Abstract
Autism spectrum disorder (ASD) is marked by both socio-communicative difficulties and abnormalities in sensory processing. Much of the work on sensory deficits in ASD has focused on tactile sensations and the perceptual aspects of somatosensation, such as encoding of stimulus intensity and location. Although aberrant pain processing has often been noted in clinical observations of patients with ASD, it remains largely uninvestigated. Importantly, the neural mechanism underlying higher-order cognitive aspects of pain processing such as pain anticipation also remains unknown. Here we examined both pain perception and anticipation in high functioning adults with ASD and matched healthy controls (HC) using an anticipatory pain paradigm in combination with functional magnetic resonance imaging (fMRI) and concurrent skin conductance response (SCR) recording. Participants were asked to choose a level of electrical stimulation that would feel moderately painful to them. Compared to HC group, ASD group chose a lower level of stimulation prior to fMRI. However, ASD participants showed greater activation in both rostral and dorsal anterior cingulate cortex during the anticipation of stimulation, but not during stimulation delivery. There was no significant group difference in insular activation during either pain anticipation or perception. However, activity in the left anterior insula correlated with SCR during pain anticipation. Taken together, these results suggest that ASD is marked with aberrantly higher level of sensitivity to upcoming aversive stimuli, which may reflect abnormal attentional orientation to nociceptive signals and a failure in interoceptive inference.
Keywords: autism spectrum disorder, functional magnetic resonance imaging, pain anticipation, anterior cingulate cortex, insular cortex
Graphical abstract
We examined pain perception and anticipation in high functioning adults with autism spectrum disorder (ASD) and matched healthy controls using an anticipatory pain paradigm in combination with fMRI and skin conductance response recording. Compared to controls, ASD participants chose lower levels of stimulation, yet showed increased activations in the anterior cingulate cortex during pain anticipation. These results indicate abnormal attentional orientation to nociceptive signals in ASD.
Introduction
Autism spectrum disorder (ASD) is a family of neurodevelopmental disorders with deficits ranging from socio-emotional to sensory problems including altered pain processing (APA, 2013). In clinical observations, for instance, self-injury behavior is often reported in individuals with ASD (Bodfish et al., 2000). However, laboratory studies, especially neuroimaging studies, have overwhelmingly focused on emotional and social deficits in ASD [for review see (Allely, 2013)]. A handful of studies have directly measured pain perception in ASD and have reported mixed results (Allely, 2013). For example, several behavioral studies have suggested that children with ASD are associated with reduced pain sensitivity, which are consistent with clinical observations (Allely, 2013). However, physiological measures of arousal indicate the opposite pattern that children with ASD have increased autonomic responses to pain (Tordjman et al., 2009). One fMRI study has shown that subjective rating of pain intensity and unpleasantness only differed between ASD and control participants when stimulation level was low, but not when it was high (Bird et al., 2010). Furthermore, group difference in neural responses related to observing others' pain was not significant (Bird et al., 2010). Thus, the specific pattern of abnormality in pain processing in individuals with ASD, particularly that of adults, remains unclear.
Pain is an important psychobiological function with high evolutionary significance, and the response to pain has been critical in the ability for biological organisms to increase their chances of survival. Not surprisingly, the pathways of pain perception have been well studied in many mammalian species, including humans. Painful stimuli administered to the body activate Aδ and C fibers which then convey pain information to brain regions including the periaqueductal gray, thalamus, anterior (AIC) and posterior insular cortex (PIC), and anterior cingulate cortex (ACC) (Jones et al., 1992; Vogt & Sikes, 2000; Craig, 2002). However, pain processing involves much more than just sensory encoding of the nociceptive stimuli, as affective and cognitive components are also involved. For instance, an individual observing another person receiving painful stimuli have the same pain-responsive regions (e.g. ACC, AIC) activated, suggesting that these regions are involved in the affective and cognitive aspects of pain perception (Corradi-Dell'Acqua et al., 2011). Importantly, anticipation of a painful stimulus can also activate brain regions overlapping with those that directly process painful stimuli like the ACC and AIC (Wager et al., 2004). It is noteworthy that the PIC is usually not activated in anticipatory or observational pain paradigms, but instead is activated during the actual delivery of nociceptive stimuli such as painful stimulations (Flynn et al., 1999; Craig, 2002; 2011). Although the neural substrates of pain perception and anticipation are relatively well described in healthy controls (HC) using neuroimaging methods, little is known about how these processes are altered in individuals with ASD, which may contribute to our lack of understanding of social-emotional processing (e.g., empathy) in this population.
In this study, we examined the neural substrates subserving both the anticipation and the encoding of painful stimulations in high-functioning adults with ASD in comparison to matched HC. We designed an anticipatory pain paradigm in which a visual cue is either associative (a stimulation cue) or not associative (a safe cue) of an electrical stimulation to be administered to the left extremity (ankle) of the participant. We used functional magnetic resonance imaging (fMRI) in combination with concurrent skin conductance response (SCR) measures to record brain and peripheral responses to stimulation cue and stimulation. Based on previous findings of heightened arousal in children with ASD (Tordjman et al., 2009), we predicted that high-functioning adults with ASD would show 1) behavioral hypersensitivity to pain; 2) increased autonomic response indexed by SCR and greater activations in brain regions involved in processing cognitive and affective aspects of pain, such as the ACC and AIC, during pain anticipation; and 3) increased SCR and hyperactivity in brain regions that encode the sensory aspects of nociceptive stimuli such as the PIC during pain perception.
Materials and Methods
Participants
We recruited 17 unmedicated high-functioning adult males with ASD and 18 matched HC participants through the Seaver Autism Center for Research and Treatment at the Icahn School of Medicine at Mount Sinai (ISMMS). One HC participant was excluded due to chance-level behavioral performance on the catch trials (see description about paradigm in next section), resulting in a final sample of 17 participants in each group (see Table 1 for participant characteristics). One additional HC participant had incomplete SCR data and was therefore excluded from the SCR analysis, yielding N = 17 for ASD and N = 16 for HC for the SCR results. Two out of 17 ASD patients and two out of 17 HC participants were excluded from the fMRI analysis due to excessive head motion, yielding 15 participants in each group for the fMRI results. Individuals in the ASD group met diagnostic criteria for autism disorder (n = 12) or Asperger syndrome (n = 5) by psychiatric interview according to the Diagnostic and Statistical Manual-IV (DSM-IV-TR) (APA, 2000) and confirmed by the Autism Diagnostic Interview-Revised [ADI-R; (Lord et al., 1994)] and Autism Diagnostic Observation Schedule-Generic [ADOS-G; (Lord et al., 2000)], except for one participant for whom ADI-R scores were unavailable and two participants for whom ADOS-G scores were unavailable. Participants who met criteria only for Pervasive Developmental Disorder not Otherwise Specified (PDD-NOS) by DSM-IV-TR were not included. Other exclusion criteria included epilepsy, history of schizophrenia, schizoaffective disorder or other Axis I mental disorders except obsessive-compulsive disorder (given the phenotypic overlap with ASD) and use of depot neuroleptic medication or other psychoactive drugs within five weeks prior to participation. For the HC group, participants were excluded based on medical illness or history in first-degree relatives of developmental disorders, learning disabilities, autism, affective disorders, and anxiety disorders. Participants from both groups with a history of substance or alcohol dependency or abuse within one year prior to participation were excluded as well. Each group had 16 right-handed and 1 left-handed male participants, measured by the Edinburgh Inventory Handedness Questionnaire (Oldfield, 1971). HCs were matched with ASD participants for age and Full-Scale Intelligence Quotient (FSIQ) measured with the Wechsler Adult Intelligence Scale [WAIS-III (Wechsler, 1997)], and had an FSIQ in the low average range or higher (>80). All participants provided written informed consent, approved by the ISMMS Institutional Review Board.
Table 1.
Participant characteristics.
| ASD (n = 17) | HC (n = 17) | P | |
|---|---|---|---|
| Age (years) | 26.2 ± 6.4 | 26.8 ± 7.8 | >0.7 |
| Handedness score | 73.5 ± 35.3 | 75.6 ± 40.5 | >0.8 |
| Parental SESa | 91.3 ± 16.8 | 92.0 ± 22.6 | >0.9 |
| Subject SES a | 27.9 ± 14.6 | 32.7 ± 15.4 | >0.3 |
| Full Scale IQ | 109.5 ± 18.0 | 113.5 ± 11.9 | >0.4 |
| ASD diagnosis (autism/Asperger) | 12/5 | ||
| ADI-R b | |||
| Social | 18.6 ± 8.0 | ||
| Verbal communication | 15.5 ± 4.9 | ||
| Repetitive behavior | 6.2 ± 3.7 | ||
| Development | 3.5 ± 1.5 | ||
| ADOS-G | |||
| Communication | 3.0 ± 1.4 | ||
| Social | 7.1 ± 2.2 | ||
| Imagination | 0.7 ± 0.5 | ||
| Stereotyped behaviors | 1.4 ± 1.5 |
Note:
SES data was not available for one ASD participant and one HC participant.
ADI-R scores were not available for one ASD participant.
ASD: autism spectrum disorder; HC: healthy control. Data are reported as means ± standard deviation.
Anticipatory pain paradigm
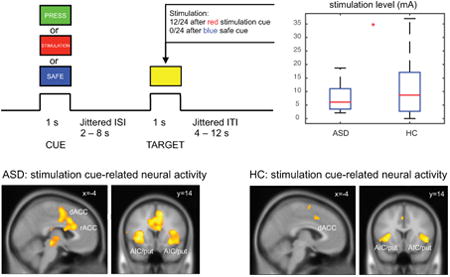
This paradigm applied uncomfortable electrodermal stimulation (low current) (LaBar et al., 1998) to the extremity (left ankle). This is a cue- target paradigm with 1 s of S1 (cue: blue or red square in Fig. 1) for safe/stimulation cue and 1 s of S2 (target: yellow square in Fig. 1) with/without stimulation, with an inter stimulus interval (ISI) of 2-8 s (mean = 3 s; Fig. 1). In addition, there were 4 catch trials with the green square and word “PRESS” to instruct participant to press a button to ensure that participants maintained an attentional set. The inter trial interval (ITI) was jittered (4-12 s, exponentially distributed, mean = 5 s). Mean duration for each trial was 10 s.
Figure 1.
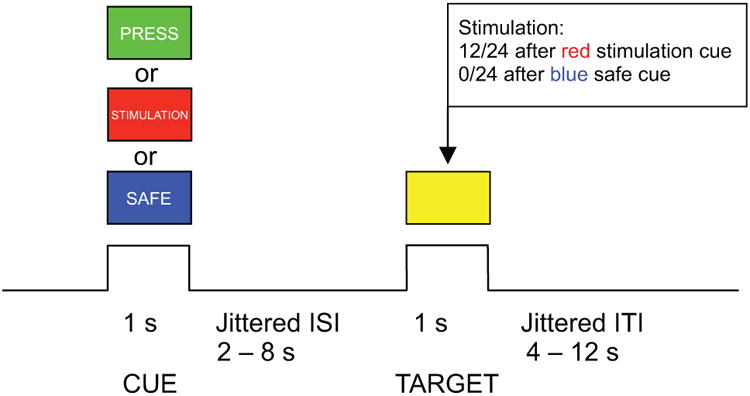
Anticipatory pain paradigm. This is an cue-target paradigm with 1 s of S1 for safe/stimulation cue and 1 s of S2 with or without stimulation, with an inter-stimulus interval (ISI) of 2-8 s. Red square with the text “STIMULATION” represents a stimulation cue, indicating a 50% chance of receiving a stimulation when the yellow square (target) appears. Blue squares of “SAFE” cues indicate there will be no stimulation when target appears. To ensure that subjects were paying attention to the cues, they were also required to press a button when they see the target following the green square / “PRESS” cue.
Pain is a complex mental function that may involve high-level cognitive factors beyond sensory encoding. It is well known that these cognitive factors, such as cognitive control and reappraisal, can have significant top-down modulatory effects on basic sensory aspects of pain (Bantick et al., 2002; Tracey et al., 2002; Salomons et al., 2004; Wager et al., 2004; Salomons et al., 2007; Tracey & Mantyh, 2007). Rating pain intensity, for example, involves more cognitive analysis of the stimuli and can activate pain-related regions even without the actual presence of painful stimuli, and thus could potentially confound the sensory encoding of pain (Kong et al., 2006). In this study, we wanted to ensure that participants focus more on the sensory aspects of pain without engaging intensive cognitive effort. Thus, participants were instructed to view visual stimulus squares (red, blue, yellow, or green) presented pseudo-randomly without rating intensity of unpleasantness on each trial. Pain intensity and unpleasantness were rated once at the end of each run to ensure that subjective pain was still matched during the fMRI session. Participants were informed that a red square with the word “STIMULATION”, when presented, indicates that stimulation may follow when a yellow square appears. They were also informed that a blue square with the word “SAFE” indicates that there was no stimulation when a yellow square appears. Each run had 48 counterbalanced trials with 24 safe trials and 24 stimulation trials. Electrodermal stimulation was given during 50% of the S2 period of the stimulation trials (12 of 24 trials) when the “STIMULATION” stimulus preceded the yellow square. We chose a probabilistic shock cue (50%) to increase uncertainty related response (Schultz et al., 1997; Fan, 2014). Previous work using salient deterministic cues (100% predictive of reward) did not find group differences in behavior (Dichter et al., 2012).
There was a 30-s resting period at the beginning and end of each run. Each run took 580 s, about 10 minutes. Two runs of the task were conducted. Electrodermal stimulations were placed to the left extremity (lower medial side above the ankle), with two MRI compatible EL508 disposable electrodes pre-gelled with a 1-cm diameter circular contact area. Electrodermal stimulations were presented at 500 ms after the onset of S2 for a duration of 100 ms (biphasic trains of 1 ms widely spaced pulses delivered at 50 Hz for 100 ms). Pain intensity and unpleasantness ratings by each participant were also collected at the end of each run with a 5-point scale using our glove response device with five buttons (BrainLogic, Psychology Software Tools, Inc.).
Calibration of stimulation level
Due to the fact that the threshold for pain perception varies markedly among individuals, the intensity of stimulation was decided and recorded individually in a separate session right before scanning. For each participant, we asked the following questions for each step of the calibration: “Was that painful?” “On a 1-5 point scale (not painful to extremely painful), how would you rate the intensity of pain for this stimulation?” and “Can you tolerate a stronger stimulation?” First, we raised the intensity by roughly 1 mA each time (starting from 0 mA), until the subject indicated that he or she did not want a stronger stimulation. Then, we gradually reduced the stimulation by approximately 1 mA each step and asked the subjects to rate the intensity of pain again. This procedure was designed to ensure that subjects were familiar with the full spectrum of stimulations and associated subjective feelings. Next, we increased the stimulation again by 1 mA each step, until the subject reported a subjective pain level of 3 (“moderate pain”). We recorded that stimulation level as the strength to be used in the fMRI session for that subject (Fig. 2A).
Figure 2.
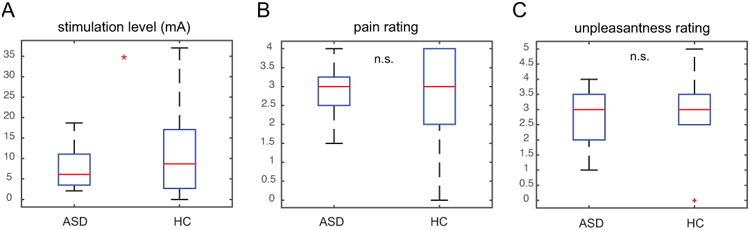
Behavioral results. (A) ASD participants chose lower levels of stimulations compared to HC participants, when subjective ratings of pain (B) and unpleasantness (C) were matched between groups. ASD: autism spectrum disorder; HC: healthy control; n.s.: non significant; *: P < 0.05.
Trait empathy measurement
To explore whether measures of pain processing would correlate with participants' social cognitive abilities such as empathy, we also assessed trait empathy using the Empathy Quotient (EQ). The EQ is a 40-item self-report questionnaire without subscales (Baron-Cohen & Wheelwright, 2004). Higher EQ scores indicate greater trait empathy. It has been shown that ASD individuals score significantly lower on EQ, indicating impaired trait empathy (Baron-Cohen & Wheelwright, 2004; Gu et al., 2015).
fMRI data acquisition and preprocessing
MRI acquisitions were obtained on a 3 T Siemens Allegra MRI system at ISMMS. Participants underwent one session with all scanning sequences (two tasks including the one reported here). The whole scan session lasted about 1.5 hours. Foam padding was used to keep participants' heads still. All images were acquired along axial planes parallel to the anterior commissure-posterior commissure (AC-PC) line. A high-resolution T2-weighted anatomical volume of the whole brain were acquired on an axial plane parallel to the AC-PC line, with a turbo spin-echo (TSE) pulse sequence with the following parameters: 40 axial 4 mm-thick slices, skip = 0 mm, repetition time (TR) = 4050 ms, echo time (TE) = 99 ms, flip angle = 170°, field of view (FOV) = 240 mm, matrix size = 448×512, voxel size = 0.47×0.47×4 mm. T2*-weighted images were acquired for fMRI. Slices were obtained corresponding to the T2-weighted images. The fMRI imaging was performed using a gradient echoplanar imaging (GE-EPI) sequence: 40 axial slices, 4 mm-thick, and skip = 0 mm, TR = 2500 ms, TE = 27 ms, flip angle = 82°, FOV = 240 mm, and matrix size = 64×64. Each run started with 2 dummy volumes before the onset of the task to allow for equilibration of T1 saturation effects. A total of two EPI runs with 232 image volumes per run for the task were acquired for each participant. Event-related analyses of the fMRI data were conducted using statistical parametric mapping (SPM12; Wellcome Department of Imaging Neuroscience, London, UK). The functional images were adjusted for slice timing, realigned to the first volume, coregistered to the T2 image, normalized to a standard template (MNI, Montreal Neurological Institute), resampled to a 2×2×2 mm voxel size, and spatially smoothed with an 8×8×8 mm full-width-at-half-maximum (FWHM) Gaussian kernel.
General linear modeling (GLM) of fMRI
GLM (Friston et al., 1995) was then conducted for the functional scans from each participant by modeling the observed event-related BOLD signals and regressors to identify the relationship between the task events and the hemodynamic response. Regressors were created by convolving a train of delta functions representing the sequence of individual events with the default SPM haemodynamic basis function (Friston et al., 1998). There were six regressors - all cues, stimulation cues, all targets, targets with stimulation cues, stimulation, and catch trials. Six parameters generated during motion correction were entered as covariates. The first-level contrast images from all participants were entered into a second-level group analysis using a standard summary statistic (random effects) analysis. A threshold of voxelwise P < 0.05 corrected for family-wise error was applied to main effects of stimulation and stimulation cue. Small volume correction with a 8 mm spherical search volume was used for group difference contrasts for regions of a priori interest, namely, left AIC ([-36,16,0]), right AIC ([32,20,-2]), dACC ([10,10,44]), and rACC ([-6,22,22]) for stimulation cue (pain anticipation); and left PIC ([-40,-4,-4]) and right PIC ([40,0,-2]) for stimulation. The peaks of these ROIs were taken from peak activations across all participants to avoid bias toward either group (see Tables 2 and 3).
Table 2. Stimulation-cue related activation.
| Region | Laterality | x | y | z | Z | p | k |
|---|---|---|---|---|---|---|---|
| Both groups | |||||||
| Anterior insula | L | -36 | 16 | 0 | 6.19 | 0 | 953 |
| Anterior insula | L | -32 | 24 | -2 | 5.99 | 0 | 0 |
| Anterior insula | L | -30 | 16 | 8 | 5.94 | 0 | 0 |
| Anterior insula | R | 32 | 20 | -2 | 6.09 | 0 | 766 |
| Rolandic operculum | R | 50 | 0 | 8 | 5.63 | 0 | 0 |
| Anterior insula | R | 40 | 8 | 12 | 5.19 | 0 | 0 |
| Thalamus | R | 10 | -8 | 2 | 5.79 | 0 | 107 |
| Dorsal anterior cingulate | R | 10 | 10 | 44 | 5.61 | 0 | 376 |
| Dorsal anterior cingulate | R | 10 | 4 | 38 | 5.44 | 0 | 0 |
| Supplementary motor area | R | 12 | 0 | 60 | 5.29 | 0 | 0 |
| Midbrain | R | 8 | -24 | -10 | 5.52 | 0 | 46 |
| Precentral gyrus | R | 42 | 0 | 38 | 5.21 | 0 | 40 |
| Rolandic operculum | R | 50 | -24 | 22 | 4.96 | 0 | 13 |
| Rostral anterior cingulate | L | -6 | 22 | 22 | 4.79 | 0 | 1 |
| Precentral gyrus | R | 48 | 0 | 48 | 4.72 | 0 | 3 |
| Postcentral gyrus | R | 18 | -38 | 68 | 4.71 | 0 | 1 |
| Supramarginal gyrus | L | -48 | -34 | 28 | 4.65 | 0 | 2 |
| ASD > HC | |||||||
| Dorsal anterior cingulate* | R | 12 | 10 | 46 | 3.51 | 0 | 79 |
| Rostral anterior cingulate* | R | -8 | 22 | 22 | 2.67 | 0 | 35 |
| HC > ASD | |||||||
| None |
P < 0.05 corrected for family-wise error;
indicates P < 0.05 small volume correction with 5mm sphere for regions of a priori interest.
ASD: autism spectrum disorder; HC: healthy control.
Table 3. Stimulation related activation.
| Region | Laterality | x | y | z | Z | p | k |
|---|---|---|---|---|---|---|---|
| Both groups | |||||||
| Insula | R | 40 | 0 | -2 | 5.31 | 0 | 87 |
| Insula | L | -40 | -4 | -4 | 5.11 | 0 | 32 |
| Superior temporal pole | R | 58 | 8 | 2 | 4.97 | 0 | 14 |
| ASD > HC | |||||||
| None | |||||||
| HC > ASD | |||||||
| None |
P < 0.05 corrected for family-wise error.
ASD: autism spectrum disorder; HC: healthy control.
Skin conductance acquisition and analysis
Skin conductance was acquired during fMRI scanning as described in our previous studies (Fan et al., 2012; Eilam-Stock et al., 2014). In brief, skin conductance was recorded using the GSR100C amplifier (BIOPAC Systems, Goleta, CA) together with the base module MP150 and the AcqKnowledge software (version 3.9.1.6). The GSR100C measures skin conductance by applying a constant voltage of 0.5 V between two electrodes that are attached to the palmar skin. Skin conductance (measured in μS) was recorded using a 2000-Hz sampling rate (gain = 2 μS/V, both high pass filters = DC, low pass filter = 10 Hz). After cleaning the skin with alcohol preps, two EL507 disposable EDA (isotonic gel) electrodes were placed on the palmar surface of the distal phalanges of the big and second toes of left foot. The signal was low-pass filtered (using the MRI-Compatible MRI CBL/FILTER System MECMRI-TRANS) to reduce radio frequency interference from the scanner. BIOPAC recording was synchronized to the E-Prime program via the parallel port of the computers. Event markers were recorded to enable precise time alignment of skin conductance recording with scan onsets and task conditions.
To analyze the SCR data, we applied general linear modeling using SCRalyze v.b2.1.7 (Bach et al., 2010). SCR data were epoched into individual runs, and the range of trimming was determined by the beginning of the first marker and the end of the last marker of each block with 30 s before and after the first and last marker for baseline. Consistent with fMRI analysis, the six vectors for onsets of the events (all cues, stimulation cues, all targets, targets with stimulation cues, stimulation, and catch trials) in seconds were extracted based on the corresponding markers recorded. The regressors were then generated by convolving the vectors with the canonical response function of the SCR (Bach et al., 2010). GLM was then performed with a band-pass filter (first-order Butterworth filter) with a high-pass filter of 0.01 Hz and low-pass filter of 0.12 Hz, similar to our previous study on SCR (Fan et al., 2012). The data were then normalized to control for between-subject differences in skin conductance response amplitude. The regression coefficients quantifying the SCR were obtained from the GLM of each participant for between-group statistical testing.
Region of interest (ROI) analysis
For regions of a priori interest, we conducted independent ROI analysis using the MarsBaR toolbox for SPM (http://marsbar.sourceforge.net/). Spherical ROIs of 5mm diameter were created for the following regions: left AIC ([-36,16,0]), right AIC ([32,20,-2]), dACC ([10,10,44]), and rACC ([-6,22,22]) for stimulation cue (pain anticipation); left PIC ([-40,-4,-4]) and right PIC ([40,0,-2]) for stimulation. The peaks of these ROIs were taken from peak activations across all participants (see Tables 2 and 3) and thus, were not biased toward either group. Parameter estimates were extracted for each participant for each ROI. The parameter estimates were then entered into a second level analysis to test for either group difference or behavior-brain relationship.
Results
Behavioral Results
As the participants were not required to make behavioral responses to stimulation or stimulation cue (except for the catch trials) in the scanner, we conducted group comparison primarily on one behavioral measurement - the actual level of stimulations participants received (measured in mA) when matched for subjective pain and unpleasantness. The ASD group had significantly lower stimulation levels than the HC group (t(32) = -2.55, P = 0.016; Fig. 2A). As predicted, there was no significant difference between groups in terms of subjective levels of pain (Fig. 2B) or unpleasantness (Fig. 2C; Ps > 0.1) rated at the end of each fMRI run. Taken together, ASD participants chose to receive lower levels of pain when subjective pain was matched between ASD and HC groups, suggesting that ASD participants have lower behavioral tolerance for pain.
Neural activates related to pain anticipation
Both ASD and HC groups showed significant activations in bilateral AIC, rostral and dorsal ACC, and putamen related to the anticipation of pain during the stimulation cue period (Fig. 3A, B and Table 2). A direct group contrast revealed stronger activation in the ACC in the ASD group during pain anticipation (Fig. 3C and Table 2). We then conducted ROI analysis on AIC, rACC, and dACC bilaterally (Fig. 3D, E). The ASD group showed significantly higher responses in both rACC (t(28) = 2.78, P = 0.010) and dACC (t(28) = 3.43, P = 0.002) during pain anticipation. Contrary to our prediction but consistent with the whole brain analysis, group differences in AIC bilaterally activation during the anticipation period were not significant (Ps > 0.1). However, left AIC, rACC, and dACC activations all negatively correlated with trait empathy measured by the EQ across all participants (Fig. 3F; r = -0.40 for left AIC, r = -0.36 for rACC, and r = -0.39 for dACC; all Ps < 0.05), suggesting that individuals with higher AIC/rACC/dACC activations (mostly ASD participants) had greater impairments in empathy.
Figure 3.
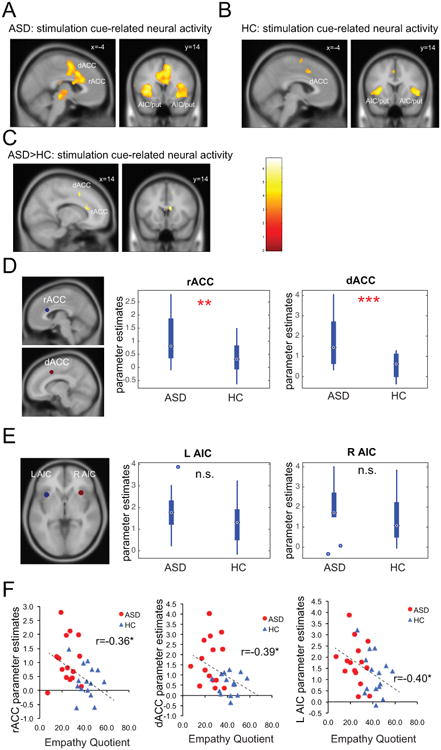
Neural activities related to pain anticipation during the stimulation cue phase. Both ASD (A) and HC (B) participants showed significant activations in the ACC and AIC (extending into putamen) in response to stimulation cue. (C) Whole brain group comparison indicates greater rACC and dACC activations in the ASD group. Region of interest analysis confirms increased rACC and dACC activations (D) but normal AIC activations (E) in the ASD group. (F) Trait empathy measured by the Empathy Quotient correlated with stimulation cue-related activations in the rACC, dACC, and left AIC. Images displayed at P < 0.001 uncorrected for visualization purpose. rACC: rostral anterior cingulate cortex; dACC: dorsal anterior cingulate cortex; AIC: anterior insular cortex; L, left; R: right; ASD: autism spectrum disorder; HC: healthy control; n.s.: non significant; *: P < 0.05.
Neural activates related to pain
In both ASD and HC groups, participants displayed robust bilateral activation in the PIC in response to stimulation (Fig. 4A, B and Table 3). Although right PIC activation also extended to mid and anterior insula in both groups, the peaks of activations were located in the posterior insula (Table 3). Direct group comparison did not reveal any significant group difference in neural activities related to stimulation even at a more liberal threshold of P < 0.001 uncorrected (Table 3). ROI analysis results were consistent with the whole brain analysis – there was no significant group difference in either left or right PIC in response to stimulation (Fig. 4C; Ps > 0.1). One possible confounding effect is the significant group difference in the actual level of stimulation participants received. Thus, we examined the relationship between PIC activation and stimulation level (mA). For both left and right PIC, this correlation was not significant in either the ASD or the HC group, or both groups combined (Fig. 4D, E; -0.1 < r < 0.1 and P > 0.1 for all correlations). This result suggests that the lack of group difference in PIC activation is unlikely to be attributable to lower stimulation levels in the ASD group.
Figure 4.
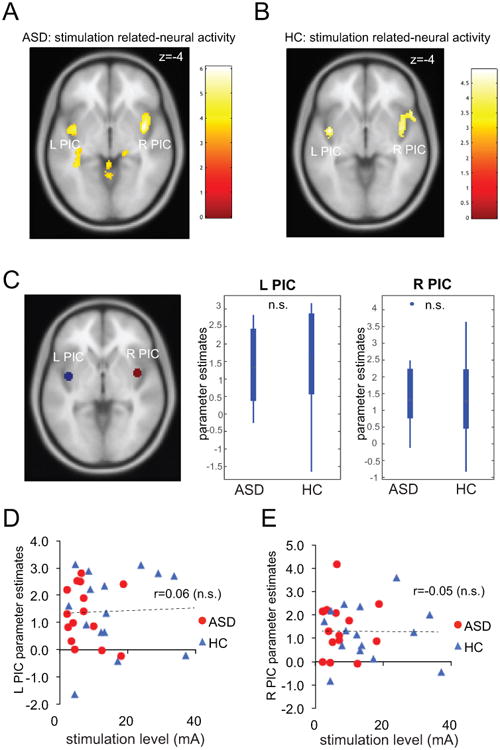
Neural activities related to pain processing during the stimulation phase. Both ASD (A) and HC (B) groups displayed significant activations in bilateral PIC. (C) Region of interest (ROI) analysis suggests that group differences are not significant for both left and right PIC ROIs. (D) Left and (E) right PIC responses to stimulation were not associated with stimulation level. Images displayed at P < 0.001 uncorrected for visualization purpose. PIC: posterior insular cortex; L, left; R: right; ASD: autism spectrum disorder; HC: healthy control; n.s.: non significant; *: P < 0.05.
Skin conductance response and SCR-fMRI relationship
For evoked SCRs related to stimulation cue (i.e. cues predicting 50% of receiving a stimulation) and stimulation, contrary to our prediction, we did not observe a significant group difference in either stimulation cue-related SCR (Fig. 5A) or stimulation-related SCR (Fig. 5B; Ps > 0.1). We also explored the correlation between peripheral measures of arousal (SCR) and brain measures (fMRI). Although ASD and HC participants did not differ statistically in either SCR or anterior insula activity during pain anticipation, there was a significant correlation between SCR and fMRI parameter estimates of right AIC related to stimulation cue (Fig. 5C; r = 0.44, P = 0.015), suggesting that higher arousal predicted greater anterior insula activation during the anticipation of pain across both groups.
Figure 5.
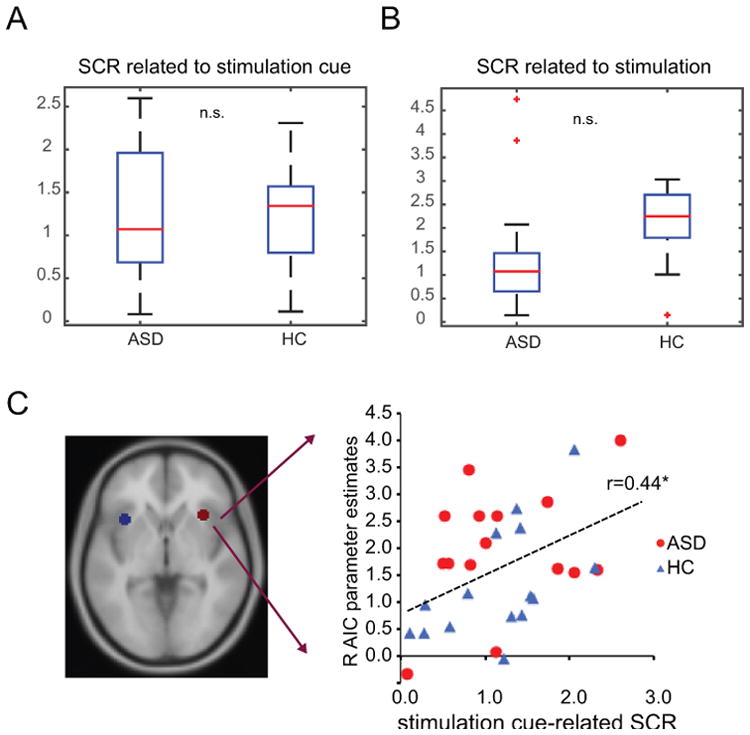
Skin conductance response (SCR). Group differences in SCR related to stimulation cue (A) and stimulation (B) did not reach statistical significance. (C) Activation in the right anterior insular cortex (AIC) correlated with SCR during the stimulation cue phase (r = 0.44). ASD: autism spectrum disorder; HC: healthy control; n.s.: non significant; *: P < 0.05.
Discussion
In this study, we examined the neural substrates underlying pain anticipation and perception in high-functioning adults with ASD. Compared to matched controls, we found increased behavioral sensitivity to anticipated pain in autistic individuals, marked by lower levels of stimulations chosen by the participant with ASD. We also found increased activation in rostral and dorsal ACC in response to pain anticipation (i.e. stimulation cue) in the ASD group. Greater ACC and insula activation correlated with lower trait empathy across groups. No group difference was found in neural responses to pain perception (i.e. stimulation). In addition, right AIC activation correlated with SCR during pain anticipation across both groups. These results suggest atypical behavioral and neural patterns associated with pain anticipation in individuals with ASD.
This is a study to systematically investigate the neural substrates of pain anticipation and perception with concurrent fMRI and SCR in high-functioning adults with ASD. Pain is an under-investigated area in research on autism. Clinical observations and case studies based on parental interviews often report pain insensitivity and self-injury behavior in ASD individuals [for review see (Allely, 2013)]. Experimental results, however, were not consistent with these observations. Behavioral expressions of increased pain sensitivity have been consistently reported in both children (Nader et al., 2004; Mandell et al., 2005) and adults (Cascio et al., 2008; Minio-Paluello et al., 2009) with ASD. In this study, we found that when asked to self-choose a stimulation level that would feel moderately painful to them (i.e. rated as 3 out of a 5-point scale), individuals with ASD chose a significantly lower level of stimulation compared to controls. This behavioral result is consistent with these previous experimental findings and further demonstrates that increased behavioral sensitivity of an upcoming painful stimulus is also evident in high-functioning adults with ASD.
In parallel with this behavioral finding, we found increased neural activation in the ACC during the stimulation cue period in the ASD group. This finding indicates hyperactivity in the ACC (both rostral and dorsal) in individuals with ASD when anticipating a painful stimulus. The ACC is involved in many affective and cognitive functions such as pain, negative affect, and cognitive control (Bush et al., 2000) and response anticipation (Fan et al., 2007). Although earlier theories propose that affective and cognitive processes can be localized in distinct subregions of the ACC (Bush et al., 2000), more recent findings suggest that these streams of information are more likely to be integrated in overlapping regions of the ACC (Shackman et al., 2011). Specifically, the part of the cingulate cortex encompassing the rACC and dACC regions defined in this study, has been proposed to subserve such integrative role (Shackman et al., 2011). In terms of pain perception, it has been shown that ACC encodes both pain anticipation (Sawamoto et al., 2000) and pain affect (Rainville et al., 1997; Wager et al., 2004). Although cognitive (i.e. expectation) and affective components (i.e. anxiety, fear) are not dissociable in our paradigm, the finding of comparable levels of SCRs in the ASD group suggest that negative affect is likely to be equivalent between groups. Thus, increased ACC activations in response to stimulation cue observed here is more likely to indicate heightened cognitive processing in the ASD group such as more mental effort or attention paid to the stimuli. One limitation of the current design is that we cannot tease apart uncertainty from saliency, because the stimulation cue is both more uncertain (50% vs. 100% predictability) and more salient (pain vs. no pain) than the safe cue. Thus, one alternative explanation is that increased ACC activation reflects enhanced uncertainty processing in ASD. This is consistent with the well-documented phenomenon of intolerance of uncertainty in ASD (Boulter et al., 2014; Wigham et al., 2015; Hodgson et al., 2016). Future studies dissociating uncertainty and salience will be required to address this question further.
Interestingly, we did not find increased AIC activation or SCR in the ASD group in response to stimulation cue as predicted. In a previous study using an empathetic pain paradigm in the same ASD sample, we observed both increased AIC activation (but not ACC) and SCR associated with observing others people's pain in the ASD group (Gu et al., 2015). Comparing these two sets of results, we believe that the specific functions and dysfunctions of ACC and AIC in ASD are dissociable. Although the ACC and AIC are often co-activated in fMRI studies such as those using empathy-for-pain paradigms, our previous studies suggest that when controlling for cognitive load, only AIC, but not ACC, is activated when observing others' pain in healthy controls (Gu et al., 2010; Gu et al., 2015). Importantly, AIC activation correlated with SCR in the current study, suggesting that AIC activity reflected arousal and autonomic response. On the other hand, ACC, but not AIC, is often implicated in uncertainty processing and effortful control (Shenhav et al., 2016). Therefore, we speculate that in the current study, ASD individuals might find the stimulation cues more uncertain and need to invest more effort in processing these aversive cues, but have normal levels of arousal. In the previous empathetic pain paradigm (Gu et al., 2015), possibly because there was no manipulation of cue uncertainty, ASD individuals did not show greater ACC response, but instead exhibited more arousal (i.e. higher SCR) and AIC activation when observing others' pain.
It is worth noting that increased behavioral sensitivity and neural responses to pain anticipation in the current study do not contradict findings of reduced behavioral sensitivity to other people's emotional states (Baron-Cohen & Wheelwright, 2004; Gu et al., 2015). One could have increased sensitivity to self-pain but yet cannot correctly evaluate other people's pain due to impaired social cognitive abilities, which is likely to be the case in ASD (Baron-Cohen & Wheelwright, 2004; Fan et al., 2014; Gu et al., 2015). One interesting finding from the current study is that trait empathy is inversely correlated with brain responses to pain anticipation in the ACC and AIC across all participants. In other words, the greater ACC/AIC response to pain anticipation (i.e. mostly ASD participants), the less empathetic the subject is behaviorally. This finding suggests that the encoding of one's own emotional states and that of another person's emotional states are two closely-related processes, but pointing to the opposite direction in the case of ASD.
Lastly, we did not find increased SCR and activation in the PIC or any other brain region associated with pain processing per se (i.e. stimulation) in the ASD group. We also examined the correlation between stimulation level and PIC activation and found no significant correlation in ASD or in HC groups. Although we matched for subjective experience of pain between the two groups, the primary afferent and subsequent nociceptive inputs were, by design, of a lower level for the ASD group relative to the control group. This could also potentially account for our failure to demonstrate significant difference in response to the two levels of stimulation used by ASD and HC groups. Nevertheless, because the stimulation levels were chosen by the participants themselves, this group difference in stimulation level suggests the sensitivity to ascending pain signals (e.g. in PIC) was greater in the ASD group relative to the HC group. These results, in combination with the differential responses to anticipatory cues, are consistent with earlier formulations of ASD in terms of impaired interoceptive inference (Lawson et al., 2014; Quattrocki & Friston, 2014). In other words, it has been previously suggested that one of the core deficits in ASD involves a failure to attenuate the precision or gain of interoceptive signals like pain (Lawson et al., 2014; Quattrocki & Friston, 2014; Gu et al., 2015). This notion is consistent with an abnormally high response to anticipatory cues that presumably reflect attentional set in relation to upcoming interoception – and a relatively greater posterior insular response to a given level of noxious stimulation. In short, ASD participants might be construed as having an abnormal attentional orientation to nociceptive signals that, in the setting of interoceptive inference and predictive coding, translates into a failure of sensory attenuation and improper gain or gating control.
In conclusion, our results support the hypothesis that high-functioning adults with ASD present with heightened behavioral and neural responses to pain anticipation. While the current study is limited by small sample size, it provides a systematic investigation into the neural, autonomic, and behavioral responses in pain anticipation and perception in high-functioning adults with ASD.
Acknowledgments
This work was supported by the National Institute of Health (NIH) Grant R21 MH083164. XG is supported by a faculty startup grant from UT Dallas and the Dallas Foundation. TJZ is supported by the National Institute of General Medical Sciences (NIGMS) T32GM074905. The funders have no role in the design of this study.
Footnotes
Data Accessibility: De-identified data from this study are currently hosted by the authors' local server and will be shared with the research community upon request.
Competing Interest: The authors declare no conflict of interest.
References
- Allely CS. Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. ScientificWorldJournal. 2013;2013:916178. doi: 10.1155/2013/916178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM-IV-TR®. American Psychiatric Association; 2000. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Pub; 2013. [DOI] [PubMed] [Google Scholar]
- Bach DR, Flandin G, Friston KJ, Dolan RJ. Modelling event-related skin conductance responses. Int J Psychophysiol. 2010;75:349–356. doi: 10.1016/j.ijpsycho.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133:1515–1525. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Boulter C, Freeston M, South M, Rodgers J. Intolerance of uncertainty as a framework for understanding anxiety in children and adolescents with autism spectrum disorders. J Autism Dev Disord. 2014;44:1391–1402. doi: 10.1007/s10803-013-2001-x. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cascio C, McGlone F, Folger S, Tannan V, Baranek G, Pelphrey KA, Essick G. Tactile perception in adults with autism: a multidimensional psychophysical study. J Autism Dev Disord. 2008;38:127–137. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi-Dell'Acqua C, Hofstetter C, Vuilleumier P. Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. J Neurosci. 2011;31:17996–18006. doi: 10.1523/JNEUROSCI.2686-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord. 2012;42:147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam-Stock T, Xu P, Cao M, Gu X, Van Dam NT, Anagnostou E, Kolevzon A, Soorya L, Park Y, Siller M, He Y, Hof PR, Fan J. Abnormal autonomic and associated brain activities during rest in autism spectrum disorder. Brain. 2014;137:153–171. doi: 10.1093/brain/awt294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. An information theory account of cognitive control. Front Hum Neurosci. 2014;8:680. doi: 10.3389/fnhum.2014.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, McCandliss BD. Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study. J Neurosci. 2007;27:2272–2282. doi: 10.1523/JNEUROSCI.3470-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Xu P, Van Dam NT, Eilam-Stock T, Gu X, Luo YJ, Hof PR. Spontaneous brain activity relates to autonomic arousal. J Neurosci. 2012;32:11176–11186. doi: 10.1523/JNEUROSCI.1172-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YT, Chen C, Chen SC, Decety J, Cheng Y. Empathic arousal and social understanding in individuals with autism: evidence from fMRI and ERP measurements. Soc Cogn Affect Neurosci. 2014;9:1203–1213. doi: 10.1093/scan/nst101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn FG, Benson DF, Ardila A. Anatomy of the insula - functional and clinical correlates. Aphasiology. 1999;13:55–78. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RSJ. Characterizing Evoked Hemodynamics with Fmri. Neuroimage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Gu X, Eilam-Stock T, Zhou T, Anagnostou E, Kolevzon A, Soorya L, Hof PR, Friston KJ, Fan J. Autonomic and brain responses associated with empathy deficits in autism spectrum disorder. Hum Brain Mapp. 2015;36:3323–3338. doi: 10.1002/hbm.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Liu X, Guise KG, Naidich TP, Hof PR, Fan J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. J Neurosci. 2010;30:3739–3744. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson AR, Freeston MH, Honey E, Rodgers J. Facing the Unknown: Intolerance of Uncertainty in Children with Autism Spectrum Disorder. J Appl Res Intellect Disabil. 2016 doi: 10.1111/jar.12245. [DOI] [PubMed] [Google Scholar]
- Jones AK, Friston K, Frackowiak RS. Localization of responses to pain in human cerebral cortex. Science. 1992;255:215–216. doi: 10.1126/science.1553549. [DOI] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lawson RP, Rees G, Friston KJ. An aberrant precision account of autism. Front Hum Neurosci. 2014;8:302. doi: 10.3389/fnhum.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mandell DS, Novak MM, Zubritsky CD. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005;116:1480–1486. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minio-Paluello I, Baron-Cohen S, Avenanti A, Walsh V, Aglioti SM. Absence of embodied empathy during pain observation in Asperger syndrome. Biol Psychiatry. 2009;65:55–62. doi: 10.1016/j.biopsych.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Nader R, Oberlander TF, Chambers CT, Craig KD. Expression of pain in children with autism. Clin J Pain. 2004;20:88–97. doi: 10.1097/00002508-200403000-00005. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Quattrocki E, Friston K. Autism, oxytocin and interoception. Neurosci Biobehav Rev. 2014;47:410–430. doi: 10.1016/j.neubiorev.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci. 2004;24:7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Shackman AJ, Davidson RJ. Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. J Cogn Neurosci. 2007;19:993–1003. doi: 10.1162/jocn.2007.19.6.993. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20:7438–7445. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. 2016;19:1286–1291. doi: 10.1038/nn.4384. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Botbol M, Brailly-Tabard S, Perez-Diaz F, Graignic R, Carlier M, Schmit G, Rolland AC, Bonnot O, Trabado S, Roubertoux P, Bronsard G. Pain reactivity and plasma beta-endorphin in children and adolescents with autistic disorder. PLoS One. 2009;4:e5289. doi: 10.1371/journal.pone.0005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Sikes RW. The medial pain system, cingulate cortex, and parallel processing of nociceptive information. Prog Brain Res. 2000;122:223–235. doi: 10.1016/s0079-6123(08)62141-x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence test. San Antonio, Tex: Psychological Corporation; 1997. [Google Scholar]
- Wigham S, Rodgers J, South M, McConachie H, Freeston M. The interplay between sensory processing abnormalities, intolerance of uncertainty, anxiety and restricted and repetitive behaviours in autism spectrum disorder. J Autism Dev Disord. 2015;45:943–952. doi: 10.1007/s10803-014-2248-x. [DOI] [PubMed] [Google Scholar]


