiffness, but when their myosin-II-based mechanical interactions with the matrix were inhibited, genes that indicate lineage specification were not expressed.
On stiff matrix as opposed to soft matrix, myosin-II in MSCs promotes the growth of focal adhesions that engage matrix, and myosin-II also favors the assembly of stress fibers, both of which influence cell morphology within hours of a cell contacting a substrate [1]. Days are required for measurable increases in matrix-dependent expression of lineage markers, which also depends on myosin-II. This separation of time scales, with very early changes in cell morphology and migration followed by later changes in lineage choices, has likewise been reported from multi-day imaging in vitro of differentiating murine hematopoietic stem and progenitor cells (HSPCs) on fibronectin-coated plastic [9]. Morphological features of greatest predictive value from phase contrast images were found to be the cell’s maximum, mean and minimum intensity as well as the cell’s perimeter and major axis length, whereas cell area and equivalent diameter proved less relevant to HSPCs.
Some adult tissues are truly regenerated in 2D processes, such as adult bone in which MSC-derived osteoblasts deposit a layer of matrix (osteoid) on top of a bone surface that is then mineralized in a process of epitaxial tissue formation [1]. However, for other tissues such as bone marrow, brain tissue, and fat, 2D cultures provide only reductionist insight into factors that could be important to 3D tissue biology. Insights into the regeneration of 3D tissues could benefit from rationally engineered 3D culture systems that eliminate apical-basal polarization while still paying attention to both cellular access of soluble nutrients and physical caging constraints on cell morphology and proliferation. Encapsulation of MSCs in 3D hydrogels of alginate (a carbohydrate commonly derived from brown seaweed) that was first modified with the adhesion tripeptide Arg-Gly-Asp (or RGD) [10] showed soft gels with elasticity from 2.5 to 5 kPa favored adipogenesis (a soft tissue lineage) whereas stiff gels (11 ∼ 30 kPa) favored osteogenesis. The results are in close agreement with 2D studies that use non-degradable PA gels (Fig. 1B–D) [1, 7]. Unfortunately, 2D cultures on top of the alginate gels were not studied and could have permitted quantitation of the effects of dimensionality (3D versus 2D) along with any changes in gel mechanics caused by cells. Degradation or extensive physical remodeling of matrix can be expected to change matrix mechanics and, therefore, requires local measurements of the mechanics of the gel around the cell. Indeed, the encapsulation of human MSCs in stiff 3D hyaluronic acid-based gels revealed that when the cell-mediated degradation of stiff gels was blocked, with cells remaining spherically encaged, osteogenesis was restricted [11]. However, activation of myosin-II -based tension in these encapsulated cells did drive osteogenesis over adipogenesis. This is consistent with theory and experiments on MSCs that showed cell shape influences cytoskeletal tension with cells of the same shape applying higher tractions to stiffer substrates [12]. Furthermore, MSCs attached and spread on ‘crossbow’ shaped patterns of equal, confining area on both soft gels and stiff gels show more myosin-II assembly on stiff gels [13], which is consistent with the observations for hematopoietic stem and progenitor cells that cell area is much less relevant than many other dynamic morphological parameters to cell lineage [9].
Mechanotransduction to the stem cell nucleus
Matrix mechanotransduction pathways that affect stem cell fate must somehow enter the nucleus and co-regulate gene expression – i.e. affect some of the many layers of transcriptional regulation. Such pathways could involve the nuclear accumulation and autocatalytic expression of basal levels of lineage-specific transcription factors [1] or perhaps more generic factors. Lamin-A,C is a nuclear envelope protein that is almost undetectable in pluripotent cells, but it is abundant in cells of stiff tissues and dominates the constitutive lamin-B isoforms [7]. Lamin-A,C is not only responsive to matrix stiffness, tending to be stabilized against phosphorylation and degradation under mechanical stress, but it also co-regulates transcription factors such as SRF (serum response factor) that controls acto-myosin cytoskeletal expression [7, 14, 15]. Current questions about lamins in cell fate choices include whether genes are regulated by the nucleoplasmic lamin-A,C, which is phosphorylated and subsequently degraded, and whether less frequent changes in lamin-B [45] can affect processes such as senescence that would tend to limit tissue regeneration.
YAP and TAZ are additional transcriptional regulators that generally affect cell growth and differentiation, appearing nuclear-localized in MSCs on stiff but not soft substrates [16, 17]. Using YAP as a marker, a memory of matrix interactions was demonstrated with human MSCs derived from bone marrow (which is soft) and then cultured for weeks on rigid polystyrene dishes (stiffness ∼ 106 kPa); this favored osteogenesis even after the cells were transferred to a soft 2 kPa gel made of polyethylene glycol (PEG) [18]. Alternatively, if culture on plastic is kept sufficiently brief, then osteogenic differentiation can be suppressed. Non-monotonic changes in YAP levels and nuclear localization with gel stiffness and tissue stiffness [7] could reflect more complicated isoform switching to TAZ or else indicate decoupling of YAP and TAZ from mechanosensing as these are also influenced by cell contact and multiple soluble factors including Wnt and TGFb [19].
Human pluripotent stem cells (hPSCs) cultured on polyacrylamide hydrogels mimicking soft brain tissue (0.75 kPa elasticity) also showed nuclear exclusion of YAP and differentiation into postmitotic neurons, whereas hPSCs on stiff gels (10 kPa) showed abundant YAP nuclear localization and maintenance of pluripotency [20]. Compared with traditional neurogenic induction methods that rely solely on soluble factors, culturing hPSCs on soft gels in the absence of neurogenic factors resulted in more rapid and efficient differentiation into neurons. Furthermore, dynamic changes in substrate stiffness have highlighted an important window of mechanosensitivity in stem cell neuronal differentiation [21]. However, the results for hPSCs on the stiff gels indicate differences from MSCs, highlighting the cell type-specific nature of mechanoresponses. Indeed, hematopoietic stem and progenitor cells (HSCPs) taken from marrow, which is soft in this respect and similar to brain tissue, or else taken from a stiffer bone niche are also mechanoresponsive to matrix elasticity, but these cells remain blood-lineage committed [22].
Matrix malleability and reorganization by stem cells
As cells tug on the adjacent matrix, they can sometimes change the local density, with such active remodeling seeming to be essential in wound healing in vivo. Indeed, the myosin-dependent contraction and migration of fibroblasts around a wound gap are primary mechanisms through which closure occurs, as opposed to the proliferation of cells [23]. The forces transmitted through cell-cell contacts have proven to be critical factors in layers of cells moving together, as in wound healing [24]. Using void-forming alginate hydrogels, murine MSCs exhibited the greatest proliferation, collagen deposition, and mineralization, with construct elasticity ranging from 20 to 60 kPa [25]. Transplantation of gels in the intermediate range of stiffness into a bone defect model showed maximal tissue regeneration, with stiffness perhaps similar to pre-calcified bone, or osteoid. More in-depth analysis is needed as other mechanisms, such as host cell infiltration and material degradation, could impact the response.
The formation of epithelial cysts also exhibits maximum polarization and lumen formation in a narrow range of ECM elasticity when using PEG hydrogels, with abnormal morphogenesis observed for softer and stiffer gels [26]. It seems that a matrix must be sufficiently rigid to provide appropriate cues to cells and yet the matrix should also be sufficiently compliant to allow cells to manipulate the matrix for migration, spreading, and proliferation in order to generate the apical--basal polarity required for lumenogenesis. Synthetic ECM technologies can thus provide insight into ECM regulation of complex morphogenetic behaviors and provide potentially useful rules for regenerative medicine.
Reprogramming the epigenetic state of primary mouse fibroblasts to induced pluripotent stem cells (iPSCs) has also been examined in 3D synthetic hydrogels with modulation of matrix stiffness, degradability, and adhesive ligand [27]. Using PEG hydrogels conjugated with adhesive peptides and crosslinked with matrix metalloproteinase-cleavable peptides, the fibroblasts were encapsulated and transduced with the four traditional Yamanaka transcription factors that initiate reprogramming in a very small subpopulation of cells. Compared with traditional reprogramming in polystyrene dishes, the gels accelerated reprogramming: with over 100 microenvironmental conditions that spanned stiffness, ligand presentation, and degradation, iPSC reprogramming efficiency was highest with gels of stiffness ∼600 Pa, high degradability, and functionalization with epithelial cell adhesion molecule (EpCAM). Matrigel produced similar efficiency but with less homogeneity in induction, which is interesting in that Matrigel lacks EpCAM. Strikingly, the optimal gels and matrigel are both very soft and within ∼2-fold of the measured elasticity for embryos [28, 29]. Such findings thus highlight the importance of the dynamic, mechanical nature of matrix to stem cells in embryonic development and in adult tissue.
Durotaxis of stem cells: effects of lateral heterogeneity
At least some cells in tissues are sufficiently dynamic to migrate between regions of different stiffness. Durotaxis refers to a tendency of a cell to migrate from soft matrix to stiff matrix [3]. Although durotaxis of stem and progenitor cells might occur in embryonic morphogenesis, early embryonic tissues are relatively soft and homogeneous compared to adult tissues in which large gradients in stiffness are present. For example, soft marrow is surrounded by rigid bone, and this soft-rigid difference could (i) prolong HSCP residence time and quiescent phenotype at the bone niche relative to a perivascular marrow niche [22], and could also (ii) influence MSCs that detach from their perivascular marrow niche to localize to the bone surface and initiate osteogenesis.
MSCs migrate and accumulate over days on the stiff regions of 2D gradient gels, and by 7 days lineage markers characteristic of the local stiffness increase (∼5–10 fold) above baseline intensity by immunofluorescence [30]. In particular, on a relatively stiff region (∼10–14 kPa) of a gradient gel, MSCs became strongly spindle-shaped by day-4 and the myogenic transcription factor MyoD was subsequently evident in nuclei by day-7, whereas MSCs on the comparatively soft region (∼1–6 kPa) of the same gel appeared more dendritic or branched, expressed a neurogenic microtubule protein, and lacked MyoD. The results are largely consistent with prior findings for MSCs on homogeneous gels with, respectively, a muscle-like stiffness or a brain-like softness [1]. Although relevance to 3D differentiation remains to be studied, durotaxis of MSCs in 3D was demonstrated with a soft collagen gel overlay on top of a 2D soft-to-stiff gradient gel: MSCs migrated from the soft gel region into the collagen overlay but never from the stiff gel region into the overlay [31].
Cytoskeletal mechanisms of durotaxis have also been elaborated in some detail with MSCs in 2D and to some extent in 3D, with studies to date revealing a key coupled role for nonmuscle myosin-II and microtubules in polarization and persistent migration. myosin-II inhibition blocks durotaxis, and because it also prevents all matrix-directed lineage specification of MSCs while still permitting soluble factor induction [1], myosin-II based sensing of matrix stiffness is necessary for both mechanosensitive differentiation and durotaxis of MSCs. More specific knockdown of the minor myosin-II isoform, nonmuscle myosin-IIB, inhibits durotaxis [31], whereas mouse embryos that lack nonmuscle myosin-IIB exhibit very few (but key) developmental defects [32], suggesting that the more abundant nonmuscle myosin-IIA has the potential to compensate and is more critical. Indeed, mouse embryos that lack nonmuscle myosin-IIA do not differentiate but can proliferate [33], and human MSCs with knockdown of nonmuscle myosin-IIA do not durotax [31]. Microtubule polarization toward the front of MSCs is essential for directed migration of isolated cells and depends on both myosin-II isoforms [13]. Thus, unlike soft matrix, stiff matrix stimulates actomyosin assembly and force-generation which pulls the nucleus backward and polarizes an abundance of microtubules that have high rigidity (millimeter persistence length) frontward [34] (Fig. 2A). This polarized, rigid cytoskeletal structure makes migration more directionally persistent on stiff matrix compared to soft matrix. Importantly, theory and simulation recently demonstrated that any gradient in persistent migration (e.g. less persistent on soft matrix and more persistent on stiff matrix) is sufficient to cause directed migration [35]. Observing durotaxis of stem cells in vivo is challenging, but critical to elucidating the role durotaxis plays in physiological differentiation and discovering if durotaxis contributes to disease states.
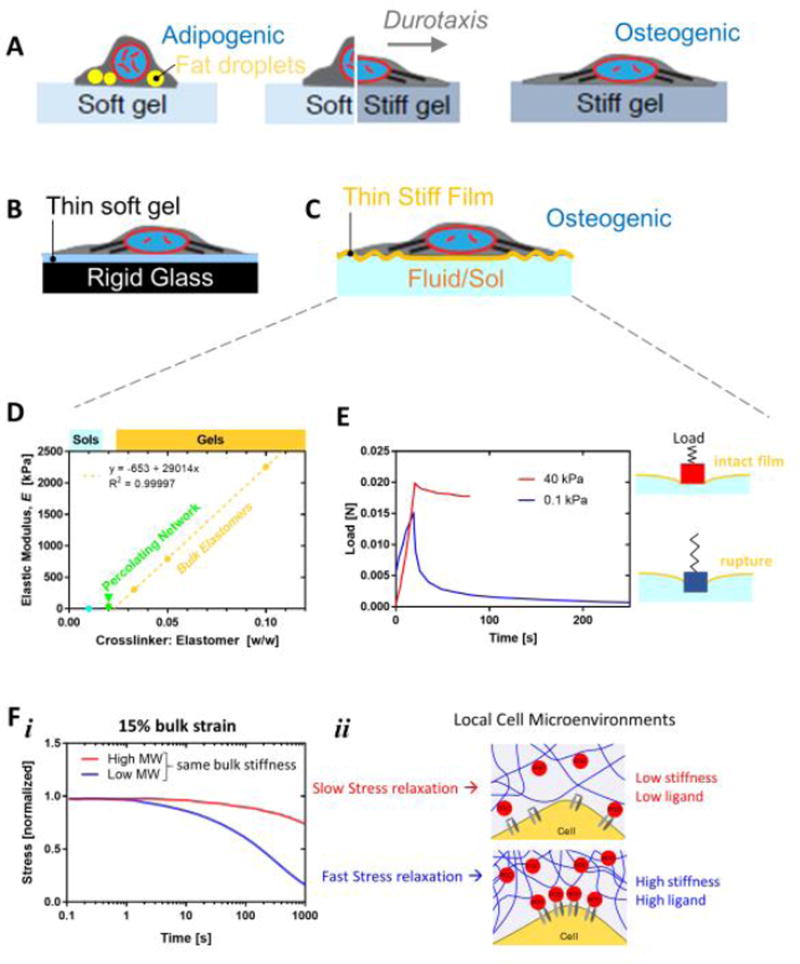
Figure 2. Heterogeneity in matrices can be lateral or vertical and affect stem cell morphology, motility, and niche localization.
A. Thick gels that are soft or stiff can be made with lateral heterogeneity to study processes such as durotaxis. MSCs are rounded and adpiogenic on soft gels, strongly spread and filled with stress fibers on osteogenic-favoring stiff gels, and directionally polarized on gels with a stiffness gradient.
B, C. Stratified materials can be laterally homogeneous and preserve adhesive ligand chemistry and density but still favor spreading and osteogenesis of MSCs. Underlying rigidity can be mechanosensed and a stiff layer can obscure underlying softness or fluidity.
D, E. Analysis of reported elastic modulus versus crosslinker content can sometimes indicate polymerization conditions insufficient for bulk gelation (data from [39]). Re-plot of the mechanical measurements reveals an instability in the softest material reported as “0.1 kPa”. The peak and decay in the load or force when this latter material is indented to a constant level is largely absent for a stiff gel, and the peak likely indicates sudden rupture through a film. The film is stiff because it initially gives a similar magnitude force peak as the stiffer material, but the subsequent force decay over tens of seconds indicates viscous losses into a fluid.
F. The stress relaxation and viscous component of a hydrogel can be modified by adjusting polymer length and interactions independent of bulk elastic stiffness. Various molecular weight chains of agarose have been coupled to a PEG spacer to control the gel relaxation time from 60–2,300 seconds under constant strain. The viscous component allows for matrix malleability but also heterogeneity:
Matrix remodeling by stem cells and thin matrix effects
Tissues and gels can, of course, possess mechanical properties far more complex than simple elasticity or linear gradients in stiffness. Some tissues, such as embryonic brain, are so soft that they creep and flow irreversibly (exhibiting plasticity) under microscale strain, whereas other tissues, such as embryonic heart, are resiliently elastic and recover completely from externally imposed strain [36]. Bulk measurements of a few isolated tissues of low/intermediate stiffness, such as liver, have also indicated that these tissues exhibit stress relaxation when exposed to an external strain of 15% [37]. Such a strain might be non-physiologically high given that a highly mechanical organ such as heart is strained by this amount or less in beating [28], but effects of matrix relaxation on stem cells could be relevant to tissue engineering as well as remodeling of blood clots (hematomas) after injury [37].
Gels with tunable stress relaxation timescales (1 min to 40 min) have been made using modified alginate with otherwise equivalent elastic moduli, ligand densities, and degradation characteristics (Fig. 2F) [37]. MSCs grown within 9 kPa gels exhibited maximal adipogenesis in slow-relaxing gels, but MSCs grown within 17 kPa gels exhibited maximum osteogenesis with fast-relaxing gels. Adhesive ligand clustering was also measured for the latter and likely indicates accumulation of gel around a cell in 3D [12]. Polymer physics tells us that an increase in local polymer density will give a locally stiffer gel, so that osteogenesis is occurring within a cocoon of gel of stiffness >17 kPa, consistent with the stiffness scale for tissue (Fig. 1A,2F). The heterogeneous mechanics that arise after cell integration in 3D gels thus requires careful, local measurements of polymer density and/or stiffness. Overlays of gels, so-called sandwich gels, have indeed revealed that human MSCs respond to the nearest rigidity: bio-derived hyaluronic acid (HA) modified for crosslink-controlled elasticity shows that a stiff overlay of HA on a soft gel drives the sandwiched MSCs to a stiff phenotype whereas a soft overlay has less of an effect (with all gels ligand-functionalized by collagen) [38]. The same studies also showed MSCs in 2D cultures respond equivalently to PA gels of the same elasticity in terms of morphologically. This further proves that mechanosensing is independent of the choice of material, provided care is taken with ligand and characterization. These studies nonetheless underscore the fundamental roles that matrix mechanics and physical properties play on stem cell fate.
Gels with stratified elasticity for 2D cultures of stem cells have been made intentionally and unintentionally in various configurations and reveal the depth of mechanosensing for morphology and differentiation. Thick gels on rigid glass is the standard, but microns-thin soft gels on glass allow cells to pull on the gel and feel the hidden rigidity below, which causes cells to spread as if on a stiff gel (Fig. 2B). Importantly, this type of structural rigidity approach can be compared to thick soft gels made with the same chemistry and ligand, which largely eliminates any concern over surface chemistry differences or ligand attachment differences between gels of different elasticity and/or different porosity [39]. Muscle stem cells (MuSCs) from mouse that are grown on such thin gels respond as if grown on rigid glass or plastic even though thick gels preserve phenotype if tuned to muscle tissue stiffness [40]. Earlier experiments with MSCs on thin soft gels likewise indicated strong spreading and focal adhesion formation as if the cells attach to glass [1], with the same approach subsequently demonstrating that nuclear lamin-A,C mechanosenses an underlying rigidity; increasing in protein level but decreasing in phosphorylation state [14]. Arrays of flexible pillars illustrate the same idea [41]; short pillars are stiffer (less bendable) than tall pillars of the same micron-scale cross section [42]. MSCs spread on top of short pillars as they sense the high rigidity and subsequently undergo osteogenesis. MSCs on tall pillars remain more rounded and undergo adipogenesis. The static fluid that is between the pillars or even above cells in 2D culture is extremely soft in that it deforms easily, and so static fluid is not mechanosensed.
MSCs on a stiff film (perhaps just a few µm’s thick) on top of a fluid likewise sense the stiff film rather than the underlying fluid, and osteogenesis would be predicted if the film is sufficiently stiff (Fig. 2C). One notable study can be re-interpreted precisely in this way as it attempted to use PDMS (poly-dimethyl-siloxane) to generate a very soft gel [39]. However, the amount of crosslinker used to make the ‘soft gel’ is shown by mechanical characterization in the same study to fall below the linear regime known from rigorous theory (see Fig. 2D and Fig. 1B). Percolation of a network across the sample is required for the phase transition from a fluid sol to a solid gel. Consistent with the ‘soft gel’ being in the sol regime, the indentation curve shows a strong peak in force that indicates rupture of a stiff film and viscosity-limited relaxation as the indenter enters the underlying fluid (Fig. 2E). Images of MSCs also show wrinkling of the film, which is probably due to cell tractions analogous to the film-wrinkling studies by Harris and coworkers that first revealed force generation by nonmuscle cells [43].
In addition to the interpretation above, Engler and colleagues explored whether cells sense nanoporosity of gels and the details of protein tethering to an underlying gel [44]. By varying the ratio of polymer to cross-linker, porosity could be changed without changing gel stiffness (Fig. 1B). MSCs on such gels respond to the underlying gel stiffness rather than porosity as they undergo osteogenesis on stiff gels and adipogenesis on soft gels regardless of composition. The results thus indicate that protein tethering to the gel is unlikely to be a critical factor, which underscores the reproducible observation that cells sense and integrate mechanical signals on a length scale much larger than a few macromolecules in size.
A stiff but porous film on top of a fluid (perhaps flowing) could be a reasonable model for the bone marrow niche that harbors MSCs and hematopoietic stem and progenitor cells (HSCPs) (Fig. 3). Marrow is perfused by leaky sinusoidal blood vessels that are sufficiently micro-porous to allow some of the differentiated marrow cells to enter the bloodstream as nearly mature blood components [45]. Importantly, the latter studies from decades ago beautifully showed that the endothelium-lined basement membrane matrix is stiffer than the maturing white and red blood cells that squeeze into the blood stream. A recent re-examination of the basic idea demonstrated that the nuclear lamins which determine nuclear rigidity correlate with marrow retention compared to egress into the blood stream [46]. In other words, MSCs in their perivascular niche and HSCPs in perivascular or bone niches seem likely to be retained in such niches because the nucleus is simply too rigid and the matrix pores too small as well as too rigid to permit egress from the marrow. If the stem cells are thus physically retained in marrow, then they are more likely to differentiate in marrow toward various blood lineages (from HSCPs) or (from MSCs) to osteoblasts that make bone and adipocytes that produce fatty marrow. Similar concepts of niche retention are likely applicable to other stem cells in adult tissues such as: (i) MSCs in their perivascular niches within many other tissues including liver (referred to as hepatic stellate cells) and muscle (referred to as fibro-adipo progenitors), and (ii) muscle stem cells that are sandwiched between basement membrane and the multi-nucleated muscle cell (when not injured – as discussed below).
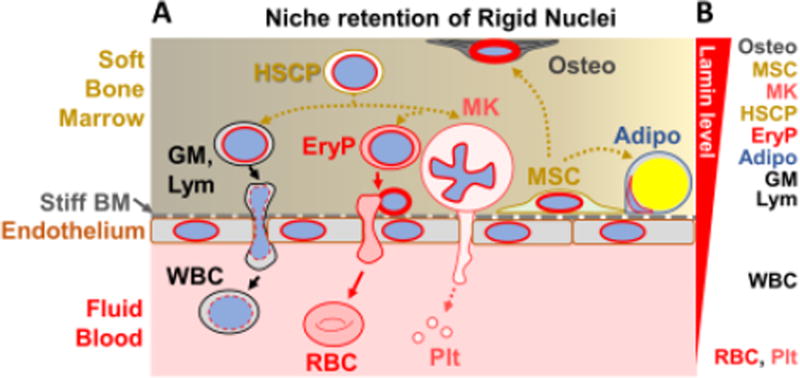
Figure 3. Tissue stratified microenvironment controls bone marrow egress depending on nuclear lamin level.
A. Tissue example of a stratified soft:stiff:fluid microenvironment. Bone marrow is soft and filled with various stem cells and differentiating cells that have high lamin levels, consistent with stiff nuclei, whereas the peripheral blood that flows through marrow contains cells with low lamin levels. Separating these two compartments is a stiff endothelial layer with basement membrane (BM) of matrix. An MSC is shown in its perivascular niche within the marrow, which can differentiate within that niche toward an osteoblast (Osteo) or adipocyte (Adipo). Hematopoietic stem cells and progenitors (HSCPs) differentiate in soft marrow toward either white blood cells (WBC) that are granulocytes, monocytes, and lymphocytes (GM, Lym) or else to red blood cells and platelets that lack nuclei and shear off respectively from nucleated erythroid progenitors (EryP) and megakaryocytes (MK).
B. Relative nuclear lamin levels are depicted. Cells that readily egress to the peripheral blood have low lamin levels, enabling them to squeeze nuclei (or lack thereof) through the basement membrane and endothelium. Results are adapted from [46].
Fibrous matrix: effects of heterogeneity on stem cells
Fibrotic tissue has a particularly heterogeneous collagenous matrix that often aligns with blood vessels and results from acute injury such as heart attack [36] as well as chronic diseases such as muscular dystrophy and liver cirrhosis [47]. Fibrosis is often referred to as a scar, which forms locally in most or all tissues of higher animals and is compositionally characterized by an abundance of crosslinked collagen-I fibers heterogeneously distributed within a fibrotic tissue. A scar tends to be locally stiff and long-lasting [36, 47]. Scar matrix seems to be made largely de novo, and a major role in the development of organ fibrosis has recently been ascribed to ubiquitous MSCs, which reside in perivascular niches of many organs including heart, liver, kidney, lung, and of course bone marrow [48]. MSCs have been well-known for decades to proliferate and to differentiate toward multiple tissue lineages (e.g. fat, bone), but genetic lineage tracing recently demonstrated that tissue-resident MSCs (specifically the Gli1+ MSCs), rather than any circulating MSCs, proliferate after organ injury to generate myofibroblast-like cells of scars. In mouse models, genetic ablation of these cells ameliorated fibrosis in multiple organs and preserved heart function following induced heart failure.
To begin to clarify the effects of fibrotic, heterogeneous matrix on MSCs, minimal matrix models of scars (MMMS) were developed by mixing soluble collagen-I subunits with acrylamide monomers plus bisacrylamide crosslinker and then polymerizing the mix into a gel [47]. Upon initiation of polymerization, collagen-I fibers phase separate from pre-gelation clusters of PA, leading to highly branched fractal fiber bundles that segregate as islands heterogeneously entrapped at the subsurface of the hydrogel – as revealed by staining with a fibrosis dye commonly used in histology (Fig. 4A). With the proper mixing ratios, a surface coverage of collagen fiber bundles of ∼30% approximates the extent of fibrosis seen, for example, in muscle cross sections [49, 50]. Importantly, collagen in the subsurface fiber bundles is not accessible for cell adhesion [47]. A uniform over-coating of matrix ligand is therefore provided for cell attachment, producing a highly heterogeneous structure with homogeneous ligand. A key cellular marker of scarring is the stress fiber associated protein α-SMA, and although α-SMA is not unique to scarring, its expression increases with contractility [51]. α-SMA increases in vivo in hepatic stellate cells (i.e. liver MSCs) in parallel with stiffening of toxin-injured liver, but α-SMA precedes the detection of fibrotic collagen in liver [52]. Despite the soft-stiff heterogeneity of MMMS gels, MSCs greatly increase expression of α-SMA compared to homogeneously low expression in MSCs on soft PA gels that lack the fiber islands (Fig. 4B). Thus heterogenous fiber matrices in soft gels are able to drive MSCs into a phenotype observed on homogenously stiff gels.
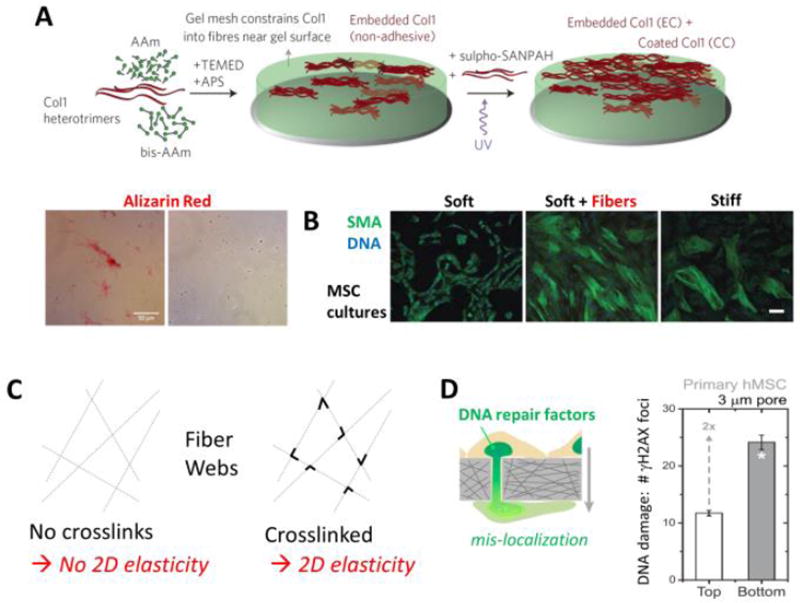
Figure 4. Fibrous composites, fiber crosslinking, and rigid pore effects on DNA damage in migration.
A. Scar-like islands of collagen-I are heterogeneously entrapped at the subsurface of the soft hydrogel in order to generate minimal matrix models of scars, MMMS. AAm (acrylamide) polymerizes and is crosslinked by bis-AAm when initiated and catalyzed by TEMED and APS; sulfo-SANPAH and UV allow uniform attachment or coating of collagen on the gel surface. Heterogeneity of the MMMS was confirmed by both immunofluorescence and staining with the histochemical dye widely used to visualize fibrosis, Sirius Red. Adapted from [47].
B. Human MSCs cultured on conventional soft or stiff homogeneous gels and separately on MMMS, which is soft + fibers; on the latter two substrates, the MSCs upregulate a common cell marker of cell tension and fibrosis, smooth muscle actin (SMA). Results are adapted from [47].
C. A web if fibers that is not crosslinked has no lateral stiffness or 2D elasticity, but addition of crosslinks or welds at fiber junctions will generate a non-zero 2D elasticity.
D. MSC migration through small pores can rupture the nuclear envelope and exhibit an increase in DNA damage measured in terms of immunostained foci of phosphorylated histone H2AX (i.e. γH2AX). Results are adapted from [54].
Sparse fibrillar matrices spun as random webs across an opening have been made with inspiration from collagen fiber based materials [53]. AFM cantilevers with colloids on the tips were used to indent single fibers and the fibrous webs, and an equivalent elastic modulus was reported analogous to soft hydrogels or stiff hydrogels. However, a fundamental problem with the claim of an equivalent elastic modulus is that crosslinking or ‘welds’ were lacking between fibers; a basic result of polymer physics (as well as structural stability) is that lateral elasticity (in 2D) and bulk elasticity (in 3D) is proportional to crosslink density – which must also percolate in 2D or 3D. A web without crosslinks has zero elastic modulus (Fig. 4C). This may explain why the authors failed to find that MSCs spread moreso on the so-called stiff webs relative to the soft webs even though the MSCs in the study did spread much more on stiff, conventional hydrogels relative to soft hydrogels. Although ligand density differences might also be important, the increased polymer that goes along with increased ligand density would also be expected to increase the local matrix stiffness. Further, when welds (i.e. crosslinks) were introduced into the system the phenotypes were altered, again revealing the need for careful attention to the most basic material physics as well as the chemistry, biochemistry, and cell biology in these highly creative experiments.
Matrix porosity and stem cell migration
Fibrous matrices are of course porous, and small pores that are about the size of the cell or its nucleus have the potential to impact the migration of motile stem cells and perhaps even affect cell fate decisions. Using model porous materials that are rigid, MSCs were found to experience rupture of the nuclear envelope and increased DNA damage (Fig. 4D) [54]. Extensive studies of cancer cells show that several ubiqutious DNA repair factors mis-localize from migrating nuclei, which makes incessant damage to DNA (e.g. in repication stress) slow to repair. DNA damage to MSCs that results from irradiation in vitro is known to promote senescence which limits proliferation and differentiation among other key cell fate decisions [55]. Thus, migration through a sufficiently dense fibrous matrix is expected to increase DNA damage and thereby alter stem cell fate.
Altered genomes are a defining feature of cancer and might also result – in part – from DNA damage in constricted migration with a further consequence possibly being the generation of cancer stem cells. Indeed, starting with a clonal population of a human derived osteosarcoma cell line, multiple rounds of migration generated a set of new clones with differencet genomes that contained losses and gains of large parts of chromosomes, including one clone that appeared more MSC-like [54]. The stem cell like clone exhibited a spindle-shaped in morphology rather than appearing round and osteoblastic like the parental clone. Genomic analyses revealed a gain in part of one chromosome (specifically chrmosome 8p) that largely explained the de-differentiated phenotype. In particular, the transcription factor GATA-4 (located on chromosome 8p) that is down-regulated in osteoblasts and expressed in MSCs was indeed increased in chromosome copy number, RNA level, and protein level in the spindle-shaped post-migration clone. Knockdown of GATA-4 in the spindle-shaped clone produced more rounded shapes while overexpression of GATA-4 in the parental clone generated more spindle-shaped cells. Transcription factors generally regulate many genes, but additional bioformatics analysis and experimental studies provided evidence that GATA-4 upregulates components of the microtubule system, which seems fully consistent with rigid microtubules driving the elongated, spindle-shaped morphology. This could play out in vivo with cells migrating through small constricting pores in the extracellular matrix to induce genomic instability, which could also cause some cells to end up with altered copy number of oncogenes that contribute (as stem cells or not) to cancer progression and/or tumor tissue heterogeneity. These studies at the interface of cancer and stem cells illustrate how various levels of matrix mechanical heterogeneity from viscous elements and multiple layers to fibers and pores can all have profound effects on stem cell phenotypes.
Embryonic cardiomyocytes show maturation is mechanosensitive
Embryos are very soft compared to nearly all adult tissues (Fig. 1A), including heart. Heart is the first organ to form from the soft embryo, and it is very interesting that cultures of embryonic and pluripotent stem cells (PSCs) are often observed to produce some spontaneously beating cardiomyocytes. Cardiomyocytes can of course serve as important models of normal and diseased hearts with potential applications for drug testing and regenerative cardiology [56, 57], but maturation is a current issue as the differentiated state is very distinct for embryonic, fetal, and adult stages. For example, most early embryo cardiomyocytes beat spontaneously when isolated as single cells and grown on sufficiently soft matrix [58] (Fig. 5A) whereas even fetal stage cells require electrical stimulation [59]. Many differentiation protocols currently seek to increase cardiomyocyte numbers but often rely on transcriptional induction alone (on rigid plastic) (Fig. 5B), which has limitations in that the resulting cardiomyocytes resemble immature or variably mature cardiomyocytes in terms of cell shape, cytoskeletal organization, and mechanical output [60]. Recent evidence suggests that such differences in maturation can be rescued at least in part by transplanting Human PSC-derived cardiomyocytes into the soft microenvironment of the neonatal rat heart [61]. It is thus becoming increasingly clear that matrix mechanics and topography as well as morphological control play key roles in advancing the structural and functional maturation of cardiomyocytes.
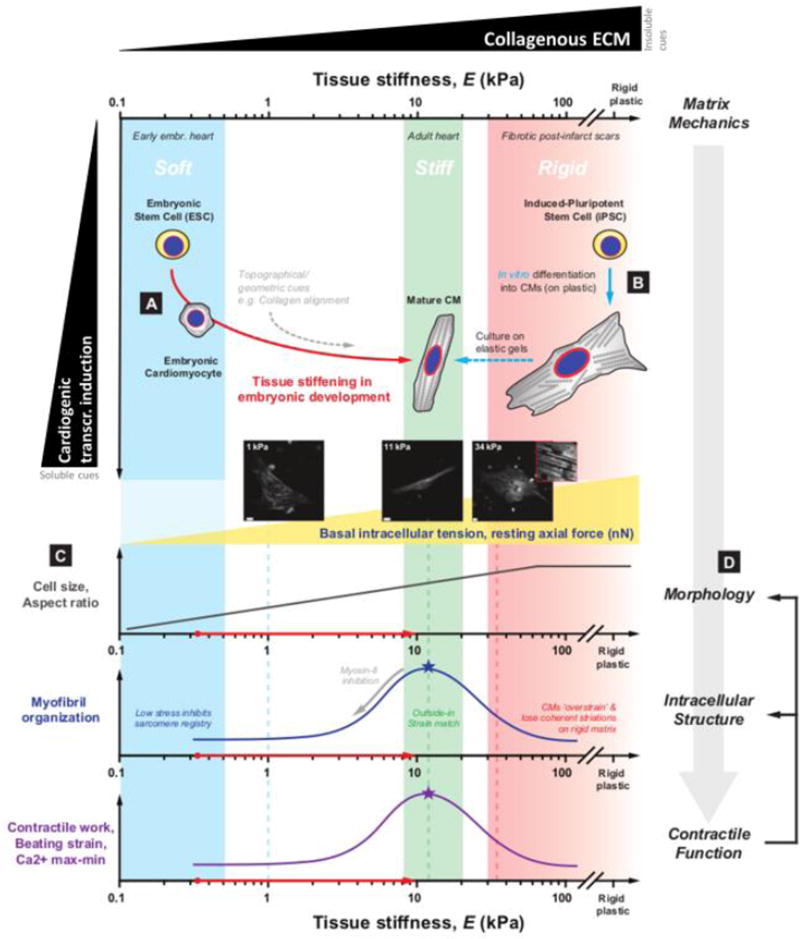
Figure 5. Mechanical cues facilitate and fine-tune cardiomyocyte (CM) differentiation and maturation from pluripotent stem cells (PSCs).
A. Early embryos are uniformly soft, but the heart stiffens rapidly as it begins to pump blood and increasing levels of collagenous ECM are deposited into the developing myocardium [28]. Rapid stiffening of heart tissue provides insoluble mechanical cues for the structural and functional maturation of CMs, orthogonal to soluble cues i.e. cardiogenic transcriptional induction by biochemical gradients.
B. The critical role of tissue mechanics is also evident in the incomplete maturation of CMs derived from iPSCs on rigid plastic: current differentiation protocols that rely solely on transcriptional programming tend to result in cells resembling immature postnatal CMs with disrupted myofibril organization, and impaired contractile function. More mature, adult CM-like phenotypes can be achieved in vitro upon differentiating and culturing cells on elastic gels that mimic the mechanical properties of native adult cardiac tissue (E ∼ 11 kPa).
C. Matrix-directed structural maturation of CMs occurs over several layers of biophysical mechanisms identified in both in vivo tissue and in vitro culture models. Basal intracellular tension (as measured by resting axial force) and cell size increase monotonically as a function of stiffness, but myofibril registry and sarcomere organization exhibit an optimum on stiff matrices with E corresponding to that of myocardial tissue. Importantly, inhibition of myosin-II (e.g. by blebbistatin treatment) also results in the loss of striations and spindle-like morphology characteristic of mature myocytes. Thus, a controlled ‘strain match’ between the cell’s actomyosin machinery and the extracellular microenvironment appears necessary for the optimal assembly of myofibrils, and ultimately CM function.
D. Such observations point to a model in which tissue mechanics -- often determined by collagen levels --sets a basal tone in intracellular stress, which regulates cell morphology, myofibril organization and, in turn, the contractile function of beating CMs.
Studies of mid-stage embryonic cardiomyocytes have demonstrated that a one day culture on very soft matrices (∼0.3–1 kPa) will suppress the beating of embryonic cardiomyocytes, which do very little work and lose their rod-like morphology as well as sarcomeric striations (Fig. 5C). However, matrices with the elasticity of mature heart (∼10 kPa) optimize the striation of cardiac myosin-II and the rhythmic work done by the cells, whereas rigid matrices that model highly fibrotic, post-infarct scars (∼25–50 kPa) disrupt myofibril striation and inhibit beating (Fig. 4C) [58, 59, 62, 63]. Pharmacological inhibition of myosin-II also disrupts striation (Fig. 5C). Thus, a suitable balance of cell stress and matrix resistance appears necessary for the conversion of peripheral striations of nonmuscle myosin-II (periodicity of ∼1 µm) to stable cardiac sarcomeres (periodicity of ∼2 µm) in a process known as myofibrillogenesis. Such sensitivity to matrix mechanics is also evident at the tissue-level using intact embryonic hearts: enzymatic softening of matrix (by collagenase) and enzymatic stiffening of matrix (by transglutaminase) impair beating within tens of minutes [28]. These observations underscore the in vivo roles of optimal matrix stiffness to a basal tone in intracellular actomyosin stress, which in turn regulates myofibril organization (i.e. striation order), cell morphology, and contractile function [28, 29] (Fig. 5D).
Transcriptional changes downstream of matrix mechanics are also likely since meta-analyses of dozens of public ‘-omics’ datasets for hearts in normal development and various diseased states show many mechano-sensitive genes are consistently correlated with changes in collagen-I expression that confers tissue stiffness [64]. Lamin-A,C is among these mechano-sensitive genes and one of the main nuclear structure proteins that regulates other pathways and increases with collagen-I levels in heart for all development stages, disease states, and species studied. Culturing of MSCs on gels of increasing stiffness likewise increases lamin-A,C, and on matrices of muscle-like stiffness (∼11 kPa) MSCs upregulate myogenic transcription factors, including Pax activators and MyoD that regulate the expression of various skeletal muscle-specific genes [1]. Matrix stiffness also regulates nuclear localization and/or activity of YAP/TAZ and MKL1/SRF, master regulators of growth and actomyosin contractility, respectively, in a variety of cell types [16, 17]. Although mechanisms of nuclear/cytoplasmic translocation of these factors remain unclear and do not seem to apply to normal tissue [7], these transcriptional regulators tend to favor entry into the nucleus in response to increased mechanical stress or stiffness in vitro. In contrast, rigid matrices promote nuclear exit of NKX-2.5, which is a crucial transcription factor in early cardiogenesis that also represses genes such as smooth muscle actin that contribute to high-tension, non-cardiogenic states [47]. Despite this handful of mechanosensitive signal transducers identified in recent years, much more can be learned about the mechanisms by which matrix stiffness and intracellular stress activate or repress specific transcriptional programs in cardiomyocyte maturation. Clarification of core mechanosensing pathways -- likely involving force-transduction to the nuclear envelope and perhaps into the genome -- will help address these broad questions and might provide fundamental insight into how mechanical stress influences cardiomyocyte fate.
In addition to rhythmic contractile stress generated by heart cells and the mechanical stress imposed by the beating heart tissue, postnatal and adult cardiomyocytes [59] are also spindle-like and highly aligned in normal mature tissue. One can therefore expect these cells to be sensitive to topographical and geometric cues from the microenvironment. Indeed, culturing cardiomyocytes on engineered substrates with nano-topographical features [65] and/or micro-patterned ligands [66] can alter contractile structure and function. For example, cardiac tissue constructs cultured on hydrogels with aligned nanoscale topographic patterns exhibit anisotropic action potential propagation and contractility that are characteristics of mature heart tissue [65]. Similarly, cardiomyocytes cultured on narrow rectangles of ligand-patterned substrates exhibit rod-like morphologies with well-aligned myofibrils that reasonably resemble adult cardiomyocytes. In contrast, cells on circular patterns or unpatterned substrates, with no anisotropic directionality cues, typically show little alignment and no orientation [66–68]. More recent efforts have explored the combined effects of such geometric constraints and substrate mechanics by examining hPSC-derived cardiomyocytes on Matrigel-micropatterned hydrogels of controlled stiffness [69]. Imposing a physiological shape while providing a physiological matrix stiffness increased the mechanical output of hPSC-cardiomyocytes and also improved calcium handling, mitochondrial organization, and transversetubule formation [69]. The findings indicate the profound effects of microenvironment mechanics are not limited to contractile activity of cardiomyocytes, but also cardiomyocyte electrophysiology. Matrix stiffness, geometry, and topography thus combine as inputs to inform cardiomyocyte differentiation and maturation.
Skeletal muscle stem cell mechanosensing from in vitro to in vivo
Constant beating of the heart relies on the long-life of heart muscle, but heart muscle seems to be rarely regenerated compared to skeletal muscle where a resident population of skeletal muscle stem cells (MuSC) provides a robust ability for skeletal muscle to adapt to mechanical demands [70]. MuSCs express the transcription factor Pax7 and stage-specific markers of differentiation: transcription factors MyoD in early phase and MyoG in later phases, as well as embryonic myosin heavy chain (MyHC) when contractile machinery is assembling. MuSCs are often termed satellite cells due to their peripheral location between the mature muscle fiber and the basement membrane that surrounds every myotube. This position provides sensitivity to mechanical changes within the muscle fiber and the surrounding matrix. The mechanical environment changes with aging and across many skeletal muscle diseases, including muscular dystrophies [50], which motivates a deeper understanding of how MuSCs respond to altered mechanical environments. Aging is associated with an increase in tissue stiffness that modulates mouse MuSCs ability to expand and differentiate [71]: aging impairs MuSC-based muscle repair, but critically, the niche has been shown to be a major determinant of aging effects rather than intrinsic impairment of mouse MuSCs [72].
MuSCs in healthy tissue are maintained in a quiescent state and upon activation signal division in one of three ways: i) symmetrically producing daughter cells that maintain stemness, ii) producing daughter cells that are both committed progenitors, or iii) asymmetric division that produces one of each type of daughter cell. In both forms of symmetric division, daughter cells maintain contact with both the basement membrane and sarcolemma, whereas in asymmetric division the self-renewed MuSC maintains connections to the basement membrane while the committed progenitor interacts with the muscle fiber [73] (Fig. 6A). The polarity induced by the unique substrate adhesion in asymmetric division is derived from non-random DNA segregation [74]. Indeed, culture systems with an asymmetric fibronectin/fibrinogen substrate at the single cell level can induce asymmetric segregation of DNA and transcription factors, thus leading to asymmetric division of mouse MuSCs [75]. Hematopoietic stem and progenitor cells can likewise divide asymmetrically on fibronectin-coated gels, using myosin-IIB to polarize the cells particularly on stiff matrices that mimic the surface of bone [22]. Such asymmetric division demonstrates that it is not only the adhesion molecule itself that determines the type of stem cell division in these critical fate decisions. To complete their task of building new muscle, MuSCs and their progeny must exit their basement membrane niche and transverse the interstitial matrix [76], which exposes MuSCs to a new mechanical microenvironment.
Figure 6. Muscle stem cell differentiation is modulated by substrate stiffness.
A, B. Adult Quiescent MuSCs (Pax7+) residing on the fiber periphery receive an activation signal and divide to form two MuSCs, two early myoblasts (MyoD+), or asymmetrically divide to form one of each. Substrates that are too stiff [40] or too soft [78] favor differentiation while asymmetric substrates support asymmetric division [74]. Early myoblasts further differentiate to form late myoblasts (MyoG+) or undergo apoptosis, the later of which is more prevalent on stiff substrates [40]. Late myoblasts fuse to form contractile myotubes or myofibers. Substrate stiffness above or below 12 kPa inhibit the ability of myoblasts to undergo terminal differentiation [77]. The effect of stiffness is indicated by the line color: softer than healthy muscle (cyan), healthy muscle (green), and stiffer than healthy muscle (red). These muscle tissue stiffness values are plotted on the kPa scale to show healthy, diseased and developing muscle.
C. After 4 weeks in differentiation media C2C12 myoblasts grown on collagen strips demonstrate a narrow range of optimal stiffness for differentiation as depicted by the presence of sarcomere striations. Quantification shows substrate stiffness between 8–15 kPa maximizes differentiation and encompasses the stiffness of healthy muscles. Adapted from [77].
MuSCs are not exposed in vivo to the extremely high rigidity of tissue culture plastic, which has long been known to rapidly diminish the stemness of plated MuSCs and make it impossible to expand these cells. Mechanical measurements of muscle tissue at the cellular scale using AFM show healthy muscle with an elastic modulus of ∼12 kPa [77]. When primary mouse MuSCs are cultured in vitro on hydrogels mimicking the stiffness of muscle they expand more readily [40] (Fig. 6A,B). This is not due to a change in proliferation rate, but instead a decrease of cell death and inhibition of differentiation. Thus, the ∼12 kPa substrate maintains the stem qualities of MuSCs with significant symmetric stem cell division that was not present on more rigid substrates. This was verified by injection of primary MuSCs cultured for 1 week back into muscle tissue showing that those cultured on muscle mimetic stiffness had the highest engraftment and even repopulated the satellite cell niche [40].
Matrix mutations cause various muscle disorders, and perhaps consistent with the many muscular dystrophies in which fibrotic collagenous matrix accumulates and tends to stiffen the tissue, the Col6a1−/− mouse has (less matrix and) more flexible muscle [78]. Mimicking in vitro the decrease from 12 kPa normal muscle stiffness to 7 kPa diseased stiffness caused normal mouse MuSC populations to lose Pax7+ MyoD-cells that maintain stemness. Culturing MuSCs for 7 days on these gels prior to engraftment in muscle indeed showed those cultured on the normal muscle stiffness could reoccupy the satellite cell niche ∼4 fold more effectively than those grown on the softer substrate [78]. Alternatively, the softer matrix of the embryonic myotome is capable of maintaining the muscle progenitor state and indeed, it has been hypothesized that stiffness directs the cells toward the myotome fate [79]. Mechanisms of such mechanosensing by MuSCs require further study, but links from matrix stiffness to the nuclear lamin-A,C as discussed above for heart [64] seem to also apply to skeletal muscle [78]. Lamin-A,C mutations can indeed cause muscular dystrophy, and knocking out or knocking down lamin-A,C in MuSCs results in impaired differentiation potential [80]. These findings demonstrate the profound impact that matrix stiffness can exert on the MuSC function in health and disease.
Muscle progenitors grown on rigid tissue culture plastic are induced to fuse and form myotubes when serum is reduced (particularly FGF in serum), but the extent of myotube development is limited and does not typically progress to produce highly contractile cells with registered sarcomeres. Myoblastic C2C12 cells grown on a flexible fibroblast layer can enhance adult myosin expression and electrically stimulated contraction whereas sarcomeric patterns were virtually non-existent on stiff gels and collagencoated glass strips [81]. This is consistent with an optimal 12 kPa stiffness for striation when using gels [77], with a high degree of sensitivity as a change of only ∼5 kPa either side of the optimal 12 kPa gels resulted in 50% decreases in striation (Fig. 6B,C). This finding is relevant to diseased muscle that often becomes more fibrotic and stiff [50]. High (transient) stiffness might thus induce differentiation, but proper muscle stiffness could be key to optimal muscle maintenance, repair, and growth.
Damage to myofibers in culture increases their stiffness and causes a marked increase in proliferative MuSCs [82]. Growing mouse MuSCs on hydrogels with matching stiffness recapitulated the proliferation of MuSCs, indicating mechanosensing as a key activator of MuSC in response to muscle fiber injury. To capture additional complexity of the MuSC niche such as myofiber dimensions, integrins, and laminin, microfluidics-based cultures are being developed that use collagen based engineered muscle fibers. Tuning the engineered muscle fiber stiffness to that observed in vivo allowed human MuSC grown in these cultures to maintain quiescence in vitro and enhanced their ability to engraft in vivo [83].
Beyond proliferation and differentiation, however, the ability of MuSC and their progeny to migrate to the appropriate site is critical and necessitates physical interactions with the surrounding environment. While MuSCs that repair or rebuild their resident fiber might meet little mechanical resistance to migration, many cells escape the basement membrane and transverse the interstitial matrix [76, 84]. This requires migratory cells to either squeeze through small pores and/or degrade the matrix in their path. Muscle progenitors have been shown to express a range of matrix metalloproteinases (MMPs) when activated [85] and inhibiting MMP degradation of ECM blocks muscle regeneration [86]. Exogenous MMP-1, an interstitial collagenase, can enhance myoblast migration in vitro and in vivo, leading to improved donor cell engraftment [87]. MMP-14, a transmembrane MMP critical for invadopodia, is required for human MuSC to penetrate collagen I matrices [88]. Reorganizing the matrix via MMPs is an important aspect of MuSC migration, and yet pore sizes these proteases open and the following stresses and deformations these cells undergo as they migrate away from their niche are largely unexplored and may well impact their ability to fuse and differentiate.
Concluding Prospectus
Stem cells of many types have been shown over the past decade to mechanosense matrix, affecting morphology, cytoskeletal organization, and migration within hours and subsequently proliferation, differentiation, and lineage maturation in days. In such fate decisions, the stiffest matrix nearby tends to dominate nearby soft media that includes static fluid media. Although molecular mechanisms are many, myosin-II generated forces are key to probing the matrix as well as internal sensory processes involving regulatory proteins such as lamin-A,C and YAP/TAZ. The former leads, in simplest terms, to stiffness matching of nucleus with matrix and also co-regulates the expression of many acto-myosin genes, whereas YAP/TAZ translocation regulates growth among other processes. Lateral heterogeneity of matrix is common in tissues bearing stem cell niches, including MSCs among the various stem cells that durotax in 2D and 3D model systems. Stratified heterogeneity of matrix is sometimes obvious and other times more subtle, but careful examination of culture models suggests considerable consensus in matrix mechanosensing in differentiation. Study of mechanosensitivity in stem cells is of particular importance within the highly contractile environment of both cardiac and skeletal striated muscle to understand disease mechanisms and eventually to create mechano-informed therapies.
Among the new ideas or effects perhaps emerging in 3D migration of stem cells is niche localization via nuclear immobilization and DNA damage in the most highly stressed stem cells and the most rigid matrix. DNA damage following migration through rigid pores has already been correlated with changes in chromosome copy number in studies of an osteosarcoma, but mutations and chromosome copy number changes are also common in cultures of pluripotent cells – which is a major concern for their application. Substrate rigidity and related mechanisms of nuclear stress might thus contribute to genomic changes in otherwise normal stem cells.
Table 1.
Definition of mechanical terms
| Term | Units | Definition |
|---|---|---|
| strain | % | Deformation of an object relative to a reference shape. |
| stress | Pa | Force per area on the surface of an object or on a plane within an object. |
| pressure | Pa | Stress in which forces are perpendicular to the surface or plane. |
| shear stress | Pa | Stress in which forces are parallel to the surface or plane. |
| elasticity | Reversible change in shape after a deformation. | |
| elastic modulus | Pa | Resistance to reversible deformation, i.e. the slope of a stress versus stain plot. |
| rigid | Object that strains very little (eg. <1 %) in response to a given force or stress. | |
| viscosity | Pa · sec | Resistance of a fluid to flow when a shear stress is applied. |
| stiffness | Resistance to deformation, often measured with elastic modulus. | |
| durotaxis | Cell migration directed by a stiffness gradient (in the absence of any chemical gradients). | |
| haptotaxis | Cell migration directed by a gradient of adhesion ligand on a surface. | |
| soft | ≤ Em or ∼ 10 kPa in the context of cell and tissue mechanics. | |
| stiff | ≥ Em or ∼ 10 kPa in the context of cell and tissue mechanics. | |
| gel | A cross-linked network within a liquid that creates a material that behaves as a solid. | |
| sol | A fluid suspension consisting of small solids within a liquid. |
Acknowledgments
The authors were supported by the US National Institutes of Health National Cancer Institute under PSOC Award Number U54 CA193417, by the National Heart Lung and Blood Institute under Award Numbers R01 HL124106 and R21 HL128187, by the National Institute of Arthritis and Musculoskeletal and Skin under Award Number K99 (L.S.), and also by the US National Science Fdn Materials Science Science and Eng’g Center grant to Penn, the US-Israel Binational Science Fdn, and the Charles Kaufman Fdn under Award Number KA2015-79197. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other granting agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86(1 Pt 1):617–28. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79(1):144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape cytoskeletal tension and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 5.Wenger MPE, Bozec L, Horton MA, Mesquida P. Mechanical Properties of Collagen Fibrils. Biophys J. 2007:1255–63. doi: 10.1529/biophysj.106.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss P, Garber B. Shape and Movement of Mesenchyme Cells as Functions of the Physical Structure of the Medium: Contributions to a Quantitative Morphology. Proc Natl Acad Sci U S A. 1952;38(3):264–80. doi: 10.1073/pnas.38.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149):1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buggenthin F, Buettner F, Hoppe PS, Endele M, Kroiss M, Strasser M, Schwarzfischer M, Loeffler D, Kokkaliaris KD, Hilsenbeck O, Schroeder T, Theis FJ, Marr C. Prospective identification of hematopoietic lineage choice by deep learning. Nat Methods. 2017 doi: 10.1038/nmeth.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9(6):518–26. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12(5):458–65. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zemel A, Rehfeldt F, Brown AE, Discher DE, Safran SA. Optimal matrix rigidity for stress fiber polarization in stem cells. Nat Phys. 2010;6(6):468–473. doi: 10.1038/nphys1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raab M, Discher DE. Matrix rigidity regulates microtubule network polarization in migration. Cytoskeleton (Hoboken) 2017;74(3):114–124. doi: 10.1002/cm.21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PC, Athirasala A, Kao YR, Cho S, Harada T, Shin JW, Discher DE. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr Biol. 2014;24(16):1909–17. doi: 10.1016/j.cub.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Discher DE, Smith L, Cho S, Colasurdo M, García AJ, Safran S. Matrix Mechanosensing: From Scaling Concepts in Omics Data to Mechanisms in the Nucleus Cancer and Regeneration. Annual Review of Biophysics. 2017;46(1) doi: 10.1146/annurev-biophys-062215-011206. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 17.Graham DM, Burridge K. Mechanotransduction and nuclear function. Curr Opin Cell Biol. 2016;40:98–105. doi: 10.1016/j.ceb.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13(6):645–52. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao B, Li L, Guan KL. Hippo signaling at a glance. J Cell Sci. 2010;123(Pt 23):4001–6. doi: 10.1242/jcs.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musah S, Wrighton PJ, Zaltsman Y, Zhong X, Zorn S, Parlato MB, Hsiao C, Palecek SP, Chang Q, Murphy WL, Kiessling LL. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc Natl Acad Sci U S A. 2014;111(38):13805–10. doi: 10.1073/pnas.1415330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rammensee S, Kang MS, Georgiou K, Kumar S, Schaffer DV. Dynamics of Mechanosensitive Neural Stem Cell Differentiation. Stem Cells. 2016 doi: 10.1002/stem.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin JW, Buxboim A, Spinler KR, Swift J, Christian DA, Hunter CA, Leon C, Gachet C, Dingal PC, Ivanovska IL, Rehfeldt F, Chasis JA, Discher DE. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell. 2014;14(1):81–93. doi: 10.1016/j.stem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakar MS, Eyckmans J, Pieters R, Eberli D, Nelson BJ, Chen CS. Cellular forces and matrix assembly coordinate fibrous tissue repair. Nat Commun. 2016;7:11036. doi: 10.1038/ncomms11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, Fredberg JJ, Trepat X. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10(6):469–75. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim WS, Alim K, Mammoto A, Ingber DE, Duda GN, Mooney DJ. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 2015;14(12):1269–77. doi: 10.1038/nmat4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enemchukwu NO, Cruz-Acuna R, Bongiorno T, Johnson CT, Garcia JR, Sulchek T, Garcia AJ. Synthetic matrices reveal contributions of ECM biophysical and biochemical properties to epithelial morphogenesis. J Cell Biol. 2016;212(1):113–24. doi: 10.1083/jcb.201506055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caiazzo M, Okawa Y, Ranga A, Piersigilli A, Tabata Y, Lutolf MP. Defined three-dimensional microenvironments boost induction of pluripotency. Nat Mater. 2016;15(3):344–52. doi: 10.1038/nmat4536. [DOI] [PubMed] [Google Scholar]
- 28.Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr Biol. 2013;23(23):2434–9. doi: 10.1016/j.cub.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasbiswas K, Majkut S, Discher DE, Safran SA. Substrate stiffness-modulated registry phase correlations in cardiomyocytes map structural order to coherent beating. Nat Commun. 2015;6:6085. doi: 10.1038/ncomms7085. [DOI] [PubMed] [Google Scholar]
- 30.Tse JR, Engler AJ. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One. 2011;6(1):e15978. doi: 10.1371/journal.pone.0015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raab M, Swift J, Dingal PC, Shah P, Shin JW, Discher DE. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J Cell Biol. 2012;199(4):669–83. doi: 10.1083/jcb.201205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci U S A. 1997;94(23):12407–12. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279(40):41263–6. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- 34.Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol. 1993;120(4):923–34. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novikova EA, Raab M, Discher DE, Storm C. Persistence-Driven Durotaxis: Generic Directed Motility in Rigidity Gradients. Phys Rev Lett. 2017;118(7):078103. doi: 10.1103/PhysRevLett.118.078103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290(6):H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 37.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15(3):326–34. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehfeldt F, Brown AE, Raab M, Cai S, Zajac AL, Zemel A, Discher DE. Hyaluronic acid matrices show matrix stiffness in 2D and 3D dictates cytoskeletal order and myosin-II phosphorylation within stem cells. Integr Biol (Camb) 2012;4(4):422–30. doi: 10.1039/c2ib00150k. [DOI] [PubMed] [Google Scholar]
- 39.Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WT. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11(7):642–9. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buxboim A, Discher DE. Stem cells feel the difference. Nat Methods. 2010;7(9):695–7. doi: 10.1038/nmeth0910-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates, jNat Methods. 2010;7(9):733–6. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208(4440):177–9. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 44.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014;13(10):979–87. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chamberlain JK, Lichtman MA. Marrow cell egress: specificity of the site of penetration into the sinus. Blood. 1978;52(5):959–68. [PubMed] [Google Scholar]
- 46.Shin JW, Spinler KR, Swift J, Chasis JA, Mohandas N, Discher DE. Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proc Natl Acad Sci U S A. 2013;110(47):18892–7. doi: 10.1073/pnas.1304996110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dingal PC, Bradshaw AM, Cho S, Raab M, Buxboim A, Swift J, Discher DE. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat Mater. 2015;14(9):951–60. doi: 10.1038/nmat4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16(1):51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1(1):21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am J Physiol Cell Physiol. 2014;306(10):C889–98. doi: 10.1152/ajpcell.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179(6):1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olsen AL, Bloomer SA, Chan EP, Gaca MD, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301(1):G110–8. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker BM, Trappmann B, Wang WY, Sakar MS, Kim IL, Shenoy VB, Burdick JA, Chen CS. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater. 2015;14(12):1262–8. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irianto J, Xia Y, Pfeifer CR, Athirasala A, Ji J, Alvey C, Tewari M, Bennett RR, Harding SM, Liu AJ, Greenberg RA, Discher DE. DNA Damage Follows Repair Factor Depletion and Portends Genome Variation in Cancer Cells after Pore Migration. Curr Biol. 2017;27(2):210–223. doi: 10.1016/j.cub.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cmielova J, Havelek R, Soukup T, Jiroutova A, Visek B, Suchanek J, Vavrova J, Mokry J, Muthna D, Bruckova L, Filip S, English D, Rezacova M. Gamma radiation induces senescence in human adult mesenchymal stem cells from bone marrow and periodontal ligaments. Int J Radiat Biol. 2012;88(5):393–404. doi: 10.3109/09553002.2012.666001. [DOI] [PubMed] [Google Scholar]
- 56.Batalov I, Feinberg AW. Differentiation of Cardiomyocytes from Human Pluripotent Stem Cells Using Monolayer Culture. Biomark Insights. 2015;10(1):71–6. doi: 10.4137/BMI.S20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121(Pt 22):3794–802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95(7):3479–87. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31(5):829–37. doi: 10.1002/stem.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho GS, Lee DI, Tampakakis E, Murphy S, Andersen P, Uosaki H, Chelko S, Chakir K, Hong I, Seo K, Chen HV, Chen X, Basso C, Houser SR, Tomaselli GF, O’Rourke B, Judge DP, Kass DA, Kwon C. Neonatal Transplantation Confers Maturation of PSC-Derived Cardiomyocytes Conducive to Modeling Cardiomyopathy. Cell Rep. 2017;18(2):571–582. doi: 10.1016/j.celrep.2016.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bajaj P, Tang X, Saif TA, Bashir R. Stiffness of the substrate influences the phenotype of embryonic chicken cardiac myocytes. J Biomed Mater Res A. 2010;95(4):1261–9. doi: 10.1002/jbm.a.32951. [DOI] [PubMed] [Google Scholar]
- 63.Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105(6):1148–60. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 64.Cho S, Irianto J, Discher DE. Mechanosensing by the nucleus: From pathways to scaling relationships. J Cell Biol. 2017;216(2):305–315. doi: 10.1083/jcb.201610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A. 2010;107(2):565–70. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton. 2008;65(8):641–51. doi: 10.1002/cm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDevitt TC, Angello JC, Whitney ML, Reinecke H, Hauschka SD, Murry CE, Stayton PS. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J Biomed Mater Res. 2002;60(3):472–9. doi: 10.1002/jbm.1292. [DOI] [PubMed] [Google Scholar]
- 68.Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91(12):e45–54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- 69.Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A. 2015;112(41):12705–10. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scharner J, Zammit PS. The muscle satellite cell at 50: the formative years. Skelet Muscle. 2011;1(1):28. doi: 10.1186/2044-5040-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lacraz G, Rouleau AJ, Couture V, Sollrald T, Drouin G, Veillette N, Grandbois M, Grenier G. Increased Stiffness in Aged Skeletal Muscle Impairs Muscle Progenitor Cell Proliferative Activity. PLoS One. 2015;10(8):e0136217. doi: 10.1371/journal.pone.0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256(6 Pt 1):C1262–6. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 73.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129(5):999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148(1–2):112–25. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 75.Yennek S, Burute M, Thery M, Tajbakhsh S. Cell adhesion geometry regulates non-random DNA segregation and asymmetric cell fates in mouse skeletal muscle stem cells. Cell Rep. 2014;7(4):961–70. doi: 10.1016/j.celrep.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 76.Ogawa R, Ma Y, Yamaguchi M, Ito T, Watanabe Y, Ohtani T, Murakami S, Uchida S, De Gaspari P, Uezumi A, Nakamura M, Miyagoe-Suzuki Y, Tsujikawa K, Hashimoto N, Braun T, Tanaka T, Takeda S, Yamamoto H, Fukada S. Doublecortin marks a new population of transiently amplifying muscle progenitor cells and is required for myofiber maturation during skeletal muscle regeneration. Development. 2015;142(1):51–61. doi: 10.1242/dev.112557. [DOI] [PubMed] [Google Scholar]
- 77.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166(6):877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA, Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 2013;4:1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136(4):677–88. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006;20(4):486–500. doi: 10.1101/gad.1364906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cooper ST, Maxwell AL, Kizana E, Ghoddusi M, Hardeman EC, Alexander IE, Allen DG, North KN. C2C12 co-culture on a fibroblast substratum enables sustained survival of contractile, highly differentiated myotubes with peripheral nuclei and adult fast myosin expression. Cell Motil Cytoskeleton. 2004;58(3):200–11. doi: 10.1002/cm.20010. [DOI] [PubMed] [Google Scholar]
- 82.Trensz F, Lucien F, Couture V, Sollrald T, Drouin G, Rouleau AJ, Grandbois M, Lacraz G, Grenier G. Increased microenvironment stiffness in damaged myofibers promotes myogenic progenitor cell proliferation. Skelet Muscle. 2015;5:5. doi: 10.1186/s13395-015-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quarta M, Brett JO, DiMarco R, De Morree A, Boutet SC, Chacon R, Gibbons MC, Garcia VA, Su J, Shrager JB, Heilshorn S, Rando TA. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat Biotechnol. 2016;34(7):752–9. doi: 10.1038/nbt.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Webster MT, Manor U, Lippincott-Schwartz J, Fan CM. Intravital Imaging Reveals Ghost Fibers as Architectural Units Guiding Myogenic Progenitors during Regeneration. Cell Stem Cell. 2016;18(2):243–52. doi: 10.1016/j.stem.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration differentiation regeneration and fibrosis. Cell adhesion & migration. 2009;3(4):337–41. doi: 10.4161/cam.3.4.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res. 2000;258(2):279–87. doi: 10.1006/excr.2000.4962. [DOI] [PubMed] [Google Scholar]
- 87.Wang W, Pan H, Murray K, Jefferson BS, Li Y. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am J Pathol. 2009;174(2):541–9. doi: 10.2353/ajpath.2009.080509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lund DK, Mouly V, Cornelison D. MMP-14 is necessary but not sufficient for invasion of three-dimensional collagen by human muscle satellite cells. Am J Physiol Cell Physiol. 2014 doi: 10.1152/ajpcell.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Discher DE, Mooney DJ, Zandstra PW. Growth factors matrices and forces combine and control stem cells. Science. 2009;324(5935):1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y-L, Discher DE. Cell Mechanics. Vol. 83. Academic Press; 2007. (Methods in Cell Biology) [Google Scholar]
- 91.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94(25):13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]