Abstract
Background
Reducing meat consumption is often advised, however inadvertent nutritional deficiencies during pregnancy may result in residual neurodevelopmental harms to offspring. This study assessed possible effects of maternal diets in pregnancy on adverse substance use among adolescent offspring.
Methods
Pregnant women and their 13 year old offspring taking part in a prospective birth cohort study, the Avon Longitudinal Study of Parents and Children (ALSPAC), provided food frequency questionnaire data from which dietary patterns were derived using Principal Components Analysis. Multivariable logistic regression models including potential confounders evaluated adverse alcohol, cannabis and tobacco use of the children at age 15 years.
Results
Lower maternal meat consumption was associated with greater problematic substance use among 15 year old offspring in dose response patterns. Comparing never to daily meat consumption after adjustment, risks were greater for all categories of problem substance use; alcohol, odds ratio OR=1.75, 95% CI = [1.23, 2.56], p < 0.001, tobacco use OR=1.85, 95% CI = [1.28, 2.63], p < 0.001 and cannabis OR=2.70, 95% CI = [1.89, 4.00], p < 0.001. Given the likelihood of residual confounding, potential causality was evaluated using stratification for maternal allelic variants that impact biological activity of cobalamin (vitamin B12) and iron. Lower meat consumption disproportionally increased the risks of offspring substance misuse among mothers with optimally functional (homozygous) variants (rs1801198) of the gene TCN2 which encodes the vitamin B12 transport protein transcobalamin 2 implicating a causal role for cobalamin deficits. Functional maternal variants in iron metabolism were unrelated to the adverse substance use. Risks potentially attributable to cobalamin deficits during pregnancy include adverse adolescent alcohol, cannabis, and tobacco use (14 %, 37% and 23% respectively).
Conclusion
Lower prenatal meat consumption was associated with increased risks of adolescent substance misuse. Interactions between TCN2 variant status and meat intake implicate cobalamin deficiencies.
INTRODUCTION
Growing evidence indicates that deficiencies in critical nutrients in utero can increase risks for adverse cognitive development and behavioural problems among otherwise healthy children (Anjos et al., 2013). Consequently it is widely accepted that nutritional deficits and toxic exposures occurring prenatally can alter early life programming and impact life time risks of psychiatric disorders through persistent epigenetic changes. Examples of such maternal deficiencies of essential nutrients, include cobalamin (vitamin B12), iron, iodine, folate, and thiamine which have all been linked to enduring neurodevelopmental deficits (Kofink et al., 2013). Epigenetic changes in the environmental programming of neurodevelopmental processes is especially relevant to nutritional deficiencies in vitamin B12 because many epigenetic modifications are dependent upon alterations in DNA methylation (Caramaschi et al 2017). Vitamin B12 is critical to the process of DNA methylation and deficiencies in Vitamin B12 can cause abnormalities in DNA methylation (Caramaschi et al 2017). The identification of such nutrients has led to successful public health prevention programs and policies, for example the fortification of foods with folate to prevent neural tube abnormalities in many countries (Anjos et al., 2013). However, the majority of published studies on neurodevelopmental outcomes in childhood have focused neither on adolescent cohorts nor substance abuse-related endpoints.
Vegetarian dietary patterns are associated with improved health outcomes among adults and, in addition, have strong ethical imperatives including promotion of sustainability, food security and reducing industrialized production of animals. However, avoidance of relatively nutrient dense meats can decrease intakes of cobalamin, iron, omega-3 fatty acids, selenium and zinc, particularly in young women of childbearing age (Fayet et al., 2014). Cobalamin is a biochemically essential nutrient available predominantly from meats and shellfish; up to 62% of vegetarians are deficient in this nutrient during pregnancy (Pawlak et al., 2013). Profound neurodevelopmental abnormalities due to severe cobalamin deficiencies were first identified among infants from Indian vegan and vegetarian mothers (Jadhav et al., 1962). Among Western populations, infants of cobalamin deficient mothers have poor brain growth, developmental regression, irritability, thrive poorly(Graham et al., 1992) and demonstrate residual deficits in cognitive and social development (Bhate et al., 2012, del Rio Garcia et al., 2009). Thus it is of great interest to determine if the lower cobalamin status of ovo-lacto vegetarian mothers, and non-vegetarians eating less meat can result in residual impairments in the neurodevelopment of their offspring (Bonilla et al., 2012, Koebnick et al., 2004). We were particularly interested in substance use outcomes, as there are few extant reports on the issue.
Here we hypothesised that maternal prenatal nutritional deficiencies were risk factors for adverse substance abuse among offspring. Our approach was to first evaluate if any dietary patterns in pregnancy were associated with adverse substance abuse among offspring. We then evaluated which specific foods comprising those patterns were associated with the increased risks. Within the vegetarian dietary pattern group, lower maternal meat consumption was identified as a risk factor. We evaluated two candidate nutrients (cobalamin and iron) that are both frequently diminished among vegetarians and for which deficiencies have adverse neurological consequences. Finally, we stratified the maternal cohort by genetic variants that affect the status and function of these nutrients, e.g. the variants affecting the bioavailability and transport of cobalamin. Within each strat of genetic variant, we examined the relationships between lower meat consumption among mothers and risks of substance use among their adolescent children.
MATERIALS and METHODS
Participants
The sample comprised participants from the Avon Longitudinal Study of Parents and Children (ALSPAC) (Boyd et al., 2013). ALSPAC is an on-going population-based prospective cohort study in the South-West of England. Pregnant women resident in the former Avon Health Authority (which included the city of Bristol), who had an estimated date of delivery between 1 April 1991 and 31 December 1992, were invited to take part, resulting in a cohort of 14,541 pregnancies which resulted in 13,976 singletons and twins alive at one year of age. Detailed information about ALSPAC is available online (http://www.bris.ac.uk/alspac) with details of all the data available at (http://www.bristol.ac.uk/alspac/researchers/data-access/data-dictionary/). Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and local Research Ethics Committees.
Substance use outcomes at age 15
At the age of approximately 15½ years, 9,979 young people were invited to a clinic and 5,246 (52.6%) attended. Median age at attendance was 15 years 5 months, inter-quartile range (IQR) = 15 years 3 months to 15 years 7 months. Of the clinic attendees, 5,228 started a session where data were collected via an electronic questionnaire that captured data on current substance use behaviour. Responses to questions on alcohol, cannabis and tobacco use and associated problems were used to derive ten substance use variables with binary classification. Five alcohol-related measures were derived, as described by Melotti et al (Melotti et al., 2013), to indicate heavy typical drinking, frequent drinking, regular binge drinking, alcohol psychosocial and alcohol behavioural problems. Three measures were created from questions which included the Cannabis Abuse Screening Test (CAST)(Piontek et al., 2008) to indicate any past-year cannabis use, moderate use and problematic cannabis use. Finally, two measures were created to indicate recent tobacco use, and current weekly tobacco use (Table 1, legend).
Table 1.
Prevalence rates for problematic substance use at age 15 in whole population and stratified by gender
| Population N (%) |
Males N (%) |
Females N (%) |
p | |
|---|---|---|---|---|
| Alcohol measures | ||||
| Heavy typical drinking | 1,024 (21.1%) | 441 (19.5%) | 583 (22.4%) | 0.011 |
| Frequent drinking | 974 (19.3%) | 487 (20.6%) | 487 (18.1%) | 0.023 |
| Regular bingeing | 523 (10.4%) | 252 (10.7%) | 271 (10.1%) | 0.497 |
| Psychosocial problems | 1,283 (25.7%) | 550 (23.6%) | 733 (27.5%) | 0.002 |
| Behavioural problems | 480 (9.5%) | 234 (10.0%) | 246 (9.1%) | 0.314 |
| Cannabis measures | ||||
| Past-year use | 984 (19.5%) | 472 (20.1%) | 512 (19.0%) | 0.335 |
| Moderate use | 485 (9.6%) | 247 (10.5%) | 238 (8.8%) | 0.050 |
| Problematic use | 194 (3.8%) | 119 (5.1%) | 75 (2.8%) | < 0.001 |
| Tobacco measures | ||||
| Recent use | 893 (17.5%) | 329 (13.8%) | 564 (20.7%) | < 0.001 |
| Weekly use | 528 (10.3%) | 195 (8.2%) | 333 (12.3%) | < 0.001 |
Table shows p-values from chi-square tests of association between gender and each binary indicator of problematic substance use.
Alcohol: heavy typical drinking (more than four drinks per occasion in the previous 6 months), frequent drinking (20 or more occasions in past six months), regular binge drinking (consuming five or more drinks in any 24-hour period in the previous two years on 20 or more occasions), alcohol psychosocial problems (any of the following events experienced on more than three occasions in the previous 2 years: ‘set a limit, drank more’; ‘felt should stop/cut back on drinking’; ‘spent a great deal of day drinking’; ‘not done things because of drinking’; ‘continued to drink despite causing problems’; ‘unable to keep up with other activities’; ‘parents/friends complained’; and ‘had a “blackout” because of drinking’) and finally alcohol behavioural problems (similar to psychosocial but based on following four events: ‘used alcohol in dangerous situations’; ‘been accidentally physically hurt while drinking’; ‘had a problem with the police’ and ‘got into fights because of drinking’).
Cannabis: any past-year cannabis use (any reported use in the last 12 months), moderate use (used cannabis at least occasionally) and finally problematic cannabis use (a report of fairly often/very often to one or more of: smoking before midday, smoking when alone, having memory problems, reproaches from family, unsuccessful attempts to quit or problems linked to cannabis consumption).
Tobacco: Two measures were created to indicate: recent tobacco use (any smoking of cigarettes in the last 30 days), and weekly tobacco use (currently smoking on a weekly basis).
Maternal diet in pregnancy
Foods consumed were assessed by a self-administered Food Frequency Questionnaire (FFQ) sent to the mothers during the third trimester of their pregnancy. A wide range of foods were listed with response options ‘never/rarely’, ‘once in two weeks’, ‘1–3 times/wk’, ‘4–7 times/wk’ and ‘more than once a day’. Median gestation at completion was 32 weeks, IQR = 32 weeks to 33 weeks gestation. Comparable measures of intake within each food category were derived in relation to the child’s own diet from FFQ responses collected at age 13 years.
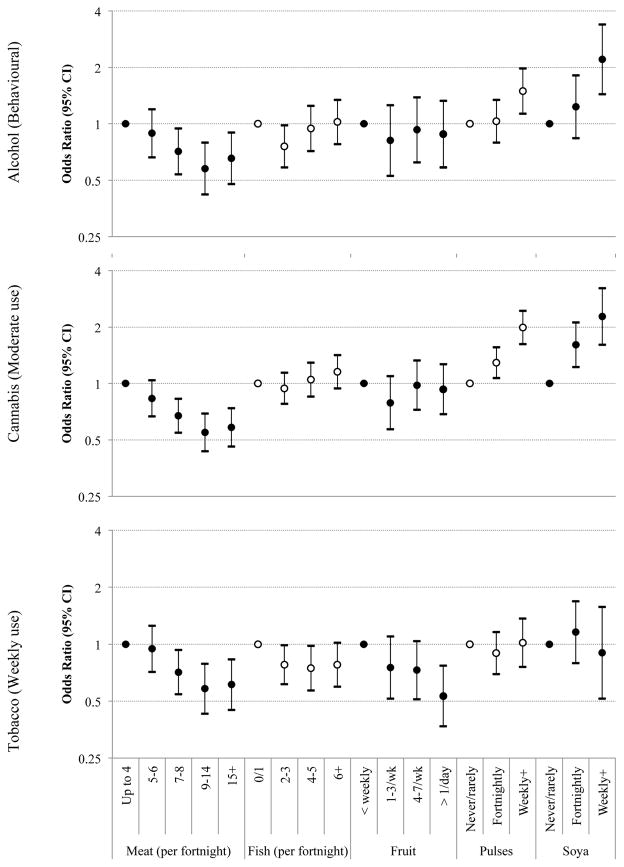
Dietary patterns. As previously reported (Northstone et al., 2008), the dietary information in pregnancy from the FFQ was used to derive scored for five continuous orthogonal dietary pattern categories using Principal Component Analysis (‘health conscious’, ‘traditional’, ‘processed’, ‘confectionary’ and ‘vegetarian’). Food categories. Ordinal measures of food consumption for food categories were derived from responses to one or more questions. Increasing frequency of meat consumption was derived from five questions covering: sausages/burger, meat pies, red meat, poultry and offal. Individual frequency data were coded as follows: ‘never/rarely’=0, ‘once in two weeks’=0.5, ‘1–3 times per week’=2.0, ‘4–7 times per week’=5.5, ‘more than once a day’=10, before summing across responses and collapsing into five large categories based on the resulting distribution. These categories are roughly interpreted as quantity of meat/fish consumption per fortnight (Figure 1). Further measures were created from single question responses including consumption of fresh fruit, soya (“Soya ‘Meat’, T V P, Vegeburgers”) and pulses (“Dried peas, beans, lentils, chick peas”). For each of these variables, we collapsed over rare response categories. Vegetarianism. Responses to ‘are you or have you ever been a vegetarian?’ included ‘yes, I am now’ (coded as 1), ‘yes, in past not now [i.e. during the pregnancy]’ and ‘no, never’ (both coded as 0).
Figure 1.
Unadjusted associations between maternal food categories and odds of substance use outcomes among their adolescent children
Circles and bars represent odds ratios and 95% confidence interval (95% CI) of unadjusted relationships between maternal consumption of selected foods and risk of substance use among their adolescent children.
Other covariates
Socio-demographic variables
Early life social and economic factors that were related to both substance use patterns at age 15 (Melotti et al., 2013) and maternal dietary patterns (Northstone et al., 2008) were identified. Data collected by questionnaire during the antenatal period included variables for housing tenure, highest maternal education level achieved, parity, parental social class, occupations, ethnicity of young person and home overcrowding at enrolment. Factors after the birth included: maternal age at delivery and when the child was a toddler; quintiles of household disposable income taking account of family size and composition and estimated housing benefits. Parent-child relationship measures. The computerized session within the 15-year clinic also contained questions from the Edinburgh Scale of Youth Transitions and Crime (Smith et al., 2001). Measures pertaining to the relationship between the young person and their parents which have been previously shown to be strong predictors of substance-use in this age group were constructed. More details can be found in the footnote to table S5.
Statistical methods
First, a series of univariable logistic regression models were estimated for combination of binary substance use outcomes and continuous standardized dietary pattern score. These models were adjusted for the potential confounding effect of the socio-demographic factors described above. We then selected those dietary patterns for which strong associations remained post-adjustment (namely ‘healthy’ and ‘vegetarian’) -- from these we determined which individual food items were strongly associated (factor loading> 0.5) with these patterns. These data (originally published in Northstone et al (Northstone et al., 2008)) are shown in table S1. Fish, non-white bread, pasta, rice, fresh fruit, meat, meat substitutes, pulses and nuts were selected for further study. Analyses were subsequently repeated for these same food categories using food frequency data on the child’s own diet at age 13. Missing data considerations are detailed in Supplementary materials. All regression modelling was carried out in Stata version 13.1 (College Station, Texas: StataCorp LP.)
Attributable risk
TCN2 genotyping was conducted as previously described (Bonilla et al., 2012). For each outcome we calculated the attributable risk, both in the whole population and stratified by TCN2 genotype. Exposed individuals are those with less than regular meat consumption, in other words, being in categories 1–4 of the 5-category meat consumption measure. This cut-off was chosen as the most conservative estimation of attributable risk of lower meat consumption. Attributable risk was calculated as the difference in risk of each outcome in those exposed and not exposed, expressed as a proportion of all exposed cases.
RESULTS
Gender differences and social patterning in rates of substance use at age 15 years
Of the 5,228 young people who commenced the clinic computing session, 5,109 (97.7%) provided sufficient information on substance use outcomes. There was moderate evidence for gender differences in substance use measures (table 1). The three outcomes of alcohol behavioural problems, moderate cannabis use and weekly tobacco use were present in approximately 10% of the cohort; they represented significant adverse use of each substance and became the focus of this report. Recent tobacco use is strongly and consistently socially patterned, whilst for the other two substances, associations are weaker and less consistent (table 2). Table S2 shows the association between social and economic factors and each dietary pattern measure. Analyses regarding the remaining seven substance use outcomes, dietary patterns and individual foods are presented in Table S3.
Table 2.
Associations between socioeconomic characteristics and the three key adverse substance use outcomes
| Risk factor | Category | Alcohol behavioural problems | Cannabis moderate use | Tobacco weekly use |
|---|---|---|---|---|
| Ethnicity | White | 454 (9.5%) | 452 (9.4%) | 497 (10.2%) |
| Non-white | 23 (10.2%) | 29 (12.8%) | 26 (11.4%) | |
| P | 0.705 | 0.090 | 0.570 | |
| Maternal age at delivery | <25 years | 88 (11.6%) | 74 (9.6%) | 120 (15.5%) |
| 25–29 | 173 (8.9%) | 157 (8.1%) | 206 (10.5%) | |
| 30–34 | 157 (9.2%) | 163 (9.5%) | 148 (8.6%) | |
| 35+ years | 62 (9.7%) | 91 (14.2%) | 54 (8.4%) | |
| P | 0.192 | < 0.001 | < 0.001 | |
| Housing tenure | Mortgaged/owned | 364 (8.8%) | 373 (9%) | 386 (9.3%) |
| Privately rented | 38 (10.7%) | 41 (11.6%) | 44 (12.2%) | |
| Subsidized housing | 62 (15.4%) | 53 (13.1%) | 74 (18.0%) | |
| P | < 0.001 | 0.012 | < 0.001 | |
| Parity | 1st child | 193 (8.1%) | 201 (8.4%) | 219 (9.0%) |
| 2nd | 186 (11.0%) | 174 (10.3%) | 181 (10.6%) | |
| 3rd or greater | 82 (10.4%) | 94 (11.8%) | 97 (12.0%) | |
| P | 0.004 | 0.008 | 0.031 | |
| Maternal education† | > O-level | 183 (8.1%) | 233 (10.3%) | 164 (7.2%) |
| O-level | 179 (10.6%) | 145 (8.5%) | 212 (12.4%) | |
| < O-level | 103 (11.0%) | 94 (10.0%) | 127 (13.4%) | |
| P | 0.007 | 0.147 | < 0.001 | |
| Equivalized income | Top 20% | 98 (9.1%) | 101 (9.3%) | 79 (7.2%) |
| Middle 60% | 243 (9.0%) | 253 (9.4%) | 278 (10.2%) | |
| Bottom 20% | 80 (12.4%) | 77 (11.9%) | 93 (14.1%) | |
| P | 0.027 | 0.131 | < 0.001 | |
| Parental social class | Professional | 52 (6.5%) | 70 (8.8%) | 43 (5.4%) |
| Managerial/technical | 197 (9.3%) | 216 (10.1%) | 212 (9.9%) | |
| Skilled non-manual | 100 (9.2%) | 87 (7.9%) | 120 (10.8%) | |
| Skilled manual or lower | 78 (13.1%) | 61 (10.2%) | 93 (15.3%) | |
| P | 0.001 | 0.172 | < 0.001 | |
| Home over-crowding | <= 1 person/room | 437 (9.4%) | 432 (9.3%) | 454 (9.6%) |
| > 1 person/room | 26 (14.6%) | 28 (15.7%) | 44 (24.4%) | |
| P | 0.020 | 0.004 | < 0.001 |
Table shows chi-square tests of association between demographic measures and each binary indicator of problematic substance use.
: O-levels were an examination taken around the age of 16 years at the end of compulsory schooling. They have been replaced by GCSE’s.
Maternal diet and adolescent substance use
Table 3 shows the results from regression analyses for the binary outcomes behavioural alcohol problems, moderate cannabis use and weekly tobacco use. (See table S3 for additional outcomes). Results show little evidence of an association between the ‘traditional’, ‘processed’ or ‘confectionary’ patterns and any of the three substance use outcomes. For the pattern labelled ‘health conscious’ there is weak evidence of an increase in the odds of alcohol behaviour problems following adjustment for confounding, strong evidence for a more substantial increase in odds of occasional cannabis use, and evidence of reduced odds of weekly tobacco use which was heavily confounded. In contrast, the dietary pattern labelled ‘vegetarian’ showed consistent and strong evidence of a moderate increase in the odds of all three substance use outcomes; associations that that were strengthened after adjustment. The ‘vegetarian’ dietary pattern was also associated with “Heavy typical drinking” and “Psychosocial alcohol problems” in unadjusted analyses (table S3). In moderate agreement with the dietary pattern results, self-reported vegetarianism was seen to be associated with increased odds of alcohol problems and cannabis use, but was not associated with weekly smoking.
Table 3.
Associations between maternal dietary measures in pregnancy and adverse substance use outcomes in offspring at age 15 years
| Alcohol (behavioural) | Cannabis (moderate) | Tobacco (weekly use) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Unadjusted | Adjusted for SES | Unadjusted | Adjusted for SES | Unadjusted | Adjusted for SES | ||
| Dietary patterns | ‘Health Conscious’ | 0.96 [0.87, 1.07] | 1.13 [0.99, 1.28] | 1.21 [1.09, 1.33] | 1.29 [1.14, 1.47] | 0.75 [0.68, 0.83] | 0.91 [0.80, 1.04] |
| p | 0.468 | 0.067 | < 0.001 | < 0.001 | < 0.001 | 0.175 | |
| ‘Traditional’ | 0.98 [0.89, 1.09] | 0.96 [0.86, 1.08] | 0.98 [0.89, 1.09] | 0.97 [0.86, 1.08] | 1.04 [0.94, 1.14] | 1.01 [0.90, 1.12] | |
| p | 0.752 | 0.486 | 0.711 | 0.574 | 0.436 | 0.978 | |
| ‘Processed’ | 1.02 [0.92, 1.13] | 0.94 [0.83, 1.07] | 1.02 [0.92, 1.13] | 1.00 [0.88, 1.14] | 1.11 [1.01, 1.22] | 0.96 [0.84, 1.08] | |
| p | 0.704 | 0.362 | 0.750 | 0.997 | 0.036 | 0.482 | |
| ‘Confectionary’ | 1.02 [0.92, 1.13] | 1.07 [0.95, 1.19] | 0.91 [0.82, 1.01] | 0.94 [0.84, 1.06] | 0.98 [0.88, 1.08] | 0.97 [0.87, 1.08] | |
| p | 0.689 | 0.259 | 0.072 | 0.306 | 0.618 | 0.584 | |
| ‘Vegetarian’ | 1.22 [1.12, 1.32] | 1.28 [1.17, 1.41] | 1.37 [1.27, 1.48] | 1.42 [1.30, 1.55] | 1.15 [1.06, 1.25] | 1.21 [1.10, 1.33] | |
| p | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.001 | < 0.001 | |
|
| |||||||
| Food categories | Meat (ref: 0–4/fortnight) | ||||||
| 5–6 per fortnight | 0.89 [0.66, 1.20] | 0.92 [0.66, 1.28] | 0.70 [0.53, 0.93] | 0.73 [0.53, 1.00] | 0.95 [0.71, 1.25] | 0.95 [0.68, 1.31] | |
| 7–8 per fortnight | 0.71 [0.54, 0.94] | 0.67 [0.49, 0.92] | 0.52 [0.40, 0.68] | 0.50 [0.37, 0.67] | 0.71 [0.54, 0.93] | 0.63 [0.46, 0.87] | |
| 9–14 per fortnight | 0.58 [0.42, 0.79] | 0.53 [0.37, 0.76] | 0.42 [0.31, 0.57] | 0.41 [0.29, 0.58] | 0.58 [0.43, 0.79] | 0.54 [0.38, 0.76] | |
| 15+ per fortnight | 0.66 [0.48, 0.90] | 0.57 [0.39, 0.81] | 0.44 [0.32, 0.60] | 0.37 [0.25, 0.53] | 0.61 [0.45, 0.83] | 0.54 [0.38, 0.78] | |
| p | 0.003 | 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Fish (ref: 0/1 per fortnight) | |||||||
| 2–3 per fortnight | 0.76 [0.59, 0.98] | 0.77 [0.57, 1.03] | 0.84 [0.65, 1.09] | 0.81 [0.60, 1.09] | 0.78 [0.62, 0.99] | 0.88 [0.67, 1.17] | |
| 4–5 per fortnight | 0.95 [0.72, 1.25] | 1.17 [0.85, 1.60] | 1.04 [0.79, 1.37] | 1.10 [0.80, 1.50] | 0.75 [0.57, 0.98] | 1.02 [0.75, 1.39] | |
| 6+ per fortnight | 1.02 [0.78, 1.34] | 1.20 [0.88, 1.64] | 1.17 [0.89, 1.53] | 1.11 [0.81, 1.51] | 0.78 [0.60, 1.02] | 1.04 [0.76, 1.42] | |
| p | 0.110 | 0.017 | 0.118 | 0.140 | 0.086 | 0.685 | |
| Fresh fruit (ref: <weekly) | |||||||
| 1–3 times a week | 0.82 [0.53, 1.26] | 0.83 [0.50, 1.36] | 0.79 [0.51, 1.24] | 0.96 [0.55, 1.67] | 0.75 [0.52, 1.10] | 1.03 [0.64, 1.66] | |
| 4–7 times a week | 0.93 [0.62, 1.39] | 1.10 [0.68, 1.76] | 1.02 [0.67, 1.53] | 1.30 [0.77, 2.19] | 0.73 [0.51, 1.04] | 1.14 [0.72, 1.79] | |
| More than once per day | 0.88 [0.59, 1.33] | 1.13 [0.69, 1.84] | 1.03 [0.68, 1.56] | 1.26 [0.74, 2.16] | 0.53 [0.37, 0.77] | 0.88 [0.55, 1.42] | |
| p | 0.732 | 0.266 | 0.279 | 0.244 | 0.002 | 0.295 | |
| Pulses (ref: never/rarely) | |||||||
| Fortnightly | 1.03 [0.79, 1.34] | 1.23 [0.91, 1.68] | 1.58 [1.24, 2.02] | 1.85 [1.39, 2.45] | 0.90 [0.69, 1.16] | 1.24 [0.92, 1.69] | |
| Weekly+ | 1.50 [1.14, 1.98] | 1.92 [1.38, 2.68] | 2.41 [1.87, 3.11] | 2.96 [2.19, 4.01] | 1.02 [0.76, 1.37] | 1.53 [1.08, 2.17] | |
| p | 0.016 | 0.001 | < 0.001 | < 0.001 | 0.691 | 0.040 | |
| Soya (ref : never/rarely) | |||||||
| Fortnightly | 1.23 [0.84, 1.82] | 1.25 [0.81, 1.93] | 1.54 [1.07, 2.20] | 1.43 [0.95, 2.14] | 1.16 [0.80, 1.69] | 1.45 [0.96, 2.19] | |
| Weekly+ | 2.21 [1.44, 3.39] | 2.48 [1.55, 3.98] | 2.98 [2.00, 4.43] | 3.11 [2.03, 4.77] | 0.90 [0.52, 1.57] | 1.14 [0.63, 2.08] | |
| p | 0.001 | 0.001 | < 0.001 | < 0.001 | 0.688 | 0.198 | |
| Vegetarianism (ref: no) | |||||||
| Yes | 1.76 [1.27, 2.44] | 1.82 [1.25, 2.66] | 1.85 [1.34, 2.54] | 1.85 [1.30, 2.64] | 1.06 [0.73, 1.53] | 1.39 [0.92, 2.09] | |
| p | 0.001 | 0.002 | < 0.001 | 0.001 | 0.769 | 0.116 | |
Table shows odds ratio estimates from univariable and multivariable regression models predicting binary indicators of problematic substance use. For continuous exposure (i.e. dietary patterns), estimates indicate odds-ratio for a 1 SD change. For categorical dietary measures odds-ratios are given relative to the stated baseline reference level.
The individual foods comprising the dietary patterns were then evaluated. Less frequent meat consumption and greater pulse and meat substitute consumption were each associated with increased risks of adolescent substance use, but no other foods showed consistent relationships (table 3, figure 1, table S3, table S4), with the exception of increased rice, pasta and nuts consumption being associated with cannabis use measures. Dietary intakes at age 13 showed similar specificities for individual foods, but less frequent meat consumption did not have a dose response pattern to substance use risk at age 15 (figure S1).
Parent-child relationships, substance abuse and maternal diets
Adverse parent-child relationships (less monitoring, greater conflict, less child disclosure) were strongly associated with increased risks of adolescent substance use (table S5). We posited that the maternal ‘vegetarian’ pattern (or low meat consumption) might be associated with a particular style of parenting that might explain the increased risks of adolescent substance use. However, parent child relationships were not related to either the ‘vegetarian’ dietary pattern or meat consumption (table S6) and henceforth we rejected this explanation.
Iron
Biomarkers of iron status included: the first haemoglobin measure taken in pregnancy, cord iron, cord blood ferritin and child haemoglobin at 7, 9 and 11 year clinics. Maternal prenatal iron supplementation was considered alone and including level of meat consumption. Interactions between maternal meat consumption and a functional single nucleotide polymorphism (SNP) variant in the hemochromatosis (HFE) gene (rs1800562) and 8 SNP variants in the Transferrin Receptor (TFRC) gene (rs9820939, rs570, rs714602, rs3817672, rs4927868, rs6583288) were evaluated. In summary, neither maternal nor child measures of iron status, nor maternal iron supplementation nor variants in iron metabolism, were robustly associated with adolescent substance abuse risk patterns.
TCN2
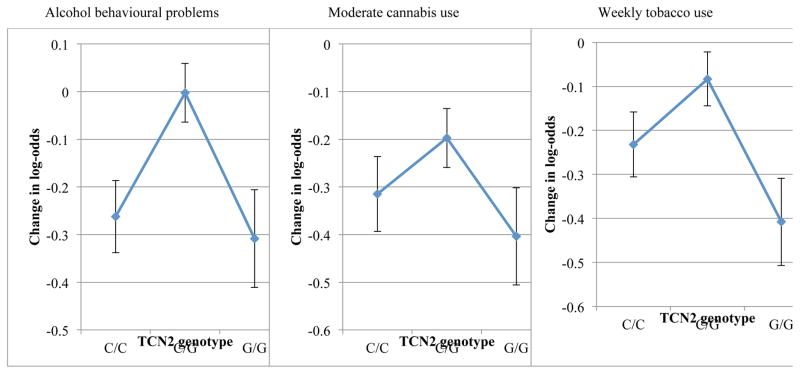
Offspring whose mothers carried homozygous alleles (CC or GG) of a sequence variant in TCN2 (rs1801198) identified by Bonilla et al. (2012) showed a lower likelihood of adverse substance use that varied directly with higher meat consumption (figure 2 and table S7). Among offspring of heterozygous CG mothers, in contrast, there was no pattern of meat consumption having any substantial modification of substance use The patterns of diet-modified response among homozygotes compared to the absence of a pattern of response among heterozygotes was common across all 10 measures of adverse substance abuse (table S7, figure S2). Risks attributable to cobalamin deficits during pregnancy for adverse adolescent alcohol, cannabis, and tobacco use were 14 %, 37% and 23% respectively (table S8).
Figure 2.
Effect of higher maternal meat consumption in pregnancy on offspring substance use outcomes differentiated by maternal genotype for TCN2 rs1801198
The effect of greater meat consumption by mothers (5 categories) and lower risk of substance abuse among their adolescents is stratified by maternal genotype for TCN2 (rs1801198). Diamonds and error bars (change in log-odds, 95% CI) indicate negative association between adverse substance use outcomes at age 15. The cytosine (C) nucleotide encodes a proline (Pro) residue at amino acid 259 in the mature protein. The guanine (G) nucleotide encodes an arginine (Arg). The TCN2 receptor is dimeric with suboptimal structural matching and transport function among heterozygous pairs, less responsive to maternal cobalamin intake. Group sizes were as follows: C/C: 30.7%, C/G: 50.1%, G/G: 19.2%.
Discussion
Adverse behavioural consequences of heavy alcohol use (9.5%), moderate cannabis use (9.6%) and weekly tobacco smoking (10.3%) are common among British 15 year olds. We found that higher scores on the maternal ‘vegetarian’ dietary pattern during pregnancy were associated with greater likelihoods of substance misuse among their 15 year old offspring. Among foods loading on to the ‘vegetarian’ diet pattern, less frequent consumption of red meat, poultry and meat products (added together) were associated with greater risks of adverse alcohol, cannabis and cigarette use OR=1.75, 95% CI = [1.23, 2.56], p < 0.001, OR=2.70, 95% CI = [1.89, 4.00], p < 0.001 OR=1.85, 95% CI = [1.28, 2.63], p < 0.001, respectively comparing consumption of ‘0–4 meat portions ‘ to ‘15+ times’ per fortnight’. Consistent dose response patterns comparing lower maternal meat consumption to increased risk were found for all measures of adverse alcohol, cannabis and tobacco use at age 15 years (see table S3.) However, many of these dose response relationships were non-significant. Greater consumption of pulses and soy protein foods (substitutes for meat proteins) were also associated with a pattern of greater risk of all adverse substance use outcomes. Maternal prenatal consumption of all other foods including fish, fruits and vegetables were unrelated to adolescent substance use risk.
Socioeconomic differences (Bonilla et al., 2012, Northstone et al., 2008) associated with less meat consumption were unlikely to explain our findings. Diet-substance use results persisted after adding terms for socioeconomic factors and inclusion of these terms strengthened diet-substance use associations and added precision to the point estimates. We had also posited that less meat consumption might be associated with more permissive parenting styles and that less parental monitoring would increase risk of substance use. In contrast, we found that less maternal meat consumption was associated with greater parental monitoring and thus would be likely to be protective. In order to more definitively rule out residual confounding from socioeconomic and other differences, we employed stratification by genetic variants. We evaluated genetic variants in transport proteins that are necessary for the biological activity for two nutrients, cobalamin and iron, that are both essential for optimal neurodevelopment and frequently insufficient among infrequent meat consumers and pregnant vegetarians (Fayet et al., 2014).
In order to evaluate causality and address problems of residual confounding, we evaluated the effects of genetic variants in genes encoding cobalamin transport proteins. Randomized controlled trials provide a close analogy; a single biological factor is isolated between groups by random assignment so as to be free from confounding differences in order to evaluate a potential causal effect. The biological factors we employ here are the genetic variants encoding transport proteins for specific nutrients disproportionally rich in meat (iron and cobalamin) (Bonilla et al., 2012) and necessary for their biological activity. For example, the impact of maternal variants coding for suboptimal transportation of cobalamin to tissue on their offspring can be compared to the impact of maternal variants coding for optimal transportation of cobalamin on their offspring. These genetic variants in transportation determine different levels of the biological activity of these nutrients in target tissues. These variants are randomly distributed throughout this population (see table S9). Thus, they are closely analogous to drug and placebo intervention trials that achieve differing tissue levels or agents, excepting that here “ adherence “ (differing levels of biological activity) is determined by genotype expression and group assignments are perfectly masked. We note of course that additive effects of MTHFR on other genotypes also influence cobalamin levels. Because these genetic variants are naturally randomized across the social, economic and other differences among this population (see table S9), these and residual confounding influences are highly unlikely to affect group comparisons based on these functional variants. Thus, a causal role can be evaluated on the basis of randomization, blinding and adherence virtually free from socioeconomic, cultural and other influences (Davey Smith and Hemani, 2014).
Functional variants in TNC2 encoding the cobalamin transport protein, transcobalamin 2
We posited that if cobalamin insufficiency had a causal role, the effects of low meat consumption would differ when comparing mothers within strata of DNA sequence variants associated with delivery of cobalamin to the nervous system. For this purpose, we evaluated functional common SNPs within ALSPAC mothers that existed in the gene encoding cobalamin transport protein transcobalamin 2 (TCN2) – these DNA variants were identified by Bonilla et al (2012). Up to 30% of cobalamin in circulation binds to TCN2 and this biologically active complex, referred to as holo-transcobalamin (holo-TC), is critical for delivery of cobalamin to all body tissues (Rothenberg and Quadros, 1995). A common TCN2 polymorphism, rs1801198, yields transition from cytosine to guanine at nucleotide base 776 (TC 776 C>G) yielding proline-to-arginine replacement of residue 259. In cohorts of Northern and Western European ancestry (HAPMAP-CEU) prevalence of the GG, CG and CC variants are estimated to be 33%, 43% and 23% respectively (dbSNP, accessed 20 Feb 2015) (Afman et al., 2001). Because TCN2 is a binding and transport protein, both polymorphisms and dietary intakes may influence circulating homocystine, holo-transcobalamin (holo-TC) levels and cobalamin concentrations (von Castel-Dunwoody et al., 2005, Zinck et al., 2015).
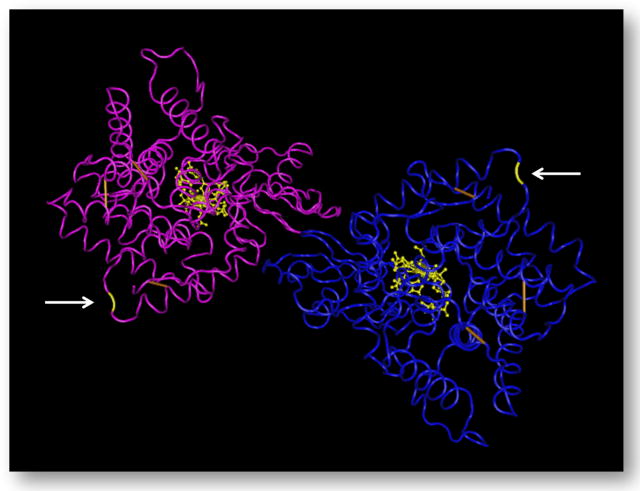
Here we found that the effects of higher meat consumption during pregnancy on adolescent outcomes were specifically mediated by a functional variant in the TCN2 allele (776C>G, rs1801198). Higher meat consumption was associated with substantially lower risk of substance use among the offspring of homozygous CC and GG mothers, but not among heterozygous CG mothers. Several investigators have reported similar biochemical patterns comparing the heterozygous to homozygous genotypes; these include: elevated homocystine;(Alessio et al., 2007, Castro et al., 2010, von Castel-Dunwoody et al., 2005) lower cobalamin(von Castel-Dunwoody et al., 2005) and lower holo-TC (von Castel-Dunwoody et al., 2005). Others have failed to find such results for homocystine, cobalamin, or holo-TC (Riedel et al., 2011). One explanation for the differences comparing homozygous to heterozygous mothers could be that the transcobalamin receptor is dimeric (figure 3) and is optimally functional only with identical subunits(Bose et al., 1996). Namor and Gueánt (McCaddon et al., 2001) suggested that the Pro/Arg heterodimer isoform resulting from 776C>G, rs1801198 manifests lower affinity for transcobalamin than either Pro/Pro or Arg/Arg homodimers and may explain differences in homocysteine levels. However, no biological substantiation of this suggestion was reported. Our findings indicate that insufficient tissue delivery of cobalamin may be both a plausible mechanism for impaired neurodevelopment consequent from less frequent maternal meat consumption and may be unaffected by residual confounding. However, we wish to emphasize that there is no consensus in the literature regarding the differences in biological activities of the transcobalamin products of TCN2 homodimers compared to those of TCN2 heterodimers and that this interpretation is therefore speculative.
Figure 3.
TCN2 dimer (holo-transcobalamin) highlighting variant at amino acid position 776 (yellow)
Wire frame structures of the transcobalamin TCN2 dimer with cobalamin ligand represented in yellow at the centre of each monomer. White arrows indicate amino acid residue 776, the site of proline to arginine transition consequent to DNA sequence variant rs1801198 (C>G). Disulfide bonds are represented in orange.
Putative mechanisms of cobalamin insufficiency in substance abuse risk
Moderate cobalamin deficiencies in pregnancy and resulting elevations of homocystine and hypermethylation (Gadgil et al., 2014) could potentially adversely impact neurodevelopmental processes relevant to substance abuse risk (Vaiserman, 2013) via homocystine toxicity to dopaminergic neurons (Lipton et al., 1997) and global changes to DNA brain methylation (Sable et al., 2015). DNA methylation of quantitative trait loci appears to underlie some of the association of genetic variation with alcohol dependence (Zhang et al., 2014). Profound impairments in myelination were observed in the earliest autopsies for severe cobalamin deficiencies and some evidence implicates impaired development of myelination in the frontal cortex as a risk factor for substance abuse (Acheson et al., 2014). Unfortunately, not enough is known about the impacts of moderate compared to severe deficits in cobalamin levels in human pregnancies on dopaminergic development, DNA methylation and myelination deficits or the contribution of these processes to residual neurodevelopmental risks for substance abuse.
Strengths and weaknesses
Although this is a well characterized longitudinal cohort representative of the British population, differential attrition among families of low socioeconomic status may bias the findings. Here we evaluate only early onset substance use at age 15 and not longitudinal addictive phenotypes. However, the similar patterns of findings in multiple domains of substance abuse suggest impacts on common underlying neurobiological mechanisms in addiction. The effects of low meat consumption on increased substance use were not isolated to pregnancy, lower meat consumption at age 13 was also associated with substance use outcomes at age 16, but without a dose response pattern and with lesser magnitude. Food frequency questionnaires are susceptible to differential misclassification expected to attenuate association measures. We have also not ruled out the potential contributions of adverse effects of pulses or soy meat substitutes. The use of Mendelian Randomization techniques permitted an evaluation of gene based causality unaffected by any social or residual confounding. Our probe set did not allow us to examine the rare variants in TCN2. We did not have measures of cobalamin, methyl-malonyl-CoA or holocobalamin among the mothers during pregnancy. Finally we did not confirm, but cannot rule out, a contribution of low iron status. We did not create a model of all factors contributing to increased adolescence substance use risk.
CONCLUSIONS
This study identifies low meat consumption in the prenatal period as potentially modifiable risk factor for adolescent substance use. In identifying vitamin B12 insufficiencies as highly likely to have a contributing role to our findings, greater meat consumption need not be advised to modify this risk. For example, fortification of foods with vegetarian sources of cobalamin and more widespread use of supplements may be low cost and readily feasible interventions.
Supplementary Material
Acknowledgments
Sources of funding
The UK Medical Research Council and the Wellcome Trust (Grant ref: 1002215/2/13/2) and the University of Bristol currently provide core support for ALSPAC. This research was also supported by the intramural program of NIAAA and the NIH. The funders played no role in the design, implementation, analysis and interpretation of the data.
We are extremely grateful to all of the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. This publication is the work of the authors who will serve as guarantors for the contents of the paper.
Abbreviations
- ALSPAC
Avon Longitudinal Study of Parents and Children
- CAST
Cannabis Abuse Screening Test
- CI
Confidence interval
- FFQ
Food Frequency Questionnaire
- holo-TC
Holo-transcobalamin
- OR
Odds ratio
- SNP
Single nucleotide polymorphism
- TCN2
Transcobalamin 2 gene
- TF
Transferrin gene
- TFRC
Transferrin Receptor gene
Footnotes
Authors’ contributions to manuscript
Contributing authors included Joseph R. Hibbeln (JRH), John Paul SanGiovanni (JPSG), Jean Golding (JG), Pauline E. Emmett (PE), Kate Northstone (KN), John M. Davis (JD), Marc Schuckit (MS) and Jon Heron (JH). JRH, JH, JPSG and JG designed the research, JRH and JH performed phenotypic analysis and JPSG genetic analysis. PE and KN provided essential dietary instruments and analyses. MS designed phenotypic characterizations. JRH, JPSG, JG, PE, KN, JD, MS and JH contributed to data interpretation and writing of the manuscript.
Conflict of Interest
The authors report no conflict of interest with regard to this research.
References
- Acheson A, Wijtenburg SA, Rowland LM, Bray BC, Gaston F, Mathias CW, Fox PT, Lovallo WR, Wright SN, Hong LE, McGuire S, Kochunov P, Dougherty DM. Combining diffusion tensor imaging and magnetic resonance spectroscopy to study reduced frontal white matter integrity in youths with family histories of substance use disorders. Human brain mapping. 2014;35:5877–5887. doi: 10.1002/hbm.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afman LA, Van Der Put NM, Thomas CM, Trijbels JM, Blom HJ. Reduced vitamin B12 binding by transcobalamin II increases the risk of neural tube defects. QJM : monthly journal of the Association of Physicians. 2001;94:159–166. doi: 10.1093/qjmed/94.3.159. [DOI] [PubMed] [Google Scholar]
- Alessio AC, Hoehr NF, Siqueira LH, Bydlowski SP, Annichino-Bizzacchi JM. Polymorphism C776G in the transcobalamin II gene and homocysteine, folate and vitamin B12 concentrations. Association with MTHFR C677T and A1298C and MTRR A66G polymorphisms in healthy children. Thrombosis research. 2007;119:571–577. doi: 10.1016/j.thromres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Anjos T, Altmae S, Emmett P, Tiemeier H, Closa-Monasterolo R, Luque V, Wiseman S, Perez-Garcia M, Lattka E, Demmelmair H, Egan B, Straub N, Szajewska H, Evans J, Horton C, Paus T, Isaacs E, van Klinken JW, Koletzko B, Campoy C, Group NR. Nutrition and neurodevelopment in children: focus on NUTRIMENTHE project. European journal of nutrition. 2013;52:1825–1842. doi: 10.1007/s00394-013-0560-4. [DOI] [PubMed] [Google Scholar]
- Bhate VK, Joshi SM, Ladkat RS, Deshmukh US, Lubree HG, Katre PA, Bhat DS, Rush EC, Yajnik CS. Vitamin B12 and folate during pregnancy and offspring motor, mental and social development at 2 years of age. Journal of developmental origins of health and disease. 2012;3:123–130. doi: 10.1017/S2040174411000778. [DOI] [PubMed] [Google Scholar]
- Bonilla C, Lawlor DA, Taylor AE, Gunnell DJ, Ben-Shlomo Y, Ness AR, Timpson NJ, St Pourcain B, Ring SM, Emmett PM, Smith AD, Refsum H, Pennell CE, Brion MJ, Smith GD, Lewis SJ. Vitamin B-12 status during pregnancy and child’s IQ at age 8: a Mendelian randomization study in the Avon longitudinal study of parents and children. PloS one. 2012;7:e51084. doi: 10.1371/journal.pone.0051084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Feix J, Seetharam S, Seetharam B. Dimerization of transcobalamin II receptor. Requirement of a structurally ordered lipid bilayer. The Journal of biological chemistry. 1996;271:11718–11725. doi: 10.1074/jbc.271.20.11718. [DOI] [PubMed] [Google Scholar]
- Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Ness A, Ring S, Davey Smith G. Cohort Profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. International journal of epidemiology. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramaschi D, Sharp GC, Nohr EA, Berryman K, Lewis SJ, Davey Smith G, Relton CL. Exploring a causal role of DNA methylation in the relationship between maternal vitamin B12 during pregnancy and child’s IQ at age 8, cognitive performance and educational attainment: A two-step Mendelian randomization study. Hum Mol Genet. 2017 Apr 27; doi: 10.1093/hmg/ddx164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro R, Barroso M, Rocha M, Esse R, Ramos R, Ravasco P, Rivera I, de Almeida IT. The TCN2 776CNG polymorphism correlates with vitamin B(12) cellular delivery in healthy adult populations. Clinical biochemistry. 2010;43:645–649. doi: 10.1016/j.clinbiochem.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Human molecular genetics. 2014;23:R89–98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio Garcia C, Torres-Sanchez L, Chen J, Schnaas L, Hernandez C, Osorio E, Portillo MG, Lopez-Carrillo L. Maternal MTHFR 677C>T genotype and dietary intake of folate and vitamin B(12): their impact on child neurodevelopment. Nutritional neuroscience. 2009;12:13–20. doi: 10.1179/147683009X388913. [DOI] [PubMed] [Google Scholar]
- Fayet F, Flood V, Petocz P, Samman S. Avoidance of meat and poultry decreases intakes of omega-3 fatty acids, vitamin B12, selenium and zinc in young women. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2014;27(Suppl 2):135–142. doi: 10.1111/jhn.12092. [DOI] [PubMed] [Google Scholar]
- Gadgil MS, Joshi KS, Naik SS, Pandit AN, Otiv SR, Patwardhan BK. Association of homocysteine with global DNA methylation in vegetarian Indian pregnant women and neonatal birth anthropometrics. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014;27:1749–1753. doi: 10.3109/14767058.2013.879702. [DOI] [PubMed] [Google Scholar]
- Graham SM, Arvela OM, Wise GA. Long-term neurologic consequences of nutritional vitamin B12 deficiency in infants. The Journal of pediatrics. 1992;121:710–714. doi: 10.1016/s0022-3476(05)81897-9. [DOI] [PubMed] [Google Scholar]
- Jadhav M, Webb JK, Vaishnava S, Baker SJ. Vitamin B12 deficiency in Indian infants. A clinical syndrome. Lancet. 1962;2:903–907. doi: 10.1016/s0140-6736(62)90682-7. [DOI] [PubMed] [Google Scholar]
- Koebnick C, Hoffmann I, Dagnelie PC, Heins UA, Wickramasinghe SN, Ratnayaka ID, Gruendel S, Lindemans J, Leitzmann C. Long-term ovo-lacto vegetarian diet impairs vitamin B-12 status in pregnant women. The Journal of nutrition. 2004;134:3319–3326. doi: 10.1093/jn/134.12.3319. [DOI] [PubMed] [Google Scholar]
- Kofink D, Boks MP, Timmers HT, Kas MJ. Epigenetic dynamics in psychiatric disorders: environmental programming of neurodevelopmental processes. Neuroscience and biobehavioral reviews. 2013;37:831–845. doi: 10.1016/j.neubiorev.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaddon A, Blennow K, Hudson P, Regland B, Hill D. Transcobalamin polymorphism and homocysteine. Blood. 2001;98:3497–3499. doi: 10.1182/blood.v98.12.3497. [DOI] [PubMed] [Google Scholar]
- Melotti R, Lewis G, Hickman M, Heron J, Araya R, Macleod J. Early life socio-economic position and later alcohol use: birth cohort study. Addiction. 2013;108:516–525. doi: 10.1111/add.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northstone K, Emmett P, Rogers I. Dietary patterns in pregnancy and associations with socio-demographic and lifestyle factors. European journal of clinical nutrition. 2008;62:471–479. doi: 10.1038/sj.ejcn.1602741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak R, Parrott SJ, Raj S, Cullum-Dugan D, Lucus D. How prevalent is vitamin B(12) deficiency among vegetarians? Nutrition reviews. 2013;71:110–117. doi: 10.1111/nure.12001. [DOI] [PubMed] [Google Scholar]
- Piontek D, Kraus L, Klempova D. Short scales to assess cannabis-related problems: a review of psychometric properties. Substance abuse treatment, prevention, and policy. 2008;3:25. doi: 10.1186/1747-597X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel BM, Molloy AM, Meyer K, Fredriksen A, Ulvik A, Schneede J, Nexo E, Hoff G, Ueland PM. Transcobalamin polymorphism 67A->G, but not 776C->G, affects serum holotranscobalamin in a cohort of healthy middle-aged men and women. The Journal of nutrition. 2011;141:1784–1790. doi: 10.3945/jn.111.141960. [DOI] [PubMed] [Google Scholar]
- Rothenberg SP, Quadros EV. Transcobalamin II and the membrane receptor for the transcobalamin II-cobalamin complex. Bailliere’s clinical haematology. 1995;8:499–514. doi: 10.1016/s0950-3536(05)80218-5. [DOI] [PubMed] [Google Scholar]
- Sable P, Randhir K, Kale A, Chavan-Gautam P, Joshi S. Maternal micronutrients and brain global methylation patterns in the offspring. Nutritional neuroscience. 2015;18:30–36. doi: 10.1179/1476830513Y.0000000097. [DOI] [PubMed] [Google Scholar]
- Smith DJ, McVie S, McAra L, Woodward A, Shute J, Flint J. The Edinburgh Study of Youth Transitions and Crime: Key Findings at Ages 12 and 13 (Edinburgh Study of Crime Research) University of Edinburgh Centre for Law & Society; 2001. [Google Scholar]
- Vaiserman AM. Long-term health consequences of early-life exposure to substance abuse: an epigenetic perspective. Journal of developmental origins of health and disease. 2013;4:269–279. doi: 10.1017/S2040174413000123. [DOI] [PubMed] [Google Scholar]
- von Castel-Dunwoody KM, Kauwell GP, Shelnutt KP, Vaughn JD, Griffin ER, Maneval DR, Theriaque DW, Bailey LB. Transcobalamin 776C->G polymorphism negatively affects vitamin B-12 metabolism. The American journal of clinical nutrition. 2005;81:1436–1441. doi: 10.1093/ajcn/81.6.1436. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang F, Kranzler HR, Yang C, Xu H, Wang Z, Zhao H, Gelernter J. Identification of methylation quantitative trait loci (mQTLs) influencing promoter DNA methylation of alcohol dependence risk genes. Human genetics. 2014;133:1093–1104. doi: 10.1007/s00439-014-1452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinck JW, de Groh M, MacFarlane AJ. Genetic modifiers of folate, vitamin B-12, and homocysteine status in a cross-sectional study of the Canadian population. The American journal of clinical nutrition. 2015;101:1295–1304. doi: 10.3945/ajcn.115.107219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.