Abstract
Background
Evidence about the effect of initiating efavirenz-containing combination antiretroviral therapy (ART) as first-line therapy on suicidal thoughts remains conflicting.
Methods
Using data from a cohort of HIV-infected adults enrolled in routine care across 5 sites in the United States, we included participants with a baseline patient-reported outcome measure and detectable viral load who initiated ART between 2011 and 2014. Participants were followed until the earliest of the following: first suicidal thoughts, discontinuation of initial ART regimen, death, loss to care (>12 months with no HIV appointments), or administrative censoring (2014 to 2015). Suicidal thoughts were measured using a Patient Health Questionnaire-9 item. We used weighted marginal structural Cox models to estimate the effect of initiating efavirenz-containing ART, versus efavirenz-free ART, on the hazard of active or passive suicidal thoughts after ART initiation, accounting for confounding by channeling bias.
Results
Overall, 597 participants were followed for a median of 19 months (13,132 total person-months); 147 (25%) initiated efavirenz-containing ART. At ART initiation, 38% of participants reported suicidal thoughts or depressive symptoms. Initiating efavirenz-based ART was associated with a hazard ratio (HR) for suicidal thoughts below the null in the crude analysis (HR 0.88 (95% CI 0.53, 1.45), and above the null in the weighted analysis (HR 1.21, 95% CI 0.66, 2.28). Among those with a prior mental health issue, the weighted HR was 1.76 (95% CI 0.45, 6.86).
Conclusions
After accounting for measured channeling bias, we observed no strong evidence that initiating efavirenz-containing ART increased the hazard of suicidal thoughts.
Keywords: HIV, efavirenz, suicidal thoughts, suicidal ideation, antiretroviral therapy
Introduction
Efavirenz, a non-nucleoside reverse transcriptase inhibitor (NNRTI), has been a mainstay of antiretroviral therapy (ART) for over 15 years. Use of efavirenz is declining in the United States (US) due to the availability and tolerability of newer agents.1 However, the World Health Organization continues to recommend efavirenz globally as first-line ART.2,3 Efavirenz is attractive given its rapid and durable viral suppression, low cost, generic formulation, and availability in a fixed-dose combination pill.4 However, serious psychiatric side effects, including suicidal thoughts, have been reported with efavirenz.5 Given its widespread use, an accurate understanding of the relationship between efavirenz use and suicidal thoughts is critical.
Evidence about the effect of efavirenz on suicidal thoughts remains conflicting. Early clinical trials suggested that efavirenz may increase the risk of depression and suicidal thoughts.6 However, side effects were thought to be rare, transient, and in most cases not life-threatening. More recently, efavirenz was associated with a two-fold increase in the hazard of a combined outcome of suicidal thoughts, behaviors, or attempted or completed suicide in a pooled analysis of four randomized controlled trials from the AIDS Clinical Trials Group (ACTG).7 Efavirenz was also associated with suicidal behavior in a secondary analysis of the START trial.8 These results were not confirmed by data from the Food and Drug Administration Adverse Event Reporting System (FAERS)9 or when the relationship between efavirenz and completed suicide was evaluated in the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study.10
Two key considerations in studying the relationship between efavirenz and suicidal thoughts are accurate measurement of suicidal thoughts and the appropriate accounting for confounding by selective prescription or discontinuation of efavirenz. Previous analyses of efavirenz’s effect on suicidal outcomes have relied on adverse event reporting 7–10 or insurance claims 11, which may capture the most severe forms of suicidal thoughts. Additionally, the potential for clinicians to prescribe efavirenz-free regimens to persons with mental health issues (channeling-in bias) or to switch persons who develop mental health issues off of efavirenz (channeling-out bias)12 is an important consideration that has not been fully accounted for in previous observational analyses.9,10
To address these questions, we utilized data from a cohort of HIV-infected adults in routine care across the US who complete routine, systematic assessments of suicidal thoughts every 4–6 months. The goal of our analysis was to estimate the effect of initiating efavirenz-containing ART as first-line therapy on time to passive or active suicidal thoughts.
Methods
Data for the present analysis come from the Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) cohort. The CNICS cohort includes over 31,000 HIV-infected adults in routine care at 8 sites in the US.13 CNICS captures comprehensive clinical data that includes standardized diagnosis, medication, laboratory, and demographic information collected through electronic medical records and institutional data systems. Participants also complete self-administered questionnaires, or Patient-Reported Outcomes (PROs), on touch-screen tablets as part of routine care. PROs are completed approximately every 4–6 months, with variation based on clinical follow-up. Participants provide written informed consent to participate in CNICS. Ethical approval for the use of routinely collected clinical data was provided by the institutional review board at each CNICS site.
Study Population
Participants in CNICS were included if they initiated ART between 2011 and 2014 (timeframe when PRO data was available and efavirenz was recommended as first-line ART in the US4,14; n=2,729), at a site collecting PRO information (n=6 of 8 sites), and had a PRO measure within the 6 months prior or one week after initiating ART (n=875). Participants already taking efavirenz when PRO collection began may have previously experienced psychiatric side effects from efavirenz that resolved, and therefore were excluded. Information on previous ART use is not always available in CNICS. Therefore, participants with incomplete data on ART use (n=40) or who had a suppressed viral load at ART initiation (indicating possible prior ART use; n=223) were excluded. Participants from 1 site where no patients initiated efavirenz (n=15 participants) were excluded, since this site may differ from others where efavirenz was prescribed. Our interest was in understanding suicidal thoughts occurring as a result of initiating efavirenz. Further, clinicians are unlikely to have access to information on a patient’s suicidal thoughts when making decisions about initial ART regimen. For these reasons, patients with suicidal thoughts at ART initiation were not excluded. Participants were followed from ART initiation until the earliest of the following dates: a PRO measure indicating suicidal thoughts, discontinuation of ART (defined as switching to an efavirenz-containing or efavirenz-free regimen, depending on initial regimen, or ART discontinuation entirely), loss to HIV care (12 months with no attended HIV appointments), or administrative censoring (October 2014 to September 2015, depending on site).
Measures
The primary exposure was initial ART regimen, categorized as efavirenz-containing or efavirenz-free. An ART regimen was defined as being on ≥ 3 antiretroviral drugs, including at least 1 NNRTI, protease inhibitor, or integrase inhibitor for ≥ 21 days.1 The outcome of interest was passive or active suicidal thoughts (hereafter suicidal thoughts) following ART initiation. Suicidal thoughts are measured as part of the CNICS PROs using one item of the Patient Health Questionnaire-9 (PHQ-9)15, which asks in a combined question whether participants had any thoughts of being better off dead (passive) or hurting themselves (active) in the previous two weeks. The PHQ-9 is a validated measure to identify suicidal thoughts, with sensitivity 65–90% and specificity 80–>90%.16,17 We did not evaluate attempted or completed suicide or ART discontinuation due to psychiatric side effects. CNICS does not collect information on reasons for ART discontinuation or suicide attempts, and cause of death information is only available for a portion of the population. Of those with cause of death information, very few were reported as suicides.
CNICS PROs collect additional patient-reported data using validated measures, including: ART adherence (AIDS Clinical Trials Unit-4 Visual Analog Scale, defined as no missed doses in the past week 18,19), depressive symptom severity (defined as a binary measure with cut-point PHQ-9 ≥ 10 and a continuous score 15), panic disorder (PHQ-5, defined as some panic disorder symptoms or panic disorder 20), high risk alcohol use (Alcohol Use Disorders Identification Test (AUDIT), defined as AUDIT score ≥ 4 for males and ≥ 3 for females 21), and current or past illicit drug use, excluding marijuana (Alcohol, Smoking and Substance Involvement Screening Test (ASSIST)22,23). Baseline depressive symptoms and suicidal thoughts were combined into a 3-level variable: no depressive symptoms or suicidal thoughts, depressive symptoms without suicidal thoughts, or suicidal thoughts (with or without depressive symptoms), due to collinearity issues in the weights model between depressive symptoms and suicidal thoughts.
Self-reported race/ethnicity was categorized as white, black, Hispanic, or other and HIV acquisition risk group as men who had sex with men (MSM), heterosexuals, intravenous drug users (IDU), or other and collected at CNICS enrollment. CNICS captures prior clinician-documented mental health or medical diagnoses in the medical record. We defined a prior medical diagnosis as any previous medical diagnosis at the time of ART initiation. A prior mental health disorder was defined as any previous diagnosis of depression, bipolar disorder, post-traumatic stress disorder (PTSD) or psychosis, as these diagnoses are most likely to affect suicidal thoughts and influence efavirenz prescription.24,25 Undetectable viral load was defined as <50 copies/mL.
Statistical Analysis
The goal of our analysis was to estimate the effect of initiating efavirenz-containing ART on time to suicidal thoughts. We used weighted Kaplan-Meier curves and marginal structural Cox models with inverse probability weights 26 to approximate a study in which individuals were randomized at baseline to initiate efavirenz-containing or efavirenz-free ART. We developed three sets of weights; inverse probability of treatment weights (IPTW) to account for potential channeling-in bias in receipt of efavirenz, inverse probability of censoring weights (IPCW) to account for bias due to loss to follow-up, and inverse probability of observation weights to account for the varying frequency of PRO measurements between participants. Additional details about the creation of the weighs are available in the Supplemental File. Directed acyclic graphs were used to identify covariates for inclusion in each set of weights.27 For all weights, restricted cubic splines28 were used for continuous variables whenever possible; any categorization was based on the functional form of the relationship between the covariate and outcome. Due to limited sample size, for both race/ethnicity and HIV acquisition participants reporting “other” were collapsed with the largest category (white and MSM) for statistical analyses.
We conducted three secondary analyses. First, we examined mental health status at baseline (defined as a prior mental health diagnosis, reported panic symptoms or disorder, depressive symptoms/suicidal thoughts, or an antidepressant prescription at ART initiation) as an effect measure modifier of the efavirenz/suicidal thoughts relationship. Second, we considered ART for ≥ 7 days as an ART regimen. Third, we examined an outcome of more frequent suicidal thoughts (defined as occurring half the days or more in the last 2 weeks). We compared crude and weighted estimates for both the primary and secondary analyses and assessed the proportional hazards assumption. All analyses were conducted in Stata version 13 (StataCorp, College Station, Texas) or SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Overall, 597 CNICS participants met our inclusion criteria (Figure 1). Characteristics between all those who initiated ART, those without a valid PRO, and the study population were similar (Table S2). At ART initiation, 147 (25%) initiated efavirenz-containing ART. Additional information on initial ART regimens in available in Table S3. Participants were followed for a median of 19 months and contributed 13,132 person-months of follow-up. Overall, participants were predominately male (90%), white non-Hispanic/other (57%), and reported contracting HIV through being MSM/other (74%; Table 1). Mental health issues were common. At ART initiation, over a third (38%) reported depressive symptoms (20%) or suicidal thoughts and/or depressive symptoms (18%), 11% were prescribed antidepressants, and 31% had a prior depression, PTSD, bipolar or psychosis diagnosis. During the follow-up period, 89 (15%) participants reported suicidal thoughts after ART initiation, 4 (1%) died, 140 (23%) were lost to follow-up, 74 (12%) discontinued ART, and 290 (49%) were administratively censored.
Figure 1.
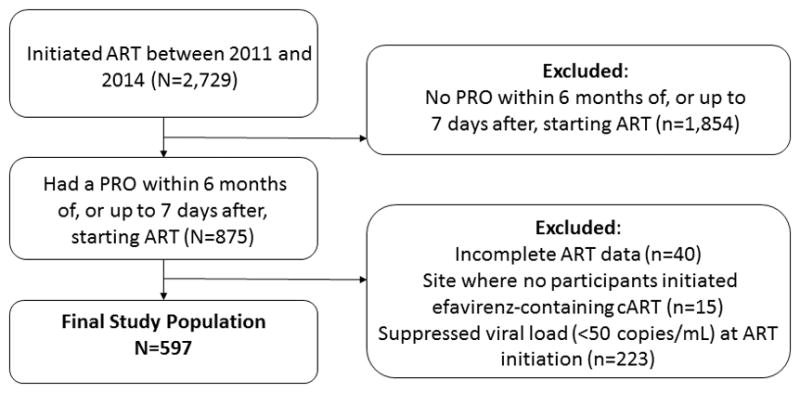
Inclusion criteria for 597 HIV-infected adults in CNICS who initiated ART between 2011 and 2014 and had a Patient Reported Outcome (PRO) within 6 months prior or up to 1 week after initiating ART and had a detectable viral load at ART initiation.
Table 1.
Clinical and demographic characteristics at ART initiation of 597 participants in CNICS: 2011–2014.
|
|
|
|
|---|---|---|
| Baseline Characteristics | EFV-free ART | EFV-containing ART |
| 450 (75.4) | 147 (24.6) | |
| N(%) or Mean (SD) | N(%) or Mean (SD) | |
|
|
|
|
| Year initiated ART | ||
| 2011 | 79 (17.6) | 76 (51.7) |
| 2012 | 121 (26.9) | 39 (26.5) |
| 2013 | 138 (30.7) | 25 (17.0) |
| 2014 | 112 (24.9) | 7 (4.8) |
| Age | 36 (28, 44) | 34 (29, 47) |
| Gender | ||
| Male | 396 (88.0) | 139 (94.6) |
| Female | 54 (12.0) | 8 (5.4) |
| Race/ethnicity | ||
| White, non-Hispanic | 219 (48.9) | 75 (51.7) |
| Black, non-Hispanic | 98 (21.9) | 37 (25.5) |
| Hispanic | 95 (21.2) | 26 (17.9) |
| Other | 36 (8.0) | 7 (4.8) |
| HIV risk group | ||
| MSM | 304 (67.6) | 114 (78.1) |
| IDU | 56 (12.4) | 13 (8.9) |
| Heterosexual | 69 (15.3) | 19 (13.0) |
| Other | 21 (4.7) | 0 (0.0) |
| Depression and suicidal ideation | ||
| No depression or suicidal ideation | 268 (59.6) | 102 (69.4) |
| Depression | 90 (20.0) | 27 (18.4) |
| Suicidal ideation, with or without depression | 92 (20.4) | 18 (12.2) |
| Antidepressant use | ||
| Not on an antidepressant | 390 (87.4) | 136 (93.8) |
| On an antidepressant | 56 (12.6) | 9 (6.2) |
| Panic Disorder | ||
| No symptoms | 279 (62.3) | 116 (80.6) |
| Some symptoms | 93 (20.7) | 16 (11.1) |
| Panic disorder | 76 (17.0) | 12 (8.3) |
| Drug use | ||
| No use | 166 (42.7) | 72 (50.7) |
| Current use | 113 (29.0) | 29 (20.4) |
| Past use | 110 (28.3) | 41 (28.9) |
| Alcohol use | ||
| Not at risk drinking | 107 (24.3) | 42 (29.2) |
| At risk drinking | 333 (75.7) | 102 (70.8) |
| Prior mental health diagnosis1 | ||
| No | 305 (67.8) | 107 (72.8) |
| Yes | 145 (32.2) | 40 (27.2) |
| Prior medical diagnosis1 | ||
| No | 386 (85.8) | 129 (87.8) |
| Yes | 64 (14.2) | 18 (12.2) |
| CD4 count, cells/mm3 | 390 (241, 545) | 417 (276, 530) |
At the time of ART initiation.
MSM: men who have sex with men, IDU: intravenous drug use. Missing data: race/ethnicity n=4, HIV risk group n=1, antidepressant use n=6, panic disorder n=5, drug use n=66, alcohol use n=13, CD4 count n=3.
In the crude analysis, those starting efavirenz-containing ART reported a slightly lower hazard of suicidal thoughts than those starting efavirenz-free ART (HR 0.88, 95% CI 0.53, 1.45; Table 2). At 36 months after ART initiation, the unadjusted risk of suicidal thoughts for persons initiating efavirenz-free ART was 21% and for persons initiating efavirenz-containing ART was 19% (Risk Difference [RD] −0.02, 95% CI −0.12, 0.06; Table 3 and Figure 2).
Table 2.
Hazard Ratios (HR) for the effect of initiating efavirenz-containing ART on time to suicidal thoughts among 597 HIV-infected adults initiating ART between 2011–2014.
| Unadjusted | Weighted | ||||
|---|---|---|---|---|---|
|
| |||||
| Initial ART Regimen | No. of Events | Person Months | Incidence Rate1 | HR (95% CI) | HR (95% CI)2 |
| Efavirenz-free ART | 69 | 9,690 | 7.12 | 1.00 | 1.00 |
| Efavirenz-containing ART | 20 | 3,442 | 5.81 | 0.88 (0.53, 1.45) | 1.21 (0.65, 2.28) |
Incidence rate per 1,000 person-months.
Weighted to account for potential channeling-in bias in efavirenz prescription, missing PRO assessments, and informative censoring due to loss to follow-up.
Mean of combined weights 0.99 (range: 0.15 to 7.67).
Table 3.
Risks and risk differences (RD) for suicicidal thoughts 6, 12, and 36 months after ART initiation by initial ART regimen among 597 HIV-infected adults initiating ART between 2011–2014.
| Crude | Weighted | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Months since ART initiation | Efavirenz-free ART | Efavirenz-containing ART | Risk Difference (95% CI)* | Efavirenz-free ART | Efavirenz-containing ART | Risk Difference (95% CI)* |
|
|
|
|||||
| 12 months | 0.12 | 0.10 | −0.02 (−0.07, 0.04) | 0.10 | 0.13 | 0.03 (−0.02, 0.10) |
| 36 months | 0.21 | 0.19 | −0.02 (−0.12, 0.06) | 0.22 | 0.25 | 0.03 (−0.10, 0.25) |
95% CI are derived from bootstraps (n=1000 for crude risk differences and n=100 for weighted risk differences, due the large size of the dataset).
Figure 2.
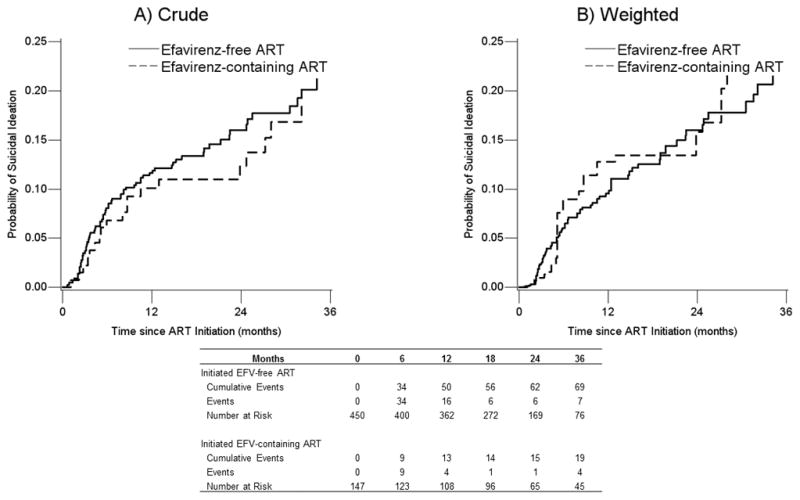
A) Unadjusted and B) Weighted Kaplan-Meier curves showing the cumulative probability of suicidal ideation for individuals who initiated efavirenz-containing ART and those who initiated efavirenz-free ART in CNICS between 2011 and 2014 through 36 months (1 event occurred after 36 months). Weighted estimates are weighted by the product of inverse probability of treatment weights to account for challenging-in bias, inverse probability of censoring weights to account for potentially informative loss to follow-up, and inverse probability of observation weights to account for not having an observed patient reported outcome assessment at least once within a 6-month period.
In the weighted analysis, those starting efavirenz-containing ART evidenced a slightly higher (but less precise) hazard of suicidal thoughts than those starting efavirenz-free ART (HR 1.21, 95% CI 0.65, 2.28; Table 2). At 36 months after ART initiation, the weighted risk of suicidal thoughts for persons initiating non-efavirenz ART was 22% compared to 25% for persons initiating efavirenz-containing ART (RD 0.03, 95% CI −0.10, 0.25; Table 3 and Figure 2). Covariates included in the IPTW were no longer associated with efavirenz initiation in the weighted analysis (Table S1). The proportional hazards assumption was met in the crude analysis (exposure by log time interaction, p-value 0.33), but not in the weighted analysis (p-value 0.02); however this is likely due to the relatively constant risk of suicidal thoughts for efavirenz users during year 2 (Figure 2).
Weighted effect estimates were similar in a secondary analysis where the minimum time on an ART regimen was 7 days (HR 1.28, 95% CI 0.67, 2.43). When baseline mental health status was considered as an effect measure modifier, effect estimates suggested that initiating efavirenz-containing ART may have a strong effect among those with mental health issues (HR 1.76, 95% CI 0.45, 6.86; HR among those without mental health issues: 0.96, 95% CI 0.23, 4.01); however confidence intervals were wide and overlapping. In a secondary analysis examining an outcome of more frequent suicidal thoughts, the HR was 0.61 (95% CI 0.10, 3.62), although there were a small number of events (n=28).
Discussion
In our cohort of HIV-infected adults initiating ART between 2011 and 2014, 25% of participants initiated ART containing efavirenz and 15% of participants reported experiencing suicidal thoughts over a median 19 months (76 weeks) of follow-up. The crude analysis indicated that those starting efavirenz-containing ART were slightly less likely to have suicidal thoughts. In contrast, in the weighted analysis those starting efavirenz-containing ART were slightly more likely to have suicidal thoughts, after accounting for channeling bias, informative censoring, and missing data. In both cases, the effect estimates were modest and 95% confidence intervals included the null. When stratified by baseline mental health status, effect estimates suggested efavirenz may have a stronger effect on suicidal thoughts among those with a prior mental health diagnosis. Our results were robust to changes in the specification of the minimum length of an ART regimen and when an outcome of more frequent suicidal thoughts was considered.
Our findings are consistent with the results of two observational analyses examining the relationship between efavirenz use and suicidal thoughts or behaviors9,11. An analysis of current efavirenz use and completed suicide, which did not account for channeling bias in efavirenz prescription, also showed no evidence of an association.10 However, a pooled analysis of 4 clinical trials found that participants randomized to an initial ART regimen with efavirenz had twice the hazard of developing suicidal thoughts, attempting, or completing suicide (composite outcome) over a median 96 weeks of follow-up, compared to those randomized to efavirenz-free ART.7 A secondary analysis of the SMART trial, which randomized participants to immediate versus delayed ART initiation, also found an increased risk of suicidal behavior among participants who initiated efavirenz in the immediate arm, compared to persons in the deferred arm before they initiated ART.8
Differences in the measurement of suicidal thoughts may lead to conflicting evidence about efavirenz’s effect on suicidal behaviors. In our analysis, suicidal thoughts were measured systematically and at regular intervals using the PHQ-9, as part of the PROs.15 In previous studies, including clinical trials, suicidal thoughts have been measured using adverse event reporting systems.7–10 Adverse event reporting systems may be more likely to capture severe active suicidal thoughts rather than the broader pool of passive or active suicidal thoughts measured by the PHQ-9.29 It is possible that efavirenz increases the more severe form of active suicidal thoughts, which may help to explain the effect of efavirenz on suicidal thoughts and behavior observed in clinical trials. However, this hypothesis has not been tested. Passive thoughts are much more common than active thoughts29, and may help to explain the attenuated result in this study.
A strength of this study is the use if IPTWs to address channeling-in bias. Reports of psychiatric side effects from efavirenz have led many clinicians to avoid prescribing efavirenz for persons with a mental health diagnosis (“channeling-in”) and to switch persons who develop mental health issues off efavirenz (“channeling-out”).12 While CNICS does not collect information on provider reasons for prescription or discontinuation, our analysis, and others, addressed channeling-in bias by balancing measured covariates between those initiating efavirenz and those not through IPTWs. 11 In our weighted data, none of the variables included in the IPTW were associated with efavirenz use (Table S1), suggesting the IPTW properly balanced the likelihood of receiving efavirenz conditional on included and measured covariates. However, residual confounding by unmeasured, and possibly difficult to measure, factors may remain. In our analysis, channeling-out bias was considered negligible due to the small number of persons who discontinued ART during the follow-up period.
In our cohort, 89 persons experienced suicidal ideation during follow-up. This was higher than the number of events reported in the pooled analysis of 4 ACTG trials (47 events in the efavirenz group and 15 events in the efavirenz-free group) that reported a HR of 2.28 for composite suicidal behaviors or suicide with efavirenz use.7 Thus, the wide confidence intervals reported in our analysis are unlikely to be driven solely by a lack of statistical power. Of note, the confidence interval for our weighted estimate did overlap with the results reported in the pooled analysis. While our sample size (n=597) may be small relative to the larger CNICS cohort, a larger source of observational data with validated measures of suicidal thoughts is unlikely to be available in the future.
As with all observational data, the internal validity of our findings rests on several assumptions. Our analysis assumes no unmeasured confounding and correct model specification.30 We note the possibility of residual or unmeasured confounding and a violation of the proportional hazards assumption in the weighted analysis as limitations. Second, we assume consistency, or the idea that initiating efavirenz represents a well-defined treatment 31; a reasonable assumption given standardized current ART treatment guidelines. Finally, our analysis assumes that there is a non-zero probability for all participants of being in either exposure category (efavirenz-containing or efavirenz-free) within all strata of measured covariates in the data; also a plausible assumption.30 Finally, we note that our analysis focuses on an outcome of suicidal ideation; evidence about efavirenz’s relationship with major depression and other neuropsychiatric side effects, which may be independent of suicidal ideation32,33, have been reported elsewhere.12
Clinicians face difficult choices about whether to prescribe efavirenz, given its possible neuropsychiatric side effects. Our results from the largest cohort of HIV-infected adults in care in the US, suggest that initiating an efavirenz-containing regimen does not meaningfully increase the risk of suicidal thoughts. For participants with a history of mental health issues, efavirenz may have a stronger effect on suicidal thoughts. Differences in our results from those seen in randomized controlled trials may stem from important differences in study populations, or more likely, from the fact that adverse event reporting systems in clinical trials are more likely to capture more sever forms of suicidal thoughts. Notably, similar proportions of participants with prior mental health diagnoses were included in our study and clinical trials,7 suggesting differences in study populations are unlikely to fully explain disparate results.
In our analysis of HIV-infected adults in routine care, initiating efavirenz-containing ART did not result in an increased hazard of suicidal thoughts. Our results come from one of the largest sources of data on efavirenz use among HIV-infected adults in the US; however, they may be less generalizable to resource-limited settings where fewer drug choices could lead to less channeling of efavirenz and mental health issues may be less likely to be recognized or treated.34,35 Given the widespread and continuing use of efavirenz globally, screening for and monitoring, suicidal thoughts among efavirenz users is recommended. Further research is needed, particularly in global settings, to clarify the relationship between efavirenz and suicidal thoughts.
Supplementary Material
Acknowledgments
BWP, RDM, CO, JJE, MMK, WCM, MJM contributed to the acquisition of the data; AMB, BWP designed the analysis; AMB drafted the manuscript; BWP, RDM, CO, EFE, JKE, JJE, MMK, WCM, KRM, MJM assisted with the interpretation of the data and critically revised the manuscript for important intellectual content. All authors take responsibility for and approve the final version of the manuscript. We thank the National Institutes of Mental Health [grant number R01MH100970] and the National Institute of Allergy and Infectious Diseases [grant numbers R24AI067039 and P30 AI50410] for their support of this work.
Funding: This work was supported by the National Institutes of Mental Health [grant number R01MH100970 to BWP] and by the National Institute of Allergy and Infectious Disease [grant numbers R24AI067039, P30AI50410].
Footnotes
Presentation: A version of this work was presented at the 20th International Workshop on HIV Observational Databases (IWHOD) April 7–9, 2016 in Budapest, Hungary.
Conflicts of Interest: K.R.M has received research support from grants awarded to UNC from Merck, AbbVie, and Gilead. E.F.E. was the recipient of a Bristol Myers-Squibb Virology Fellows Research Grant. Grants from Merck have been received by University of Alabama at Birmingham (UAB) on behalf of E.F.E. The other authors report no conflicts of interest.
References
- 1.Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2016 http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 2.Raffi F, Pozniak AL, Wainberg MA. Has the time come to abandon efavirenz for first-line antiretroviral therapy? The Journal of antimicrobial chemotherapy. 2014 Jul;69(7):1742–1747. doi: 10.1093/jac/dku058. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach. 2010. [PubMed] [Google Scholar]
- 4.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2015 Available at https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL003400.pdf.
- 5.Kryst J, Kawalec P, Pilc A. Efavirenz-Based Regimens in Antiretroviral-Naive HIV-Infected Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One. 2015;10(5):e0124279. doi: 10.1371/journal.pone.0124279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Squibb B-M, editor. Efavirenz [Package insert] Princeton, NJ: 2013. [Google Scholar]
- 7.Mollan KR, Smurzynski M, Eron JJ, et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med. 2014 Jul 1;161(1):1–10. doi: 10.7326/M14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arenas-Pinto Alejandro, Grund Birgit, Sharma Shweta, et al. Increased risk of suicidal behaviour with use of efavirenz: Results from the START Trial. Paper presented at: AIDS; 2016; Durban, South Africa. 2016. [Google Scholar]
- 9.Napoli AA, Wood JJ, Coumbis JJ, Soitkar AM, Seekins DW, Tilson HH. No evident association between efavirenz use and suicidality was identified from a disproportionality analysis using the FAERS database. J Int AIDS Soc. 2014;17:19214. doi: 10.7448/IAS.17.1.19214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith C, Ryom L, Monforte A, et al. Lack of association between use of efavirenz and death from suicide: evidence from the D:A:D study. J Int AIDS Soc. 2014;17(4 Suppl 3):19512. doi: 10.7448/IAS.17.4.19512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nkhoma ET, Coumbis J, Farr AM, et al. No Evidence of an Association Between Efavirenz Exposure and Suicidality Among HIV Patients Initiating Antiretroviral Therapy in a Retrospective Cohort Study of Real World Data. Medicine. 2016 Jan;95(3):e2480. doi: 10.1097/MD.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of efavirenz. AIDS Behav. 2011 Nov;15(8):1803–1818. doi: 10.1007/s10461-011-9939-5. [DOI] [PubMed] [Google Scholar]
- 13.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008 Oct;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. 1999 https://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL05051999011.pdf.
- 15.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altura KC, Patten SB, Fiest KM, Atta C, Bulloch AG, Jette N. Suicidal ideation in persons with neurological conditions: prevalence, associations and validation of the PHQ-9 for suicidal ideation. Gen Hosp Psychiatry. 2016 Sep-Oct;42:22–26. doi: 10.1016/j.genhosppsych.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Uebelacker LA, German NM, Gaudiano BA, Miller IW. Patient health questionnaire depression scale as a suicide screening instrument in depressed primary care patients: a cross-sectional study. The primary care companion for CNS disorders. 2011;13(1) doi: 10.4088/PCC.10m01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000 Jun;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 19.Amico KR, Fisher WA, Cornman DH, et al. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquir Immune Defic Syndr. 2006 Aug 1;42(4):455–459. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 21.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT, The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Care. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 22.The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002 Sep;97(9):1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 23.Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008 Jun;103(6):1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 24.Akiskal HS, Benazzi F. Psychopathologic correlates of suicidal ideation in major depressive outpatients: is it all due to unrecognized (bipolar) depressive mixed states? Psychopathology. 2005 Sep-Oct;38(5):273–280. doi: 10.1159/000088445. [DOI] [PubMed] [Google Scholar]
- 25.Ashrafioun L, Pigeon WR, Conner KR, Leong SH, Oslin DW. Prevalence and correlates of suicidal ideation and suicide attempts among veterans in primary care referred for a mental health evaluation. J Affect Disord. 2016 Jan 1;189:344–350. doi: 10.1016/j.jad.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology (Cambridge, Mass) 2000;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999 Jan;10(1):37–48. [PubMed] [Google Scholar]
- 28.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr Splines for trend analysis and continuous confounder control. Epidemiology (Cambridge, Mass) 2011;22(6):874–875. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinlivan EB, Gaynes BN, Lee JS, et al. Suicidal Ideation is Associated with Limited Engagement in HIV Care. AIDS Behav. 2016 Jul 5; doi: 10.1007/s10461-016-1469-8. [DOI] [PubMed] [Google Scholar]
- 30.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology (Cambridge, Mass) 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 31.VanderWeele TJ. Concerning the consistency assumption in causal inference. Epidemiology. 2009 Nov;20(6):880–883. doi: 10.1097/EDE.0b013e3181bd5638. [DOI] [PubMed] [Google Scholar]
- 32.Cuijpers P, de Beurs DP, van Spijker BA, Berking M, Andersson G, Kerkhof AJ. The effects of psychotherapy for adult depression on suicidality and hopelessness: a systematic review and meta-analysis. J Affect Disord. 2013 Jan 25;144(3):183–190. doi: 10.1016/j.jad.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Weitz E, Hollon SD, Kerkhof A, Cuijpers P. Do depression treatments reduce suicidal ideation? The effects of CBT, IPT, pharmacotherapy, and placebo on suicidality. J Affect Disord. 2014;167:98–103. doi: 10.1016/j.jad.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 34.Patel V. Mental health in low- and middle-income countries. Br Med Bull. 2007;81–82:81–96. doi: 10.1093/bmb/ldm010. [DOI] [PubMed] [Google Scholar]
- 35.Semrau M, Evans-Lacko S, Alem A, et al. Strengthening mental health systems in low- and middle-income countries: the Emerald programme. BMC Med. 2015 Apr 10;13:79. doi: 10.1186/s12916-015-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. American Journal of Epidemiology. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ., Jr Selection Bias Due to Loss to Follow Up in Cohort Studies. Epidemiology. 2016 Jan;27(1):91–97. doi: 10.1097/EDE.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernan MA, McAdams M, McGrath N, Lanoy E, Costagliola D. Observation plans in longitudinal studies with time-varying treatments. Stat Methods Med Res. 2009 Feb;18(1):27–52. doi: 10.1177/0962280208092345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


