Abstract
Existing studies suggest that dehydroepiandrosterone (DHEA) may be important for human brain development and cognition. For example, molecular studies have hinted at the critical role of DHEA in enhancing brain plasticity. Studies of human brain development also support the notion that DHEA is involved in preserving cortical plasticity. Further, some, though not all, studies show that DHEA administration may lead to improvements in working memory in adults. Yet these findings remain limited by an incomplete understanding of the specific neuroanatomical mechanisms through which DHEA may impact the CNS during development. Here we examined associations between DHEA, cortico-hippocampal structural covariance, and working memory (216 participants [female=123], age range 6–22 years old, mean age: 13.6 +/−3.6 years, each followed for a maximum of 3 visits over the course of 4 years). In addition to administering performance-based, spatial working memory tests to these children, we also collected ecological, parent ratings of working memory in everyday situations. We found that increasingly higher DHEA levels were associated with a shift toward positive insular-hippocampal and occipito-hippocampal structural covariance. In turn, DHEA-related insular-hippocampal covariance was associated with lower spatial working memory but higher overall working memory as measured by the ecological parent ratings. Taken together with previous research, these results support the hypothesis that DHEA may optimize cortical functions related to general attentional and working memory processes, but impair the development of bottom-up, hippocampal-to-cortical connections, resulting in impaired encoding of spatial cues.
Keywords: DHEA, Androgen, Structural Magnetic Resonance Imaging, Cortical Thickness, Puberty, Adolescence, Brain Development, Attention
1. INTRODUCTION
Dehydroepiandrosterone (DHEA) rises steeply in children at the intersection of a critical cognitive developmental period, middle childhood (i.e. 6–8 years of age) and an important endocrine event, adrenarche (Campbell, 2011; Remer et al., 2005). This hormone remains at high levels throughout adolescence and young adulthood, only starting to decrease past the third decade of life (Adams, 1985). All this points toward a critical role for DHEA in both physical maturation and neurodevelopment throughout middle childhood and adolescence (Campbell, 2011). In fact, only humans, the great apes and certain types of Old World monkeys exhibit adrenarche, a key developmental event leading to increased production of DHEA (Remer et al., 2005), further bolstering the evolutionary importance of this hormone in primate-specific development. Recent genetic evidence confirms this hypothesis by demonstrating that DHEA affects gene expression relevant to energy allocation and homeostasis, alertness, and cell survival in the central nervous system (CNS) (Mo et al., 2009).
Existing studies of human brain development support the notion that DHEA may be involved in preserving cortical plasticity in brain networks involved in cognitive control, such as the left dorsolateral prefrontal cortex, right temporoparietal junction, right premotor and right entorhinal cortex, particularly in children age 4–13 years old (Nguyen et al., 2013b). Reports of DHEA administration in adults also outline beneficial effects on cognition overall, with a certain specificity for working memory and attention (Alhaj et al., 2006; Davis et al., 2008; de Menezes et al., 2016; do Vale et al., 2014). Recent findings from our group support some of these ‘pro-cognition’ effects of DHEA by showing that DHEA ameliorates visual attention processes by shaping amygdala-dependent cortical plasticity from childhood to young adulthood (Nguyen et al., 2016). More specifically, DHEA was found to be associated with structural covariance between the amygdala and the anterior cingulate, somatosensory and primary visual cortex (Nguyen et al., 2016). In turn, this DHEA-related structural covariance was related to improved performance on tests of visual awareness, visuo-motor dexterity and general level of attention (Nguyen et al., 2016). Still, not all studies are consistent with regards to the association between DHEA and different aspects of declarative, episodic and working memory, and sex and age differences in the cognitive effects of DHEA have been reported (de Menezes et al., 2016). In addition, there have been no studies, to our knowledge, examining the relationship between DHEA, brain structure, and working memory during development.
Yet, DHEA is likely to impact working memory in a developmentally sensitive manner, with possibly more prominent effects during adrenarche and middle childhood, when there is a steep rise in DHEA levels (Remer et al., 2005). Though levels of DHEA do not tend to show significant sex differences, some, though not all, studies suggest that sex could moderate the impact of adrenarche on brain function and mental health vulnerability, and the physical changes of adrenarche tend to occur at an earlier age in girls compared to boys (Byrne et al., 2017). DHEA may stimulate neurogenesis and protect against neuronal injury by opposing the neurotoxic effects of glucocorticoids in hippocampal as well as cortical structures (Jin et al., 2016; Karishma and Herbert, 2002; Kimonides et al., 1998; Nguyen et al., 2013b). These neurotoxic effects of glucocorticoids, which can affect both the cortex and hippocampus, may be especially detrimental during childhood and adolescence, as the brain experiences an accelerated increase in metabolic activity and cerebral perfusion throughout the pubertal maturation process (Campbell, 2011; Satterthwaite et al., 2014).
Cortical networks involved in cognitive control are known to regulate executive function, including working memory (Mazoyer et al., 2001). Cortico-hippocampal connections may also play a role in working memory, in particular for the maintenance of novel items in new contextual situations (Axmacher et al., 2007; Fuentemilla et al., 2010; Leszczynski, 2011; Poch et al., 2011; Ranganath and D'Esposito, 2001). More specifically, hippocampal-to-cortical afferents may be instrumental in the process of spatial encoding and spatial working memory (Spellman et al., 2015). In turn, DHEA may be involved in bolstering both cortical and hippocampal plasticity (Jin et al., 2016; Karishma and Herbert, 2002; Kimonides et al., 1998; Nguyen et al., 2013b), and DHEA levels or administration have been linked to improved attention and working memory in some, though not all, studies (do Vale et al., 2014; Nguyen et al., 2016; Ritsner et al., 2006; Strous et al., 2001; Wolf et al., 1998). Taken together, the current literature thus suggests that DHEA could significantly impact working memory during childhood and adolescence through an alteration in cortico-hippocampal networks.
Interestingly, there is accumulating evidence that these CNS effects could be directly related to DHEA itself rather than to other steroid hormones derived from its metabolism (Labrie et al., 2008; Labrie et al., 2005). In fact, major metabolites of DHEA in the hippocampus have been found to be essentially devoid of androgenic or estrogenic activity (Jellinck et al., 2005; Lu et al., 2003; Mo et al., 2004; Mo et al., 2006; Morfin and Starka, 2001), highlighting the critical role of DHEA itself in the survival of hippocampal neurons.
In summary, the current literature suggests that, during childhood and adolescence, DHEA may significantly affect cortical and hippocampal plasticity, in particular cortico-hippocampal structural covariance, and is likely to play a critical role in the development of working memory processes. Developmental coupling has previously been demonstrated between structural covariance and functional connectivity networks (Raznahan et al., 2011), reflecting the progressive integration of these networks between 5 and 18 years of age (Zielinski et al., 2010), and establishing structural covariance as a valid measure of developmental brain changes (Alexander-Bloch et al., 2013). Our investigation was based on data from 216 typically developing children and adolescents 6 to 22 years of age who were each followed longitudinally for a maximum of 4 years with repeated measurement of hormonal, cognitive and neuroimaging data every 2 years, in the context of the National Institutes of Health MRI Study of Normal Brain Development (NIHPD), a multi-site longitudinal study that aimed to provide a normative database to characterize healthy brain maturation. First, we tested for associations between DHEA and cortico-hippocampal structural covariance over time, looking at covariance between hippocampal volumes and cortical thickness across the whole brain. Second, we examined the relationship between DHEA-related cortico-hippocampal covariance and two tests of working memory: (1) a laboratory, clinical performance test of spatial working memory (Cambridge Neuropsychological Test Automated Battery [CANTAB] (Luciana, 2003); and (2) a parent-rated measure of everyday working memory exhibited by the child/adolescent in real-world situations (Behavior Rating Inventory of Executive Function [BRIEF] (Gioia et al., 2002b)). Third, we tested whether the relationship between DHEA and working memory was mediated by cortico-hippocampal structural covariance. Finally, because DHEA previously showed age-related associations with cortical thickness (Nguyen et al., 2013b), and because sex may moderate both the impact of DHEA on brain structure and working memory (Byrne et al., 2017), we tested for age and sex interaction effects in all of the above relationships. We hypothesized that DHEA levels would be associated with cortico-hippocampal structural covariance, particularly in regions involved in cognitive control. In turn, we expected these cortico-hippocampal networks to mediate the relationship between DHEA and working memory, and for that effect to be moderated by age and sex.
2. METHODS AND MATERIALS
2.1 Sampling and Recruitment
Participants were recruited across the United States with a population-based sampling method seeking to achieve a representative sample in terms of income level, race and ethnicity (Evans, 2006). All experiments on human participants were conducted in accordance with the Declaration of Helsinki. All procedures were carried out with the adequate understanding and written parental consent, as well as assent of the participants (or consent, if >=18 years old). Participants underwent repeated magnetic resonance brain imaging (MRI) every 2 years. For the subjects included in this study, age at first visit was between 6 and 18 years old, with each child then followed longitudinally for a maximum of 3 visits, over the course of 4 years (i.e. full age span 6–22 years, one to three visits per child). The sample was limited to developmentally healthy children with rigorous exclusion criteria, described in detail elsewhere (Evans, 2006). In particular, any children with a current or past treatment for language disorder (simple articulation disorders not exclusionary); and a lifetime history of Axis I psychiatric disorder (except for simple phobia, social phobia, adjustment disorder, oppositional defiant disorder, enuresis, encopresis, nicotine dependency) were excluded from the study. After strict quality control of MRI data (see section 2.2) and the exclusion of scans without hormonal measurements or behavioral parameters, 216 participants were included in hormonal-related analyses (324 scans) and 187 to 188 participants (245 to 257 scans) in cognitive analyses, depending on data available for each cognitive test (see Table 1 for more details). All longitudinal MRI data for each child were used to the extent that it passed quality control (see section 2.2 below) and could be paired with hormonal and cognitive data collected at the same time point.
TABLE 1.
Sample Characteristics
| Visit | DHEA | BRIEF Working Memory |
CANTAB Spatial Working Memory |
|
|---|---|---|---|---|
|
| ||||
| # Scans per Visit # | 1 | n = 121 scans (F = 69) | n = 119 scans (F = 68) | n = 119 scans (F = 68) |
|
| ||||
| 2 | n = 110 scans (F = 71) | n = 91 scans (F = 57) | n = 97 scans (F = 62) | |
|
| ||||
| 3 | n = 93 scans (F = 50) | n = 35 scans (F = 21) | n = 41 scans (F = 25) | |
| Total = 324 (F = 190) | Total = 245 (F = 146) | Total = 257 (F = 155) | ||
|
| ||||
| # Participants per # Scans Completed | 1 scan | n =129 participants (F = 70) | n = 132 participants (F = 75) | n = 126 participants (F = 71) |
|
| ||||
| 2 scans | n = 66 participants (F = 39) | n = 52 participants (F = 31) | n = 55 participants (F = 33) | |
|
| ||||
| 3 scans | n = 21 participants (F = 14) | n = 3 participants (F = 3) | n = 7 participants (F = 6) | |
| Total = 216 (F = 123) | Total = 187 (F = 109) | Total = 188 (F = 110) | ||
|
| ||||
| Testosterone (ug/dL) | 1 | n = 121, mean = 99.20, SD = 74.14 | n = 119, mean = 99.12, SD = 74.76 | n = 119, mean = 98.65, SD = 74.53 |
|
| ||||
| 2 | n = 110, mean = 74.00, SD = 45.83 | n = 91, mean = 71.84, SD = 43.97 | n = 97, mean = 72.75, SD = 42.68 | |
|
| ||||
| 3 | n = 93, mean = 105.23, SD = 101.34 | n = 35, mean = 88.33, SD = 99.01 | n = 41, mean = 93.11, SD = 93.04 | |
|
| ||||
| Estradiol (ug/dL) | 1 | n = 121, mean = 10.87, SD = 5.63 | n = 119, mean = 10.86, SD = 5.67 | n = 119, mean = 10.64, SD = 5.12 |
|
| ||||
| 2 | n = 110, mean = 12.11, SD = 6.19 | n = 91, mean = 12.42, SD = 6.16 | n = 97, mean = 12.60, SD = 6.13 | |
|
| ||||
| 3 | n = 93, mean = 16.82, SD = 10.01 | n = 35, mean = 19.45, SD = 10.93 | n = 41, mean = 19.68, SD = 10.27 | |
|
| ||||
| DHEA (ug/dL) | 1 | n = 121, mean = 103.07, SD = 100.03 | n = 119, mean = 103.90, SD = 100.66 | n = 119, mean = 103.39, SD = 100.82 |
|
| ||||
| 2 | n = 110, mean = 188.84, SD = 171. 85 | n = 91, mean = 173.19, SD = 157.78 | n = 97, mean = 181.13, SD = 164.22 | |
|
| ||||
| 3 | n = 93, mean = 197.28, SD = 164.48 | n = 35, mean = 147.44, SD = 138.07 | n = 41, mean = 168.69, SD = 141.59 | |
|
| ||||
| Season of sampling | 1 | Spring = 0 | Spring = 0 | Spring = 0 |
| Summer = 11 | Summer = 11 | Summer = 10 | ||
| Fall = 110 | Fall = 108 | Fall = 109 | ||
| Winter= 0 | Winter = 0 | Winter = 0 | ||
| Total = 121 | Total = 119 | Total = 119 | ||
|
| ||||
| 2 | Spring = 0 | Spring = 0 | Spring = 0 | |
| Summer = 0 | Summer = 0 | Summer = 0 | ||
| Fall = 108 | Fall = 89 | Fall = 95 | ||
| Winter = 2 | Winter = 2 | Winter = 2 | ||
| Total = 110 | Total = 91 | Total = 97 | ||
|
| ||||
| 3 | Spring = 0 | Spring = 0 | Spring = 0 | |
| Summer = 0 | Summer = 0 | Summer = 0 | ||
| Fall = 3 | Fall = 0 | Fall = 0 | ||
| Winter = 90 | Winter = 35 | Winter = 41 | ||
| Total = 93 | Total = 35 | Total = 41 | ||
|
| ||||
| Collection time (min after midnight) | 1 | n = 121, mean = 682.58, SD = 139.42 | n = 119, mean = 684.90, SD = 139.43 | n = 119, mean = 682.52, SD = 139.31 |
|
| ||||
| 2 | n = 110, mean = 710.63, SD = 115.73 | n = 91, mean = 713.27, SD = 109.35 | n = 97, mean = 715.28, SD = 115.53 | |
|
| ||||
| 3 | n = 93, mean = 702.38, SD = 123.07 | n = 35, mean = 699.03, SD = 132.12 | n = 41, mean = 694.93, SD = 139.75 | |
|
| ||||
| Age (years) | 1 | n = 121, mean = 12.71, SD = 3.22 | n = 119, mean = 12.63, SD = 3.19 | n = 119, mean = 12.69, SD = 3.21 |
| Age range = 6.09 to 18.24 | Age range = 6.08 to 18.24 | Age range = 6.09 to 18.24 | ||
|
| ||||
| 2 | n = 110, mean = 13.48, SD = 3.66 | n = 91, mean = 12.80, SD = 3.31 | n = 97, mean = 13.29, SD = 3.64 | |
| Age range = 6.79 to 20.17 | Age range = 6.79 to 18.90 | Age range = 6.79 to 20.05 | ||
|
| ||||
| 3 | n = 93, mean = 14.50, SD = 3.77 | n = 35, mean = 12.76, SD = 2.64 | n = 41, mean =14.15, SD = 3.95 | |
| Age range = 9.08 to 22.26 | Age range = 9.07 to 17.91 | Age range = 9.07 to 22.26 | ||
|
| ||||
| Sex F = female M = male | 1 | F = 69, M = 52 | F = 68, M = 51 | F = 68, M = 51 |
|
| ||||
| 2 | F = 71, M = 39 | F = 57, M = 34 | F = 62, M = 35 | |
|
| ||||
| 3 | F = 50, M = 43 | F = 21, M = 14 | F = 25, M = 16 | |
|
| ||||
| Pubertal stage | 1 | n = 121, mean = 2.53 SD = 1.47 | n = 119, mean = 2.51 SD = 1.478 | n = 119, mean = 2.52, SD = 1.478 |
|
| ||||
| 2 | n = 110, mean = 2.52, SD = 1.48 | n = 91, mean = 2.46, SD = 1.463 | n = 97, mean = 2.42, SD = 1.45 | |
|
| ||||
| 3 | n = 93, mean = 3.05, SD = 1.492 | n = 35, mean = 3.03, SD = 1.445 | n = 41, mean = 2.95, SD = 1.465 | |
|
| ||||
| Handedness | 1 | L = 8, R = 113 Total = 121 | L = 8, R = 111 Total = 119 | L = 8, R = 111 Total = 119 |
|
| ||||
| 2 | L = 9, R = 101 Total = 110 | L = 8, R = 83 Total = 91 | L = 7, R = 90 Total = 97 | |
|
| ||||
| 3 | L = 8, R = 85 Total = 93 | L = 3, R = 32 Total = 35 | L = 3, R = 38 Total = 41 | |
|
| ||||
| Total brain volume (cm3) | 1 | n = 121, mean = 1270.55 SD = 123.26 | n = 119, mean = 1270.89, SD = 123.94 | n = 119, mean = 1273.23, SD = 122.19 |
|
| ||||
| 2 | n = 110, mean = 1268.22, SD = 126.26 | n = 91, mean = 1275.79, SD = 126.51 | n = 97, mean = 1273.20, SD = 125.64 | |
|
| ||||
| 3 | n = 93, mean = 1285.88, SD = 140.12 | n = 35, mean = 1286.54, SD = 139.63 | n = 41, mean = 1272.81, SD = 130.80 | |
|
| ||||
| Left hippocampus (mm3) | 1 | n = 121, mean = 2954.43 SD = 322.78 | n = 119, mean = 2955.73, SD = 324.86 | n = 119, mean = 2961.26, SD = 320.13 |
|
| ||||
| 2 | n = 110, mean = 2971.405, SD = 296. 595 | n = 91, mean = 2964.77, SD = 304.25 | n = 97, mean = 2966.92, SD = 305.61 | |
|
| ||||
| 3 | n = 93, mean = 3071.97, SD = 356. 90 | n = 35, mean = 2992.22, SD = 354.68 | n = 41, mean = 2992.48, SD = 336.63 | |
|
| ||||
| Right hippocampus (mm3) | 1 | n = 121, mean = 3045.01 SD = 349.12 | n = 119, mean = 3043.366, SD = 351,70 | n = 119, mean = 3048.76, SD = 349.36 |
|
| ||||
| 2 | n = 110, mean = 3064.97, SD = 330. 436 | n = 91, mean = 3064. 55, SD = 343.96 | n = 97, mean = 3059.62, SD = 343.18 | |
|
| ||||
| 3 | n = 93, mean = 3140.50, SD = 380. 25 | n = 35, mean = 3054.88, SD = 361.08 | n = 41, mean = 3059.113, SD = 339.81 | |
Each column shows the characteristics of the sample used for each set of analyses (DHEA-related analyses, BRIEF and CANTAB analyses). The data are divided into 3 visits (as each child was followed longitudinally up to 3 times, every 2 years, for a total of 4 years), and the subdivisions ‘1, 2, and 3’ for each row represent each of these visits, except for the row ‘# Participants per # of Scans completed’, for which each row represents the number of scans completed. F=female; M=male
2.2 Neuroimaging Measures
A three-dimensional T1-weighted (T1W) Spoiled Gradient Recalled (SPGR) echo sequence from 1.5 Tesla scanners was obtained on each participant, with 1mm isotropic data acquired sagittally from the entire head for most scanners. In addition, T2-weighted (T2W) and proton density-weighted (PDW) images were acquired using a two-dimensional (2D) multi-slice (2mm) dual echo fast spin echo (FSE) sequence.
Fully automated analysis of whole-brain cortical thickness was done through the CIVET pipeline, developed at the Montreal Neurological Institute (MNI) (Ducharme et al., 2016). First, a multistage quality control process was implemented, as described previously, excluding participants with white or gray matter artifacts (Ducharme et al., 2016; Nguyen et al., 2013a; Nguyen et al., 2013b). All quality-controlled MR images were subsequently processed through the CIVET pipeline. These processing steps have been described at length in other publications (Ducharme et al., 2016; Nguyen et al., 2013a; Nguyen et al., 2013b).
Volumetric measures of the hippocampus were obtained from MRI data using a fully automated segmentation method validated in human participants (Collins and Pruessner, 2010). This method utilizes a large MRI dataset (n = 80) of young healthy adults that serves as a template library of manually-labeled hippocampal volumes (Pruessner et al., 2001). The manual segmentation was done by four different raters, and intra-class intra-rater and inter-rater reliability varied between r=0.83 for the right and r=0.95 for the left hippocampus (Pruessner et al., 2000). From this manual segmentation, a fully automated method was derived, characterized by label fusion techniques that combine segmentations from a subset of ‘n’ most similar templates. Specifically, each template is used to produce an independent segmentation of the participant using the ANIMAL pipeline (Collins and Evans, 1997), followed by a thresholding step to eliminate cerebrospinal fluid, which results in ‘n’ different segmentations. To fuse the segmentations at each voxel, a voting strategy is used; the label with the most votes from the ‘n’ templates is assigned to the voxel. Combining multiple segmentations minimizes errors and maximizes consistency between segmentations. When using n = 11 templates, the label fusion technique has been shown to yield an optimal median Dice Kappa of 0.886 and Jaccard similarity of 0.796 for the hippocampus (Collins and Pruessner, 2010). Of note, even though the template library of manually labeled hippocampal volumes consisted of data from healthy young adults, using the ANIMAL pipeline combined with this template library results in a method fairly resistant to developmental volumetric deviations, as shown by its high Dice Kappa and Jaccard similarity values (Collins and Pruessner, 2010). In addition, previous comparisons between pediatric and adult structural MRI brain templates detected no systematic bias in comparisons between adults and children over 6 years of age in our NIHPD dataset (Fonov et al., 2011).
2.3 Hormonal and Pubertal Measures
Salivary sampling measures the unbound, biologically active portions of circulating hormonal levels, which freely crosses the blood-brain barrier and is therefore more relevant to studies of brain-hormone associations than total plasma hormonal levels (Khan-Dawood et al., 1984; Worthman et al., 1990). DHEA, compared to its sulfated hydrophilic form DHEAS, is more easily measured in saliva and crosses the blood-brain barrier due to its lipophilicity (Stanczyk, 2006; Vining and McGinley, 1987). In contrast to the reported local CNS production of DHEA(S) in the rat brain, de novo synthesis of DHEA in the human brain is unlikely to be significant given the low/negligible levels of P450c17 and cytochrome b5 in the human CNS (Baulieu, 1998; Compagnone and Mellon, 2000). Should local CNS production of DHEA still proceed, it would nonetheless be dwarfed by the high net influx of peripheral DHEA into the CNS, the major portion of which is produced in the adrenal cortex (Compagnone and Mellon, 2000; Maninger et al., 2009). Further evidence supporting this model of DHEA synthesis comes from a study of human participants in which serum DHEA levels were shown to correlate with cerebrospinal fluid DHEA levels (Kancheva et al., 2011). DHEA rises steeply during middle childhood and adrenarche (i.e. 6–8 years of age), and remains at high levels throughout adolescence and young adulthood, only starting to decrease past the third decade of life (Adams, 1985; Campbell, 2011; Remer et al., 2005). Therefore, peripheral DHEA levels, as measured by salivary sampling, may be especially representative of DHEA in the CNS and relevant to the study of brain developmental changes (Compagnone and Mellon, 2000). These characteristics of DHEA are not shared by its sulfated form DHEAS, which is not easily measured in saliva and does not easily cross the blood-brain barrier, in addition to being actively transported out of the CNS (Stanczyk, 2006; Vining and McGinley, 1987) -and therefore, is not expected to correlate closely with CNS levels. Of note, the levels of DHEA, as well as those of other steroid hormones, have been shown to follow diurnal and, to a certain extent, seasonal patterns in response to the pulsatile release of adrenocorticotropic hormone and gonadotropin-releasing hormone (Matchock et al., 2007). To control for these patterns, we have included season and time of collection as covariates in hormonal-related analyses (see section 2.5).
During each MRI visit, children provided two separate 1–3 cm3 samples of saliva, collected on the day of the scan, which were assayed by enzyme-linked immunosorbent assay (ELISA) methods, and the average results used as a measure of hormonal levels. The intra-assay and inter-assay coefficients of variation (COVs) were 6.5% and 16.2% for DHEA, 6.1% and 13.5% for testosterone, and 4.1% and 9.1% for estradiol, respectively (Salimetrics ELISA, State College, PA; Salimetrics Salivary ELISA Kit, State College, PA). At the next MRI, a similar procedure was followed and the child again provided two separate saliva samples for hormonal measurements. Therefore, within the constraints of missing or poor-quality data that did not allow hormonal quantification, all longitudinal hormonal measurements were included (with a maximum of 3 hormonal measurements, every 2 years, over the course of 4 years, for each individual child).
Pubertal maturation was measured using the Pubertal Development Scale (PDS), which was administered by a physician to all participants included in this study, at each visit (Petersen et al., 1988). This scale has been shown to have good reliability (Cronbach’s alpha coefficient: 0.77) (Petersen et al., 1988). In addition, moderate to high correlations (r2=0.61–0.67) between PDS scores and physical examinations by a physician (i.e. the gold standard test for pubertal staging) have been reported, thereby establishing the validity of this scale (Brooks-Gunn et al., 1987). In this study, we attempted to further increase the reliability and validity of the PDS by having it administered by a physician during an interview with the child/adolescent. Total scores from the PDS were then converted to a puberty variable consisting of 5 stages, representing increasing levels of physical maturity similar to Tanner staging, previously described (Nguyen et al., 2013a). All longitudinal pubertal measurements were included whenever available, and attempts were made to collect this data in each child during each of their 3 visits, every 2 years, for a maximum of 4 years.
2.4 Cognitive Measures
Cognitive measures were administered each time the participant underwent a scan, and by extension, each time hormonal measures were collected (during each of their 3 visits, every 2 years, for a maximum of 4 years). The debate about the optimal way to assess executive function, including working memory, is still ongoing (Gioia et al., 2000a; Gioia et al., 2002a; Gioia et al., 2002b; Gioia et al., 2001; Goldman-Rakic, 1987; Welsh et al., 1991). Some have supported the use of direct, performance-based testing, whereas others have suggested that most performance-based tests are not entirely adequate because they attempt to separate integrated functions into component parts, and do not take into account the complexity of real-life situations (Burgess, 1997; Goldberg and Podell, 2000; Shallice and Burgess, 1991). To address this issue, we selected both a laboratory, clinical performance-based test of spatial working memory (Cambridge Neuropsychological Test Automated Battery [CANTAB] (Luciana, 2003)) and a parent- and teacher-rated measure of everyday working memory exhibited by the child in real-world situations (Behavior Rating Inventory of Executive Function [BRIEF] (Gioia et al., 2002b)).
The CANTAB spatial working memory subtest is part of a computerized neuropsychological test battery that includes only nonverbal geometric designs or simple shapes, with minimal required language proficiency. The validity of CANTAB for assessing brain-behavior relations in adults has been established, and results of tests in pediatric populations have shown that children can be tested with the same item sets that are employed in adult studies. Reliability is high in pediatric populations (Cronbach’s alpha coefficients=0.73 for reaction time, and 0.95 for performance on the spatial working memory test) (Luciana, 2003). Test-retest stability coefficients are moderate in magnitude and range from 0.6–0.7, and construct validity has been established in pediatric populations (Luciana, 2003). Here we are using the CANTAB Spatial Working Memory subtest (use of self-guided spatial search strategies) as a measure of spatial working memory.
In contrast to the performance-based CANTAB, the BRIEF uses parent ratings of executive function in the context of everyday problem solving. It measures executive function (including working memory) in an integrated, relativistic way, outlining the complex, priority-based decision-making that is demanded in real-world situations (Gioia et al., 2000a; Gioia et al., 2002a; Gioia et al., 2002b; Gioia et al., 2001). The main strength of the BRIEF lies in its use of ecologically valid measurements, which allow a ‘real-world’ snapshot of working memory that includes aspects of complex, everyday problem-solving demands (Gioia et al., 2000a; Gioia et al., 2002b). The BRIEF has demonstrated high test-retest reliability (r ≈0.82 for parent ratings) and high internal consistency (Cronbach’s alphas ≈ .80 – .98) (Gioia et al., 2000b). Here we are using the BRIEF Working Memory scale, which measures the ‘on-line representational memory’, i.e. the capacity to hold information in mind for the purpose of completing a task, encoding information, or generating goals, plans and sequential steps to achieving goals. In other words, this scale measures the ability to sustain working memory for appropriate lengths of time in order to sustain performance and attention.
Of note, previous findings from our group outlined the relationship between higher DHEA levels, the development of cortico-amygdalar structural covariance and improved performance on measures of visual attention (visual awareness, visuo-motor dexterity and global attention levels) (Nguyen et al., 2016). Because of this, we also tested the relationship between DHEA-related cortico-hippocampal networks and visual attention. The previous DHEA/cortico-amygdalar study included measures related to visual awareness (Woodcock-Johnson (WJ III) Letter-Word Identification) and visuo-motor dexterity (WJ III Coding) as well as a measure of global attention (Child Behavior Checklist (CBCL) Attention subscale). Additional details on these cognitive measures have been published elsewhere (Nguyen et al., 2016).
2.5 Statistical Analyses
Statistical analyses were done using SurfStat (Matlab toolbox; http://www.math.mcgill.ca/keith/surfstat/) and SPSS 21.0 (SPSS, Inc., Chicago, Illinois). Please see Table 2 for more details on statistical models used in this section.
Table 2.
Description of statistical models
| Methods section | Statistical model |
|---|---|
| 2.5.1 DHEA & Cortico-Hippocampal Structural Covariance |
|
| 2.5.2 Cortico-Hippocampal Structural Covariance & Cognitive Tests |
|
| 2.5.3 Mediation |
|
The specific statistical term of interest is underlined in each model; the rest of the terms represent control variables.
‘id’ refers to a specific participant’s identification number: this term is included in order to identify and link all longitudinal data from the same participant
‘I’ to the identity matrix of the mixed effects model
‘CTh’ in section 2.5.2 refers to average cortical thickness of the brain regions found to be significant in section 2.5.1
2.5.1 DHEA-Related Cortico-Hippocampal Structural Covariance
Mixed effects designs take into account the within- and between- individual variances in this longitudinal sample, i.e. they allow the modeling of the trajectory of brain structural changes within a single child (within-individual) as well as the cumulative trajectory of the group of children over time (between-individuals). These mixed effects models were used to examine the relationship between DHEA and covariance of the hippocampus with whole-brain, native-space cortical thickness (CTh), controlling for the effects of age, sex, total brain volume, scanner, handedness, and time of salivary sampling (the latter, coded as a continuous variable: number of minutes after midnight). In addition, estradiol, testosterone, pubertal stage and season of collection (coded as a categorical variable: spring, summer, fall, winter) were also included as control variables in additional DHEA-related models, to examine any distinct effects of DHEA above and beyond those related to its main androgenic metabolite, testosterone, or its main estrogenic metabolite, estradiol, as well as to disentangle DHEA’s effects from those related to pubertal maturation or to the variation in hormonal levels due to seasonal changes (see Table 2 for more details).
All continuous variables were centered using their respective means. A correction for multiple comparisons across the whole brain, using random field theory (RFT, p<0.05), was applied to all analyses (Worsley et al., 1992). To examine associations between DHEA and structural covariance of the hippocampus, we examined the significance of the term ‘DHEA*Hippocampus’ on whole-brain cortical thickness, while controlling for all the aforementioned control variables.
Because age and sex effects may also impact the relationship between DHEA and cortico-hippocampal covariance, we also tested the terms ‘DHEA*Hippocampus*Age’ and ‘DHEA*Hippocampus*Sex’ for significance. We examined DHEA-related structural covariance between whole-brain CTh and mean hippocampal volume, defined as the average volume of the left and right hippocampi.
2.5.2 Cortico-Hippocampal Structural Covariance and Working Memory
To examine associations between DHEA-related cortico-hippocampal covariance and cognitive measures, we averaged CTh of brain regions found to be significant in section 2.5.1 (see Table 2 for more details) and examined the impact of cortico-hippocampal covariance on cognitive measures, while controlling for age, sex, total brain volume, scanner, and handedness. More specifically, we tested for associations between cortico-hippocampal structural covariance and working memory (as measured by the CANTAB and the BRIEF), i.e. testing for the significance of the interaction term ‘CTh*Hippocampus’ on working memory (‘CTh’ referring here to average cortical thickness of the brain areas identified in section 2.5.1, see Table 2 for more details).
2.5.3 Mediation Effects of Cortico-Hippocampal Structural Covariance
We tested whether cortico-hippocampal structural covariance (covariance between the brain areas identified in section 2.5.1 and mean hippocampal volumes) mediated the relationship between DHEA and working memory. To examine the relationship between DHEA and cortico-hippocampal covariance, we extracted the beta coefficients and standard errors of the significant interaction term ‘DHEA*Hippocampus’ for the peak vertex i.e. the vertex with the highest coefficient (in the context of the mixed effects statistical model already described in section 2.5.1). Similarly, to examine the relationship between DHEA-related cortico-hippocampal covariance and working memory, we extracted the beta coefficients and standard errors of the significant interaction term ‘CTh*Hippocampus’ (in the context of the mixed effects statistical model already described in section 2.5.2). These beta coefficients and standard errors were entered in the Sobel-Goodman test calculator to formally test mediation effects (http://quantpsy.org/sobel/sobel.htm). This more traditional approach to test mediation effects, using Baron-Kenny’s criteria and augmented by a formal Sobel’s test, was preferred by our group to more recent methods that include bootstrapping. This decision is due to the complexity of our longitudinal data (multiple scans per participants and different number of scans per participant). The traditional method treats each relationship (between predictor and moderator, and then between moderator and outcome) separately, allowing us to model the longitudinal component of the data. The same set of control variables (as listed in Methods, 2.5.1) was used for the mediation analyses.
3. RESULTS
3.1 Sample Characteristics
Table 1 details sample characteristics, including number of longitudinal scans and covariates of interest. The sample used for DHEA-related analyses included 216 participants (female=123) and 324 scans (female=190), with an age range of 6 to 22 years (mean=13.6 years, SD=3.6 years). The sample used for BRIEF analyses included 187 participants (female=109) and 245 scans (female=146), with an age range of 6 to 19 years (mean=12.7 years, SD=3.1 years). Finally, the sample used for CANTAB analyses included 188 participants (female=110), 257 scans (female=155), with an age range of 6 to 22 years (mean=13.4 years, SD=3.6 years).
*Of note, for all of the results described below (sections 3.2–3.4), each dataset was analyzed as a whole (DHEA levels and scores on working memory tests included as continuous variables, with no splitting into high/low categories). However, for visualization purposes, data are shown in binary fashion in all the figures, i.e. lower/higher DHEA levels and lower/higher working memory scores.
3.2 DHEA-Related Cortico-Hippocampal Structural Covariance
As shown in Figures 1 and 2, whole-brain analyses, controlling for the effects of age, sex, total brain volume, scanner, handedness and collection time of salivary samples, revealed that DHEA levels were significantly associated with the structural covariance between mean hippocampal volume and:
-
(1)
CTh of the right insular cortex (linear mixed models, Brodmann area 13, r=0.18, SE=0.045, cluster-level p=0.01, peak vertex id 53158 [x=38.3, y=−2.8, z=−5.4]);
-
(2)
CTh of the left occipital pole (linear mixed models, Brodmann area 17, r=0.21, SE=0.056, cluster-level p=0.04, peak vertex id 33180 [x=−13.3, y=−104.6, z=−1.0]).
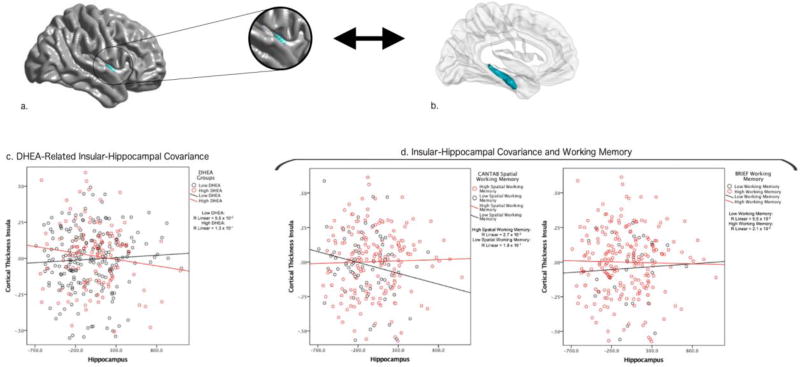
Figure 1. Insular-Hippocampal Structural Covariance and Working Memory.
This figure shows the associations between DHEA, insular-hippocampal structural covariance and working memory. The negative insular-hippocampal covariance seen at higher DHEA levels was associated with lower scores on a performance-based test of spatial working memory (CANTAB) and higher scores on a ‘real-world’ parent rating of overall working memory (BRIEF).
Brain figures A and B show the DHEA-related structural covariance between mean hippocampus and the region of the right insular cortex found to be significant in whole-brain analyses, corrected for multiple comparisons using random field theory (p<0.05).
Graph C shows that higher DHEA levels were associated with a negative covariance between mean hippocampal volume and cortical thickness of the right insular cortex, while lower DHEA levels were associated with a positive covariance between these regions.
Graphs under the heading D show that negative insular-hippocampal covariance, akin to the one seen at higher DHEA levels, was associated with lower spatial working memory (left-hand side) and higher overall working memory (right-hand side).
All analyses consisted of mixed effects models that included all longitudinal data, as well as continuous measures of DHEA and cognitive scores. However, in order to clearly visualize the direction of the interaction, the graphs display DHEA and cognitive scores as dichotomous groups, and each child’s longitudinal structural covariance trajectory is not included. Please note the Y axes of graphs list standardized residuals of cortical thickness (accounting for the effects of age, sex, handedness, scanner, and total brain volume in all analyses, as well as collection time for DHEA-related analyses).
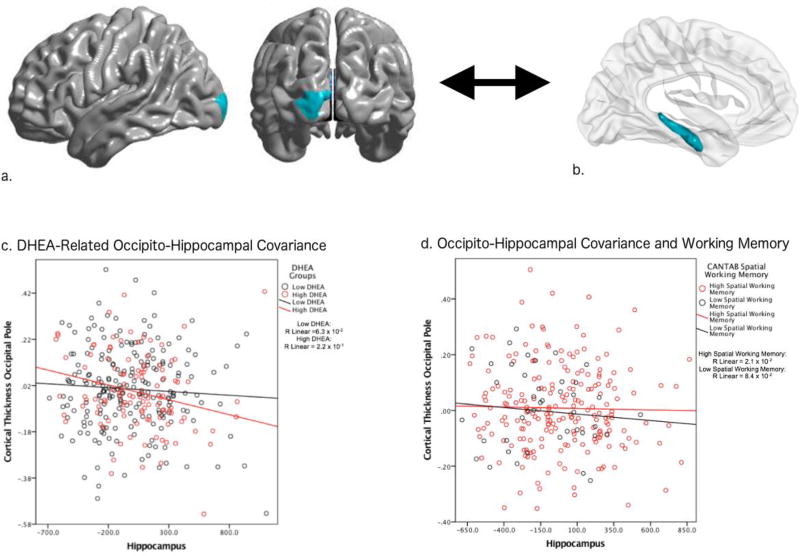
Figure 2. Occipital-Hippocampal Structural Covariance and Working Memory.
This figure shows the associations between DHEA, occipito-hippocampal structural covariance and working memory. The more pronounced negative occipito-hippocampal covariance seen at higher DHEA levels tended to be associated with lower scores on a performance-based test of spatial working memory (CANTAB).
Brain figures A and B show the DHEA-related structural covariance between mean hippocampus and the region of the left occipital pole (primary visual cortex) found to be significant in whole-brain analyses, corrected for multiple comparisons using random field theory (p<0.05).
Graph C shows that higher DHEA levels were associated with a more pronounced negative covariance between mean hippocampal volume and cortical thickness of the left occipital pole, compared to the covariance seen at lower DHEA levels.
Graph D shows that a more pronounced negative insular-hippocampal covariance, akin to the one seen at higher DHEA levels, tended to be associated with lower CANTAB spatial working memory.
Similar to Figure 1, for the purposes of visualization, the graphs display DHEA and cognitive scores as dichotomous groups, each child’s longitudinal structural covariance trajectory is not included, and the Y axes of graphs list standardized residuals of cortical thickness.
For both insular and occipital cortical areas, higher DHEA levels were associated with a negative cortico-hippocampal covariance, and lower DHEA levels, with positive cortico-hippocampal covariance across the entire sample (including both within- and between- individual comparisons). No other brain region met the threshold for significance (RFT, p<0.05). Adding testosterone, estradiol, pubertal stage, and season of sampling as control variables (one at a time, to limit decreases in power) did not result in any significant change in the findings. Finally, there were no significant interactions between DHEA, sex and age on cortico-hippocampal covariance (no significant effects of ‘DHEA*Hippocampus*Age’ and ‘DHEA*Hippocampus* Sex’ on CTh).
3.3 Cortico-Hippocampal Structural Covariance and Working Memory
As shown in Figure 1, analyses controlling for the effects of age, sex, total brain volume, scanner and handedness, revealed an effect of insular-hippocampal covariance on both the performance-based CANTAB spatial working memory (r=−0.0023, SE=0.0011, p=0.030) and the ecological, real-world focused BRIEF working memory subscales (r=0.0089, SE=0.004, p=0.026). More specifically, lower CANTAB Spatial Working Memory and higher BRIEF Working Memory scores were associated with a negative covariance between insular-hippocampal covariance (similar to the covariance seen with higher DHEA levels). In contrast, higher CANTAB Spatial Working Memory and lower BRIEF Working Memory scores were associated with a positive insular-hippocampal covariance (similar to the covariance seen at lower DHEA levels). A similar relationship was found for occipito-prefrontal covariance (lower CANTAB Spatial Working Memory scores associated with the negative occipito-prefrontal covariance seen at higher DHEA levels -see Figure 2), though this trend did not reach significance (r=−0.002; SE=0.0012; p=0.089). On the other hand, there was no significant association or near-significant trends between DHEA-related cortico-hippocampal structural covariance and any of the visual attention measures previously linked to DHEA-related cortico-amygdalar covariance (WJ III Letter-Word, WJ III Coding, CBCL Attention, p>0.1).
3.4 Mediation Effects of Cortico-Hippocampal Structural Covariance
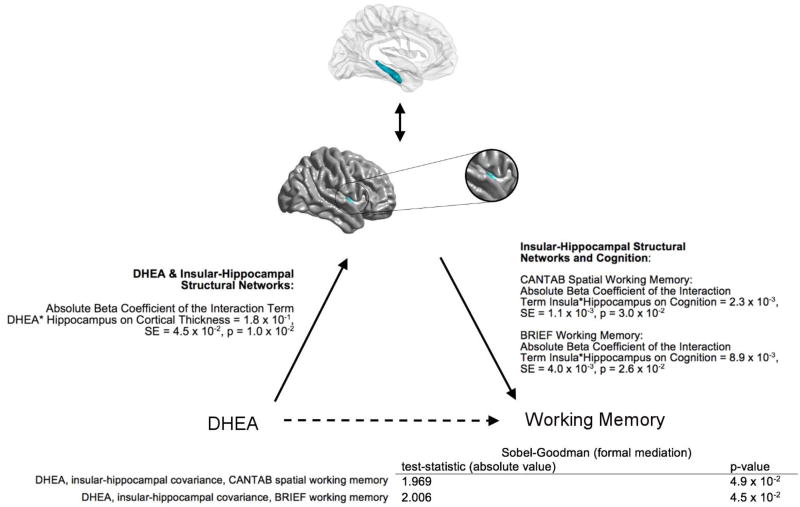
Insular-hippocampal structural covariance was found to significantly mediate the relationship between DHEA and working memory, as measured by both the CANTAB Spatial Working Memory (Sobel-Goodman test statistic: 1.969; p=0.049) and the BRIEF Working Memory tests (Sobel-Goodman test statistic: 2.006; p=0.045; see Figure 3 for more details).
Figure 3. Insular-Hippocampal Structural Covariance Mediates the Relationship between DHEA levels and Working Memory.
This figure displays the relationship between DHEA levels, insular-hippocampal structural covariance, and working memory.
The beta coefficients and standard errors, extracted from previous analyses corrected for multiple comparisons using random field theory (see Methods, sections 2.5.1 and 2.5.2 for more details), are displayed in the center of the figure:
(1) On the left, for the relationships between DHEA and insular-hippocampal covariance;
(2) On the right, for the relationship between insular-hippocampal covariance and working memory.
In addition, test statistics and p-values for the formal Sobel-Goodman mediation tests are displayed at the bottom of the figure.
4. DISCUSSION
Results from this study show significant associations between DHEA, the development of cortico-hippocampal structural covariance, and working memory. The negative covariance between insular and occipital brain regions and hippocampal volumes, seen at higher DHEA levels, was associated with lower spatial working memory as measured by a performance-based test (CANTAB), but higher overall working memory as measured by ecological parent ratings of the child’s function in situations of everyday life (BRIEF). Similar to findings from a previous study from our group on DHEA-related cortico-amygdalar networks (Nguyen et al., 2016), these effects of DHEA did not vary according to sex or age (no significant sex or age interactions). In other words, these DHEA-related effects were present in both boys and girls and remained into effect throughout the developmental period examined (i.e. from 6–22 years old). Of note, previous analyses of DHEA-cortical thickness yielded associations with brain regions other than those found significant in cortico-hippocampal structural covariance analyses (Nguyen et al., 2013), and DHEA-hippocampal volume analyses yielded no significant associations. Taken together, these findings suggest that DHEA is more significantly involved in changing the developmental relationship between the cortex and hippocampus (in other words, cortico-hippocampal structural covariance), rather than in unrelated, independent modifications of the cortex and the hippocampus.
DHEA rises steeply in children during a critical developmental stage, middle childhood (i.e. 6–8 years of age) and remains at high levels throughout adolescence and young adulthood (Campbell, 2011; Remer et al., 2005). Further, the blood-brain barrier is highly permeable to the peripheral levels of DHEA produced by the adrenals (Kancheva et al., 2011; Kancheva et al., 2010). This, coupled with decreased uptake of DHEA into adipose tissue during childhood and adolescence, makes DHEA more available for CNS uptake (Benfield et al., 2008; Campbell, 2011; Dalla Valle et al., 2006). As a result, DHEA’s neuroprotective effects may play a particularly important role in favoring CNS plasticity during childhood and adolescence, particularly in late-developing parts of the cortex.
The insula, for example, exhibits elevated glucose utilization relative to the rest of the cortex, suggesting a prolonged period of synaptogenesis in this region (Van Bogaert et al., 1998), and making it a primary target for the effects of DHEA, as has been previously hypothesized (Campbell, 2011). In particular, the right insula, as identified in our results, is known to integrate somatosensory and emotional information to create a sense of interoceptive awareness, regulating subjective bodily awareness during situations of social threat or unfairness (Craig, 2009a, b). Bilateral connections between the insula and the hippocampus have been demonstrated (Zonjy et al., 2014). Hippocampal-to-insula connections may modulate the memory of feeling and bodily states in social settings, for example by bringing forward the memory of negative affects and somatic symptoms related to certain faces (Immordino-Yang and Singh, 2013; Paulus and Stein, 2006; Singer, 2007; Tsukiura et al., 2013). In sum, when taken together with the present findings, current evidence raise the possibility that DHEA may improve overall working memory by decreasing the impact of affect-laden stimuli (including negative feelings and adverse bodily states), and that it may do so by decreasing the structural connectivity between the hippocampus and the insula. In other words, DHEA may decrease bottom-up influences from the hippocampus to the insula.
In contrast, the same DHEA-related, negative insular-hippocampal covariance was also associated with lower performance on a test of spatial working memory. This difference (lower spatial working memory, but overall better working memory) could be explained, perhaps, by the central role of hippocampal-to-cortical connections in the process of encoding spatial cues (Spellman et al., 2015) and in working memory (Broadbent et al., 2004). If, as hypothesized, DHEA inhibits the development of bottom-up influences from the hippocampus to the cortex, this would be expected to cause impairment in the encoding of spatial cues and in turn, lower performance on a test of spatial working memory. Our finding that a more pronounced negative, DHEA-related occipito-hippocampal covariance tends to be associated with lower spatial working memory further supports this theory. Similar to the insular cortex, the primary visual cortex has been shown to exhibit extended postnatal plasticity during the preadolescent period (Selemon, 2013), a time when there is a steep rise in DHEA levels in the context of adrenarche. Interestingly, sensory plasticity in the visual cortex has been shown to affect spatial information processing in the hippocampus (Tsanov and Manahan-Vaughan, 2008), and existing evidence supports the existence of visual cortical neurons functionally coupled with hippocampal place cells for spatial processing and the maintenance of spatial memory (Haggerty and Ji, 2015). In sum, current evidence suggests that DHEA may worsen spatial working memory by interfering with the hippocampal-based maintenance and consolidation of spatial memories, and that it may do so by decreasing the structural connectivity between the hippocampus, the insula, and the primary visual cortex.
Our results are reminiscent of previous findings from our group regarding the relationship between higher DHEA levels, the development of cortico-amygdalar structural covariance and improved performance on visual awareness, visuo-motor dexterity and global attention levels (Nguyen et al., 2016). Taken together, these results support the concept that both DHEA-related cortico-amygdalar and cortico-hippocampal structural covariance networks may enhance executive function, but through distinct and non-overlapping pathways, i.e. affecting different brain regions and cognitive parameters (visual attention for cortico-amygdalar networks and working memory for cortico-hippocampal networks). Previous clinical studies have also shown a positive relationship between DHEA(S) levels and attention level in a sample of children suffering from attention-deficit hyperactivity disorder (Strous et al., 2001). In addition, some, though not all, studies in adults have shown positive associations between DHEA(S) levels and overall working memory (Alhaj et al., 2006; Davis et al., 2008). In contrast, elevated levels of DHEA or DHEAS have also been associated with: (1) social disinhibition and higher externalizing symptoms in girls with premature adrenarche (Dorn et al., 2008; Whittle et al., 2015); (2) more psychosocial difficulties and peer problems in boys with premature adrenarche (Mundy et al., 2015); and (3) more symptom burden (e.g. oppositional-defiant disorder, aggressive behaviour) in children with conduct disorder (Pajer et al., 2006; van Goozen et al., 1998; van Goozen et al., 2000). There are also reports of potentially decreased emotional salience with increasing DHEA levels (Sripada et al., 2013; Whittle et al., 2015).
A potentially unifying theory would be that DHEA improves both attention and overall working memory during development by decreasing the influence of amygdalar and hippocampal afferents on cortical functions. For example, DHEA may inhibit amygdalar-based functions (e.g. the detection of emotional stimuli), and hippocampal-based functions (e.g. encoding and processing of spatial and social cues). This inhibition could allow for more purely cognitive functions, like attention and overall working memory, to proceed unencumbered, at the cost of a DHEA-related impairment in interoceptive awarenesss, social understanding and emotional processing/empathy. This hypothesis would be consistent with the higher rates of disruptive behaviors seen with increased DHEA levels, an effect may be particularly striking in the context of an abnormal, premature timing of adrenarche (Dorn et al., 2008; Pajer et al., 2006; van Goozen et al., 1998; van Goozen et al., 2000; Whittle et al., 2015).
4.1 Strengths and Limitations
Strengths of our study include the large, longitudinal developmental dataset, including the repeated collection of hormonal, neuroimaging and cognitive data and the use of two different complementary measures of working memory. However, one notable limitation is the use of 1.5T MRI scanners, which have lower resolution compared to 3T models. This could lead to decreased accuracy and misclassification of white vs. gray matter. Still, all quality-controlled structural MR images were processed using the highest standards (see section 2.2). Missing data were biased to a certain extent by differences in age (e.g. younger children were less likely than older children to be included because of motion artifacts frequently leading to exclusion of data in younger children). However, given the large sample size, it is unlikely that these differences have biased or confounded findings in a systematic manner. In addition, our use of mixed effects designs minimized this bias, given that these statistical models are generally robust to systematic biases introduced by missing data, and can accommodate data that are not homogeneous across participants (i.e. different number of scans per participant).
An additional concern in hormone-brain association studies is the presence of intra-individual hormonal variation due to known, or unknown, causes, and the interaction between steroid hormones on brain structure/function. Reassuringly, androgen levels have been shown to remain highly correlated for several days, weeks and possibly even an entire year, and to reliably correlate with stable measures of personality (Dabbs Jr, 1990; Granger et al., 2004; Sellers et al., 2007). Still, to limit any systematic bias related to intra-individual hormonal variation, we have controlled for sex, diurnal and seasonal variation, with no significant changes in the results. We also controlled for testosterone and estradiol in all analyses, to ensure that the DHEA-related effects outlined in the study were above and beyond those related to these other steroid hormones. Still, it is possible that testosterone-related effects on cortico-hippocampal structural networks on different aspects of executive function (monitoring and shifting abilities), outlined in a previous study from our group, may interact with those of DHEA on cortico-hippocampal networks and working memory. However, we could not demonstrate interactive effects of testosterone and DHEA on either cortico-hippocampal structural covariance or working memory in the current study, and therefore these remain to be confirmed by future investigations.
Another limitation may be the large age span over which data collection proceeded (6 to 22 years old). Although this may have increased the variance in our data, the broad age range also increases the generalizability of the results and may be seen as a major strength of the study as well. Several longitudinal neuroimaging studies similar to ours have demonstrated that the adolescent brain continues to mature well into the 20s (Johnson et al., 2009), supporting our inclusion of adolescents and young adults between the ages of 19–22 years old.
The Baron-Kenny approach used to test mediation effects also has two additional limitations: low power (thus low likelihood of detecting a significant effect) and the assumption that we can infer the indirect effect (the effect of the mediator) by evaluating only the direct effects (from independent variable to mediator, and from mediator to outcome). The first limitation could be viewed as a strength in the context of this study because it makes this approach more conservative (and increases the confidence one may have in the actual results). The second limitation may be difficult to avoid because of the complexity of our data (longitudinal data, multiple scans per participant, not the same number of scans for each participant), which renders the use of structural equation modeling approaches challenging.
Finally, the discrepancy between the results for the performance-based spatial working memory test and ecological parent ratings of working memory may be, at least in part, related to inherent differences, or limitations, in the methodology of these tests. In particular, while CANTAB scores may be limited by the lack of ecological validation in real-world settings, while BRIEF scores result from parent-ratings, which can therefore be more vulnerable to the subjective understanding of each parent. For example, the results obtained with BRIEF could be strongly influenced by the child’s outward behavior (as witnessed by the parent, e.g. level of hyperactivity, rule compliance, diagnosis of oppositional defiant disorder). However, our study of a typically developing sample was not designed to confirm or infirm the validity of one type of test over the other, and was not adequately powered to demonstrate associations between DHEA and clinical disorders such as oppositional defiant disorder. Instead, our findings underline the complexity of working memory, and support the differentiation of spatial, vs. non-spatial working memory processes within the context of structural brain networks.
4.2 Conclusions
In this study, we report evidence that, in addition to its effects on cortico-amygdalar structural covariance (Nguyen et al., 2016), DHEA affects the development of cortico-hippocampal covariance, more specifically that of insular-hippocampal and occipito-hippocampal connections. This DHEA-related cortico-hippocampal covariance was found to be associated with both improved overall working memory and lower spatial working memory. Taken together with previous research, these results point toward beneficial effects of DHEA on more purely cortical functions such as attention and working memory processes, potentially to the detriment of amygdalar- and hippocampal-based functions related to the encoding and processing of emotional, spatial and social cues.
HIGHLIGHTS.
Insular-hippocampal structural covariance varies as a function of DHEA levels
Occipito-hippocampal structural covariance varies as a function of DHEA levels
Working memory varied according to the development of cortico-hippocampal networks
DHEA-related cortico-hippocampal networks may play a role in in working memory
Acknowledgments
This work was supported by Federal funds from the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, −2315, −2316, −2317, −2319 and −2320).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Adams JB. Control of secretion and the function of C19-delta 5-steroids of the human adrenal gland. Molecular Cellular Endocrinology. 1985;41:1–17. doi: 10.1016/0303-7207(85)90138-8. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14:322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaj HA, Massey AE, McAllister-Williams RH. Effects of DHEA administration on episodic memory, cortisol and mood in healthy young men: a double-blind, placebo-controlled study. Psychopharmacology. 2006;188:541–551. doi: 10.1007/s00213-005-0136-y. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Cohen MX, Elger CE, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:7807–7816. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Benfield LL, Fox KR, Peters DM, Blake H, Rogers I, Grant C, Ness A. Magnetic resonance imaging of abdominal adiposity in a large cohort of British children. Int J Obes (Lond) 2008;32:91–99. doi: 10.1038/sj.ijo.0803780. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. P Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child development. 1987;58:829–841. [PubMed] [Google Scholar]
- Burgess P. Theory and methodology in executive function research. Psychology Press; East Sussex, UK: 1997. [Google Scholar]
- Byrne ML, Whittle S, Vijayakumar N, Dennison M, Simmons JG, Allen NB. A systematic review of adrenarche as a sensitive period in neurobiological development and mental health. Dev Cogn Neurosci. 2017;25:12–28. doi: 10.1016/j.dcn.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BC. Adrenarche and middle childhood. Hum Nat. 2011;22:327–349. doi: 10.1007/s12110-011-9120-x. [DOI] [PubMed] [Google Scholar]
- Collins DL, Evans AC. Animal: validation and applications of nonlinear registration-based segmentation. International Journal of Pattern Recognition and Artificial Intelligence. 1997;11:1271–1294. [Google Scholar]
- Collins DL, Pruessner JC. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. Neuroimage. 2010;52:1355–1366. doi: 10.1016/j.neuroimage.2010.04.193. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Frontiers in Neuroendocrinology. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2009a;364:1933–1942. doi: 10.1098/rstb.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009b;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dabbs JM., Jr Salivary testosterone measurements: Reliability across hours, days, and weeks. Physiology & Behavior. 1990;48:83–86. doi: 10.1016/0031-9384(90)90265-6. [DOI] [PubMed] [Google Scholar]
- Dalla Valle L, Toffolo V, Nardi A, Fiore C, Bernante P, Di Liddo R, Parnigotto PP, Colombo L. Tissue-specific transcriptional initiation and activity of steroid sulfatase complementing dehydroepiandrosterone sulfate uptake and intracrine steroid activations in human adipose tissue. J Endocrinol. 2006;190:129–139. doi: 10.1677/joe.1.06811. [DOI] [PubMed] [Google Scholar]
- Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. The Journal of clinical endocrinology and metabolism. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- de Menezes KJ, Peixoto C, Nardi AE, Carta MG, Machado S, Veras AB. Dehydroepiandrosterone, its sulfate and cognitive functions. Clinical practice and epidemiology in mental health : CP & EMH. 2016;12:24–37. doi: 10.2174/1745017901612010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Vale S, Selinger L, Martins JM, Gomes AC, Bicho M, do Carmo I, Escera C. The relationship between dehydroepiandrosterone (DHEA), working memory and distraction - a behavioral and electrophysiological approach. Plos One. 2014:9. doi: 10.1371/journal.pone.0104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Rose SR, Rotenstein D, Susman EJ, Huang B, Loucks TL, Berga SL. Differences in endocrine parameters and psychopathology in girls with premature adrenarche versus on-time adrenarche. Journal of pediatric endocrinology & metabolism : JPEM. 2008;21:439–448. doi: 10.1515/jpem.2008.21.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Nguyen TV, Hudziak JJ, Mateos-Perez JM, Labbe A, Evans AC, Karama S. Trajectories of cortical thickness maturation in normal brain development--The importance of quality control procedures. Neuroimage. 2016;125:267–279. doi: 10.1016/j.neuroimage.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentemilla L, Penny WD, Cashdollar N, Bunzeck N, Duzel E. Theta-coupled periodic replay in working memory. Current biology : CB. 2010;20:606–612. doi: 10.1016/j.cub.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2000a;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L, Baron IS. Test review: Behavior rating inventory of executive function. Child Neuropsychology. 2000b;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Kenworthy L, Barton RM. Profiles of everyday executive function in acquired and developmental disorders. Child Neuropsychology. 2002a;8:121–137. doi: 10.1076/chin.8.2.121.8727. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Retzlaff PD, Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2002b;8:249–257. doi: 10.1076/chin.8.4.249.13513. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Retzlaff PD, Pratt BM. Modeling executive functions with everyday behaviors: A unitary or fractionated system? Brain Cognition. 2001;47:203–207. [Google Scholar]
- Goldberg E, Podell K. Adaptive decision making, ecological validity, and the frontal lobes. Journal of clinical and experimental neuropsychology. 2000;22:56–68. doi: 10.1076/1380-3395(200002)22:1;1-8;FT056. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. Oxford University Press; NY: 1987. [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Haggerty DC, Ji D. Activities of visual cortical and hippocampal neurons co-fluctuate in freely moving rats during spatial behavior. eLife. 2015:4. doi: 10.7554/eLife.08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino-Yang MH, Singh V. Hippocampal contributions to the processing of social emotions. Human brain mapping. 2013;34:945–955. doi: 10.1002/hbm.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinck PH, Croft G, McEwen BS, Gottfried-Blackmore A, Jones G, Byford V, Bulloch K. Metabolism of dehydroepiandrosterone by rodent brain cell lines: Relationship between 7-hydroxylation and aromatization. J Steroid Biochem. 2005;93:81–86. doi: 10.1016/j.jsbmb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Jin RO, Mason S, Mellon SH, Epel ES, Reus VI, Mahan L, Rosser RL, Hough CM, Burke HM, Mueller SG, Wolkowitz OM. Cortisol/DHEA ratio and hippocampal volume: A pilot study in major depression and healthy controls. Psychoneuroendocrinology. 2016;72:139–146. doi: 10.1016/j.psyneuen.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kancheva R, Hill M, Novak Z, Chrastina J, Kancheva L, Starka L. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience. 2011;191:22–27. doi: 10.1016/j.neuroscience.2011.05.054. [DOI] [PubMed] [Google Scholar]
- Kancheva R, Hill M, Novak Z, Chrastina J, Velikova M, Kancheva L, Riha I, Starka L. Peripheral neuroactive steroids may be as good as the steroids in the cerebrospinal fluid for the diagnostics of CNS disturbances. The Journal of steroid biochemistry and molecular biology. 2010;119:35–44. doi: 10.1016/j.jsbmb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. The European journal of neuroscience. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Khan-Dawood FS, Choe JK, Dawood MY. Salivary and plasma bound and “free” testosterone in men and women. American journal of obstetrics and gynecology. 1984;148:441–445. [PubMed] [Google Scholar]
- Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Cusan L, Gomez JL, Martel C, Berube R, Belanger P, Chaussade V, Deloche C, Leclaire J. Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem. 2008;110:1–9. doi: 10.1016/j.jsbmb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Leszczynski M. How does hippocampus contribute to working memory processing? Front Hum Neurosci. 2011:5. doi: 10.3389/fnhum.2011.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SF, Mo QX, Hu S, Garippa C, Simon NG. Dehydroepiandrosterone upregulates neural androgen receptor level and transcriptional activity. Journal of neurobiology. 2003;57:163–171. doi: 10.1002/neu.10260. [DOI] [PubMed] [Google Scholar]
- Luciana M. Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB) Journal of child psychology and psychiatry, and allied disciplines. 2003;44:649–663. doi: 10.1111/1469-7610.00152. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neurosci-Switz. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiol Int. 2007;24:969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Research Bulletin. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Mo Q, Lu S, Garippa C, Brownstein MJ, Simon NG. Genome-wide analysis of DHEA- and DHT-induced gene expression in mouse hypothalamus and hippocampus. The Journal of steroid biochemistry and molecular biology. 2009;114:135–143. doi: 10.1016/j.jsbmb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Mo QM, Lu SF, Hu S, Simon NG. DHEA and DHEA sulfate differentially regulate neural androgen receptor and its transcriptional activity. Mol Brain Res. 2004;126:165–172. doi: 10.1016/j.molbrainres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Mo QX, Lu SF, Simon NG. Dehydroepiandrosterone and its metabolites: Differential effects on androgen receptor trafficking and transcriptional activity. J Steroid Biochem. 2006;99:50–58. doi: 10.1016/j.jsbmb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Morfin R, Starka L. Neurosteroid 7-hydroxylation products in the brain. Int Rev Neurobiol. 2001;46:79–95. doi: 10.1016/s0074-7742(01)46059-4. [DOI] [PubMed] [Google Scholar]
- Mundy LK, Romaniuk H, Canterford L, Hearps S, Viner RM, Bayer JK, Simmons JG, Carlin JB, Allen NB, Patton GC. Adrenarche and the Emotional and Behavioral Problems of Late Childhood. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2015;57:608–616. doi: 10.1016/j.jadohealth.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Gower P, Albaugh MD, Botteron KN, Hudziak JJ, Fonov VS, Collins L, Ducharme S, McCracken JT. The developmental relationship between DHEA and visual attention is mediated by structural plasticity of cortico-amygdalar networks. Psychoneuroendocrinology. 2016;70:122–133. doi: 10.1016/j.psyneuen.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S. Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 2013a;23:1424–1432. doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken JT, Ducharme S, Cropp BF, Botteron KN, Evans AC, Karama S. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013b;33:10840–10848. doi: 10.1523/JNEUROSCI.5747-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajer K, Tabbah R, Gardner W, Rubin RT, Czambel RK, Wang Y. Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology. 2006;31:1245–1256. doi: 10.1016/j.psyneuen.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Poch C, Fuentemilla L, Barnes GR, Duzel E. Hippocampal theta-phase modulation of replay correlates with configural-relational short-term memory performance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7038–7042. doi: 10.1523/JNEUROSCI.6305-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. Journal of Neuroscience. 2001;21:194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lerch Jason P, Lee N, Greenstein D, Wallace Gregory L, Stockman M, Clasen L, Shaw Phillip W, Giedd Jay N. Patterns of Coordinated Anatomical Change in Human Cortical Development: A Longitudinal Neuroimaging Study of Maturational Coupling. Neuron. 2011;72:873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remer T, Boye KR, Hartmann MF, Wudy SA. Urinary Markers of Adrenarche: Reference Values in Healthy Subjects, Aged 3–18 Years. Journal of Clinical Endocrinology & Metabolism. 2005;90:2015–2021. doi: 10.1210/jc.2004-1571. [DOI] [PubMed] [Google Scholar]
- Ritsner MS, Gibel A, Ratner Y, Tsinovoy G, Strous RD. Improvement of sustained attention and visual and movement skills, but not clinical symptoms, after dehydroepiandrosterone augmentation in schizophrenia - A randomized, double-blind, placebo-controlled, crossover trial. J Clin Psychopharm. 2006;26:495–499. doi: 10.1097/01.jcp.0000237942.50270.35. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, Ruparel K, Calkins ME, Roalf DR, Gennatas ED, Jackson C, Erus G, Prabhakaran K, Davatzikos C, Detre JA, Hakonarson H, Gur RC, Gur RE. Impact of puberty on the evolution of cerebral perfusion during adolescence. P Natl Acad Sci USA. 2014;111:8643–8648. doi: 10.1073/pnas.1400178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Translational psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers JG, Mehl MR, Josephs RA. Hormones and personality: Testosterone as a marker of individual differences. Journal of Research in Personality. 2007;41:126–138. [Google Scholar]
- Shallice T, Burgess P. Higher-order cognitive impairments and frontal lobe lesions in man. Oxford University Press; NY: 1991. [Google Scholar]
- Singer T. The neuronal basis of empathy and fairness. Novartis Foundation symposium. 2007;278:20–30. discussion 30–40, 89–96, 216–221. [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rajaram N, Garfinkel SN, Abelson JL, Liberzon I. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1798–1807. doi: 10.1038/npp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk FZ. Measurement of androgens in women. Semin Reprod Med. 2006;24:078–085. doi: 10.1055/s-2006-939566. [DOI] [PubMed] [Google Scholar]
- Strous RD, Spivak B, Yoran-Hegesh R, Maayan R, Averbuch E, Kotler M, Mester R, Weizman A. Analysis of neurosteroid levels in attention deficit hyperactivity disorder. Int J Neuropsychoph. 2001;4:259–264. doi: 10.1017/S1461145701002462. [DOI] [PubMed] [Google Scholar]
- Tsanov M, Manahan-Vaughan D. Synaptic plasticity from visual cortex to hippocampus: systems integration in spatial information processing. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2008;14:584–597. doi: 10.1177/1073858408315655. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Shigemune Y, Nouchi R, Kambara T, Kawashima R. Insular and hippocampal contributions to remembering people with an impression of bad personality. Social cognitive and affective neuroscience. 2013;8:515–522. doi: 10.1093/scan/nss025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bogaert P, Wikler D, Damhaut P, Szliwowski HB, Goldman S. Regional changes in glucose metabolism during brain development from the age of 6 years. Neuroimage. 1998;8:62–68. doi: 10.1006/nimg.1998.0346. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Kettenis PT, Thijssen JHH, van Engeland H. Adrenal androgens and aggression in conduct disorder prepubertal boys and normal controls. Biological psychiatry. 1998;43:156–158. doi: 10.1016/S0006-3223(98)00360-6. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, van den Ban E, Matthys W, Cohen-Kettenis PT, Thijssen JHH, van Engeland H. Increased adrenal androgen functioning in children with oppositional defiant disorder: a comparison with psychiatric and normal controls. J Am Acad Child Psy. 2000;39:1446–1451. doi: 10.1097/00004583-200011000-00020. [DOI] [PubMed] [Google Scholar]
- Vining R, McGinley R. The measurement of hormones in saliva: possibilities and pitfalls. J Steroid Biochem. 1987;27:81–94. doi: 10.1016/0022-4731(87)90297-4. [DOI] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF, Groisser DB. A normative developmental study of executive function - a window on prefrontal function in children. Dev Neuropsychol. 1991;7:131–149. [Google Scholar]
- Whittle S, Simmons JG, Byrne ML, Strikwerda-Brown C, Kerestes R, Seal ML, Olsson CA, Dudgeon P, Mundy LK, Patton GC, Allen NB. Associations between early adrenarche, affective brain function and mental health in children. Social cognitive and affective neuroscience. 2015;10:1282–1290. doi: 10.1093/scan/nsv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology. 1998;23:617–629. doi: 10.1016/s0306-4530(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Stallings JF, Hofman LF. Sensitive salivary estradiol assay for monitoring ovarian function. Clinical chemistry. 1990;36:1769–1773. [PubMed] [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci U S A. 2010;107:18191–18196. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonjy B, Alkhachroum A, Kahriman E, Lacuey N, Miller J, Luders H. Functional Connectivity between Insula, Hippocampus, and Amygdala Investigated Using Cortico-Cortical Evoked Potentials (CCEPs) (S50.005) Neurology. 2014:82. [Google Scholar]