Abstract
Obstructive sleep apnea (OSA) has been linked to increased mortality in pulmonary fibrosis. Its key feature, chronic intermittent hypoxia (CIH), can lead to oxidative stress and inflammation, known to lead to fibrotic pathology in other organs. We tested the effects of CIH in an animal model of bleomycin-induced lung fibrosis. Sprague Dawley rats were instilled intratracheally with bleomycin (Blm) or saline (Sal), and exposed to CIH or normal air (Norm) for 9 or 30 days. Pulmonary function was tested and lungs were harvested for histological and molecular analyses.
In Blm-treated animals, 30 days of CIH compared to Norm increased total lung collagen content (p=0.008) and reduced Quasi-static lung compliance (p=0.04). CIH upregulated lipid peroxidation and increased NF-κB activation, IL-17 mRNA and Col1α1 mRNA expression. Our results indicate that following Blm-induced lung injury, CIH amplifies collagen deposition via oxidative and inflammatory pathways, culminating in stiffer lungs. Thus, OSA may augment fibrosis in patients with interstitial lung disease.
Keywords: sleep apnea, obstructive, hypoxia, intermittent, fibrosis, lung/immunology/metabolism/pathology
Introduction
Idiopathic pulmonary fibrosis (IPF) poses an increasing public health burden. Owing to population aging in the US, both IPF prevalence and annual incidence have escalated, currently estimated at 43–63 cases per 100,000 people and 16.3–17.4 per 100,000 people, respectively (Lee et al., 2014). Despite years of research, mortality in IPF remains high (Lee et al., 2014), with very few, if any, effective therapies, emphasizing the need for new approaches. IPF is believed to be caused by repetitive cycles of epithelial cell injury and activation, linked to fibroblast activation and uncontrolled proliferation, and deposition of extra-cellular matrix (Yang and Schwartz, 2015). Following injury, two factors are known to be universal mediators of fibrogenesis: transforming growth factor β (TGF-β), and connective tissue growth factor (CCN2) (Wang et al., 2011) which is expressed in response to TGF-β (Leask et al., 2003; Leask et al., 2008). TGF-β production depends on the presence of IL-17, establishing a pivotal role for this cytokine in the development of pulmonary fibrosis (Gasse et al., 2011). A major step in fibrosis development is fibroblast differentiation into myofibroblasts, which express α-smooth muscle actin (α-SMA) and lay down the collagen.
Contrary to frequent anecdotal perception of snoring/obstructive sleep apnea (OSA) as nothing more than a nuisance in patients with IPF, accumulating evidence deleteriously links OSA with IPF. OSA is highly common among patients with IPF and is frequently unrecognized (Lancaster et al., 2009; Mermigkis et al., 2007; Pihtili et al., 2013). In the first study that prospectively assessed OSA prevalence by laboratory polysomnography (PSG) in patients with IPF, 44 out of 50 (88%) patients were diagnosed with OSA, of which only 10 (23%) had a prior OSA diagnosis (Lancaster et al., 2009). Most importantly, OSA may adversely influence progression of IPF and related mortality. Treatment for OSA with continuous positive airway pressure (CPAP) in patients with IPF improved their activity and quality of life, and reduced mortality (Mermigkis et al., 2015; Mermigkis et al., 2013).
The mechanisms whereby OSA worsens IPF have not been studied, but several possibilities exist. Apart from promoting gastro-esophageal reflux, accelerating progression of systemic cardiovascular and pulmonary vascular pathology, OSA’s features could directly amplify the fibrotic parenchymal process. OSA’s hallmark, chronic intermittent hypoxia (CIH) is associated with oxidative stress and inflammation, known to lead to fibrosis in the systemic vasculature and myocardium (Marcus et al., 2014; Ramos et al., 2014), liver (Cannito et al., 2014), and the kidney (Pan et al., 2013). In the lung, we recently showed in the allergic rat, that CIH exposure led to airway fibrosis with extention to the sorrounding parenchyma, concurrent with airflow limitation (Broytman et al., 2015). These data highlight the potential of CIH to promote fibrosis in multiple organs, including the lung associated with functional deficits.
In this study, we aimed to test the effects of CIH on lung fibrosis in a rat model of bleomycin (Blm)-induced fibrosis and to explore putative mechanisms. We hypothesized that following Blm, CIH will worsen the lung fibrosis and physiologic deficits, via oxidative stress and inflammatory-mediated pathways; moreover, that these effects will be most prominent with longer CIH exposure. Initial results of this study were published in abstract form (Braun et al., 2015; Clithero et al., 2015; Modi et al., 2015).
2. Methods
2. 1. Animals
The Laboratory Animal Resources facilities of the UW-Madison School of Medicine and Public Health are accredited by the American Association for the Accreditation of Laboratory Animal Care. All procedures were approved by the University’s Institutional Animal Care and Use Committee and adhered to the NIH Guide for the Care and Use of Laboratory Animals. Male 200 – 220 gram Sprague Dawley rats were purchased from Harlan (Indianapolis, IN). The animals were housed 2 to 4 per cage, in environmentally controlled rooms at 22°C and 50% to 55% humidity, on a 12/12-hour light/dark cycle, with lights on at 06:00 hours. Standard rat chow and water were provided ad libitum. The animals were allowed to acclimate for 5 days before the start of experiments.
2.2. Experimetal treatments
Bleomycin instillation
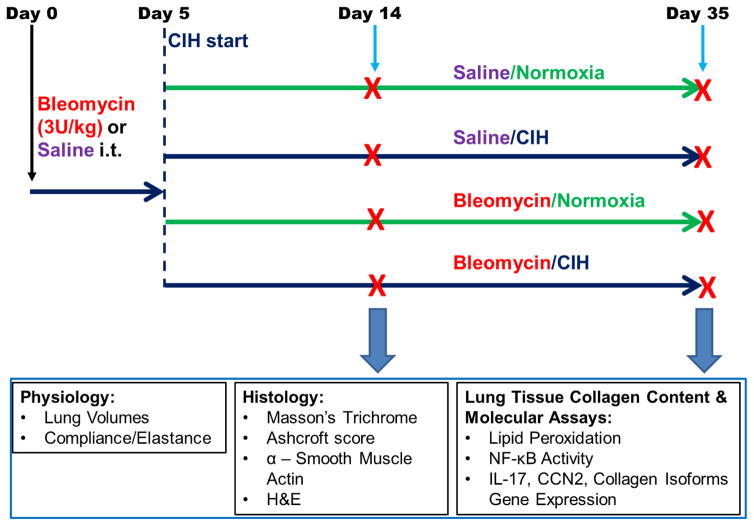
Fig. 1 presents the study protocol. Bleomycin sulfate from Streptomyces verticillus (Sigma, St. Louis, MO) was dissolved in sterile normal saline at 4 USP Units (U)/ml and instilled intratracheally at a dose of 3U/kg using a MicroSprayer® Aerosolizer (Penn-Century, Inc., Wyndmoor, PA). Control rats received an equal volume of normal saline (Sal).
Figure 1. Experimental Protocol.
Animals were given a single intratracheal instillation of Blm or saline on Day 0. CIH or Normoxia exposures commenced on Day 5 after Blm. Endpoints were collected on Day 14 or Day 35 after Blm instillation. i.t. = intratracheal instillation of Blm.
Chronic intermittent hypoxia exposure
CIH or normoxia (Norm) exposures (10% FIO2, 30 episodes/h, 10h during daytime) were initiated on day 5 following Blm. The exposure was performed essentially as described in (Broytman et al., 2015) and detailed in the Supplement. Data were acquired on Day 14 or Day 35 after Blm (Fig. 1); therefore, animals were exposed to either 9 or 30 days of CIH after Blm instillations.
2.3. Physiologic studies
Pulmonary function testing (PFT)
Lung Volumes
Lung volumes were measured as in (Broytman et al., 2015). Detailed methods are available in the Supplement.
Pulmonary Tissue Elastance and Quasi-Static Compliance
Following lung volumes measurements, animals were transferred to a FlexiVent system (Scireq, Montreal, QC, Canada). Pulmonary tissue quasi-static compliance (Cst) and elastance (H) were obtained from pressure-volume loops, as described (Sorkness et al., 2013) and presented in the Supplement.
2.4. Sample preparation and lung histology
After PFTs, rats were euthanized by exsanguination and both lungs were removed en block, as described in the Supplement. The left lung was used for Hematoxylin and Eosin (H&E) and Masson’s Trichrome staining, and for immunostaining with anti-α-Smooth Muscle Actin (α-SMA) antibody, as detailed in the Supplement. Modified Ashcroft scoring was determined per published methods using the Masson’s Trichrome stained tissue (Hubner et al., 2008). The right lung was flash frozen in liquid nitrogen and ground to powder to use for the following molecular assays.
2.5. Molecular assays
Collagen assay
Right lung tissue powder was used for determining the concentration of hydroxyproline, as per established methods (Creemers et al., 1997), and detailed in the Supplement.
Lipid Peroxidation
Lipid peroxidation was determined by measuring malondialdehyde (MDA) levels, as detailed in the Supplement.
NfκB activity assay
Nuclear factor κB (NfκB) activity was measured by an electrophoretic mobility shift assay (EMSA), as previously published (Miyamoto et al., 1994; Yuan et al., 2015) and detailed in the Supplement.
RT-PCR
Samples were collected and stored in Tri-reagent and later analyzed for IL-17, CCN2, Collagen 5α1 (Col5α1) and Collagen 1α1 (Col1α1), as detailed in the Supplement.
2.6. Statistics
For representative Pressure – Volume (PV) loops (Fig. 5A, B), slope of the inflation curve was estimated at half-maximum volume, to illustrate the lung compliance during inflation (Salazar and Knowles, 1964). Relative gene expression is presented in arbitrary units (A.U.), as fold difference over the mean expression among the Sal/Norm animals. Each value for relative gene expression was determined in duplicate.
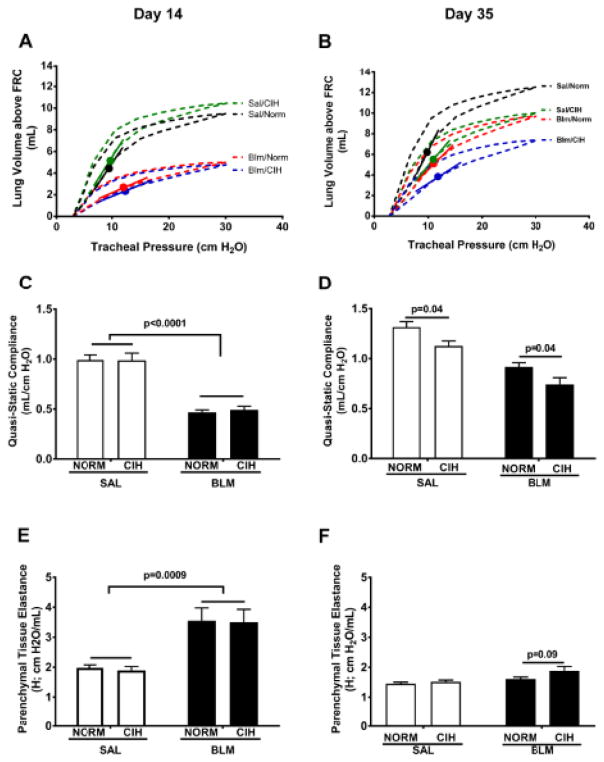
Figure 5. CIH leads to pulmonary function deficits following Blm injury.
(A, B): Representative Pressure-Volume loops from Cst measurement in the indicated treatment groups; line segments indicate slope of the inspiratory curve at the point of half-maximum inflation (circles). (D–F): Quasi-static compliance (Cst) (C, D) and Parenchymal Tissue Elastance (H) (E, F) were measured on the indicated days after Blm instillation.
Data are presented as group mean ± SEM. Groups were compared using two-way analysis of variance (ANOVA) with post-hoc comparisons using Holm-Sidak’s multiple comparison test using Prism software (version 7.01; GraphPad Software, San Diego, CA). Multiple comparison-adjusted p-values are reported. Effects were considered significant when p < 0.05. A trend towards significance was noted for 0.05 < p ≤0.20.
3. Results
3.1. Chronic intermittent hypoxia increased total lung collagen
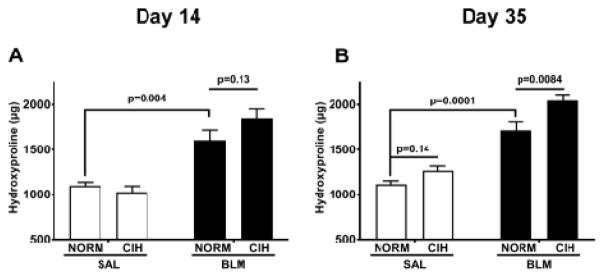
Blm under normoxic condition induced the expected increase in total right lung collagen (Fig. 2), both at day 14 (1,608±226 in Blm/Norm vs. 1,100±76 μg in Sal/Norm group, p=0.004; Fig. 2A) and day 35 (1,717±188 μg in Blm/Norm vs. 1,116±74 μg in Sal/Norm group, p=0.0001; Fig. 2B). However, under CIH, at day 35 after Blm, the Blm-induced elevation in collagen content was further increased (Fig. 2B) (2,047±69 μg in the Blm/CIH vs. 1,717±188 μg in the Blm/Norm group, p=0.008). This effect appears to have emerged, with a non-significant trend, at day 14 after Blm (1,849±102 μg in the Blm/CIH vs. 1,608±113 μg in the Blm/Norm group, p=0.13). In the Sal-treated animals, at day 35, CIH increased the amount of hydroxyproline slightly (1,266±52 μg in the Sal/CIH vs. 1,116±38 μg in the Sal/Norm group), but the increase did not reach statistical significance (p=0.14); no CIH effect was noted among Sal-treated animals, at the earlier timepoint.
Figure 2. 30 days of CIH and Blm increased total right lung collagen content.
Total hydroxyproline content (μg) in lung tissue at day 14 (A) and day 35 following Blm (B).
3.2. CIH enhanced the Bleomycin – induced parenchymal fibrosis and induced “emphysema-like” changes in lung tissue
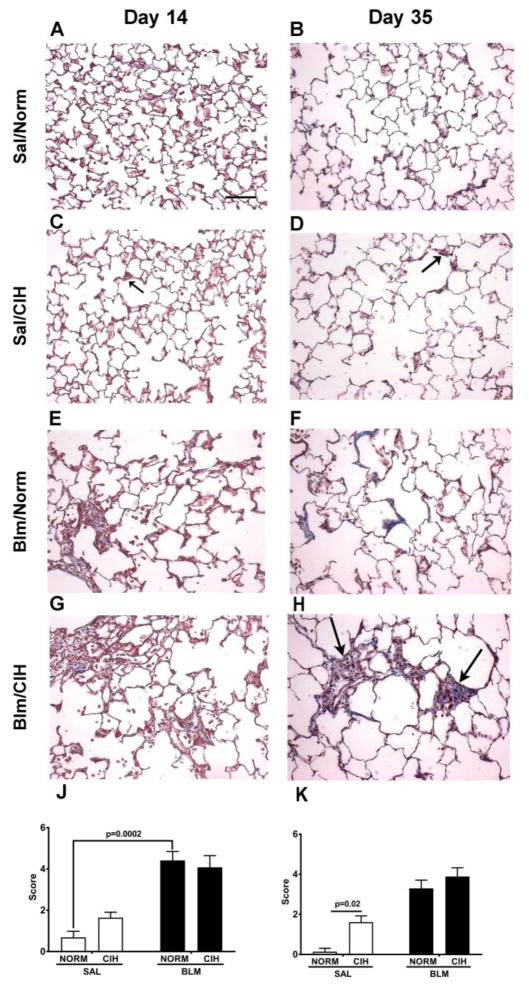
Qualitative analysis of Masson’s Trichrome stained sections (Fig. 3), revealed that in Sal-treated animals, compared to Norm (Fig. 3A, B), at both time points, CIH led to scattered fibrotic changes in alveolar walls and some deterioration in alveolar structure (Fig 3C, D; see black arrows in representative sections). In Blm injured lungs, relative to Norm, CIH at day 14 did not appear to affect parenchymal collagen deposition (Fig. 3E vs. G); at day 35, in the CIH exposed animals the parenchymal collagen deposition was greater (Fig. 3H vs. F; see black arrows). Modified Ashcroft scoring (Fig. 3J, K) demonstrated that the overall injury and fibrosis scores were significantly higher in the Sal/CIH than in the Sal/Norm group at day 35 (p=0.02), while at day 14, a non-sgnificant trend was noted (p=0.23). In the Blm-treated animals, CIH did not significantly alter the Ashcroft scores, at any time points (Fig. 3J, K).
Figure 3. Blm and 30 days of CIH increased fibrotic lung pathology.
(A–H): Representative Masson Trichrome stain images (20×) of lung parenchyma in Sal/Norm (A, B), Sal/CIH (C, D), Blm/Norm (E, F) and Blm/CIH – treated (G, H) animals on Day 14 (A, C, E, G) and Day 35 (B, D, F, H) following Blm instillation; black arrows pointing towards areas of alveolar wall thickening (blue colour) in Sal/CIH – treated animals at Day 14 (C) and Day 35 (D), as well as dense fibrotic areas in Blm/CIH, 35 days after Blm injury (H). (J, K) Ashcroft scores of Masson Trichrome stained sections on Day 14 (J) and Day 35 following Blm instillation (K). Scale bar (Panel A): 100 μm.
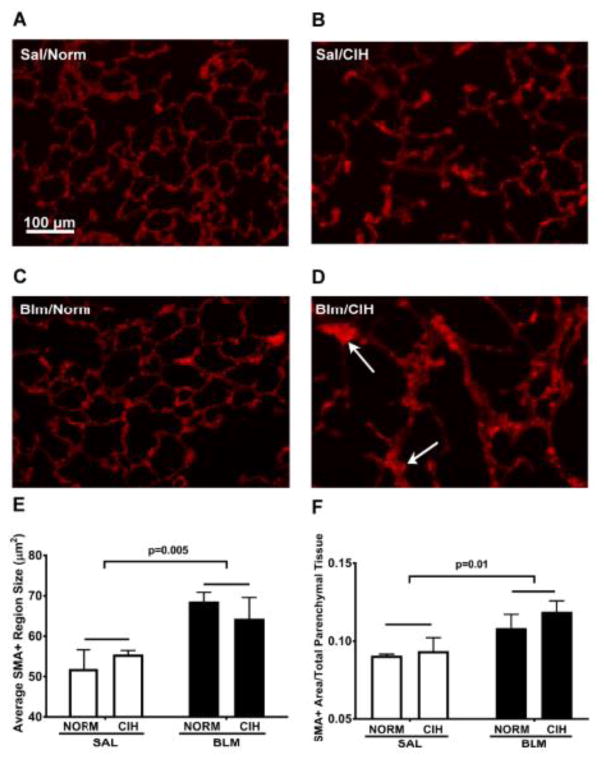
The persistent elevation in total collagen accumulation at day 35 in Blm/CIH treated animals was paralleled by an increase in α-SMA immunofluorescence in lung parenchyma (i.e. thickened alveolar septa characterized by bright red fluorescence indicative of α-SMA overexpression). As shown in the representative lung tissue sections (Fig. 4, A–D), no fibrotic areas were present in the Sal/Norm condition (Fig. 4A), whereas, just as shown by Ashcroft scoring (Fig. 3K), such areas became apparent with CIH alone (Fig. 4B) and Blm alone (Fig. 4C). These foci of thickened parenchymal tissue with high α-SMA expression had the largest cross-sectional area in the Blm/CIH treated animals (Fig. 4D), where along with the fibrosis, we also noted the presence of “emphysema-like” areas (Fig. 3; 4 A–D), similar to observations in the allergic rat (Broytman et al., 2015). On group data, the average area of the α-SMA-positive regions was higher in Blm-treated animals than the Sal - treated controls (p=0.005), but no significant difference was seen between Blm/CIH and Blm/Norm (p=0.7; Fig. 4E). However, because the average size of α-SMA+ regions could have been affected by the concurrent emphysema as noted above, we normalized the average area of α-SMA+ regions, in each field, to the total area of parenchymal tissue present in each field (Fig. 4F). Blm-treated animals had significantly larger α-SMA+ regions as a fraction of total parenchymal tissue, compared to Sal-treated controls (p=0.01; Fig. 4F), and again no difference was seen between Blm/Norm and Blm/CIH (p=0.5). Corroborating with the total collagen results, these data suggest that Blm and CIH together may lead to larger areas of myofibroblast proliferation or aggregation, in combination with “emphysema-like” tissue loss, ie a mixed phenotype of fibrosis and “emphysema-like” pattern.
Figure 4. Thirty days of CIH following Blm injury increased average area of α-Smooth Muscle Actin positive staining relative to total lung parenchymal tissue area.
(A–D): Representative α-SMA immunostained images of Sal/Norm (A), Sal/CIH (B), Blm/Norm (C) and Blm/CIH (D) animals, 35 days after Sal or Blm treatment (20×). Emphysema-like changes are present in CIH treated animals (B, D) and increased foci of α-SMA+ staining (D; arrows) are seen in the lungs of Blm/CIH treated rats. Scale bar (shown in Panel A): 100 μm. (E): Average α-SMA+ region area (μm2). (F): Average SMA+ region area divided by total parenchymal tissue area in the microscope field, to account for presence of “emphysema-like” areas. Each symbol represents the average of all images (≥15) from one rat.
On H&E staining, in comparison to Sal/Norm controls (Fig. E1, A&B), in Sal/CIH lungs we observed some thickening of alveolar septa (Fig. E1, C&D, black arrows and insets). Blm treatment resulted in severe parenchymal fibrosis at day 14, which was largely resolved by day 35 (Fig. E1, E&F). CIH however, prevented the resolution of this Blm effect, resulting in persistent fibrosis and paracicatricial emphysema on Day 35 (Figure E1, H).
3.3. CIH led to impaired pulmonary function
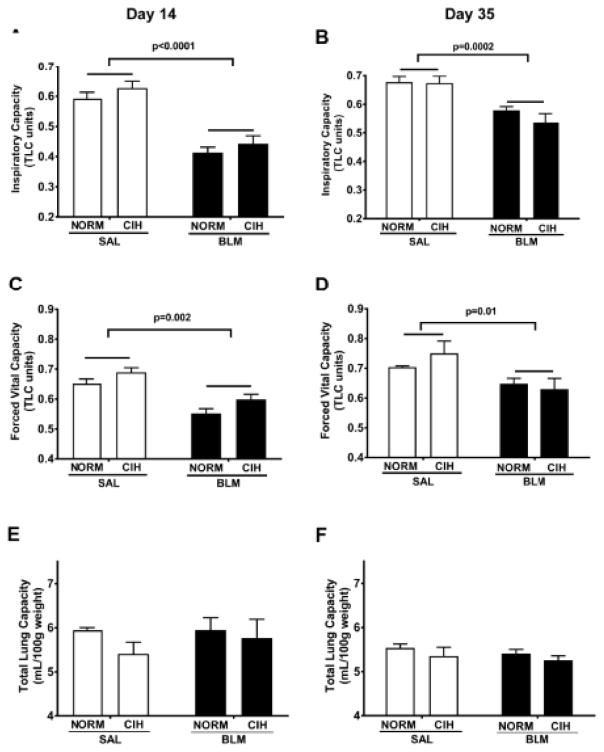
At day 14 after Blm, representative pressure-volumes loops show the expected Blm-induced decline in the slope of the inflation (lower) limb, and in the volume at the maximum inflation pressure tested (Fig. 5A), suggesting reduced tissue compliance (Cst) compared to Sal-treated rats. At day 35, there is also separation among the Blm-treated animals, with higher impairment noted in the Blm/CIH treated rats compared to Blm/Norm rats (Fig. 5B). On group averages at day 14, Cst was significantly decreased by Blm (p<0.0001) and unaffected by CIH (p=0.8) (Fig. 5C). At day 35, however, CIH significantly decreased Cst in both Sal- (p=0.04) and Blm–treated (p=0.04) animals (Fig. 5D); Blm/CIH animals had the lowest Cst among all the treatment groups. Likewise, parenchymal tissue elastance (H) was considerably increased by Blm at day 14 (p=0.0009), but no difference due to CIH was observed (p=0.85; Fig. 5E). In Blm/Norm animals compared to Sal/Norm, the increase noted earlier was abolished at day 35, while in Blm/CIH animals, H tended to remain elevated (p=0.09 vs. Blm/Norm; Fig. 5F).
On lung volumes, CIH did not affect the inspiratory capacity (IC) expressed as a fraction of total lung capacity (TLC), whereas the Blm treatment reduced it both at Day 14 (p<0.0001; Fig. 6A) and at day 35 (p=0.0002, Fig. 6B). A similar pattern of changes, with no effects of CIH was observed on forced vital capacity (FVC) expressed as a fraction of TLC, at both time points (Fig. 6C and D); at day 35, the Blm-induced reduction in this parameter persisted (Fig. 6D). Neither Blm nor CIH significantly affected total lung capacity (TLC) (Fig. 6E&F) at any time points.
Figure 6. CIH did not affect the Blm-induced decline in lung volumes.
Inspiratory Capacity (A, B) and Forced Vital Capacity (C, D) as a fraction of total lung capacity (TLC), and TLC (E, F) normalized to rat body weight were measured at the indicated days after bleomycin.
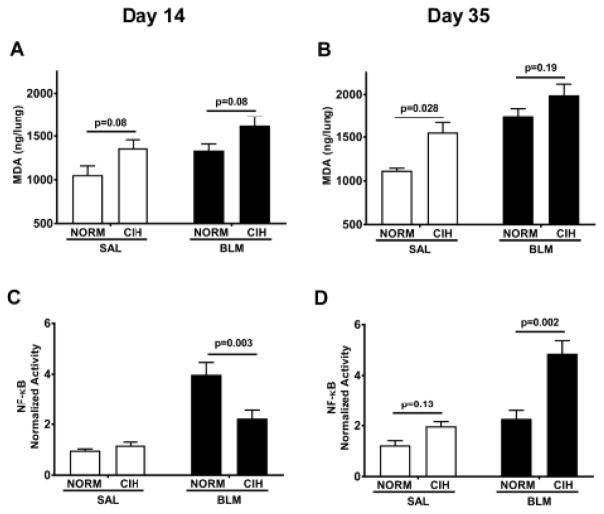
3.4. CIH amplified lipid peroxidation following Blm injury
Blm and CIH each create reactive oxygen species which lead to lipid peroxidation (Hay et al., 1991; Yang et al., 2016; Yuan et al., 2015). One of the most prevalent by-products used as a biomarker of lipid peroxidation is malondialdehyde (MDA). Indeed, at day 14, either CIH (p=0.01) or Blm (p=0.02) alone increased the amount of MDA in the lung (overall effects, 2-way ANOVA; Fig. 7A). On post-hoc analyses, CIH tended to increase the amount of MDA in the lung, in both Sal and Blm conditions (p=0.08, Fig. 7A). With the prolonged exposure, at day 35, the CIH effect was more firmly established, (p=0.004 for overall CIH effect, 2-way ANOVA; Fig. 7B), and in the post-hoc analyses, the effect became significant in the Saline – treated animals (p=0.028). The Blm/CIH group maintained a non-significant trend vs. Blm/Norm group (p=0.19), and demonstrated the highest level among all groups (p=0.028vs. Sal/CIH; p=0.0002 vs. Sal/Norm).
Figure 7. Prolonged CIH enhances Blm – induced oxidative stress and NF-κB activation in lung tissue.
Total malondialdehyde (MDA) content (A, B) and NF-κB activity (C, D) were measured in lung tissue on day 14 (A, C) or day 35 (B, D) after Blm instillation. 30 days of CIH exposure enhanced Blm – induced increase in MDA content and led to increased NF-κB activity.
3.5. CIH prolonged the activation of NF-κB
At day 14, the Blm/Norm group showed significantly higher activation of NF-κB in lung tissue compared to all other groups (p<0.001 for all, Fig. 7C). However, by day 35, CIH increased the NF-κB activation in the Blm-treated rats (p=0.002, Fig. 7D); whereas in the Blm/Norm, NF-κB activation returned to that of Sal/Norm, as the inflammatory phase subsided. Also, this more prolonged CIH exposure appeared to start eliciting an effect in the Sal treated rats as well, as a trend appeared to emerge vs. Norm exposed rats (p=0.13, Fig. 7D).
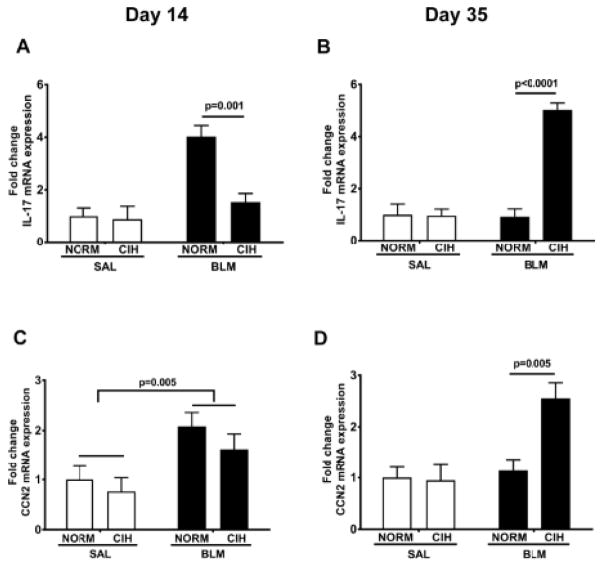
3.6. CIH prolonged IL-17 and CCN-2 gene expression
IL-17 has been strongly associated with the detrimental effects of Blm in the rodent lungs (Gasse et al., 2011; Wilson et al., 2010). At day 14, IL-17 expression was elevated 4-fold in Blm/Norm animals compared to Saline-treated controls, but remained unaltered in Blm/CIH – treated rats (p=0.001 vs. Blm/Norm; Fig. 8A). At day 35, whereas IL-17 mRNA expression was not different in the Blm/Norm relative to Sal/Norm, in Blm/CIH-treated animals it was elevated ~5-fold compared to any other treatment group (p < 0.0001 for each comparison, Fig. 8B).
Figure 8. Prolonged CIH iIncreased IL-17 and CCN-2 mRNA expression.
IL-17 (A, B) and CCN-2 (C, D) mRNA expression in lung tissue were measured by qPCR on day 14 (A, C) or day 35 (B, D) after Blm instillation. A.U: Arbitrary Units.
Connective tissue growth factor (CTGF, also known as CCN2) is an important pro-fibrotic cytokine in the Blm injury model (Barrientos et al., 2008; Ponticos et al., 2009). At day 14 after Blm, CCN2 mRNA expression in lung tissue was increased on average ~3.5 fold in Blm–treated compared to Sal-treated animals (p=0.005; Fig. 8C), without an effect of CIH noted. At day 35, CCN2 expression in Blm/Norm animals was indistinguishable from that of Sal-treated animals, whereas in Blm/CIH animals, CCN2 expression was 2.5-fold elevated compared to every other treatment group (p<0.005 for each comparison, Fig. 8D).
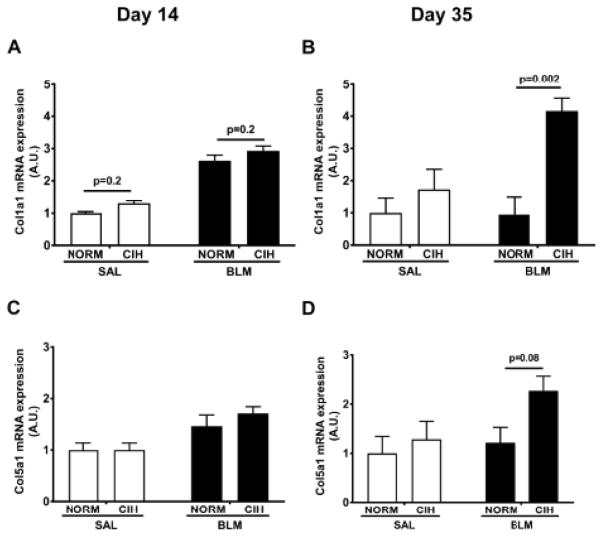
3.7. CIH prolonged the Blm-induced increase in Collagen 1 and Collagen 5 gene expression
Consistent with the increase in total collagen content (Fig. 2), the prolonged combined Blm and CIH treatments enhanced gene expression of Collagen 1 and 5 isoforms in lung tissue. At day 14, Blm had increased expression of Col1α1 mRNA compared to Sal (p<0.0001 for overall effect of Bleomycin; Fig 9A), but Blm/CIH were no different (p=0.20) from Blm/Norm animals. However, at day 35, CIH firmly established the increase in the Col1α1 mRNA expression in the Blm/CIH group compared to the Blm/Norm (p=0.002), Sal/Norm (p=0.02) and the Sal/CIH (p=0.03) groups, with no difference among the latter 3 groups (Fig. 9B). A similar pattern was seen with Col5α1, where at day 35 Blm tended to increase overall mRNA expression compared to Sal-treated animals (p=0.09 for overall Blm effect, 2-way ANOVA) and in post-hoc analyses, Blm/CIH rats trended towards increased expression compared to Blm/Norm (p=0.08, Fig 9D).
Figure 9. CIH increased Col1α1 and Col5α1 mRNA expression.
Col1α1 (A, B) and Col5α1 (C, D) mRNA expression in lung tissue was measured by qPCR on day 14 (A, C) or day 35 (B, D) after Blm instillation. A.U: Arbitrary Units.
4. Discussion
Our study tested the effects of CIH on the progression of pulmonary fibrosis induced by Blm in rats. Thirty days of CIH relative to normoxia exposure led to: 1) a rise in total lung collagen content (Fig. 2); 2) increased fibrosis and pathological thickening of alveolar septa (Figs. 3 and 4); 3) increased parenchymal stiffness (demonstrated by decreased compliance and tendency to increased elastance) (Fig, 5); 4) an increase in lipid peroxidation (Fig. 7A, B); 5) an increase in NF-κB protein activation (Fig. 7C, D); and 6) upregulation of IL-17 and CCN-2 (Fig. 8), and of Col1α1 and Col5α1 isoforms mRNA expression (Fig. 9) in lung tissue. Some of these effects (collagen content, Fig 2A; col1α1 expression in Fig. 9A) emerged with non-significant trends after 9 days of CIH exposure, while most effects noted were firmly established with the longer, 30-day CIH exposure, following Blm. These data highlight the potential of OSA – a years-long chronic condition – to promote and perpetuate fibrosis, leading to functional deficits in the injured lung. Additionally, we also observed that CIH alone, independent of Blm, caused some pathological changes to the lung parenchyma with the prolonged 30-day exposure. These consisted of a tendency to increase the total lung collagen content (Fig. 2B), a significant increase in the Ashcroft score (Fig. 3K) and a decrease in Cst (Fig. 5D), concurrent with an increase in lipid peroxidation (Fig 7B) in uninjured, Sal-treated lungs, suggesting potential long-term pathogenic effects of OSA in otherwise “healthy” individuals.
To the authors’ knowledge, this study is the first to report on effects of CIH, modeling OSA, on lung fibrosis following Blm. Prior studies in the context of Blm-lung injury, have used various protocols of hypoxic preconditiong which exerted protective effects against lung fibrosis (Berend, 1984; Lan et al., 2015). However, this “protective” paradigm, much like those used as therapy in other disease entities (Mateika et al., 2015; Mateika and Komnenov, 2017; Navarrete-Opazo and Mitchell, 2014), is markedly different from the CIH of OSA used herein, which has established detrimental effects in multiple other organ systems (Navarrete-Opazo and Mitchell, 2014). Along these lines, in our study, CIH appears to perpetuate and/or increase lung fibrosis following a single Blm instillation. A single intra-tracheal dose of Blm is known to induce interstitial and intra-alveolar inflammation with peribronchiolar fibrosis. Collagen gene expression is upregulated within a few days and fibrosis appears between days 7 to 28. However, a single dose of Blm does not induce a progressive disease, as inflammation and most fibrosis resolve within 6 weeks (Chung et al., 2003; Degryse et al., 2010) after injury. This time-course of development and resolution of Blm–induced fibrosis is evident in our Norm–exposed animals. At day 35, recovery is evident in Blm/Norm rats, as typically reported, since parenchymal elastance (Fig. 5F) and CCN2 expression (Fig. 8D) are indistinguishable from Sal-treated animals. However, this recovery is prevented by CIH treatment: 30 days of CIH increased the lipid peroxidation and collagen deposition in Blm-treated animals.
CIH seems to produce a more heterogenous type of remodeling to the lung parenchyma, a phenomenon we also recently reported in the allergic rat (Broytman et al., 2015). Along with an increased fibrosis, in the current study, we noted “emphysema-like” changes, as shown on trichorome (Fig. 3A–D) and α-SMA (Fig. 4A–D) stains. Intratracheal Blm is known to induce peribronchiolar fibrosis accompanied by paracicatricial emphysema – a morphology that matches some of the human IPF cases (Borzone et al., 2001). Corroborating with the increased fibrosis following Blm (Figs. 2, 3 and 4), we reason that CIH may enhance both these processes (ie, parenchymal fibrosis and “emphysema-like”) that have mutually antagonistic functional correlates. This may explain our lack of observed group differences among Blm-treated animals in the FVC and TLC (Fig. 6, C – F). Thus, more precise characterization of these structural changes with functionally antagonistic effects via imaging, as well as elucidating their molecular substrates, are paramount. Owing to the high prevalence and low recognition of OSA in IPF (Lancaster et al., 2009), this finding becomes highly relevant to the Combined Pulmonary Fibrosis and Emphysema (CPFE), the pathophysiology of which remains poorly understood (Cottin, 2013).
CIH appeared to also modulate the inflammatory process triggered by Blm. At day 14, Blm induced NF-κB activity to a much greater extent in Norm exposed animals than in animals exposed to CIH (Fig. 7C). However, at day 35, this pattern was reversed with elevated levels of NF-κB, suggesting ongoing inflammation and tissue stress, while in Blm/Norm animals the NF-κB activity had returned to control levels (Fig. 7D). NF-κB is an oxidant-sensitive transcription factor whose activation is known to promote inflammation and tissue injury in response to CIH (da Rosa et al., 2012; Dyugovskaya et al.) and in the context of lung disease (McNicholas, 2009). Neutrophil NF- κB activity is correlated to OSA severity (Htoo et al., 2006). Our data indicate that 9 days of CIH inhibits the NF-κB activation in the lung induced by Blm injury. However, this inhibition does not correlate with reduced oxidative stress, collagen deposition or CTGF expression. Therefore, even at this early time point, it is unlikely that CIH is exerting a “protective” effect and that our more intense daily hypoxic paradigm exerts similar effects to those reported in other models (Mateika et al., 2015; Mateika and Komnenov, 2017). Rather, it is possible that 9 days of CIH shifts Blm-driven intracellular signaling from NF-κB to other transcription factors, such as the HIF family (Gu et al., 2013; Yuan et al., 2008).
Increased IL-17 mRNA expression was also preserved in the lung tissue of Blm–injured animals at day 35 post-injury. Autoimmunity and production of IL-17 have been strongly linked with IPF pathogenesis (Feghali-Bostwick et al., 2007). Antibodies and TH-17 cell reactivity against collagen type 5 (Col5) are commonly found in IPF patients (Parra et al., 2006), while tolerance to Col5 prevents fibrosis in Blm-treated animals (Braun et al., 2010). The ongoing tissue damage and increased Col5 expression in CIH-treated animals may contribute to the induction of an autoimmune response, thereby creating a profibrotic microenvironment and driving pulmonary fibrosis. Further, more direct approaches to evaluate the effects of CIH on presence and extent of autoimmunity, as well as confirm the target collagen, are required. At day 14 post-injury CIH significantly inhibited the expression of IL-17 mRNA, but this inhibition did nothing to prevent bleomycin induced tissue injury. This again suggests that the short duration of CIH was not protective against the inflammation and injury caused by Bleomycin, but rather changed the signaling pathways through which the inflammation and injury were expressed in lung tissue.
Despite its novelty, this study has several limitations. We used a relatively mild intensity of exposures to model both diseases – a single (and relatively low dose) of Blm and no more than 30 days of CIH exposure. Multiple instillations of Blm induce repetitive injury and a far more severe and progressive phenotype, better modeling IPF in humans. Thirty days of CIH exposure in our model may not accurately reflect the extended exposure in OSA patients. OSA, and the negative effects thereof, often go undiagnosed for years (Young et al., 1997), if not for life. Most of our metrics do indicate a progressive increase with the longer duration of CIH tested. This suggests that the effects of oxidative stress and inflammatory response have likely not reached their peak. NFκB activation and lipid peroxidation values might reach significance with a longer timeframe of CIH. Future studies should consider extending the CIH exposure, to better model the human disease. Second, in selecting the CIH intensity, we aimed to replicate as best as possible the human disease. In the study of Lancaster et al, 77% of the 44 patients with IPF and PSG-diagnosed OSA had moderate-severe OSA, with desaturation indices on average of 35/hour and minimum SpO2 of 78% (Lancaster et al., 2009). Guided by these data, we used a moderate CIH severity paradigm of 30 desaturations/hour to an FIO2 of 10%, corresponding to a SpO2=80% (unpublished data). However, some of the patients had more severe OSA (desaturations index mean±SD = 35±19/hour) (Lancaster et al., 2009), warranting further animal studies employing more intense CIH protocols. Thirdly, one could posit that the rise in levels of some of the inflammatory markers, such as the NF-kB activation, in the Blm/CIH group could be the result of FlexiVent-induced trauma and alveolar damage. However, all the groups underwent the same FlexiVent testing arguing against this supposition. Finally, while we were able to collect compelling evidence of augmentation in lung fibrosis by CIH for most clinically relevant outcomes we set up to study, larger groups of animals would have likely solidified some of our mechanistic findings that we aimed to explore; also, larger samples are needed to allow disentangling the additive vs. synergistic nature of the CIH effects following Blm.
In conclusion, our clinically-relevant rodent model provides novel insights into effects and mechanisms that may underpin the deleterious outcomes of OSA in IPF. Our findings warrant further investigations to better understand the consequences of OSA/CIH on IPF progression and severity, and to relieve the effects of OSA in IPF. Additionally, these findings may be relevant to other lung diseases that involve fibrotic injury and autoimmune inflammatory responses, such as sarcoidosis, collagen vascular disease-associated and bronchiolitis obliterans syndrome.
Supplementary Material
Highlights.
Following bleomycin-induced lung injury, chronic intermittent hypoxia:
increased the total lung collagen content
induced heterogeneous structural changes that led to stiffer lungs
augmented oxidative and inflammatory pathways, culminating in enhanced collagen expression.
Acknowledgments
Financial Support: This study was supported by pilot research funds from the Department of Medicine, University of Wisconsin School of Medicine and Public Health (M. Teodorescu), and by grants NIH-NHLBI R01 – HL115061 (M. Eldridge) and NIH-NHLBI R01 Supplement HL1150613 (M. Eldridge).
The authors wish to thank Will Burlingham, Oleg Nicolaev, Natalie Morel, Nicolas Fesser, Drew Roennenburg for their help with experiments and scientific discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Berend N. Protective effect of hypoxia on bleomycin lung toxicity in the rat. The American review of respiratory disease. 1984;130:307–308. doi: 10.1164/arrd.1984.130.2.307. [DOI] [PubMed] [Google Scholar]
- Borzone G, Moreno R, Urrea R, Meneses M, Oyarzun M, Lisboa C. Bleomycin-induced chronic lung damage does not resemble human idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2001;163:1648–1653. doi: 10.1164/ajrccm.163.7.2006132. [DOI] [PubMed] [Google Scholar]
- Braun FM, Braun RK, Broytman O, Pegelow DF, Eldridge MW, Teodorescu M. LUNG INJURY, REPAIR, AND FIBROSIS: THE PLOT THICKENS FOR THREE’S COMPANY. American Thoracic Society; 2015. Chronic Intermittent Hypoxia Amplifies Oxidative Stress and Pulmonary Fibrosis Following Bleomycin-Induced Injury, A57; pp. A2080–A2080. [Google Scholar]
- Braun RK, Martin A, Shah S, Iwashima M, Medina M, Byrne K, Sethupathi P, Wigfield CH, Brand DD, Love RB. Inhibition of bleomycin-induced pulmonary fibrosis through pre-treatment with collagen type V. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2010;29:873–880. doi: 10.1016/j.healun.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Broytman O, Braun RK, Morgan BJ, Pegelow DF, Hsu PN, Mei LS, Koya AK, Eldridge M, Teodorescu M. Effects of Chronic Intermittent Hypoxia on Allergen-Induced Airway Inflammation in Rats. American journal of respiratory cell and molecular biology. 2015;52:162–170. doi: 10.1165/rcmb.2014-0213OC. [DOI] [PubMed] [Google Scholar]
- Cannito S, Paternostro C, Busletta C, Bocca C, Colombatto S, Miglietta A, Novo E, Parola M. Hypoxia, hypoxia-inducible factors and fibrogenesis in chronic liver diseases. Histology and histopathology. 2014;29:33–44. doi: 10.14670/HH-29.33. [DOI] [PubMed] [Google Scholar]
- Chung MP, Monick MM, Hamzeh NY, Butler NS, Powers LS, Hunninghake GW. Role of repeated lung injury and genetic background in bleomycin-induced fibrosis. American journal of respiratory cell and molecular biology. 2003;29:375–380. doi: 10.1165/rcmb.2003-0029OC. [DOI] [PubMed] [Google Scholar]
- Clithero A, Braun FM, Broytman O, Braun RK, Pegelow DF, Eldridge MW, Teodorescu M. BEAST IS INSIDE: WHAT CAUSES THE ADVERSE OUTCOMES OF SLEEP DISORDERED BREATHING. American Thoracic Society; 2015. Chronic Intermittent Hypoxia (CIH) Alters Macrophage Subpopulations and Collagen Gene Expression in the Lung, B30; pp. A2702–A2702. [Google Scholar]
- Cottin V. Syndrome of combined pulmonary fibrosis and emphysema: understanding the functional profile. Revue des maladies respiratoires. 2013;30:173–175. doi: 10.1016/j.rmr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Creemers LB, Jansen DC, van Veen-Reurings A, van den Bos T, Everts V. Microassay for the assessment of low levels of hydroxyproline. BioTechniques. 1997;22:656–658. doi: 10.2144/97224bm19. [DOI] [PubMed] [Google Scholar]
- da Rosa DP, Forgiarini LF, Baronio D, Feijo CA, Martinez D, Marroni NP. Simulating sleep apnea by exposure to intermittent hypoxia induces inflammation in the lung and liver. Mediators of inflammation. 2012;2012:879419. doi: 10.1155/2012/879419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. American journal of physiology. Lung cellular and molecular physiology. 2010;299:L442–452. doi: 10.1152/ajplung.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyugovskaya L, Polyakov A, Ginsberg D, Lavie P, Lavie L. Molecular pathways of spontaneous and TNF-{alpha}-mediated neutrophil apoptosis under intermittent hypoxia. American journal of respiratory cell and molecular biology. 45:154–162. doi: 10.1165/rcmb.2010-0025OC. [DOI] [PubMed] [Google Scholar]
- Feghali-Bostwick CA, Tsai CG, Valentine VG, Kantrow S, Stoner MW, Pilewski JM, Gadgil A, George MP, Gibson KF, Choi AM, Kaminski N, Zhang Y, Duncan SR. Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis. J Immunol. 2007;179:2592–2599. doi: 10.4049/jimmunol.179.4.2592. [DOI] [PubMed] [Google Scholar]
- Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, Fick L, Charron S, Lagente V, Eberl G, Le Bert M, Quesniaux VF, Huaux F, Leite-de-Moraes M, Ryffel B, Couillin I. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PloS one. 2011;6:e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu CJ, Li M, Li QY, Li N. Chronic intermittent hypoxia increases beta cell mass and activates the mammalian target of rapamycin/hypoxia inducible factor 1/vascular endothelial growth factor A pathway in mice pancreatic islet. Chinese medical journal. 2013;126:2368–2373. [PubMed] [Google Scholar]
- Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Archives of toxicology. 1991;65:81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- Htoo AK, Greenberg H, Tongia S, Chen G, Henderson T, Wilson D, Liu SF. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep & breathing = Schlaf & Atmung. 2006;10:43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- Hubner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. BioTechniques. 2008;44:507–511. 514–507. doi: 10.2144/000112729. [DOI] [PubMed] [Google Scholar]
- Lan YW, Choo KB, Chen CM, Hung TH, Chen YB, Hsieh CH, Kuo HP, Chong KY. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem cell research & therapy. 2015;6:97. doi: 10.1186/s13287-015-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Milstone AP, Collard HR, Malow BA. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136:772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. The Journal of biological chemistry. 2003;278:13008–13015. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- Leask A, Shi-Wen X, Khan K, Chen Y, Holmes A, Eastwood M, Denton CP, Black CM, Abraham DJ. Loss of protein kinase Cepsilon results in impaired cutaneous wound closure and myofibroblast function. Journal of cell science. 2008;121:3459–3467. doi: 10.1242/jcs.029215. [DOI] [PubMed] [Google Scholar]
- Lee AS, Mira-Avendano I, Ryu JH, Daniels CE. The burden of idiopathic pulmonary fibrosis: An unmet public health need. Respiratory medicine. 2014;108:955–967. doi: 10.1016/j.rmed.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Current hypertension reports. 2014;16:411. doi: 10.1007/s11906-013-0411-y. [DOI] [PubMed] [Google Scholar]
- Mateika JH, El-Chami M, Shaheen D, Ivers B. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J Appl Physiol (1985) 2015;118:520–532. doi: 10.1152/japplphysiol.00564.2014. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Komnenov D. Intermittent hypoxia initiated plasticity in humans: A multipronged therapeutic approach to treat sleep apnea and overlapping co-morbidities. Exp Neurol. 2017;287:113–129. doi: 10.1016/j.expneurol.2016.05.011. [DOI] [PubMed] [Google Scholar]
- McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. American journal of respiratory and critical care medicine. 2009;180:692–700. doi: 10.1164/rccm.200903-0347PP. [DOI] [PubMed] [Google Scholar]
- Mermigkis C, Bouloukaki I, Antoniou K, Papadogiannis G, Giannarakis I, Varouchakis G, Siafakas N, Schiza SE. Obstructive sleep apnea should be treated in patients with idiopathic pulmonary fibrosis. Sleep and Breathing. 2015;19:385–391. doi: 10.1007/s11325-014-1033-6. [DOI] [PubMed] [Google Scholar]
- Mermigkis C, Bouloukaki I, Antoniou KM, Mermigkis D, Psathakis K, Giannarakis I, Varouchakis G, Siafakas N, Schiza SE. CPAP therapy in patients with idiopathic pulmonary fibrosis and obstructive sleep apnea: does it offer a better quality of life and sleep? Sleep & breathing = Schlaf & Atmung. 2013;17:1137–1143. doi: 10.1007/s11325-013-0813-8. [DOI] [PubMed] [Google Scholar]
- Mermigkis C, Chapman J, Golish J, Mermigkis D, Budur K, Kopanakis A, Polychronopoulos V, Burgess R, Foldvary-Schaefer N. Sleep-related breathing disorders in patients with idiopathic pulmonary fibrosis. Lung. 2007;185:173–178. doi: 10.1007/s00408-007-9004-3. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Schmitt MJ, Verma IM. Qualitative changes in the subunit composition of kappa B-binding complexes during murine B-cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5056–5060. doi: 10.1073/pnas.91.11.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi DA, Braun RK, Broytman O, Pegelow DF, Eldridge MW, Teodorescu M. MULTI-FACETED EFFECTS OF INTERMITTENT HYPOXIA. American Thoracic Society; 2015. Chronic Intermittent Hypoxia (CIH) Exposure Leads to Progressive Pulmonary Dysfunction in Adult Sprague-Dawley Rats, C98; pp. A5152–A5152. [Google Scholar]
- Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1181–1197. doi: 10.1152/ajpregu.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan SY, Chang YT, Lin SL. The role of hypoxia-inducible factors in renal fibrosis. Journal of the Formosan Medical Association = Taiwan yi zhi. 2013;112:587–588. doi: 10.1016/j.jfma.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Parra ER, Teodoro WR, Velosa AP, de Oliveira CC, Yoshinari NH, Capelozzi VL. Interstitial and vascular type V collagen morphologic disorganization in usual interstitial pneumonia. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2006;54:1315–1325. doi: 10.1369/jhc.6A6969.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihtili A, Bingol Z, Kiyan E, Cuhadaroglu C, Issever H, Gulbaran Z. Obstructive sleep apnea is common in patients with interstitial lung disease. Sleep & breathing = Schlaf & Atmung. 2013;17:1281–1288. doi: 10.1007/s11325-013-0834-3. [DOI] [PubMed] [Google Scholar]
- Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, Hoyles RK, Bou-Gharios G, Black CM, Denton CP, Abraham DJ, Leask A, Lindahl GE. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis and Rheumatism. 2009;60:2142–2155. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- Ramos P, Rubies C, Torres M, Batlle M, Farre R, Brugada J, Montserrat JM, Almendros I, Mont L. Atrial fibrosis in a chronic murine model of obstructive sleep apnea: mechanisms and prevention by mesenchymal stem cells. Respiratory research. 2014;15:54. doi: 10.1186/1465-9921-15-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar E, Knowles JH. AN ANALYSIS OF PRESSURE-VOLUME CHARACTERISTICS OF THE LUNGS. Journal of applied physiology. 1964;19:97–104. doi: 10.1152/jappl.1964.19.1.97. [DOI] [PubMed] [Google Scholar]
- Sorkness RL, Szakaly RJ, Rosenthal LA, Sullivan R, Gern JE, Lemanske RF, Jr, Sun X. Viral Bronchiolitis in Young Rats Causes Small Airway Lesions that Correlate with Reduced Lung Function. American journal of respiratory cell and molecular biology. 2013;49:808–813. doi: 10.1165/rcmb.2013-0096OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Usinger W, Nichols B, Gray J, Xu L, Seeley TW, Brenner M, Guo G, Zhang W, Oliver N, Lin A, Yeowell D. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. The Journal of experimental medicine. 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Zhuang WL, Shen YJ, Lai CJ, Kou YR. NADPH Oxidase-Derived ROS Induced by Chronic Intermittent Hypoxia Mediates Hypersensitivity of Lung Vagal C Fibers in Rats. Frontiers in physiology. 2016;7:166. doi: 10.3389/fphys.2016.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IV, Schwartz DA. Epigenetics of idiopathic pulmonary fibrosis. Translational research: the journal of laboratory and clinical medicine. 2015;165:48–60. doi: 10.1016/j.trsl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. Journal of cellular physiology. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Guo X, Deng Y, Zhu D, Shang J, Liu H. Chronic intermittent hypoxia-induced neuronal apoptosis in the hippocampus is attenuated by telmisartan through suppression of iNOS/NO and inhibition of lipid peroxidation and inflammatory responses. Brain research. 2015;1596:48–57. doi: 10.1016/j.brainres.2014.11.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.