Abstract
Objective
In a clinical trial examining daily clonidine as an adjunct to buprenorphine treatment for opioid dependence, we found that clonidine increased opioid abstinence and decoupled stress from craving. From a personalized-medicine perspective, the next step is to identify people for whom clonidine would be beneficial. To that end, using data from the same clinical trial, we examined the associations of daily-life activities with treatment success.
Methods
Outpatients (N=118) received clonidine (0.3 mg/day) or placebo during 18 weeks of buprenorphine treatment. Participants carried a smartphone that randomly prompted them 4 times per day to report their moods and activities. Using generalized linear mixed models, we assessed the likelihoods of different types of daily activity as a function of clonidine vs. placebo, days of longest continuous opioid abstinence, and their interaction.
Results
Participants in the buprenorphine-only (placebo plus clonidine) control group who engaged in more responsibilities (work and child/elder care) had longer streaks of abstinence, while those who engaged in more unstructured-time activities had shorter streaks of abstinence. Conversely, for participants in the buprenorphine-plus-clonidine group, longer streaks of abstinence were associated with higher frequencies of activities associated with unstructured time.
Conclusions
The study replicates findings that engaging in responsibilities is related to positive treatment outcomes in standard opioid agonist therapy. The pattern of results also suggests that clonidine helped participants engage in unstructured-time activities with less risk of craving or use than they might otherwise have had.
Keywords: Opioid Treatment, Ecological Momentary Assessment, Clonidine, Buprenorphine Treatment
Introduction
In a recently published clinical trial, we tested clonidine as an adjuvant medication for opioid relapse prevention during buprenorphine treatment, and we found that participants randomized to clonidine took longer to lapse and achieved greater durations of opioid abstinence (Kowalczyk et al. 2015). Using ecological momentary assessment (EMA) data from the same participants, we showed that clonidine helped to reduce the impact of stress on heroin craving. Participants given clonidine in conjunction with their buprenorphine reported less heroin craving at moderate levels of stress than did those taking placebo with their buprenorphine. However, like most medications, clonidine was not helpful for everyone who took it. This can be clearly seen in figure 2 of our original report (Kowalczyk et al. 2015) which displays the longest period of continuous abstinence for each participant. From these data points we can calculate that the number needed to treat (NNT) for prolonged opioid abstinence with clonidine was roughly 4.0. In what is being called an age of personalized medicine (Vaidyanathan 2012), a good next step would be to identify who is most likely to respond to clonidine.
The characteristics of treatment success have been explored within the context of standard opioid agonist maintenance. For example, patients report that positive outcomes are facilitated and sustained by greater engagement in familial and employment responsibilities (Notley et al. 2013). Using EMA data, we showed patients spent more time with their significant other and children when abstinent from cocaine (Epstein and Preston 2010). We have also shown that having a closer marital relationship predicts achievement of abstinence from opioids and cocaine during opioid agonist treatment (Heinz et al. 2009). Employment has been consistently predictive of success during opioid agonist treatment (Brewer et al. 1998) and can actually be used as an incentive for abstinence (Silverman et al. 2002, DeFulio et al. 2009). This is not just a byproduct of the need for income: our EMA data have shown that patients reported less cocaine and heroin craving, less stress, and better mood while they were at work than at all other times (Epstein and Preston 2012).
The complement to these findings is that unstructured time—time spent in behavior with no strict schedule or urgent goal—was associated with more craving, more stress, and worse mood, a phenomenon that we have begun to call “the risk of unstructured time.” This phrase suggests unidirectional causation, which we acknowledge is unlikely to be the case, but patients who relapse during treatment have frequently cited boredom, or unstructured time, as a cause (Bradley et al. 1989, McKay et al. 2006, Notley et al. 2015), and in our EMA data, we have shown prospectively that reports of having felt bored in the past hour increase linearly in the five hours leading up to cocaine use and heroin craving (Epstein et al. 2009). Taken together, these findings support the idea that unstructured time can cause problems during treatment.
In the current analyses, using data from our recent clinical trial (Kowalczyk et al. 2015), we sought to replicate the finding that good outcomes during standard opioid agonist therapy are associated with spending more time on responsibilities, and that the converse is true for unstructured time (Epstein et al. 2009, Epstein and Preston 2010, Epstein and Preston 2012). We also sought to determine whether randomization to adjuvant clonidine would change those associations, possibly reducing the risk of unstructured time.
Materials and Method
Participants
Participants were treatment-seeking heroin-dependent or prescription-opioid-dependent outpatient volunteers. Inclusion criteria were physical dependence on opioids and age between 18 and 60 years. Exclusion criteria were a current psychotic disorder, a history of bipolar disorder or schizophrenia, current major depression, current dependence on alcohol or sedatives, cognitive impairment that would preclude informed consent or valid self-report, pregnancy or breastfeeding, use of contraindicated medications, such as beta-blockers, and medical illness that would compromise participation.
Study Design
A more detailed description of the study design can be found in our report of the main outcomes (Kowalczyk et al. 2015). The study was an efficacy trial that tested clonidine as an adjuvant medication to buprenorphine for opioid relapse prevention, conducted at our outpatient treatment-research clinic in Baltimore. Participants attended the clinic 7 days per week for sublingual buprenorphine (buprenorphine alone: 8–24 mg/day), attended weekly counseling sessions for 28 weeks, and, thrice weekly, provided urine samples under observation that were tested for opioids, cocaine, marijuana, amphetamines, benzodiazepines, and barbiturates. Participants who demonstrated opioid abstinence in weeks 5 and 6 (six consecutive negative urine screens) were randomized to receive clonidine or placebo (p.o.) in conjunction with their daily buprenorphine. Participants randomized to clonidine were inducted onto a dose of up to 0.3mg over 2 weeks, and then maintained on that dose for a 12-week intervention phase. Participants continued on buprenorphine treatment for another 8 weeks (maintenance phase) after the end of the intervention phase, beginning with 2 weeks in which participants were slowly tapered off of clonidine. Participants then either transferred to another opioid agonist maintenance program or were tapered off of buprenorphine.
Ecological Momentary Assessment
During the second week of the induction phase, participants were issued a portable electronic device (PalmOne Zire 21, Palm Tungsten E2, or HTC TyTN II smartphone) to be used to capture EMA data. Participants completed prompts through the 12-week intervention phase and the 8-week maintenance phase. The device issued alerts at four randomly selected times during participants’ waking hours each day, prompting the participants to complete a series of questions about mood and craving (i.e., a random prompt entry). Each random prompt included the question “What were you doing when the beep occurred?” Participants were instructed to select all applicable responses. For the analyses reported here, we classified some responses as responsibilities (Working, Child/Elder Care, Chores/Hygiene) some as unstructured-time activities (Watching TV/Videos/DVD, Listening to Music, Talking Socializing, Talking on the Phone, Sports/Games/Recreation, Reading, Internet, Thinking, Resting/Sleeping, Waiting), and the rest as ambiguous (Shopping/Errands, Walking/Riding/Traveling, Eating or Preparing Food). These categories were not part of the way the activity items were presented to participants.
Data Analysis
To examine the relationship between activities and treatment outcome, we operationalized treatment outcome as the longest period of continuous opioid abstinence from induction onto placebo or clonidine through the intervention phase. The longest period of abstinence was calculated as the maximum number of days between positive urines in the intervention phase, or from the beginning of clonidine/placebo administration to the first positive urine, or from the first negative urine to the end of the intervention phase, whichever was longer.
In generalized linear mixed models (SAS Proc Glimmix), we used treatment group (clonidine or placebo) and days of continuous abstinence to predict a repeated dichotomous outcome—reporting yes or no to indicate engaging in a given activity. We first analyzed the concomitants of engaging in any activity of a category (i.e., any of the responsibilities, any of the unstructured-time activities, or any of the ambiguous activities). In these analyses, we used a Bonferroni correction to control for our making three comparisons, with differences considered significant when p ≤ 0.0167. In subsequent post-hoc analyses, we examined each of the individual activities for the categories with a significant interaction. This was done to determine which of the specific activities in the category were driving the effect and whether there were other patterns within those behaviors that might suggest additional hypotheses. As these analyses were exploratory, we did not correct for multiple comparisons.
All analyses used a first-order autoregressive error structure and included a control term for the number of random prompts completed by each participant. The models included all the data from the 12-week intervention phase. For all analyses, we used a two-tailed alpha of 0.05, except where Bonferroni-corrected as described above.
Results
Participants
Participants completed a total of 7,705 days of EMA and answered 24,322 random prompts in the intervention phase. This yields on average 3.16 prompts completed out of the 4 intended on average each day for a compliance percentage of 79%. Of the 118 participants randomized, ten did not provide data for this analysis (7 placebo and 3 clonidine) because they dropped out of the study either during or shortly after clonidine/placebo induction and never received a device for EMA. As reported in earlier work, the two randomized groups did not differ on any relevant factors assessed before randomization (Table 1: Kowalczyk et al. 2015) and this holds true for the slightly smaller sample (n = 108) who provided data for this analysis. Especially relevant to the groups’ access to unstructured activities, we found no pre-study differences in their number of paid work days in the last 30 (Clonidine 8.7 ± 9.0; Placebo 8.4 ± 9.2, t(1,106) = 0.21, p = 0.84) or in marital status (Never Married: Clonidine 65.5%; Placebo 66.0%, χ2 =0.003, p = 0.96). The two groups also did not differ in years of education (Clonidine 11.9 ± 1.3; Placebo 12.0 ± 2.1, t (1,80) = −0.24, p = 0.81). In this restricted sample, the average length of longest abstinence was 30.3 ± 24.5 days. The difference between clonidine and placebo participants remained in the same direction as the full sample, but was no longer statistically significant (Clonidine 36.6 ± 28.2; Placebo 29.0 ± 19.6, t (102) = 1.64, p = .10).
The effect of study-drug group and days of continuous opioid abstinence on activity categories
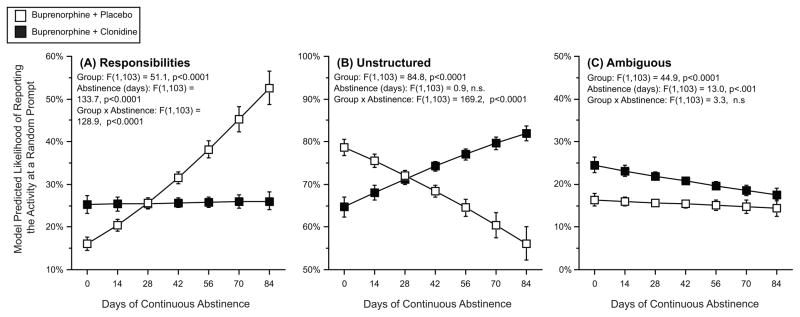
Figure 1 shows how often participants reported engaging in activities we categorized as responsibilities, unstructured, or ambiguous. There was a group by abstinence interaction for engaging in responsibilities (fig 1, panel A), whereby responsibilities were related to increased abstinence for placebo participants, but unrelated to abstinence for clonidine participants. There was a group by abstinence interaction for engaging in unstructured-time activities (fig 1, panel B). In the placebo group, we saw the “risk of unstructured time” effect: participants who were more likely to engage in unstructured-time activities had fewer days of continuous abstinence. This association was reversed in the clonidine group: participants treated with clonidine who were more likely to engage in unstructured activities had more days of continuous abstinence. There was no interaction effect with the category of ambiguous activities (fig 1, panel C); for both study-drug groups, increased days of abstinence were related to engaging in this category of activities less.
Figure 1. The effect of the longest period of continuous opioid abstinence and treatment group on model-predicted likelihood of reporting engaging in each of 3 categories of activity.
The y-axis represents the model-predicted likelihood of engaging in a category of activity at any random prompt. The x-axis represents the longest streak, in days, of opioid abstinence. In each panel, open squares are participants randomized to buprenorphine plus placebo, and the closed squares are participants randomized to buprenorphine plus clonidine. Error bars are 95% confidence intervals.
The effect of study-drug group and days of continuous opioid abstinence on specific responsibility activities
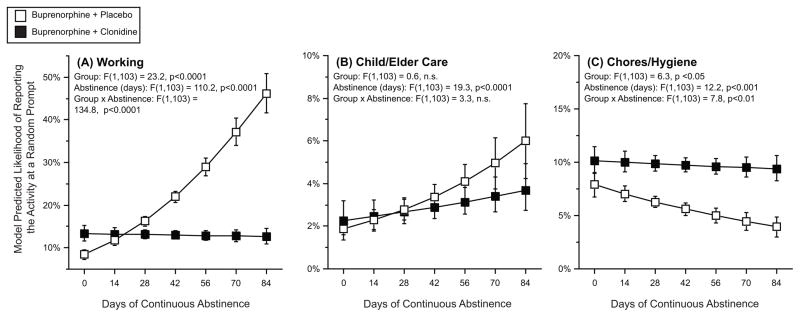
Figure 2 shows how often participants reported specific activities related to responsibilities. Reports of engaging in work (fig 2, panel A) were related to more days of abstinence, but this effect was only seen for the participants in the control condition. Reports of engaging in child or elder care (fig 2, panel B) were associated with more days of abstinence, but this effect was not different between the two groups. Reports of engaging in chores or hygiene (fig 2, panel C) were associated with fewer days of abstinence, but this effect was only seen in the participants in the placebo group.
Figure 2. The effect of the longest period of continuous opioid abstinence and treatment group on model-predicted likelihood of reporting engaging in specific responsibility activities.
The y-axis represents the model-predicted likelihood of engaging in a specific responsibility at any random prompt. The x-axis represents the longest streak, in days, of opioid abstinence. In each panel, open squares are participants randomized to buprenorphine plus placebo, and the closed squares are participants randomized to buprenorphine plus clonidine. Error bars are 95% confidence intervals.
The effect of study-drug group and days of continuous opioid abstinence on specific unstructured-time activities
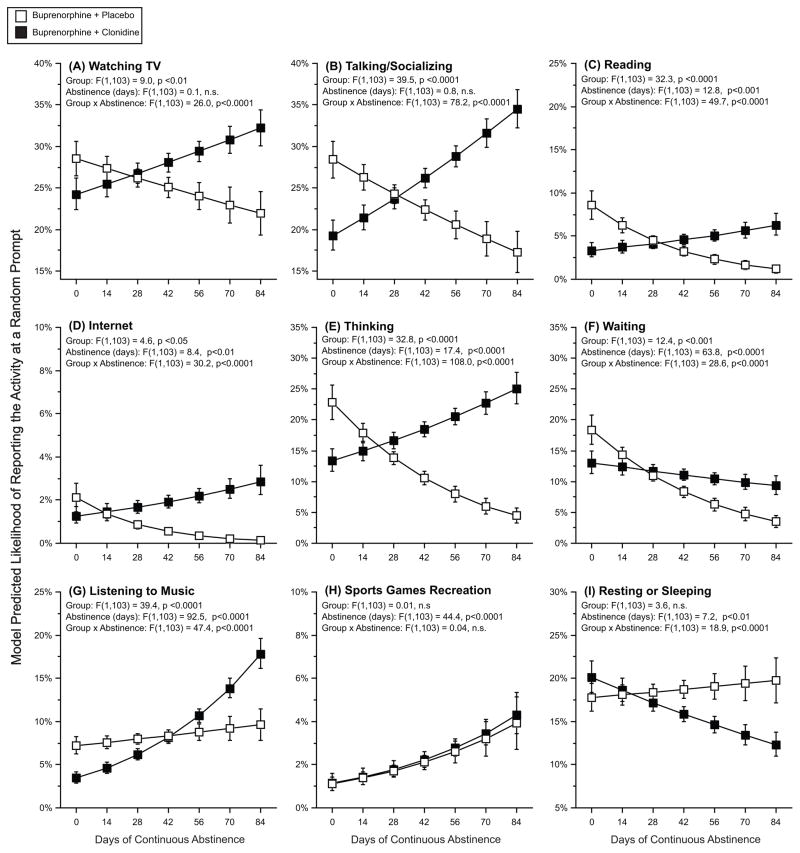
Specific activities classified as unstructured uses of time are displayed in figure 3. For these activities, days of abstinence generally interacted with study-drug group, such that participants in the placebo group showed a “risk of unstructured time” effect and participants in the clonidine group showed the reverse, with more unstructured use of time related to longer streaks of abstinence. This group by abstinence interaction was seen for reports of watching TV (fig 3, panel A), talking/socializing (fig 3, panel B), reading (fig 3, panel C), internet usage (fig 3, Panel D), and thinking (fig 3, panel E). Waiting (fig 3, panel F) was associated with fewer days of abstinence. Listening to music (fig 3, panel G) was associated with more days of abstinence for both placebo and clonidine groups, but the association was greater within the clonidine group. There was no significant group by abstinence interaction for sports/games/recreation (fig 3 panel H). Finally, reports of resting or sleeping (fig 3, panel I) showed the opposite pattern, with clonidine-group participants having a negative association between abstinence and reporting resting or sleeping and placebo-group participants having a positive relationship.
Figure 3. The effect of the longest period of continuous opioid abstinence and treatment group on model-predicted likelihood of reporting engaging in specific unstructured activities.
The y-axis represents the model-predicted likelihood of engaging in a specific unstructured activity at any random prompt. The x-axis represents the longest streak, in days, of opioid abstinence. In each panel, open squares are participants randomized to buprenorphine plus placebo, and the closed squares are participants randomized to buprenorphine plus clonidine. Error bars are 95% confidence intervals.
Discussion
The present study replicated our previous findings (Epstein and Preston 2012) that for participants receiving standard opioid agonist therapy there appears to be a risk associated with spending time in unstructured ways: those who spent more time watching TV or socializing did more poorly in treatment. Notably, these associations extended beyond recreational activities, occurring also with activities such as waiting and thinking, but did not extend to resting and sleeping.
We suggest two reasons for this “risk of unstructured time,” both of which could contribute to clonidine’s reversal of the effect. One of them, as we noted in the introduction, is boredom. In these analysis, we did not directly assess boredom, but in prior analyses, we showed that past hour boredom increases linearly in the five hours prior to cocaine use and heroin craving (Epstein et al. 2009). As Marlatt discussed in his classic taxonomy of relapse triggers, unstructured time might lead to boredom, which patients might try to alleviate with drug use (Marlatt 1996). A recent study of injection drug users in Baltimore City found high levels of boredom in one-third of the sample (German and Latkin 2012). Earlier studies comparing opioid-users with users of other drugs suggest that feelings of boredom or emptiness (Malow et al. 1989) or boredom susceptibility (O’Connor et al. 1995) may be more common among opioid-users. In both quantitative and qualitative studies, opioid users frequently attribute their use to boredom, both in terms of ongoing use and lapses during quit attempts (e.g. Powell 1973, Mullen and Hammersley 2006, Phillips et al. 2014, Winstock et al. 2014). Boredom during unstructured time (“leisure boredom”), in particular, has also been associated with drug use: in a nonclinical but resource-poor sample of adolescents, leisure boredom predicted greater use of alcohol, cigarettes, and marijuana (Sharp et. al., 2011; see also IsoAhola and Crowley, 1991). Even in the general population, leisure time can lead to problematic increases in boredom (Iso-Ahola and Weissinger 1987, Haller et al. 2013).
A second more insidious possibility is that, during the using career of a recovering drug user, leisure activities may have become, or come to include, conditioned stimuli for drug use. This would be especially troublesome because addiction treatment, including treatment in our clinic, usually includes encouragement for patients to engage in non-drug-related activities (Kadden 1995, Carroll 1998). For a patient with a long history of continuous use, there may be very few truly non-drug-related activities. This possibility is highlighted by findings from a recent EMA study of “substance-specific” and “person-specific” drug cues; whereas substance-specific cues encompassed items traditionally (or broadly) considered drug-associated (e.g., syringes, bottles), person-specific cues included items that were seemingly innocuous and nearly unavoidable (e.g., “being in my backyard”) (Fatseas et al. 2015). Many such person-specific cues could be embedded into leisure/unstructured-time activities like the ones assessed here. Thus, when counselors prescribe non-drug-related activities during recovery, they should probably confer with patients about the possibility of “person-specific” drug cues.
Whatever accounts for the association between unstructured time and shorter continuous abstinence, we found that the association was reduced or reversed by clonidine. Clonidine’s effects on boredom and cues could help account for this reversal. In the initial report for this clinical trial, we found that clonidine not only weakened the connection between stress and heroin craving, but also between the more general state of negative affect and heroin craving (Kowalczyk et al. 2015). Negative affect encompasses negatively valanced states that are not necessarily high in arousal—one of which is boredom. We found (see fig S3: Kowalczyk et al. 2015) that participants randomized to clonidine had less heroin craving at moderate levels of boredom, only reaching the same levels of craving as the placebo group at the highest level of boredom.
At the same time, clonidine may have also mitigated the effects of drug-associated cues, including person-specific cues. In human laboratory studies, alpha-2 agonists block cue-induced craving (Sinha et al. 2007, Jobes et al. 2011, Fox et al. 2012), and the alpha-2 antagonist yohimbine can enhance craving, especially in women (Sinha et al. 1999). This effect may depend on CNS-mediated changes in the incentive motivational effects of the cues (Economidou et al. 2011). Nonetheless, given that drug-cue exposure in humans can produce sympathetic activation accompanied by increases in anxiety (Sinha et al. 1999, Sinha et al. 2000), clonidine may also exert benefits by reducing autonomic responses to cues, thereby reducing their perceived salience. Such reductions in perceived cue salience could be mediated by central actions of clonidine as well (Ventura et al. 2008). Future work with clonidine as an adjunct medication to buprenorphine for opioid treatment should consider carefully the combination’s impact on boredom and cues to help describe the mechanisms behind the treatment effect.
While most activities grouped as unstructured were associated with a reversal or a diminishment of the risk of unstructured time in the clonidine group, one behavior bucked this trend. Higher frequency of resting or sleeping was associated with fewer days of abstinence in the clonidine group, but more days of abstinence in the placebo group. The clearest distinction between resting/sleeping and the other unstructured activities is that resting/sleeping implies some diminution of alertness, possibly to the point of unconsciousness, whereas all the other unstructured activities were conscious. There are both behavioral and pharmacological reasons that this difference might be important. It is possible that the greater likelihood of reporting work in the placebo participants with longer streaks of abstinence may have led to more need for rest. It is possible that the clonidine-treated participants with longer streaks of abstinence had less daytime sleepiness as opioid use (Angarita, Emadi, Hodges, & Morgan, 2016) and stress (Hirotsu, Tufik, & Andersen, 2015) can be associated with sleep disturbances. Additionally increases in opioid use may have compounded clonidine’s sedating side effects in those with more opioid use (Stone, German, Kitto, Fairbanks, & Wilcox, 2014). Future studies of clonidine as an adjuvant medication for opioid use might examine reasons for resting and sleeping to clarify these findings.
Results from the participants randomized to standard buprenorphine treatment replicated prior findings, by our group and others, that time spent on job and familial responsibilities is associated with good treatment outcomes (Brewer et al. 1998, Silverman et al. 2002, Epstein and Preston 2012). Examining the individual behaviors highlight job and familial responsibilities, as this pattern was seen for working and child/eldercare but not for chores/hygiene. Surprisingly, participants in the clonidine condition did not have the same treatment benefits seen with engaging in responsibilities. Looking at the individual behaviors we can see that this diminishment was related to a lack of the usual positive association between work-related activities and opioid abstinence. This dampening did not extend to child/eldercare responsibilities, which maintained a positive relationship statistically equal to that of the control group. Thus, the finding with work in the clonidine group may be spurious. However, it is certainly worth examining in any follow-up study.
Our analyses have significant limitations. First, our data are from a clinical trial that we had not designed specifically to address the relationship between poorer outcomes and unstructured time. A more rigorous approach to the question would require randomization to different ways of spending time. Although we think we have a sufficient convergence of evidence from multiple studies to refer to a risk of unstructured time, we cannot make airtight inferences about causation from a data set in which participants self-selected into activities. This limitation is offset by the fact that comparisons between the randomized clonidine and placebo groups can be discussed in causal terms.
Second, like the trial itself, the EMA questions were not designed to separate responsibilities from free time, or structured from unstructured time, as has sometimes been done with items such as, “I wanted to do it / I had to do it / I had nothing else to do” (Csikszentmihalyi and Graef 1980). Therefore, we had to make assumptions about how different classes of activities were experienced, probably reducing the sensitivity of our analyses.
Conclusions
Our findings in the standard buprenorphine group are consistent with an old proverb that, in one variant, says idle hands are the devil’s workshop. For participants in this group, engaging in more activities related to responsibilities for others (work and family) was associated with better outcomes, while more unstructured uses of time were associated with poorer outcomes. Clonidine seemed to protect participants from this effect—a finding that suggests it should be helpful not just for people who are undergoing moderate stress, but also for clients in recovery who, due to underemployment or social isolation, face possibly risky amounts of unstructured time. A randomized or micro-randomized trial (Klasnja et al. 2015), with some experimental control over momentary activities, would be needed to confirm this suggestion.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIDA.
References
- Angarita GA, Emadi N, Hodges S, Morgan PT. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addict Sci Clin Pract. 2016;11(1):9. doi: 10.1186/s13722-016-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Phillips G, Green L, Gossop M. Circumstances surrounding the initial lapse to opiate use following detoxification. Br J Psychiatry. 1989;154:354–9. doi: 10.1192/bjp.154.3.354. [DOI] [PubMed] [Google Scholar]
- Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction. 1998;93:73–92. [PubMed] [Google Scholar]
- Carroll KM. Therapy Manuals for Drug Addiction, Manual 1: A Cognitive-Behavioral Approach: Treating Cocaine Addiction. National Institute on Drug Abuse. 1998 [Google Scholar]
- Csikszentmihalyi M, Graef R. The experience of freedom in daily life. American Journal of Community Psychology. 1980;8:401–414. [Google Scholar]
- DeFulio A, Donlin WD, Wong CJ, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: a randomized controlled trial. Addiction. 2009;104:1530–8. doi: 10.1111/j.1360-0443.2009.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Dalley JW, Everitt BJ. Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry. 2011;69:266–74. doi: 10.1016/j.biopsych.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Daily life hour by hour, with and without cocaine: an ecological momentary assessment study. Psychopharmacology. 2010;211:223–32. doi: 10.1007/s00213-010-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. TGI Monday?: drug-dependent outpatients report lower stress and more happiness at work than elsewhere. Am J Addict. 2012;21:189–98. doi: 10.1111/j.1521-0391.2012.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatseas M, Serre F, Alexandre JM, Debrabant R, Auriacombe M, Swendsen J. Craving and substance use among patients with alcohol, tobacco, cannabis or heroin addiction: a comparison of substance- and person-specific cues. Addiction. 2015;110:1035–42. doi: 10.1111/add.12882. [DOI] [PubMed] [Google Scholar]
- Fox HC, Seo D, Tuit K, et al. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol. 2012;26:958–72. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German D, Latkin CA. Boredom, depressive symptoms, and HIV risk behaviors among urban injection drug users. AIDS Behav. 2012;16:2244–50. doi: 10.1007/s10461-012-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller M, Hadler M, Kaup G. Leisure Time in Modern Societies: A New Source of Boredom and Stress? Soc Indic Res. 2013;111:403–434. [Google Scholar]
- Heinz AJ, Wu J, Witkiewitz K, Epstein DH, Preston KL. Marriage and relationship closeness as predictors of cocaine and heroin use. Addict Behav. 2009;34:258–63. doi: 10.1016/j.addbeh.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu C, Tufik S, Andersen ML. Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 2015;8(3):143–152. doi: 10.1016/j.slsci.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso-Ahola SE, Weissinger E. Leisure and boredom. Journal of social and clinical psychology. 1987;5:356–364. [Google Scholar]
- Isoahola SE, Crowley ED. Adolescent Substance-Abuse and Leisure Boredom. J Leisure Res. 1991;23:260–271. [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology (Berl) 2011;218:83–8. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadden R. Cognitive-behavioral coping skills therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. DIANE Publishing; 1995. [Google Scholar]
- Klasnja P, Hekler EB, Shiffman S, et al. Microrandomized trials: An experimental design for developing just-in-time adaptive interventions. Health Psychology. 2015;34:1220. doi: 10.1037/hea0000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk WJ, Phillips KA, Jobes ML, et al. Clonidine Maintenance Prolongs Opioid Abstinence and Decouples Stress From Craving in Daily Life: A Randomized Controlled Trial With Ecological Momentary Assessment. Am J Psychiatry. 2015;172:760–7. doi: 10.1176/appi.ajp.2014.14081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow RM, West JA, Williams JL, Sutker PB. Personality disorders classification and symptoms in cocaine and opioid addicts. J Consult Clin Psychol. 1989;57:765–7. doi: 10.1037//0022-006x.57.6.765. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Taxonomy of high-risk situations for alcohol relapse: evolution and development of a cognitive-behavioral model. Addiction. 1996;91(Suppl):S37–49. [PubMed] [Google Scholar]
- McKay JR, Franklin TR, Patapis N, Lynch KG. Conceptual, methodological, and analytical issues in the study of relapse. Clin Psychol Rev. 2006;26:109–27. doi: 10.1016/j.cpr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Mullen K, Hammersley R. Attempted cessation of heroin use among men approaching mid-life. Drug-Educ Prev Polic. 2006;13:77–92. [Google Scholar]
- Notley C, Blyth A, Maskrey V, Craig J, Holland R. The experience of long-term opiate maintenance treatment and reported barriers to recovery: a qualitative systematic review. Eur Addict Res. 2013;19:287–98. doi: 10.1159/000346674. [DOI] [PubMed] [Google Scholar]
- Notley C, Blyth A, Maskrey V, Pinto H, Holland R. Exploring the Concepts of Abstinence and Recovery Through the Experiences of Long-Term Opiate Substitution Clients. Subst Abus. 2015;36:232–9. doi: 10.1080/08897077.2014.941085. [DOI] [PubMed] [Google Scholar]
- O’Connor LE, Berry JW, Morrison A, Brown S. The drug-of-choice phenomenon psychological differences among drug users who preferred different drugs. Int J Addict. 1995;30:541–55. doi: 10.3109/10826089509048743. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Epstein DH, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Substance use and hepatitis C: an ecological momentary assessment study. Health Psychol. 2014;33:710–9. doi: 10.1037/hea0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DH. A pilot study of occasional heroin users. Arch Gen Psychiatry. 1973;28:586–94. doi: 10.1001/archpsyc.1973.01750340104016. [DOI] [PubMed] [Google Scholar]
- Sharp EH, Coffman DL, Caldwell LL, et al. Predicting substance use behavior among South African adolescents: The role of leisure experiences across time. Int J Behav Dev. 2011;35:343–351. doi: 10.1177/0165025411404494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Wong CJ, Hampton J, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: three-year abstinence outcomes. Exp Clin Psychopharmacol. 2002;10:228–40. doi: 10.1037//1064-1297.10.3.228. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–51. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–8. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology (Berl) 2007;190:569–74. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Stone LS, German JP, Kitto KF, Fairbanks CA, Wilcox GL. Morphine and clonidine combination therapy improves therapeutic window in mice: synergy in antinociceptive but not in sedative or cardiovascular effects. PLoS One. 2014;9(10):e109903. doi: 10.1371/journal.pone.0109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan G. Redefining clinical trials: the age of personalized medicine. Cell. 2012;148:1079–80. doi: 10.1016/j.cell.2012.02.041. [DOI] [PubMed] [Google Scholar]
- Ventura R, Latagliata EC, Morrone C, La Mela I, Puglisi-Allegra S. Prefrontal norepinephrine determines attribution of “high” motivational salience. PLoS One. 2008;3:e3044. doi: 10.1371/journal.pone.0003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Borschmann R, Bell J. The non-medical use of tramadol in the UK: findings from a large community sample. Int J Clin Pract. 2014;68:1147–1151. doi: 10.1111/ijcp.12429. [DOI] [PubMed] [Google Scholar]