Abstract
The development of broad-spectrum, host-acting antiviral therapies remains an important but elusive goal in anti-infective drug discovery. To replicate efficiently, viruses not only depend on their hosts for an adequate supply of pyrimidine nucleotides, but also up-regulate pyrimidine nucleotide biosynthesis in infected cells. In this review, we outline our understanding of mammalian de novo and salvage metabolic pathways for pyrimidine nucleotide biosynthesis. The available spectrum of experimental and FDA-approved drugs that modulate individual steps in these metabolic pathways is also summarized. The logic of a host-acting combination antiviral therapy comprised of inhibitors of dihydroorotate dehydrogenase and uridine/cytidine kinase is discussed.
Introduction
Pyrimidine nucleosides are heterocyclic aromatic metabolites that include uridine, cytidine and thymidine. In addition to their fundamental role in nucleic acid biosynthesis, they are required for carbohydrate and lipid metabolism. For example, a number of glycosyltransferases utilize UDP-sugars, while CDP-diacylglycerol is an intermediate in the biosynthesis of glycerophospholipids. Although pyrimidine analogs such as azidothymidine (AZT), 5-fluorouracil (5-FU), and arabinosylcytosine (ara-C) have been used to target HIV reverse transcriptase or as anti-cancer chemotherapeutic drugs for decades, the potential for rationally targeting human pyrimidine nucleoside metabolism for antiviral chemotherapy has not been generally recognized. Here we review the rationale for such a chemotherapeutic strategy as well as the relevant features of mammalian pyrimidine nucleoside metabolism and its regulation.
Pyrimidine nucleotide biosynthesis through de novo and salvage pathways
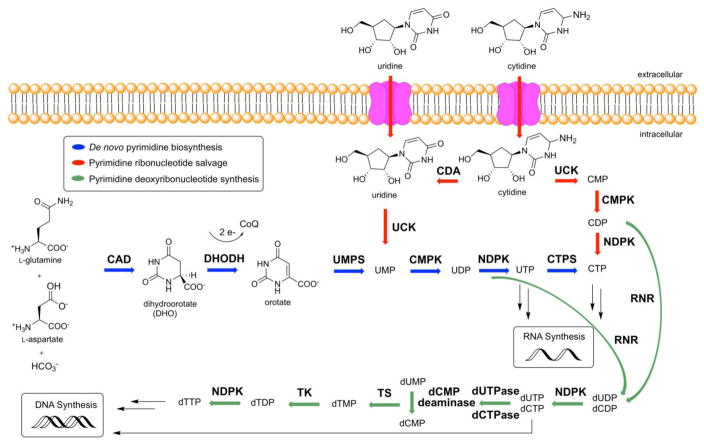
Mammalian cells derive pyrimidine nucleotides through a combination of de novo biosynthesis and salvage [1]. De novo biosynthesis is initiated by a multifunctional enzyme (CAD) harboring carbamoyl phosphate synthase, aspartate transcarbamoylase, and dihydroorotase activities [2]. CAD uses an equivalent of L-glutamine, aspartate, and bicarbonate along with two equivalents of ATP to make dihydroorotate (DHO) (Figure 1). A mitochondrial membrane protein, dihydroorotate dehydrogenase (DHODH), then reduces DHO to orotic acid while transferring 2e− to Coenzyme Q (CoQ, ubiquinone) [3]. Not only does DHODH catalyze the first committed step in de novo pyrimidine nucleoside biosynthesis, but it also links this pathway to the electron transport chain of aerobic respiration. Orotic acid is converted into uridine monophosphate (UMP) by a bifunctional protein, uridine monophosphate synthetase (UMPS). The N-terminal domain of UMPS transforms orotic acid into orotidylate (OMP) using phospho-α-Dribosyl-1-pyrophosphate (PRPP) as a cosubstrate, while its C-terminal OMP decarboxylase converts OMP into UMP [4]. UDP and UTP are synthesized by cytidine monophosphate kinase (CMPK) and nucleoside-diphosphate kinase (NDPK), respectively [5,6]. UTP is converted into CTP by CTP synthetase (CTPS) in an ATP dependent reaction that uses glutamine as an amine donor [7]. Alternatively, UDP and CDP are deoxygenated into deoxy-UDP (dUDP) and dCDP, respectively, by ribonucleotide reductase (RNR), and further phosphorylated by NDPK [8]. To avoid misincorporation into DNA, dUTP is rapidly broken down by dUTPase into dUMP. dUMP is a substrate of thymidylate synthase, yielding deoxy-TMP (dTMP) that can be phosphorylated into dTTP [9]. Thus, the de novo biosynthetic pathway in mammals is capable of supplying all pyrimidine ribonucleotides (CTP, UTP) and deoxyribonucleotides (dCTP, dTTP) for RNA and DNA biosynthesis, respectively.
Figure 1. De novo and salvage biosynthesis of pyrimidine nucleotides in humans.
For details, see text
In addition to de novo biosynthesis, pyrimidine nucleotides can also be salvaged from intracellular nucleic acid degradation or from extracellular nucleosides, which circulate in the bloodstream. The latter pathway depends on several nucleoside transport channels and pumps in mammalian cells. The relative importance of de novo biosynthesis and salvage varies from organ to organ and is also highly dependent on the physiological state of cells. RNA catabolism yields UMP and CMP, which can be converted into the corresponding NTPs via the successive action of CMPK1 and NDPK. With a plasma concentration of ~5 μM, uridine is the dominant circulatory nucleoside in mammals [10]; the plasma concentrations of all other pyrimidine nucleosides are at least an order of magnitude lower [11], and are therefore insufficient to support cellular demands of the corresponding nucleotides via direct salvage. Uridine/cytidine kinase (UCK) converts transported pyrimidine nucleosides into the corresponding NMPs, which can be further phosphorylated and modified as discussed above. Since both de novo biosynthesis as well as intracellular and extracellular salvage require CMPK1 activity, this enzyme is essential for pyrimidine utilization in all cells.
As an alternative to salvage, pyrimidine nucleosides can also be irreversibly degraded. Uridine and cytidine catabolism is initiated by the action of uridine phosphorylase (UPase) and cytidine deaminase, respectively, giving rise to uracil, while thymidine phosphorylase releases thymine from thymidine. In principle, these phosphorylases can also catalyze the reverse reactions to convert circulatory bases into nucleosides (as in OMP biosynthesis), although mammals appear to predominantly utilize these enzymes in the catabolic direction [12].
Intracellular regulation of pyrimidine nucleotide biosynthesis
The multifunctional CAD protein is the primary site for regulation of de novo pyrimidine biosynthesis. Transcription factors such as Myc are known to induce its gene expression [13]. The enzyme is activated by MAP kinase-catalyzed phosphorylation before the S-phase of the cell cycle, and is inhibited by protein kinase A-catalyzed phosphorylation at a distinct site at the end of S-phase [14,15]. CAD is also activated by phosphorylation at a third site by the mammalian target of rapamycin complex 1 (mTORC1) or the ribosomal protein S6 kinase 1 (p70S6K), thus enabling post-translational control in response to increased anabolic activity in the cell [16,17].
The importance of coordinately regulating intracellular pyrimidine nucleotide biosynthesis at multiple sites is underscored by our recent observation that genetic knockout of a negative regulator of mTORC1 activity sensitizes cells to pharmacological inhibition of DHODH with the small molecule GSK983 [3]. Similarly, the activities of mTORC1 and p70S6K are post-translationally regulated in response to the extracellular availability of uridine [18].
The activity of UCK, which plays a pivotal role in pyrimidine nucleoside salvage, is also subject to both negative regulation by CTP and UTP (i.e., the ultimate pathway products) and positive regulation by ATP. Such dual control is achieved through changes in the quaternary structure of UCK; CTP and UTP are competitive inhibitors (Ki ~ 6 μM [19]) that stabilize its inactive monomeric state, whereas ATP allosterically stabilizes UCK as an active tetramer [20].
Finally, CMPK1, which sits at the crossroads between de novo biosynthesis and intracellular/extracellular salvage is subject to feedback regulation of its activity by CTP, UTP and dCTP, but not dTTP [5]. Moreover, in vitro analysis has revealed a need for reducing agents to maintain its catalytic activity, suggesting that the intracellular redox potential may also play a significant role in metabolic flux control at this step [5].
Regulation of uridine concentration in the bloodstream
As discussed above, uridine plays a unique role as a reservoir of circulating pyrimidine nucleosides in mammals. Its plasma concentration is therefore subject to tight regulation. Indeed, plasma uridine levels are maintained within a narrow range in healthy humans even after fasting [21] or uridine administration [22]. The role of plasma uridine as a system-wide control variable is further underscored by two observations. First, oral administration of large doses of CDP-choline, a bioavailable form of cytidine, increased plasma uridine levels without significantly altering those of either cytidine or choline [22]. Second, a sharp increase in systemic uridine demand has a relatively modest effect on the concentration of plasma uridine, presumably due to its replenishment from reservoir organ(s). For example, blocking de novo pyrimidine synthesis by DHODH inhibition (in mice [23]) or CAD inhibition (in humans [24]) results in markedly higher use of the salvage pathway but only modest perturbation of plasma uridine levels.
The liver has been suggested as a potential site for regulating plasma uridine based on the observation that uridine is cleared in a single pass through the liver and is replaced by de novo synthesized uridine also from the liver [25], but the mechanistic logic of this unusual exchange process is unknown. Oral administration of glucose also increases the levels of uridine in the bloodstream [26], presumably due to the acute need for UDP-glucose during glycogen synthesis in the liver and muscle [27].
Plasma uridine levels are also regulated by the degradative activity of UPase, as well as by cellular uptake mechanisms that involve both facilitated diffusion and Na+-dependent active transport. Genetic and pharmacological inhibition of UPase in mice led to a major elevation of uridine concentrations in the blood (6-fold), lung and gut (5 to 6-fold), and liver and kidney (2 to 3-fold) [12]. Cellular uptake of uridine can be promoted via several nucleoside transporters [28].
Pharmacological tools to modulate pyrimidine nucleotide biosynthesis in humans
Due to their diverse metabolic roles, pyrimidine nucleotide biosynthesis inhibitors have been used to treat a variety of diseases. Many such drugs are nucleoside analogs. Once transported into the cells by facilitated diffusion, they are phosphorylated and either incorporated into DNA or RNA, or they can inhibit host or pathogen enzymes such as polymerases. Table 1 lists approved or experimental drugs that modulate de novo biosynthetic or salvage pathways in humans.
Table 1. Modulators of Pyrimidine Nucleotide Biosynthesis.
Acronyms are defined in the text.
| Drug name | Mode of action | Status | On- & off-target effects | Clinical use |
|---|---|---|---|---|
| De novo pathway | ||||
|
| ||||
| N-phosphonacetyl-L-aspartate (PALA) | Inhibits CAD | Not approved | Inhibits DNA synthesis | - |
| Leflunomide | Inhibits DHODH | FDA-approved | Inhibits DNA synthesis, liver problems, flu symptoms, diarrhea | Rheumatoid arthritis and multiple sclerosis |
| Brequinar | Inhibits DHODH | FDA-approved | Inhibits DNA synthesis, leukocytopenia, thrombocytopenia | Rheumatoid arthritis and multiple sclerosis |
| Pyrazofurin | Inhibits OMP decarboxylase | Not approved | Inhibits DNA synthesis, myelosuppression, stomatitis | Phase I/II clinical trials for various cancers |
|
| ||||
| Shared salvage | ||||
|
| ||||
| Dipyridamole | Inhibits nucleoside transporters (ENT1–4) & phosphodiesterase | FDA-approved | Increases cAMP and cGMP levels in platelets, vasodilation | Anti-platelet |
| Dilazep | Inhibits nucleotide transporter (ENT1) | Disturbances, allergic reactions, mouth ulcers, headache | Stroke | |
|
| ||||
| NTP-specific | ||||
|
| ||||
| Cyclopentenyl cytosine (CPE-C) | Inhibitor of CTP synthetase | Not approved (Phase I) | The depletion of CTP and dCTP pools | Anticancer, antiviral |
| Diazo-5-oxo-L-norleucine (DON) | Inhibitor of CAD, CTP synetthase, GMP synthetase | Not approved (Phase I/II) | Inhibits glutaminolysis, uric acid synthesis | Anticancer |
|
| ||||
| dNTP-specific | ||||
|
| ||||
| Fludarabine | Inhibits RNR, DNA polymerase, primase | FDA-approved | Inhibits DNA synthesis, causes lymphopenia | Acute leukemias, lymphoproliferative disorders |
| Cladribine | Inhibits RNR, DNA polymerase | FDA-approved for cancer | Inhibits DNA synthesis, myelosuppression, rashes, and nausea | Acute leukemia, lymphoproliferative disorders |
| Gemcitabine | Inhibits RNR, DNA synthesis | FDA-approved | Inhibits DNA synthesis, bone marrow suppression, nausea, fever, hair loss | Ovarian, breast, non-small cell lung, pancreatic cancer |
| Clofarabine | Inhibits RNR, DNA polymerases | FDA-approved | Tumor lysis, inflammation, dehydration, low blood pressure | Acute lymphoblastic leukemia |
| Fluorouracil (5-FU, prodrug capecitabine & floxuridine) | Inhibits thymidylate synthase | FDA-approved | Toxicity in patients with DPD deficiency, nausea, vomiting & diarrhea | Colon, esophageal, gastric, pancreatic, breast, & cervical cancers |
| Trifluridine | Inhibits thymidylate synthase (TS) and DNA synthesis | FDA-approved as eye-drops, | Transient burning, stinging, local irritation of the eyelids | Herpes simplex virus, vaccinia virus in eye |
| TAS-114 | dUTPase inhibitor, DPD inhibitor | Not approved (Phase I/II) | Enhancer of fluoropyrimidines | Non-small cell lung cancer |
Modulators of the de novo pathway
Although there are no FDA-approved inhibitors of CAD, N-phosphonacetyl-L-aspartate (PALA) is a bisubstrate analog inhibitor of aspartyl transcarbamoylase that has been introduced into human clinical trials. It failed to show efficacy as monotherapy or in combination with other agents in Phase II clinical trials on cancer patients [29–31]. In contrast, DHODH inhibitors such as teriflunomide (or its prodrug, leflunomide) and brequinar have been successfully used as immunosuppressive agents in rheumatoid arthritis and multiple sclerosis patients. The clinical benefit of DHODH inhibitors is thought to arise from reduced proliferation of activated T and B lymphocytes by decreasing pyrimidine pools in both cell types [32]. Prolonged administration of both leflunomide and brequinar causes hepatic microvesicular steatosis (a.k.a., lipid accumulation in the liver), a condition that can also be induced by artificially manipulating plasma uridine levels and is reversed by exogenous uridine administration [33,34]. DHODH inhibitors were also recently shown to initiate differentiation in multiple acute myeloid leukemia (AML) subtypes [35].
Inhibitors of CAD and DHODH have broad-range anticancer and antiviral effects in vitro [3,36], and have been tested in a range of clinical trials [37,38]. However, in vitro efficacy has not, for the most part, been translated in vivo, presumably due to the ability of the body to maintain a robust and relatively constant uridine supply for cellular salvage of pyrimidine nucleotides. The reason why uridine salvage is unable to neutralize the immunosuppressant effects of DHODH inhibitors is unknown. It is possible that the salvage pathway is less dominant in lymphocytes compared to the de novo synthesis pathway.
Pyrazofurin (PZF) is a nucleoside analog that inhibits OMP decarboxylase in the de novo pathway. It has been tested in clinical trials for the treatment of various cancers but failed to proceed beyond phase II trials due to toxicity and lack of efficacy [39].
Modulators of shared (d)NTP salvage steps
Nucleoside transport through membrane channels and pumps is the first step in pyrimidine salvage. Because these transporters recognize all four bases, their inhibition disturbs both dNTP and NTP salvage. FDA-approved nucleoside channel inhibitors such as dipyridamole (DP) [40] and dilazep [41] have been used to treat stroke due to their ability to block adenosine uptake by platelets, endothelial cells, and erythrocytes [42].
Modulators of the NTP-specific steps
Cyclopentenyl cytosine (CPE-C), a pyrimidine analogue, is an inhibitor of CTP synthetase, and has both antiviral and anticancer activity. Because CTP synthetase activity is upregulated in many cancers [43], CPE-C was tested in a Phase I clinical trial for solid tumors [44], where 5 out of 26 patients experienced unexplained cardiotoxicity. CPE-C has also shown significant activity against both DNA and RNA viruses in vitro including HSV and influenza virus (Hong Kong flu) [45].
Diazo-5-oxo-L-norleucine (DON) is a glutamine mimic that inhibits several enzymes involved in nucleotide biosynthesis including CAD, CTP synthetase, and guanosine monophosphate synthetase. Although DON was tested in phase I/II clinical trials for the treatment of cancer [46,47], its therapeutic index was inadequate for further development.
Notwithstanding the potential to modulate pyrimidine nucleotide biosynthesis by targeting the kinases in the salvage pathway, to our knowledge none of these enzymes (UCK, CMPK, NDPK) have inhibitors that have entered human clinical trials.
Modulators of the dNTP specific steps
Ribonucleotide reductase (RNR) catalyzes a crucial step of de novo DNA synthesis by converting ribonucleoside diphosphates (ADP, GDP, UDP, CDP) into their deoxyribonucleoside counterparts. Because tight control of the dNTP pool is essential for cellular homeostasis, RNR inhibitors have been widely used to treat cancers. They include fludarabine [48–50], cladribine [51], gemcitabine [52–54], and clofarabine [55], although these compounds also block other steps in DNA synthesis in addition to RNR activity (Table 1).
Thymidylate synthase, followed by nucleoside diphosphate kinase and UTPase [56], has been targeted by the widely used nucleoside analog 5-fluorouracil (5-FU) and its prodrug, capecitabine [57]. Inside the cell 5-FU is processed into 5-fluoro-2′-deoxyuridine monophosphate (FdUMP), a covalent inhibitor of thymidylate synthase. Drug toxicity is mitigated with a recently FDA-approved uridine prodrug, uridine triacetate [58]. Alternatively, clinical trials have also been conducted to mitigate the toxicity of 5-FU by co-administration with dipyridamole [59]. A related molecule, trifluorothymidine, is also believed to inhibit thymidylate synthase [60] in addition to blocking viral replication or cell growth by incorporation into viral or host DNA, respectively [61]. It is used in eye drops for the treatment of herpes virus, and is also undergoing clinical trials for metastatic colorectal, colon cancers, and solid tumors.
Thymidine kinase, which converts dTMP into dTDP, is also an important therapeutic target, because it facilitates the incorporation of unnatural thymidine analogs into DNA. Examples of clinically useful thymidine kinase inhibitors include AZT and stavudine (anti-HIV), and idouridine (anti-herpes).
The enzyme dUTPase, which converts dUTP into dUMP and pyrophosphate, is inhibited by TAS-114, a first-in-class oral fluoropyrimidine that prevents the degradation of another fluoropyrimidine used in combination [62]. TAS-114 also moderately inhibits dihydropyrimidine dehydrogenase, the initial step in pyrimidine catabolism. Analogously, tipiracil, a thymidine phosphorylase inhibitor, is also clinically used to prevent the catabolism of other fluoropyrimidine nucleoside drugs including trifluorothymidine [63]. Curiously, analogous drugs that block the catabolism of therapeutic cytidine analogs have not yet been developed [64].
Implications for antiviral chemotherapy
Whereas most drugs that block pyrimidine nucleotide biosynthesis are targeted at cancer chemotherapy or immunosuppression, a deeper understanding of these metabolic pathways in humans could also be the foundation for the design of novel antiviral therapies. When viruses infect host cells, they up-regulate nucleotide biosynthetic flux [65]. Therefore, not only would inhibitors of nucleotide biosynthesis have the potential to neutralize a wide range of viruses, but their likelihood of eliciting drug-resistant mutants may also be lower than drugs targeted at viral proteins.
Although inhibitors of de novo pyrimidine nucleotide synthesis are known to exhibit broad-spectrum antiviral activity in vitro [66,67], they are ineffective in vivo due to efficient salvage of exogenous uridine. In this regard, our recent discovery that blocking the UCK isozyme, UCK2, sharply sensitizes cells toward DHODH inhibitors in the presence of a non-limiting uridine supply opens a new door for designing a combination antiviral agent comprised of a DHODH and a UCK2 inhibitor antiviral [3]. Inhibition of both the de novo and the salvage pyrimidine synthesis could be particularly effective at limiting the fast proliferation of RNA viruses. In fact, a combination regimen containing PALA and dipyridamole has been tested in clinical trials for the treatment of cancer, albeit with limited efficacy [68]. Weak activity could be due to inefficient inhibition of de novo pyrimidine synthesis by a CAD inhibitor as opposed to a DHODH inhibitor.
Since CMPK1 inhibition was also shown to sensitize cells to DHODH inhibitors [3], a similar outcome might also be achieved with a CMPK1 inhibitor. Indeed, given the location of CMPK1 at the convergence point of de novo biosynthesis and salvage, a sufficiently potent CMPK1 inhibitor could also be an effective form of monotherapy. However, unlike a DHODH/UCK2 combination agent, a CMPK1 inhibitor would also be expected to block the salvage of CMP and UMP derived from RNA degradation, and may therefore have a narrower therapeutic window. Future studies along either direction must await the development of medicinally appropriate small molecule inhibitors of UCK2 and CMPK1.
Highlights.
Human pyrimidine nucleotide biosynthesis has been targeted for the treatment of many diseases.
Chemotherapy combining DHODH and UCK inhibitors can be a broad-spectrum antiviral.
Targeting the host cell, such an antiviral therapy could mitigate resistant viruses.
Acknowledgments
Research in the authors’ laboratories on host-acting antiviral therapies is supported by a grant from the National Institutes of Health (U19 AI109662) to C.K. and M.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans DR, Guy HI. Mammalian pyrimidine biosynthesis: Fresh insights into an ancient pathway. The Journal of biological chemistry. 2004;279(32):33035–33038. doi: 10.1074/jbc.R400007200. [DOI] [PubMed] [Google Scholar]
- 2.Simmer JP, Kelly RE, Scully JL, Grayson DR, Rinker AG, Bergh ST, Evans DR. Mammalian aspartate transcarbamylase (atcase): Sequence of the atcase domain and interdomain linker in the cad multifunctional polypeptide and properties of the isolated domain. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(12):4382–4386. doi: 10.1073/pnas.86.12.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *3.Deans RM, Morgens DW, Okesli A, Pillay S, Horlbeck MA, Kampmann M, Gilbert LA, Li A, Mateo R, Smith M, Glenn JS, et al. Parallel shrna and crispr-cas9 screens enable antiviral drug target identification. Nature chemical biology. 2016;12(5):361–366. doi: 10.1038/nchembio.2050. Using parallel genome-wide high-coverage short hairpin RNA (shRNA) and clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 screens, the authors identify cellular target of small molecule GSK983 to be a pyrimidine biosynthesis enzyme dihydroorotate dehydrogenase (DHODH) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wittmann JG, Heinrich D, Gasow K, Frey A, Diederichsen U, Rudolph MG. Structures of the human orotidine-5′-monophosphate decarboxylase support a covalent mechanism and provide a framework for drug design. Structure (London, England : 1993) 2008;16(1):82–92. doi: 10.1016/j.str.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Liou JY, Dutschman GE, Lam W, Jiang Z, Cheng YC. Characterization of human ump/cmp kinase and its phosphorylation of d- and l-form deoxycytidine analogue monophosphates. Cancer Research. 2002;62(6):1624–1631. [PubMed] [Google Scholar]
- 6.Webb PA, Perisic O, Mendola CE, Backer JM, Williams RL. The crystal structure of a human nucleoside diphosphate kinase, nm23-h2. Journal of molecular biology. 1995;251(4):574–587. doi: 10.1006/jmbi.1995.0457. [DOI] [PubMed] [Google Scholar]
- *7.Martin E, Palmic N, Sanquer S, Lenoir C, Hauck F, Mongellaz C, Fabrega S, Nitschke P, Esposti MD, Schwartzentruber J, Taylor N, et al. Ctp synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature. 2014;510(7504):288–292. doi: 10.1038/nature13386. The authors showed that the disfunction of CTP synthase activity in humans was found to cause life-threatening immunodeficiency, characterized by an impaired capacity of activated T and B cells to proliferate in response to antigen receptor-mediated activation. This gene might represent a therapeutic target of immunosuppressive drugs that could specifically dampen lymphocyte activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotruvo JA, Stubbe J. Class i ribonucleotide reductases: Metallocofactor assembly and repair in vitro and in vivo. Annual review of biochemistry. 2011;80:733–767. doi: 10.1146/annurev-biochem-061408-095817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreras CW, Santi DV. The catalytic mechanism and structure of thymidylate synthase. Annual review of biochemistry. 1995;64:721–762. doi: 10.1146/annurev.bi.64.070195.003445. [DOI] [PubMed] [Google Scholar]
- 10.Zilly M, Langmann P, Winzer R, Benesic A, Schirmer D, Walker UA, Klinker H. Liquid chromatographic method for the determination of uridine in human serum. Journal of Chromatography B. 2004;803(2):345–351. doi: 10.1016/j.jchromb.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Traut TW. Physiological concentrations of purines and pyrimidines. Molecular and cellular biochemistry. 1994;140(1):1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 12.Cao DLJ, McCabe J, Kim B, Pizzorno G. Abnormalities in uridine homeostatic regulation and pyrimidine nucleotide metabolism as a consequence of the deletion of the uridine phosphorylase gene. J Biol Chem. 2005;280(22):21169–75. doi: 10.1074/jbc.M412343200. [DOI] [PubMed] [Google Scholar]
- 13.Boyd KE, Farnham PJ. Myc versus usf: Discrimination at the cad gene is determined by core promoter elements. Molecular and Cellular Biology. 1997;17(5):2529–2537. doi: 10.1128/mcb.17.5.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotsis DH, Masko EM, Sigoillot FD, Di Gregorio R, Guy-Evans HI, Evans DR. Protein kinase a phosphorylation of the multifunctional protein cad antagonizes activation by the map kinase cascade. Molecular and cellular biochemistry. 2007;301(1–2):69–81. doi: 10.1007/s11010-006-9398-x. [DOI] [PubMed] [Google Scholar]
- 15.Sigoillot FD, Kotsis DH, Masko EM, Bame M, Evans DR, Evans HI. Protein kinase c modulates the up-regulation of the pyrimidine biosynthetic complex, cad, by map kinase. Frontiers in bioscience : a journal and virtual library. 2007;12:3892–3898. doi: 10.2741/2358. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mtor and s6k1. Science (New York, NY) 2013;339(6125):1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Robitaille AM, Christen S, Shimobayashi M, Cornu M, Fava LL, Moes S, Prescianotto-Baschong C, Sauer U, Jenoe P, Hall MN. Quantitative phosphoproteomics reveal mtorc1 activates de novo pyrimidine synthesis. Science (New York, NY) 2013;339(6125):1320–1323. doi: 10.1126/science.1228771. By using quantitative phosphoproteomics, the authors showed mTORC1 indirectly phosphorylates CAD-S1859 through S6 kinase (S6K) and activates the de novo pyrimidine synthesis to control cell proliferation. [DOI] [PubMed] [Google Scholar]
- 18.Urasaki Y, Pizzorno G, Le TT. Uridine affects liver protein glycosylation, insulin signaling, and heme biosynthesis. PloS one. 2014;9(6):e99728. doi: 10.1371/journal.pone.0099728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson EP, Brockman RW. Feedback inhibition of uridine kinase by cytidine triphosphate and uridine triphosphate. Biochimica et biophysica acta. 1964;91:380–386. doi: 10.1016/0926-6550(64)90067-2. [DOI] [PubMed] [Google Scholar]
- 20.Cheng N, Payne RC, Traut TW. Regulation of uridine kinase. Evidence for a regulatory site. The Journal of biological chemistry. 1986;261(28):13006–13012. [PubMed] [Google Scholar]
- 21.Hamada T, Mizuta E, Yanagihara K, Kaetsu Y, Sugihara S, Sonoyama K, Yamamoto Y, Kato M, Igawa O, Shigemasa C, Inokuchi T, et al. Plasma levels of uridine correlate with blood pressure and indicators of myogenic purine degradation and insulin resistance in hypertensive patients. Circulation journal : official journal of the Japanese Circulation Society. 2007;71(3):354–356. doi: 10.1253/circj.71.354. [DOI] [PubMed] [Google Scholar]
- 22.Wurtman RJ, Regan M, Ulus I, Yu L. Effect of oral cdp-choline on plasma choline and uridine levels in humans. Biochemical pharmacology. 2000;60(7):989–992. doi: 10.1016/s0006-2952(00)00436-6. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q-Y, Bushell S, Qing M, Xu HY, Bonavia A, Nunes S, Zhou J, Poh MK, Florez de Sessions P, Niyomrattanakit P, Dong H, et al. Inhibition of dengue virus through suppression of host pyrimidine biosynthesis. Journal of Virology. 2011;85(13):6548–6556. doi: 10.1128/JVI.02510-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karle JM, Anderson LW, Erlichman C, Cysyk RL. Serum uridine levels in patients receiving n-(phosphonacetyl)-l-aspartate. Cancer Research. 1980;40(8 Pt 1):2938–2940. [PubMed] [Google Scholar]
- 25.Gasser T, Moyer JD, Handschumacher RE. Novel single-pass exchange of circulating uridine in rat liver. Science. 1981;213(4509):777–778. doi: 10.1126/science.7256279. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, Koyama H, Kurajoh M, Shoji T, Tsutsumi Z, Moriwaki Y. Biochemistry of uridine in plasma. Clinica Chimica Acta. 2011;412(19–20):1712–1724. doi: 10.1016/j.cca.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Gibson JB, Berry GT, Mazur AT, Palmieri MJ, Reynolds RA, Segal S. Effect of glucose and galactose loading in normal subjects on red and white blood cell uridine diphosphate sugars. Biochemical and molecular medicine. 1995;55(1):8–14. doi: 10.1006/bmme.1995.1025. [DOI] [PubMed] [Google Scholar]
- 28.Pizzorno G, Cao D, Leffert JJ, Russell RL, Zhang D, Handschumacher RE. Homeostatic control of uridine and the role of uridine phosphorylase: A biological and clinical update. Biochimica et biophysica acta. 2002;1587(2–3):133–144. doi: 10.1016/s0925-4439(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 29.Paridaens R, Mouridsen HT, Palshof T, Cocconi G, Van Oosterom A, Rotmensz N, Sylvester R, Heuson JC, Rozencweig M. N-(phosphonacetyl)-l-aspartate (pala) in advanced breast cancer: A phase ii trial of the eortc breast cancer cooperative group. European journal of cancer & clinical oncology. 1982;18(1):67–70. doi: 10.1016/0277-5379(82)90026-8. [DOI] [PubMed] [Google Scholar]
- 30.Wadler S, Gleissner B, Hilgenfeld RU, Thiel E, Haynes H, Kaleya R, Rozenblit A, Kreuser ED. Phase ii trial of n-(phosphonacetyl)-l-aspartate (pala), 5-fluorouracil and recombinant interferon-α-2b in patients with advanced gastric carcinoma. European Journal of Cancer. 1996;32(7):1254–1256. doi: 10.1016/0959-8049(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 31.Kleeberg UR, Mulder JH, Rümke P, Thomas D, Rozencweig M. N-(phosphonacetyl)-l-aspartate (pala) in advanced malignant melanoma: A phase ii trial of the eortc malignant melanoma cooperative group. European Journal of Cancer and Clinical Oncology. 1982;18(8):723–726. doi: 10.1016/0277-5379(82)90069-4. [DOI] [PubMed] [Google Scholar]
- 32.Palmer AM. Teriflunomide, an inhibitor of dihydroorotate dehydrogenase for the potential oral treatment of multiple sclerosis. Current opinion in investigational drugs (London, England : 2000) 2010;11(11):1313–1323. [PubMed] [Google Scholar]
- 33.Le TT, Ziemba A, Urasaki Y, Hayes E, Brotman S, Pizzorno G. Disruption of uridine homeostasis links liver pyrimidine metabolism to lipid accumulation. Journal of lipid research. 2013;54(4):1044–1057. doi: 10.1194/jlr.M034249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le TT, Urasaki Y, Pizzorno G. Uridine prevents tamoxifen-induced liver lipid droplet accumulation. BMC pharmacology & toxicology. 2014;15:27. doi: 10.1186/2050-6511-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Sykes DB, Kfoury YS, Mercier FE, Wawer MJ, Law JM, Haynes MK, Lewis TA, Schajnovitz A, Jain E, Lee D, Meyer H, et al. Inhibition of dihydroorotate dehydrogenase overcomes differentiation blockade in acute myeloid leukemia. Cell. 2016;167(1):171–186. e115. doi: 10.1016/j.cell.2016.08.057. Acute myeloid leukemia (AML), which is characterized by the arrest of leukemic myeloblasts at an immature stage of development, may be overcome by DHODH inhibitors such as brequinar (BRQ). The authors showed that BRQ treatment slowed tumor growth and extended survival while promoting cellular differentiation in leukemia mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyde PR, Moore DK, Pimentel DM, Blough HA. Evaluation of the antiviral activity of n-(phosphonoacetyl)-l-aspartate against paramyxoviruses in tissue culture and against respiratory syncytial virus in cotton rats. Antiviral research. 1995;27(1–2):59–69. doi: 10.1016/0166-3542(94)00080-r. [DOI] [PubMed] [Google Scholar]
- 37.Schwartsmann G, Dodion P, Vermorken JB, ten Bokkel Huinink WW, Joggi J, Winograd B, Gall H, Simonetti G, van der Vijgh WJF, van Hennik MB, Crespeigne N, et al. Phase i study of brequinar sodium (nsc 368390) in patients with solid malignancies. Cancer Chemotherapy and Pharmacology. 1990;25(5):345–351. doi: 10.1007/BF00686235. [DOI] [PubMed] [Google Scholar]
- 38.Natale R, Wheeler R, Moore M, Dallaire B, Lynch W, Carlson R, Grillo-Lopez A, Gyves J. Multicenter phase ii trial of brequinar sodium in patients with advanced melanoma. Annals of oncology : official journal of the European Society for Medical Oncology. 1992;3(8):659–660. doi: 10.1093/oxfordjournals.annonc.a058298. [DOI] [PubMed] [Google Scholar]
- 39.Lake-Lewin D, Myers J, Lee BJ, Young CW. Phase ii trial of pyrazofurin in patients with multiple myeloma refractory to standard cytotoxic therapy. Cancer treatment reports. 1979;63(8):1403–1404. [PubMed] [Google Scholar]
- 40.Kim HH, Liao JK. Translational therapeutics of dipyridamole. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(3):s39–42. doi: 10.1161/ATVBAHA.107.160226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takenaga M, Kitagawa H, Hirai A, Tamura Y, Yoshida S. Mechanism of anti-platelet aggregating action of dilazep. Journal of pharmacobio-dynamics. 1985;8(2):77–83. doi: 10.1248/bpb1978.8.77. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Zhou G, Zhou X, Zhou S. The efficacy and safety of aspirin plus dipyridamole versus aspirin in secondary prevention following tia or stroke: A meta-analysis of randomized controlled trials. Journal of the Neurological Sciences. 2013;332(1–2):92–96. doi: 10.1016/j.jns.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 43.Kizaki H, Williams JC, Morris HP, Weber G. Increased cytidine 5′-triphosphate synthetase activity in rat and human tumors. Cancer Research. 1980;40(11):3921–3927. [PubMed] [Google Scholar]
- 44.Politi PM, Xie F, Dahut W, Ford H, Kelley JA, Bastian A, Setser A, Allegra CJ, Chen AP, Hamilton JM, Arbuck SF, et al. Phase i clinical trial of continuous infusion cyclopentenyl cytosine. Cancer Chemotherapy and Pharmacology. 1995;36(6):513–523. doi: 10.1007/BF00685802. [DOI] [PubMed] [Google Scholar]
- 45.Shigeta S, Konno K, Yokota T, Nakamura K, De Clercq E. Comparative activities of several nucleoside analogs against influenza a, b, and c viruses in vitro. Antimicrobial agents and chemotherapy. 1988;32(6):906–911. doi: 10.1128/aac.32.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Earhart RH, Amato DJ, Chang AY, Borden EC, Shiraki M, Dowd ME, Comis RL, Davis TE, Smith TJ. Phase ii trial of 6-diazo-5-oxo-l-norleucine versus aclacinomycin-a in advanced sarcomas and mesotheliomas. Investigational new drugs. 1990;8(1):113–119. doi: 10.1007/BF00216936. [DOI] [PubMed] [Google Scholar]
- 47.Lynch G, Kemeny N, Casper E. Phase ii evaluation of don (6-diazo-5-oxo-l-norleucine) in patients with advanced colorectal carcinoma. American journal of clinical oncology. 1982;5(5):541–543. [PubMed] [Google Scholar]
- 48.Ricci F, Tedeschi A, Morra E, Montillo M. Fludarabine in the treatment of chronic lymphocytic leukemia: A review. Therapeutics and Clinical Risk Management. 2009;5:187–207. doi: 10.2147/tcrm.s3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christopherson RI, Mactier S, Almazi JG, Kohnke PL, Best OG, Mulligan SP. Mechanisms of action of fludarabine nucleoside against human raji lymphoma cells. Nucleosides, nucleotides & nucleic acids. 2014;33(4–6):375–383. doi: 10.1080/15257770.2013.863334. [DOI] [PubMed] [Google Scholar]
- 50.Wisitpitthaya S, Zhao Y, Long MJ, Li M, Fletcher EA, Blessing WA, Weiss RS, Aye Y. Cladribine and fludarabine nucleotides induce distinct hexamers defining a common mode of reversible rnr inhibition. ACS chemical biology. 2016;11(7):2021–2032. doi: 10.1021/acschembio.6b00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leist TP, Weissert R. Cladribine: Mode of action and implications for treatment of multiple sclerosis. Clinical neuropharmacology. 2011;34(1):28–35. doi: 10.1097/WNF.0b013e318204cd90. [DOI] [PubMed] [Google Scholar]
- 52.Artin E, Wang J, Lohman GJS, Yokoyama K, Yu G, Griffin RG, Bar G, Stubbe J. Insight into the mechanism of inactivation of ribonucleotide reductase by gemcitabine 5′-diphosphate in the presence or absence of reductant. Biochemistry. 2009;48(49):11622–11629. doi: 10.1021/bi901590q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Lohman GJ, Stubbe J. Mechanism of inactivation of human ribonucleotide reductase with p53r2 by gemcitabine 5′-diphosphate. Biochemistry. 2009;48(49):11612–11621. doi: 10.1021/bi901588z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H, Faber C, Uchiki T, Racca J, Dealwis C. Structures of eukaryotic ribonucleotide reductase i define gemcitabine diphosphate binding and subunit assembly. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(11):4028–4033. doi: 10.1073/pnas.0600440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aye Y, Stubbe J. Clofarabine 5′-di and -triphosphates inhibit human ribonucleotide reductase by altering the quaternary structure of its large subunit. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(24):9815–9820. doi: 10.1073/pnas.1013274108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu C-M, Yeh M-T, Tsao N, Chen C-W, Gao Q-Z, Chang C-Y, Lee M-H, Fang J-M, Sheu S-Y, Lin C-J, Tseng M-C, et al. Tumor cells require thymidylate kinase to prevent dutp incorporation during DNA repair. Cancer Cell. 2012;22(1):36–50. doi: 10.1016/j.ccr.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 57.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nature Reviews Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 58.Ison G, Beaver JA, David McGuinn W, Palmby TR, Dinin J, Charlab R, Marathe A, Jin R, Liu Q, Chen XH, Ysern X, et al. Fda approval: Uridine triacetate for the treatment of patients following fluorouracil or capecitabine overdose or exhibiting early-onset severe toxicities following administration of these drugs. Clinical Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-0638. [DOI] [PubMed] [Google Scholar]
- 59.Grem JL. Biochemical modulation of fluorouracil by dipyridamole: Preclinical and clinical experience. Seminars in oncology. 1992;19(2 Suppl 3):56–65. [PubMed] [Google Scholar]
- 60.Suzuki N, Emura T, Fukushima M. Mode of action of trifluorothymidine (tft) against DNA replication and repair enzymes. International journal of oncology. 2011;39(1):263–270. doi: 10.3892/ijo.2011.1003. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki N, Nakagawa F, Nukatsuka M, Fukushima M. Trifluorothymidine exhibits potent antitumor activity via the induction of DNA double-strand breaks. Experimental and therapeutic medicine. 2011;2(3):393–397. doi: 10.3892/etm.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saito K, Nagashima H, Noguchi K, Yoshisue K, Yokogawa T, Matsushima E, Tahara T, Takagi S. First-in-human, phase i dose-escalation study of single and multiple doses of a first-in-class enhancer of fluoropyrimidines, a dutpase inhibitor (tas-114) in healthy male volunteers. Cancer Chemotherphy Pharmacology. 2014;73(3):577–583. doi: 10.1007/s00280-014-2383-2. [DOI] [PubMed] [Google Scholar]
- 63.Longo-Munoz F, Argiles G, Tabernero J, Cervantes A, Gravalos C, Pericay C, Gil-Calle S, Mizuguchi H, Carrato-Mena A, Limon ML, Garcia-Carbonero R. Efficacy of trifluridine and tipiracil (tas-102) versus placebo, with supportive care, in a randomized, controlled trial of patients with metastatic colorectal cancer from spain: Results of a subgroup analysis of the phase 3 recourse trial. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2016 doi: 10.1007/s12094-016-1528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Llona-Minguez S, Höglund A, Jacques SA, Johansson L, Calderón-Montaño JM, Claesson M, Loseva O, Valerie NCK, Lundbäck T, Piedrafita J, Maga G, et al. Discovery of the first potent and selective inhibitors of human dctp pyrophosphatase 1. Journal of Medicinal Chemistry. 2016;59(3):1140–1148. doi: 10.1021/acs.jmedchem.5b01741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munger J, Bennett BD, Parikh A, Feng X-J, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nature Biotechnology. 2008;26(10):1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffmann H-H, Kunz A, Simon VA, Palese P, Shaw ML. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(14):5777–5782. doi: 10.1073/pnas.1101143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang QY, Bushell S, Qing M, Xu HY, Bonavia A, Nunes S, Zhou J, Poh MK, Florez de Sessions P, Niyomrattanakit P, Dong H, et al. Inhibition of dengue virus through suppression of host pyrimidine biosynthesis. Journal of virology. 2011;85(13):6548–6556. doi: 10.1128/JVI.02510-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Markman M, Chan TCK, Cleary S, Howell SB. Phase i trial of combination therapy of cancer with n-phosphanacetyl-l-aspartic acid and dipyridamole. Cancer Chemotherapy and Pharmacology. 1987;19(1):80–83. doi: 10.1007/BF00296262. [DOI] [PubMed] [Google Scholar]