Abstract
HIV patients who are not retained in medical care risk viral resistance, disease progression to AIDS, and mortality. Numerous interventions have been tested to improve retention, but they are limited by their resource-intensive approaches and lack of focus on new patients, who are at highest risk for drop-out. Data show that acceptance and disclosure of HIV status might impact retention, yet these variables have not been targeted in previous interventions. In this pilot randomized controlled trial, we assessed feasibility, acceptability, and preliminary efficacy of a brief, 2-session Acceptance Based Behavior Therapy (ABBT), relative to treatment-as-usual (TAU), in 34 new-to-care HIV patients. ABBT attendance was high and patient feedback was positive. Relative to TAU, ABBT had significant positive effects on retention, as well as putativ mechanisms of action, including experiential avoidance of HIV, willingness to make and actual disclosures of HIV status, and perceived social support. Further testing of ABBT is warranted.
Keywords: HIV/AIDS, retention, acceptance, disclosure
Introduction
Retention in HIV medical care goes beyond linkage, which typically consists of initiating care after a positive test result. Retention represents a patient's longitudinal engagement in care as evidenced by consistency in attending appointments with medical providers. For People Living with HIV (PLWH), the U.S. Department of Health and Human Services (DHHS) recommends a follow-up visit at least once every 3-6 months (1) after initiating HIV care. For patients who recently started antiretroviral treatment (ART), the expected appointment frequency may be increased. However, drop-out rates in the months following initiation of HIV care can run as high as 50% (2), with 31-46% of patients dropping out after the first visit (3). Unfortunately, up to 50% of the more than one million individuals (4) infected with HIV in the U.S. are not regularly retained in medical care (5, 6); only 45-55% of PLWH complete at least one visit every six months (6, 7).
PLWH who do not receive consistent, ongoing medical care risk a number of critical consequences to their health and quality of life, and are at increased risk of transmitting HIV (8). Most notably, patients who have difficulty engaging in care have a reduced likelihood of timely ART initiation (9). Once ART is initiated, patients who are not retained in care are also less likely to be medication adherent, increasing the risk for viral drug resistance (10) and HIV transmission (11). Many HIV patients in the U.S. fail to achieve sustained viral suppression, in part because of gaps in retention in care (12). Patients who are not retained in care also have reduced CD4 counts; greater risk of opportunistic infections (13); greater risk of disease progression to AIDS (11); and, increased mortality risk (14). In sum, retention in HIV care is essential for the long-term health and well-being of PLWH. As such, the present study aimed to improve retention via a brief, behaviorally-based intervention.
In the past decade, there have been approximately 20 intervention studies aimed at improving HIV retention in care (summarized in (15-17)). Although several are promising, many of these interventions focused on retaining patients who showed sporadic engagement in care in the past (e.g., women who had recently missed an appointment or had not attended an appointment in the past four months (18)). Only two studies sought to improve retention in patients who were seeking HIV medical care for the first time. In a study by Naar-King et al. (19), a case management approach was used to address structural, as well as psychological barriers to care. Although results were encouraging, the intervention was highly intensive and lengthy. Over the 12-month intervention period, the average monthly contact was 4.90 hours per patient. This approach simply is not feasible at most HIV community-based clinics. Similarly, Wohl et al.'s intervention (20), although effective at increasing retention among youth new to care, was also extremely resource intensive, with participants meeting weekly with a case manager for the first two months and monthly for the next 22 months of care. In sum, extant data show that retention can improve with interventions that are complex, resource intensive, and prolonged.
A number of environmental and structural barriers have been shown to reduce entry and future retention in care for PLWH, including housing instability and lack of transportation (5). In addition to these challenges, new patients must also overcome a number of psychological hurdles to fully engage in care, including depression (21) and coping with the psychosocial aspects of their HIV diagnosis. Two well-documented retention barriers relevant to psychological coping with HIV are: poor social support and non-disclosure. Social support has consistently been shown to predict retention in care. For instance, in a study of PLWH that controlled for AIDS diagnosis, insurance, gender, and substance abuse, increased number of social network supports was predictive of retention in care (22).
A factor closely linked to social support is disclosure of HIV status. Undoubtedly, disclosure can provoke anxiety, shame, fear of stigmatization, fear of abandonment, and, sometimes, fear of violence (23, 24). For women, accusations of infidelity and the risk of violence are often cited as acute fears associated with disclosure (25). By practicing informed decision-making about disclosure, in which patients explore the safety risks and other possible outcomes of disclosure while rehearsing various potential conversation outcomes (26), steps can be taken to mitigate these risks and maximize the likelihood of positive reactions to disclosure. As a consequence, PLWH can improve their social support and increase the likelihood of positive HIV health outcomes via careful and thoughtful disclosure (27, 28). Specifically, we suggest that disclosure allows patients to obtain the practical social support (e.g., rides to appointments) and emotional social support (e.g., encouragement to attend appointments) that they need to engage in care. Informed disclosure has been specifically linked to receiving adequate medical care (29), perhaps because disclosers are, in general, more open and receptive to care (30). Wohl et al. (27) reported that disclosure was a robust predictor of retention among HIV-positive minority populations in care (27). Among mostly single, minority, impoverished mothers, disclosure to children predicted fewer missed medical appointments (31). In another study, 66% of patients who disclosed their serostatus were retained in care for up to two years, whereas only 11% of patients who did not disclose were retained during the same period (32). Despite the growing literature supporting the positive relationship of disclosure and retention in care, only 50% of patients receive input from care providers on the benefits and risks of disclosure and how best to share their serostatus, and these discussions are not necessarily initiated early in care (33). Further, to our knowledge, none of the previously tested retention interventions specifically targeted facilitating or increasing disclosure.
We suggest that acceptance of HIV status is an important precursor to disclosure. Initial contact with medical treatment providers is the first step toward self-acceptance of the diagnosis and its related stresses (e.g., stigmatization (34, 35)), learning the implications of infection on one's future, and considering a change in behaviors to fit the needs of maintaining healthy living. Attempts to avoid distressing experiences (e.g., anxious bodily sensations, depressive feelings, fearful thoughts of rejection) can be broadly defined as “experiential avoidance (36).” Wegner and Zanakos (37) found that suppression of emotional thoughts magnified the emotionality and accompanying physiological reaction to the suppressed thoughts. Further, attempts at thought suppression often exacerbate symptoms, producing a “rebound effect” in which thought frequency increases (38-40). Thus, applied to PLWH, those who attempt to ignore or suppress the stresses of living with HIV via experiential avoidance are at risk for feeling worse about their illness, having a worsened overall psychological state, and, potentially, poor retention in medical care.
A growing body of research supports the use of behaviorally-based interventions to reduce experiential avoidance and promote acceptance. These interventions, often described as acceptance-based behavior therapy (ABBT), stem from Acceptance and Commitment Therapy (ACT; (41)). ACT encourages patients to “defuse” distressing psychological experiences and to adopt an accepting stance towards one's experience as it unfolds in real time. This contrasts with traditional cognitive therapy, which is predicated on the assumption that therapeutic effects are mediated by changes in cognitions, including thoughts, beliefs, and schemas. ACT also stresses exercises aimed at identifying and crystallizing key personal values, translating these values into specific behavioral goals, and designing and implementing behavior change strategies to realize those goals through “committed action.” The research supporting ACT's efficacy is growing, with a number of studies showing significant between-group effect sizes (42) on a variety of problems (see (43) for review). There is also a growing body of literature supporting ACT's efficacy in behavioral medicine populations (see (44) for review). For example, ABBT effectively promoted adherence behaviors in adults with diabetes (45). In a small sample, Skinta and colleagues showed that an acceptance-based intervention could reduce self-stigma in PLWH (46). Thus, it appears that acceptance-based interventions might impact variables relevant to medical care retention, but more research is needed among PLWH.
The purpose of this pilot randomized controlled trial was to determine whether further testing of acceptance-based behavioral therapy (ABBT) to promote retention in care among PLWH newly seeking medical care was feasible and acceptable to patients, and to provide preliminary effect sizes. The current study built on our previous open trial in which we developed and tested ABBT in a small sample of patients who were new to care (47). We targeted reducing experiential avoidance (and increasing acceptance of HIV) as the primary mechanism to mitigate the effects of barriers to HIV care by promoting self-care and encouraging disclosure, as a way of leveraging social supports. By promoting acceptance and reducing experiential avoidance of HIV, we hypothesized that ABBT, relative to a control condition, would lead to increased willingness to seek social support because of informed disclosure, and ultimately, increased willingness to attend medical appointments.
Methods
Setting
This study occurred in two academically-affiliated HIV primary care, hospital-based, outpatient clinics from August 2014 to May 2016. The first site, in Providence, R.I., is the largest comprehensive HIV primary and specialty care clinic in R.I., treating 85% of PLWH in the state. The patient panel is primarily male (70%), with the following ethno-racial backgrounds: 62% non-Latino White, 34% African American, and 25% Latino. The second site, in New Orleans, L.A., is the largest HIV clinic in the area. Patients are primarily African American (76%) and male (63%), with very few being ethnically Latino (<5%).
Participants
Inclusion criteria were
(1) HIV+; (2) between 18 and 60 years old; (3) entering HIV medical care services for the first time (that is, not transferring HIV care from another location); and, (4) have telephone access. Telephone access was important because participants had the option to complete follow-up assessments by telephone to reduce transportation burden and to reduce potentially confounding our primary outcome of medical appointment attendance. Among patients screened at our recruitment sites, 97% had mobile phones. The only exclusion criterion was cognitive impairment assessed by the International HIV Dementia Scale (48).
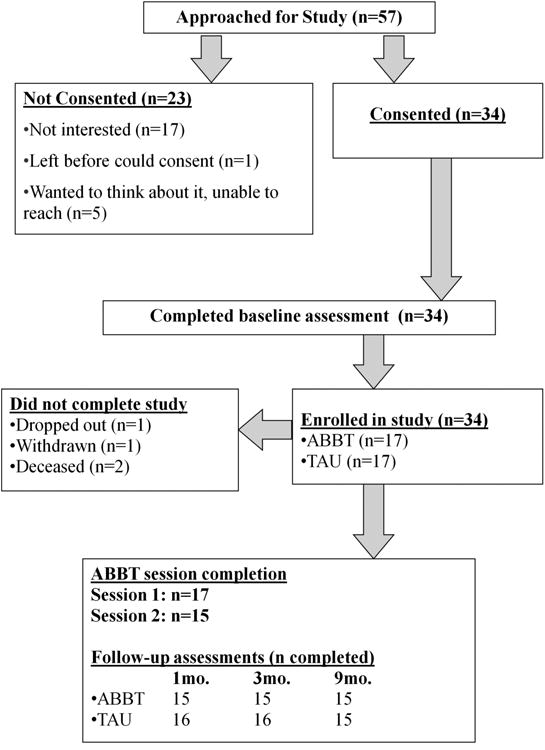
Fifty-seven participants across both sites were screened and eligible to participate. Thirty percent (n=17) of eligible patients declined participation in the study when they were approached; another 11% (n=6) expressed some interest in participation but either left the clinic prior to consenting or could not be reached to schedule a research visit. The final sample consisted of 34 patients. The majority of participants (88%; n=30) completed endpoint, 9-month follow-up data collection procedures.
Procedures
Study procedures were approved by the Institutional Review Boards at each of the recruitment sites. Research assistants recruited and screened eligible participants during routine intake procedures for new patients at each site with the priority of enrolling participants before their first appointment with a medical provider. Informed consent was obtained and participants signed a release of information form so that study staff could review their medical records. Participants were compensated $25 for completion of each assessment, which occurred at baseline, 1-, 3-, and 9-months post-baseline. As needed, cab rides/bus vouchers were provided for travel to the clinic if participants preferred to complete assessments in person. After completing baseline assessments, participants were randomized to ABBT or treatment-as-usual (TAU). Stratified permuted block randomization was used to ensure groups were balanced for active substance use (alcohol use, defined as hazardous drinking by Alcohol Use Disorders Identification Test-C (49); or, use of heroin, cocaine, or methamphetamine during the previous month).
Study interventions
ABBT
The ABBT intervention was administered using a manualized, structured protocol that was previously developed and revised in a small open trial (47). Interventionists were three licensed clinical psychologists who provided mental health services at each site but were not typically involved in the early stages of new patient care. A four-hour didactic training workshop for the interventionists educated them on the challenges of engaging PLWH in care, acceptance-based perspectives on health behavior change, skills to help patients make informed disclosure choices, plus role-plays of standard, challenging, and emergency situations. To overcome geographical barriers, interventionists had weekly telephone supervision during the trial. Supervision was provided by one of the study's principal investigators, a doctoral-level clinical psychologist. All sessions were audiotaped and 50% of sessions were randomly selected for fidelity evaluation. These sessions equally came from the two study sites and were evenly split between the first and second sessions. The three study interventionists had sessions coded for fidelity by a non-study rater. According to this rater, interventionists' adherence to the intervention manual was 96%. We delivered the intervention at the time of the first two clinic appointments. Session 1 of ABBT lasted 20-25 minutes and session 2, which occurred approximately two weeks later, was 10-15 minutes. The intervention was designed to be short and feasible in a busy clinical setting. Whenever possible, these visits were linked to regular clinical appointments. The primary goal of ABBT was to facilitate acceptance of HIV status by examining the problems associated with struggling with the HIV diagnosis, promoting a willingness to accept the stressors that living with HIV entail, and setting goals to help improve quality of life. As discussed in an earlier paper (47), we sought to create a foundation for HIV acceptance at the first session via experiential exercises (36) and built on this in the second session via practice of acceptance-based coping skills and development of a behavioral plan to target retention barriers identified in the first session. To aid patients in assessing the benefits and risks of seeking social support via disclosure, part of our second intervention session (and the first when appropriate) was used to develop informed decision-making skills about disclosure as described by Kalichman and Nachimson (26). The intervention was designed to acknowledge the real risks associated with disclosure and the common fears PLWH have about disclosure. Interventionists discussed disclosure-related concerns with participants and as appropriate, helped participants balance fears of negative reactions with motivation to disclose to personally-relevant, participant-identified disclosure targets.
TAU
The control condition was each clinic's treatment-as usual procedure which did not include any elements of ABBT. All participants received TAU services that included referrals to psychosocial treatment, substance abuse counseling, clinic- and community-based HIV support groups, and referrals to clinic case managers, as needed. Mental health services were available at both study sites and were used by participants depending on needs, preferences, and on-site clinician availability. TAU also consisted of each clinic's already established procedures for retaining new patients in care. Specifically, both sites employed individuals whose primary job responsibility was to maintain contact with patients and to support their retention by addressing barriers whenever possible (e.g., offering bus vouchers to overcome transportation barriers).
Assessments
Feasibility and acceptability outcomes included ABBT attendance rates at medical appointments, responses to qualitative interviews with participants, and satisfaction ratings at treatment completion (measured by a study-specific exit measure, with item scores ranging from 1-7, with 7 indicating highest agreement; and the Client Satisfaction Questionnaire-Revised (CSQ-8-R; (50)), which is an 8-point measure with a score of 32 indicating highest satisfaction). We assessed the primary outcome of interest, medical appointment attendance, via review of participants' electronic medical records. We also assessed ABBT's proposed mechanisms of action via self-report measures. The Multidimensional Scale of Perceived Social Support (MSPSS; (51, 52)) was used to measure perceived social support; reliability of this measure in our sample was 0.856. No measure of willingness to make informed disclosure in general exists; the closest approximation is the Brief HIV Disclosure and Safer Sex Self-Efficacy Scales (BHD) (26, 53). We used the HIV disclosure subscale from the BHD to assess for willingness to make informed disclosure and for actual disclosure; reliability was 0.835. We also assessed the raw number of individuals disclosed to at each assessment. HIV experiential avoidance was assessed by the Acceptance and Action Questionnaire-2 (AAQ-2; (54)), using slight modifications to two items measuring HIV acceptance/experiential avoidance. The widely-used AAQ-2 is a 10-item measure assessing an individual's ability to accept undesirable internal events while otherwise continuing to pursue desired goals (55); reliability to the slightly modified AAQ-2 was 0.925 in our study. Finally, to further characterize the sample, we assessed experiences of stigmatization via the shortened version (56) of the HIV Stigma Scale (57), a well-validated measure of HIV-related experiences and perceptions of internal and external stigmatization that showed adequate reliability in our sample (0.888).
Data Analysis
Descriptive statistics were used to characterize the sample. We used t tests and χ2 tests for between group comparisons on continuous and categorical variables, respectively. We estimated intervention effects as the between-group difference in change scores between baseline and the 1-, 3-, and, 9-month assessments. We analyzed change scores as they speak directly to the amount of within subject change over time, allowing us to transparently convey the substantive magnitude of between group differences. We present Cohen's d (58) with 95 % confidence intervals (CIs). Effect sizes of 0.2 are considered small, 0.5 medium, and 0.8 large (58). We used a change score analysis in this study because it (a) examines within subject change, (b) controls for time-invariant between-group differences, and (c) unlike more complex analytical models, is not based on statistical assumptions that may not be reasonable with small sample sizes.
Results
Participant characteristics
Participants were mostly male (n=27; 82%), mostly of minority ethno-racial backgrounds (African American: n=21; 64%; Latino: n=2; 6%), and were, on average, 34.4 years old (SD=11.3). The average lag time between HIV diagnosis and first seeking medical care was 37.9 days (SD=28.1). The sample was predominantly single (n=18; 55%), employed full-time (n=15; 46%), and 58% reported being men who have sex with men. Mean baseline CD4+ count was 335.3 cells/mm3 (SD=196.4 cells/mm3). See Table 1 for demographic summary, comparing the two intervention groups. There were no significant baseline differences on demographic or clinical variables between intervention groups.
Table 1. No ABBT vs. TAU differences in demographic or clinical characteristics at baseline (n=34).
| Descriptive statistics | Comparison between groups at baseline | ||
|---|---|---|---|
|
| |||
| TAU (n = 17) | AABT (n = 17) | t or χ2 (p = ) | |
| Age, mean (SD) | 34.6 (10.9) | 34.3 (12.0) | 0.07 (.947) |
| Male, n (%) | 13 (81.3%) | 14 (82.4%) | 0.01 (.935) |
| Non-Latino White, n (%) | 5 (31.3%) | 3 (17.7%) | 0.83 (.362) |
| Single, n (%) | 8 (50.0%) | 10 (58.8%) | 2.06 (.560) |
| Employed part- or full-time, n (%) | 8 (50.0%) | 12 (70.6%) | 9.12 (.057) |
| Days HIV+, mean (SD) | 39.6 (71.2) | 59.1 (88.7) | -0.69 (.494) |
| Method of HIV transmission | |||
| Men who have sex with men, n (%) | 13 (81.3%) | 10 (58.8%) | 0.31 (.579) |
| Heterosexual, n (%) | 3 (18.8%) | 7 (41.2%) | 1.96 (.161) |
| Intravenous drug use, n (%) | 1 (6.3%) | 0 (0.0%) | 1.10 (.295) |
| Unknown, n (%) | 1 (6.3%) | 0 (0.0%) | 1.10 (.295) |
| CD4+ (cells/mm3), mean (SD) | 351.9 (255.2) | 319.7 (124.7) | 0.47 (.645) |
| HIV Stigmatization, mean (SD) | 27.8 (5.1) | 23.8 (6.1) | 2.03 (.051) |
| Experiential avoidance of HIV, mean (SD) | 21.3 (10.6) | 24.5 (14.6) | -0.73 (.469) |
| HIV Disclosure willingness, mean (SD) | 18.1 (4.9) | 20.5 (5.6) | -1.32 (.197) |
| # of HIV disclosures, mean (SD) | 3.8 (5.8) | 6.9 (10.3) | -1.09 (.285) |
| Perceived social support, mean (SD) | 5.1 (1.6) | 5.2 (1.3) | -0.15 (.884) |
Acceptability and feasibility
In general, retention rates were high for ABBT and the study assessments (see Figure 1). Of the participants randomized to ABBT (n=17), 100% attended the first session and 88% attended both sessions. One individual who did not attend the second ABBT session withdrew from the study; the second individual did not respond to study outreach following the first session. Exit feedback was generally positive: (a) “I plan to continue to use and practice what I learned at these meetings;” mean=6.92 (SD=0.29); (b) “Discussion of barriers to attending my medical appointments was useful;” mean=6.75 (SD=0.45); and, (c) “Discussion of the difficulties caused by struggling with HIV was useful;” mean=6.67 (SD=0.65). Moreover, results from the CSQ-8-R were very high: mean=29.9 (SD=2.78).
Figure 1. Participant flow chart in RCT.
Treatment effects over 9 months
At all follow-up time-points, effects favored ABBT relative to TAU, with most effects being medium to large for: experiential avoidance of HIV distress, willingness to disclose HIV status, actual disclosures of serostatus, and perceived social support (see Table 2). With respect to our primary outcome of interest, medical care retention, only 6.7% of ABBT participants dropped out of medical care (i.e., did not attend any medical appointments) over nine months compared to 26.7% in the TAU condition.
Table 2. Effects favor ABBT at 1-, 3-, and, 9-Month Follow-Ups.
| Mean Change | |||
|---|---|---|---|
|
| |||
| Month 1 - Baseline | TAU (n = 13) | AABT (n = 15) | Cohen's d (95%CI) |
| Experiential avoidance of HIV | -1.62 (10.1) | -6.20 (15.2) | -.35 (-1.10; 0.40) |
| HIV Disclosure willingness | 1.08 (3.25) | 2.00 (6.12) | .18 (-0.56; 0.92) |
| # of HIV Disclosures | 0.04 (0.18) | 0.24 (0.28) | .84 (0.06; 1.61) |
| Perceived social support | -.30 (1.29) | 0.25 (1.27) | .43 (0.32; 1.18) |
| Month 3 - Baseline | (n = 12) | (n = 15) | |
|
|
|||
| Experiential avoidance of HIV | -0.17 (12.8) | -7.87 (13.1) | -.59 (-1.36; 0.19) |
| HIV Disclosure willingness | 1.25 (3.60) | 4.00 (6.22) | .53 (-0.25; 1.29) |
| # of HIV Disclosures | 0.10 (0.32) | 0.29 (0.33) | .58 (-0.20; 1.35) |
| Perceived social support | 0.17 (0.93) | 0.54 (1.14) | .35 (0.42; 1.11) |
| Month 9 - Baseline | (n = 13) | (n = 14) | |
|
|
|||
| Experiential avoidance of HIV | -1.85 (11.20) | -7.07 (12.3) | -.44 (-1.20; 0.33) |
| HIV Disclosure willingness | 2.38 (4.91) | 3.14 (6.65) | .13 (-0.62; 0.88) |
| # of HIV disclosures | 0.18 (0.42) | 0.30 (0.33) | .32 (-0.44; 0.48) |
| Perceived social support | 0.00 (1.50) | 0.40 (1.22) | .29 (0.47; 1.05) |
Discussion
The primary aim of this pilot trial was to assess the feasibility and acceptability of ABBT for new-to-care HIV patients. The brief intervention is specifically designed to be implemented in busy clinical settings. Results supported the acceptability and feasibility of this intervention, given the successful recruitment rate and good intervention attendance rate at two very different clinical sites. In addition, persons assigned to ABBT were more likely to be longitudinally retained in care. Although not powered for efficacy, this is among the first interventions to demonstrate successful improvement in retention in care among new-to-care HIV patients.
The content of the ABBT intervention was rated positively by participants who reported that skills taught in ABBT were useful and worth continuing to implement in their daily lives. This is particularly notable given ABBT's emphasis on disclosure as a natural extension of HIV acceptance. HIV providers might be hesitant to encourage disclosure due to fear that it might lead to physical and/or emotional harm for patients. However, Mansergh and colleagues (59) demonstrated that experiences of disclosure among PLWH are generally more positive than expected and that family, friends and partners are generally supportive in response to disclosure. Our study also suggests that the experience of disclosing to significant others can be a positively reinforcing experience in which PLWH realize that acceptance of their HIV status is possible and might not be as stressful as expected. However, we do note that it is important that patients should be careful in choosing to whom they disclose.
Between group differences in clinical and mechanistic outcomes were directionally consistent with the hypothesized effects of ABBT, and were relatively robust given the small sample size. ABBT, compared to TAU, reduced experiential avoidance of HIV, increased willingness to disclose HIV status, increased the number of disclosures completed, and led to improved perceived social support. Notably, results showed that ABBT had its largest effects in the first three months post-intervention. We interpret these results as participants developing a significant and persistent acceptance of their serostatus following the two early intervention sessions. We also suggest that ABBT “jump started” the disclosure process and by three months, individuals who received the intervention had disclosed to many, if not all, of the appropriate individuals in their social network and therefore the 9-month outcomes in these domains were somewhat attenuated.
This study had several limitations. First, we had a modest sample size given this was a pilot study. Therefore, despite the notable effect sizes, Cohen's d values should be interpreted with caution and only within the relevant confidence intervals. Indeed, only 60% of patients approached agreed to participate in this study. There may have been a sampling bias in that patients who were too busy or distressed declined to participate in this study. Second, we did not match for attention in the control condition, relative to ABBT, as TAU consisted of standard clinic practices, which likely varied widely depending on patients' needs. However, all ABBT participants also received TAU services at these sites. Third, the intervention was delivered by doctoral-level clinicians. It is possible that lower level clinicians, such as social workers or case managers, might have difficulty administering ABBT. However, we note that this intervention is manualized and data show that acceptance-based interventions can be effectively administered by clinicians with a wide range of skills, including graduate students (60). Finally, we do not know if the improved retention had clinical implications such as greater rates of ART initiation or adherence, or the delay of HIV symptoms.
Conclusions
A brief, scalable ABBT intervention may significantly improve rates of retention in care, a critical step in the HIV care continuum. Suboptimal retention in care is associated with significant patient morbidity and mortality, as well as potential HIV transmission. This pilot intervention addressed barriers to retention in care by encouraging disclosure of status as a pathway to acceptance of one's diagnosis to facilitate retention. Our preliminary findings lay the groundwork for future testing of ABBT which, if proven efficacious, will be scalable to a variety of HIV care settings.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R34 MH098694 to Drs. Moitra and Stein. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Services HaH., editor. DHHS. Guidelines for the use of antiretroviral agents in HIVinfected adults and adolescents. 2016. [Google Scholar]
- 2.Rumptz MH, Tobias C, Rajabiun S, Bradford J, Cabral H, Young R, et al. Factors associated with engaging socially marginalized HIV-positive persons in primary care. AIDS Patient Care STDS. 2007;21(1):S30–9. doi: 10.1089/apc.2007.9989. [DOI] [PubMed] [Google Scholar]
- 3.Marks G, Gardner LI, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. Aids. 2010;24(17):2665–78. doi: 10.1097/QAD.0b013e32833f4b1b. [DOI] [PubMed] [Google Scholar]
- 4.Campsmith ML, Rhodes P, Hall HI, Green T. HIV Prevalence Estimates-United States, 2006 (Reprinted from MMWR, vol 57, pg 1073-1076, 2008) Jama-J Am Med Assoc. 2009;301(1):27–9. [Google Scholar]
- 5.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The Spectrum of Engagement in HIV Care and its Relevance to Test-and-Treat Strategies for Prevention of HIV Infection. Clinical Infectious Diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in Care of Adults and Adolescents Living With HIV in 13 US Areas. J Acquir Immune Defic Syndr. 2012;60(1):77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 7.Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA, Network HR. Establishment, Retention, and Loss to Follow-Up in Outpatient HIV Care. Jaids-J Acq Imm Def. 2012;60(3):249–59. doi: 10.1097/QAI.0b013e318258c696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, et al. Human Immunodeficiency Virus Transmission at Each Step of the Care Continuum in the United States. Jama Intern Med. 2015;175(4):588–96. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 9.Ulett KB, Willig JH, Lin HY, Routman JS, Abroms S, Allison J, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23(1):41–9. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parienti JJ, Massari V, Descamps D, Vabret A, Bouvet E, Larouze B, et al. Predictors of virologic failure and resistance in HIV-Infected patients treated with nevirapineor efavirenz-based antiretroviral therapy. Clinical Infectious Diseases. 2004;38(9):1311–6. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 11.Metsch LR, Pereyra M, Messinger S, del Rio C, Strathdee SA, Anderson-Mahoney P, et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clinical Infectious Diseases. 2008;47(4):577–84. doi: 10.1086/590153. [DOI] [PubMed] [Google Scholar]
- 12.Hull MW, Wu ZY, Montaner JSG. Optimizing the engagement of care cascade: a critical step to maximize the impact of HIV treatment as prevention. Curr Opin Hiv Aids. 2012;7(6):579–86. doi: 10.1097/COH.0b013e3283590617. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg BE, Davidson AJ, Burman WJ. Epidemiology of Pneumocystis carinii pneumonia in an era of effective prophylaxis: the relative contribution of non-adherence and drug failure. Aids. 2000;14(16):2559–66. doi: 10.1097/00002030-200011100-00019. [DOI] [PubMed] [Google Scholar]
- 14.Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed Visits and Mortality among Patients Establishing Initial Outpatient HIV Treatment. Clinical Infectious Diseases. 2009;48(2):248–56. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to Improve Retention in HIV Primary Care: A Systematic Review of US Studies. Current HIV/AIDS reports. 2012;9(4):313–25. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liau A, Crepaz N, Lyles CM, Higa DH, Mullins MM, DeLuca J, et al. Interventions to Promote Linkage to and Utilization of HIV Medical Care Among HIV-diagnosed Persons: A Qualitative Systematic Review, 1996-2011. Aids and Behavior. 2013;17(6):1941–62. doi: 10.1007/s10461-013-0435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higa DH, Crepaz N, Mullins MM, Project PRS. Identifying Best Practices for Increasing Linkage to, Retention, and Re-engagement in HIV Medical Care: Findings from a Systematic Review, 1996-2014. Aids and Behavior. 2016;20(5):951–66. doi: 10.1007/s10461-015-1204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen M, Hockman E, Smereck G, Tinsley J, Milfort D, Wilcox R, et al. Retaining women in HIV medical care. J Assoc Nurse Aids C. 2007;18(3):33–41. doi: 10.1016/j.jana.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Naar-King S, Bradford J, Coleman S, Green-Jones M, Cabral H, Tobias C. Retention in care of persons newly diagnosed with HIV: Outcomes of the outreach initiative. Aids Patient Care St. 2007;21:S40–S8. doi: 10.1089/apc.2007.9988. [DOI] [PubMed] [Google Scholar]
- 20.Wohl AR, Garland WH, Wu JH, Au CW, Boger A, Dierst-Davies R, et al. A youth-focused case management intervention to engage and retain young gay men of color in HIV care. Aids Care-Psychological and Socio-Medical Aspects of Aids/Hiv. 2011;23(8):988–97. doi: 10.1080/09540121.2010.542125. [DOI] [PubMed] [Google Scholar]
- 21.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment - Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 22.Knowlton AR, Hua W, Latkin C. Social support networks and medical service use among HIV-positive injection drug users: Implications to intervention. Aids Care-Psychological and Socio-Medical Aspects of Aids/Hiv. 2005;17(4):479–92. doi: 10.1080/0954012051233131314349. [DOI] [PubMed] [Google Scholar]
- 23.Stein MD, Freedberg KA, Sullivan LM, Savetsky J, Levenson SM, Hingson R, et al. Sexual ethics - Disclosure of HIV-positive status to partners. Arch Intern Med. 1998;158(3):253–7. doi: 10.1001/archinte.158.3.253. [DOI] [PubMed] [Google Scholar]
- 24.Klitzman R. Self-Disclosure of HIV Status to Sexual Partners: A Qualitative Study of Issues Faced by Gay Men. Journal of the Gay and Lesbian Medical Association. 1999;3(2):39–49. [Google Scholar]
- 25.Maman S, Medley A, Garcia-Moreno C, McGill S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. B World Health Organ. 2004;82(4):299–307. [PMC free article] [PubMed] [Google Scholar]
- 26.Kalichman SC, Nachimson D. Self-efficacy and disclosure of HIV-positive serostatus to sex partners. Health Psychol. 1999;18(3):281–7. doi: 10.1037//0278-6133.18.3.281. [DOI] [PubMed] [Google Scholar]
- 27.Wohl AR, Galvan FH, Myers HF, Garland W, George S, Witt M, et al. Do Social Support, Stress, Disclosure and Stigma Influence Retention in HIV Care for Latino and African American Men Who Have Sex with Men and Women? AIDS and Behavior. 2011;15(6):1098–110. doi: 10.1007/s10461-010-9833-6. [DOI] [PubMed] [Google Scholar]
- 28.Williams B, Amico KR, Konkle-Parker D. Qualitative Assessment of Barriers and Facilitators to HIV Treatment. Journal of the Association of Nurses in AIDS care. 2011;22(4):307–12. doi: 10.1016/j.jana.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathews C, Kuhn L, Fransman D, Hussey G, Dikweni L. Disclosure of HIV status and its consequences. S Afr Med J. 1999;89(12):1238. [PubMed] [Google Scholar]
- 30.Obermeyer CM, Baijal P, Pegurri E. Facilitating HIV Disclosure Across Diverse Settings: A Review. Am J Public Health. 2011;101(6):1011–23. doi: 10.2105/AJPH.2010.300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellins CA, Havens JF, McCaskill EO, Leu CS, Brudney K, Chesney MA. Mental health, substance use and disclosure are significantly associated with the medical treatment adherence of HIV-infected mothers. Psychology, Health & Medicine. 2002;7(4):451–60. [Google Scholar]
- 32.Halperin J, Pathmanathan I, Richey LE. Disclosure of HIV Status to Social Networks Is Strongly Associated with Increased Retention Among an Urban Cohort in New Orleans. Aids Patient Care St. 2013;27(7):375–7. doi: 10.1089/apc.2013.0037. [DOI] [PubMed] [Google Scholar]
- 33.Marks G, Richardson JL, Crepaz N, Stoyanoff S, Milam J, Kemper C, et al. Are HIV care providers talking with patients about safer sex and disclosure?: A multi-clinic assessment. Aids. 2002;16(14):1953–7. doi: 10.1097/00002030-200209270-00013. [DOI] [PubMed] [Google Scholar]
- 34.Madru N. Stigma and HIV: Does the Social Response Affect the Natural Course of theEpidemic. Journal of the Association of Nurses in AIDS care. 2003;14:39–48. doi: 10.1177/1055329003255112. [DOI] [PubMed] [Google Scholar]
- 35.Schuster MA, Collins R, Cunningham WE, Morton SC, Zierler S, Wong M, et al. Perceived discrimination in clinical care in a nationally representative sample of HIV-infected adults receiving health care. J Gen Intern Med. 2005;20(9):807–13. doi: 10.1111/j.1525-1497.2005.05049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes SC, Wilson KG, Strosahl K. Acceptance and commitment therapy: an experiential approach to behavior change. New York: Guilford Press; 1999. [Google Scholar]
- 37.Wegner DM, Zanakos S. Chronic Thought Suppression. J Pers. 1994;62(4):615–40. doi: 10.1111/j.1467-6494.1994.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 38.Clark DM, Ball S, Pape D. An Experimental Investigation of Thought Suppression. Behav Res Ther. 1991;29(3):253–7. doi: 10.1016/0005-7967(91)90115-j. [DOI] [PubMed] [Google Scholar]
- 39.Clark DM, Winton E, Thynn L. A Further Experimental Investigation of Thought Suppression. Behav Res Ther. 1993;31(2):207–10. doi: 10.1016/0005-7967(93)90074-5. [DOI] [PubMed] [Google Scholar]
- 40.Zeitlin SB, Netten KA, Hodder SL. Thought suppression: an experimental investigation of spider phobics. Behav Res Ther. 1995;33(4):407–13. doi: 10.1016/0005-7967(94)00054-n. [DOI] [PubMed] [Google Scholar]
- 41.Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2nd. New York: Guilford; 2012. [Google Scholar]
- 42.A-Tjak JGL, Davis ML, Morina N, Powers MB, Smits JAJ, Emmelkamp PMG. A Meta-Analysis of the Efficacy of Acceptance and Commitment Therapy for Clinically Relevant Mental and Physical Health Problems. Psychotherapy and Psychosomatics. 2015;84(1):30–6. doi: 10.1159/000365764. [DOI] [PubMed] [Google Scholar]
- 43.Hayes SC, Levin ME, Plumb-Vilardaga J, Villatte JL, Pistorello J. Acceptance and Commitment Therapy and Contextual Behavioral Science: Examining the Progress of a Distinctive Model of Behavioral and Cognitive Therapy. Behav Ther. 2013;44(2):180–98. doi: 10.1016/j.beth.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gundy JM, Woidneck MR, Pratt KM, Christian AW, Twohig MP. Accetpance and Commitment Therapy: State of Evidence in the Field of Health Psychology. The Scientific Review of Mental Health Practice. 2011;8(2):23–35. [Google Scholar]
- 45.Gregg JA, Callaghan GA, Hayes SC, Glenn-Lawson JL. Improving diabetes self-management through acceptance, mindfulness, and values: A randomized controlled trial. J Consult Clin Psych. 2007;75(2):336–43. doi: 10.1037/0022-006X.75.2.336. [DOI] [PubMed] [Google Scholar]
- 46.Skinta MD, Lezama M, Wells G, Dilley JW. Acceptance and Compassion-Based Group Therapy to Reduce HIV Stigma. Cogn Behav Pract. 2015;22(4):481–90. [Google Scholar]
- 47.Moitra E, Chan PA, Stein MD. Open Trial of an Acceptance-based Behavior Therapy Intervention to Engage Newly Diagnosed HIV Patients in Care: Rationale and Evidence of Feasibility and Acceptability. Behav Modif. 2015;39:670–90. doi: 10.1177/0145445515590977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, et al. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. Aids. 2005;19(13):1367–74. [PubMed] [Google Scholar]
- 49.Babor T, Grant M. From clinical research to secondary prevention: International collaboration in the development of the Alcohol Use Disorders Identification Test (AUDIT) Alcohol Health & Research World. 1989;13:371–4. [Google Scholar]
- 50.Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann. 1979;2(3):197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- 51.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. J Pers Assess. 1988;52(1):30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 52.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric Characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess. 1990;55(3-4):610–7. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 53.Kalichman SC, Rompa D, DiFonzo K, Simpson D, Kyomugisha F, Austin J, et al. Initial Development of Scales to Assess Self-Efficacy for Disclosing HIV Status and Negotiating Safer Sex in HIV-Positive Persons. AIDS and Behavior. 2001;5:291–6. [Google Scholar]
- 54.Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, et al. Preliminary Psychometric Properties of the Acceptance and Action Questionnaire-II: A Revised Measure of Psychological Inflexibility and Experiential Avoidance. Behav Ther. 2011;42(4):676–88. doi: 10.1016/j.beth.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Bond FW, Bunce D. The role of acceptance and job control in mental health, job satisfaction, and work performance. J Appl Psychol. 2003;88(6):1057–67. doi: 10.1037/0021-9010.88.6.1057. [DOI] [PubMed] [Google Scholar]
- 56.Wright K, Naar-King S, Lam P, Templin T, Frey M. Stigma scale revised: Reliability and validity of a brief measure of stigma for HIV plus youth. J Adolescent Health. 2007;40(1):96–8. doi: 10.1016/j.jadohealth.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: Psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24(6):518–29. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- 58.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 59.Mansergh G, Marks G, Simoni JM. Self-Disclosure of Hiv-Infection among Men Who Vary in Time since Seropositive Diagnosis and Symptomatic Status. Aids. 1995;9(6):639–44. doi: 10.1097/00002030-199506000-00017. [DOI] [PubMed] [Google Scholar]
- 60.Lappalainen R, Lehtonen T, Skarp E, Taubert E, Ojanen M, Hayes SC. The impact of CBT and ACT models using psychology trainee therapists - A preliminary controlled effectiveness trial. Behav Modif. 2007;31(4):488–511. doi: 10.1177/0145445506298436. [DOI] [PubMed] [Google Scholar]


