Abstract
Inflammation occurs after HIV infection and persists despite highly active antiretroviral therapy (HAART). Diffusion tensor imaging (DTI), measures HIV associated white matter changes, but can be confounded by inflammation. Currently, the influence of inflammation on white matter integrity in well-controlled HIV+ patients remains unknown. We used diffusion basis Spectrum imaging (DBSI) derived cellularity to isolate restricted water diffusion associated with inflammation separated from the anisotropic diffusion associated with axonal integrity. Ninety-two virologically suppressed HIV+ patients on HAART and 66 HIV uninfected (HIV-) controls underwent neuropsychological performance (NP) testing and neuroimaging. NP tests assessed multiple domains (memory, psychomotor speed, and executive functioning). DTI and DBSI derived fractional anisotropy (FA) maps were processed with tract based spatial statistics for comparison between both groups. Cellularity was assessed with regards to age, HIV status, and NP. Within the HIV+ cohort, cellularity was compared to clinical (HAART duration) and laboratory measures of disease (e.g. CD4 cell current and nadir). NP was similar for both groups. DTI derived FA was lower in HIV+ compared to HIV- individuals. In contrast, DBSI derived FA was similar for both groups. Instead, diffuse increases in cellularity were present in HIV+ individuals. Observed changes in cellularity were significantly associated with age, but not NP, in HIV+ individuals. A trend level association was seen between cellularity and HAART duration. Elevated inflammation, measured by cellularity, persists in virologically well-controlled HIV+ individuals. Widespread cellularity changes occur in younger HIV+ individuals and diminish with aging and duration of HAART.
Keywords: Cellularity, Diffusion tensor imaging (DTI), Diffusion basis Spectrum imaging (DBSI), HIV, Inflammation
Introduction
Advances in highly active antiretroviral therapy (HAART) have prolonged the lifespan for HIV-infected (HIV+) patients.1 This has increased the incidence of older (> 50 years old) HIV+ individuals in the United States (US).2 Most HIV+ individuals in the US have access to HAART and a majority virologically well-controlled.3 Despite these advances, cognitive impairment and systemic inflammation persist in the HAART era.4,5
Currently, HIV associated cognitive impairment is quantified by neuropsychological performance (NP) testing and ability to perform activities of daily living.6 However, NP may be insensitive to neurodegenerative changes associated with chronic low-level neuro-inflammation that persist in virologically well-controlled HIV+ patients.7,8 Systemic inflammation can exacerbate immune dysfunction and lead to conformational changes in cellular expression that are similar to “premature aging”.9
The advent of HAART has led to increased focus on mechanisms that reduce chronic systemic inflammation and improve prognostic outcome.10 Peripheral blood measures and/or cerebrospinal fluid (CSF) markers have traditionally been used to quantify residual inflammation. Peripheral markers of inflammation (e.g. neopterin, C-reactive protein, sCD163, sCD14 and subpopulations of monocytes and CD4 and CD8 T lymphocytes) are often elevated prior to initiation of HAART and remain abnormal even after viral suppression with HAART.5,7 These measures are regarded as proxies for disease severity and immune activity.11,12 The focus of ongoing studies has shifted to diminishing residual inflammation that remains in virologically well-controlled individuals receiving HAART.10 However, current investigations have not focused on the spatial extent of inflammatory changes that continue to persist in the brain. Neuroimaging of neuro-inflammation may provide important localization of residual inflammatory changes in HIV+ patients receiving HAART.
Diffusion tensor imaging (DTI) is a non-invasive magnetic resonance imaging (MRI) method that detects changes in white matter microstructural integrity.13,14 DTI, which assess the diffusion of water molecules, has become increasingly popular for assessing HIV-associated white matter changes within HIV+ patients with detectable15-17 and undetectable viral load.18 However, DTI is incapable of distinguishing underlying pathophysiological changes beyond axonal integrity19 and can be confounded by inflammation.20
Diffusion basis Spectrum imaging (DBSI) employs a novel data-driven multiple-tensor modeling approach to disentangle biological entities within a particular voxel.20 This method preserves the characteristics of DTI by assuming Gaussian diffusion but avoids using high b values.21 In DBSI, the apparent diffusion coefficient is further differentiated into anisotropic (representing fiber tracts) and isotropic (representing infiltrating cells (as measured by cellularity), edema, and tissue loss) components. Thus, DBSI provides additional information concerning underlying white matter dysfunction and surrounding pathological changes.20-22
Here we compared DBSI derived cellularity, a correlate of neuroinflammation, in a cohort of aviremic HIV+ patients and HIV- controls. We predict that cellularity will be higher in virologically well-controlled HIV+ patients compared to HIV- controls as this metric will reflect subtle inflammation that may continue to persist after viral suppression is attained with HAART. First, we compared standard DTI measures to DBSI metrics for both groups. Next, we focused on changes in cellularity with respect to aging and NP testing. Finally, we studied associations between cellularity and laboratory variables within the HIV+ cohort.
Materials and Methods
Subjects
Participants were recruited from the Washington University School of Medicine (WUSM) Infectious Disease Clinic, the WUSM AIDS Clinical Trial Unit (ACTU), and the Supportive Positive Opportunities with Teens (SPOT). Participants provided informed written consent that was approved by the Institutional Review Board at WUSM. Each participant was screened for the following exclusion criteria: current or past history of confounding neurological disorders, ≥29 on the Beck Depression Inventory II,23 current alcohol or substance abuse, head injury with loss of consciousness greater than 30 minutes, claustrophobia or seizures, or fewer than 8 years of education. Current alcohol and substance misuse information were collected via self-report for the past month prior to NP testing. Confirmation of serologic status was performed for HIV- participants using a rapid oral HIV test. All HIV+ individuals were virally suppressed (<20 copies/ml) and on a stable HAART regiment for at least 3 months prior to time of assessment. Demographic information on both groups is provided in Table 1. For the HIV+ cohort, clinical disease variables (e.g. duration of infection, medication status, etc.) were obtained from medical records or self-reported when records were not available. All HIV+ participants had laboratory evaluations (peripheral blood CD4 cell count and HIV RNA viral load) within one year of neuroimaging.
Table 1.
Demographics of HIV infected and HIV uninfected individuals
| HIV- (N=66) | HIV+ (N=92) | p-value | |
|---|---|---|---|
|
| |||
| Age in Years (SD) | 38.6 (17.3) | 49.1 (12.4) | <0.001** |
|
| |||
| Gender (% Male) | 53% | 76% | 0.003** |
|
| |||
| Ethnicity | 0.03* | ||
| % Caucasian | 50% | 33% | |
| % African American | 48% | 67% | |
| % Asian | 2% | 0% | |
|
| |||
| Education in Years (SD) | 13.6 (1.8) | 13.1 (2.9) | 0.2 |
|
| |||
| Current Mean Plasma CD4 Cell Count (SD) | Not applicable | 636.8 (315.3) | --- |
|
| |||
| Nadir Mean Plasma CD4, (SD) | Not applicable | 185.6 (176.5) | --- |
|
| |||
| Duration of Infection (Years) | Not applicable | 13.4 (8.9) | --- |
|
| |||
| Duration of Highly Active Antiretroviral Therapy (HAART) in Years (SD) | Not applicable | 10.3 (7.1) | --- |
|
| |||
| CPE | Not applicable | 7.54 (1.69) | --- |
|
| |||
| Substance Abuse HIV- | |||
| N = 35 | |||
| HIV+ N = 78 | |||
|
| |||
| Tetrahydrocannabinol | 9/35 | 23/78 | 0.68 |
|
| |||
| Cocaine | 3/35 | 10/78 | 0.52 |
|
| |||
| Opiate | 3/35 | 5/78 | 0.68 |
|
| |||
| Methamphetamine | 1/35 | 2/78 | 0.93 |
|
| |||
| Barbiturates | 1.35 | 1/78 | 0.56 |
|
| |||
| Benzodiazapene | 2/35 | 7/78 | 0.56 |
|
| |||
| Phencyclidine | 1/35 | 1/78 | 0.56 |
|
| |||
| Alcohol | 26/35 | 50/78 | 0.29 |
|
| |||
| Smoking | 20/51 | 38/91 | 0.77 |
| HIV- N = 51 | |||
| HIV+ N = 91 | |||
p<0.05
p<0.01
CPE=CNS Penetration Effectiveness
Neuropsychological performance
Cognition was evaluated across a standard battery that encompasses three domains: executive, motor, and verbal learning memory.24 Raw NP scores were converted to standardized T-scores that were adjusted for age, gender, and education by subtracting the appropriate normative mean from the raw score and then dividing by the normative standard deviation. The three domains tested included:
Executive function -- Letter Number Sequencing, Trail Making Test-B25, and Verbal Fluency26
Verbal learning and memory -- Hopkins Verbal Learning Test (HVLT) learning and recall.27
Psychomotor speed -- Grooved Peg Board28 and Trail Making Test-A.25
NP scores were categorized into their respective domains and averaged to yield a domain specific value. A composite neuropsychological summary T-score was calculated by averaging T-scores across domains.29-31 These NP tests have previously been shown to be affected by HIV.32
Imaging Acquisition
Imaging was performed on the same 3T Siemens Tim TRIO for all participants (Siemens AG, Erlangen Germany). High-resolution 3D magnetization-prepared rapid acquisition of gradient echo (MP-RAGE) images were collected in the sagittal plane using a 12-channel head coil. A total of 176 slices, 1.0-mm slice thickness, and voxel dimensions of 1.0×1.0×1.0mm were acquired. Two sequential diffusion-weighted scans were obtained (2 × 2 × 2 mm voxels, TR=9,900 ms, TE=102 ms, flip angle = 90°, 23 directions, b-values ranging from 0 to 1400 s/mm2), and one non-diffusion weighted image.33
Image Pre-Processing for DTI and DBSI
Preprocessing included correction for motion and eddy current distortions followed by skull stripping using FSL 5.0.9.34 Rigorous motion inspection was applied after eddy current correction. Subjects that moved more than 3.5 mm were excluded. Tensor calculation for FA was derived using FMRIB software library (FSL) for preparation of tract based spatial statistics.35
DTI Processing
Fractional anisotropy (FA) measurements were generated in FSL with DTIFIT.36,37 This single diffusion model represents the overall displacement of water molecules primarily oriented by the hydrophobic properties of myelin. This model depicts axonal projections but can be insensitive to subtle patterns of diffusion that reflect underlying pathological changes due to persistent inflammation (Jones et al., 2013). In single diffusion models FA values reflect the diffusion signal (Figure 1 adapted from Chiang et al., 2014) but do not dissociate anisotropic from isotropic diffusion.
Figure 1.

Comparison between diffusion tensor imaging (DTI) and diffusion basis Spectrum imaging (DBSI). Voxels are relatively large with regard to the underlying complex biology. DBSI is capable of differentiating amongst cellularity (green), non-restricted diffusion (blue), and axons (red) whereas DTI cannot dissociate these various measures.
DBSI Processing
The multiple tensor model that is utilized by DBSI allows for increased sensitivity at detecting underlying biological changes that can disrupt standard DTI metrics. Inflammation, as detected by DBSI, has been shown to cause spurious results regarding white matter damage.21 Extracting the cellularity component from traditional measures of structural integrity (FA) could clarify whether HIV+ pathology can also compromise structural integrity. We expected an increase in cellularity in virologically well-controlled HIV+ patients compared to HIV- controls as this metric signifies subtle inflammation even in virally suppressed HIV+ individuals receiving HAART.
DBSI metrics were generated using in-house software scripted in MATLAB.20 The overall diffusion signal (Sk) measured by DBSI is comprised of both anisotropic (Ak) and isotropic components (Ik; Equation 1). The anisotropic signal depicts elliptical diffusion but does not quantify the degree of anisotropy (red wedge Figure 1). DBSI derived FA is generated on the isolated anisotropic signal, which excludes isotropic diffusion (blue and green wedges Figure 1). For DTI, FA quantifies the degree of anisotropy for the entire signal while DBSI estimates this metric only for anisotropic patterns of diffusion (red wedge Figure 1).
| Equation 1 |
| Equation 2 |
In Equation 2 the anisotropic component is derived from the axial (λ∥_i) diffusivity, radial diffusivity (λ⊥_i), and the angle (ψik) between the kth diffusion gradient and the principal direction of the ith anisotropic tensor. The pure anisotropic signal reflects water diffusion in and around axons. FA was derived from Ak using previously described methods.20
| Equation 3 |
Isotropic diffusion is comprised of both restricted diffusion (cellularity), and non-restricted isotropic diffusion patterns (extracellular space). In this equation, the integral, demarcated by a and b represent the boundaries for the isotropic diffusion spectrum. Cellularity is defined by an upper boundary (b) of 0.3 μm2/ms while extracellular diffusion is calculated when the boundary is greater than 0.3 μm2/ms.20-22
Voxel-Wise Processing
Post-processing of DTI and DBSI was conducted with tract based spatial statistics (TBSS).35 TBSS alleviates registration artifacts and isolates voxels within the white matter.35 Briefly, all post processed images were warped into common space using a combination of linear and non-linear alignments.35 White matter skeletons were generated from voxels within core white matter by searching for the local FA maximum in each voxel.35
Statistical Analyses
Demographic and Clinical Measures
Neuropsychological performance differences in cognitive domains and composite scores were compared between HIV- and HIV+ participants. Correlations were also performed between NP values (domain and composite scores) and cellularity for both HIV+ and HIV- controls. Additionally, associations were performed between clinical variables (current CD4, nadir CD4, duration of infection, and duration of treatment) and cellularity for HIV+ participants after adjusting for gender and age.
Voxel-Wise Analysis
A voxel-wise analysis of both DBSI and DTI data was performed using Randomise, a statistical package in FSL.38 A threshold-free cluster enhancement approach was utilized to correct for multiple comparisons with a family-wise error rate derived from 5,000 Monte Carlo permutations.39 For the preceding voxel-wise analyses only, residual maps were created after adjusting for age and gender at the voxel level using a general linear model in FSL.
Aging Analysis
Since microglial activity can occur throughout the brain in HIV,40 an average skeletal value for cellularity was derived and used for subsequent analyses. The effect of age on cellularity was assessed for HIV+ patients and HIV- controls. Linear regression models assessed the relationship between cellularity and age for both HIV+ and HIV- groups separately, and jointly. For this regression model gender was treated as a covariate at the voxel level prior to quantifying a cellularity value. The potential interaction between age and HIV status on cellularity was also evaluated. For the HIV+ group, we performed a multiple linear regression of cellularity as a function of age after adjusting for duration of infection and duration of treatment. Residuals were calculated in the HIV+ cohort for age and cellularity after adjusting for either duration of infection or duration of treatment. These residuals were subsequently compared to the HIV- cohort to determine if an interaction between age and HIV status was present.
Results
Demographic and Clinical Variables
Overall, the HIV+ group (n=92) was significantly older (p<0.001) and had a higher proportion of males (p=0.003) compared to the HIV- cohort (n=66). The two groups were similar with regards to education. No differences were seen between cohorts for substance misuse including: cannabis (p=0.68), cocaine (p=0.52), opiates (p=0.68), methamphetamine (p=0.93), barbiturates (p=0.56), benzodiazepines (p=0.56), phencyclidine (p=0.56), alcohol (p=0.29), or smoking (p=0.77). Observed differences in age and gender were included within subsequent analyses.
Voxel-Wise Comparison for DTI and DBSI
DTI-derived FA, that does not exclude isotropic patterns of diffusion, was significantly different between HIV+ and HIV- individuals. Differences were predominantly seen within the left inferior longitudinal fasciculus, parietal regions, external capsule, anterior corona radiate, and bilateral superior temporal regions (Supplemental Figure 1). However, FA derived from DBSI was not significantly different between the two groups. DBSI FA exclusively evaluates the anisotropic component (red wedge; Figure 1) independently from isotropic diffusion.
Cellularity was the only isotropic measure that was significantly different between the two groups (p<0.05 corrected). Differences in cellularity were seen diffusely throughout the brain (Figure 2). Due to the large age difference between our two populations we repeated this voxel-wise analysis on a smaller subset of 40 HIV- and 40 HIV+ individuals that were age-gender matched (Supplemental Figure 2). Similar diffuse changes were seen with HIV+ participants having higher cellularity than HIV- individuals. Observed differences between the two diffusion methods may reflect that DTI measures are confounded by isotropic patterns of diffusion that appear as a loss in white matter integrity.
Figure 2.
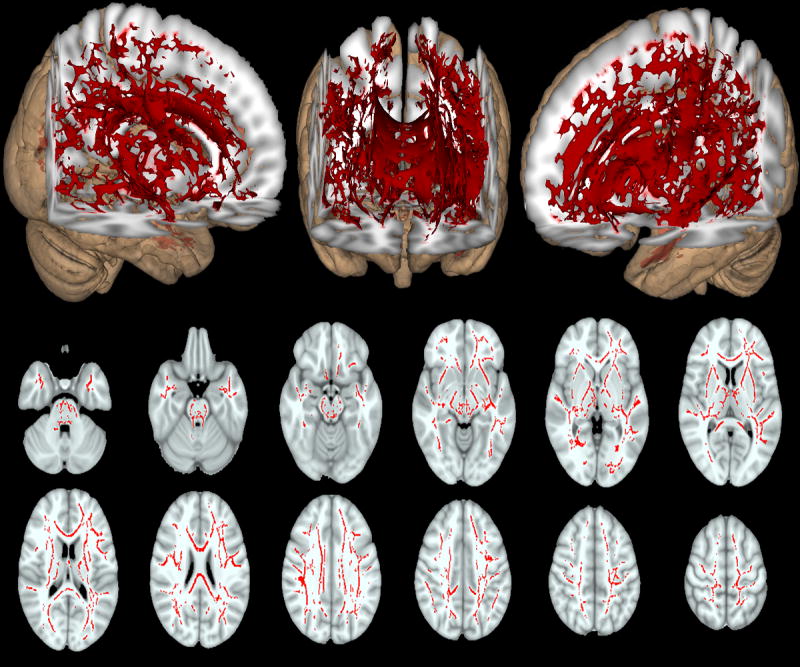
Differences in cellularity between HIV infected (HIV+) compared to HIV uninfected (HIV-) controls (red voxels). Higher cellularity was diffusely seen in HIV+ patients compared to HIV- controls after controlling for differences in age and gender between the two groups. Overall, diffuse increases in cellularity were seen for both 3D representation (top row) and select 2D axial slices (middle and bottom rows). Significant voxels were corrected for age and gender (p<0.05).
Skeletal Average Comparison for DTI and DBSI
Due to the disperse cellularity findings, skeletal averages were acquired for subsequent analyses. Skeletal averages revealed similar findings to those observed using a voxel-wise analysis (Figure 3). In particular, FA derived from DTI was significantly diminished for HIV+ patients compared to HIV- controls. In contrast, significant differences were seen for average cellularity, but not FA, for DBSI.
Figure 3.

Comparisons between HIV+ (red) and HIV- (green) for average diffusion imaging metrics (both DTI and DBSI). FA derived from DTI was significantly reduced in HIV+ patients (red) compared to HIV- controls (green). In contrast, FA derived from DBSI was not significantly different with cellularity instead significantly higher in HIV+ individuals compared to HIV- controls. Average diffusion measures were normalized for visual purposes. All values were corrected for age and gender.
* = p<0.05
** = p<0.01
Relationships Between DBSI and NP or Clinical Variables
NP was not significantly different between HIV+ and HIV- participants for either composite (p=0.76) or domain specific scores (p=0.23 Memory; p=0.88 Psychomotor Speed; p=0.47 Executive). Cellularity did not correlate with cognitive measures (data not shown). Cellularity also did not correlate with laboratory measures (including log nadir CD4 (r=0.218; p=0.087), log current CD4 (r=0.083; p=0.48)), or clinical measures (duration of infection (r=-0.137, p=0.23)). A trend towards a negative association was observed between years on medication and cellularity (r=-0.202; p=0.072).
Effects of aging on cellularity in HIV+ and HIV- individuals
In an analyses that included all participants, a significant interaction was observed between age and HIV status for cellularity (p=0.025). This negative association was primarily driven by HIV+ (r=-0.310; p=0.003) and not HIV- controls (r<0.001; p=0.99) (Figure 4).When duration of infection was included as a covariate for the HIV+ group, the association between age and cellularity remained unchanged (r=-0.240; p=0.035) but the interaction between HIV status and age was only at a trend level (p=0.076). After adjusting for duration on HAART neither the relationship between cellularity and age (r=-0.197; p=0.0785; Figure 4) nor interaction with HIV status were significant (p=0.154).
Figure 4.

The effects of aging on cellularity in HIV+ and HIV- individuals. (A) A negative relationship between cellularity and age was observed for HIV+ (r=0.-31; p=0.003) individuals but not HIV- controls (r<0.001, p=0.999) after regressing for gender. (B) After adjustment for gender and duration of HAART the relationship was no longer significant (blue regression line; r=-0.197, p=0.079).
Discussion
DBSI has been previously utilized to assess inflammation in other neurological diseases (e.g. Multiple Sclerosis).22 We extended its’ application to virologically well-controlled HIV+ individuals. Our data shows that typical DTI measurements may be confounded by the presence of inflammation, which is not the case for DBSI. Diffuse increases in cellularity were seen in HIV+ individuals compared to HIV- controls. In particular, cellularity was greatest in younger HIV+ individuals and was modulated by duration on HAART. Observed changes in cellularity did not correlate with clinical laboratory variables or degree of cognitive impairment.
NP has often been used to measure the effects of HIV in the brain.6 NP testing may be insensitive to subtle inflammation that persists in virologically well-controlled HIV+ individuals in the HAART era.7 Additional neuroimaging metrics that measure inflammation may provide important information concerning residual reservoirs.41 Our results suggest that diffuse inflammation, as measured by cellularity, may still occur in HIV+ patients regardless of their degree of impairment. Additional adjunctive measures to reduce inflammation may be needed for virologically well-controlled HIV+ individuals on HAART.
Advanced non-invasive neuroimaging modalities have focused on HIV associated changes in white matter. In particular, diffusion weighted imaging has increasingly been utilized within HIV+ individuals.42 Often decreases in FA have been seen in HIV+ individuals compared to HIV- controls.15,18,33,43-46 While we observed a decrease in FA for HIV+ individuals using DTI, DBSI revealed that observed changes in DTI may in fact reflect the continued presence of inflammation. Inflammation can appear as axonal loss from reduced diffusion along the primary direction yielding a concurrent reduction in FA.20 When FA was derived independently from other isotropic factors using DBSI no difference was seen between HIV+ and HIV- individuals. These results suggest caution when investigating typical DTI measures such as FA in this population.
Cellularity is sensitive to cellular presence and may reflect microglial activity in the brain.22 Our results complement previous pathological findings that show increased level of microglial activation in HIV+ individuals.47 Additionally, a recent positron emission tomography (PET) study also revealed similar diffuse spatial patterns of microglial activation in aviremic cognitively normal HIV+ patients compared to HIV- controls.40 Our current results using DBSI derived metric of cellularity nicely complement the aforementioned study. What is unique about DBSI is that this technique can be performed on most conventional MRI scans, does not require the synthesis of a tracer, and does not require genetic studies to confirm receptor binding capacity.
An increasing older HIV+ population is now present as the HIV infection has become a more chronic condition. We observed a negative relationship between aging and cellularity for the HIV+ group with younger HIV+ individuals having the greatest cellularity. In contrast, cellularity was constant across a spectrum of ages for HIV- controls. This unique aging relationship for HIV+ individuals was influenced by HAART. After accounting for duration of HAART, a decrease in inflammation was seen with long-term treatment. This implies that persistent inflammation can partially recede with aging, which is in agreement with prior studies that monitor peripheral markers of immune activation.10,48
Inflammation is present even after HAART using in vivo neuroimaging. A reduction of inflammation is possible but may require protracted HAART administration before normalization. Neuroimaging markers paired with other markers of inflammation could therefore assist in better characterizing a patient’s treatment trajectory. Viral reservoirs are the proposed instigators of subtle inflammation and cellularity may directly monitor spatial topography of viral reservoir activity or indirectly by a secondary inflammatory process. These results would suggest that early initiation of HAART with continued maintenance is important for reducing inflammation associated with HIV. However, our current study is cross-sectional and longitudinal inquiries are needed.
Microglial and macrophages assist in CNS injury repair and are the main sources of proinflammatory cytokines.49 Initiation of HAART can reduce immune mediated changes but microglial and macrophage activation remains elevated.4,12,50 In addition, latent HIV viral antigen can instigate further immune activation leading to a proliferation of microglia and reactivation of virus.51 Cellularity is sensitive to cellular proliferation and may reflect prominent microglial and macrophage induced inflammation that occurs in response to injury.20 This would explain the lack of a relationship with absolute and nadir CD4 T-cells that was observed in this study. Our current study focused on peripheral cellular markers that are routinely acquired in the clinic. We did not investigate soluble plasma and cerebrospinal (CSF) inflammatory markers or specific subpopulations of peripheral cells that could provide additional insight on cellular and immunologic correlates of cellularity. Further studies are needed to determine if there is an association between cellularity with naïve, effector, central or effector memory T cells, subpopulations of monocytes and soluble plasma and CSF inflammatory markers.
In summary, our data demonstrate a novel neuroimaging approach to detect residual inflammation in virologically well-controlled HIV+ individuals. DBSI has the potential to distinguish different etiologies that influence the patterns of diffusion within an HIV+ cohort. Elevated levels of cellularity were diffusely seen in HIV+ individuals compared to HIV- controls, with younger HIV+ patients having the greatest cellularity changes. After adjusting for duration of HAART the association between age and cellularity in HIV+ individuals was no longer significant suggesting that prolonged treatment may reduce inflammation. These studies suggest a potential non-invasive method for assessing potential brain viral reservoirs in HIV+ individuals on HAART.
Supplementary Material
Acknowledgments
We would like to express our sincerest gratitude to our study participants that helped make this work possible.
Funding:
This work was funded by the National Institute of Health Grants R01-NR012907 (BA), R01-NR012657 (BA), R01-NR014449 (BA), and R25MH080663 (LB). Research was conducted and supported by the Washington University Institute of Clinical and Translational Sciences (UL1 TR000448 from the National Center for Advancing Translational Sciences).
Footnotes
Author Contributions:
Jeremy F Strain: Processed, analyzed, contributed to interpretation of the data and participated in writing the manuscript. Tricia H. Burdo: Contributed to the design of the study, consulted on the analyses, contributed to the interpretation of the data, and participated in writing the manuscript. Sheng-Kwei Song: Involved in the processing of the data, interpretation of the data, and participated in writing the manuscript. Peng Sun: Involved in the processing of the data, interpretation of the data, and participated in writing the manuscript. Omar El-Ghazzawy: Involved in the processing of the data, interpretation of the data, and participated in writing the manuscript. Brittany Nelson: Involved in subject recruitment, acquisition and preparation of the data, consulted on the design of the study, and participated in writing the manuscript. Elizabeth Westerhaus: Involved in subject recruitment, supervised and constructed the neuropsychological battery that was administered to our subjects, consulted on the design of the study, and participated in writing the manuscript. Laurie Baker: Involved in subject recruitment, acquisition and preparation of the data, consulted on the design of the study, and participated in writing the manuscript. Florin Vaida:Contributed to the design of the study, offered critical revisions of the manuscript, contributed to the interpretation of the data, and participated in writing the manuscript. Beau Ances: Involved in the conception and design of the study, provided revisions and assistance in manuscript preparation, contributed to the interpretation of the data, administrative duties and obtained funding for this project.
References
- 1.Lohse N, Hanse AE, Gerstoft J, Obel N. Improved survival in HIV-infected persons: consequences and perspectives. Journal of Antimicrobial Chemotherapy. 2007;3:461–463. doi: 10.1093/jac/dkm241. [DOI] [PubMed] [Google Scholar]
- 2.Luther VP, Wilkin AM. HIV Diseases in Older Adults. Clinics in Geriatric Medicine. 2007;23:567–583. doi: 10.1016/j.cger.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, et al. Epidemiology of Human Immunodeficiency Virus-Associated Opportunistic Infections in the United States in the Era of Highly Active Antiretroviral Therapy. Clinical Infectious Diseases. 2000;30:S5–S14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdo TH, Lentz MR, Autisser P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antinori A, Marcotullio S, Andreoni M, Ammassari A, Monforte AA, Galli M, et al. Italian guidelines for the use of antiretroviral agents and the diagnostic-clinical management of HIV-1 infected persons. Update December 2014. New Microbiol. 2015;38:299–328. [PubMed] [Google Scholar]
- 7.Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar ES, et al. Persistence of HIV- Associated Cognitive Impairment, Inflammation and Neuronal Injury in era of Highly Active Antiretroviral Treatment. AIDS. 2011;25:625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtfuss GF, Cheng W, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham, et al. Virologically Suppressed HIV Patients Show activation of NK cells and Persistent Innate Immune Activation. J Immunol. 2012;189:1491–1499. doi: 10.4049/jimmunol.1200458. [DOI] [PubMed] [Google Scholar]
- 9.Nasi M, Pinti M, Biasi SD, Gibellini, Ferraro D, Mussini C, et al. Aging with HIV infection: A journey to the center of inflammAIDS, immunosenescence and neuroHIV. Immunol Lett. 2014;162:329–333. doi: 10.1016/j.imlet.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Funderburg NT, Boucher M, Sattar A, Kulkarni M, Labbato D, Kinley BI, et al. Rosuvastatin decreases intestinal fatty acid binding protein (I-FABP), but does not alter zonulin or lipopolysaccharide binding protein (LBP) levels, in HIV-infected subjects on antiretroviral therapy. Pathog Immun. 2016;1:118–128. doi: 10.20411/pai.v1i1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuler PJ, Macatangay BJC, Saze Z, Jackson EK, Riddler SA, Buchanan WG, et al. CD4+CD73+ T cells are associated with lower T-cell activation and C reactive protein levels and are depleted in HIV-1 infection regardless of viral suppression. AIDS. 2013;27:1545–1555. doi: 10.1097/QAD.0b013e328360c7f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27:1387–1395. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;1:259–67. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Chiro DG. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 15.Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: A diffusion tensor imaging study. Psychiatry Res. 2001;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB. Diffusion Alterations in Corpus Callosum of Patients with HIV. AJNR. 2006;27:656–660. [PMC free article] [PubMed] [Google Scholar]
- 17.Hoare J, Jean-Paul F, Nicole P, Joska J, Paul R, Donald KA, et al. White matter micro-structural changes in ART-naïve and ART-treated children and adolescents infected with HIV in South Africa. AIDS. 2015;29:1793–1801. doi: 10.1097/QAD.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 18.Su T, Caan MWA, Wit FWNM, Schouten J, Geurtsen GJ, Cole JH, et al. White matter structure alterations in HIV-1-infected men with sustained suppression of viraemia on treatment. AIDS. 2016;30:311–322. doi: 10.1097/QAD.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 19.Jones DK, Knosche TR, Turner White matter integrity, fiber count, and other fallacies: The do’s and dont’s of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Wang Q, Haldar JP, Yeh FC, Xie M, Sun P, et al. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134:3590–601. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang C, Wang Y, Sun P, Lin T, Trinkaus K, Cross AH, et al. Quantifying white matter tract diffusion parameters in the presence of increased extra-fiber cellularity and vasogenic edema. NeuroImage. 2014;101:310–319. doi: 10.1016/j.neuroimage.2014.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Sun P, Wang Q, Trinkaus K, Schmidt RE, Naismith RT, et al. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain. 2015;138:1223–38. doi: 10.1093/brain/awv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and –II in psychiatric out-patients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 24.Baker LM, Robert PH, Heap-Woodruff JM, Chang JY, Ortega M, Margolin Z, et al. The effect of central nervous system penetration effectiveness of highly active antiretroviral therapy on neuropsychological performance and neuroimaging in HIV infected individuals. J Neuroimmune Pharmacol. 2015;10:487–492. doi: 10.1007/s11481-015-9610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skill. 1958;8:271–276. [Google Scholar]
- 26.Benton AL, Hamsher K. Multilingual aphasia examination: Manual of instruction. Iowa City: University of Iowa; 1976. [Google Scholar]
- 27.Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5:125–142. [Google Scholar]
- 28.Baser CN, Ruff RM. Construct validity of the San Diego Neuropsychological Test Battery. Arch Clin Neuropsychol. 1987;2:13–32. [PubMed] [Google Scholar]
- 29.De Santi S, Pirraglia E, Barr W, James B, Schantel W, Rogers K, et al. Robust and conventional neuropsychological norms: Diagnosis and prediction of age-related cognitive decline. Neuropsychol. 2008;22:469–484. doi: 10.1037/0894-4105.22.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for Letter and Category Fluency: Demographic Corrections for Age, Education, and Ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 31.Ruff RM, Parker SB. Gender-and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 32.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright PW, Vaida FF, Fernandez RJ, Rutlin J, Price RW, Evelyn L, et al. Cerebral white matter integrity during primary HIV infection. AIDS. 2015;29:433–442. doi: 10.1097/QAD.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multisubject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Hrabe J, Kaur G, Guilfoyle DN. Principles and limitations of NMR diffusion measurements. J Med Phys. 2007;32:34–42. doi: 10.4103/0971-6203.31148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behrens TEJ, Woolrigh MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 38.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;J15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, et al. Neuroinflammation in treated HIV-positive individuals. Neurology. 2016;86:1425–1432. doi: 10.1212/WNL.0000000000002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13:907. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masters MC, Ances BM. Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol. 2014;34:89–102. doi: 10.1055/s-0034-1372346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ragin AB, Wu Y, Gao Y, Keating S, Du H, Sammet C, et al. Brain alterations within the first 100 days of HIV infection. Ann Clin Transl Neurol. 2015;2:12–21. doi: 10.1002/acn3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filippi CG, Ulug AM, Ryan E, Ferrando SJ, Van Gorp W. Diffusion tensor imaging of patients with HIV and Normal-appearing White Matter on MR Images of the Brain. AJNR Am J Neuroradiol. 2001;22:277–283. [PMC free article] [PubMed] [Google Scholar]
- 45.Du H, Wu Y, Ochs R, Edelman RR, Epstein LG, McArthur J, et al. A comparative evaluation of quantitative neuroimaging measurements of brain status in HIV infection. Psychiatry Res. 2012;203:95–99. doi: 10.1016/j.pscychresns.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, An H, Zhu H, Stone T, Smith JK, Hall C, et al. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage. 2009;47:1154–1162. doi: 10.1016/j.neuroimage.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tauber SC, Staszewki O, Prinz M, Weis J, Nolte K, Bunkowski S, et al. HIV encephalopathy: glial activation and hippocampal neuronal apoptosis, but limited neural repair. HIV Medicine. 2015;17:143–151. doi: 10.1111/hiv.12288. [DOI] [PubMed] [Google Scholar]
- 48.Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol. 2016;14:55–60. doi: 10.1038/nrmicro.2015.5. [DOI] [PubMed] [Google Scholar]
- 49.Colonna M, Butovsky O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol. 2017 doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, et al. Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis. 2017;64:124–131. doi: 10.1093/cid/ciw683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tompkins L, Dukhovlinova E, Swanstrom R. HIV Reservoirs in the Central Nervous System. 2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


