Abstract
Background
A Grm2 cys407* stop codon mutation, which results in a loss of the metabotropic glutamate 2 (mGlu2) receptor protein, was identified as being associated with high alcohol drinking by alcohol preferring (P) rats. The objectives of the current study were to characterize the effects of reduced levels of mGlu2 receptors on glutamate transmission and alcohol drinking.
Methods
Quantitative no-net-flux microdialysis was used to test the hypothesis that basal extracellular glutamate levels in the prelimbic (PL) cortex and nucleus accumbens shell (NACsh) will be higher in P than Wistar rats. A lentiviral-delivered short-hairpin RNA (shRNA)-mediated knockdown was used to test the hypothesis that reduced levels of mGlu2 receptors within the PL cortex will increase voluntary alcohol drinking by Wistar rats. A linear regression analysis was used to test the hypothesis that there will be a significant correlation between the Grm2 cys407* mutation and level of alcohol intake.
Results
Extracellular glutamate concentrations within the PL cortex (3.6 ± 0.6 vs 6.4 ± 0.6 μM) and NACsh (3.2 ± 0.4 vs 6.6 ± 0.6 μM) were significantly lower in female P than female Wistar rats. Western blot detected the presence of mGlu2 receptors in these regions of female Wistar rats, but not female P rats. Micro-infusion of shRNAs into the PL cortex significantly reduced local mGlu2 receptor levels (by 40%), but did not alter voluntary alcohol drinking in male Wistar rats. In addition, there was no significant correlation between the Grm2 mutation and alcohol intake in 36 rodent lines (r = 0.29, p > 0.05).
Conclusions
Collectively, these results suggest a lack of association between the loss of mGlu2 receptors and glutamate transmission in the NACsh and PL cortex of female P rats, and between the level of mGlu2 receptors in the PL cortex and alcohol drinking of male Wistar rats.
Keywords: Alcohol-preferring P rat, glutamate, mGlu2 receptor, prelimbic cortex
Introduction
Brain glutamate transmission has been implicated in the neurobiology of alcohol use disorder (AUD; Gass & Olive 2008, Koob & Volkow 2010). Acute and repeated alcohol exposure can alter extracellular glutamate levels within the mesocorticolimbic (MCL) system (Ding et al 2012, Kapasova & Szumlinski 2008, Moghaddam & Bolinao 1994, Selim & Bradberry 1996). Chronic alcohol drinking and dependence induce a hyperglutamatergic state characterized by increased extracellular glutamate levels within the MCL system in human alcoholics and rodents (Ding et al 2013, Griffin 3rd et al 2014, Hermann et al 2012). In the wake of these findings, efforts have been invested in the development of pharmacotherapy targeting glutamate receptors in both preclinical and clinical research (Olive 2009, Olive et al 2012).
A recent genomic sequencing study identified a Grm2 cys407* stop codon mutation in the alcohol preferring P rat, which results in a loss of functional metabotropic glutamate 2 (mGlu2) receptors (Zhou et al 2013), suggesting a possible association between the loss of mGlu2 receptors and a predisposition for high alcohol preference and consumption in the P rat. Such a notion was further supported by a follow-up study showing that Grm2 knockout mice exhibited higher voluntary alcohol consumption of 15–17% (but not of 3–13%) ethanol compared to wild-type mice (Zhou et al 2013). In addition, alcohol dependence is associated with reduced Grm2 mRNA expression in the infralimbic cortex and rescue of this deficit with virus-mediated overexpression attenuated heightened alcohol seeking in dependent male Wistar rats (Meinhardt et al 2013). Collectively, these findings suggest that the Grm2/mGlu2 receptor may play a significant role in alcohol drinking and seeking.
The mGlu2 receptor is mainly a presynaptic auto-receptor that provides feedback inhibitory regulation of synaptic glutamate release (Bell et al 2016, Moussawi & Kalivas 2010). Loss of these receptors would hypothetically result in increased glutamate release, leading to increased basal extracellular glutamate levels. Since alcohol drinking and dependence are associated with increased glutamate levels within the brain (Ding et al 2013, Griffin 3rd et al 2014, Hermann et al 2012), it is possible that high alcohol drinking in the P rat compared to the Wistar rat may be due to greater basal extracellular glutamate levels in the P rat than the Wistar rat due to the loss of mGlu2 receptors. Such possible neurochemical differences may be manifested most likely in brain systems strongly implicated in the development of AUD, e.g., the MCL system (Gonzales et al 2004, Koob et al 1998). Therefore, the current study measured basal extracellular glutamate levels in Wistar and P rats using a quantitative microdialysis procedure in key regions of the MCL system, i.e., the prelimbic (PL) cortex and nucleus accumbens shell (NACsh).
Altered glutamate transmission within the medial prefrontal cortex (mPFC) has been linked to alcohol dependence in alcoholics and rodents (Hermann et al 2012). The PL cortex of the mPFC projects extensively to and regulates activities of various key MCL regions, including the NAC and ventral tegmental area, among others (Heidbreder & Groenewegen 2003, Vertes 2004). Accumulating evidence suggests an important role of the PL cortex in the development of AUD, as pharmacological manipulations of the PL cortex can regulate alcohol self-administration and drinking (Faccidomo et al 2016, Samson & Chappell 2001, Seif et al 2013, Warnault et al 2016). Therefore, it is possible that the PL cortex may be an anatomical site underlying a possible association between the loss of mGlu2 receptors and elevated alcohol drinking. The current study investigated this possibility by examining the effects of genetic manipulation of mGlu2 receptor expression, using lentiviral-mediated, short hairpin RNA (shRNA)-induced RNA interference, on voluntary alcohol consumption by Wistar rats.
A recent study by Wood and colleagues reported that the Grm2 cys407* mutation was prevalent in some strains of Wistar rats of Hannover origin and rats selectively bred for particular behaviors, with various frequencies of the mutant allele (Wood et al 2017). If there is an association between the Grm2 genetic variations and level of alcohol drinking, then rats carrying this stop codon mutation should exhibit higher alcohol consumption compared to rats without this mutation. Since the levels of ethanol intake were not provided in the Wood et al study, the current study searched the literature for levels of ethanol intake for these rodents and examined the relation between the Grm2 cys407* allele frequency and alcohol drinking using a linear regression procedure.
The hypotheses tested in the current study were a) basal extracellular glutamate levels within key MCL regions will be greater in P than Wistar rats; b) reduction of mGlu2 receptors within the PL cortex will increase voluntary alcohol drinking by Wistar rats; and c) there will be a significant correlation between the Grm2 cys407* mutant allele frequency and alcohol intake levels.
Materials and Methods
Animals
Adult P (Indiana University) and Wistar rats (Harlan Inc. Indianapolis, Indiana) were housed in pairs in temperature- and humidity-controlled rooms maintained on a regular 12-hr light-dark cycle (light on at 7:00 a.m.) with food and water available ad libitum. Female rats were used in the microdialysis study as previously described (Ding et al 2012, 2013, Engleman et al 2011). The estrous cycle was not monitored. Microdialysis was conducted in one side of the brain on one day and the other side on the next day in a counterbalanced manner. Therefore, any effect of a given phase of the estrous cycle should be distributed across cohorts. Adult male HAD1&2 and LAD1&2 rats were genotyped for Grm2 alleles. Protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. All experiments were conducted in accordance with principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council 2011).
Quantitative no-net-flux (NNF) microdialysis and histology
Alcohol naïve female P and Wistar rats (n=5–8/group) were compared in the microdialysis study. Alcohol non-preferring (NP) rats were not used because these rats have a blood-clotting deficiency that results in excessive bleeding following surgeries. Therefore, Wistar rats were used for comparisons with P rats when stereotaxic surgeries are required (Rodd et al 2004, Engleman et al 2009, Katner et al 2011).
Rats were stereotaxically implanted with dual guide cannula (18-gauge; Plastics One, Inc., Roanoke, VA, USA), one aimed at the PL cortex (AP +3.0 mm, ML +0.7 mm, DV −5.0 mm) and the other at the contralateral NACsh (AP +1.7 mm, ML +2.3 mm, DV −8.5 mm; 10° angle away from the vertical line) according to the brain atlas of Paxinos & Watson (Paxinos & Watson 1998). The selection of these two regions was based on evidence indicating dense projections from the PL cortex to the NACsh (Vertes 2004) and their implication in the AUD (Koob & Volkow 2010). Rats were individually housed following surgery, and were allowed to recover for at least 5 days. Microdialysis probes (active membrane length 1.0 mm, inner diameter 200 μm, molecular weight cut-off: 13,000, Spectrum Laboratories, Inc, Rancho Dominguez, CA, USA) were constructed and inserted into the target regions 16–18 hr prior to the sample collection.
Microdialysis was conducted over two consecutive days with one side at a time, counter-balanced, as previously described (Ding et al 2012, 2013). Briefly, rats were connected to a Harvard pump with PE20 tubing (inner diameter 0.38 mm, Becton Dickinson & Co., MD, USA). Microdialysis started with a 90-min wash-out period during which artificial cerebrospinal fluid (aCSF: 140.0 mM NaCl, 3.0 mM KCl, 1.2 mM CaCl2, 2.0 mM Na2HPO4·7H2O, 1.0 mM MgCl2, pH 7.2–7.4) was perfused through the probes, followed by collection of 4–5 baseline samples. Then, four different concentrations of glutamate (1, 5, 10 or 20 μM in aCSF) were perfused through the probes in a random order. Each concentration was perfused for 25 min. After the perfusion of the last glutamate concentration, the perfusion medium was changed to aCSF for an additional 20 min. Samples were collected every 5 min at a flow rate of 2.0 μl/min. Samples were frozen at −70° C until glutamate analysis.
At the end of microdialysis, rats were euthanized with an overdose of CO2 inhalation, and 1% bromophenol blue was perfused through probes. Brains were quickly removed and frozen immediately on dry ice and stored at −70° C. Sections (40 μm) were sliced on a cryostat microtome and stained with cresyl violet for verification of probe placements.
Glutamate analysis
Dialysate glutamate was analyzed with a reversed-phase high performance liquid chromatography system with electrochemical detection, as previously described (Ding et al 2012, 2013, 2016). Briefly, precolumn derivatization with o-phthalaldehyde (Pickering Laboratory, Inc, CA, USA) was performed using an ESA Model 542 auto-sampler. The mobile phase consisted of 25% methanol (v/v), 100 mM Na2HPO4·7H2O, pH 6.75 (Sigma, MO, USA) and was delivered by an ESA 582 solvent delivery system. Dialysis samples were injected onto a reversed-phase column (BDS Hypersil C18 Pioneer, 150 × 2 mm, Thermo Fisher Scientific, IL, USA) and glutamate was separated and detected with a BAS LC-4C amperometric detector with the oxidation potential set at +550 mV and the sensitivity set at 0.2 μA. The output from the detector was sent to a ChromPerfect chromatography data analysis system (Justice Laboratory Software, Denville, NJ, USA). The concentration of glutamate was quantified by comparing the area under the peak with an external standard curve.
Generation and verification of lentiviral vectors
The siDesign Center program (Thermo Fisher Scientific, CO, USA) was used to identify three 19-nucleotide small interfering RNAs targeting the rat Grm2 transcript (NCBI accession number NM_001105711). DNA oligonucleotides encoding shRNAs containing the 19-nucleotide targeting sequences were synthesized and cloned into the lentiviral vector pLL3.7 as previously described (Lasek et al 2007). To validate the effectiveness of the shRNAs, lentiviral plasmids were co-transfected with a plasmid encoding the Grm2 cDNA (graciously provided by Dr. Wolfgang Sommer, Central Institute of Mental Health, Mannheim, Germany) into Neuro-2a cells and Grm2 mRNA expression was measured using quantitative real-time PCR (qPCR) two days after transfection. The two most effective shRNAs, i.e., shGrm2-972 and shGrm2-2344, contained the targeting sequences 5′-GCAGAAACCCAGTGCCCGT-3′ and 5′-GTGGTGACATTGCGCTGTA-3′, respectively. Lentiviruses were produced from these constructs in 293FT cells by co-transfection with packaging plasmids. Control (scrambled) lentivirus was the same as we have used before and is not predicted to target any gene in the rat genome (Lasek et al 2007). Viral titers were obtained using the p24 antigen ELISA kit (Zeptometrix, NY, USA) and ranged from 2 × 107 to 3 × 107 pg/ml.
Microinjection of lentiviral vectors
Surgery followed a similar procedure previously described with an adaptation of the cannulation procedure (Lasek & Azouaou 2012). Specifically, the infusion cannula system in the study of Lasek & Azouaou study (2012) consisted of a 33-gauge hypodermic tube inserted into a 26-gauge tube; the system was then attached to the insertion tool and was lowered into the target brain region during surgery. This procedure could be readily adapted to our stereotaxic instruments. Briefly, a 26-gauge guide cannula was inserted, aimed 1 mm above the target region and was secured with dental cement on the surface of skull. Then, a 33-gauge hypodermic tube was lowered into the target region through the guide cannula for virus infusion. The guide cannula was removed following the completion of infusion. This procedure has been successfully implemented in our previous studies involving microinjection of lentiviral vectors into discrete brain regions (Franklin et al 2015a, b). In the present study, alcohol naïve adult male Wistar rats were implanted with dual guide cannulae (26-gauge; Plastics One, Inc., Roanoke, VA, USA) aimed 1 mm above the contralateral PL cortex (AP +3.0 mm, ML +2.0 mm, DV −5.3 mm, with a 20° angle away from the vertical line). Stainless steel hypodermic tubes (33-gauge; Plastics One, Inc., Roanoke, VA, USA), connected to 25 μl Hamilton syringes with PE-20 tubing, were then inserted through the guide cannulas into the PL cortex. The syringes were placed in a micro-infusion pump for controlled delivery of lentiviral vectors. The vector solution was infused for 1.5 μl/side at a rate of 0.2 μl/min. The 33-gauge tubes were left in place for another 10 min before being slowly removed. The guide cannulae were then removed and the scalp were closed and stapled with stainless steel wound clips. The rats were randomly assigned to one of three groups (the scrambled, shGrm2-972, and shGrm2-2344; n=9–10/group). Following the delivery of the vectors, rats were quarantined for 72 hr followed by general housing for 6 weeks before alcohol drinking was initiated. Previous studies suggested that significant effects typically occurred 5–6 weeks after the shRNA infusion (Franklin et al 2015a, b).
Voluntary ethanol consumption
Following the quarantine, male Wistar rats were transferred to metal hanging cages and were individually housed throughout the experiment. After one week of acclimation, rats received 24-hr continuous access to ethanol with a three-bottle choice paradigm (10% and 15% ethanol versus water) for 5 weeks followed by 24-hr intermittent access on Mondays, Wednesdays and Fridays for 3 weeks. The ethanol solution was made by mixing 95% ethanol (McCormick Distilling, Weston, MO, USA) with water to the final concentrations. The intermittent access is a validated procedure to achieve high alcohol consumption in outbred rats (Carnicella et al 2014, Simms et al 2008). The positions of ethanol and water bottles were randomly alternated every week, and fluid intake was recorded to the nearest 0.1 g by weighing bottles three times a week, at the same time body weights were taken. Ethanol intake was converted to grams of ethanol per kilogram of body weight (grams per kilogram per day). Following the last drinking session, rats were euthanized by CO2 overdose; brains were quickly removed and immediately frozen at −70° C until Western blot analysis.
Western blot
Basal levels of mGlu2 receptors were examined within the PL cortex and NACsh of alcohol-naïve female Wistar and P rats (n=3/group). In addition, mGlu2 receptors were determined in the PL cortex of male Wistar rats from the viral vector experiment. Brain tissue was micro-punched from the target regions as previously described (Ding et al 2013, McBride et al 2009). Tissue was transferred to a RIPA protein extraction buffer (Thermo Fisher Scientific, IL, USA) containing protease inhibitors (Roche Diagnostics, IN, USA), homogenized and centrifuged at 12,000 rpm at 4°C for 20 min. The supernatant was separated and protein concentrations were measured using the Pierce BCA assay. Western blot for the mGlu2 receptor and GAPDH was conducted as previously described (Ding et al 2013). Briefly, approximately 10 μg of protein were loaded and separated on Bolt 4–12% Bis-Tris mini gels (Thermo Fisher Scientific, IL, USA), followed by transfer onto polyvinylidene difluoride membranes (Merck Millipore Ltd., CO, USA). Blots were probed with mouse anti-mGlu2 receptor (ab15672, 1:1000, Abcam, MA, USA), or mouse anti-GAPDH (G8795, 1:10,000, Sigma-Aldrich, MO) antibody. The blots were then probed with a goat anti-mouse IRDye® 800CW secondary antibody (92532210, 1:15,000, LI-COR®, Lincoln, NE, USA) and detected with an ODYSSEY® CLx (LI-COR®, Lincoln, NE, USA) fluorescent imaging system. The signal was quantified using Image Studio™ software (LI-COR®, Lincoln, NE, USA).
Genotyping of HAD1/2 and LAD1/2 rats
Blood samples were genotyped at the Alcohol Research Center at Indiana University as previously described (Hendershot et al 2009). Briefly, genomic DNA was isolated with the “HotSHOT” method followed by a multiplexed, end-point, allelic discrimination assay using TaqMan probes (Applied BioSystems, Foster City, CA). Each assay mix contained two different TaqMan probes, labeled with VIC or FAM fluorescent reporter dye, which bind preferentially to one of the alleles. Each reaction contained 5 μl of 2× TaqMan Universal PCR Mastermix, No AmpErase UNG, 3.75 μl of water, 0.25μl of 40× Assay Mix, and 1μl of DNA sample. Eight or eleven control samples were included in each 96-well plate: 2 no template controls, 2 or 3 heterozygous samples, and 2 or 3 of each of the homozygous samples. After PCR, the PCR products were analyzed in an ABI PRISM® 7300 Sequence Detection System (SDS) instrument. The raw data were converted with SDS Software 1.3.1 for plots of the allelic discrimination.
Statistical analysis
NNF data were analyzed with multiple linear regressions, as previously described (Ding et al 2012, 2013). [Gluin] and [Gluout] were defined as glutamate concentrations perfused and obtained from dialysates, respectively. [Gluin-Gluout], defined as the net gain or loss of glutamate, was plotted on the ‘y’ axis with [Gluin] on the ‘x’ axis. The slope (the extraction fraction, Ed) and the x-intercept (the NNF point as the extracellular glutamate concentration) were determined by multiple linear regressions modeling and analyzed with ANOVA using the SAS System for Windows, version 8.02. Alcohol drinking data were analyzed with a time × treatment ANOVA. Western blot data were analyzed with a one-way ANOVA or student t-tests. Linear regression was used to analyze the correlation between Grm2 mutant allele frequency and alcohol intake. Post-hoc tukey’s b analysis was conducted where appropriate.
Results
Basal extracellular glutamate levels and mGlu2 receptors within the PL cortex and NACsh
In the PL cortex, extracellular glutamate concentrations (no-net-flux points) were 6.4 ± 0.6 μM in female Wistar rats and 3.6 ± 0.6 μM in female P rats. Wistar rats had significantly higher basal glutamate levels than P rats (F1, 199 = 3.94, p < 0.05; Fig. 1A&1B). The extraction fractions (Eds, slopes of the regression lines) were 32 ± 5% in Wistar rats, and 42 ± 5% in P rats (Fig. 1A&1B). There was no significant difference in the Eds between groups (F1, 198 = 1.91, p = 0.16).
Figure 1.
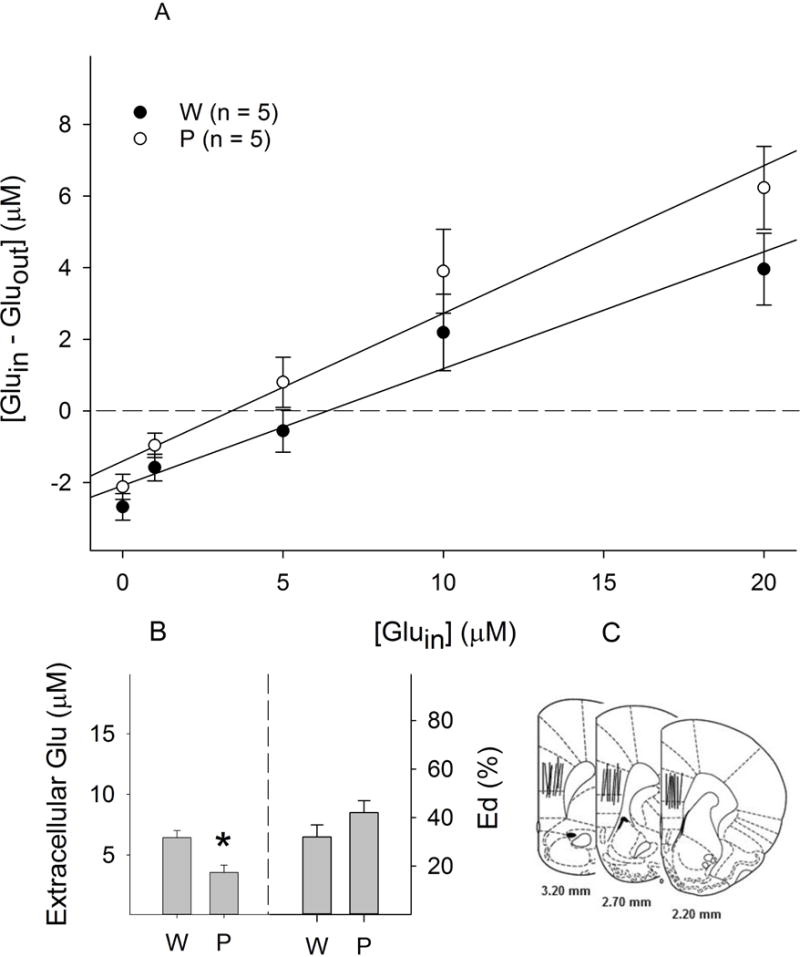
Basal extracellular glutamate concentrations within the prelimbic cortex of female Wistar (W) and P rats (n = 5/group). A: Linear regression plots of glutamate for Wistar and P rats; B: Extracellular glutamate concentrations (the points of no-net-flux, left panel) and clearance (Ed values; the slope of the plots, right panel). * p < 0.05, significantly different from the ‘Wistar’ group; C: Representative placements of microdialysis probes within the prelimbic cortex. For illustration purpose, all placements were shown in one side of the brain.
In the NACsh, extracellular glutamate concentrations were 6.6 ± 0.6 μM for Wistar rats, and 3.2 ± 0.4 μM for P rats, with significantly higher concentrations in Wistar than P rats (F1, 222 = 14.6, p < 0.001; Fig. 2A & 2B). On the other hand, the Eds were significantly greater in P rats than Wistar rats (45 ± 3% vs 28 ± 3%, respectively; F1, 221 = 15.3, p < 0.001; Fig. 2A & 2B).
Figure 2.
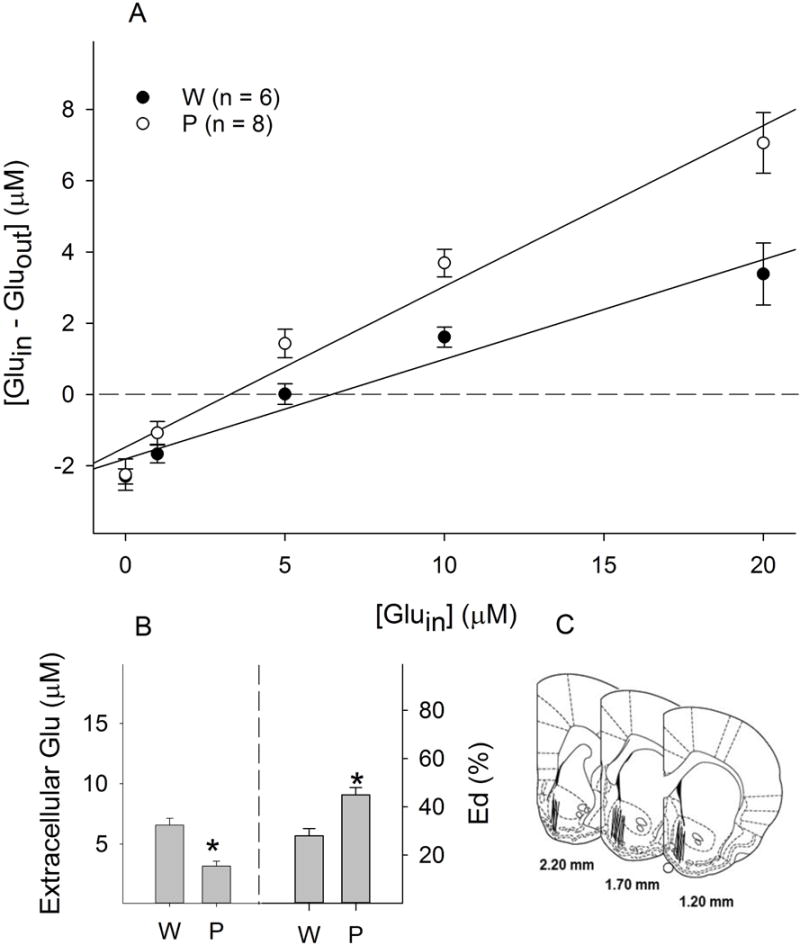
Basal extracellular glutamate concentrations within the nucleus accumbens shell of female Wistar (W) and P rats (n = 6–8/group). A: Linear regression plots of glutamate for Wistar and P rats; B: Extracellular glutamate concentrations (the points of no-net-flux, left panel) and clearance (Ed values; the slope of the plots, right panel). * p < 0.05, significantly different from the ‘Wistar’ group; C: Representative placements of microdialysis probes within the nucleus accumbens shell. For illustration purpose, all placements were shown in one side of the brain.
Representative placements of probes within the PL cortex and NACsh are shown in Fig. 1C & 2C. To be included, the probes should have at least 75% of the active membrane within the target area. Approximately 85% of rats fulfilled the criteria and were included in the analysis.
Basal protein levels of mGlu2 receptors in the NACsh and PL cortex were assessed in separate groups of rats and the results are presented in Fig. 3. The mGlu2 receptor protein was expressed in both regions of female Wistar rats. On the other hand, neither region of female P rats had detectable levels of mGlu2 receptor expression, confirming the finding reported in Zhou et al study (2013).
Figure 3.
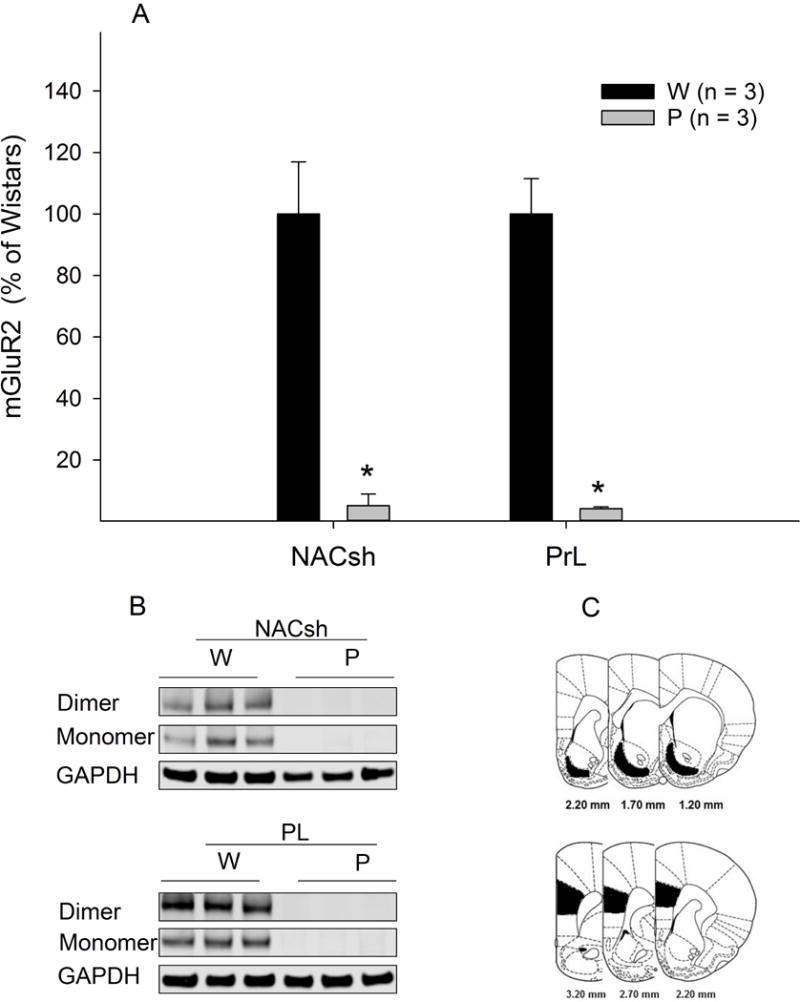
Basal mGlu2 receptor (mGluR2) protein levels in the nucleus accumbens shell and prelimbic cortex of female Wistar (W) and P rats (n = 3/group). * p < 0.05. Tissues were punched from both sides of the brain (shaded areas). For illustration purpose, only one side of the brain is shown.
Grm2 shRNAs and voluntary alcohol consumption
Alcohol drinking following micro-infusion of lentiviruses expressing either the scrambled or Grm2 shRNAs into the PL cortex of male Wistar rats is shown in Fig. 4A. With continuous access, average daily intake was ~ 1 g/kg/d during week 1 and gradually increased to ~ 3 g/kg/d at the end of week 5. During intermittent access, averaged daily intake was ~ 4.5 g/kg/d during week 6 and reached ~ 5.5 g/kg/d at the end of week 8. Repeated measures ANOVA revealed a significant effect of time (F7, 19 = 14.8, p < 0.001), but no significant effect of treatment (F2, 25 = 0.2, p = 0.82) or time × treatment interaction (F14, 40 = 1.3, p = 0.26). The significant effect of time was due in part to the significant increase in alcohol intake when rats were switched from continuous access to intermittent access. Neither Grm2 shRNA had a significant effect on alcohol intake.
Figure 4.
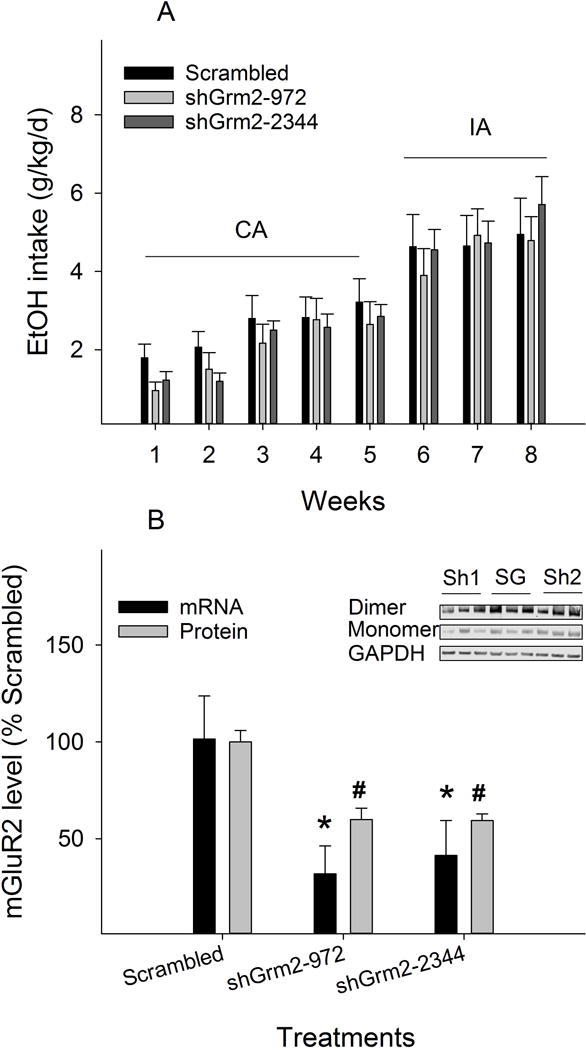
A: Effects of microinjection of lentiviral shRNAs into the prelimbic cortex on alcohol drinking in male Wistar rats (n = 9–10/group) given continuous access (CA) for 5 weeks and intermittent access (IA) for 3 weeks to alcohol under a three-bottle choice condition (10% & 15% ethanol vs water). B: Effects of shRNAs on expression levels of Grm2 mRNA in vitro and mGlu2 receptor (mGluR2) protein in vivo. * p < 0.05, significantly lower than the ‘scrambled’ group. Inset: SG: scrambled; Sh1: shGrm2-972; Sh2: shGrm2-2344.
Brain tissues from the PL cortex were micro-punched following the cessation of the last drinking session and levels of mGlu2 receptor protein were analyzed with Western blots. The results indicate that both Grm2 shRNAs reduced mGlu2 receptor expression by ~ 40% compared to the scrambled shRNA (Fig. 4B; F2, 25 = 20.6, p < 0.001).
These shRNAs were also validated in vitro on Grm2 mRNA expression. Both Grm2 shRNAs significantly reduced mRNA expression in cultured cells (F2, 6 = 12.5, p = 0.007). shGrm2-972 and shGrm2-2344 reduced mRNA expression to 32 ± 14% and 41 ± 18% of levels seen with the scrambled shRNA, respectively (Fig. 4B). There were no significant differences between the two Grm2 shRNAs in this regard.
Linear regression of the Grm2 mutant allele frequency and alcohol intake
Table 1 depicts genotypic data for the Grm2 cys407* allele frequency and the corresponding alcohol intake levels in rodents obtained from a literature search. There were a total of 36 different strains, with 34 rat strains and 2 mouse strains. Twelve strains did not carry the Grm2 cys407* stop codon mutation: ANA, NP, LAD1, sNP, ACI/N, WKY/N, F344/N, BN/Ssn, 407C/C, Grm2+/+, Hsd:WI, and RLA-1. Average alcohol intake ranged from 0.3 to 3.2 g/kg/d. Fifteen strains were homozygous for the Grm2 cys407* mutation: P, HAD1/2, LAD2, BUF/N, M520/N, MR/N, WN/N, 407*/*, Grm2−/−, BkI/WI, HsdHan:W, RHA-I, HAB, and LAB. Average alcohol intake ranged from 0.5 to 5.7 g/kg/d. Among these, seven strains exhibited average alcohol intake less than 2.0 g/kg/d. In addition, there were nine strains in which the mutant allele frequency varied between 0.04 and 0.99: AA, CrL:WI, RjHan:WI, WLP, WHP, 407C/*, sP, HanTac:W, and RccHan:W. Average alcohol intake ranged from 0.4 to 7.8 g/kg/d.
Table 1.
Depicts the allele frequency of Grm2 cys407* stop codon mutant and the level of ethanol intake in rodents
| Common name | Nomenclature | N | Frequency | Ethanol (g/kg/d) |
|---|---|---|---|---|
| Alko Alcohol-Perferring | AA | 12 | 0.04A | 6.0C |
| Alko Non Alcohol-Preferring | ANA | 12 | 0A | 1.0C |
| Alcohol Preferring | P | 125 | 1B | 5.0D |
| Alcohol Non-Preferring | NP | 59 | 0B | 1.0D |
| High Alcohol Drinking Replicate line 1 | HAD1 | 8 | 1 | 5.0D |
| Low Alcohol Drinking Replicate line 1 | LAD1 | 4 | 0 | 1.0D |
| High Alcohol Drinking Replicate line 2 | HAD2 | 4 | 1 | 5.0D |
| Low Alcohol Drinking Replicate line 2 | LAD2 | 4 | 1 | 1.0D |
| Sardinian alcohol Preferring | sP | 10 | 0.65A | 6.5E |
| Sardinian alcohol Non-Preferring | sNP | 10 | 0A | 0.5E |
| Warsaw Alcohol High-Preferring | WHP | 5 | 0.5A | 7.8F |
| Warsaw Alcohol Low-Preferring | WLP | 5 | 0.4A | 0.5F |
| Buffalo | BUF/NN | 1 | 1 | 1.6G |
| Marshall 520 | M520/NN | 1 | 1 | 4.8G |
| Maudsley Reactive | MR/NN | 1 | 1 | 5.7G |
| Inbred Wistar NIH | WN/NN | 1 | 1 | 2.3G |
| A X C 9935 Irish | ACI/NN | 1 | 0 | 0.3G |
| Wistar Kyoto NIH | WKY/NN | 1 | 0 | 1.8G |
| Fischer 344 NIH | F344/NN | 1 | 0 | 1.4G |
| BN/Ssn | BN/SsnN | 1 | 0 | 2.2G |
| iP X iNP F2 Heterozygous | 407 C/* | 87 | 0.5B | 3.0B |
| iP X iNP F2 Mutant Homozygous | 407 */* | 46 | 1B | 4.1B |
| iP X iNP F2 Non-Mutant | 407 C/C | 247 | 0B | 3.2B |
| Wild Type CD1 mice | Grm2 +/+ | 8 | 0B | 2.0H |
| Grm2 Knockout CD1 mice | Grm2 −/− | 8 | 1B | 5.0H |
| B&K Wistar | Bkl:WI | 14 | 1A | 1.8I |
| CR Wistar | Crl:WI | 10 | 0.25A | 2.3I |
| Harlan Wistar | Hsd:WI | 48 | 0A | 3.1I |
| Taconic Han Wistar | HanTac:WH | 15 | 0.87A | 3.1I |
| HSD Han Wistar | HsdHan:WIST | 50 | 1A | 0.5J |
| Janvier Han Wistar | RjHan:WI | 10 | 0.3A | 0.4J |
| RCC Han Wistar | RccHan:WIST | 50 | 0.99A | 1.7J |
| Roman High Avoidance | RHA-I | 6 | 1A | 1.0K |
| Roman Low Avoidance | RLA-I | 6 | 0A | 1.0K |
| High Anxiety-related Behavior | HAB | 8 | 1A | 1.5M |
| Low Anxiety-related Behavior | LAB | 9 | 1A | 2.0M |
Sommer et al 2006;
McBride & Li 1998, general selection criterion;
Colombo et al 2006, 40th generation;
Dyr & Kostowski 2008, 37th generation, males/females average;
Li & Lumeng 1984, males/females average;
Zhou et al 2013, 17% ethanol;
Palm et al 2011, median at week 6;
Goepfrich et al 2013;
Manzo et al 2012, 10% ethanol;
Henniger et al 2002, males average;
Progenitor inbred rats for HAD/LAD line.
Figure 5 shows the linear regression analysis between the mutant allele frequency and average alcohol intake. The analysis revealed no significant correlation between alcohol intake levels and the mutant allele frequency (F1, 35 = 3.1, p = 0.09), with the correlation coefficient (R) value at 0.29 and the coefficient of determination (R2) value at 0.09. The data suggest that the mutant allele frequency cannot predict alcohol intake levels.
Figure 5.
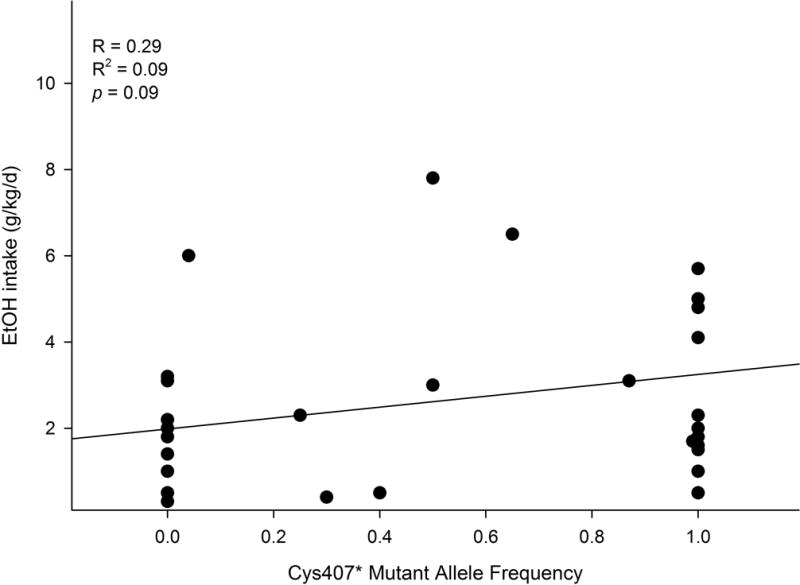
Linear regression analysis between Grm2 cys407* mutant allele frequency and ethanol intake levels in rodent lines. There was no significant correlation between the mutation and alcohol drinking.
Discussion
The major findings of the current study indicate 1) basal extracellular glutamate levels within the PL cortex and NACsh were lower in female P rats than female Wistar rats; 2) lentiviral-mediated shRNA-induced knockdown of mGlu2 receptors within the PL cortex did not significantly alter voluntary alcohol consumption in male Wistar rats; and 3) no significant correlation was found between the Grm2 cys407* mutation and alcohol drinking in rodents. These results do not support our original hypotheses. Instead, they suggest that there is no significant association between the loss of Grm2/mGlu2 receptors and glutamate transmission, and between reduced levels of mGlu2 receptors in the PL cortex and alcohol drinking.
Protein analysis confirmed a lack of mGlu2 receptor expression within the NACsh and PL cortex of female P rats, but not of female Wistar rats (Fig. 3). Due to its auto-receptor nature, the loss of mGlu2 receptors would hypothetically lead to higher extracellular glutamate concentrations within these regions. However, the results indicate the extracellular glutamate levels were significantly lower in these two regions of P than Wistar rats (Fig. 1 & 2). The basal glutamate concentrations within the NACsh were consistent with a previous study conducted in P rats (Ding et al 2013). These results suggest that the loss of mGlu2 receptors may not be associated with high extracellular glutamate transmission. It should be noted that extracellular glutamate levels are regulated not only by the mGlu2 receptor, but also by other factors, e.g., glutamate transporters that are responsible for glutamate clearance (Danbolt 2001), glutamate release from neurons following depolarization, and glutamate release from glial cells through the cystine-glutamate antiporter, which contributes to majority of extracellular glutamate levels (Baker et al 2002). Compensatory changes in these processes may also contribute to lower extracellular glutamate levels in P rats. For example, the lower extracellular glutamate levels in P rats are accompanied by greater Ed values in the NACsh (Fig. 2B), which suggests that enhanced glutamate clearance may have overcome the effects produced by the loss of mGlu2 receptors in this region. In addition, reduced glutamate release may also contribute to lower the extracellular glutamate levels in the PL cortex.
A previous study indicated that basal extracellular glutamate levels within the prefrontal cortex and NAC were lower in Lewis than Fischer 344 rats (Selim & Bradberry 1996). Ethanol has been shown to be more reinforcing in Lewis than Fischer 344 rats, and Lewis rats self-administer more ethanol than Fischer 344 rats (Cadoni 2016). These findings, along with the current results, suggest that extracellular glutamate levels may be associated with alcohol self-administration and drinking. The physiology of differential extracellular glutamate levels between P and Wistar rats and their contributions to the predisposition to alcohol preference and consumption remain unknown. It is possible that the lower basal glutamate transmission in P rats may render the glutamate system in these rats more susceptible to perturbations induced by alcohol, resulting in heightened alcohol-glutamate interactions that drive and precipitate further alcohol drinking.
The lentiviral-mediated shRNAs significantly decreased Grm2 mRNA expression by ~ 60–70% in cultured cells and reduced mGlu2 receptor protein levels by ~ 40% within the PL cortex of male Wistar rats (Fig. 4B). Nonetheless, there were no significant differences in voluntary alcohol drinking between rats treated with the scrambled shRNA and rats treated with the active Grm2 shRNAs during either continuous or intermittent ethanol access (Fig. 4A). It is noted that a previous study demonstrated that antagonism of mGlu2/3 receptors with LY341495 escalated alcohol self-administration in Wistar rats (Zhou et al 2013). Differences in experimental procedures may have contributed to the different results, e.g., systemic vs central administrations, and genetic vs pharmacological manipulations. Another possibility for the current findings is that alteration of glutamate transmission within the PL cortex may not be involved in alcohol drinking. However, previous studies demonstrated an important role of the PL cortex, in general (Faccidomo et al 2016, Samson & Chappell 2001, Warnault et al 2016), and glutamate transmission within the PL cortex, in particular (Seif et al 2013), in alcohol drinking. Furthermore, the magnitude of mGlu2 receptor knock-down (~ 40%) may not be sufficient to alter drinking behavior. Such a possibility cannot be tested at the current stage due to technical limitations. In the foreseeable future, however, new technology may evolve and enable such a study, e.g., conditional knockout of Grm2/mGlu2 receptor powered by the latest genetic editing tools such as CRISPR-Cas9. It should be noted that the loss of mGlu2 receptors may have induced compensatory changes during development, e.g., reduced glutamate release and/or increased glutamate re-uptake, which may contribute to the high alcohol preference and consumption in P rats. Whereas the knockdown of mGlu2 receptors during adulthood in Wistar rats may not allow similar compensatory changes to develop, thus preventing enhanced alcohol drinking. Alternatively, some studies suggest that the involvement of the mGlu2 receptor may be dependent on the stage of AUD. For example, Augier et al (2016) reported that the selective mGlu2 receptor positive allosteric modulator AZD8529 significantly reduced reinstatement of alcohol seeking with only marginal effects on alcohol self-administration. In addition, Meinhardt and colleagues (2013) found that alcohol dependence reduced Grm2 mRNA expression in the infralimbic cortex and rescue of this deficit attenuated the reinstatement of alcohol seeking specifically in dependent, but not non-dependent, rats.
A shortcoming of the current study was that female rats were used in the microdialysis experiments and male rats were used in the shRNA experiment. Female rats were used in the microdialysis experiments because female P rats maintain their head size over longer periods better than male P rats, which allows for more precise stereotaxic placements of microdialysis probes. The male Wistar rats were used in the viral vector experiment to match male Wistar rats used in the study by Meinhardt and colleagues (2013).
Similar to P vs NP rats, there appeared to be an association between Grm2 and alcohol drinking in sP vs sNP rats (Colombo et al 2006, Wood et al 2017). However, this association did not appear to generalize to other strains of rodents (Table 1), as the linear regression analysis found no significant correlation between Grm2 mutant allele frequency and alcohol intake levels by different rodent lines (Fig. 5). In particular, the Low Alcohol Drinking Replicate line 2 (LAD2) is homozygous for the Grm2 cys407* mutation, whereas only a fraction of the Alko Alcohol-Preferring rats (AA) carried the mutant allele (frequency: 0.04). These results suggest that the loss of Grm2/mGlu2 receptors alone may not predict high alcohol drinking.
Collectively, the current experiments do not support a significant association between the loss of the Grm2/mGlu2 receptor and higher glutamate transmission in the PL cortex and NACsh of female P rats, and between the reduction of mGlu2 receptor protein in the PL cortex and alcohol drinking in male Wistar rats. This latter finding is in contrast to findings by Zhou and colleagues (Zhou et al 2013). However, the Zhou et al study (2013) reported on the effects of a total loss of mGlu2 receptors throughout all tissues, whereas only the PL cortex was examined in the present study. Furthermore, numerous other genetic, neurochemical and molecular differences have been found between P and NP rats (Bell et al 2012, McBride & Li 1998, Murphy et al 2002). Genetic studies also point to the genetic heterogeneity of alcoholism with potential involvement of a cluster of genes (Crabbe et al 2006, Reilly et al 2017). Therefore, it is likely that the Grm2/mGlu2 receptor, as a contributing rather than determining factor, works in concert with a host of genetic and molecular substrates to shape the bi-directional drinking phenotypes manifested in P and NP rats.
In summary, the results of the current study suggest a lack of association between the loss of the Grm2/mGlu2 receptor and glutamate transmission within key corticolimbic regions of female P rats, reduction of the levels of mGlu2 receptors within the PL cortex and alcohol drinking by male Wistar rats, and no correlation between frequency of the Grm2 cys407* stop codon mutation and the level of alcohol intake in rodents. However, there is evidence suggesting the involvement of the mGlu2 receptors in the reinstatement of alcohol seeking, particularly following dependence (Augier et al 2016, Meinhardt et al 2013). These are particularly relevant to these two stages of the conceptualized addiction cycle: withdrawal/negative affect, and preoccupation/anticipation stages (Koob & Volkow 2016). Therefore, future studies will need to examine the involvement of the Grm2/mGlu2 receptor in these two stages using appropriate animal models; such work will increase our knowledge regarding the role of the Grm2/mGlu2 receptor in the development of AUD and aid in the development of medications targeting the Grm2/mGlu2 receptor.
Acknowledgments
This study was supported, in part, by research grants AA007611, AA012262, AA013522 (INIA), and AA016654 (INIA). We thank Dr. Tiebing Liang from the Indiana Alcohol Research Center for genotyping the HAD/LAD rats. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
Footnotes
There is no conflict of interest.
References
- Augier E, Dulman RS, Rauffenbart C, Augier G, Cross AJ, Heilig M. The mGluR2 Positive Allosteric Modulator, AZD8529 and Cue-Induced Relapse to Alcohol Seeking in Rats. Neuropsychopharmacology. 2016;41:2932–2940. doi: 10.1038/npp.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Hauser SR, McClintick JN, Rahman S, Edenberg HJ, Szumlinski KK, McBride WJ. Ethanol-associated changes in glutamate reward neurocircuitry: A minireview of clinical and preclinical genetic findings. Prog Mol Biol Transl Sci. 2016;137:41–85. doi: 10.1016/bs.pmbts.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav. 2012;103:119–155. doi: 10.1016/j.pbb.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C. Fischer 344 and Lewis Rat Strains as a Model of Genetic Vulnerability to Drug Addiction. Front Neurosci. 2016;10:13. doi: 10.3389/fnins.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and -non-preferring (sNP) rats. Addict Biol. 2006;11:324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Engleman EA, Rodd ZA, McBride WJ. Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcohol Clin Exp Res. 2012;36:633–640. doi: 10.1111/j.1530-0277.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Ingraham CM, Rodd ZA, McBride WJ. Alcohol drinking increases the dopamine-stimulating effects of ethanol and reduces D2 auto-receptor and group II metabotropic glutamate receptor function within the posterior ventral tegmental area of alcohol preferring (P) rats. Neuropharmacology. 2016;109:41–48. doi: 10.1016/j.neuropharm.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol preferring (P) rats. Addict Biol. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33:2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Keen EJ, Tilford SS, Thielen RJ, Morzorati SL. Ethanol drinking reduces extracellular dopamine levels in the posterior ventral tegmental area of nondependent alcohol-preferring rats. Alcohol. 2011;45:549–557. doi: 10.1016/j.alcohol.2011.02.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Reid GT, Agoglia AE, Ademola SA, Hodge CW. CaMKII inhibition in the prefrontal cortex specifically increases the positive reinforcing effects of sweetened alcohol in C57BL/6J mice. Behav Brain Res. 2016;298:286–290. doi: 10.1016/j.bbr.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, Bell RL, McBride WJ. Involvement of Purinergic P2X4 Receptors in Alcohol Intake of High-Alcohol-Drinking (HAD) Rats. Alcohol Clin Exp Res. 2015a;39:2022–2031. doi: 10.1111/acer.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, McClintick J, Ding ZM, McBride WJ, Bell RL. Reduction of alcohol drinking of alcohol-preferring (P) and high-alcohol drinking (HAD1) rats by targeting phosphodiesterase-4 (PDE4) Psychopharmacology. 2015b;232:2251–2262. doi: 10.1007/s00213-014-3852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Collins SE, George WH, Wall TL, McCarthy DM, Liang T, Larimer ME. Associations of ALDH2 and ADH1B genotypes with alcohol-related phenotypes in Asian young adults. Alcohol Clin Exp Res. 2009;33:839–847. doi: 10.1111/j.1530-0277.2009.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Katner SN, Oster SM, Ding ZM, Deehan GA, Jr, Toalston JE, Hauser SR, McBride WJ, Rodd ZA. Alcohol-preferring (P) rats are more sensitive than Wistar rats to the reinforcing effects of cocaine self-administrered directly into the nucleus accumbens shell. Pharmacol Biochem Behav. 2011;99:688–695. doi: 10.1016/j.pbb.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek AW, Janak PH, He L, Whistler JL, Heberlein U. Downregulation of mu opioid receptor by RNA interference in the ventral tegmental area reduces ethanol consumption in mice. Genes Brain Behav. 2007;6:728–735. doi: 10.1111/j.1601-183X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- Lasek AW, Azouaou N. Virus-delivered RNA interference in mouse brain to study addiction-related behaviors. Methods Mol Biol. 2012;602:283–298. doi: 10.1007/978-1-60761-058-8_17. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li T-K. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Schultz JA, Kimpel MW, McClintick JN, Wang M, You J, Rodd ZA. Differential effects of ethanol in the nucleus accumbens shell of alcohol-preferring (P), alcohol-non-preferring (NP) and Wistar rats: A proteomics study. Pharmacol Biochem Behav. 2009;92:304–313. doi: 10.1016/j.pbb.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci. 2013;33:2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci Lett. 1994;178:99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGluR2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Reilly MT, Noronha A, Goldman D, Koob GF. Genetic studies of alcohol dependence in the context of the addiction cycle. Neuropharmacology. 2017;122:3–21. doi: 10.1016/j.neuropharm.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li TK, Murphy JM, McBride WJ. Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring and Wistar rats. Alcohol Clin Exp Res. 2004;28:1212–1219. doi: 10.1097/01.alc.0000134401.30394.7f. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A. Muscimol injected into the medial prefrontal cortex of the rat alters ethanol self-administration. Physiol Behav. 2001;74:581–587. doi: 10.1016/s0031-9384(01)00607-2. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex between the Lewis and Fischer 344 rat strains. Brain Res. 1996;716:157–164. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise RA, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Morisot N, Phamluong K, Wilbrecht L, Massa SM, Longo FM, Ron D. The BDNF valine 68 to methionine polymorphism increases compulsive alcohol drinking in mice that is reversed by tropomyosin receptor kinase B activation. Biol Psychiatry. 2016;79:463–473. doi: 10.1016/j.biopsych.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CM, Nicolas CS, Choi SL, Roman E, Nylander I, Fernandez-Teruel A, Kiianmaa K, Bienkowski P, de Jong TR, Colombo G, Chastagnier D, Wafford KA, Collingridge GL, Wildt SJ, Conway-Campbell BL, Robinson ES, Lodge D. Prevalence and influence of cys407* Grm2 mutation in Hannover-derived Wistar rats: mGlu2 receptor loss links to alcohol intake, risk taking and emotional behaviour. Neuropharmacology. 2017;115:128–138. doi: 10.1016/j.neuropharm.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Karlsson C, Liang T, Xiong W, Kimura M, Tapocik JD, Yuan Q, Barbier E, Feng A, Flanigan M, Augier E, Enoch MA, Hodgkinson CA, Shen PH, Lovinger DM, Edenberg HJ, Heilig M, Goldman D. Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc Natl Acad Sci U S A. 2013;110:16963–16968. doi: 10.1073/pnas.1309839110. [DOI] [PMC free article] [PubMed] [Google Scholar]


