Abstract
Background
Bowel function in long-term rectal cancer survivors with anastomosis has not been characterized adequately. We hypothesized that bowel function is associated with patient, disease, and treatment characteristics.
Methods
The cohort study included Kaiser Permanente members who were long-term (≥5 years) rectal cancer survivors with anastomosis. Bowel function was scored using the self-administered, 14-item Memorial Sloan-Kettering Cancer Center Bowel Function Index. Patient, cancer, and treatment variables were collected from the electronic medical charting. We used multiple regression to assess the relationship of patient- and treatment-related variables with the bowel function score.
Results
The study included 381 anastomosis patients surveyed an average 12 years after their rectal cancer surgeries. The total bowel function score averaged 53 (standard deviation, 9; range, 31 to 70, higher scores represent better function). Independent factors associated with worse total bowel function score included receipt of radiation therapy (yes vs. no: 5.3-unit decrement, p<0.0001), tumor distance from the anal verge (≤6 cm vs. >6 cm: 3.2-unit decrement, p<0.01), and history of a temporary ostomy (yes vs. no: 4.0-unit decrement, p<0.01). One factor measured at time of survey was also associated with worse total bowel function score: ever smoking (2.3-unit decrement, p<0.05). The regression model explained 20% of the variation in the total bowel function score.
Conclusions
Low tumor location, radiation therapy, temporary ostomy during initial treatment, and history of smoking were linked with decreased long-term bowel function following an anastomosis. These results should improve decision-making about surgical options.
Keywords: Rectal Cancer, Cancer Survivors, Anastomosis, Oncology, Bowel Function
INTRODUCTION
In the past 40 years, the five-year survival rate following rectal cancer has increased from about 50% to 70%.1,2 This improvement has resulted from early screening and diagnosis, as well as implementation of newer surgical techniques and therapies.3 In some patients with low rectal cancers, a low anterior resection can be performed so that the anal sphincter is spared allowing the patient to maintain their bowel function and continuity. In other patients with low rectal cancer, an abdominoperineal resection is necessary. These patients must use an ostomy bag to collect their stool. Some patients may also be given a “temporary ostomy” with the goal of restoring bowel function continuity later with a second surgery. Among those who receive sphincter-sparing surgery, many have impaired bowel function, or “low anterior resection syndrome”4–7 that can lead to a reduction in health-related quality of life.4, 6, 8–12
When the probabilities of cancer recurrence and overall survival are similar for patients considering sphincter-sparing surgery or ostomy, it is essential that patients have access to high-quality information about long-term bowel function outcomes.13 Information about long-term outcomes is important for the primary care providers who manage long-term cancer survivor’s health care as well.13
We conducted a secondary analysis of a cohort study to understand the relationship of patient and clinical factors known at the time of treatment planning with risk of long-term bowel dysfunction. We also sought to understand the role of long-term health status on long-term bowel function.
MATERIALS AND METHODS
This secondary analysis of a cohort study included both longitudinal and cross-sectional components and was approved by Institutional Review Boards at the University of Arizona Cancer Center and Kaiser Permanente. Our methods are detailed in our earlier reports14, 15 and summarized here.
Study Population
The primary study was set within the membership of Kaiser Permanente in Northern California and Oregon/southwest Washington. Kaiser Permanente is an integrated healthcare delivery system. In these two regions, it offers comprehensive, capitated care. Health plan members were eligible if they received a diagnosis of rectal or rectosigmoid cancer, had survived their cancer diagnosis by least 5 years as of recruitment in 2010, and had received an anastomosis with or without a temporary ostomy that was reversed as part of their initial phase of rectal cancer treatment.
Data Collection
The data used in this secondary analysis was obtained from responses to a mailed survey, from patient and clinical data recorded into an electronic health record (EHR) and cancer registry, and from chart review of the bowel surgery operative report.
A survey was mailed to eligible health plan members in 2010–2011. The survey included the Memorial Sloan-Kettering Cancer Center Bowel Function Index (BFI),16 the City of Hope Quality of Life Colorectal Cancer questionnaire (COH-QOL-CRC),17 and the Short-Form 12 Health Survey version 2 (SF-12v2).18 It also included questions asking the date of surgery, whether patient received a temporary ostomy at time of surgery (yes/no), physical activity (minutes/week), and self-reported general health (5-point Likert scale, excellent to poor).
The BFI contains 14 items (Table 1).16 For item 1, the patient wrote in the estimated number of bowel movements per day. The number of bowel movements per day was recoded to 1 to 5 (< 2, 2, 3, 4 – 5, and ≥ 6 per day, respectively). The remaining items are coding on a Likert scale ranging from 1 (always) to 5 (never). Following the BFI scoring instructions, [Temple et al. 2005], scores were inverted for two items (#2 and #6) so that ‘1’ represented worst bowel function and ‘5’ the best bowel function for all items. The items were added together for a “total BFI score,” with range 31 to 70, where high score indicates better bowel function.
Table 1.
MSKCCa Bowel Function Index
| Over the past 4 weeks… |
|---|
| FREQUENCY |
| How many bowel movements did you generally have in a 24-hour day?b |
| How often did you get to the toilet in time?c |
| How often did you use medicines to decrease the number of bowel movements (drugs like Imodium® or Lomotil ®)?d |
| How often did you have diarrhea (no form, watery stool)?d |
| How often did you have loose stool (slight form, but mushy)?d |
| How often were you able to wait 15 minutes to get to the toilet when you felt like you were going to have a bowel movement?c |
| URGENCY |
| How often have you had soilage (leakage of stool) of your undergarments during day?d |
| How often have you had soilage (leakage of stool) of your undergarments when you go to bed?d |
| How often did you use a tissue, napkin, and/or pad in your undergarments in case of stool leakage?d |
| How often have you had to alter activities because of bowel function?d |
| DIETARY |
| How often did certain foods that you ate increase your number of bowel movements in a day?d |
| How often did certain liquids that you drank increase your number of bowel movements in a day?d |
| How often did limiting the types of solid foods you ate help you to control your bowel movements?d |
| How often did limiting the types of liquids your drink help you control you bowel movements?d |
Memorial Sloan-Kettering Cancer Center
BM in 24 hours: Best is < 2 and worst is ≥ 6 (intermediate categories: 2, 3, 4–5)
Worst is never, best is always
Worst is always, best is never
The BFI has three subscales: Frequency, Urgency, and Dietary (Table 1). The Frequency subscale includes 6 of the 14 items (range: 6–30), those concerning stool consistency, ability to get to the toilet on time, and the number of bowel movements in 24 hours. The Urgency subscale includes 4 items (range: 4–20) concerning fecal leakage and the impact of bowel function on changes in activities. The Dietary subscale includes 4 items (range: 4–20) concerning the impact of solid foods and liquids on bowel control. We previously reported the psychometric properties of the BFI in our study population.14
Patient age, sex, and race (White, Asian-American, other) were obtained from the EHR. Use of opiates during the year prior to survey was obtained from pharmacy dispensing information. Charlson-Deyo comorbidity scores were computed from encounter diagnoses.19 Receipt of pre- and post-operative radiation and chemotherapy at the time of the initial treatment was ascertained from the cancer registry. Distance of the tumor from the anal verge was ascertained from chart review of the operative report or the report of gastroenterologists’ preoperative reports.
Statistical Analysis
For 27 patients with one missing value among the 14 BFI items, the missing value was assigned using the average of the patient’s non-missing items from the same subscale. Ten cases with ≥2 missing items on the BFI were removed from the analysis. The BFI subscales (Frequency, Urgency, and Dietary) were grouped into tertiles (low, medium, high) for analysis of subscale results. The number of years between the date of surgery and the date of survey was dichotomized into ≤10 or >10 years. Age was dichotomized into <65 or ≥65 years. The distance of the tumor from the anal verge was dichotomized as ≤6 or >6 cm, separating the lower rectum from middle and upper rectum.20
We conducted two types of analyses. For the first type of analysis, we estimated the association of predictors with total BFI score using multiple linear regression with total BFI score entered as a continuous variable. For the second type of analysis, we estimated the adjusted odds ratios (ORs) and 95% confidence intervals (95% CI) for the associations of patient, disease, and treatment characteristics with BFI subscales (coded in tertiles) using ordinal logistic regression. This model estimated an average adjusted odds ratio that can be interpreted as the odds of having a risk factor in the highest tertile of BFI relative to having the risk factor in the medium tertile of BFI, as well as medium tertile of BFI relative to the lowest tertile of BFI. We used the Score test to evaluate the proportional odds assumption. All statistical analyses were performed in SAS® version 9.3.
Results
The number of subjects identified for the primary study was 1119, of which 782 (70%) had anastomosis and 337 (30%) had ostomy. The present secondary analysis focused on 674 eligible patients with an anastomosis who were invited to respond to a mailed survey. Another 313 patients who underwent ostomy were also sent the survey, but are not the subjects of this study. Of the 674, the number who completed the survey was 394 (response rate 58.5%), which is comparable to response rates in other survey research studies. 21 Among these 394 cases, 10 patients had information missing for 2 or more BFI questions and 3 had missing information on the type of surgery (anastomosis). These 13 were excluded from the study. Final analysis included 381 anastomosis patients. In our primary study 14, we compared responders with non-responders, including both anastomosis and ostomy patients. Compared with non-responders, responders were on average 2 years younger (p=0.01) and more likely non-Hispanic white (p<0.001). Responders and non-responders did not differ significantly on time since diagnosis, sex, Hispanic ethnicity, or tumor stage.
Characteristics of the Study Population
Characteristics of the study participants are shown in Table 2. Sixty-five percent of the population was under age 65 at surgery, and 57% were male. The tumor was within 6 cm of the anal verge for 20% of participants; 35% had radiation therapy, and 17% had a temporary ostomy. The mean number of years since surgery was 12.4. About half of the participants were ever smokers, and 29% used opiates in the year prior to survey.
Table 2.
Patient Demographic and Clinical Characteristics in 381 Patients with Anastomosis with an Average 12 Years of Follow-up after Surgery.
| Patient Characteristics
| |||
|---|---|---|---|
| At time of surgery | % | At time of survey | % |
| Age at surgery, years | Period of surgery | ||
| <65 | 65 | Before 2000 | 59 |
| >65 | 35 | 2000–2005 | 41 |
| Gender | Years since surgery | ||
| Female | 43 | 5–10 | 40 |
| Male | 57 | ≥10 | 60 |
| Race/ethnicity | Charlson-Deyo Comorbidity | ||
| White | 83 | ≤2 | 93 |
| Asian-American | 9 | >2 | 7 |
| Other | 8 | Body mass index, kg/m2 | |
| Tumor distance from anal verge, cm | <24.9 | 36 | |
| 25.0 – 29.9 | 36 | ||
| >6 | 80 | ≥30 | 28 |
| ≤6 | 20 | Use of prescribed opiates | |
| Tumor stage | No | 71 | |
| Localized | 52 | Yes | 29 |
| Regional | 47 | Physical activity, min/week | |
| Metastatic or Systemic | 1 | ≥210.0 | 32 |
| Temporary ostomy | 20.0 – 209.9 | 32 | |
| No | 80 | ≤19.9 | 37 |
| Yes | 20 | General health | |
| Chemotherapy | Excellent, very good | 46 | |
| No | 52 | Good | 37 |
| Yes, before surgery | 14 | Fair, poor | 17 |
| Yes, after surgery | 33 | ||
| Radiation | |||
| No | 65 | ||
| Yes, before surgery | 13 | ||
| Yes, after surgery | 22 | ||
| Smoking history | |||
| Never | 44 | ||
| Ever | 56 | ||
Bowel Function Scores
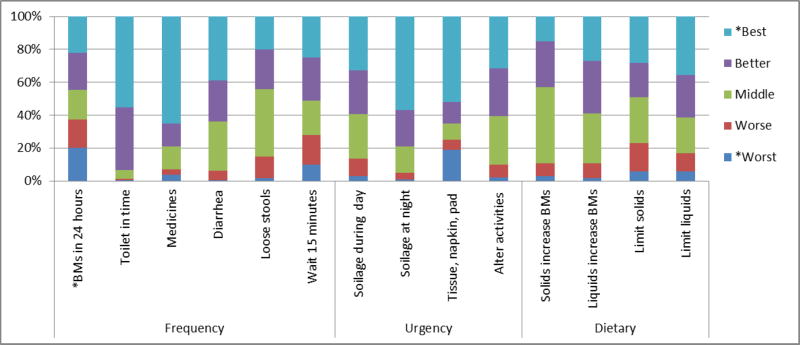
Responses to the 14 bowel function questions are shown in Figure 1. Twenty percent of the patients reported 6 or more bowel movements in 24 hours, and 10% reported never being able to wait 15 minutes before using the toilet. Forty-one percent reported having soilage during the day at least sometimes; 19% always used pads in case of stool leakage; and 39% reported altering their activities at least sometimes. The total BFI score ranged from 31 to 70, with higher scores representing better function. The average total BFI score was 53, with a standard deviation [SD] of 9. The average scores for the subscales were as follows: Frequency (average 22.7; SD 4.1), Urgency (average 15.6; SD 3.7), and Dietary (average 14.4; SD 3.4).
Figure 1. Distribution of Bowel Function Scores Across 14 Items.
*Cutpoints for scores are provided in the footnotes to Table 1.
Associations with Total BFI Score
Table 3 shows the adjusted differences in mean total BFI score in relation to patient and treatment characteristics. The reference group was defined as those with none of the risk factors identified in Table 3. Key characteristics of patients in the reference group included no history of radiation for their rectal cancer, tumor located >6 cm above the anal verge, no temporary ostomy, no history of smoking and localized stage of tumor. Among the patients in the reference group, the mean total BFI score was 66.2 (95% CI, 60.8 to 71.7). Initial treatment with radiation was associated with an average 5.3-unit lower total BFI score (p<0.0001). In other words, a person in the reference group had a total BFI score of 66.2, while a person who differed only by exposure to initial treatment with radiation had a total BFI score of 60.9. Tumor distance ≤6 cm (compared with >6 cm) was associated with a 3.2-unit lower average total BFI score (p<0.01). Similarly, a history of temporary ostomy (compared with none) was associated with a 4.0-unit lower average total BFI score (p<0.01). A history of ever smoking, which was measured at the time of survey, was also associated with worse bowel function score, by 2.3 units (p<0.05). The variables listed in Table 3 explained 20% of variation in the total BFI scores.
Table 3.
Adjusted Change in Mean Total BFI Score in Relation to Patient and Treatment Characteristics in 381 Patients with Anastomosis with an Average 12 Years of Follow-up after Surgery.
| Decline in mean total BFI score | 95% CI | p value | |
|---|---|---|---|
| Age at surgery, years | |||
| <65 | Ref* | ||
| ≥65 | −0.5 | −2.4, 1.3 | 0.57 |
| Gender | |||
| Male | Ref | ||
| Female | −0.06 | −1.8, 1.7 | 0.95 |
| Race | |||
| White | Ref | ||
| Asian | −2.9 | −6.2, 0.3 | 0.08 |
| Other | −1.9 | −5.2, 1.3 | 0.23 |
| Chemotherapy | |||
| No | Ref | ||
| Yes | 0.76 | −1.9, 3.4 | 0.76 |
| Radiation | |||
| No | Ref | ||
| Yes | −5.3 | −7.7, −3.0 | <0.0001 |
| Tumor distance, cm | |||
| >6 cm | Ref | ||
| ≤6 cm | −3.2 | −5.4, −0.9 | <0.01 |
| Temporary ostomy | |||
| No | Ref | ||
| Yes | −4.0 | −6.4, −1.7 | <0.01 |
| Tumor Stage | |||
| Localized | Ref | ||
| Regional | −1.5 | −3.8, 0.8 | 0.20 |
| Smoking history | |||
| Never | Ref | ||
| Ever | −2.3 | −4.1, −0.5 | <0.05 |
The reference group included younger white men with no cancer treatment, tumors above 6 cm, etc. The mean total BFI in the reference group was 66.2 (95% CI, 60.8 to 71.7).
Associations with Frequency, Urgency, and Dietary Subscales
Adjusted associations of patient, disease, and treatment characteristics with each of the three BFI subscales are provided in Table 4. A worse score on the Frequency subscale was significantly associated with Asian race as compared to white race, initial treatment with radiation, and history of a temporary ostomy. A worse score on the Urgency subscale was significantly associated with receiving radiation, distance of tumor from anal verge, temporary ostomy, regional stage of tumor and smoking history. A worse score on the Dietary subscale was significantly associated with gender, radiation, tumor distance, and smoking history.
Table 4.
Adjusted odds ratio (OR) and 95% confidence interval (CI) for the association of patient, disease, and treatment characteristics with BFI subscale scores (coded in tertiles) in 381 Patients with Anastomosis with an Average 12 Years of Follow-up after Surgery.
| Frequency subscale | Urgency subscale | Dietary subscale | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Adj. OR | 95% CI | Adj. OR | 95% CI | Adj. OR | 95% CI | |
| Age at surgery, years | ||||||
| <65 | Ref | Ref | Ref | |||
| ≥65 | 0.7 | 0.5, 1.1 | 1.3 | 0.8, 2.0 | 1.1 | 0.7, 1.7 |
| Gender | ||||||
| Male | Ref | Ref | Ref | |||
| Female | 1.0 | 0.6, 1.4 | 0.7 | 0.5, 1.1 | 1.4* | 1.0, 2.2 |
| Race | ||||||
| White | Ref | Ref | Ref | |||
| Asian-American | 2.2* | 1.0, 4.8 | 1.3 | 0.6, 2.8 | 1.5 | 0.7, 3.2 |
| Other | 1.2 | 0.6, 2.5 | 1.2 | 0.6, 2.7 | 1.5 | 0.7, 3.2 |
| Chemotherapy | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 1.2 | 0.7, 2.3 | 0.9 | 0.5, 1.7 | 0.7 | 0.4, 1.3 |
| Radiation | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 2.7¶ | 1.6, 4.7 | 2.3¶ | 1.3, 4.0 | 1.7* | 1.0, 2.9 |
| Tumor distance, cm | ||||||
| >6 | Ref | Ref | Ref | |||
| ≤6 | 1.0 | 0.6, 1.7 | 2.2¶ | 1.3, 3.9 | 1.9^ | 1.1, 3.2 |
| Temporary ostomy | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 2.7¶ | 1.5, 4.7 | 3.6§ | 1.9, 6.5 | 1.3 | 0.8, 2.3 |
| Tumor stage | ||||||
| Localized | Ref | Ref | Ref | |||
| Regional | 1.4 | 0.8, 2.4 | 1.6* | 0.9, 2.9 | 1.3 | 0.7, 2.2 |
| Smoking history | ||||||
| Never | Ref | Ref | Ref | |||
| Ever | 1.4 | 0.9, 2.2 | 2.4§ | 1.5, 3.6 | 1.5^ | 1.0, 2.3 |
P<0.10;
P<0.05;
P<0.01;
P<0.001
DISCUSSION
We sought to characterize bowel function in long-term rectal cancer survivors with anastomosis, and to identify risk factors for poor bowel function. We observed that the total BFI score averaged 53, relative to the range of 31 (worst function) to 70 (best function), with a standard deviation of 9. Factors associated with reduced total BFI score included initial treatment with radiation, tumor distance ≤6 cm, a history of temporary ostomy, and a history of ever smoking. The regression model explained about one-fifth of the variation in the total BFI score among study participants.
Synthesis of this study with past reports is difficult because of differences in the definition of poor bowel function. We used the Memorial Sloan-Kettering Bowel Function Index because it was the only validated instrument available at the time we planned our survey. Study populations differ as well, with respect to the year of surgery, type of surgery, time since surgery, and presence of comorbidities. Downing and colleagues22 obtained self-reported outcomes from >3,000 rectal cancer patients with anastomosis seen in England during 2010–2011 surviving at least 12 months. Thirteen percent of patients reported having no control of their bowels, 10% a little control, and 17% moderate control, with 60% reporting quite a bit or very much control. In a cohort of 399 patients randomized to total mesorectal excision with or without preoperative radiotherapy (1996–99) and followed for 5 years, fecal incontinence was reported by 62% of patients who had radiotherapy and 39% of patients those who did not (p<0.001).23 Wells and colleagues12 examined the records of 277 rectal cancer patients seen at the Auckland City Hospital, 2002–2012. Six bowel symptoms were ascertained from review of clinical notes: fecal incontinence, urgency, increased frequency (≥4 bowel movements per day), constipation, sensation of incomplete evacuation, and changes to stool consistency. The prevalence of having ≥1 of these symptoms was 43% at 5 years. In multivariate analysis, temporary stoma (65% compared with 29%, p<0.01) and tumor distance from the anal verge (>10 vs ≤5 cm: 74% compared with 36%, p<0.01) were predictive of the outcome at 4 years after diagnosis. Findings from our study add to our understanding of the role of radiation therapy, the location of the tumor, and the occurrence of adverse bowel symptoms in long-term rectal cancer survivors.
Two studies have assessed bowel function using the standardized Low Anterior Resection Score questionnaire of Emmertsen and colleagues.24 The total possible score ranges from 0–42, and major bowel dysfunction is defined as a score of ≥30. Ekkarat and colleagues25 obtained questionnaires from 129 rectal cancer patients seen at a Thai hospital, 2004–2013, and had at least 12 months of follow-up. At the time of survey, the prevalence of major bowel dysfunction was 28% in those with low anterior resection. In univariate analyses, temporary ostomy, chemotherapy, radiation therapy, and the operation (extended low vs. low anterior resection) were associated with major bowel dysfunction. Bregendahl and colleagues26 evaluated 938 rectal cancer patients who underwent low anterior resection in Denmark, 2001–2007. The prevalence of major bowel dysfunction was 41%. In multivariate analysis, the use of neoadjuvant therapy, total vs. partial mesorectal excision, younger age, and female gender were associated with major bowel dysfunction, as measured using the Lower Anterior Resegment Syndrome questionnaire.
Although these studies are difficult to compare, the results we report add to the evidence on bowel dysfunction in survivors of rectal cancer treatment. All of the studies observed a relatively high prevalence of bowel dysfunction. Moreover, all identified radiation as an important risk factor. In addition, studies have been consistent in identifying temporary ostomy and tumor distance as risk factors for worse prognosis. Past studies have not examined cigarette smoking, which we found to have an association with long-term bowel function. This may be related to chronic comorbid conditions that are associated with smoking or to microvascular changes.27
Our study fills a knowledge gap by assessing patients an average 12 years after their surgery. These surgeries may not represent contemporary approaches, and we did not have information on whether the anastomosis was stapled or hand-sewn, end-to-end, side-to-side, or J-pouch. These limitations should be considered when using the data to project future outcomes. We recommend that surgical oncologists discuss with patients the possible outcomes of an anastomosis with tumors near the anal verge using words from validated bowel function questionnaires. Patients should understand the problems of managing poor bowel function before they refuse an ostomy.
SYNOPSIS.
The study evaluates the risk factors for reduced long-term bowel function in rectal cancer survivors. By studying bowel function in patients with certain disease and treatment characteristics, choices on surgical approaches can be optimized.
Acknowledgments
We thank Mary Wagner, Administrative Assistant, University of Arizona Cancer Center, for help with this work.
Funding Sources: Supported by Grant R01 CA106912, “Health-Related Quality of Life in Colorectal Cancer Survivors With Stomas,” from the National Cancer Institute, National Institutes of Health, in collaboration with resources and the use of facilities provided at the Southern Arizona Veterans Affairs Health Care System, Tucson, Arizona, and Kaiser Permanente.
Footnotes
Disclaimer
The views expressed in this report are those of the authors and do not necessarily represent the views of the University of Arizona or Kaiser Permanente.
Conflicts of Interest
Dr. Herrinton has had research contracts in the past three years with Medimmune that was unrelated to this study. The other authors have no disclosures.
Details of Ethics Committee Review
All studies were approved by the Institutional Review Boards of the University of Arizona and Kaiser Permanente.
References
- 1.American Cancer Society. [accessed June 29-2016];Fact Sheet Colorectal Cancer Facts & Figures 2011–2013. Available: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028312.pdf.
- 2.Altekruse S, Kosary C, Krapcho M, et al., editors. SEER Cancer Statistics Review 1975–2007. Bethesda, MD: National Cancer Institute; Apr, 2010. 2010. [Google Scholar]
- 3.Kosinski L, Habr-Gama A, Ludwig K, Perez R. Shifting concepts in rectal cancer management: a review of contemporary primary rectal cancer treatment strategies. CA Cancer J Clin. 2012 May-Jun;62(3):173–202. doi: 10.3322/caac.21138. [DOI] [PubMed] [Google Scholar]
- 4.Juul T, Ahlberg M, Biondo S, et al. Low anterior resection syndrome and quality of life: an international multicenter study. Dis Colon Rectum. 2014;57:585–591. doi: 10.1097/DCR.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 5.Emmertsen KJ, Laurberg S. Bowel dysfunction after treatment for rectal cancer. Acta Oncologica. 2008;47(6):994–1003. doi: 10.1080/02841860802195251. [DOI] [PubMed] [Google Scholar]
- 6.Emmertsen KJ, Laurberg S. Rectal Cancer Function Study Group. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg. 2013;100:1377–1387. doi: 10.1002/bjs.9223. [DOI] [PubMed] [Google Scholar]
- 7.Lai X, Wong FK, Ching SS. Review of bowel dysfunction of rectal cancer patients during the first five years after sphincter-preserving surgery: a population in need of nursing attention. Eur J Oncol Nurs. 2013;17:681–692. doi: 10.1016/j.ejon.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Chen TY, Wiltink LM, Nout RA, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer. 2015;14:106–114. doi: 10.1016/j.clcc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Desnoo L, Faithfull S. A qualitative study of anterior resection syndrome: the experiences of cancer survivors who have undergone resection surgery. Eur J Cancer Care (Engl) 2006;15:244–251. doi: 10.1111/j.1365-2354.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 10.Knowles G, Haigh R, McLean C, et al. Long term effect of surgery and radiotherapy for colorectal cancer on defecatory function and quality of life. Eur J Oncol Nurs. 2013 Oct;17(5):570–7. doi: 10.1016/j.ejon.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Pucciarelli S, Del Bianco P, Efficace F, et al. Health-related quality of life, faecal continence and bowel function in rectal cancer patients after chemoradiotherapy followed by radical surgery. Support Care Cancer. 2010;18:601–8. doi: 10.1007/s00520-009-0699-y. [DOI] [PubMed] [Google Scholar]
- 12.Wells CI, Vather R, Chu MJ, Robertson JP, Bissett IP. Anterior resection syndrome—a risk factor analysis. J Gastrointest Surg. 2015;19:350–359. doi: 10.1007/s11605-014-2679-x. [DOI] [PubMed] [Google Scholar]
- 13.Herrinton LJ, Altschuler A, McMullen CK, et al. Conversations for providers caring for patients with rectal cancer: Comparison of long-term patient-centered outcomes for patients with low rectal cancer facing ostomy or sphincter-sparing surgery. CA Cancer J Clin. 2016 Mar 21; doi: 10.3322/caac.21345. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendel CS, Grant M, Herrinton L, et al. Reliability and validity of a survey to measure bowel function and quality of life in long-term rectal cancer survivors. Qual Life Res. 23:2831–2840. doi: 10.1007/s11136-014-0724-6. 014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun V, Grant M, Wendel CS, et al. Dietary and Behavioral Adjustments to Manage Bowel Dysfunction After Surgery in Long-Term Colorectal Cancer Survivors. Ann Surg Oncol. 2015 Dec;22(13):4317–24. doi: 10.1245/s10434-015-4731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Temple LK, Bacik J, Savatta SG, et al. The development of a validated instrument to evaluate bowel function after sphincterpreserving surgery for rectal cancer. Dis Colon Rectum. 2005;48:1353–1365. doi: 10.1007/s10350-004-0942-z. [DOI] [PubMed] [Google Scholar]
- 17.Grant M, Ferrell B, Dean G, et al. Revision and psychometric testing of the City of Hope Quality of Life - Ostomy Questionnaire. Qual Life Res. 2004;13:1445–1457. doi: 10.1023/B:QURE.0000040784.65830.9f. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. SF-12v2™: How to Score Version 2 of the SF-12® Health Survey. Lincoln, RI: QualityMetric Incorporated; 2002. [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Kenig J, Richter P. Definition of the rectum and level of the peritoneal reflection - still a matter of debate? Wideochir Inne Tech Maloinwazyjne. 2013;8:183–6. doi: 10.5114/wiitm.2011.34205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fincham JE. Response Rates and Responsiveness for Surveys, Standards and the Journal. Am J Pharm Educ. 2008;72(2):43. [Google Scholar]
- 22.Downing A, Morris EJ, Richards M, et al. Health-related quality of life after colorectal cancer in England: a patient-reported outcomes study of individuals 12 to 36 months after diagnosis. J Clin Oncol. 2015;33:616–24. doi: 10.1200/JCO.2014.56.6539. [DOI] [PubMed] [Google Scholar]
- 23.Lange MM, den Dulk M, Bossema ER, et al. Risk factors for fecal incontinence after rectal cancer treatment. Brit J Surg. 2007;94:1278–1284. doi: 10.1002/bjs.5819. [DOI] [PubMed] [Google Scholar]
- 24.Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255:922–8. doi: 10.1097/SLA.0b013e31824f1c21. [DOI] [PubMed] [Google Scholar]
- 25.Ekkarat P, Boonpipattanapong T, Tantiphlachiva K, Sangkhathat S. Factors determining low anterior resection syndrome after rectal cancer resection: a study in Thai patients. Asian J Surg. 2015 Sep 1; doi: 10.1016/j.asjsur.2015.07.003. pii: S1015-9584(15)00086-X. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. 2013;15:1130–1139. doi: 10.1111/codi.12244. [DOI] [PubMed] [Google Scholar]
- 27.De Bruin AF, Schouten SB, de Kort PP, Gosselink MP, van der Harst E. The impact of chronic smoking on rectal mucosal blood flow. Tech Coloproctol. 2009 Dec;13(4):269–72. doi: 10.1007/s10151-009-0529-8. [DOI] [PubMed] [Google Scholar]