Abstract
Defect in the complex I of the mitochondrial electron-transport chain is a characteristic of Parkinson’s disease (PD) which is thought to play a critical role in the disease pathogenesis. Mutations in vacuolar sorting protein 35 (VPS35) cause autosomal dominant PD and we recently demonstrated that pathogenic VPS35 mutations cause mitochondrial damage through enhanced mitochondrial fragmentation. In this study, we aimed to determine whether pathogenic VPS35 mutation impacts the activity of complex I and its underlying mechanism. Indeed, VPS35 D620N mutation led to decreased enzymatic activity and respiratory defects in complex I and II in patient fibroblasts. While no changes in the expression of the complex I and II subunits were noted, the level of assembled complex I and II as well as the supercomplex was significantly reduced in D620N fibroblasts. Importantly, inhibition of mitochondrial fission rescued the contents of assembled complexes as well as the functional defects in complex I and II. Overall, these results suggest that VPS35 D620N mutation-induced excessive mitochondrial fission leads to the defects in the assembled complex I and supercomplex and causes bioenergetics deficits.
Keywords: Parkinson’s disease, Mitochondrial fission, VPS35, complex I deficits, Blue native gel electrophoresis
1. Introduction
Parkinson’s disease (PD) is a chronic neurodegenerative disease and was first described as “shaking palsy” by James Parkinson in 1817 [1]. It is the second most common neurodegenerative disease after Alzheimer’s disease [2]. The prevalence of PD increases with age, affecting more than 1% of the population over the age of 60 and about 4% at the age of 80 [3]. Sporadic PD accounts for the majority of PD cases with less than 10% of all cases being familial. Mutations in α-synuclein, leucine-rich repeat kinase 2 (LRRK2), PTEN-induced kinse 1 (PINK1), parkinson protein 7 (PARK7, commonly known as DJ-1), parkin RBR E3 ubiquitin protein ligase (PARK2, commonly known as Parkin) have been associated with familial forms of PD (fPD) [4–9].
Although the pathogenic mechanism of PD remains elusive, it is well documented that mitochondrial dysfunction represents a critical event during the course of PD [10]. Significant deficits in the expression and activity of mitochondrial electron transport chain complex I were consistently found in the substantia nigra of PD patients [11–17] and specific inhibition of complex I by MPTP or rotenone caused Parkinsonism in rodents, primates and humans [10]. Importantly, fPD-related proteins such as alpha-synuclein, PINK1, Parkin, DJ-1 and LRRK2 are localized to mitochondria and impact mitochondrial function through regulation of mitochondrial dynamics and quality control [18]. Recent studies demonstrated that PINK1 or DJ-1 deficiency may impact complex I activity by altering the mitochondrial respiratory chain organization [19].
Recently, it has shown that mutation of VPS35 causes a rare, autosomal dominant form of PD and the clinical phenotype is similar to sporadic PD [20, 21]. The frequency of the D620N mutation is estimated to be about 1.5% in familial PD [22]. VPS35 is a subunit of the cargo-recognition subcomplex of the large protein complex retromer, a master conductor of endosomal sorting and trafficking involved in the endosome-to-Golgi and endosome-to-plasma membrane retrieval of membrane proteins [23, 24]. We recently found that VPS35 and retromer could recognize mitochondrial dynamin like protein 1 (DLP1) complex by binding to DLP1 through a conserved FLV domain at the C-terminus and plays an essential role in the recycling of this fission-inhibitory mitochondrial DLP1 complex via mitochondria-derived vesicle-dependent trafficking and lysosomal degradation [25, 26]. Furthermore, the pathogenic VPS35 mutant increased VPS35-DLP1 interaction and enhanced mitochondrial fission which leads to mitochondrial fragmentation and dysfunction [25, 26]. In this study, we aimed to determine whether pathogenic VPS35 mutation impacts the activity of complex I and its underlying mechanism.
2. Materials and Methods
2.1 Cell culture
Primary human fibroblast from a PD patient with the VPS35 D620N mutation was generated and characterized as previously published [27]. Primary human fibroblasts from a gender- and age-matched normal subject (normal human fibroblasts, NHFs) was obtained from Coriell Institute for Medical Research. Primary fibroblasts were maintained and treated with 10 µM mdivi-1 or vehicle (i.e., DMSO) for 24 hr as we previously published [25, 26].
2.2 Chemical and antibodies
Unless specified, all the chemicals were purchased from Sigma. Mdivi-1 was purchase from Enzo. Mouse anti-OXPHOS (Abcam, ab110413) and mouse anti-VDAC1 (Abcam, ab14734) were used in this study.
2.3 Mitochondria isolation
Mitochondria were isolated as described previously [28], then the mitochondria fraction was resuspended in mitochondria resuspension buffer (250 mM mannitol, 5 mM HEPES and 0.5 mM EGTA, pH 7.4) and used for enzymatic activity and protein assays.
2.4 Measurement of respiratory activity
Cultured fibroblasts were collected into prewarmed mitochondria respiration medium (110 mM sucrose, 60 mM potassium lactobionate, 0.5 mM EGTA, 3 mM MgCl2 ·6H2O, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, and 2 mg/ml bovine serum albumin, pH 7.1). Oxygen consumption rate (OCR) was measured with an Oxygraph-2K system (Oroboros, Austria) as described previously [29]. A 2-mL cell suspension (600,000cells/ml) was added to each chamber, and intact cellular respiration as well as oxygen consumption after addition of indicated substrates or inhibitors was measured. Final concentrations of substrates and inhibitors were as described previously [29]. At step 5 [29], complex II substrate (succinate) was injected in addition to the three complex I substrates, then combined complex I and II substrate oxidation was measured. At step 6 [29], multiple titrations in a stepwise increment of FCCP were performed, which evokes the maximum oxidative capacity. At step 7, rotenone is added to inhibit complex I. Antimycin A is then added to inhibit complex III (step 8). The residual oxygen consumption is considered to be non-mitochondrial. Rotenone sensitive-complex I and rotenone insensitive-complex II rate could be calculated using the rate after FCCP and rotenone treatment.
2.5 Enzyme activity assay
The enzymatic activity of Complex I, II and citrate synthase was determined as described [30]. The same amounts of isolated mitochondria resuspended in 25 mM potassium phosphate buffer were frozen and thawed for 3 cycles. The spectrophotometric kinetic assays were performed at 37 °C using a microplate reader (Synergy H1). All activities were calculated as nmol min−1 per mg protein, and expressed as a percentage of control activity.
2.6 Blue-Native PAGE and immunoblot analysis
Blue-native gel electrophoresis was performed with NativePAGE Bis-Tris Gel system (Life Technologies,). Briefly, 10 µg of isolated mitochondrial proteins were resuspended in sample buffer and solubilized with 2% digitonin (Sigma-Aldrich) for 30 min on ice. Insolubilized pellets were removed by centrifugation for 30 min at 18,000g. The supernatant was collected, and 5% G-250 sample additive was added. Samples were loaded to 3–12% precast Bis-Tris gradient gels (Life Technologies), followed by electrophoresis at 4°C and transferred to immobilon. For regular immunoblot analysis, purified mitochondria were lysed with RIPA lysis buffer (Abcam) plus 1× protease inhibitor cocktail (Roche, Nutley, NJ). Equal amounts of 10 µg total protein extract were resolved by SDS–PAGE and transferred to membrane (Millipore). After blocking with 5% nonfat dry milk or 5% BSA, primary and secondary antibodies were applied as recommended by producer, and the blots were developed with Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA).
3. Results
3.1 VPS35 D620N impairs complex I and II respiration and enzymatic activity
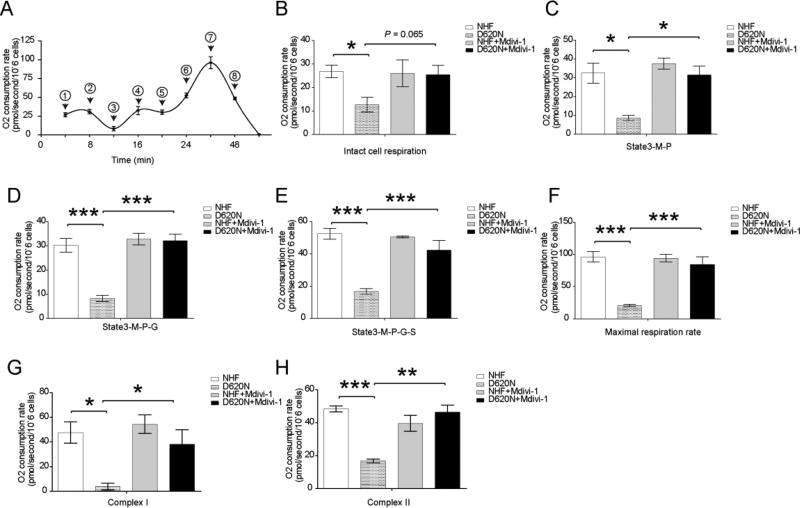
We previously reported that human fibroblasts from PD patient bearing VPS35 D620N mutation (D620N fibroblast) demonstrated bioenergetics deficits as evidenced by impaired respiratory control ratio and decreased spare respiratory capacity compared with normal human fibroblasts from age-matched controls (NHFs) [25]. To investigate exactly where the respiratory impairment occurred, we followed a protocol developed by Hoppel with sequential injection of complex I and II substrates, FCCP and inhibitors to sequentially measure complex I and II respiration in digitonin-permeabilized fibroblasts [29]. Consistent with the prior results [25], the intact cell respiration rate, state 3 respiration rate and the maximum oxidation capacity of D620N fibroblasts was significantly decreased compared to that of NHFs (Fig. 1A–F). Importantly, both the uncoupled complex I respiration rate (i.e., the rotenone-sensitive rate) (Fig. 1G) and the uncoupled complex II respiration rate (i.e., the rotenone-insensitive rate) (Fig. 1H) were significantly decreased in the D620N fibroblasts compared with that of NHFs.
Figure.1.
Impaired complex I and II respiration in VPS35 D620N fibroblasts and the rescue by mitochondrial fission inhibitor, mdivi-1. (A) A schematic tracing of the oxygen consumption rate following protocol 1 as reported in [29] with reagents being added at sequential steps marked with a numeric symbol: 1 = 2mM malate and 2.5mM pyruvate; 2 = 2µg/ml digitonin; 3 = 2.5mM ADP; 4 = 10mM glutamate; 5 = 10mM succinate; 6 = 0.5µM increment of FCCP; 7 = 75nM rotenone; 8 = 125nM antimycin. (B–H) Quantification of OXPHOS profiles of fibroblasts. (C) State 3 rate of malate (M) + pyruvate (P). (D) State 3 rate of M + P + glutamate (G). (E) State 3 rate of succinate (S) on top of M + P + glutamate (G). (F) maximal respiration rate. (G) rotenone-sensitive complex I respiration rate. (H) Rotenone-insensitive complex II respiration rate. NHF, normal human fibroblasts. D620N, fibroblasts from the patient bearing VPS35 D620N mutant. Data are presented as mean ± SEM from 3 independent experiments. Asterisks indicate statistical significance. *p<0.05, **p<0.01, ***p<0.001.
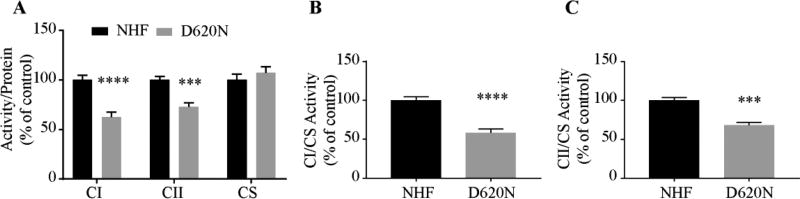
To corroborate this finding, we also used spectrophotometry to measure complex I and II enzymatic activities in D620N fibroblasts (Fig. 2). To account for possible variations in mitochondrial content due to isolation procedures, all of the measurements of individual complex activities were normalized by the activity of citrate synthase (CS), an enzyme of the tricarboxylic acids cycle, since CS activity remains unchanged between D620N fibroblasts and NHFs (Fig. 2A). Indeed, we found significant decrease of complex I and II activities in D620N fibroblasts (CI/protein, 37%; CII/protein, 28%; CI/CS, ~40%; CII/CS, 32%). as compared with NHFs when corrected by protein concentration or CS activity (Fig. 2A–C).
Figure.2.
Decreased mitochondrial enzymatic activity in VPS35 D620N fibroblasts. Specific enzymatic activities of complex I (CI) and II (CII) and citrate synthase (CS) were measured in isolated mitochondria from fibroblasts. (A) Enzymatic activity normalized to protein concentration. (B, C) Enzymatic activity normalized to citrate synthase activity. Data are presented as mean ± SEM from 3 independent experiments. Asterisks indicate statistical significance. ***p<0.001, ****p<0.0001.
We previously demonstrated that inhibition of mitochondrial fission by mdivi-1 alleviated bioenergetics deficits in D620N fibroblasts [25], therefore, we further determined whether mdivi-1 could alleviate deficits in complex I and II respiration in D620N fibroblasts. Indeed, mdivi-1 treatment could restore intact cell respiration rate, state 3 respiration rate and the maximum oxidation capacity as well as the uncoupled complex I and II respiration rate in D620N fibroblasts to a value that was comparable to NHFs (Fig. 1), indicating that mdivi-1 could rescue complex I and II respiration in D620N fibroblasts.
3.2 VPS35 D620N decreases levels of assembled Complex I and II
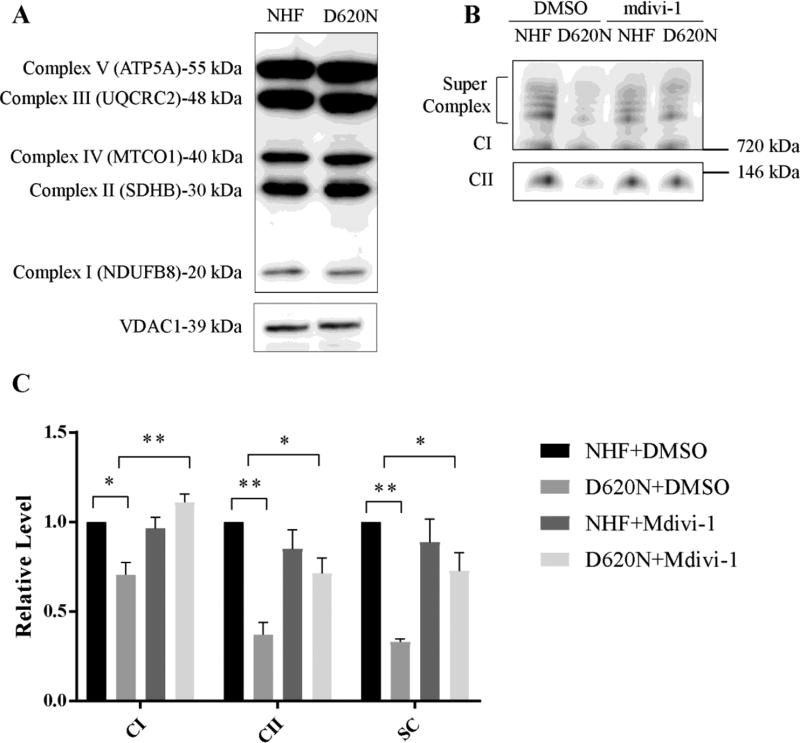
To explore the mechanism(s) underlying the VPS35 D620N mutation-induced deficits in complex I and II, we investigated the expression of oxidative phosphorylation (OXPHOS) proteins by western blot analysis of a selection of CI, CII, CII, CIV and CV subunits. The expression level of the all the subunits of OXPHOS complexes analyzed remained unaltered in mitochondrial fraction from patient fibroblasts, in comparison with the NHFs’ mitochondria (Fig. 3A), suggesting that there is no overall changes in the expression of OXPHOS proteins.
Figure.3.
VPS35 D620N decreases levels of assembled Complex I and II which is rescued by mdivi-1 treatment. (A) Mitochondria isolated from fibroblasts were subjected to SDS-PAGE and probed with anti-OXPHOS antibody. VDAC1 was used as an internal loading control. (B) Mitochondria isolated from fibroblasts were subjected to BN-PAGE. CI, complex I; CII, complex II. (C) Quantitative analysis of band intensity normalized to the level of NHF+DMSO. Data corresponds to mean ± SEM from 3 independent experiments. Asterisks indicate statistical significance. *p<0.05, **p<0.01.
OXPHOS proteins are organized into large complexes located on the mitochondrial inner membrane, and the function of OXPHOS complexes is dependent on their proper assembly [31]. To determine the integrity of assembled complex I and II, mitochondria purified from fibroblasts were subjected to blue native gel electrophoresis (BN-PAGE) followed by Western blot analysis. As shown in Figure 3B, there was a significant decrease in the total level of assembled complex I and supercomplex in D620N fibroblasts. Levels of assembled Complex II was also decreased in D620N fibroblasts. Since mdivi-1 could restore the complex I and II respiration in D620N fibroblasts (Fig. 1), we further determined whether mdivi-1 could rescue the levels of complex I and II in D620N fibroblasts. Indeed, the level of assembled complex I, supercomplex and complex II were rescued to a level comparable to NHFs (Fig. 3B,C) after mdivi-1 treatment.
4. Discussion
In this study, we found that VPS35 D620N mutation caused significantly decreased enzymatic activity and respiratory defects in both complex I and II of the electron transport chain in the fibroblasts from PD patient with VPS35 D620N mutation compared with age-matched NHFs. While there was no change in the expression of OXPHOS complexes, the levels of assembled complex I, II and supercomplex were significantly reduced in the D620N fibroblasts which likely underlies the functional defects of these complexes. Importantly, the inhibition of mitochondrial fission by mdivi-1 rescued levels of these complexes and functional defects of complex I and II in D620N fibroblasts.
While VPS35 is largely involved in the retrograde transport of membrane proteins from endosome to the trans-Golgi network and to the plasma membrane as the recognition subunit of retromer [23, 24], increasing evidence also indicated a critical role of VPS35 and retromer in the regulation of mitochondrial function. This was initially demonstrated by the involvement of VPS35 in the formation of mitochondria-derived vesicles (MDVs) and transport of mitochondria-anchored protein ligase from the mitochondria to the peroxisomes [32]. More recently, Tang et al. and our group demonstrated that VPS35 D620N mutation caused excessive mitochondrial fission and impaired mitochondrial function [25, 33], although there was discrepancy on the detailed mechanism involved. In the present study, we extended our prior studies by demonstrating that VPS35 D620N mutation specifically caused defects in the enzymatic activity and respiration in both complex I and II. It is of importance to note that defects in complex I is a hallmark of PD and inhibition of complex I causes Parkinsonism in human [10]. There was ample evidence that other PD genetic factors could also impair complex I activity [18]. For example, abundant evidence shows that complex I enzymatic activity is reduced in PINK1-deficient mice and flies [34, 35]. Therefore, our study indicated that pathogenic VPS35 mutations could cause complex I defects.
Another important finding of the current study is the significantly reduced levels of assembled complex I, II and supercomplex in the D620N fibroblasts and its rescue by the treatment of mdivi-1. The reduced complex I, II and supercomplex could be the structural basis that underlies the functional defects of complex I and II induced by D620N mutation. It remains to be determined whether the reduced complex I, II and supercomplex is due to defects in assembly or increased instability or disorganization. In this regard, it is of interest to note that increased mitochondrial respiratory chain disorganization was most recently reported in the fibroblasts from patients bearing Pink1 mutations [19]. We further found that the reduction in assembled complex I, II and supercomplex in the D620N fibroblasts could be rescued by the treatment of mdivi-1. In a prior study, we have clearly demonstrated that D620N caused mitochondrial fragmentation in both neuronal cells and human PD patient fibroblasts and the treatment of mdivi-1, a widely-used inhibitor of mitochondrial fission, could reverse D620N-induced mitochondrial fragmentation [25]. Our current results thus suggest that it is the D620N-induced mitochondrial fragmentation that likely caused reduced complex I, II and supercomplex either through impaired assembly or increased disorganization. In this regard, it has been reported that the PINK1 knockout also caused defective assembly of mitochondrial OXPHOS complexes which is associated with impairment in mitochondrial fission in flies [36], indicating that changes in mitochondrial fission/fusion balance could impact the assembly of mitochondrial OXPHOS complexes. A more recent study suggests that cristae shape determines the assembly and stability of supercomplex and hence mitochondrial respiratory efficiency which places the proper assembly of supercomplex as the critical link between mitochondrial morphology and function [37]. Therefore, our study supports the notion that VPS35 D620N mutation-induced excessive mitochondrial fission reduced the assembled complex I and supercomplex which leads to the bioenergetics defects. Nevertheless, the possibility that VPS35 D620N may impact complex I indirectly through effects on α-synulcein may not be ruled out since VPS35 D620N mutant causes accumulation and aggregation of a-synuclein [38] and mitochondrial accumulated α-synuclein interacts with complex I [39].
In conclusion, we demonstrated that VPS35 D620N mutation led to decreased enzymatic activity and respiratory defects in complex I and II along with reduced levels of assembled complex I and II in the patient fibroblasts. The inhibition of mitochondrial fission by mdivi-1 rescued content of these complexes as well as the functional defects of complex I and II. Overall, these results suggest that VPS35 D620N mutation-induced excessive mitochondrial fission impairs complex I complex and supercomplex which leads to the bioenergetics defects which support the critical pathogenic role of VPS35 mutation-induced mitochondrial dynamic deficits.
Highlights.
VPS35 D620N mutation caused decreased enzymatic activity of complex I and II
VPS35 D620N mutation caused respiratory deficits in complex I and II
VPS35 D620N mutation caused decreased levels of assembled complex I and II
Mdivi-1 rescued assembled complex levels and functional defects of complex I and II
Acknowledgments
This work is partly supported by NIH grants NS083385 and NS083498 (to X.Z.), by Chinese Overseas, Hong Kong and Macao Scholars Collaborated Research Fund Grant 81228007 to X. Z., by the Dr. Robert M. Kohrman Memorial Fund (to X.Z.), and by grants from the National Natural Science Foundation of China to J.L. (81471287, 81071024, 81171202, 81371407, 30872729, 30870879) and the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (20152201 to J.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
X.Z. is a paid consultant of Sierra Research Group LLC.
References
- 1.Parkinson J. An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 2002;14:223–236. doi: 10.1176/jnp.14.2.223. discussion 222. [DOI] [PubMed] [Google Scholar]
- 2.Massano J, Bhatia KP. Clinical approach to Parkinson's disease: features, diagnosis, and principles of management. Cold Spring Harb. Perspect. Med. 2012;2:a008870. doi: 10.1101/cshperspect.a008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 4.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 6.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 8.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 9.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson's disease pathogenesis. Biochim. Biophys. Acta. 2009;1792:651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann. Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 12.Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 13.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J. Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 14.Krige D, Carroll MT, Cooper JM, Marsden CD, Schapira AH. Platelet mitochondrial function in Parkinson's disease. The Royal Kings and Queens Parkinson Disease Research Group. Ann. Neurol. 1992;32:782–788. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- 15.Janetzky B, Hauck S, Youdim MB, Riederer P, Jellinger K, Pantucek F, Zochling R, Boissl KW, Reichmann H. Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson's disease. Neurosci. Lett. 1994;169:126–128. doi: 10.1016/0304-3940(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 16.Mann VM, Cooper JM, Daniel SE, Srai K, Jenner P, Marsden CD, Schapira AH. Complex I, iron, and ferritin in Parkinson's disease substantia nigra. Ann. Neurol. 1994;36:876–881. doi: 10.1002/ana.410360612. [DOI] [PubMed] [Google Scholar]
- 17.Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults CW. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson's disease. Ann. Neurol. 1995;37:714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- 18.Bose A, Beal MF. Mitochondrial dysfunction in Parkinson's disease. J. Neurochem. 2016;139(Suppl 1):216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Fabuel I, Martin-Martin L, Resch-Beusher M, Azkona G, Sanchez-Pernaute R, Bolanos JP. Mitochondrial respiratory chain disorganization in Parkinson's disease-relevant PINK1 and DJ1 mutants. Neurochem. Int. 2017 doi: 10.1016/j.neuint.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C, Reinthaler E, Harutyunyan A, Kralovics R, Peters A, Zimprich F, Brucke T, Poewe W, Auff E, Trenkwalder C, Rost B, Ransmayr G, Winkelmann J, Meitinger T, Strom TM. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, Behrouz B, Melrose HL, Hentati E, Puschmann A, Evans DM, Conibear E, Wasserman WW, Aasly JO, Burkhard PR, Djaldetti R, Ghika J, Hentati F, Krygowska-Wajs A, Lynch T, Melamed E, Rajput A, Rajput AH, Solida A, Wu RM, Uitti RJ, Wszolek ZK, Vingerhoets F, Farrer MJ. VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 2011;89:162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujioka S, Ogaki K, Tacik PM, Uitti RJ, Ross OA, Wszolek ZK. Update on novel familial forms of Parkinson's disease and multiple system atrophy. Parkinsonism Relat. Disord. 2014;20(Suppl 1):S29–34. doi: 10.1016/S1353-8020(13)70010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J. Cell. Biol. 2004;165:111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivan G, Weisberg AS, Americo JL, Moss B. Retrograde transport from early endosomes to the trans-Golgi network enables membrane wrapping and egress of vaccinia virus virions. J. Virol. 2016;90:8891–8905. doi: 10.1128/JVI.01114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Wang X, Fujioka H, Hoppel C, Whone AL, Caldwell MA, Cullen PJ, Liu J, Zhu X. Parkinson's disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat. Med. 2016;22:54–63. doi: 10.1038/nm.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Ma X, Zhou L, Liu J, Zhu X. A conserved retromer sorting motif is essential for mitochondrial DLP1 recycling by VPS35 in Parkinson's disease model. Hum. Mol. Genet. 2017;26:781–789. doi: 10.1093/hmg/ddw430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGough IJ, Steinberg F, Jia D, Barbuti PA, McMillan KJ, Heesom KJ, Whone AL, Caldwell MA, Billadeau DD, Rosen MK, Cullen PJ. Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease-linked VPS35(D620N) mutation. Curr. Biol. 2014;24:1670–1676. doi: 10.1016/j.cub.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat. Protoc. 2009;4:1582–1590. doi: 10.1038/nprot.2009.151. [DOI] [PubMed] [Google Scholar]
- 29.Ye F, Hoppel CL. Measuring oxidative phosphorylation in human skin fibroblasts. Anal. Biochem. 2013;437:52–58. doi: 10.1016/j.ab.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012;7:1235–1246. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- 31.Stroud DA, Surgenor EE, Formosa LE, Reljic B, Frazier AE, Dibley MG, Osellame LD, Stait T, Beilharz TH, Thorburn DR, Salim A, Ryan MT. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature. 2016;538:123–126. doi: 10.1038/nature19754. [DOI] [PubMed] [Google Scholar]
- 32.Braschi E, Goyon V, Zunino R, Mohanty A, Xu L, McBride HM. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr. Biol. 2010;20:1310–1315. doi: 10.1016/j.cub.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 33.Tang FL, Liu W, Hu JX, Erion JR, Ye J, Mei L, Xiong WC. VPS35 deficiency or mutation causes dopaminergic neuronal loss by impairing mitochondrial fusion and function. Cell Rep. 2015;12:1631–1643. doi: 10.1016/j.celrep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc. Natl. Acad. Sci. U S A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, Vanbrabant M, Haddad D, Frezza C, Mandemakers W, Vogt-Weisenhorn D, Van Coster R, Wurst W, Scorrano L, De Strooper B. Parkinson's disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol. Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W, Acin-Perez R, Geghman KD, Manfredi G, Lu B, Li C. Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proc. Natl. Acad. Sci. U S A. 2011;108:12920–12924. doi: 10.1073/pnas.1107332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155:160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang FL, Erion JR, Tian Y, Liu W, Yin DM, Ye J, Tang B, Mei L, Xiong WC. VPS35 in dopamine neurons Is required for endosome-to-Golgi retrieval of Lamp2a, a receptor of chaperone-mediated autophagy that is critical for alpha-synuclein degradation and prevention of pathogenesis of Parkinson's disease. J. Neurosci. 2015;35:10613–10628. doi: 10.1523/JNEUROSCI.0042-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]