Abstract
ROS1 is a validated therapeutic target in non-small cell lung cancer (NSCLC). In a phase I study, the multi-targeted MET/ALK/ROS1 inhibitor crizotinib demonstrated remarkable efficacy in ROS1-rearranged NSCLCs, and consequently gained approval by the United States Food and Drug Administration as well as the European Medicines Agency in 2016. However, similar to other oncogene-driven lung cancers, ROS1-rearranged lung cancers treated with crizotinib eventually acquire resistance, leading to disease relapse. Novel ROS1 inhibitors and therapeutic strategies are therefore needed. Insights into the mechanisms of resistance to ROS1-directed tyrosine kinase inhibitors (TKIs) are now beginning to emerge and are helping to guide the development of new ROS1 inhibitors. This review discusses the biology and diagnosis of ROS1-rearranged NSCLC, and current and emerging treatment options for this disease. Future challenges in the field are highlighted.
Keywords: ROS1 rearrangement, non-small cell lung cancer, crizotinib, ROS1 inhibitor, resistance
Introduction
Chromosomal rearrangements involving the ROS proto-oncogene 1, receptor tyrosine kinase (ROS1) gene were first described in non-small cell lung cancer (NSCLC) in 2007 (1). Since then, oncogenic ROS1 rearrangements have become an established therapeutic target in lung cancer. ROS1 rearrangements are identified in 1–2% of NSCLC patients (2). While this prevalence may seem low at first glance, the high incidence of lung cancer cases in the United States (U.S.) means that 2,000 to 4,500 patients will be newly diagnosed with ROS1-rearranged NSCLC each year (3).
In March 2016, crizotinib, an anaplastic lymphoma kinase (ALK)/ROS1/MET inhibitor, became the first targeted agent approved by the U.S. Food and Drug Administration (FDA) for the treatment of advanced ROS1-rearranged NSCLC. This approval was based on the efficacy and safety data from the expansion cohort of the phase I crizotinib study (PROFILE 1001), which demonstrated an objective response rate (ORR) of 72% and median progression-free survival (PFS) of 19.2 months in advanced ROS1-rearranged NSCLC (4). Despite often durable responses, the majority of patients ultimately experience disease relapse due to acquired resistance. To date, crizotinib is the only targeted therapy approved for ROS1-rearranged NSCLC, underscoring the urgent need to develop novel ROS1-directed therapies.
In this review, we provide an up-to-date overview of the biology and treatment of ROS1-rearranged NSCLC, with an emphasis on current and emerging targeted therapy options. We discuss early insights into mechanisms of crizotinib resistance, and how these mechanisms may help guide the development of new therapeutic strategies on the horizon.
ROS1 Function
The ROS1 gene was originally discovered as a homolog of the transforming sequence of the avian sarcoma RNA virus UR2 (5, 6). Located on chromosome 6q22.1 (7, 8), ROS1 encodes a receptor tyrosine kinase (RTK) containing a large N-terminal extracellular domain, a hydrophobic single-pass transmembrane region, and a C-terminal intracellular tyrosine kinase domain (9). Phylogenic sequence analysis has revealed that ROS1 is related to the ALK/LTK and insulin receptor RTK families (10). The homology to ALK has been particularly relevant in the development of ROS1-directed therapies; several (but importantly, not all) ALK tyrosine kinase inhibitors (TKIs) harbor dual inhibitory activity against ALK and ROS1 (see discussion below).
ROS1 is an evolutionarily conserved RTK in C. elegans, D. melanogaster, and vertebrates. In Drosophila, the ROS1 homologue, Sevenless, is activated by the binding of its ligand Bride of sevenless (Boss) and contributes to photoreceptor cell differentiation (9). In chicken, mouse, and rats, ROS1 expression has been detected in the epithelial cells of the kidneys, male reproductive organs, small intestines, heart, and lungs (11, 12). ROS1-deficient male mice are notably healthy but infertile, with defective sperm maturation secondary to the impaired differentiation of epididymal epithelial cells; female ROS1-deficient mice develop normally without any detectable abnormalities (13). The biological role of native ROS1 in humans has not yet been defined, and it remains an orphan RTK without a known ligand (9).
ROS1 Gene Fusions in Cancer
ROS1 gene fusions were first identified in a human glioblastoma cell line (Figure 1) (14). In this cell line, the 3′ region of ROS1 was found fused to the 5′ region of the fused in glioblastoma gene (FIG; also called golgi associated PDZ and coiled coil motif containing, GOPC) via the interstitial deletion of 240 kilobases on chromosome 6q21, resulting in a constitutively active fusion kinase (15, 16). A CEP85L-ROS1 rearrangement has also been reported in an adult glioblastoma tumor (17). Since the initial description in glioblastoma, ROS1 fusions have been detected in a range of malignancies including inflammatory myofibroblastic tumor (18, 19), cholangiocarcinoma (20), ovarian cancer (21), gastric cancer (22), colorectal cancer (23), angiosarcoma (24), spitzoid melanoma (25), and NSCLC (1, 4, 26–38).
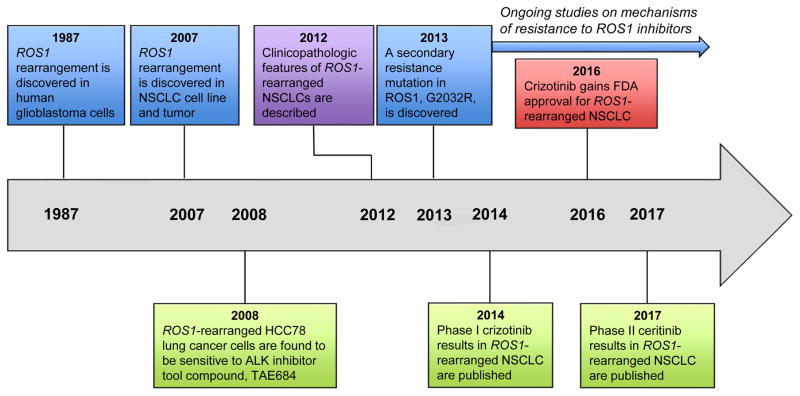
Figure 1.
Timeline of key advances in targeting ROS1 in lung cancer. Abbreviations: NSCLC, non-small cell lung cancer; ALK, anaplastic lymphoma kinase; FDA, Food and Drug Administration.
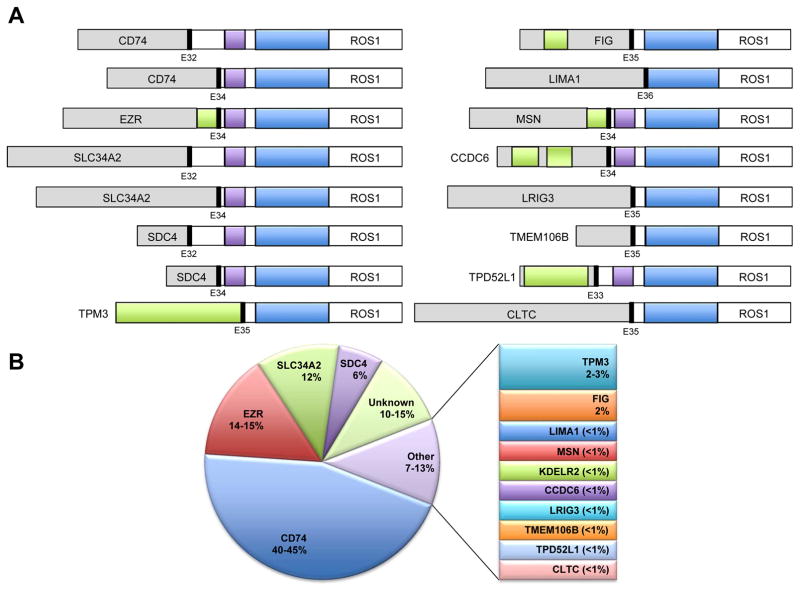
NSCLC was the second solid tumor found to harbor ROS1 rearrangements. In a study of 41 NSCLC cell lines and over 150 NSCLC tumors, ROS1 was identified as a highly activated kinase in one cell line and one tumor sample (Figure 1) (1). Sequencing analysis of these samples revealed a novel SLC34A2-ROS1 fusion in the HCC78 cell line, and a CD74-ROS1 fusion in the tumor specimen (1). A total of 14 different ROS1 fusion partner genes have now been reported in lung cancer, including CD74 (1, 4, 26–29), SLC34A2 (1, 4, 26–28), SDC4 (4, 26, 27), EZR (4, 26, 30, 31), FIG (28, 32), TPM3 (4, 26), LRIG3 (26), KDELR2 (33), CCDC6 (34), MSN (4, 35), TMEM106B (36), TPD52L1 (37), CLTC (38), and LIMA1 (4) (Figure 2). Of these, the CD74-ROS1 occurs most frequently in NSCLC (Figure 2B). All ROS1 fusions retain the entire ROS1 kinase domain (26).
Figure 2.
ROS1 rearrangements in non-small cell lung cancer (NSCLC). (A) Schematic representations of ROS1 fusion proteins described to date. Note that KDELR2-ROS1 is not shown, as the genomic structure of this fusion has not been published. Blue, ROS1 tyrosine kinase domain; purple, ROS1 transmembrane domain; green, coiled-coil domain. (B) Distribution of ROS1 fusion proteins by the reported frequencies in NSCLC. Each fusion protein listed under the ‘other’ category likely occurs in <1% of ROS1-rearranged NSCLCs, unless otherwise indicated. [Figure updated/modified from ref. 103.]
A number of studies have demonstrated the oncogenic potential of ROS1 fusions. Expression of ROS1 fusions results in transformation of NIH3T3 and Ba/F3 cells in vitro, and tumorigenicity in vivo (16, 20, 26, 27, 30, 39). Transgenic mice expressing EZR-ROS1 in the lung alveolar epithelium develop bilateral lung adenocarcinomas (30). The exact mechanism of ROS1 kinase activation in the fusion proteins has not been established. Interestingly, in contrast to ALK rearrangements in which the fusion partners provide dimerization domains that induce constitutive kinase activation, the majority of the known ROS1 fusion partners do not contain such domains (Figure 2A) (26). Furthermore, localization of the ROS1 fusion proteins varies, ranging from the plasma membrane to the Golgi apparatus to the cytoplasm (28, 40). Once activated, ROS1 signals through the MAPK/ERK, PI3K/AKT, JAK/STAT3, and SHP1/2 pathways to promote cell growth and survival (20, 27, 39, 41). Whether distinct ROS1 fusions confer differential levels of expression, kinase activation and oncogenicity is unknown.
Clinicopathologic Characteristics of ROS1-Rearranged Lung Cancer
Across studies, the reported frequency of ROS1 rearrangements in NSCLC has ranged from 0.9–2% (2, 17, 26–29, 38). Similar to ALK, ROS1 rearrangements are associated with younger age, never or light smoking history, and adenocarcinoma histology (2, 26). Rarely though, ROS1 rearrangements have been detected in NSCLC with large cell or squamous cell histology (28, 40). Notably, not all clinicopathologic features are shared between ALK- and ROS1-rearranged NSCLCs. For example, one recent series comparing 39 ROS1-rearranged and 196 ALK-rearranged NSCLC patients found that ROS1 rearrangements were associated with a significantly lower rate of extrathoracic and brain metastases at the time of diagnosis, in addition to a lower cumulative incidence of brain metastases (42). Although the number of patients included in this series was limited, particularly for the ROS1-rearranged subset, the findings suggest that patterns of metastases may be distinct for ROS1- versus ALK-rearranged lung cancer. The biological basis for these differences in metastatic patterns has yet to be determined.
Generally, oncogenic drivers in NSCLC, such as KRAS, EGFR, and ALK, are mutually exclusive (43). An early analysis of 1,073 NSCLC tumor specimens demonstrated no overlap between ROS1 and ALK rearrangements (2). However, conflicting findings have subsequently been reported, with some later studies suggesting a co-occurrence of ROS1 rearrangements and mutations in EGFR (28, 44), KRAS (44), or BRAF (44). In the most recent and largest series to date, a total of 220 cases of ROS1-rearranged NSCLCs were examined (45). Amongst these tumors, ROS1 fusions did not overlap with ALK fusions, and rarely co-occurred with oncogenic EGFR mutations (0.5%; 1/220) or KRAS mutations (1.8%; 4/220) (45). Therefore, ROS1 rearrangements generally define a unique molecular subset of NSCLC.
Clinical Detection of ROS1 Fusions
Diagnostic methods used for the detection of ROS1 fusions largely mirror those used for ALK, with a few differences. Crizotinib was initially approved in 2011 for the treatment of advanced ALK-rearranged NSCLCs, with ALK fluorescence in situ hybridization (FISH) as the companion diagnostic test. Four years later, ALK immunohistochemistry (IHC; Ventana D5F3 antibody) also received FDA approval as a companion diagnostic (46). In contrast to ALK, there are currently no approved companion assays for ROS1-rearranged NSCLC. Based on experiences with ALK, commonly used methods for ROS1 fusion detection have included FISH, IHC, reverse transcription polymerase chain reaction (RT-PCR), and next-generation sequencing (NGS).
FISH positivity was required in the registration trials of crizotinib, and FISH is commonly utilized as the standard diagnostic assay for ALK rearrangement in NSCLC. Early ROS1 studies also employed this technique for fusion detection (2, 26, 27). ROS1 FISH utilizes a dual break-apart probe design, with red and green fluorescent labels for the 3′ and 5′ portions flanking the ROS1 breakpoint (2). A normal ROS1 gene without a rearrangement yields the fused, yellow signal. A rearranged ROS1 gene yields the ‘classic’ split red and green signals, or the ‘atypical’ isolated 3′ red signal. A tumor is considered ‘FISH positive’ if at least 15% of evaluated tumor cells contain split or isolated 3′ signals. While FISH can be performed on a small amount of formalin-fixed, paraffin-embedded (FFPE) tissue, the assay can be challenging to interpret, particularly for fusions arising from small intrachromosomal deletion events. Therefore, false-negative and false-positive FISH results can occur (45).
IHC serves as an alternative diagnostic tool, and is often faster to perform than FISH. The ROS1 D4D6 rabbit monoclonal antibody (Cell Signaling Technology, MA) is commercially available and exhibits sensitivity nearing 100% and specificity ranging between 92–100% (28, 47, 48). However, the ROS1 IHC readout can be more difficult to interpret and operator-dependent compared to ALK IHC for several reasons. ROS1 staining patterns may vary due to different intracellular localization of the ROS1 fusions (28). Moreover, benign pneumocytes and alveolar macrophages, and, in bone metastatic lesions, osteoclast-type giant cells can all express ROS1, generating more background staining than is seen with ALK (47, 48). IHC results may also be falsely positive due to aneuploidy leading to aberrant expression. Thus, albeit useful as a screening tool, ROS1 IHC by itself may be insufficient to diagnose ROS1-rearranged NSCLC, and testing typically requires confirmation using an orthogonal method such as FISH or NGS (45).
A unique advantage of NGS is that it enables multiplex testing and allows for the detection of known as well as novel fusions. Indeed, a number of ROS1 fusions were discovered using NGS (33, 36, 37). This may become particularly relevant if specific ROS1 variants are found to impact tumor biology and clinical outcomes, as has been proposed in ALK-rearranged lung cancers (49–51). By comparison, RT-PCR requires a priori knowledge of fusions for the design and incorporation of fusion-specific primers, and will thus miss the detection of previously unknown fusions. NGS is being increasingly utilized because of these advantages, although there are limitations including higher cost than FISH or IHC, the need for more tissue, and slower turnaround time. Further studies are needed to assess the performance of different NGS platforms; however, in one study, an anchored multiplex PCR enrichment method to detect gene rearrangements in 319 FFPE samples achieved 100% sensitivity and 100% specificity, compared to FISH reference assays (35).
ROS1-Targeted Therapies in Lung Cancer
ROS1-rearranged lung cancers are dependent on (i.e., “addicted” to) ROS1 for growth and survival. Rikova et al. demonstrated that the HCC78 lung cancer cells bearing the SLC34A2-ROS1 fusion undergo apoptosis upon short interfering RNA-mediated knockdown of ROS1 (1). Subsequently, pharmacologic inhibition of ROS1 was shown to induce growth inhibition in a number of ROS1-rearranged cell line models (2, 27, 28, 52). This finding was later validated in patients (2, 4), spurring efforts to develop ROS1-directed TKIs.
Crizotinib
One year after the initial description of ROS1 fusions in NSCLC, McDermott et al. fortuitously discovered that HCC78 was the only non-ALK-rearranged cell line (in a screen of 602 human cancer cell lines) sensitive to the ALK inhibitor compound TAE684 (52). The authors speculated that this sensitivity could potentially arise from the inhibition of ROS1 by TAE684, based on the homology between ROS1 and ALK (52). Indeed, these two RTKs share a 49% amino acid sequence identity in the kinase domain and 77% identity in the adenosine triphosphate (ATP)-binding site (4), providing a structural basis for the activity of ALK TKIs against ROS1.
Crizotinib was originally developed as a MET inhibitor and subsequently approved for the treatment of advanced ALK-rearranged NSCLCs (53, 54). Further preclinical studies demonstrated that crizotinib also potently inhibits ROS1 (2, 4, 27, 28). In biochemical assays, crizotinib had an IC50 of 8 nM against MET, 40–60 nM against ALK, and 60 nM against ROS1 (55). Based on the available preclinical data, the phase I PROFILE 1001 study of crizotinib was amended to include ROS1-rearranged NSCLC patients in the expansion cohort. Early responses to crizotinib were marked and reminiscent of responses in ALK-rearranged patients (Figure 3). Among 50 patients with ROS1-rearranged NSCLCs in this trial cohort, the ORR to crizotinib was 72%, with disease control rate (DCR) of 90%. The median PFS reached 19.2 months (4). Based on the efficacy and safety demonstrated in this study, crizotinib was granted full approval by the FDA for the treatment of advanced ROS1-rearranged NSCLC in March 2016. Crizotinib has also received approval by the European Medicines Agency (EMA) for metastatic ROS1-rearranged NSCLC.
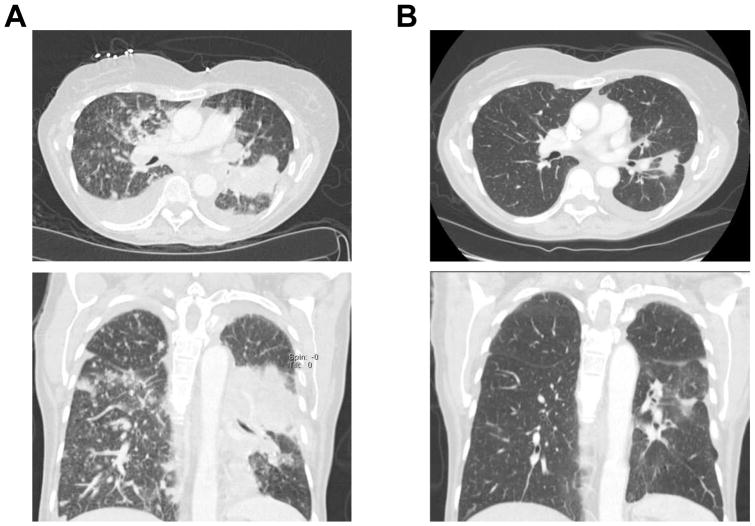
Figure 3.
Clinical response of a ROS1-rearranged patient to crizotinib. (A) Axial (top) and coronal (bottom) computed tomography images of the chest before crizotinib. (B) Axial (top) and coronal (bottom) computed tomography images of the chest after 6 weeks of crizotinib, demonstrating a dramatic improvement in the left lung mass, bilateral pulmonary nodules and pleural effusions.
It is worth noting that subsequent studies have suggested a shorter PFS estimate for crizotinib in ROS1-rearranged NSCLC. In the French phase II study (56) and in the EUROS1 retrospective study (57) of crizotinib for ROS1-rearranged NSCLC, median PFS was 9–10 months, although both of these studies enrolled only ~30 patients. In a larger East Asian phase II study of crizotinib, median PFS among 127 ROS1-rearranged lung cancer patients was 13.4 months (58). Each study included patients who had received varying numbers of prior lines of systemic therapy, although for all of these patients, crizotinib remained the first ROS1-directed TKI. A number of phase II studies of crizotinib are currently underway and will help generate more efficacy data for this group of patients.
Resistance to Crizotinib
TKI resistance represents a major hurdle to achieving durable responses to targeted therapy in virtually every context, including EGFR-mutant and ALK-rearranged NSCLC (59). Crizotinib resistance in ROS1-rearranged NSCLC is no exception, and causes the vast majority of patients to eventually progress on therapy. We are still in the early stages of elucidating clinical patterns of crizotinib resistance [e.g., frequency of oligoprogression or central nervous system (CNS)-only progression] as well as molecular mechanisms of resistance, but emerging data offer helpful insights to guide drug development efforts and inform the clinical use of ROS1 inhibitors beyond crizotinib.
Broadly speaking, acquired resistance to crizotinib arises secondary to “on target” (e.g., secondary acquired mutations in the ROS1 kinase domain) and “off target” (e.g., bypass signaling track activation or phenotypic change) mechanisms. Mutations within the ROS1 kinase domain occur in ~50–60% of crizotinib-resistant tumors (42). This is higher than the frequency of ALK kinase domain mutations (~20–25%) observed in crizotinib-resistant ALK-rearranged lung cancers (60, 61), and may reflect differences in crizotinib binding characteristics or its higher potency against ROS1 versus ALK (55).
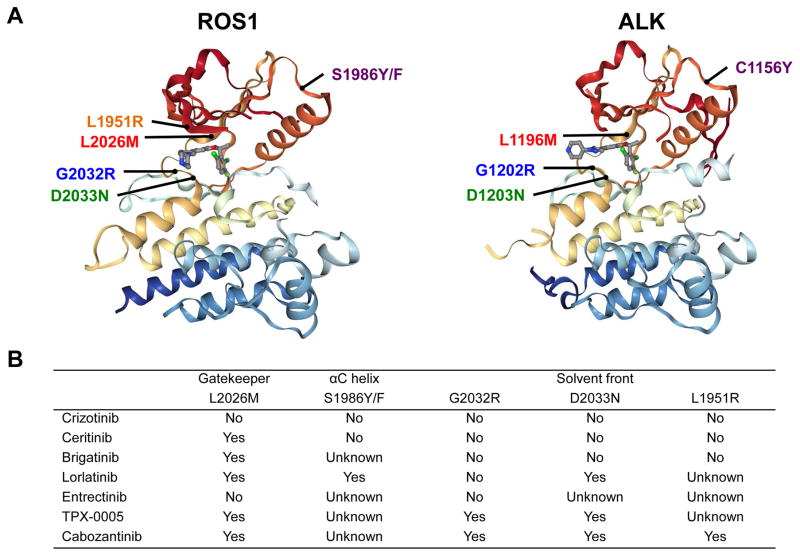
The most frequently observed resistant mutation has been the ROS1 G2032R mutation in the solvent front (i.e., solvent-exposed region of the kinase), analogous to ALK G1202R (Figure 4A) (42, 62, 63). G2032R was the first crizotinib-resistant mechanism reported in a patient with ROS1-rearranged lung adenocarcinoma (62). Multiple tumor metastatic sites examined at autopsy all harbored this mutation, suggesting it arose early in the evolution of resistance (62). Based on structural and cellular analyses, G2032R causes steric hindrance to the drug binding and does not alter the oncogenic kinase activity (62). In a recent series examining 16 ROS1-rearranged NSCLC patients with 17 post-crizotinib tumor biopsies, G2032R was identified in as many as 41% of the resistant biopsies (42), underscoring the importance of developing ROS1 inhibitors with potent activity against G2032R.
Figure 4.
Crizotinib-resistant ROS1 mutations. (A) Crizotinib-resistant secondary ROS1 mutations reported to date, mapped on the structure data of ROS1 kinase domain (left) in complex with crizotinib (PDB:3ZBF). Analogous ALK resistance mutations are mapped on the ALK kinase domain in complex with crizotinib (PDB:2XP2) on the right, revealing structural similarities. Note: The ALK mutation analogous to ROS1 L1951R has not been reported and is therefore not shown. (B) The activity of ROS1-directed tyrosine kinase inhibitors against known crizotinib-resistant ROS1 mutations. This table is based on the available preclinical data, not all of which have been validated in the clinic.
Another solvent front mutation, D2033N (analogous to ALK D1203N), has also been detected in crizotinib-resistant tumors (Figure 4A) (42, 64). This mutation affects the key electrostatic interaction between the D2033 residue and the piperidine moiety of crizotinib and also affects the neighboring residues at the surface of the ATP-binding pocket (64). As demonstrated in Figure 4A, additional mutations reported in clinical samples include: S1986Y/F (a mutation affecting the αC helix of the kinase domain which causes steric interference with drug binding; analogous to ALK C1156Y) (42, 65), L2026M (a ‘gatekeeper’ mutation in the ATP-binding pocket which hinders drug binding; analogous to ALK L1196M) (66), and L1951R (a solvent front mutation; no known analogous mutation in ALK) (66). The L1951R resistance mutation also emerged in an N-ethyl-N-nitrosourea (ENU) mutagenesis screen with CD74-ROS1-transformed Ba/F3 cells (67). Interestingly, along with G2032R, L1951R conferred the highest level crizotinib-resistant phenotype in vitro compared to other mutations including L2026M (67).
Off-target mechanisms of crizotinib resistance have been reported for ROS1, but are thus far (in the clinic) limited to isolated case reports. Tumor cells may activate an alternative signaling pathway (“bypass pathway”) and acquire resistance. As an example, an activating KIT mutation, D816G, was detected in a crizotinib-resistant ROS1-rearranged lung tumor, but not in the treatment-naïve tumor (68). In this resistant tumor specimen, other genes including ROS1, EGFR, KRAS, and BRAF did not have detectable alterations (68). Upregulation of EGFR signaling—not mediated by activating EGFR mutations at the DNA level—has been reported as a bypass mechanism in HCC78 cells made resistant to crizotinib (63, 69), although not yet validated in the clinic. Interestingly, in ROS1-rearranged and other fusion kinase-driven cell line models, EGFR activation and signaling appears to serve as an important early adaptive survival response to TKI exposure (70). Another preclinical study has suggested a KRAS G12C mutation as a potential crizotinib resistance mechanism in ROS1-rearranged lung cancer (71).
Finally, phenotypic changes such as epithelial mesenchymal transition (EMT) may contribute to crizotinib resistance. In one crizotinib-resistant, ROS1-rearranged lung tumor, no alterations were detected in ROS1, EGFR, ALK, KRAS, or MET. Instead, EMT-like changes were observed with decrease in E-cadherin and increase in vimentin (63). Similar changes were noted in two crizotinib-resistant clones derived from HCC78 cells with the acquisition of spindle-shaped morphology. However, these clones contained a concomitant ROS1 L2155S mutation, shown to confer crizotinib resistance in cell lines (63) [but not identified in mutagenesis screens (65) or patient samples (42, 62–66)]. Histologic transformation from adenocarcinoma to small-cell lung cancer (SCLC)—a known phenomenon in EGFR and ALK TKI resistance (59)—has not yet been reported in the context of ROS1. Nonetheless, we anticipate that SCLC transformation may eventually be observed as more patients are treated with sequential, increasingly potent ROS1 inhibitors. Further studies are needed to comprehensively characterize the landscape of TKI resistance mechanisms in ROS1-driven lung cancer.
Other ROS1 Inhibitors in Development
Table 1 lists additional ROS1 inhibitors in development. These TKIs each inhibit a different spectrum of kinase targets and ROS1 resistance mutations (Figure 4B), and exhibit unique toxicity profiles and activity in the central nervous system (CNS). Collectively, all of these factors will influence which TKI is used, and in what context, in the clinic. Below, we summarize the available data for the ROS1 inhibitors in development, and then discuss our approach to the sequential use of ROS1 TKIs in the clinic at the present time.
Table 1.
Ongoing clinical trials for ROS1-rearranged NSCLC.
| Study drug | Company | Phase | Target kinases | Primary outcome | Study location | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|
| Crizotinib | Pfizer | II | ROS1, ALK, MET | ORR | France | NCT02034981 |
| Crizotinib | Pfizer | II | ROS1 | ORR | Europe | NCT02183870 |
| Crizotinib | Pfizer | II | ROS1, ALK, MET | ORR | UK | NCT02664935 |
| Crizotinib | Pfizer | II | ROS1, MET | ORR | Italy | NCT02499614 |
| Ceritinib | Novartis | II | ROS1 | ORR | ROK | NCT01964157 |
| Ceritinib | Novartis | II | ROS1, ALK | ORR | China | NCT02276027 |
| Lorlatinib | Pfizer | II | ROS1, ALK | ORR | Global | NCT01970865* |
| Lorlatinib | Pfizer | II | ROS1, ALK | Intracranial DCR | USA | NCT02927340 |
| Entrectinib | Ignyta | II | ROS1, ALK, TRK A/B/C | ORR | Global | NCT02568267 |
| Cabozantinib | Exelixis | II | ROS1, TRK A/B/C, RET, MET, AXL | ORR | USA | NCT01639508 |
| DS-6051b | Daiichi Sankyo | I | ROS1, TRK A/B/C | Toxicity profile, ORR | USA | NCT02279433 |
| DS-6051b | Daiichi Sankyo | I | ROS1, TRK A/B/C | Toxicity profile | Japan | NCT02675491 |
| TPX-0005 | TP Therapeutics | I | ROS1, ALK, TRK A/B/C | Toxicity profile, ORR | USA, ROK | NCT03093116 |
This trial is now closed to accrual for ROS1 patients.
Note: While there are currently no open clinical trials of brigatinib in ROS1-rearranged NSCLC, brigatinib is also known to have anti-ROS1 activity.
Abbreviations: NSCLC, non-small cell lung cancer; ORR, objective response rate; DCR, disease control rate; UK, United Kingdom; USA, United States of America; ROK, Republic of Korea.
Ceritinib
Ceritinib is a potent and selective ALK inhibitor, active in crizotinib-naïve and - pretreated ALK-rearranged NSCLC (72–75). Ceritinib also inhibits ROS1 with a cellular IC50 of 180 nM in Ba/F3 cells expressing ROS1 rearrangement (as compared to cellular IC50 of 27–35 against ALK), and 50 nM in HCC78 cells harboring SLC34A2-ROS1 (76).
In a Korean phase II study, 32 patients with ROS1-rearranged advanced NSCLC were treated with ceritinib, dosed 750 mg daily fasting. Of note, in the two patients who had received prior crizotinib, no clinical response was observed. The trial was subsequently amended to include only crizotinib-naïve patients. Among crizotinib-naïve patients, the ORR was 67%, with DCR of 87%. The median PFS was 9.3 months for the entire cohort, and reached 19.3 months for crizotinib-naïve patients (76). Eight patients in the study had known brain metastases; intracranial ORR was 25% with intracranial DCR of 63% (76).
While ceritinib is clinically active in crizotinib-naïve ROS1-rearranged NSCLC, these findings also suggest that the role of ceritinib may be much more limited in the crizotinib-resistant setting. Indeed, ceritinib can inhibit the ROS1 L2026M gatekeeper mutation in vitro (68), but not G2032R (62, 67), D2033N (64), L1951R (67), or S1986Y/F (65) (Figure 4B). Furthermore, the efficacy of ceritinib must be weighed against its toxicities. Consistent with prior experiences (72–75), the most common ceritinib-associated adverse events (AEs) in the phase II study were diarrhea (78%), nausea (59%), anorexia (56%), and vomiting (53%) (76)—at higher frequencies overall than observed with crizotinib (4). Alternative ceritinib dosing with food may help ameliorate these side effects going forward (77). In the ASCEND-8 study, the 450 mg daily dosing of ceritinib with meals was much better tolerated as compared to 750 mg daily dosing fasting, yet reached similar steady-state plasma levels based on pharmacokinetic studies (77). Confirmation that antitumor efficacy is comparable between the two dosing regimens remains to be established. Larger, global studies will help better assess the systemic and intracranial efficacy of ceritinib in ROS1-rearranged lung cancers, particularly in crizotinib-naïve patients.
Brigatinib
Brigatinib recently received accelerated FDA approval for use in advanced ALK-rearranged NSCLC (78, 79). Like crizotinib and ceritinib, it also has anti-ROS1 activity based on preclinical studies, with an IC50 of 7.5 nM (versus anti-ALK IC50 of 9.8 nM) in CD74-ROS1-expressing Ba/F3 cells (80). In a recently reported phase 1/2 study of brigatinib, 3 patients with ROS1-rearranged NSCLC were enrolled. Two crizotinib-pretreated patients had stable disease and progressive disease, whereas a crizotinib-naïve patient had a partial response (78). The activity of brigatinib against resistant ROS1 mutants has thus far appeared comparable to ceritinib based on Ba/F3 models. In vitro, brigatinib inhibits the L2026M mutation, but not G2032R, D2033N, or L1951R (64, 67, 80, 81), raising the concern that similarly to ceritinib, it likely has limited activity against crizotinib-resistant ROS1-driven tumors (Figure 4B). The most common treatment-emergent AEs for brigatinib have consisted of nausea, diarrhea, headache, and cough; however, it has notably been associated with early pulmonary events including radiographic changes consistent with pneumonitis (78, 79).
Lorlatinib
Lorlatinib is a highly potent, small-molecule oral TKI optimized for selective ALK/ROS1 inhibition and robust CNS penetration (82). In vivo experiments in rats suggest a 30–40% drug exposure in the brain as compared to plasma; consistent with this, lorlatinib inhibits ROS1-driven glioblastoma tumor growth in mice (82). Moreover, in patients treated at the standard 100 mg daily dosing, lorlatinib achieves an even higher cerebrospinal fluid to plasma ratio ranging 61–96% (compared to the 30–40% seen in rats), consistent with excellent CNS penetration (83).
Recently, preliminary results from the phase I study of lorlatinib in ALK- and ROS1-rearranged NSCLCs were presented. Among 12 patients with ROS1-rearranged lung adenocarcinomas, the ORR was 50% with median PFS of 7 months (84). Among 5 patients with intracranial target lesions, 4 (80%) achieved intracranial responses (84). Lorlatinib was generally well tolerated, with the most frequent AEs of hypercholesterolemia (72%), hypertriglyceridemia (39%), peripheral neuropathy (39%), and peripheral edema (39%) (81).
Of note, lorlatinib has in vitro activity against several crizotinib-resistant mutations including L2026M (65, 82), S1986Y/F (65), and D2033N (64) (Figure 4B). Facchinetti et al. reported on a patient with EZR-ROS1-rearranged, crizotinib-resistant NSCLC with the S1986Y/F mutation, who achieved a dramatic and durable disease response to lorlatinib (65). However, lorlatinib’s activity against the ROS1 G2032R mutation may be limited in the clinic. Based on preclinical studies, G2032R appears to significantly reduce the cellular potency of lorlatinib, with IC50 ranging from 177 nM to 508 nM in ROS1-rearranged Ba/F3 models (as compared to cellular IC50 of 0.5–1 nM against wild-type ROS1) (65, 80, 81). Therefore, lorlatinib could be clinically active in select patients post-crizotinib, but may have limited efficacy in the subset of patients with tumors known to harbor G2032R. A global phase I/II trial of this agent in ALK- and ROS1-rearranged NSCLC has completed accrual (NCT01970865).
Entrectinib
Entrectinib is an oral inhibitor with low nanomolar potency against ALK, ROS1, and TRK kinases in enzymatic assays (85), and is able to effectively penetrate the blood-brain barrier (86). Its cellular IC50 against ROS1 in Ba/F3 models is ~5 nM (86). Notably, entrectinib has failed to demonstrate preclinical activity against the ROS1 L2026M or G2032R resistance mutations (81), suggesting it likely has little role in treating crizotinib-resistant ROS1-rearranged NSCLC. In two phase I studies of entrectinib (ALKA-372-001 and STARTRK-1), 14 patients with crizotinib-naïve ROS1-rearranged tumors (13 NSCLCs, 1 melanoma) were evaluated. The ORR to entrectinib was 86% (intracranial ORR, 63%), and median PFS was 19 months (87). Of note, 6 patients with prior crizotinib did not respond to entrectinib, consistent with the aforementioned preclinical studies. An ongoing phase II basket trial of entrectinib (NCT02568267) is enrolling patients with ROS1-rearranged NSCLCs; prior crizotinib is allowed only if patients have CNS-only disease progression.
Cabozantinib
Cabozantinib is an inhibitor of multiple tyrosine kinases including MET, VEGFR2, RET, and KIT. This multitargeted TKI is approved for use in medullary thyroid cancer and advanced renal cell carcinoma after prior anti-angiogenic therapy. Recent studies have demonstrated that cabozantinib additionally harbors anti-ROS1 activity (67, 80–82). In particular, cabozantinib has been shown across multiple studies to be active against solvent front resistance mutations in ROS1, including G2032R and D2033N (Figure 4B) (67, 80–82). The IC50 for cabozantinib in Ba/F3 cells expressing G2032R ranges from 13.5 nM (67) to 26 nM (81), and the IC50 in Ba/F3 expressing D2033N is 0.8 nM (64). Cabozantinib induced a near complete response in a patient with ROS1-rearranged NSCLC, who progressed on crizotinib with an acquired D2033N mutation (64).
Cabozantinib could thus represent a therapeutic option for crizotinib-pretreated patients with a G2032R mutation against which other available ROS1-targeted agents described above have limited activity. However, the significant toxicities seen with this agent due to its lack of selectivity—including palmar-plantar erythrodysesthesia, gastrointestinal toxicities and cardiovascular toxicities such as hypertension—will likely hinder development of this TKI for ROS1-rearranged NSCLC (88). In a phase II study of cabozantinib in 26 patients with RET-rearranged lung adenocarcinomas, 73% required dose reductions because of intolerable drug-related toxicities, consistent with experiences in other solid tumors (88). Alternative dosing regimens may need to be explored in order to mitigate toxicities. A phase II trial of cabozantinib is ongoing in patients with NSCLC harboring RET/ROS1/NTRK fusions or increased MET/AXL activity, and will help to define its activity in the ROS1 subset (NCT01639508).
DS-6051b
DS-6051b is an oral ROS1/TRK inhibitor currently in phase I testing (NCT02279433). Preliminary results from a Japanese phase I study of DS-6051b were recently presented (89). Among 13 patients with ROS1-rearranged NSCLCs, of whom 8 were evaluable, the ORR was 62.5% with DCR of 100%. Among the 3 crizotinib-pretreated patients in this cohort, no objective responses were seen. The most common treatment-emergent AEs included transaminitis, diarrhea, nausea, and constipation (89). More data is needed in order to evaluate the safety and activity of DS-6051b in ROS1-rearranged NSCLC.
TPX-0005
TPX-0005 is a potent ALK/ROS1/TRK inhibitor specifically designed to overcome the gatekeeper and solvent front resistance mutations in the respective kinases (90). Preliminary preclinical data suggest that it is active against the solvent front mutations including ROS1 G2032R, and also inhibits kinases implicated in bypass signaling such as SRC and FAK (90). A phase I/II study of TPX-0005 in advanced solid tumors with ALK/ROS1/NTRK1-3 rearrangements (TRIDENT-1) is now enrolling (NCT03093116).
Our current approach
Deciding which ROS1 inhibitor to use, at what juncture in the course of a patient’s treatment, is complex—particularly given the limited data available for some of the newer agents. Figure 5 summarizes our current evidence-based approach to the treatment of ROS1-rearranged NSCLC. An important caveat is that this approach will evolve over time as additional data emerge on new TKIs and resistance mechanisms.
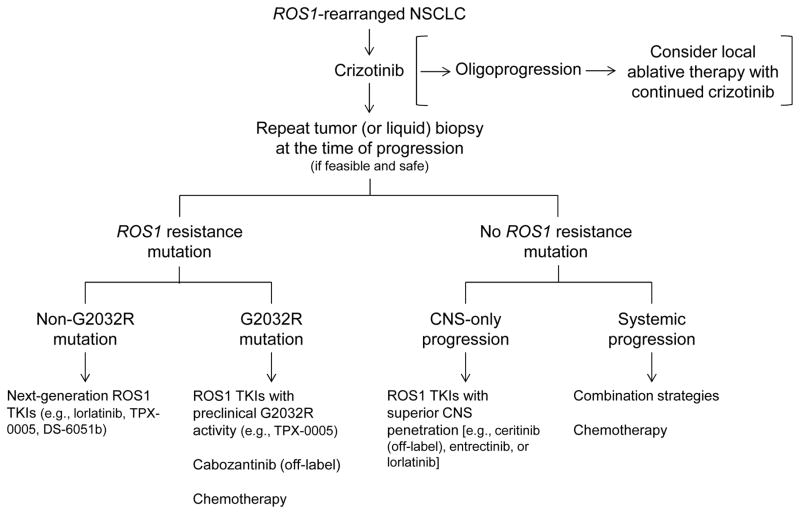
Figure 5.
Our approach to the treatment of advanced ROS1-rearranged lung cancer. After progression on first-line crizotinib, a repeat tumor biopsy is strongly recommended if feasible and safe, in order to determine the mechanism of crizotinib resistance. Liquid biopsy may be an alternative option if tumor biopsy is not feasible. The detection of a secondary ROS1 resistance mutation can inform the selection of next-line therapy. For example, in the case of a G2032R mutation, ROS1 inhibitors that have limited activity against G2032R based on preclinical data (see Figure 4B) should be avoided. In the absence of a ROS1 resistance mutation, options could include combination regimen trials or chemotherapy. Progression on/after crizotinib limited only to the central nervous system (CNS) may be effectively treated with a ROS1 inhibitor that has improved CNS penetration, such as entrectinib or lorlatinib. Of note, in the case of “oligoprogression” (i.e., progression in a limited number of metastatic sites) on first-line crizotinib, local ablative therapy could be considered with the continuation of crizotinib (see ref. 61).
For now, crizotinib remains the standard of care first-line TKI for the treatment of advanced ROS1-rearranged NSCLC. Once patients progress on or after crizotinib, we strongly recommend a repeat tumor biopsy if feasible and safe, in order to characterize the mechanism(s) of resistance and determine the presence of any ROS1 resistance mutations. If a tumor biopsy is not feasible, a “liquid biopsy” using a circulating tumor DNA (ctDNA) NGS-based assay may serve as an alternative, albeit less sensitive than the former. Of note, liquid biopsies have not yet been validated for the detection of ROS1 mutations; and furthermore, it can be more technically challenging to detect gene fusions as compared to point mutations using ctDNA.
The presence of a ROS1 resistance mutation in the repeat biopsy specimen suggests that the tumor may still be ROS1-dependent, and the patient should be directed toward clinical trials of new ROS1 inhibitors such as lorlatinib or TPX-0005, with activity against the detected mutations. In the particular case of G2032R, lorlatinib may be less effective; therefore, trials of other agents such as TPX-0005, or off-label use of cabozantinib (albeit with concerns regarding its toxicities), may be needed. In the absence of a ROS1 resistance mutation post-crizotinib, novel ROS1 TKIs may still be tried; however, these patients may derive greater benefit from chemotherapy or a combination strategy (e.g., a trial of a ROS1 TKI combined with another targeted agent or chemotherapy). A number of studies have suggested that patients with ROS1-rearranged lung cancer may be particularly responsive to pemetrexed-based therapies, similar to what has been observed in ALK-rearranged lung cancer (91–94).
The potential role of immunotherapy in the treatment of ROS1-rearranged NSCLC post-ROS1 TKIs is unclear. At least in EGFR-mutant and ALK-rearranged NSCLC, there appears to be limited benefit derived from checkpoint inhibitor monotherapy (61, 95–97). Further studies are needed to evaluate the role of immunotherapy in ROS1-rearranged lung cancer, and to assess the presence of potential biomarkers of response to immunotherapy—including the level of PD-L1 expression, inflammation in the tumor microenvironment, and tumor mutational burden—in this subset of patients.
Conclusions and Future Directions
Ten years have passed since the initial report of ROS1 rearrangements in NSCLC (Figure 1). ROS1-rearranged lung cancers are dependent on ROS1 for survival, and are thus sensitive to treatment using ROS1-targeted TKIs. Today, the National Comprehensive Cancer Network (NCCN) guidelines recommend testing for ROS1—along with EGFR, ALK, and PD-L1—at the time of diagnosis of metastatic NSCLC (98). Systematic diagnostic testing for ROS1 in metastatic NSCLC is not yet recommended by the European Society of Medical Oncology (ESMO) guidelines, although it is suggested (99). While crizotinib is currently the only FDA- and EMA-approved agent for the treatment of ROS1-rearranged NSCLC, a number of additional ROS1 inhibitors are undergoing clinical testing, with several showing early signals of clinical activity in the post-crizotinib setting.
As more ROS1 inhibitors are developed, further research will be critical to defining the role of each TKI in the clinic. Paramount to addressing this question will be the rigorous evaluation of each agent’s (1) CNS activity, (2) potency and clinical activity against resistant ROS1 mutant kinases, particularly G2032R, and (3) toxicity profile. Current data suggest that G2032R is the most frequent ROS1 resistance mutation emerging post-crizotinib (42). Therefore, development of agents that effectively target the G2032R mutation and are safe/tolerable in patients should be of highest priority in the ROS1 field.
In ALK-rearranged NSCLC for which multiple FDA-approved TKIs are available, the question of optimal sequencing of ALK TKIs has taken center stage. The recently reported data from J-ALEX and the global ALEX studies comparing alectinib with crizotinib in the front-line setting demonstrate that alectinib—a more potent and CNS-penetrant ALK TKI—is superior to crizotinib in advanced ALK-rearranged NSCLC (100, 101). The large magnitude of benefit seen with alectinib suggests that upfront use may be superior to the current sequential approach of crizotinib followed by alectinib. Extrapolating to ROS1-rearranged lung cancer, upfront use of a more potent and CNS-penetrant ROS1 TKI may confer greater benefit than crizotinib and possibly sequential treatment, although this remains to be evaluated. Future preclinical and clinical studies will help inform the optimal first-line therapy for ROS1-rearranged NSCLC.
Similar to what we have observed in EGFR-mutant and ALK-rearranged NSCLC (59), combination strategies (e.g., combining a ROS1 TKI with another targeted agent or chemotherapy) need to be developed and evaluated as potential strategies to overcome resistance to ROS1 inhibitors. In the case of resistance driven by off-target mechanisms such as bypass signaling activation, the use of a ROS1 inhibitor as monotherapy may be ineffective. There are currently no actively enrolling combination trials for ROS1-rearranged NSCLC. However, the knowledge of bypass resistance mechanisms outlined above suggests potential therapeutic avenues one could pursue. For instance, an approach of co-targeting ROS1 and EGFR could be explored given the preclinical evidence for the role of EGFR in driving acquired resistance as well as adaptive survival response to ROS1-targeted therapy (70, 71). Additionally, an entrectinib-based regimen may soon enter early-phase clinical testing, combining the blockade of ALK/ROS1 and MEK, a critical downstream survival and proliferation pathway (71, 102). For any new combination regimen, safety and dosing schedule will need to be carefully evaluated, as additive and/or unexpected toxicities may arise. Further advances in ROS1-based combination strategies will require an enhanced understanding of the bypass and downstream signaling tracks involved in ROS1 TKI resistance. Ultimately, upfront use of combination regimens may help delay and even prevent TKI resistance from emerging, and hence have a more transformative impact on the natural history of the cancer.
Finally, efforts are needed to standardize the diagnosis and treatment of ROS1-rearranged lung cancer at the global level. Clinicians, researchers, regulatory agencies, and patient advocates will need to come together in this endeavor, in order to ensure that patients across communities have access to the diagnostic tools and emerging therapy options. It is our hope that with continued research into the biology of ROS1-driven lung cancers, development of new therapeutic strategies, and enhanced patient access to these advances, we can further extend and improve the lives of patients with ROS1-rearranged lung cancer.
Acknowledgments
Grant Support
This work was supported by a grant from the National Cancer Institute (5R01CA164273, to ATS), by Be a Piece of the Solution, and by LungStrong.
Footnotes
Disclosures: JJL has served as a compensated consultant or received honoraria from Boehringer-Ingelheim and Chugai. ATS has served as a compensated consultant or received honoraria from Pfizer, Novartis, Genentech/Roche, Ariad, Ignyta, Blueprint Medicines, KSQ Therapeutics, and Foundation Medicine.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 2.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushime H, Wang LH, Shibuya M. Human c-ros-1 gene homologous to the v-ros sequence of UR2 sarcoma virus encodes for a transmembrane receptorlike molecule. Mol Cell Biol. 1986;6:3000–3004. doi: 10.1128/mcb.6.8.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birchmeier C, Birnbaum D, Waitches G, et al. Characterization of an activated human ros gene. Mol Cell Biol. 1986;6:3109–3116. doi: 10.1128/mcb.6.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagarajan L, Louie E, Tsujimoto Y, et al. The human c-ros gene (ROS) is located at chromosome region 6q16----6q22. Proc Natl Acad Sci U S A. 1986;83:6568–6572. doi: 10.1073/pnas.83.17.6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh H, Yoshida MC, Matsushime H, et al. Regional localization of the human c-ros-1 on 6q22 and flt on 13q12. Jpn J Cancer Res. 1987;78:772–775. [PubMed] [Google Scholar]
- 9.Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta. 2009;1795:37–52. doi: 10.1016/j.bbcan.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenberg E, Gödecke A, Walter B, et al. Transient and locally restricted expression of the ros1 protooncogene during mouse development. EMBO J. 1991;10:3693–3702. doi: 10.1002/j.1460-2075.1991.tb04937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tessarollo L, Nagarajan L, Parada LF. c-ros: the vertebrate homolog of the sevenless tyrosine kinase receptor is tightly regulated during organogenesis in mouse embryonic development. Development. 1992;115:11–20. doi: 10.1242/dev.115.1.11. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg-Riethmacher E, Walter B, Riethmacher D, et al. The c-ros tyrosine kinase receptor controls regionalization and differentiation of epithelial cells in the epididymis. Genes Dev. 1996;10:1184–1193. doi: 10.1101/gad.10.10.1184. [DOI] [PubMed] [Google Scholar]
- 14.Birchmeier C, Sharma S, Wigler M. Expression and rearrangement of the ROS1 gene in human glioblastoma cells. Proc Natl Acad Sci U S A. 1987;84:9270–9274. doi: 10.1073/pnas.84.24.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charest A, Lane K, McMahon K, et al. Fusion of FIG to the receptor tyrosine kinase ROS in a glioblastoma with an interstitial del(6)(q21q21) Genes Chromosomes Cancer. 2003;37:58–71. doi: 10.1002/gcc.10207. [DOI] [PubMed] [Google Scholar]
- 16.Charest A, Kheifets V, Park J, et al. Oncogenic targeting of an activated tyrosine kinase to the Golgi apparatus in a glioblastoma. Proc Natl Acad Sci U S A. 2003;100:916–921. doi: 10.1073/pnas.242741799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4:889–895. doi: 10.1158/2159-8290.CD-14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto H, Yoshida A, Taguchi K, et al. ALK, ROS1 and NTRK3 gene rearrangements in inflammatory myofibroblastic tumours. Histopathology. 2016;69:72–83. doi: 10.1111/his.12910. [DOI] [PubMed] [Google Scholar]
- 20.Gu TL, Deng X, Huang F, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One. 2011;6:e15640. doi: 10.1371/journal.pone.0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birch AH, Arcand SL, Oros KK, et al. Chromosome 3 anomalies investigated by genome wide SNP analysis of benign, low malignant potential and low grade ovarian serous tumours. PLoS One. 2011;6:e28250. doi: 10.1371/journal.pone.0028250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Lee SE, Kang SY, et al. Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer. 2013;119:1627–1635. doi: 10.1002/cncr.27967. [DOI] [PubMed] [Google Scholar]
- 23.Aisner DL, Nguyen TT, Paskulin DD, et al. ROS1 and ALK fusions in colorectal cancer, with evidence of intratumoral heterogeneity for molecular drivers. Mol Cancer Res. 2014;12:111–118. doi: 10.1158/1541-7786.MCR-13-0479-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacomini CP, Sun S, Varma S, et al. Breakpoint analysis of transcriptional and genomic profiles uncovers novel gene fusions spanning multiple human cancer types. PLoS Genet. 2013;9:e1003464. doi: 10.1371/journal.pgen.1003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 27.Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18:4570–4579. doi: 10.1158/1078-0432.CCR-12-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimkunas VM, Crosby KE, Li D, et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res. 2012;18:4449–4457. doi: 10.1158/1078-0432.CCR-11-3351. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Fang R, Sun Y, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One. 2011;6:e28204. doi: 10.1371/journal.pone.0028204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arai Y, Totoki Y, Takahashi H, et al. Mouse model for ROS1-rearranged lung cancer. PLoS One. 2013;8:e56010. doi: 10.1371/journal.pone.0056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida A, Kohno T, Tsuta K, et al. ROS1-rearranged lung cancer: a clinicopathologic and molecular study of 15 surgical cases. Am J Surg Pathol. 2013;37:554–562. doi: 10.1097/PAS.0b013e3182758fe6. [DOI] [PubMed] [Google Scholar]
- 32.Suehara Y, Arcila M, Wang L, et al. Identification of KIF5B-RET and GOPC-ROS1 fusions in lung adenocarcinomas through a comprehensive mRNA-based screen for tyrosine kinase fusions. Clin Cancer Res. 2012;18:6599–6608. doi: 10.1158/1078-0432.CCR-12-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo JS, Ju YS, Lee WC. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res. 2012;22:2109–2119. doi: 10.1101/gr.145144.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 36.Ou SH, Chalmers ZR, Azada MC, et al. Identification of a novel TMEM106B-ROS1 fusion variant in lung adenocarcinoma by comprehensive genomic profiling. Lung Cancer. 2015;88:352–354. doi: 10.1016/j.lungcan.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Zhu VW, Upadhyay D, Schrock AB, et al. TPD52L1-ROS1, a new ROS1 fusion variant in lung adenosquamous cell carcinoma identified by comprehensive genomic profiling. Lung Cancer. 2016;97:48–50. doi: 10.1016/j.lungcan.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charest A, Wilker EW, McLaughlin ME, et al. ROS fusion tyrosine kinase activates a SH2 domain-containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling axis to form glioblastoma in mice. Cancer Res. 2006;66:7473–7481. doi: 10.1158/0008-5472.CAN-06-1193. [DOI] [PubMed] [Google Scholar]
- 40.Davies KD, Doebele RC. Molecular pathways: ROS1 fusion proteins in cancer. Clin Cancer Res. 2013;19:4040–4045. doi: 10.1158/1078-0432.CCR-12-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jun HJ, Johnson H, Bronson RT, et al. The oncogenic lung cancer fusion kinase CD74-ROS activates a novel invasiveness pathway through E-Syt1 phosphorylation. Cancer Res. 2012;72:3764–3774. doi: 10.1158/0008-5472.CAN-11-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gainor JF, Tseng D, Yoda S, et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small cell lung cancer. J Clin Oncol Precision Oncol. 2017 doi: 10.1200/PO.17.00063. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:4273–4281. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiesweg M, Eberhardt WE, Reis H, et al. High prevalence of concomitant oncogene mutations in prospectively identified patients with ROS1-positive metastatic lung cancer. J Thorac Oncol. 2017;12:54–64. doi: 10.1016/j.jtho.2016.08.137. [DOI] [PubMed] [Google Scholar]
- 45.Lin JJ, Ritterhouse LL, Ali SM, et al. ROS1 fusions rarely overlap with other oncogenic drivers in non-small cell lung cancer. J Thorac Oncol. 2017;12:872–877. doi: 10.1016/j.jtho.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dagogo-Jack I, Shaw AT. Screening for ALK rearrangements in lung cancer: time for a new generation of diagnostics? Oncologist. 2016;21:662–663. doi: 10.1634/theoncologist.2016-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bubendorf L, Büttner R, Al-Dayel F, et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch. 2016;469:489–503. doi: 10.1007/s00428-016-2000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sholl LM, Sun H, Butaney M, et al. ROS1 immunohistochemistry for detection of ROS1-rearranged lung adenocarcinomas. Am J Surg Pathol. 2013;37:1441–1449. doi: 10.1097/PAS.0b013e3182960fa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heuckmann JM, Balke-Want H, Malchers F, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. 2012;18:4682–4690. doi: 10.1158/1078-0432.CCR-11-3260. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida T, Oya Y, Tanaka K, et al. Differential crizotinib response duration among ALK fusion variants in ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34:3383–3389. doi: 10.1200/JCO.2015.65.8732. [DOI] [PubMed] [Google Scholar]
- 51.Woo CG, Seo S, Kim SW, et al. Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann Oncol. 2017;28:791–797. doi: 10.1093/annonc/mdw693. [DOI] [PubMed] [Google Scholar]
- 52.McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68:3389–3395. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 53.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 54.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 55.Shaw AT, Camidge DR, Engelman JA, et al. Clinical activity of crizotinib in advanced non-small cell lung cancer (NSCLC) harboring ROS1 gene rearrangement. J Clin Oncol. 2012;23(suppl):2091a. [Google Scholar]
- 56.Moro-Sibilot D, Faivre L, Zalcman G, et al. Crizotinib in patients with advanced ROS1-rearranged non-small cell lung cancer (NSCLC). Preliminary results of the ACSé phase II trial. J Clin Oncol. 2015;33(suppl) abstr 8065. [Google Scholar]
- 57.Mazières J, Zalcman G, Crinò L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33:992–999. doi: 10.1200/JCO.2014.58.3302. [DOI] [PubMed] [Google Scholar]
- 58.Goto K, Yang JC, Kim DW, et al. Phase II study of crizotinib in East Asian patients (pts) with ROS1-positive advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2016;34(suppl) doi: 10.1200/JCO.2017.75.5587. abstr 9022. [DOI] [PubMed] [Google Scholar]
- 59.Lin JJ, Shaw AT. Resisting resistance: targeted therapies in lung cancer. Trends Cancer. 2016;2:350–364. doi: 10.1016/j.trecan.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Awad MM, Katayama R, McTigue M, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;368:2395–2401. doi: 10.1056/NEJMoa1215530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song A, Kim TM, Kim DW, et al. Molecular changes associated with acquired resistance to crizotinib in ROS1-rearranged non-small cell lung cancer. Clin Cancer Res. 2015;21:2379–2387. doi: 10.1158/1078-0432.CCR-14-1350. [DOI] [PubMed] [Google Scholar]
- 64.Drilon A, Somwar R, Wagner JP, et al. A novel crizotinib-resistant solvent-front mutation responsive to cabozantinib therapy in a patient with ROS1-rearranged lung cancer. Clin Cancer Res. 2016;22:2351–2358. doi: 10.1158/1078-0432.CCR-15-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Facchinetti F, Loriot Y, Kuo MS, et al. Crizotinib-resistant ROS1 mutations reveal a predictive kinase inhibitor sensitivity model for ROS1- and ALK-rearranged lung cancers. Clin Cancer Res. 2016;22:5983–5991. doi: 10.1158/1078-0432.CCR-16-0917. [DOI] [PubMed] [Google Scholar]
- 66.McCoach CE, Le AT, Aisner D, et al. Resistance mechanisms to targeted therapies in ROS1+ and ALK+ non- small cell lung cancer. J Clin Oncol. 2016;34(suppl) doi: 10.1158/1078-0432.CCR-17-2452. abstr 9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katayama R, Kobayashi Y, Friboulet L, et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion positive cancer. Clin Cancer Res. 2015;21:166–174. doi: 10.1158/1078-0432.CCR-14-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dziadziuszko R, Le AT, Wrona A, et al. An activating KIT mutation induces crizotinib resistance in ROS1-positive lung cancer. J Thorac Oncol. 2016;11:1273–1281. doi: 10.1016/j.jtho.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davies KD, Mahale S, Astling DP, et al. Resistance to ROS1 inhibition mediated by EGFR pathway activation in non-small cell lung cancer. PLoS One. 2013;8:e82236. doi: 10.1371/journal.pone.0082236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaishnavi A, Schubert L, Rix U, et al. EGFR mediates responses to small-molecule drugs targeting oncogenic fusion kinases. Cancer Res. 2017;77:3351–3363. doi: 10.1158/0008-5472.CAN-17-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cargnelutti M, Corso S, Pergolizzi M, et al. Activation of RAS family members confers resistance to ROS1 targeting drugs. Oncotarget. 2015;6:5182–5194. doi: 10.18632/oncotarget.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crinò L, Ahn MJ, De Marinis F, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol. 2016;34:2866–2873. doi: 10.1200/JCO.2015.65.5936. [DOI] [PubMed] [Google Scholar]
- 74.Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–463. doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 76.Lim SM, Kim HR, Lee JS, et al. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol. 2017 doi: 10.1200/JCO.2016.71.3701. [DOI] [PubMed] [Google Scholar]
- 77.Cho BC, Kim DW, Bearz A, et al. ASCEND-8: A randomized phase I study of ceritinib 450 mg or 600 mg taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic-lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC) J Thorac Oncol. 2017 doi: 10.1016/j.jtho.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:1683–1696. doi: 10.1016/S1470-2045(16)30392-8. [DOI] [PubMed] [Google Scholar]
- 79.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017 doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 80.Davare MA, Vellore NA, Wagner JP, et al. Structural insight into selectivity and resistance profiles of ROS1 tyrosine kinase inhibitors. Proc Natl Acad Sci U S A. 2015;112:E5381–5390. doi: 10.1073/pnas.1515281112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chong CR, Bahcall M, Capelletti M, et al. Identification of existing drugs that effectively target NTRK1 and ROS1 rearrangements in lung cancer. Clin Cancer Res. 2017;23:204–213. doi: 10.1158/1078-0432.CCR-15-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou HY, Li Q, Engstrom LD, et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci U S A. 2015;112:3493–3498. doi: 10.1073/pnas.1420785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Solomon BJ, Bauer TM, Felip E, et al. Safety and efficacy of lorlatinib (PF-06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC) J Clin Oncol. 2016;34(suppl) abstr 9009. [Google Scholar]
- 84.Felip E, Bauer TM, Solomon B, et al. Safety and efficacy of lorlatinib (PF-06463922) in patients with advanced ALK+ or ROS1+ non-small-cell lung cancer (NSCLC) J Thorac Oncol. 2017;12:S383–S384. [Google Scholar]
- 85.Menichincheri M, Ardini E, Magnaghi P, et al. Discovery of entrectinib: a new 3-aminoindazole as a potent anaplastic lymphoma kinase (ALK), c-ros oncogene 1 kinase (ROS1), and pan-tropomyosin receptor kinases (Pan-TRKs) inhibitor. J Med Chem. 2016;59:3392–3408. doi: 10.1021/acs.jmedchem.6b00064. [DOI] [PubMed] [Google Scholar]
- 86.Ardini E, Menichincheri M, Banfi P, et al. Entrectinib, a pan-TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol Cancer Ther. 2016;15:628–639. doi: 10.1158/1535-7163.MCT-15-0758. [DOI] [PubMed] [Google Scholar]
- 87.Drilon A, Siena S, Ou SI, et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372–001 and STARTRK-1) Cancer Discov. 2017;7:400–409. doi: 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17:1653–1660. doi: 10.1016/S1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nosaki K, Fujiwara Y, Takeda M, et al. Phase I study of DS-6051b, a ROS1/NTRK inhibitor, in Japanese subjects with advanced solid tumors harboring either a ROS1 or NTRK fusion gene. J Thorac Oncol. 2017;12:S1069. [Google Scholar]
- 90.Cui JJ, Zhai D, Deng W, et al. TPX-0005: A multi-faceted approach to overcoming clinical resistances from current ALK or ROS1 inhibitor treatment in lung cancer. J Thorac Oncol. 2017;12:S1164–S1165. [Google Scholar]
- 91.Riess JW, Padda SK, Bangs CD, et al. A case series of lengthy progression-free survival with pemetrexed-containing therapy in metastatic non-small cell lung cancer patients harboring ROS1 gene rearrangements. Clin Lung Cancer. 2013;14:592–595. doi: 10.1016/j.cllc.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liang Y, Wakelee HA, Neal JW. Relationship of driver oncogenes to long-term pemetrexed response in non-small-cell lung cancer. Clin Lung Cancer. 2015;16:366–373. doi: 10.1016/j.cllc.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen YF, Hsieh MS, Wu SG, et al. Efficacy of pemetrexed-based chemotherapy in patients with ROS1 fusion-positive lung adenocarcinoma compared with in patients harboring other driver mutations in East Asian populations. J Thorac Oncol. 2016;11:1140–1152. doi: 10.1016/j.jtho.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 94.Song Z, Su H, Zhang Y. Patients with ROS1 rearrangement-positive non-small-cell lung cancer benefit from pemetrexed-based chemotherapy. Cancer Med. 2016;5:2688–2693. doi: 10.1002/cam4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 97.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.National Comprehensive Cancer Network. [Accessed May 26, 2017];Non-small cell lung cancer (Version 6.2017) https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 99.Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v1–v27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 100.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 101.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017 doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 102.Hrustanovic G, Olivas V, Pazarentzos E, et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med. 2015;21:1038–1047. doi: 10.1038/nm.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist. 2013;18:865–875. doi: 10.1634/theoncologist.2013-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]