Abstract
Metastatic cancer cells invading through dense tumor stroma experience internal and external forces that are sensed through a variety of mechanosensory proteins that drive adaptations for specific environments. Alpha-actinin-4 (ACTN4) is a member of the α-actinin family of actin crosslinking proteins that is upregulated in several types of cancers. It shares 86% protein similarity with α-actinin-1, another non-muscle ACTN isoform, which appears to have a more modest role, if any, in cancer progression. While they share regulatory mechanisms, such as phosphorylation, calcium binding, phosphatidyl inositol binding, and calpain cleavage, α-actinin- 4 exhibits a unique mechanosensory regulation that α-actinin-1 does not. This behavior is mediated, at least in part, by each protein’s actin-binding affinity as well as the catch-slip-bond behavior of the actin binding domains. We will discuss currently known modes of ACTN4 regulation, their interactions, and how mechanosensation may provide major therapeutic targeting potential for cancer metastasis.
1. Introduction
Mechanosensation is the cellular process in which cells sense and respond to physical forces of their environment. It is crucial for a number of developmental and homeostatic cellular processes, such as the generation of epithelial cell-to-cell junctions [1], axonal guidance [2], and fibroblast migration [3] as well as pathological conditions such as cancer metastasis [4]. Understanding the cellular mechanisms driving metastasis will allow us to develop effective therapies to mitigate the dangers of late diagnoses often seen in many cancer types. During cancer cell invasion, a cell must sense its environment and actively rearrange its actin cytoskeleton to allow for efficient leading edge protrusion formation [5], retraction of the trailing edge [6] and nuclear translocation [7]. It is becoming evident that a well-regulated network of proteins is central to mechanosensation that allow a cell to quickly respond and adapt. The mechanical and mechanosensory roles of the α-actinin proteins have been studied using a variety of techniques including traction force microscopy [8], magnetic and optical tweezers [9], atomic force microscopy [10], and the application of physical stress by micropipette aspiration (MPA) [11]. This review will discuss how the tumor environment affects the mechanosensing protein, α-actinin 4 (ACTN4), and the various modes of regulation that enhance or inhibit its activity, thereby influencing a tumor cell’s metastatic behavior.
2. Physical environment of a tumor
The tumor stroma plays a large role in the development [12], differentiation [13] and dissemination [14, 15] of the metastatic cells of a variety of solid tumors [16]. The stroma is defined as the components of the tumor that are not cancer cells themselves, and can have a different composition depending on the cancer-type [12]. However, it usually includes immune cells, neurons, blood vessels, cancer-associated fibroblasts (CAFs), and the extra cellular matrix deposited by CAFs [12]. As an example of the importance of the stroma, the hallmark diagnostic of breast cancer is a palpable mass, created by the deposition of a dense fibrillary collagen matrix deposited by CAFs [17]. In fact, breast tumor density is a strong negative prognostic indicator of survivability [18]. In addition, glioblastoma cells invade along blood vessels which have much higher rigidity than neurons, indicating a sensation and preference for stiffer substrates [19]. In pancreatic tumors, the pancreatic stromal cells (PSCs) are thought to deposit the dense collagen type I network seen in pancreatic cancer [20]. A feedback loop exists in which a cancer cell stimulates PSCs to deposit more collagen [21], and the collagen matrix stimulates the proliferation and dissemination of tumor cells [22, 23], driving increased stromal density as the tumor grows. To successfully disseminate from the primary tumor, the metastatic cancer cell must adapt to become efficient at migrating through the dense and rigid stromal network of three-dimensional collagen. The increased extracellular matrix deposition, as well as increased hydrostatic pressures, creates a physical stress on tumor cells [24]. Exactly how the interplay between extracellular environment and intracellular cytoskeletal dynamics enhance cancer proliferation and metastasis is still unclear. What is becoming evident is that targeting one aspect of tumor biology, such as cell division, may be insufficient and both tumor cell and stroma need to be addressed for optimal survivability [25]. ACTN4 is a key link between the two tumor components, because it directs the coupling of the actin cytoskeleton inside the cell to the integrins that directly bind the stromal ECM [26], and is capable of sensing and responding to externally-applied force [27]. For this reason, its mechanisms of regulation may provide insight into methods of uncoupling the two tumor components, and thus inhibiting invasion.
3. Alpha-Actinin in Metastatic Cancer
Mammals have four α-actinin isoforms: ACTN2 and ACTN3 are considered to be specific to muscle, while ACTN1 and ACTN4 are expressed in non-muscle cells [28]. ACTN4 is also upregulated and crucial for the development and progression of breast [29], colorectal [30], ovarian [31], and pancreatic [32, 33] cancers. While their primary biochemical function is actin crosslinking, it remains unclear whether the non-muscle paralogs play distinct or redundant roles in organizing the actin cytoskeleton. ACTN4, for example, plays multiple roles in influencing cellular morphology, yet the mechanisms by which it contributes to increased cancer invasion has not been resolved. Previous studies found that ACTN4 plays a major role in destabilizing focal adhesions in multiple cell types [34, 35]. The focal adhesion protein zyxin binds to the rod domain of ACTN4 and recruits it to nascent focal adhesions [26]. Overexpression of ACTN4 in melanoma cancer cell lines drives cellular morphology from a more mesenchymal type to an amoeboid type by reducing focal adhesion size [35]. In ACTN4 knockdown mouse fibroblasts, focal adhesions are fewer, yet larger, and spreading is increased, consistent with mesenchymal type cells [36]. Both examples show that ACTN4 affects the formation and maturation of focal adhesions, which are critical for the assembly of actin stress fibers and the migratory behavior of cells [37]. Driving cells to a more amoeboid-like migratory behavior has major implications for the metastatic potential of cancers [38]. Mesenchymal cells are dependent on the formation of focal adhesions and integrin signaling, require matrix metalloprotease activity for invasion through collagen [39], and invade at a rate of 0.1–0.5 µm/min [40]. Conversely, amoeboid-like cells have a much higher rate of invasion, from 2 µm/min [41] to 25 µm/min [42] due to their independence from integrin signaling and matrix metalloproteases. Amoeboid cells lack stress fibers, and instead use high myosin II-mediated contraction in the cell cortex which drives the cell to invade through a blebbing mechanism [38]. The amoeboid phenotype of ACTN4-overexpressing cells [35] might explain their dramatic increase in invasion and metastasis through the dense stroma of tumors. Understanding this alteration and enhancement of invasion remains critical to developing therapeutics to mitigate metastasis, and ACTN4 presents itself as an intriguing target.
4. Regulation of α-actinin-4
The structure of all members of the α-actinin family of proteins consist of three major domains (Fig 1). The highly conserved actin-binding domain (ABD), consisting of two calponin homology (CH) domains CH1 and CH2, resides at the N-terminus. Following a small flexible neck region, a long rod shaped domain is formed by a series of spectrin repeats. Finally, a calmodulin-like domain (CaM) is located at the C-terminal region [43]. Monomers associate to form dimers with very high affinity (KD = 10 pM) [44] in anti-parallel orientation creating a dumbbell shape with ABDs on each end. Actin binding is primarily mediated through the ABD, where the separate CH domains exist in a closed, low affinity state or an open, high affinity state [45]. The actin-binding affinities for the ABDs of ACTN1 and ACTN4 are strikingly different with ACTN1 having a much higher affinity (KD = 0.36 µM) [46, 47] than ACTN4 (KD = 32 µM) [45]. This 90-fold difference in ABD affinities suggests that the two isoforms play different roles in maintaining cytoskeletal dynamics. It is important to note that the stated affinities have been determined through analysis of binding kinetics of individual ABDs and not full-length protein. We find these values are more useful for understanding properties of actin crosslinkers for two reasons. First, apparent actinbinding affinities of full-length crosslinking proteins have been observed to decrease with increasing actin concentration likely due to the actin filaments becoming less accessible as they are buried into an actin bundle [48]. Further, crosslinker-actin dynamics are observed to be very stable in the absence of free crosslinker. However, when present, the free crosslinker competes with the bound crosslinkers in the actin bundle, allowing for very fast exchange. The underlying mechanism for this comes from protein avidity where in the absence of free crosslinker, one ABD from the bound crosslinker unbinds, but then is more likely to rebind before the opposite ABD releases, thus, the crosslinker fails to escape the bundle. On the other hand, if present, free crosslinker competes with the bound crosslinker, displacing it from the actin bundle and allowing it to escape [49]. Thus, the individual ABD affinities and kinetics are essential for discerning the behaviors of actin crosslinkers, especially in the context of the cell where free crosslinkers can exist and the system is subjected to mechanical stresses (section 4.5below).
Figure 1. Structure of α-actinin dimmers.
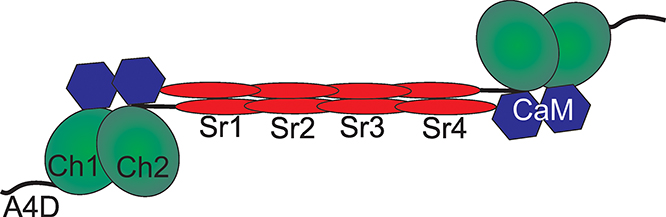
The active homodimer form of α-actinin consists of a pair of subunits in an anti-parallel arrangement. Three main functional domains exist in the monomer. At the N-terminus, two CH domains (CH1 and CH2) comprise an actin binding domain (ABD). A series of four spectrin repeats (SR1-4) form the long rod-shaped domain and is the main region of monomer association. At the C-terminus, a pair of EF domains comprise the calmodulinlike domain (CaM). A4D denotes 19 amino acid region of the N-terminus that is specific to the α- actinin-4 protein.
Structurally, α-actinin-4 contains an additional 19 amino acids at the N-terminus, which are not present on α-actinin-1 and which are crucial for enhanced regulation of α-actinin-4’s actin-binding ability. Activity of the protein is additionally regulated by multiple mechanisms (Fig 2) that fine tune ACTN’s ability to bind actin. Historically, four separate mechanisms have been described: phosphorylation of specific residues, calcium modulation, cleavage by calpain, and binding of lipids such as PIP3. Recent evidence suggests that mechanical stress results in a fifth mode of α-actinin regulation [11, 27]. The following sections will describe how each individual mode of regulation affects actin binding and discuss how these mechanisms are combined to fine tune the activity of ACTN4.
Figure 2. Modes of α-actinin-4 regulation.
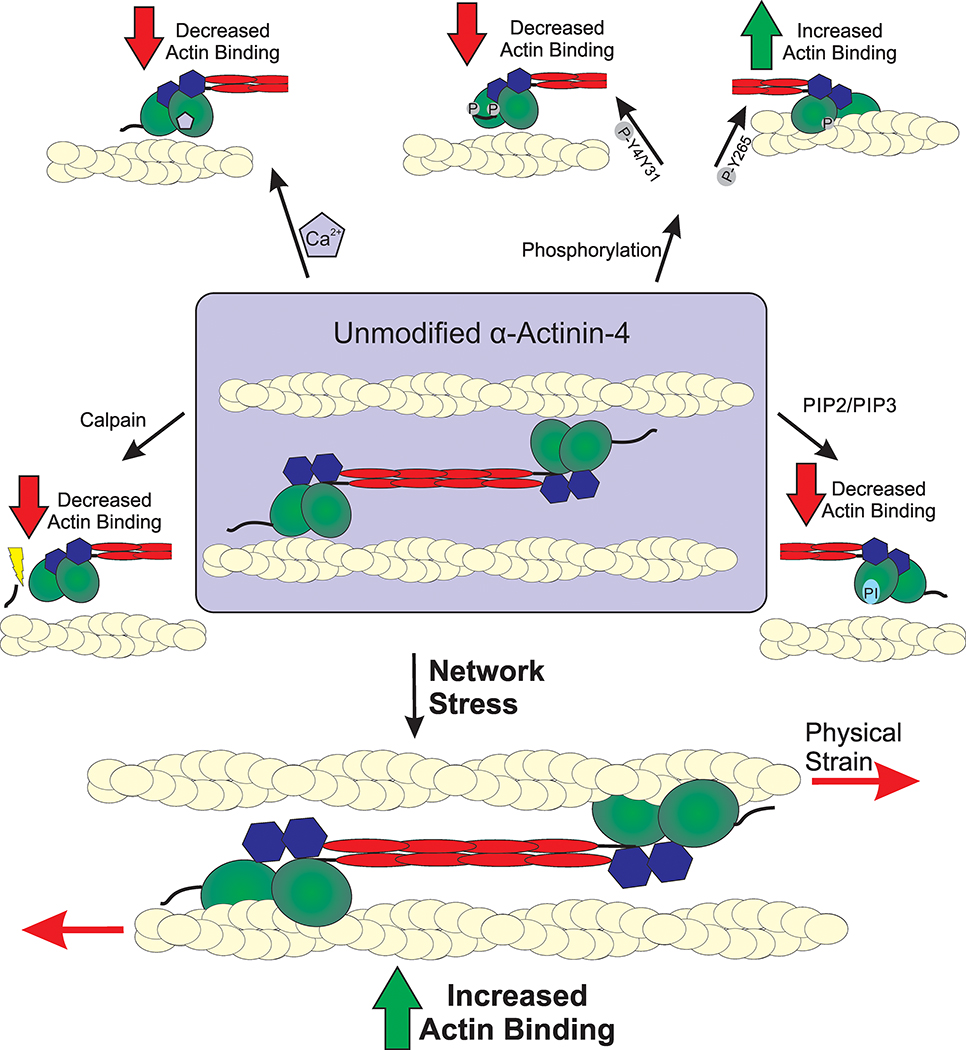
Actin binding activity of α-actinin-4 is mediated through a variety of regulatory mechanisms. The full dumb-bell shape dimer is displayed in the center with branching modes of regulation, showing only one half of a full dimer. Actin filaments are depicted as yellow cables. Calpain cleavage removes a regulatory N-terminal fragment that results in the reduction of actin-binding ability. Phosphorylation of α-actinin-4 results in both increased and decreased actin binding. Phosphorylation of Y4/Y31 residues closes the ABD and lowers actin affinity. A secondary phosphorylation site at Y265 disrupts the interaction between the CH domains and locks the ABD in a high affinity open state. Actin binding is decreased through binding of PIP2/PIP3 binding of the CH domains as well as Ca2+ binding of the C-terminal CaM domain, which lock the ABD in a closed state. The fifth regulatory mechanism of α-actinin-4 is sensation of mechanical stress on the actin cytoskeleton, which results in rapid accumulation to sites of external stress.
4.1 Phosphorylation
Alpha-actinin-4 contains three tyrosine residues (Y4, Y31, and Y265), which are known to be phosphorylated, affecting α-actinin-4’s actin-binding behavior. Upon stimulation with Epidermal Growth Factor (EGF) in NR6WT fibroblast cells, Y4 and Y31 are the primary phosphorylated residues [50]. Phosphorylation occurs through p38 MAPK and Src protein-tyrosine kinase [50], downstream of the receptor tyrosine kinase AXL, and independent of FAK [51]. Initial phosphorylation of Y4 is required to expose the Y31 residue for phosphorylation, resulting in a reduction of actin binding [51]. Phosphorylation of the Y12 in ACTN1, which is homologous to Y31 in ACTN4 and dependent on FAK, results in a similar reduction of actin binding [52], suggesting a conserved mechanism of regulation between the proteins. The added complexity of a dual phosphorylation mechanism of ACTN4 highlights the protein’s critical role in maintaining proper cellular function involvement in critical cellular processes and how dysregulation could enhance the metastatic potential of many carcinomas. It is interesting to note that ACTN1 does not contain the first 19 amino acids at the N-terminus, which includes the Y4 residue of ACTN4 [53].
The third tyrosine residue that has been shown to affect actin dynamics upon phosphorylation is the Y265 residue, which lies within the CH2 region of the ABD [50]. This phosphorylation acts to ease opening of the ABD, resulting in higher actin-binding affinity [54]. Phosphomimetic studies using Y265E constructs shows a loss of proper cellular localization [54], possibly due to the increase in affinity leading to a loss of dynamic turnover to nascent actin filaments. Interestingly, actin binding reduces the ability of Y265 to be phosphorylated and conversely, EGF-stimulated phosphorylation results in decreased actin binding [50].
4.2 Calcium Modulation
The C-terminal region of the α-actinin proteins consist of a calcium binding, Calmodulin-like domain (CaM) consisting of two EF domains. The two muscle specific isoforms, ACTN2 and ACTN3, have evolutionarily lost the ability to bind calcium [55, 56], while the non-muscle isoforms, ACTN1 and ACTN4, retain Ca2+ binding [55, 57, 58]. In vitro studies demonstrate an abolishment of actin-binding at Ca2+ concentrations greater than 0.1 µM [58, 59]. Structural studies show that the C-terminal CaM domain of one antiparallel monomer of the pair interacts with the neck region of the N-terminal ABD of the dimer partner [54, 60]. This interaction results in a closed ABD and reduced actin binding affinity [54].
4.3 Calpain Cleavage
Calpains are a ubiquitous family of intracellular proteases that play key roles in cellular migration processes [61, 62]. Alpha-actinin-1 and α-actinin-4 have been shown to be targets of cleavage that serves to reduce actin binding affinity and increase focal adhesion turnover [53, 63]. Alphaactinin- 4 contains an m-calpain cleavage site between Y13 and G14, which when cleaved, removes the regulatory Y4 phosphorylation site and reduces actin-binding ability [53].
4.4 Lipid Binding
The presence of a phosphoinositide binding site was originally identified in ACTN1 [64, 65]. The CH2 region of the ABD contains a binding site for phosphoinositide in which phosphatidylinositol 4,5 bisphosphate (PIP2) or phosphatidylinositol 3,4,5-triphosphate (PIP3) bind to regulate actin dynamics and focal adhesion assembly [65–67]. The regulatory subunit of phosphatidylinositol-3 kinase (PI3K), p85, binds directly to α-actinin [68] which allows for rapid phosphorylation of PIP2 to PIP3. PIP2 and PIP3 differentially regulate α-actinin dynamics, where PIP2 regulates the actin bundling activity of α-actinin turnover in actin filaments, while PIP3 disrupts α-actinin-focal adhesion association, resulting in increased focal adhesion turnover [67].
4.5 Mechanosensory Regulation
A cell must sense internal and external physical forces and quickly mount an appropriate response, a process known as mechanosensation [69]. Alpha-actinin-4 has been shown to be critical for sensing extracellular matrix stiffness during focal adhesion maturation [70]. Mechanical stress causes a conformational change in the actinin rod-domain resulting in stabilizing interactions with the focal adhesion protein, vinculin [71]. Within a tumor, cells are subjected to increased mechanical pressure through increased extracellular matrix and hydrostatic pressures. This increased mechanical stress has been shown to upregulate expression of ACTN4 [24], further implicating its role in mechanosensation. Physical stress can be transferred to ACTN4 through the actin cytoskeleton, and indeed, alteration of ACTN4’s actin-binding affinity has been shown to be linked to disease states [72]. A single point mutation (K255E) in ACTN4 contributes to focal segmental glomerulosclerosis, which increases the ABD’s actin affinity by ~5 fold (KD = 7 µM) [45] and leads to the mislocalization of α-actinin-4 to the perinuclear and posterior regions of the cell [45]. Within the second CH domain of ACTN4, two actin binding sites are present, one of which is normally sterically blocked by a salt bridge formed between tryptophan 147 in the CH1 domain and lysine 255 in the CH2 domain [45]. The K255 residue is close to the phosphoryla table Y265 residue, which when phosphorylated, results in a similar increase of actin affinity brought on by an opening of the ABD [54], similar to the proposed mechanism of K255E. Disruption of this salt bridge exposes the second actin-binding sequence, allowing it to bind, and thereby increasing α-actinin-4’s actin affinity [45]. This shift in binding affinity upon conformational change could be the molecular mechanism underlying α-actinin’s mechanoresponse, defined as the ability of α-actinin to accumulate in response to applied mechanical stress [11, 27]. The mechanoresponsiveness implicates catch-slip bond behavior between α-actinin and actin [11]. A catch-slip bond is one in which mechanical stress on the bond increases the binding lifetime up to a threshold of mechanical stress. Beyond this threshold, the bond ruptures, decreasing the bond lifetime. In fact, the loss of the salt bridge in the ACTN4 K255E mutant disrupts α-actinin- 4’s mechanoresponsiveness, further supporting this molecular explanation for the catch-slip bond function necessary for mechanosensitive accumulation [27]. The mechanosensitive accumulation appears to depend on catch-slip bond formation as well as an optimal actin-binding affinity range, which is observed for wild type ACTN4 (KD = 32 µM) [45] and not ACTN1 (KD = 0.36 µM) [46] (Fig 3). Without the catch-slip bond, no diffusion-mediated mechanoaccumulation is observed, as is the case with the ACTN4 K255E mutant which has a higher affinity to actin (KD = 7 µM) [45], but predicted to have no catch bond activity [27]. On the other hand, if the actin-binding affinity is too high, then mechanoresponsiveness is abolished by the absence of an available free pool of α-actinin, as seen with the high affinity paralog, ACTN1. These protein behaviors are predicted computationally and observed experimentally [11, 27] and point towards a key strategy by which nature has engineered cells to have a mechanically responsive, tunable system.
Figure 3. Mechanoaccumulation of α-actinin-4 during micropipette aspiration.
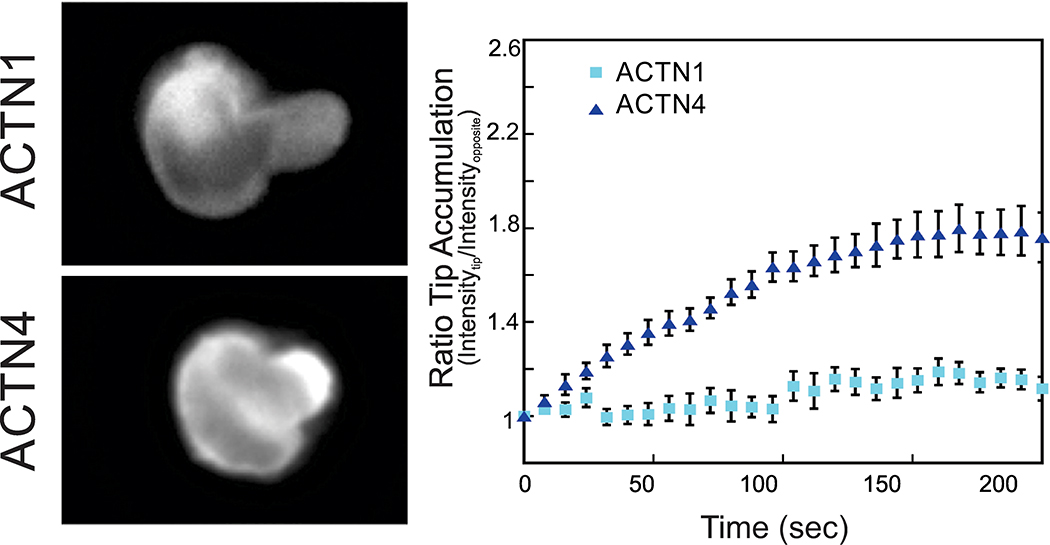
Representative images of Jurkat cells expressing GFP-ACTN1 or GFP-ACTN4 are shown on the left. As mechanical stress is applied to the cells, α-actinin-4 accumulates in the region of dilational stress, while α-actinin-1 does not. Quantification of the ratio of GFP fluorescence intensity at the tip to the opposite end of the cortex demonstrates a rapid accumulation of α-actinin-4. Adapted from Schiffhauer E., et al, 2016 [27].
4.6 Regulatory Interactions
The complexity of α-actinin-4’s regulation is further modified by the interplay between the mechanisms described previously. It is through these interactions that regulatory mechanisms can be fine-tuned for the optimal response to a specific input. For example, phosphorylation of Y4 of α-actinin-4 is the primary site of phosphorylation and essential to allow phosphorylation of Y31, which is the actin-binding modulating residue. Cleavage of α-actinin-4 between Y13 and G14 by m-calpain target site can remove the regulatory Y4 residue, resulting in the reduction of actin binding and increase of focal adhesion turnover. Binding of PIP2 reduces susceptibility of m-calpain cleavage, increasing phosphorylation at Y4 [53] while PIP3 increases susceptibility of m-calpain [63]. Actin binding itself is a modulator of regulatory mechanisms. Phosphorylation of Y4/Y31 results in a dissociation of α-actinin-4 from actin, however, a mutant Y265E that has a very high actin affinity results in a reduction of phosphorylation of Y4 as well as resistance to cleavage by m-calpain [50]. Furthermore, the α-actinin-4 K255E mutant greatly increases actin binding actin affinity, which ablates sensitivity to regulation by Ca2+ [45] as well as the mechanoaccumulation seen by wild type α-actinin-4 [27].
5. Conclusion
Cancer metastasis is a process that involves an interconnectedness between cancerous cells and normal cells within the environment of a solid tumor. Tumor cells signal to stromal cells, which enhance their deposition of extracellular matrix. The increased rigidity and structure of the extracellular matrix then stimulates various signaling pathways, upregulating activity and expression of a variety of proteins that enhance the invasive ability of cancer cells. Understanding the roles and mechanisms of these upregulated proteins and how they are regulated is critical for a more complete understanding of tumor metastasis and eventual directed development of therapeutics. A major component of tumor metastasis is the cellular machinery that enhances invasion by modifying cytoskeletal dynamics. Alpha-actinin-4 is highly overexpressed and implicated in the metastasis of a variety of cancers [72]. For efficient migration through tumor stroma, cytoskeletal structures and focal adhesions must remain dynamic, yet structurally sound enough to exert forces on a dense environment.
Tumor cells must sense their environment and quickly alter their behavior. To do this, they must maintain the machinery to sample their extracellular environment. While intracellular signaling pathways are a vital aspect to cellular migration, we propose that a much more immediate, mechanosensory response is utilized by invading cells. Like the reflex responses of the nervous system, where quick, involuntary decisions are made before the brain can sense and respond to a stimulus, cells can sense a mechanical stress and exhibit an immediate response prior to a more thorough remodeling of cytoskeletal architecture. Alpha-actinin-4 is likely to be a part of the cellular reflex system that mechanoaccumulates at areas of physical stress. This immediate localization allows the cell to mount a more specific response, whether it be through calcium signaling, PIP3 synthesis, or downstream of chemokine signaling. Evidence for this hierarchical pathway already exists with the K255E mutant seen in kidney disease. The K255E mutation results in a loss of mechanoresponsiveness [27] brought on by an increased actin affinity and a presumed loss of the catch bond [45]. This mutant is also rendered insensitive to regulation by calcium binding [45]. Increased actin affinity is also responsible for a decrease in the regulatory phosphorylation of the Y4/Y31 residues as seen in the Y265E phosphomimetic mutant [50, 54]. While there is clear evidence that α-actinin-4 plays a specific role in the metastasis of cancer and is functionally distinct from the α-actinin-1 paralog, many differential molecular properties between the two proteins remain to be described. Many studies in the field do not differentiate between the two paralogs making it unclear which protein exhibits the described property. With growing evidence that the two proteins have separate and distinct functions in cell biology, it is increasingly important that further studies focus on their different biochemical properties. Understanding the complete mechanism of mechanosensation and how it incorporates into the broader scope of cellular signaling may be a key factor in developing therapeutic strategies around the α-actinins.
Acknowledgments
We thank Eric Schiffhauer and Priyanka Kothari for comments on the manuscript. Our work is supported by NIH grants (R01 GM66817 and R01 GM109863).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12(6):533–42. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 2.Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, Svoboda H, Viana M, Costa LD, Guck J, Holt CE, Franze K. Mechanosensing is critical for axon growth in the developing brain. Nat Neurosci. 2016;19(12):1592–1598. doi: 10.1038/nn.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raab M, Swift J, Dingal PC, Shah P, Shin JW, Discher DE. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J Cell Biol. 2012;199(4):669–83. doi: 10.1083/jcb.201205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaman MH. The role of engineering approaches in analysing cancer invasion and metastasis. Nat Rev Cancer. 2013;13(8):596–603. doi: 10.1038/nrc3564. [DOI] [PubMed] [Google Scholar]
- 5.Bisi S, Disanza A, Malinverno C, Frittoli E, Palamidessi A, Scita G. Membrane and actin dynamics interplay at lamellipodia leading edge. Curr Opin Cell Biol. 2013;25(5):565–73. doi: 10.1016/j.ceb.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Petrie RJ, Yamada KM. Fibroblasts Lead the Way: A Unified View of 3D Cell Motility. Trends Cell Biol. 2015;25(11):666–74. doi: 10.1016/j.tcb.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DG, Yenepalli A, Denais CM, Rape A, Beach JR, Wang YL, Schiemann WP, Baskaran H, Lammerding J, Egelhoff TT. Non-muscle myosin IIB is critical for nuclear translocation during 3D invasion. J Cell Biol. 2015;210(4):583–94. doi: 10.1083/jcb.201502039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrlicher AJ, Krishnan R, Guo M, Bidan CM, Weitz DA, Pollak MR. Alpha-actinin binding kinetics modulate cellular dynamics and force generation. Proc Natl Acad Sci U S A. 2015;112(21):6619–24. doi: 10.1073/pnas.1505652112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roca-Cusachs P, del Rio A, Puklin-Faucher E, Gauthier NC, Biais N, Sheetz MP. Integrindependent force transmission to the extracellular matrix by alpha-actinin triggers adhesion maturation. Proc Natl Acad Sci U S A. 2013;110(15):E1361–70. doi: 10.1073/pnas.1220723110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson WM, Jaasma MJ, Baik AD, Keaveny TM. Over-expression of alpha-actinin with a GFP fusion protein is sufficient to increase whole-cell stiffness in human osteoblasts. Ann Biomed Eng. 2008;36(10):1605–14. doi: 10.1007/s10439-008-9533-9. [DOI] [PubMed] [Google Scholar]
- 11.Luo T, Mohan K, Iglesias PA, Robinson DN. Molecular mechanisms of cellular mechanosensing. Nat Mater. 2013;12(11):1064–71. doi: 10.1038/nmat3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson CM, Tien J. Microstructured extracellular matrices in tissue engineering and development. Curr Opin Biotechnol. 2006;17(5):518–23. doi: 10.1016/j.copbio.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 14.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178(3):1221–32. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riching KM, Cox BL, Salick MR, Pehlke C, Riching AS, Ponik SM, Bass BR, Crone WC, Jiang Y, Weaver AM, Eliceiri KW, Keely PJ. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys J. 2014;107(11):2546–58. doi: 10.1016/j.bpj.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200(4):429–47. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 17.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21(1):33–9. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 19.Lathia JD, Gallagher J, Myers JT, Li M, Vasanji A, McLendon RE, Hjelmeland AB, Huang AY, Rich JN. Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells. PLoS One. 2011;6(9):e24807. doi: 10.1371/journal.pone.0024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6(4):1186–97. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 21.Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128(4):907–21. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong T, Packham G, Murphy LB, Bateman AC, Conti JA, Fine DR, Johnson CD, Benyon RC, Iredale JP. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10(21):7427–37. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 23.Kikuta K, Masamune A, Watanabe T, Ariga H, Itoh H, Hamada S, Satoh K, Egawa S, Unno M, Shimosegawa T. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403(3-4):380–4. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 24.Downey C, Craig DH, Basson MD. Isoform-specific modulation of pressure-stimulated cancer cell proliferation and adhesion by alpha-actinin. Am J Surg. 2011;202(5):520–3. doi: 10.1016/j.amjsurg.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhard M, Zumbrunn J, Jaquemar D, Kuhn M, Walter U, Trueb B. An alpha-actinin binding site of zyxin is essential for subcellular zyxin localization and alpha-actinin recruitment. J Biol Chem. 1999;274(19):13410–8. doi: 10.1074/jbc.274.19.13410. [DOI] [PubMed] [Google Scholar]
- 27.Schiffhauer ES, Luo T, Mohan K, Srivastava V, Qian X, Griffis ER, Iglesias PA, Robinson DN. Mechanoaccumulative Elements of the Mammalian Actin Cytoskeleton. Curr Biol. 2016;26(11):1473–9. doi: 10.1016/j.cub.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda K. The biological role of actinin-4 (ACTN4) in malignant phenotypes of cancer. Cell Biosci. 2015;5:41. doi: 10.1186/s13578-015-0031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kallioniemi A, Kallioniemi OP, Piper J, Tanner M, Stokke T, Chen L, Smith HS, Pinkel D, Gray JW, Waldman FM. Detection and mapping of amplified DNA sequences in breast cancer by comparative genomic hybridization. Proc Natl Acad Sci U S A. 1994;91(6):2156–60. doi: 10.1073/pnas.91.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda K, Yamada T, Hayashida Y, Idogawa M, Sato S, Hasegawa F, Ino Y, Ono M, Hirohashi S. Actinin-4 increases cell motility and promotes lymph node metastasis of colorectal cancer. Gastroenterology. 2005;128(1):51–62. doi: 10.1053/j.gastro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto S, Tsuda H, Honda K, Kita T, Takano M, Tamai S, Inazawa J, Yamada T, Matsubara O. Actinin-4 expression in ovarian cancer: a novel prognostic indicator independent of clinical stage and histological type. Mod Pathol. 2007;20(12):1278–85. doi: 10.1038/modpathol.3800966. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi S, Honda K, Tsuda H, Hiraoka N, Imoto I, Kosuge T, Umaki T, Onozato K, Shitashige M, Yamaguchi U, Ono M, Tsuchida A, Aoki T, Inazawa J, Hirohashi S, Yamada T. Expression and gene amplification of actinin-4 in invasive ductal carcinoma of the pancreas. Clin Cancer Res. 2008;14(17):5348–56. doi: 10.1158/1078-0432.CCR-08-0075. [DOI] [PubMed] [Google Scholar]
- 33.Welsch T, Keleg S, Bergmann F, Bauer S, Hinz U, Schmidt J. Actinin-4 expression in primary and metastasized pancreatic ductal adenocarcinoma. Pancreas. 2009;38(8):968–76. doi: 10.1097/MPA.0b013e3181b28d6f. [DOI] [PubMed] [Google Scholar]
- 34.Fukumoto M, Kurisu S, Yamada T, Takenawa T. alpha-Actinin-4 enhances colorectal cancer cell invasion by suppressing focal adhesion maturation. PLoS One. 2015;10(4):e0120616. doi: 10.1371/journal.pone.0120616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao H, Li S, Watkins SC, Wells A. alpha-Actinin-4 is required for amoeboid-type invasiveness of melanoma cells. J Biol Chem. 2014;289(47):32717–28. doi: 10.1074/jbc.M114.579185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao H, Wang JH, Pollak MR, Wells A. alpha-actinin-4 is essential for maintaining the spreading, motility and contractility of fibroblasts. PLoS One. 2010;5(11):e13921. doi: 10.1371/journal.pone.0013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10(9):1039–50. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pankova K, Rosel D, Novotny M, Brabek J. The molecular mechanisms of transition between mesenchymal and amoeboid invasiveness in tumor cells. Cell Mol Life Sci. 2010;67(1):63–71. doi: 10.1007/s00018-009-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85(5):683–93. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 40.Friedl P, Zanker KS, Brocker EB. Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech. 1998;43(5):369–78. doi: 10.1002/(SICI)1097-0029(19981201)43:5<369::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5(8):711–9. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 42.Friedl P, Noble PB, Shields ED, Zanker KS. Locomotor phenotypes of unstimulated CD45RAhigh and CD45ROhigh CD4+ and CD8+ lymphocytes in three-dimensional collagen lattices. Immunology. 1994;82(4):617–24. [PMC free article] [PubMed] [Google Scholar]
- 43.Blanchard A, Ohanian V, Critchley D. The structure and function of alpha-actinin. J Muscle Res Cell Motil. 1989;10(4):280–9. doi: 10.1007/BF01758424. [DOI] [PubMed] [Google Scholar]
- 44.Flood G, Kahana E, Gilmore AP, Rowe AJ, Gratzer WB, Critchley DR. Association of structural repeats in the alpha-actinin rod domain. Alignment of inter-subunit interactions. J Mol Biol. 1995;252(2):227–34. doi: 10.1006/jmbi.1995.0490. [DOI] [PubMed] [Google Scholar]
- 45.Weins A, Schlondorff JS, Nakamura F, Denker BM, Hartwig JH, Stossel TP, Pollak MR. Disease-associated mutant alpha-actinin-4 reveals a mechanism for regulating its Factin-binding affinity. Proc Natl Acad Sci U S A. 2007;104(41):16080–5. doi: 10.1073/pnas.0702451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldmann WH, Isenberg G. Analysis of filamin and alpha-actinin binding to actin by the stopped flow method. FEBS Lett. 1993;336(3):408–10. doi: 10.1016/0014-5793(93)80847-n. [DOI] [PubMed] [Google Scholar]
- 47.Ferrer JM, Lee H, Chen J, Pelz B, Nakamura F, Kamm RD, Lang MJ. Measuring molecular rupture forces between single actin filaments and actin-binding proteins. Proc Natl Acad Sci U S A. 2008;105(27):9221–6. doi: 10.1073/pnas.0706124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girard KD, Chaney C, Delannoy M, Kuo SC, Robinson DN. Dynacortin contributes to cortical viscoelasticity and helps define the shape changes of cytokinesis. EMBO J. 2004;23(7):1536–46. doi: 10.1038/sj.emboj.7600167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Courson DS, Rock RS. Actin cross-link assembly and disassembly mechanics for alpha-Actinin and fascin. J Biol Chem. 2010;285(34):26350–7. doi: 10.1074/jbc.M110.123117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shao H, Wu C, Wells A. Phosphorylation of alpha-actinin 4 upon epidermal growth factor exposure regulates its interaction with actin. J Biol Chem. 2010;285(4):2591–600. doi: 10.1074/jbc.M109.035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Travers T, Shao H, Joughin BA, Lauffenburger DA, Wells A, Camacho CJ. Tandem phosphorylation within an intrinsically disordered region regulates ACTN4 function. Sci Signal. 2015;8:ra51. doi: 10.1126/scisignal.aaa1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izaguirre G, Aguirre L, Hu YP, Lee HY, Schlaepfer DD, Aneskievich BJ, Haimovich B. The cytoskeletal/non-muscle isoform of alpha-actinin is phosphorylated on its actin-binding domain by the focal adhesion kinase. J Biol Chem. 2001;276(31):28676–85. doi: 10.1074/jbc.M101678200. [DOI] [PubMed] [Google Scholar]
- 53.Shao H, Travers T, Camacho CJ, Wells A. The carboxyl tail of alpha-actinin-4 regulates its susceptibility to m-calpain and thus functions in cell migration and spreading. Int J Biochem Cell Biol. 2013;45(6):1051–63. doi: 10.1016/j.biocel.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Travers T, Shao H, Wells A, Camacho CJ. Modeling the assembly of the multiple domains of alpha-actinin-4 and its role in actin cross-linking. Biophys J. 2013;104(3):705–15. doi: 10.1016/j.bpj.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noegel A, Witke W, Schleicher M. Calcium-sensitive non-muscle alpha-actinin contains EF-hand structures and highly conserved regions. FEBS Lett. 1987;221(2):391–6. doi: 10.1016/0014-5793(87)80962-6. [DOI] [PubMed] [Google Scholar]
- 56.Beggs AH, Byers TJ, Knoll JH, Boyce FM, Bruns GA, Kunkel LM. Cloning and characterization of two human skeletal muscle alpha-actinin genes located on chromosomes 1 and 11. J Biol Chem. 1992;267(13):9281–8. [PubMed] [Google Scholar]
- 57.Burridge K, Feramisco JR. Non-muscle alpha actinins are calcium-sensitive actin-binding proteins. Nature. 1981;294(5841):565–7. doi: 10.1038/294565a0. [DOI] [PubMed] [Google Scholar]
- 58.Witke W, Hofmann A, Koppel B, Schleicher M, Noegel AA. The Ca(2+)-binding domains in non-muscle type alpha-actinin: biochemical and genetic analysis. J Cell Biol. 1993;121(3):599–606. doi: 10.1083/jcb.121.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Condeelis J, Vahey M. A calcium- and pH-regulated protein from Dictyostelium discoideum that cross-links actin filaments. J Cell Biol. 1982;94(2):466–71. doi: 10.1083/jcb.94.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, Taylor DW, Taylor KA. A 3-D reconstruction of smooth muscle alpha-actinin by CryoEm reveals two different conformations at the actin-binding region. J Mol Biol. 2004;338(1):115–25. doi: 10.1016/j.jmb.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 61.Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JS, Elce JS, Huttenlocher A. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem. 2001;276(51):48382–8. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- 62.Shiraha H, Glading A, Chou J, Jia Z, Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol. 2002;22(8):2716–27. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sprague CR, Fraley TS, Jang HS, Lal S, Greenwood JA. Phosphoinositide binding to the substrate regulates susceptibility to proteolysis by calpain. J Biol Chem. 2008;283(14):9217–23. doi: 10.1074/jbc.M707436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fukami K, Furuhashi K, Inagaki M, Endo T, Hatano S, Takenawa T. Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature. 1992;359(6391):150–2. doi: 10.1038/359150a0. [DOI] [PubMed] [Google Scholar]
- 65.Fukami K, Endo T, Imamura M, Takenawa T. alpha-Actinin and vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase. J Biol Chem. 1994;269(2):1518–22. [PubMed] [Google Scholar]
- 66.Greenwood JA, Theibert AB, Prestwich GD, Murphy-Ullrich JE. Restructuring of focal adhesion plaques by PI 3-kinase. Regulation by PtdIns (3,4,5)-p(3) binding to alpha-actinin. J Cell Biol. 2000;150(3):627–42. doi: 10.1083/jcb.150.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fraley TS, Pereira CB, Tran TC, Singleton C, Greenwood JA. Phosphoinositide binding regulates alpha-actinin dynamics: mechanism for modulating cytoskeletal remodeling. J Biol Chem. 2005;280(15):15479–82. doi: 10.1074/jbc.M500631200. [DOI] [PubMed] [Google Scholar]
- 68.Shibasaki F, Fukami K, Fukui Y, Takenawa T. Phosphatidylinositol 3-kinase binds to alpha-actinin through the p85 subunit. Biochem J. 1994;302(Pt 2):551–7. doi: 10.1042/bj3020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldmann WH. Mechanosensation: a basic cellular process. Prog Mol Biol Transl Sci. 2014;126:75–102. doi: 10.1016/B978-0-12-394624-9.00004-X. [DOI] [PubMed] [Google Scholar]
- 70.Meacci G, Wolfenson H, Liu S, Stachowiak MR, Iskratsch T, Mathur A, Ghassemi S, Gauthier N, Tabdanov E, Lohner J, Gondarenko A, Chander AC, Roca-Cusachs P, O'Shaughnessy B, Hone J, Sheetz MP. alpha-Actinin links extracellular matrix rigidity-sensing contractile units with periodic cell-edge retractions. Mol Biol Cell. 2016;27(22):3471–3479. doi: 10.1091/mbc.E16-02-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shams H, Golji J, Mofrad MR. A molecular trajectory of alpha-actinin activation. Biophys J. 2012;103(10):2050–9. doi: 10.1016/j.bpj.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Otey CA, Carpen O. Alpha-actinin revisited: a fresh look at an old player. Cell Motil Cytoskeleton. 2004;58(2):104–11. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]


