Abstract
The ability to rapidly switch behaviors in dynamic environments is fundamental to survival across species. Recognizing when an ongoing behavioral strategy should be replaced by an alternative one requires the integration of a diverse number of cues both internal and external to the organism including hunger, stress, or the presence of reward predictive cues. Increasingly sophisticated behavioral paradigms coupled with state of the art electrophysiological and pharmacological approaches have delineated a brain circuit involved in behavioral flexibility. However, how diverse contextual cues are integrated to influence strategy selection on a trial by trial basis remains largely unknown. One promising candidate for integration of internal and external cues to determine whether an ongoing behavioral strategy is appropriate is the lateral habenula (LHb). The LHb receives input from many brain areas that signal both internal and external environmental contexts and in turn projects to areas involved in behavioral monitoring and plasticity. This review examines how these connections, combined with recent pharmacological and electrophysiological results reveal a critical role for the LHb in behavioral flexibility in dynamic environments. This proposed role extends the known contributions of the LHb to motivated behaviors and suggests that the fundamental role of the LHb in these behaviors goes beyond signaling rewards and punishments to dopaminergic systems.
1. Introduction
Learning to choose and reliably execute appropriate behaviors in complex environments that optimize goals such as obtaining rewards or avoiding punishments is a fundamental process across many species. Action selection relies on a number of different behavioral processes. Specifically, animals must learn about behaviors or cues that are likely to lead to rewards or avoid punishments. They must then reliably recall these behaviors and cue associations when encountered in their environment. Changes in the context of the environment can occur unexpectedly, making behavioral selection a rapid, continuous, and dynamic process. The ability to switch behaviors when contexts change, or to switch from an ongoing behavior to a new one, is commonly referred to as behavioral flexibility. For the purposes of this review the word behavior(s) is used to denote a general course of actions an animal makes in order to achieve a goal. It does not mean a specific action or series of actions that are temporally ordered aimed to accomplish the overall behavior.
Behavioral flexibility has been assessed using many tasks in both animal research and in clinical settings. For example, in the go/no-go task the subject must inhibit the normal behavioral response when a cue is presented on a minority of trials. In a reversal learning task, the subject must cease to perform a rule, e.g. always choose the left, do the opposite (go right) when it is no longer rewarded. There are many more instances in which varied stimuli, behavioral requirements, and outcome conditions require subjects to utilize behavioral flexibility. Common to these conditions, animals must be able determine whether the ongoing behavior/strategy is appropriate based on the current emotional state or internal signals (e.g. hunger, thirst, stress, and threat), and whether the current behavior should be replaced by an alternative one. This requires that the subject is able to compare the most recent context-specific response with the expected outcome. Proper behavioral selection, therefore, must integrate these diverse motivational systems with movement systems in the midbrain and striatum. One mechanism that has been proposed to serve this function is the dorsal diencephalic conduction system (Figure 1) and in particular the habenular complex (Sutherland 1982).
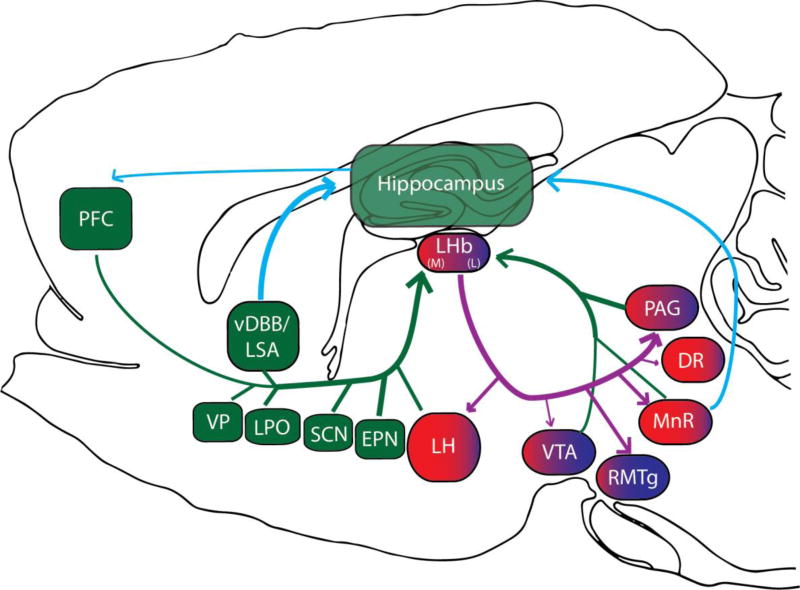
Figure 1.
The dorsal diencephalic conduction system. Projections to the lateral habenula (LHb), shown in green, include the medial prefrontal cortex (mPFC), vertical limb of the diagonal band of Broca (vDBB), lateral septal area (LSA), ventral pallidum (VP), lateral preoptic area (LPO), entopeduncular nucleus (EPN), suprachiasmatic nucleus (SCN), ventral tegmental area (VTA), median raphe nuclei (MnR), periaqueductal gray (PAG), and lateral hypothalamus (LH). It is not well known whether these afferent connections are topographically organized. LHb efferent projections contain topographically organized, although somewhat overlapping, projections to midbrain areas. The medial portion of the LHb (in red) projects to the LH, MnR, and dorsal raphe (DR). The lateral portion of the LHb (in blue) projects mainly to the rostromedial tegmental nucleus (RMTg) and to a lesser extent the VTA. The periaqueductal gray (PAG) receives a prominent projection from the whole of the LHb. Functional evidence supports a connection between the hippocampus and the LHb. A proposed circuit connecting these structures is shown in blue. The relative strength of projections is represented by the line thickness.
Recently the lateral habenula (LHb) has been implicated in translating reward and aversive signals from forebrain areas to the dopamine and other monoamine systems particularly within emotional contexts (Bromberg-Martin and Hikosaka 2011; Proulx et al. 2014; Zhao et al. 2015). Both outcomes and the cues that predict them are signaled in the LHb and seem to represent valence on a trial by trial basis (Kawai et al. 2015). Other studies have suggested that the LHb monitors specific behaviors in complex environments to guide choices (Stopper and Floresco 2014; Baker et al. 2015). Evidence from lesion and pharmacological manipulations suggest that the LHb is required for switching behaviors when environmental cues or reward feedback indicate that behavior should be changed, particularly in dynamic environments (Thornton and Evans 1984; Lecourtier et al. 2004; Baker et al. 2016b). This suggests that the LHb integrates a broad range of internal and external cues to monitor behaviors and assign valence to them. This review will focus on the breadth of salient information that the LHb is sensitive to and how these data support a role for the LHb in monitoring ongoing behaviors to determine whether they should be replaced with alternative ones across a wide range of behavioral flexibility tasks.
2. Interactions between learning, memory and behavioral flexibility functions in the LHb
Fundamental to behavioral flexibility is the ability to formulate and reliably execute appropriate learned responses to salient cues in the environment. The LHb has long been considered critical for normal learning and memory as evidenced by the deficits observed in habenula (both the medial and lateral portion) lesioned animals performing hippocampal-dependent water maze tasks in which the animal must find a hidden platform to escape (Thornton and Davies 1991; Lecourtier et al. 2004). A close examination of these results suggests that habenula lesions resulted in an inability to modify ongoing behaviors relative to an initial strategy. For example, if rats were first trained to escape to either side of a visually cued rectangular tank and then the escape platform was restricted to the non-preferred side, habenula lesioned animals committed more errors than sham lesioned animals (Thornton and Davies 1991).
More recent studies using pharmacological manipulation and biochemical techniques further elaborate the interaction between the LHb and learning and memory processes. For example,Flagel et al. (2011) found increased expression of the immediate early gene c-fos, a marker of neural activity, in rats that learned to attribute salience to the presentation of a conditioned stimulus but not in rats which associated the goal location with salience. Thus, the LHb is important for signaling learned salient cues animals have associated with behavioral goals. Similarly, rats that learned to discriminate odors to receive reinforcement showed increased c-fos expression in the LHb but not in the medial habenula (MHb) following a retrieval test (Tronel and Sara 2002). The same increase was not observed following the training session, although there was a trend, suggesting that the LHb is especially involved when learned cue discriminations must be recalled and utilized in working memory.
Pharmacological manipulation of the LHb has revealed a role for the LHb in encoding and retrieval processes. Using a water maze task, it was found that inactivation of the LHb with the GABA-A receptor agonist muscimol or injection of the glutamatergic AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) impaired performance when injected before training on the escape platform location but not when injected soon after training sessions, i.e. during the consolidation period (Mathis et al. 2015). In contrast, for well-trained rats, injection of either drug disrupted retrieval of the platform location. In both cases of deficit, animals increased the amount of time engaged in thigmotaxis, or attempting to escape by climbing the walls of the arena, prior to switching strategies and searching for the hidden platform. The fact that rats preferentially engaged in the default thigmotaxis escape strategy complicates a clear interpretation of the LHb role in this task. For example, LHb inactivation may have impaired working memory, or alternatively, LHb inactivation could have prevented switching from an ongoing to alternative strategy. The latter explanation is supported by other findings that indicate rats are able to perform simple discriminations such as a spatial/egocentric discrimination or a reward magnitude discrimination following LHb inactivation (Stopper and Floresco 2014; Baker et al. 2015). However, recent findings do support a role for the LHb in performing mPFC dependent working memory functions as LHb inactivation with the GABA-A receptor agonist muscimol or disconnection of the medial prefrontal cortex (mPFC) and LHb were shown to impair a learned non-match to sample spatial working memory task (Mathis et al. 2016). Further work should examine whether the LHb also contributes to similar working memory tasks in naïve animals that have not learned the task prior to inactivation to determine the interaction between working memory and performance of a learned strategy to obtain rewards.
In vivo recordings from the LHb in freely behaving rodents lend additional insight into the nature of its contributions to learning and memory functions. When rats were allowed to randomly forage for food rewards distributed within an open arena, a relatively small proportion (10%) of neurons in the LHb showed correlated firing with movement velocity (Sharp et al. 2006). In a separate experiment in which animals were trained over many consecutive days to navigate down maze arms within a large, spatially extended environment to collect rewards at specific locations, a much higher proportion (66%) of LHb neurons were correlated with velocity (Baker et al. 2015). Taken together these data support the findings discussed above that suggest the LHb is preferentially involved in encoding learned goal-directed behaviors. Specifically, it suggests that the LHb becomes increasingly involved in working memory tasks as specific components or actions within the task are learned to more effectively obtain the goals.
Based on the findings summarized above, one possibility is that the LHb interacts with memory in order to determine whether the ongoing behavior or strategy results in the desired outcome. In order for this to be the case, the LHb would likely need to be functionally linked with the hippocampus. The hippocampus plays a central role in context-dependent memories (Hirsh 1974; Mizumori et al. 1999) which could be utilized to evaluate the extent to which context features predict current behavioral goals.
While no direct connection between the LHb and hippocampus is known to exist, they share a common afferent connection, the diagonal band of Broca, and the LHb indirectly projects to the hippocampus via the raphe nuclei (Lecourtier and Kelly 2007; Quina et al. 2015). Additionally, the mPFC functionally connects the LHb and hippocampus via a hippocampus-mPFC-LHb projection that also likely contributes to memory related functions (Kim and Lee 2012; Mathis et al. 2016). Functional connection between brain areas has been shown to be governed by network oscillatory activity even between areas without a direct connection. (Adachi et al. 2012; Harris and Gordon 2015). Lesions of the LHb alter hippocampal theta rhythms, an effect which is dependent on an intact median raphe (Aizawa et al. 2013). When animals perform an object recognition task, theta frequency synchrony between the LHb and hippocampus correlates with behavioral performance (Goutagny et al. 2013). While evidence clearly links the hippocampus and LHb in behaviors dependent on both structures, the mechanism and target of this interaction is poorly understood. Additional research should target the interaction between these structures to determine whether their interaction is necessary for behaviors including behavioral flexibility.
3. LHb sensitivity to context and salient cues
Despite a clear role for the LHb in learning and memory processes as discussed above, the neural mechanism controlling its contribution remains largely unknown. In awake, anesthetized, and sleeping rats LHb neurons are phase locked to hippocampal theta (Aizawa et al. 2013; Goutagny et al. 2013). This phase locking raises the possibility that context specific information is also signaled in the LHb. Context can refer to a wide range of environmental traits both internal (including hunger, level of threat, timing, etc.) and external (spatial orientation, auditory cues, lighting, etc.) to the subject . Many brain areas that are sensitive to context project to the LHb including the lateral hypothalamus (hunger/thirst), suprachiasmatic nucleus (circadian rhythm), the prefrontal cortex (current strategy), and other areas. This suggests that the LHb is privy to a wide range of environmental and internal contexts that could be integrated to then signal to the monoamine nuclei of the midbrain to ultimately influence appropriate behavioral selection.
There is a range of evidence that supports LHb sensitivity to many different contexts. In pre-pulse inhibition (PPI) testing an animal’s reaction to a loud sound is directly preceded by a quieter sound that decreases the startle response to the loud sound. Animals under stress normally show a potentiation of sensitivity in PPI. LHb lesioned rats under stress conditions behave similarly to unstressed controls in that they show lower PPI than stressed control rats (Heldt and Ressler 2006). This indicates an insensitivity to stress potentiated PPI. LHb sensitivity to stressors appears to be widespread as indicated by a similar increase in c-fos expression in response to open field exposure, lithium chloride induced illness, and restraint (Wirtshafter et al. 1994). Chronic exposure to stress leads to a degradation of the LHb, further supporting its role in signaling stress (Jacinto et al. 2016).In addition to ethological stressors, the LHb has also been strongly linked with cocaine and ethanol withdrawal stress responses (Neumann et al. 2014; Meye et al. 2016; Kang et al. 2017). Alterations in inhibitory tone in the LHb selectively during drug withdrawal drive negative symptoms and may contribute to continued drug seeking (Meye et al. 2016; Kang et al. 2017). How these pathological states alter normal behavioral flexibility processes within the LHb remains unclear.
Circadian rhythms and light exposure have also been shown to affect LHb neural firing in a context dependent manner. During the light phase of a 12h light-dark cycle, LHb neurons have a higher average firing rate than in the dark phase, an effect that is maintained in vitro for two light-dark cycles (Zhao and Rusak 2005). Further, about half of recorded LHb neurons responded with short latency to retinal photo stimulation particularly during the dark phase when light exposure would be less expected. Additional support for a role for the LHb in circadian rhythm signaling comes from findings that lesions of the LHb caused a reduction in sleep rebound time following six hours of sleep deprivation (Zhang et al. 2016a). The authors argue that these findings support a role for the LHb in sleep homeostasis. This in turn suggests the LHb contributes to signaling context in the circadian rhythm such as whether light exposure is expected or unexpected or whether the animal is in the light or dark portion of the day.
Owing to the fact that the LHb receives input from brain areas that signal a diverse range of internal and external contexts, it is not surprising that there is evidence supporting a role for the LHb in the integration of these contexts to shape behaviors. Indeed, the interaction between stress and thirst reveals integration of stimuli to influence behavior. Animals that were water deprived 24 hours prior to exposure to a cat showed decreased c-fos expression in the LHb, and this was likely due to a vasopressin positive projection from the paraventricular hypothalamus onto GABAergic neurons in its medial portion (Zhang et al. 2016b). The decreased c-fos expression was accompanied by decreased freezing suggesting thirst may be sufficient to drive risky behavior even in potentially life-threatening situations. Interactions between effort and aversive shock avoidance have also been observed in LHb lesioned animals. When either the intensity of the shock was increased or the effort required to escape the shock was increased, LHb lesioned rats were impaired in jumping from a shock grid to the escape platform (Thornton and Bradbury 1989).
These examples of context related processing in the LHb provide convincing evidence to not only support its role in signaling contexts such as circadian cycle or thirst, but also in integrating them to guide behavioral selection in a wide range of tasks. Tasks traditionally used to assess behavioral flexibility have relied in large part on rather discrete proactively signaled (visual, auditory, tactile) or retroactively signaled (reward or punishment feedback) cues that inform the subject of an impending change in task conditions. The following section will outline what has been learned from careful examination of learning and error patterns in these forms of behavioral flexibility tasks.
4. LHb contributions to behavioral flexibility
Significant evidence supports the hypothesis that the LHb is particularly important for signaling emotional (and in particular negative) stimuli to guide behaviors (Okamoto et al. 2012; Proulx et al. 2014). For example, when rats were required to reverse arm choices in a t-shaped maze in order to press a lever to stop shock, habenula lesions resulted in continual choices for the previously correct arm (Nielson and McIver 1966). In studies in which water mazes were utilized, changing the location of the hidden escape platform also led to impairments in escape (Thornton and Davies 1991; Lecourtier et al. 2004). However, neurons in the LHb show changes in neural firing patterns to both aversive and rewarding stimuli indicating a more broad role in switching behaviors without associated negative or strong emotional valence (Matsumoto and Hikosaka 2009). Further, responses of a subset of LHb neurons also track outcome informative cues which could relate to behavioral planning based on those cues (Bromberg-Martin and Hikosaka 2011). Thus, the LHb may be more broadly tuned to regulate behaviors when salient cues, emotional or otherwise, can guide decisions.
Subjective decisions, such as those made between small immediate rewards and larger but delayed rewards in delay discounting tasks, reveal contributions to choice behavior when either choice may be considered “correct”. This allows for the separation of choice from objective errors or aversive (no reward) experiences. When the LHb is inactivated, delay discounting performance drops to chance levels even at short delays (Stopper and Floresco 2014). This surprising result indicates that the LHb contributes to choice behavior even when there is no objectively correct choice. In risk based decisions in which the larger reward is instead given at increasingly less frequent probabilities across the session, LHb inactivation also led to similar chance performance (Stopper and Floresco 2014). Interestingly, if the small and large rewards are directly compared with no time delay or probability of receiving the reward, rats were able to discriminate similarly to controls. These findings demonstrate that the role of the LHb in subjective decisions is not primarily to signal reward (or aversive) outcomes to guide future decisions. Rather, these data suggest that the LHb is required when the animal must track a learned strategy or choice pattern as it changes across the session based on the current delay or probability of the large reward. This is another example of evidence that supports a broad and more fundamental role for the LHb in tasks that require flexible responses.
For behaviors in which there is an objectively correct choice, the requirement for the LHb to guide correct choices appears strikingly similar to its role in subjective decisions. Using a probabilistic reversal learning task in which the reward contingencies of arms on a figure eight maze were reversed once rats began to reliably choose the correct arm, LHb inactivation led to poor performance compared to controls (Baker et al. 2015). One means of determining the specific behavioral contribution of a brain area is to analyze in detail the pattern of errors that the animal commits (Dalley et al. 2004; Ragozzino 2007). During reversal learning, LHb inactivated rats showed increased errors once the prior choice pattern had been abandoned (regressive errors) indicating an inability to maintain the currently relevant choice (Baker et al. 2015). Thus, LHb may play a critical role in maintaining newly adopted choices. In addition, both a decrease to chance performance in the probability of choosing the correct choice when it was rewarded on the prior trial (win-stay) and an increase to chance performance in the probability of choosing the incorrect choice if a prior correct choice was not rewarded (lose-shift) was observed. The win-stay and lose-shift results in particular suggest that the LHb is critical for monitoring the current behavior on a trial by trial basis to inform future decisions. In monkeys, neurons recorded from the LHb in a saccade based version of probabilistic reversal learning showed short latency responses to outcomes that were not modulated by the number of consecutive reward experiences (either positive or negative) that the monkey had experienced (Kawai et al. 2015). These neural results support the hypothesis that the LHb is sensitive to trial by trial changes in outcome expectations, an idea that is consistent with those found from both reversal learning and subjective decision making inactivation studies outlined above.
The LHb has also been shown to be critical for behavioral flexibility when cues reliably signal the need to switch behavior or to avoid negative outcomes. In such a proactive switching task, rats learned to rely on auditory cues to guide arm choices on a figure eight maze. LHb inactivation led to chance performance with an increase in the likelihood that rats chose a single arm choice for the entire session (Baker et al. 2016b). Support for LHb involvement in cue guided behavioral flexibility comes from electrophysiological recordings in monkeys in which a subset of LHb neurons encoded outcome predictive cues (Bromberg-Martin and Hikosaka 2011). Without this cue information signaled in the LHb animals choose a random strategy such as preferentially entering a single arm for the entire session despite only receiving reward half of the time on average.
The findings from subjective decision making, reversal learning, and proactive switching tasks all show a major contribution of the LHb to behavioral selection when choices must be dynamic and responsive to changes in outcomes or cues that predict optimal choices. The full extent of LHb contributions to behavioral flexibility remains to be discovered as additional tasks are employed including go/no-go tasks and set-shifting tasks. Based on the available evidence, however, it is likely that the LHb is broadly tuned to guide decisions on a trial by trial basis in any task in which switching behaviors is a requirement. This raises the question of where the LHb sits in the overall neural circuity governing behavioral flexibility.
5. The LHb within a broader neural circuitry underlying behavioral flexibility
Studies of the neural circuitry of behavioral flexibility have long focused on dissociations between brain areas based on task attributes or switching requirements (Goldman-Rakic 1996; Wise et al. 1996; Ragozzino and Baker 2016). From this work, it is generally accepted that the mPFC is required for switching rules (e.g. choose based on an odor and ignore spatial location or choose based on spatial location and ignore odor) while the orbitofrontal cortex is required for reversing choices within a rule (Birrell and Brown 2000; McAlonan and Brown 2003). Neurotransmitters including dopamine, serotonin, and noradrenaline have similarly been identified to differentially contribute to behavioral flexibility in various brain regions (Kehagia et al. 2010; Izquierdo et al. 2016). For example, serotonergic lesions using 5,7-dihydroxytryptamine in the prefrontal cortex of monkeys impairs reversal learning but does not affect set-shifting (Clarke et al. 2005). Alternatively, norepinephrine transporter blockade in the PFC facilitates set-shifting but has no effect on reversal learning in adolescent rats (Cain et al. 2011). It has also been suggested that specific brain areas may be specialized to broadly integrate different domains of information to guide behavior. For example, Wassum and Izquierdo (2015) have suggested that the basolateral amygdala is critical for attending to reward value, cost, and history of reward. These reward aspects are integrated to dynamically transmit action values to its output structures which in turn influence choices. We propose a similar broad influence of the LHb in behavioral flexibility. However, rather than signaling specific values of choices, the evidence summarized in prior sections points to the LHb as integrating diverse sensory and internal state information to determine whether the current response pattern or strategy is appropriate, and if not, engaging circuitry to change response patterns. This adaptive process is particularly important in dynamic or cognitively demanding circumstances. Below we suggest a mechanism by which the LHb might regulate choices under these conditions (Figure 2).
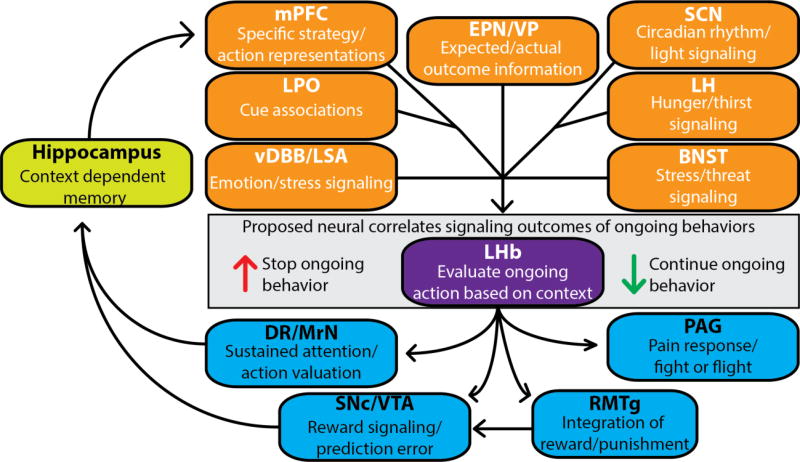
Figure 2.
Summary of the proposed input and output circuitry of the lateral habenula (LHb). The LHb receives a diverse range of context related information. This information is integrated to evaluate whether the ongoing behavior should be continued or replaced by an alternative one. Arrows adjacent to the LHb represent the proposed direction of neural responses signaling whether to continue or replace the ongoing behavior. This evaluative signal influences midbrain areas important for attention, stress, pain, and reward evaluation to influence behavior/strategy selection. Abbreviations are as follows: medial prefrontal cortex (mPFC), lateral preoptic area (LPO), vertical limb of the diagonal band of Broca (vDBB), lateral septal area (LSA), entopeduncular nucleus (EPN), ventral pallidum (VP), suprachiasmatic nucleus (SCN), lateral hypothalamus (LH), bed nucleus of the stria terminalis (BNST), dorsal raphe (DR), median raphe (MrN), substantia nigra pars compacta (SNc), ventral tegmental area (VTA), , rostromedial tegmental nucleus (RMTg), and the periaqueductal gray (PAG).
In order for the LHb to be broadly tuned to integrate internal and external contexts, it must have access to a broad range of input that reflects these contexts. Of particular relevance to its contributions to behavioral flexibility is its connection with the mPFC in rodents (Kim and Lee 2012). The mPFC is a key mediator of executive function including working memory, strategy selection, and attention (Chudasama 2011; Kesner and Churchwell 2011). In a study which disconnected the mPFC and LHb using contralateral pharmacological inactivation, working memory was dramatically disrupted (Mathis et al. 2016). Based on these data, behavioral flexibility tasks which rely on both the LHb and mPFC, such as proactive switching (Baker and Ragozzino 2014a; Baker et al. 2016b), may also rely on an intact mPFC-LHb connection.
The basal ganglia is also a key mediator of behavioral flexibility (Floresco et al. 2009; Eagle and Baunez 2010). A prominent afferent connection to the LHb emerges from a specialized region of the entopeduncular nucleus (EPN), or internal globus pallidus in primates (Shabel et al. 2012; Yetnikoff et al. 2015; Stephenson-Jones et al. 2016). A subset of EPN neurons (termed the GPh) project exclusively to the LHb and co-release both GABA and glutamate (Meye et al. 2016). These LHb projecting neurons have been found to encode the valence of stimuli in a similar manner to signals observed in the LHb with sucrose resulting in decreased firing and aversive air puffs in the eye resulting in increased firing (Stephenson-Jones et al. 2016). Additionally, artificially driving or inhibiting these neurons during a task in which rewards are given probabilistically and occasionally are reversed resulted in decreased sensitivity to negative and positive outcomes respectively suggesting a role in outcome evaluation for this pathway (Stephenson-Jones et al. 2016). Indirect influences from other basal ganglia areas have also been probed for contributions to LHb function. The GPh receives GABAergic input from both striosomal and matrix compartments of the striatum (Stephenson-Jones et al. 2016) and stimulation across the striatum results in varying and complex responses in the LHb (Hong and Hikosaka 2013). High frequency stimulation of the subthalamic nucleus changes LHb cell firing and alters serotonin signaling, suggesting that the LHb may mediate changes in serotonin signaling in therapeutic interventions (Hartung et al. 2011; Hartung et al. 2016). Further, both the subthalamic nucleus and LHb have been implicated in sustained attention, delay discounting, and behavioral flexibility (Baunez and Robbins 1999; Winstanley et al. 2005; Baker and Ragozzino 2014b) suggesting that the link between these areas may have functional implications across a number of behaviors. Stimulation of the subthalamic nucleus increases GPh firing making this pathway a likely mediator of subthalamic – LHb coordination (Stephenson-Jones et al. 2016). Additional basal ganglia input to the LHb arises from the ventral pallidum (VP) (Groenewegen et al. 1993). However, LHb neuron responses to VP stimulation are exclusively inhibitory and have responses at three times the latency (5ms vs. 15ms) of GPh neurons raising the question of how these two inputs might function in differing ways (Hong and Hikosaka 2013).
The most likely means by which the LHb contributes to behavioral flexibility is through its efferent connections to the dopamine, serotonin, and to a lesser extent norepinephrine systems. The dopamine and serotonin systems are thought to contribute to behavioral flexibility via dissociable mechanisms. Variations in the dopamine (DAT1) and serotonin (SERT) transporters in human subjects were associated with changes in perseveration on the prior strategy and increased lose-shift responses in a probabilistic reversal learning task (den Ouden et al. 2013). Additionally, dorsal raphe neurons recorded from primates during a saccade guided task in which reward contingencies are occasionally reversed, neurons predominately show tonic increases in activity during behavioral performance and also encode reward information (Bromberg-Martin et al. 2010). This tonic task related activity may relate to velocity correlated activity observed in the LHb during task performance (Sharp et al. 2006; Baker et al. 2015), a possibility that should be explored as a mechanism for tracking ongoing behavior and relating it to outcomes. Others have reviewed in detail connections of the LHb with the primate dopamine reward prediction error signals first described by Schultz (1998) (Proulx et al. 2014; Baker et al. 2016a). The role of the LHb in contributing to reward prediction error signals in dopamine neurons is likely a contributor to its role in behavioral flexibility tasks.
A key question will be to understand the nature of the contributions of these LHb monoamine outputs to different aspects of behavioral flexibility tasks. One important fact is that the outputs to the dopamine and serotonin systems largely arise from separate areas of the LHb. The medial portion of the LHb preferentially projects to the serotonin containing nuclei while the lateral portion project to the dopamine and associated nuclei (Proulx et al. 2014). One interesting observation is that over one third of mPFC –LHb projecting neurons go to the medial portion of the LHb, a much higher proportion than other cortical areas (Kim and Lee 2012). This suggests that the mPFC-LHb connection may preferentially influence LHb control of serotonin containing areas. Regardless, individually manipulating outputs to the dopamine and serotonin containing nuclei during task performance coupled with careful analysis of resultant error patterns will be particularly useful in understanding their respective roles.
6. Conclusion
As studies continue to elucidate the role of the LHb across measures of motivated behavior, the extent to which the LHb is fundamental in monitoring ongoing behaviors across appetitive, aversive, and emotional states is becoming increasingly apparent. These functions appear to be governed by both direct and indirect connections to a wide range of brain areas including the hippocampus, basal ganglia, hypothalamus, midbrain monoamine structures and others. Examination of additional prominent connections with other brain areas such as the basal forebrain and the periaqueductal grey area will likely offer added insight into the role of the LHb in behaviors such as pain, attention, and other domains. While a great deal of research has elucidated the role of the LHb-dopamine pathway in reward prediction error signaling, the role of the LHb-serotonin pathway remains somewhat unclear.
Owing to the difficulty in recording from the LHb in awake and freely navigating animals, there is also a paucity of data connecting the various functions of the LHb with neural correlates of these behaviors. To this point, the neural data obtained in freely navigating rodents appears quite distinct from those obtained in head fixed primates with the former mostly showing velocity correlates and the later showing reward correlates. New technologies such as calcium imaging may begin to address this gap in the literature and add insight into how these two seemingly disparate correlates may be connected. In addition, connecting findings from optogenetic interventions with neural correlates of behaviors such as active and passive avoidance will further clarify how the LHb encodes behaviors aimed at obtaining rewards and avoiding punishments.
The LHb is a critical node in the overall neural circuity governing behavioral flexibility. Its apparent role in monitoring ongoing behaviors to determine whether the current response is appropriate makes it critical across a wide range of contexts and behavioral situations. This function is facilitated through a broad range of efferent and afferent connections to cortical and subcortical areas. It also clarifies the LHb connection with various psychopathologies including depression, addiction and anxiety. Specifically, dysfunction in an organism’s ability to determine whether an ongoing behavior is appropriate could lead to disorganized behaviors or an inability to switch to appropriate behaviors as is observed in depression and anxiety (Channon 1996; Han et al. 2016). This would likely play out broadly across efferent structures also implicated these psychopathologies increasing the need to understand these additional LHb connections to improve human health.
Highlights.
The lateral habenula monitors and regulates trial by trial strategy selection.
Lateral habenula afferent and efferent connections clarify this role in behavior.
Behavioral flexibility tests reveal essential lateral habenular contributions.
Lateral habenula behavioral flexibility functions may underlie psychopathology.
Acknowledgments
Funding:
This work was supported by NIH grants MH58755 and MH109796, and the University of Washington Research Royalty Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
The authors have no conflicts of interest to declare.
References
- Adachi Y, Osada T, Sporns O, Watanabe T, Matsui T, Miyamoto K, Miyashita Y. Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex. 2012;22:1586–1592. doi: 10.1093/cercor/bhr234. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Yanagihara S, Kobayashi M, Niisato K, Takekawa T, Harukuni R, McHugh TJ, Fukai T, Isomura Y, Okamoto H. The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation. J Neurosci. 2013;33:8909–8921. doi: 10.1523/JNEUROSCI.4369-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Jhou T, Li B, Matsumoto M, Mizumori SJY, Stephenson-Jones M, Vicentic A. The Lateral Habenula Circuitry: Reward Processing and Cognitive Control. The Journal of Neuroscience. 2016a;36:11482–11488. doi: 10.1523/JNEUROSCI.2350-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Oh SE, Kidder KS, Mizumori SJ. Ongoing behavioral state information signaled in the lateral habenula guides choice flexibility in freely moving rats. Front Behav Neurosci. 2015;9:295. doi: 10.3389/fnbeh.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Ragozzino ME. Contralateral disconnection of the rat prelimbic cortex and dorsomedial striatum impairs cue-guided behavioral switching. Learn Mem. 2014a;21:368–379. doi: 10.1101/lm.034819.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Ragozzino ME. The prelimbic cortex and subthalamic nucleus contribute to cue-guided behavioral switching. Neurobiol Learn Mem. 2014b;107:65–78. doi: 10.1016/j.nlm.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Raynor SA, Francis NT, Mizumori SJ. Lateral habenula integration of proactive and retroactive information mediates behavioral flexibility. Neuroscience. 2016b doi: 10.1016/j.neuroscience.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of transient inactivation of the subthalamic nucleus by local muscimol and APV infusions on performance on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 1999;141:57–65. doi: 10.1007/s002130050806. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O. Lateral habenula neurons signal errors in the prediction of reward information. Nat Neurosci. 2011;14:1209–1216. doi: 10.1038/nn.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O, Nakamura K. Coding of task reward value in the dorsal raphe nucleus. J Neurosci. 2010;30:6262–6272. doi: 10.1523/JNEUROSCI.0015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain RE, Wasserman MC, Waterhouse BD, McGaughy JA. Atomoxetine facilitates attentional set shifting in adolescent rats. Developmental cognitive neuroscience. 2011;1:552–559. doi: 10.1016/j.dcn.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon S. Executive dysfunction in depression: the Wisconsin Card Sorting Test. J Affect Disord. 1996;39:107–114. doi: 10.1016/0165-0327(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Chudasama Y. Animal models of prefrontal-executive function. Behav Neurosci. 2011;125:327–343. doi: 10.1037/a0023766. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- den Ouden HE, Daw ND, Fernandez G, Elshout JA, Rijpkema M, Hoogman M, Franke B, Cools R. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80:1090–1100. doi: 10.1016/j.neuron.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Goutagny R, Loureiro M, Jackson J, Chaumont J, Williams S, Isope P, Kelche C, Cassel JC, Lecourtier L. Interactions between the lateral habenula and the hippocampus: implication for spatial memory processes. Neuropsychopharmacology. 2013;38:2418–2426. doi: 10.1038/npp.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–142. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Han G, Helm J, Iucha C, Zahn-Waxler C, Hastings PD, Klimes-Dougan B. Are Executive Functioning Deficits Concurrently and Predictively Associated with Depressive and Anxiety Symptoms in Adolescents? Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2016;45:44–58. doi: 10.1080/15374416.2015.1041592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Gordon JA. Long-range neural synchrony in behavior. Annu Rev Neurosci. 2015;38:171–194. doi: 10.1146/annurev-neuro-071714-034111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H, Tan SK, Steinbusch HM, Temel Y, Sharp T. High-frequency stimulation of the subthalamic nucleus inhibits the firing of juxtacellular labelled 5-HT-containing neurones. Neuroscience. 2011;186:135–145. doi: 10.1016/j.neuroscience.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Hartung H, Tan SK, Temel Y, Sharp T. High-frequency stimulation of the subthalamic nucleus modulates neuronal activity in the lateral habenula nucleus. Eur J Neurosci. 2016;44:2698–2707. doi: 10.1111/ejn.13397. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Lesions of the habenula produce stress- and dopamine-dependent alterations in prepulse inhibition and locomotion. Brain Res. 2006;1073–1074:229–239. doi: 10.1016/j.brainres.2005.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behav Biol. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Diverse sources of reward value signals in the basal ganglia nuclei transmitted to the lateral habenula in the monkey. Frontiers in human neuroscience. 2013;7:778. doi: 10.3389/fnhum.2013.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. The neural basis of reversal learning: An updated perspective. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto LR, Mata R, Novais A, Marques F, Sousa N. The habenula as a critical node in chronic stress-related anxiety. Exp Neurol. 2016 doi: 10.1016/j.expneurol.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Kang S, Li J, Zuo W, Fu R, Gregor D, Krnjevic K, Bekker A, Ye JH. Ethanol Withdrawal Drives Anxiety-Related Behaviors by Reducing M-type Potassium Channel Activity in the Lateral Habenula. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Yamada H, Sato N, Takada M, Matsumoto M. Roles of the Lateral Habenula and Anterior Cingulate Cortex in Negative Outcome Monitoring and Behavioral Adjustment in Nonhuman Primates. Neuron. 2015;88:792–804. doi: 10.1016/j.neuron.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Kim U, Lee T. Topography of descending projections from anterior insular and medial prefrontal regions to the lateral habenula of the epithalamus in the rat. Eur J Neurosci. 2012;35:1253–1269. doi: 10.1111/j.1460-9568.2012.08030.x. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur J Neurosci. 2004;19:2551–2560. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- Mathis V, Barbelivien A, Majchrzak M, Mathis C, Cassel J-C, Lecourtier L. The Lateral Habenula as a Relay of Cortical Information to Process Working Memory. Cerebral Cortex. 2016 doi: 10.1093/cercor/bhw316. [DOI] [PubMed] [Google Scholar]
- Mathis V, Cosquer B, Avallone M, Cassel JC, Lecourtier L. Excitatory Transmission to the Lateral Habenula Is Critical for Encoding and Retrieval of Spatial Memory. Neuropsychopharmacology. 2015;40:2843–2851. doi: 10.1038/npp.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Meye FJ, Soiza-Reilly M, Smit T, Diana MA, Schwarz MK, Mameli M. Shifted pallidal co-release of GABA and glutamate in habenula drives cocaine withdrawal and relapse. Nat Neurosci. 2016;19:1019–1024. doi: 10.1038/nn.4334. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Ragozzino KE, Cooper BG, Leutgeb S. Hippocampal representational organization and spatial context. Hippocampus. 1999;9:444–451. doi: 10.1002/(SICI)1098-1063(1999)9:4<444::AID-HIPO10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Neumann PA, Ishikawa M, Otaka M, Huang YH, Schluter OM, Dong Y. Increased excitability of lateral habenula neurons in adolescent rats following cocaine self-administration. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2014;18 doi: 10.1093/ijnp/pyu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson HC, McIver AH. Cold stress and habenular lesion effects on rat behaviors. J Appl Physiol. 1966;21:655–660. doi: 10.1152/jappl.1966.21.2.655. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Agetsuma M, Aizawa H. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Developmental neurobiology. 2012;72:386–394. doi: 10.1002/dneu.20913. [DOI] [PubMed] [Google Scholar]
- Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci. 2014;17:1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quina LA, Tempest L, Ng L, Harris JA, Ferguson S, Jhou TC, Turner EE. Efferent pathways of the mouse lateral habenula. J Comp Neurol. 2015;523:32–60. doi: 10.1002/cne.23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Baker PM. The Neurobiological Basis of Memory. Springer International Publishing; 2016. Prefrontal Cortex and Basal Ganglia Attributes Underlying Behavioral Flexibility. In; pp. 241–260. [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–481. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PE, Turner-Williams S, Tuttle S. Movement-related correlates of single cell activity in the interpeduncular nucleus and habenula of the rat during a pellet-chasing task. Behav Brain Res. 2006;166:55–70. doi: 10.1016/j.bbr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Yu K, Ahrens S, Tucciarone JM, van Huijstee AN, Mejia LA, Penzo MA, Tai LH, Wilbrecht L, Li B. A basal ganglia circuit for evaluating action outcomes. Nature. 2016;539:289–293. doi: 10.1038/nature19845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB. What's better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat Neurosci. 2014;17:33–35. doi: 10.1038/nn.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Thornton EW, Bradbury GE. Effort and stress influence the effect of lesion of the habenula complex in one-way active avoidance learning. Physiol Behav. 1989;45:929–935. doi: 10.1016/0031-9384(89)90217-5. [DOI] [PubMed] [Google Scholar]
- Thornton EW, Davies C. A water-maze discrimination learning deficit in the rat following lesion of the habenula. Physiol Behav. 1991;49:819–822. doi: 10.1016/0031-9384(91)90324-h. [DOI] [PubMed] [Google Scholar]
- Thornton EW, Evans JA. The effects of lesions of the habenula nucleus on lever press behaviour during a tandem operant schedule with contrasting response requirements. Behav Brain Res. 1984;12:327–334. doi: 10.1016/0166-4328(84)90158-x. [DOI] [PubMed] [Google Scholar]
- Tronel S, Sara SJ. Mapping of olfactory memory circuits: region-specific c-fos activation after odorreward associative learning or after its retrieval. Learn Mem. 2002;9:105–111. doi: 10.1101/lm.47802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev. 2015;57:271–283. doi: 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Baunez C, Theobald DE, Robbins TW. Lesions to the subthalamic nucleus decrease impulsive choice but impair autoshaping in rats: the importance of the basal ganglia in Pavlovian conditioning and impulse control. Eur J Neurosci. 2005;21:3107–3116. doi: 10.1111/j.1460-9568.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE, Pitzer MR. Dopamine agonists and stress produce different patterns of Foslike immunoreactivity in the lateral habenula. Brain Res. 1994;633:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA, Gerfen CR. The frontal cortex-basal ganglia system in primates. Crit Rev Neurobiol. 1996;10:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Yetnikoff L, Cheng AY, Lavezzi HN, Parsley KP, Zahm DS. Sources of input to the rostromedial tegmental nucleus, ventral tegmental area, and lateral habenula compared: A study in rat. J Comp Neurol. 2015;523:2426–2456. doi: 10.1002/cne.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Gao Y, Li Y, Yang J, Zhao H. Sleep Deprivation Influences Circadian Gene Expression in the Lateral Habenula. Behavioural neurology. 2016a;2016:7919534. doi: 10.1155/2016/7919534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hernandez VS, Vazquez-Juarez E, Chay FK, Barrio RA. Thirst Is Associated with Suppression of Habenula Output and Active Stress Coping: Is there a Role for a Non-canonical Vasopressin-Glutamate Pathway? Frontiers in neural circuits. 2016b;10:13. doi: 10.3389/fncir.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132:519–528. doi: 10.1016/j.neuroscience.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhang BL, Yang SJ, Rusak B. The role of lateral habenula-dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behav Brain Res. 2015;277:89–98. doi: 10.1016/j.bbr.2014.09.016. [DOI] [PubMed] [Google Scholar]