Abstract
Cognitive functioning difficulties in breast cancer patients receiving chemotherapy are common, but not all women experience these impairments. Exposure to childhood trauma may impair cognitive functioning following chemotherapy, and these impairments may be mediated by dysregulation of hypothalamic-pituitary-adrenal (HPA) axis function and cortisol slope. This study evaluated the association between childhood trauma exposure, cortisol, and cognition in a sample of breast cancer survivors. 56 women completed measures of trauma exposure (the Traumatic Events Survey), salivary cortisol, and self-reported cognitive functioning (the Functional Assessment of Cancer Therapy – Cognitive). We examined correlations between childhood trauma exposure and cognitive functioning, then used linear regression to control for factors associated with cognition (age, education, time since chemotherapy, depression, anxiety, and insomnia), and the MacArthur approach to test whether cortisol levels mediated the relationship between trauma and cognitive functioning. 57.1% of the sample had experienced at least one traumatic event in childhood, with 19.6% of the sample witnessing a serious injury, 17.9% experiencing physical abuse, and 14.3% experiencing sexual abuse. Childhood trauma exposure and cognitive functioning were moderately associated (r=−0.29). This association remained even when controlling for other factors associated with cognition; the final model explained 47% of the variance in cognitive functioning. The association between childhood trauma and cognitive functioning was mediated by steeper cortisol slope (partial r=0.35, p=0.02). Childhood trauma exposure is associated with self-reported cognitive functioning among breast cancer survivors and is mediated by cortisol dysregulation. Trauma should be considered, among other factors, in programs aiming to address cognition in this population.
Keywords: Breast Cancer, Cortisol, HPA axis, Childhood Trauma, Cognitive functioning
Background
Breast cancer can have a pervasive impact on a woman’s cognitive functioning (Fitch, Armstrong, & Tsang, 2008). Cognitive functioning difficulties in breast cancer patients receiving chemotherapy are well-documented in the research literature, and many women report concerns about experiencing cognitive difficulties during the breast cancer trajectory (Janelsins et al., 2016). Several theories have been proposed to explain the prevalence and onset of impaired cognitive functioning among breast cancer patients and survivors. Most commonly, either the direct effect of chemotherapy on the brain (Matsuda et al., 2005) or systemic inflammatory processes due to chemotherapy or radiation treatments are thought to underlie cancer-related impairments in cognitive functioning (Ahles & Saykin, 2007). Other research has shown that some cognitive functioning difficulties may begin even before the initiation of cancer treatments and could, therefore, be related to tumor pathophysiology (Adams, Packer, Palesh, & Kesler, 2016). However, not all women experience cognitive functioning difficulties during treatment, and not all women continue to report these impairments following the cessation of treatment (Christie et al., 2012; Fitch et al., 2008; Von Ah, Habermann, Carpenter, & Schneider, 2013). Much work still needs to be done in order to understand the mechanism of cognitive functioning difficulties in breast cancer patients. Identifying factors that can predict women at risk of cognitive functioning difficulties after breast cancer treatment would allow researchers and clinicians to develop and implement early interventions to improve long-term cognitive outcomes.
Early exposure to traumatic events could put women at risk of impaired cognitive functioning. In the population at large, exposure to traumatic events in childhood (before age 18) may result in persistent alteration of hypothalamic-pituitary-adrenal (HPA) activity (Heim, Newport, et al., 2000; Meinlschmidt & Heim, 2005; Shea, Walsh, Macmillan, & Steiner, 2005). A review by Lupien and colleagues (2009) suggests that depending on a person’s age when exposed to trauma, he or she may develop long-term HPA suppression or hyperactivity, causing the HPA axis to be easily activated by stress and to continue to produce glucocorticoids even after a threat has passed (Lupien, McEwen, Gunnar, & Heim, 2009; McEwen, 1998). This overproduction of glucocorticoids, including cortisol, has a direct impact on cognitive functioning among people in general (McEwen, 1998; McEwen & Stellar, 1993) and in the specific context of cancer (Andreotti, Root, Ahles, McEwen, & Compas, 2015), indicating that exposure to childhood trauma may also put women at risk of cognitive functioning difficulties after breast cancer diagnosis and treatment.
Childhood trauma may have a pervasive impact on cognitive functioning because glucocorticoid receptors are found throughout the brain, including the hippocampus. The hippocampus is particularly vulnerable during childhood as this brain area is developing. Prolonged excessive secretion of glucocorticoids in the hippocampus, especially during childhood, may lead to a reduction of hippocampal volume, and thereby restrict capacity for learning and memory formation (Lupien et al., 1998; Sapolsky, Krey, & McEwen, 1986). Multiple studies have shown that memory and learning are impaired in survivors of childhood trauma (Bremner & Narayan, 1998; Charney & Manji, 2004; Weniger, Lange, Sachsse, & Irle, 2009), and that reduced hippocampal volume in trauma survivors is associated with increased arousal under stress (Gilbertson et al., 2002). The nature of the glucocorticoid response when exposed to stress, however, has yet to be fully characterized in medically ill populations who have been exposed to trauma. Some studies examining cortisol slopes in medical and psychiatric illness (Heim, Ehlert, & Hellhammer, 2000; McEwen, 1998; Sephton, 1998), including trauma (Heim, Ehlert, et al., 2000; Yehuda, 1997), have shown that flatter slopes, indicating a blunted stress response, are likely to emerge after longstanding exposure to stress. However, some studies have found a steeper cortisol slope, indicating a more pronounced stress response, in individuals experiencing health and illness related anxiety (Edwards, Hucklebridge, Clow, & Evans, 2003; Ferguson, 2008). For example, previous studies have indicated that steeper diurnal cortisol slopes were significantly related to increased anxiety about nonspecific health symptoms in healthy adults (Ferguson, 2008) and to increased awareness of one’s medical symptoms (Edwards et al., 2003). Similarly, another study among 274 women with breast cancer found that steeper diurnal cortisol levels predicted greater fatigue and depression (Palesh, 2009).
Given that hippocampal degeneration has also been found in cancer patients following chemotherapy (Christie et al., 2012), exposure to early childhood trauma could predispose cancer survivors to experience increased stress-related arousal and poorer cognitive functioning in the context of cancer treatment. Breast cancer patients who experienced childhood trauma may have dysregulated HPA axis function; their cortisol secretion patterns may have already been dysregulated by early life stress and could be further dysregulated by the introduction of inflammatory chemotherapies to their vulnerable neurocognitive systems. At present, few studies have explored the link between childhood trauma exposure, cancer treatment, dysregulation of HPA axis and cortisol secretion, and cognitive functioning among women with breast cancer. The current study addresses this gap. Our hypotheses are:
H1: Exposure to one or more traumatic events in childhood will be associated with greater impairment in cognitive functioning following breast cancer treatment. This relationship will remain even after controlling for type of breast cancer treatment, time since treatment, depression, anxiety, and sleep disturbance.
H2: Greater impairment in cognitive functioning will be mediated by dysregulation in cortisol levels in those patients who were exposed to one or more traumatic events in childhood.
Methods
Participants
This was a secondary analysis of data collected in the context of a randomized clinical trial. Participants were recruited between March, 2011 and April, 2012 through the Stanford Cancer Center and Love/Avon Army of Women (AOW), an online recruitment resource designed to partner women with the breast cancer research community (Stanton et al., 2013). Participants were approached, recruited, and consented into a study of acupuncture for treatment of insomnia in breast cancer survivors. In order to be eligible for this study, participants had to: 1) be diagnosed with breast cancer but not currently undergoing cancer treatment (with the exception of hormonal treatment), 2) have completed their last cancer treatment ≥ 2 weeks prior to screening, 3) have a habitual sleep phase between 9:00 pm and 11:00 am, 4) meet DSM-IV criteria for insomnia with duration ≥ 1 month, 5) have a Karnofsky Performance Status scale score ≥ 70, 6) have an Insomnia Severity Index (ISI) score ≥ 8, 7) be at least 21 years of age, 8) be able to understand written and spoken English, and 9) be able to travel to the study site. Participants could not: 1) have an unstable medical or psychiatric illness (determined by the Mini-International Neuropsychiatric Interview; Sheehan et al., 1998) within the last 5 years, 2) have had exposure to acupuncture within six months prior to screening, 3) be using sleep aids (e.g., over-the-counter, prescription, naturopathic), 4) be currently pregnant or nursing, 5) have history of substance abuse or meet criteria for current alcohol abuse or dependence, 6) have a Center for Epidemiological Studies Depression Scale (CES-D) score >27 (Hann, Winter, & Jacobsen, 1999), 7) meet DSM-IV criteria for restless legs syndrome, Circadian Rhythm Sleep Disorder, or probable sleep apnea, or 8) have had major surgery within four weeks prior to first acupuncture treatment. A total of 68 participants were screened and found eligible to participate in the parent study. Of these, 12 opted not to complete measures of trauma and could not be included in the current study. The 12 women who opted not to complete the measure of trauma did not differ in demographic characteristics from the 56 who did. The final sample for the current study consisted of 56 women with breast cancer.
Design
Participants in the study were randomized by a study statistician to receive either real (N=34) or sham (N=34) acupuncture. Participants and study staff were blinded to the randomization arm. Participants completed assessments at baseline (prior to any intervention procedures), 3 weeks (mid-treatment), 6 weeks (end of acupuncture or sham treatment), and 10 weeks (follow-up). Because we were interested in associations between childhood trauma exposure and perceived cognitive function in the current study, we only used data from the baseline assessment period from the 56 women who completed the measure of trauma exposure before any intervention procedures. All study procedures were approved by the Stanford University Institutional Review Board.
Measures
Demographics and medical variables
Demographic information and medical history were obtained by self-report measures as well as extraction from medical records
Childhood trauma exposure
Exposure to traumatic events in childhood was assessed with the Traumatic Events Survey (TES; Briere, Woo, McRae, Foltz, & Sitzman, 1997; Elliott & Briere, 1992). The TES is a face-valid measure that is widely accepted and utilized in the field of trauma psychology. See Briere (1997) for a detailed examination on psychological measures used to assess posttraumatic states, including a thorough description of the TES. This survey assesses a range of traumatic experiences in both childhood and adulthood. Of the 30 traumatic experiences assessed, 10 are specifically childhood traumas: experienced natural disaster, had a life-threatening illness, witnessed serious injury to someone else, witnessed someone being killed, witnessed someone being sexually assaulted, was physically assaulted, been sexually assaulted, was victim of a violent crime, was tortured or witnessed torture, was kidnapped or held captive. For each traumatic event, participants are asked how many times the event happened, who perpetrated the event (for interpersonal traumas), and how helpless and horrified the participant felt during the event. We calculated childhood trauma exposure as a count of the number of traumatic events experienced in childhood (range: 0–10) and also dichotomized exposure as experienced any trauma vs. experienced no trauma to account for the relatively low base rate of childhood trauma.
Self-reported cognitive functioning
Cognitive functioning was assessed using items from Version 3 of the Functional Assessment of Cancer Therapy – Cognitive (FACT-Cog; Wagner, 2009). In the current study, at the recommendation of the measure’s publisher, we used a subset of 29 items organized into three subscales: perceived cognitive abilities (18 items; e.g., concentration, verbal and nonverbal memory, verbal fluency), perceived cognitive impairment (7 items; e.g., cognitive deficits in daily activities), and impact of cognitive impairment on quality of life (4 items). Items are rated on a 0–4 scale of severity. Subscale scores were added together to create a sum score, representing overall cognitive functioning; higher scores on each subscale and on the sum scale indicate better cognitive functioning. The FACT-Cog has excellent psychometric characteristics in general, and has been psychometrically validated with a sample of 204 cancer patients undergoing chemotherapy (Wagner, 2009). The sum score created for this study similarly displayed excellent internal consistency (α=0.88).
Cortisol
We assessed cortisol as a marker of HPA axis function and stress response (Clow, Hucklebridge, Stalder, Evans, & Thorn, 2010; Evans et al., 2011). Participants were asked to collect their saliva three times a day (T1: waking, before getting out of bed; T2: 30 minutes after waking; T3: at 9:00 pm) for two days. Immediately after participants collected their saliva, samples were frozen at −20° C. Following completion of two days of saliva collection, the samples were transported to a −70° C freezer. Salivary cortisol was analyzed in duplicates using an enzyme immunoassay (Salimetrics, PA, USA). The minimal level of sensitivity to distinguish from zero cortisol is 0.007 µg/dL and average intra- and inter-assay coefficients of variation are 4.6% and 6% respectively. The following seven variables were considered as mediators: Log Cortisol at T1, Log Cortisol at T2, Log Cortisol at T3, Slope (LogT1–T2), Slope (LogT2–T3), Slope (LogT1–T3), and area under the curve (LogT1,T2,T3).
Mental health covariates
As participants were selected into the parent study on the basis of insomnia scores, we used these scores as control variables in our analyses. Insomnia was assessed using the total score on the Insomnia Severity Index questionnaire (ISI), a well-validated measure of sleep that has been found to be reliable and valid in cancer patients and survivors (Savard, Savard, Simard, & Ivers, 2005). The ISI has acceptable internal consistency (Cronbach’s alpha = 0.74) and moderate item-total correlations with sleep diaries (correlations ranging from 0.35 – 0.38 for the domains of early morning awakenings, sleep onset latency, and wake time after sleep onset; Bastien, Vallieres, & Morin, 2001). Both depression and anxiety are associated with cognitive functioning; accordingly, we controlled for both anxiety and depression in our analyses. Depression was assessed using the Center for Epidemiological Studies – Depression scale (CESD), a commonly-used 20 item measure of depression (Hann et al., 1999). The CESD has been shown to be a valid and reliable measure with cancer patients. Internal consistency is good (Cronbach’s alpha > 0.85) and test-retest correlations range from 0.51 – 0.57. Furthermore, the CESD has been found to correlate well with other tests of depression (Hann et al., 1999). Anxiety was assessed with the State-Trait Anxiety Inventory (STAI) – State subscale, a 20-item measure of anxiety with good internal consistency, reliability, and convergent validity (Spielberger, Sydeman, Owen, & Marsh, 1999).
Statistical Analyses
We examined descriptive statistics for sample demographic characteristics, childhood trauma exposure, and self-reported cognitive functioning. Given that most individuals with exposure to childhood trauma experienced only one traumatic event, we categorized individuals into two categories: those with one or more childhood trauma exposures (trauma) and those without childhood trauma exposure (no trauma). To test associations between trauma exposure and cognitive functioning, we used t-tests to assess mean differences between those with trauma and no trauma in baseline self-reported cognitive functioning, looking both at the FACT-Cog sum score and at the subscale scores. We also examined mean differences between those with trauma and no trauma in mental health covariates, and correlations between cognitive functioning and mental health covariates. Finally, we regressed cognitive function onto childhood trauma exposure while controlling for insomnia, depression, anxiety, and demographic variables such as age and education that have been associated with cognitive functioning in previous literature (e.g., Hayat et al., 2016).
For hypothesis 2, we theorized that childhood trauma exposure affects HPA axis functioning, operationalized as elevated salivary cortisol levels and a dysregulation in diurnal cortisol slope. Under this theoretical framework, although our data are cross-sectional, we assume that childhood trauma exposure precedes change in cortisol and change in cortisol precedes change in cognitive functioning. Assuming this temporal precedence, we explored the mediating role of cortisol patterns using the MacArthur approach for mediator analysis (Kraemer, Kiernan, Essex, & Kupfer, 2008). All cortisol measures were log-transformed before the analysis. Using a two-sample t-test, we first compared individuals with and without trauma in terms of cortisol patterns. Cortisol variables that showed significant group differences, meeting the eligibility criteria for mediators, were then examined in a subsequent linear regression analysis to identify potential mediators. In this second step of mediation analysis, trauma exposure, cortisol variables and the interaction between the two were modeled as predictors of self-reported cognitive functioning. Mediators were centered at their means and trauma exposure was centered at +0.5 and −0.5. In line with the analytical criteria for mediators in the MacArthur approach, a cortisol variable that showed a significant main effect or an interaction effect on cognitive functioning was identified as a potential mediator (Kraemer et al., 2008). Regression coefficients for these analyses were standardized and therefore each predictor’s coefficient can be interpreted as correlation conditional on the other predictors in the model. We used SPSS version 22 to conduct analyses.
Results
Participant characteristics
Of the 332 patients who were assessed for eligibility, 84 were eligible and consented to the study, 68 were randomized, and 12 opted not to respond to the Traumatic Events Survey. The mean age of the final sample of 56 breast cancer patients was 54 years. The modal level of education was 4 year college or university (39.3%, n = 22), modal yearly household income was over $100,000 (62.5%, n=35), and the majority were married (78.6%, n=44) and non-Hispanic White (94.6%, n=53). In subsequent analyses, we dichotomized education into college education or higher (76.8%, n=43) or less than college education (23.2%, n=13). The modal breast cancer stage was Stage II (41.1%, n = 23). See Table 1 for demographic factors for the sample.
Table 1.
Survivor demographics at baseline (n=56).
| Age, M (SD) | 53.6 (9.8) |
|
| |
| Education, n (%) | |
| Graduate training | 21 (37.5%) |
| Standard college/university | 22 (39.3%) |
| Partial college or less | 13 (23.2%) |
|
| |
| Household income, n (%) | |
| <$20,000–$59,999 | 7 (12.5%) |
| $60,000–$99,999 | 13 (23.2%) |
| >$100,000 | 35 (62.5%) |
| Missing | 1 (1.8%) |
|
| |
| Employment status, n (%) | |
| Disability Leave | 1 (1.8%) |
| Retired | 16 (28.6%) |
| Employed | 16 (28.6%) |
| None of the above | 23 (41.1%) |
|
| |
| Marital status, n (%) | |
| Married & living with spouse | 44 (78.6%) |
| Divorced | 6 (10.7%) |
| Widowed | 1 (1.8%) |
| Long-term committed relationship | 3 (5.4%) |
| Single | 2 (3.6%) |
|
| |
| Race, n (%) | |
| White | 53 (94.6%) |
| Asian/Asian American | 2 (3.6%) |
| Other | 1 (1.8%) |
|
| |
| Ethnicity, n (%) | |
| Hispanic or Latino | 2 (3.6%) |
| Not Hispanic or Latino | 54 (96.4%) |
|
| |
| Stage, n (%) | |
| DCIS | 9 (16.1%) |
| I | 11 (19.6%) |
| II | 23 (41.1%) |
| III | 9 (16.1%) |
| IV | 2 (3.6%) |
| Missing | 2 (3.6%) |
|
| |
| Weeks since chemotherapy, M (SD) | 235 (225) |
Childhood trauma exposure and cognitive functioning
In total, 57.1% (n=32) of the sample had experienced at least one traumatic event in childhood; 41.1% (n=23) had experienced only one event, 10.7% (n=6) had experienced two, 3.6% (n=2) had experienced three, and one participant had experienced four events. The most commonly experienced event was witnessing a serious injury (19.6%, n=11), followed by experiencing physical abuse (17.9%, n=10), and experiencing sexual abuse (14.3%, n=8).
The mean self-reported cognitive functioning score at baseline as measured by the FACT-Cog sum score was 74.62 (standard deviation = 20.63). Individuals with childhood trauma exposure had significantly lower FACT-Cog sum scores than those without childhood trauma (t=2.09, p=0.04). Individuals with childhood trauma exposure had significantly lower FACT-Cog subscale scores for perceived cognitive abilities (t=−2.09, p=0.04) and between-group differences approached significance for perceived cognitive impairments (t=−1.87, p=0.06). Individuals with and without trauma did not differ on mental health covariates. However, self-reported cognitive functioning was significantly negatively correlated with insomnia as measured by the Insomnia Severity Index (r=−0.50, p<0.001) and anxiety as measured by the STAI-State subscale (r=−0.45, p<0.001).
Modeling perceived cognitive functioning
To test hypothesis 1, we regressed the FACT-Cog sum score onto the dichotomous variable for childhood trauma exposure, controlling for age, college education or higher (dichotomous), time since last chemotherapy treatment, depression, anxiety, and insomnia. Childhood trauma exposure was significantly associated with self-reported cognitive functioning as measured by the FACT-Cog sum score in this multivariate model (β=−0.22, p=0.04). Anxiety and insomnia were also independently associated with cognitive functioning, while age, education, time since chemotherapy treatment, and depression were not significantly associated with cognitive functioning.
In an exploratory analysis, we tested for moderation of the effect of exposure to traumatic events in childhood by education, anxiety, or insomnia, using interaction terms. None of the interactions between other factors and exposure to traumatic events in childhood were significant.
To test hypothesis 2, we examined the sample statistics of individuals with and without childhood trauma exposure and compared differences in cortisol patterns. Among the seven cortisol-related candidate mediators, the two groups showed statistically significant differences in waking cortisol (Log cortisol T1: d=0.75, p=.010) and in the cortisol slope between morning and evening (SlopeLogT1T3: d=0.68, p=.019). We additionally examined college education, anxiety, depression and insomnia as possible mediators. Among these, only college education showed difference between those with and without trauma (χ2=4.30, p=0.04). As these three candidate mediators (waking cortisol, cortisol slope between morning and evening, college education) met the eligibility criteria for mediators, we proceeded with the next step of mediation analysis.
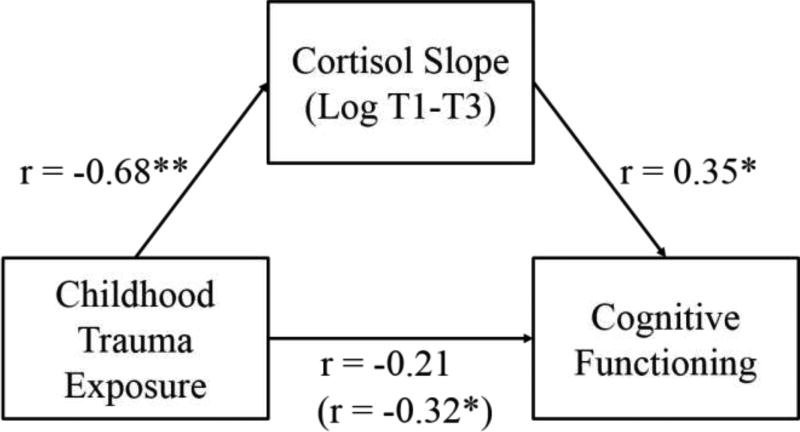
To conduct mediation analysis in line with the MacArthur approach, we used a linear regression model treating self-reported cognitive functioning as the outcome (Kraemer et al., 2008). We tested log-transformed waking cortisol, the diurnal slope between the waking and evening time cortisol, and college education (dichotomous yes/no) as potential mediators of the effect of childhood trauma on cognitive functioning. These models were run independently, in keeping with the MacArthur approach (Kraemer et al., 2008). We also included trauma and the interaction between trauma and the candidate variables in the model as predictors. In this final model, only the cortisol slope between morning (waking) and evening (9:00 pm) was identified as a potential mediator. The estimated correlation between cognitive functioning and the cortisol slope between morning and evening (with the outcome conditional on the other predictors in the model) was significant (partial r=0.35, p=0.02), meaning that the cortisol slope meets the analytical criteria for a mediator assuming that temporal precedence holds.
Discussion
In this present study we found a significant relationship between exposure to childhood trauma and self-reported cognitive functioning in a sample of breast cancer survivors following treatment. In addition to childhood trauma, education, anxiety, and insomnia were also significantly related to cognitive functioning. The relationship between childhood trauma and cognitive functioning remained significant even after controlling for age, education, time since chemotherapy treatment, insomnia, anxiety, and depression. These results suggest that childhood trauma may play an important role in cognitive functioning experienced after cancer treatments and should be taken into consideration in developing interventions and support services for this population.
The present study is novel in its examination of cortisol as an explanatory mechanism for the effects of childhood trauma on cognitive functioning in breast cancer survivors. Results of our study suggest that those who had experienced trauma had significantly higher morning cortisol levels and steeper cortisol slopes than those who had not experienced trauma. In regression analyses predicting cognitive scores, steeper diurnal cortisol slopes found in the trauma group were significantly related to poorer self-reported cognitive functioning. As such, our data suggest that traumatic experiences during childhood may result in changes in cortisol response that continue into adulthood, which may in turn be associated with more significant perceived cognitive difficulties.
Our findings of a significant association between poor self-reported cognitive functioning and stress in early life are consistent with previous literature suggesting that trauma experienced during childhood can be detrimental to neurological development and, thereby, impact cognitive functioning (Lupien et al., 2009), even in non-clinical samples of healthy adults (Majer, Nater, Lin, Capuron, & Reeves, 2010). Interestingly, in this sample of breast cancer survivors, the relationship between trauma and cognitive functioning was mediated by a steeper diurnal cortisol slope. Such findings are in contrast to previous research studies that implicated flatter cortisol slopes in populations exposed to trauma (Heim, Ehlert, et al., 2000; Yehuda, 1997). This finding is consonant, however, with research showing that a steeper cortisol slope indicates a more pronounced stress response in the context of medical illness (Edwards et al., 2003; Ferguson, 2008). The inconsistent link between cortisol slope, stress, and trauma has been the subject of scientific discussion for decades (Abercrombie et al., 2004; Heim, Ehlert, et al., 2000; Lupien et al., 2009; Yehuda, 1997). Part of the difficulty in establishing a consistent base of evidence regarding cortisol can be attributed to lack of consistency in measurement methods used (e.g., blood, saliva, or urine), interpretation of data, and implications of findings (Levine, Zagoory-Sharon, Feldman, Lewis, & Weller, 2007; Pollard, 1995). Conceivably, cognitive functioning difficulties may not be attributable directly to the steepness or flatness of the cortisol slope, but instead to how the overall system responds to a particular endogenous or exogenous challenge. The findings of the current study on cortisol in the context of cancer should be replicated to better assess the impact of stress response on cognitive function among cancer survivors exposed to childhood trauma.
Though the lack of longitudinal data makes causal relationships impossible to determine, the relationship between dysregulated diurnal cortisol slopes and impaired cognitive functioning following cancer diagnosis and treatment may be the result of a preexisting vulnerability due to exposure to childhood trauma (Lupien et al., 2009; Miller, Chen, & Zhou, 2007). In that regard, trauma should be considered, among other factors, in treatments aiming to address difficulties with cognitive functioning in cancer population. Future research should assess mechanisms leading from trauma exposure to cognitive functioning difficulties and possible intervention approaches. For example, interventions may place greater emphasis on enhancing cancer survivors’ coping strategies (specifically using cognitive behavioral techniques). Such interventions have been shown not only to effect psychological and emotional well-being in breast cancer patients, but also to alter physiological functioning, including HPA functioning (Luecken & Compas, 2002). Such interventions may also be beneficial for cancer survivors who have experienced childhood trauma.
Limitations
There are several limitations to the study. First, the structure of the original study from which these data were drawn limits both generalizability and power. Our relatively small sample size of breast cancer survivors was selected on the basis of insomnia symptoms and may be different from breast cancer survivors in general. While we are unable to state, given our data, whether these results might be replicated in a population without any sleep problems, current research suggests that sleep problems are ubiquitous in this population, with over two-thirds of breast cancer survivors suffering from chronic sleep disturbance (Palesh et al., 2013; Palesh et al., 2012). Our small sample size also limits our power to detect statistically significant associations between exposure to childhood trauma and other psychological outcomes, such as depression and anxiety. We were unable in this dataset to measure the impact of particular types and dosages of chemotherapy on cognitive functioning. Participants also self-reported both their trauma history and their cognitive functioning. Recall bias may impact self-report of trauma experiences, particularly self-report of childhood trauma by adults. Our measure of cognitive functioning was derived from a subset of items from the FACT-Cog, and so may not match established norms and is best treated as a general indicator of cognitive functioning. Standardized and objective measures of cognitive functioning would allow for a more nuanced assessment of various domains of cognition, and should be implemented in other studies in this area.
Although is impossible to draw causal inferences in this research, our hypotheses are based on plausible findings from previous research studies in non-cancer populations. The results of the current study should be cautiously interpreted as initial support for our hypothesis that childhood trauma affects cortisol secretion, and that this physiological dysregulation is associated with lower cognitive functioning. Future research should use temporal precedence and repeated measures of cortisol and cognitive functioning to more accurately determine longitudinal relationships. Furthermore, researchers should replicate current findings with cortisol data collected throughout a 24-hour time period, as our cortisol samples were only collected during the daytime. Cortisol can behave differently throughout the day and night, and thus collecting samples throughout a 24-hour day on multiple occasions will strengthen the accuracy of cortisol test data.
Overall, the current study indicates that childhood trauma exposure is uniquely associated with self-reported cognitive functioning among breast cancer survivors. Future studies should further investigate the complex relationship between trauma and HPA axis dysregulation and explore interventions that could address exposure to childhood trauma in this patient population. This study could serve as a stepping stone for future observational research and treatment studies to consider exposure to childhood trauma as a potential marker for poor cancer-related outcomes among breast cancer survivors.
Figure 1.
Mediation of the relationship between childhood trauma exposure and cognitive functioning by cortisol slope (n=56).
Note: All standardized associations are presented as correlations. * statistically significant at the 0.05 level; ** statistically significant at the 0.01 level.
Table 2.
Sample means (and standard deviations) of study measures by childhood trauma exposure (n=56).
| Variable | Total (n=56) |
Trauma (n=32) |
No Trauma (n=24) |
t-test | Cohen's d | |||
|---|---|---|---|---|---|---|---|---|
| Cognitive functioning (FACT-Cog) | 74.13 | (20.57) | 68.80 | (21.90) | 80.79 | (16.93) | p=0.03 | −0.60 |
| Insomnia (ISI) | 13.70 | (3.83) | 13.72 | (3.93) | 13.67 | (3.77) | p=0.96 | 0.01 |
| Depression (CES-D) | 8.27 | (7.51) | 7.91 | (7.86) | 8.75 | (7.15) | p=0.68 | −0.11 |
| Anxiety (STAI) | 30.32 | (8.91) | 30.84 | (10.08) | 29.63 | (7.20) | p=0.62 | 0.14 |
| Log Cortisol T1 | −1.41 | (0.55) | −1.24 | (0.53) | −1.63 | (0.49) | p=0.01 | 0.75 |
| Log Cortisol T2 | −1.10 | (0.59) | −1.01 | (0.46) | −1.22 | (0.73) | p=0.26 | 0.34 |
| Log Cortisol T3 | −3.48 | (0.59) | −3.55 | (0.60) | −3.39 | (0.58) | p=0.33 | −0.28 |
| Slope (LogT1–T2) | 0.57 | (1.03) | 0.44 | (0.78) | 0.74 | (1.29) | p=0.35 | −0.28 |
| Slope (LogT2–T3) | −0.18 | (0.05) | −0.18 | (0.05) | −0.17 | (0.05) | p=0.25 | −0.33 |
| Slope (LogT1–T3) | −0.14 | (0.05) | −0.16 | (0.05) | −0.13 | (0.03) | p=0.02 | −0.68 |
| AUC (LogT1,T2,T3) | −32.11 | (6.78) | −32.38 | (7.04) | −31.77 | (6.62) | p=0.76 | −0.09 |
Abbreviations: THQ = Trauma History Questionnaire; FACT-Cog = Functional Assessment of Cancer Therapy – Cognitive; ISI = Insomnia Severity Index; CESD-D = Center for Epidemiologic Studies – Depression; STAI = State-Trait Anxiety Inventory; T1 = waking cortisol before getting out of bed; T2 = 30 minutes after awakening; T3 = 9:00 pm; AUC = area under the curve.
Table 3.
Simultaneous linear regression predicting variance in cognitive functioning (n=56).
| Variable | B | SE B |
|---|---|---|
| Age | 0.28 | 0.24 |
| Education | 5.61 | 5.41 |
| Weeks since chemotherapy | >0.01 | 0.01 |
| Depression | 0.16 | 0.32 |
| Anxiety | −0.87** | 0.26 |
| Insomnia | −2.08** | 0.60 |
| Childhood trauma exposure | −10.16* | 4.28 |
|
| ||
| R2 = 0.47** | ||
Abbreviations: SE = Standard error
Note:
statistically significant at the 0.05 level;
statistically significant at the 0.01 level.
Table 4.
Simultaneous linear regression testing mediation of the relationship between childhood trauma exposure and cognitive functioning by cortisol slope (n=56).
| Predictors of Cognition | Association (r) | p-value |
|---|---|---|
| Trauma | −0.210 | 0.17 |
| Cortisol Slope (LogT1–T3) | 0.352 | 0.02 |
| Trauma × Slope (LogT1–T3) | −0.079 | 0.61 |
Abbreviations: T1= waking cortisol before getting out of bed, T3= cortisol collected at 9pm.
Acknowledgments
Conflict of interest: This study was supported by National Cancer Institute grants K07 CA190529, UG1 CA189961, R01 CA126968, R01CA181659 and 5R21CA185678.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29(8):1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Adams M, Packer MM, Palesh O, Kesler S. Cognitive Status of Newly Diagnosed Patients with Breast Cancer at Pre-Surgery Baseline; Paper presented at the The Diagnosis, Mechanisms, & Management of Cancer Treatment-Induced Neurotoxicity's: Neuropathy, Fatigue and Cognitive Impairment Conference, MD Anderson Cancer Center.2016. [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti C, Root JC, Ahles TA, McEwen BS, Compas BE. Cancer, coping, and cognition: a model for the role of stress reactivity in cancer-related cognitive decline. Psycho oncology. 2015;24(6):617–623. doi: 10.1002/pon.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M. The effects of stress on memory and the hippocampus throughout the life cycle: implications for childhood development and aging. Dev Psychopathic. 1998;10(4):871–885. doi: 10.1017/s0954579498001916. [DOI] [PubMed] [Google Scholar]
- Briere J. Psychological assessment of adult posttraumatic states. Washington, DC: American Psychological Association; 1997. [Google Scholar]
- Briere J, Woo R, McRae B, Foltz J, Sitzman R. Lifetime victimization history, demographics, and clinical status in female psychiatric emergency room patients. J Nerve Mint Dies. 1997;185(2):95–101. doi: 10.1097/00005053-199702000-00005. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sic STKE. 2004;2004(225):re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Christie LA, Chary MM, Pariah VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18(7):1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35(1):97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Edwards S, Hucklebridge F, Clow A, Evans P. Components of the diurnal cortisol cycle in relation to upper respiratory symptoms and perceived stress. Psychosom Med. 2003;65(2):320–327. doi: 10.1097/01.psy.0000033123.70631.8e. [DOI] [PubMed] [Google Scholar]
- Elliott DM, Briere J. Sexual abuse trauma among professional women: validating the Trauma Symptom Checklist-40 (TSC-40) Child abuse & neglect. 1992;16(3):391–398. doi: 10.1016/0145-2134(92)90048-v. [DOI] [PubMed] [Google Scholar]
- Evans PD, Fredhoi C, Loveday C, Hucklebridge F, Aitchison E, Forte D, Clow A. The diurnal cortisol cycle and cognitive performance in the healthy old. Int J Psychophysiol. 2011;79(3):371–377. doi: 10.1016/j.ijpsycho.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Ferguson E. Health anxiety moderates the daytime cortisol slope. J Psychosom Res. 2008;64(5):487–494. doi: 10.1016/j.jpsychores.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Fitch MI, Armstrong J, Tsang S. Patients' experiences with cognitive changes after chemotherapy. Can Oncol Nurs J. 2008;18(4):180–192. doi: 10.5737/1181912x184180185. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46(5):437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Hayat SA, Luben R, Dalzell N, Moore S, Anuj S, Matthews FE, Khaw KT. Cross Sectional Associations between Socio-Demographic Factors and Cognitive Performance in an Older British Population: The European Investigation of Cancer in Norfolk (EPIC-Norfolk) Study. PloS one. 2016;11(12):e0166779. doi: 10.1371/journal.pone.0166779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG, Morrow GR. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.5826. JCO2016685856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008;27(2S):S101–108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiol Behav. 2007;90(1):43–53. doi: 10.1016/j.physbeh.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Compas BE. Stress, coping, and immune function in breast cancer. Ann Behav Med. 2002;24(4):336–344. doi: 10.1207/S15324796ABM2404_10. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Majer M, Nater UM, Lin JM, Capuron L, Reeves WC. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurol. 2010;10:61. doi: 10.1186/1471-2377-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Takayama T, Tashiro M, Nakamura Y, Ohashi Y, Shimozuma K. Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients--evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer. 2005;12(4):279–287. doi: 10.2325/jbcs.12.279. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sic. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology. 2005;30(6):568–576. doi: 10.1016/j.psyneuen.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Palesh O. Fatigue, Depression and Cortisol in Cancer, Older Adults and Caregivers; Paper presented at the International Symposium Supportive Care in Cancer; Italy, Rome. 2009. [Google Scholar]
- Palesh O, Aldridge-Gerry A, Ulusakarya A, Ortiz-Tudela E, Capuron L, Innominato PF. Sleep disruption in breast cancer patients and survivors. J Natl Compr Canc Netw. 2013;11(12):1523–1530. doi: 10.6004/jnccn.2013.0179. [DOI] [PubMed] [Google Scholar]
- Palesh O, Peppone L, Innominato PF, Janelsins M, Jeong M, Sprod L, Mustian K. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sic Sleep. 2012;4:151–162. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TM. Use of cortisol as a stress marker: Practical and theoretical problems. American Journal of Human Biology. 1995;7(2):265–274. doi: 10.1002/ajhb.1310070217. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psycho oncology. 2005;14(6):429–441. doi: 10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Absence of diurnal cortisol variation predicts early breast cancer mortality. Psychoneuroendocrinology. 1998;23(S51) [Google Scholar]
- Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30(2):162–178. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Spielberger CD, Sydeman SJ, Owen AE, Marsh BJ. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI) In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment. 2. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 1999. pp. 993–1021. [Google Scholar]
- Stanton AL, Morra ME, Diefenbach MA, Miller SM, Slevin Perocchia R, Raich PC, Marcus AC. Responding to a significant recruitment challenge within three nationwide psychoeducational trials for cancer patients. J Cancer Surviv. 2013;7(3):392–403. doi: 10.1007/s11764-013-0282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Ah D, Habermann B, Carpenter JS, Schneider BL. Impact of perceived cognitive impairment in breast cancer survivors. Eur J Oncol Nurs. 2013;17(2):236–241. doi: 10.1016/j.ejon.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Wagner L, Sweet J, Butt Z, Lai J, Cella D. Measuring Patient Self-Reported Cognitive Function: Development of the Functional Assessment of Cancer Therapy-Cognitive Function Instrument. Journal of Supportive Oncology. 2009;7:32–39. [Google Scholar]
- Weniger G, Lange C, Sachsse U, Irle E. Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. J Psychiatry Neurosci. 2009;34(5):383–388. [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Stress and glucocorticoid. Science. 1997;275(5306):1662–1663. doi: 10.1126/science.275.5306.1662. [DOI] [PubMed] [Google Scholar]