Abstract
Cytotoxic lymphocytes encompass natural killer lymphocytes (cells) and cytotoxic T cells that include CD8+ T cells, natural killer (NK) T cells, γ, δ (γδ)‐T cells and human CD4 + CD28− T cells. These cells play critical roles in inflammatory diseases and in controlling cancers and infections. Cytotoxic lymphocytes can be activated via a number of mechanisms that may involve dendritic cells, macrophages, cytokines or surface proteins on stressed cells. Upon activation, they secrete pro‐inflammatory cytokines as well as anti‐inflammatory cytokines, chemokines and cytotoxins to promote inflammation and the development of atherosclerotic lesions including vulnerable lesions, which are strongly implicated in myocardial infarctions and strokes. Here, we review the mechanisms that activate and regulate cytotoxic lymphocyte activity, including activating and inhibitory receptors, cytokines, chemokine receptors‐chemokine systems utilized to home to inflamed lesions and cytotoxins and cytokines through which they affect other cells within lesions. We also examine their roles in human and mouse models of atherosclerosis and the mechanisms by which they exert their pathogenic effects. Finally, we discuss strategies for therapeutically targeting these cells to prevent the development of atherosclerotic lesions and vulnerable plaques and the challenge of developing highly targeted therapies that only minimally affect the body's immune system, avoiding the complications, such as increased susceptibility to infections, which are currently associated with many immunotherapies for autoimmune diseases.
Linked Articles
This article is part of a themed section on Targeting Inflammation to Reduce Cardiovascular Disease Risk. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.22/issuetoc and http://onlinelibrary.wiley.com/doi/10.1111/bcp.v82.4/issuetoc
Abbreviation
- TCR
T cell receptor
- MHC
major histocompablity complex
- NKG2D
natural‐killer group 2, member D
- TRAIL
TNF‐related apoptosis‐inducing ligand
- DNAM‐1
DNAX accessory molecule‐1
- Clec9A
C‐type lectin domain family 9 member A
Introduction
Atherosclerosis is a disease of large elastic and muscular arteries that is responsible for most myocardial infarctions (MIs) including angina, ischaemic strokes and peripheral vascular disease. Collectively, MIs and strokes are the leading cause of global death, responsible for 248 deaths per 100 000 persons in 2013, representing 85.4% of all cardiovascular deaths and 28.2% of all mortalities (Barquera et al., 2015; Mortality and Causes of Death C, 2015). Without significant new interventions, these statistics are predicted to worsen with the world‐wide increase in type 2 diabetes mellitus associated with obesity (Dutton and Lewis, 2015; Munnee et al., 2016), as obesity and type 2 diabetes mellitus are independent risk factors for MIs and strokes (Kalofoutis et al., 2007; Kernan et al., 2013). Atherosclerosis is initiated by the subendothelial accumulation of low‐density lipoproteins rich in cholesterol and apolipoprotein B at sites of disturbed flow, mostly at vessel bends and branch points, where diffuse intimal thickenings develop (Nakashima et al., 2008). Apoptotic and necrotic cells are characteristic features of human and mouse atherosclerotic lesions, which increase with lesion progression (Otsuka et al., 2015). In vulnerable atherosclerotic lesions, the necrotic core is composed of necrotic cells, cell debris and lipid and frequently constitutes more than 40% of a lesion; it is a significant contributor to plaque instability. Necrotic cells are largely the consequence of apoptotic cells undergoing secondary necrosis due at least in part to impaired efferocytosis, with apoptosis initiated by cytotoxins (Froelich et al., 2004) and cytokines such as TNF‐α, largely derived from cytotoxic cells (Tay et al., 2016) and with secondary necrosis recently shown to be mediated by caspase 3 (Rogers et al., 2017). Apoptosis of smooth muscle cells within inflamed fibrous caps covering large necrotic cores is also a significant contributor to lesion instability, as their loss results in collagen reduction, leading to fibrous cap thinning (Chen et al., 2016; Yahagi et al., 2016).
Recent evidence indicates that cytotoxic lymphocytes play important roles in the pathology of atherosclerosis utilizing cytotoxic mechanisms to promote vulnerable plaque development and progression. Here, we highlight the role of cytotoxic lymphocytes in atheroma development, including the development of inflamed and unstable atheromas, focusing on the major cytotoxic lymphocyte populations, invariant NKT (iNKT) cells, natural killer (NK) cells, γδ‐T cells, CD8+ T cells and human CD4 + CD28− T cells. We first review their basic immunological characteristics including their activating and inhibitory receptors and their production of cytotoxic factors and cytokines, highlighting aspects of knowledge that has the potential to advance our understanding of atheroma development, progression and provide the theoretical basis of future therapies. We then review the current knowledge on their involvement in atherosclerosis and finally consider pharmacological intervention strategies to prevent atheromas and vulnerable plaque development.
Immunological characteristics of cytotoxic lymphocytes
Major lymphocytes with cytotoxic effector function comprise NK cells, γδ‐T cells, NKT cells, CD8 T cells and human CD4 + CD28− T cells. Despite having similar haemopoietic origins, NK and γδ‐T cells do not require antigen presentation for their activation and effector function; instead, they are activated by innate receptors. Also, γδ‐T cells and NKT cells are considered to bridge the innate and adaptive immune systems. Here, we highlight the basic aspects of the immunology of cytotoxic lymphocytes (Table 1), much of which has not been applied to atherosclerosis but is likely to impact on our understanding as to how they exert their pro‐atherosclerotic effects, with potential for translation.
Table 1.
Comparison of general characteristics of different cytotoxic lymphocytes
| NK cellsa | γδ‐T cellsb | iNKT cells | CD8+ T cellsc | CD4+ CD28−T cellsd | |
|---|---|---|---|---|---|
| Immune response | Innate | Innate/?adaptiveb | Adaptive | Adaptive | Adaptive |
| Antigen | Not required | Not required | Lipid | Peptide | Peptide |
| Tissue residence | SLO, Spleen | Mucosa, Epithelium | SLO, Liver/spleen | SLO | SLO |
| Signature surface markers | NK1.1, TCR− | TCRγδ | TCR Vα24‐Jα18 (h) TCR Vα14‐Jα18 (m) NK1.1 | TCRαβ CD8 | TCRαβ CD4 |
| Activating or inhibiting | NKG2D, NKp46, NKp30, NKp44, KIR (h), Ly49 (m), DNAM, FcγRIII | NKG2D, NKp44, DNAM, FcγRIII | NKG2D, NKp30, NK046, KIR (h), Ly49 (m), FcγRIII | TCR‐dependent antigens, NKG2D, KIR (h), Ly49 (m), | TCR‐dependent antigens, NKG2D, DNAM |
| Chemokine receptors | CXCR1, CXCR3, CXCR4, CCR7, CCR9 | CCR7, CCR10, CXCR5 | CCR4, CCR5, CCR6, CXCR3, CXCR4 | CCR4, CCR5, CCR7, CCR9, CDR10, CXCR3 | CCR5, CCR7, CXCR4, CX3CR1 |
| Effector functions | |||||
| *cytotoxins | + | + | + | + | + |
| *Fas | + | + | + | + | ? |
| *TRAIL | + | + | + | + | ? |
| *cytokines | + | + | + | + | + |
| Cell‐to‐cell interaction | CD4 T cells | NK cells, monocytes | MZ B cells | Monocytes, dendritic cells, macrophages | NA |
NK cells
NK cells largely function as part of the innate immune system. These cytotoxic cells develop independently of the thymus and reside in peripheral lymphoid organs. NK cell activity is regulated by activating and inhibitory receptors (Pegram et al., 2011). Human NK cell inhibitory receptors are mainly killer cell immunoglobulin‐like receptors (KIR) recognizing major histocompablity complex (MHC)‐I molecules whereas in mouse, Ly49 receptors perform similar functions. Activating receptors include NKp46, NKp30 and NKp44 as well as activating versions of KIR and Ly49 receptors (Pegram et al., 2011). The activating receptor natural‐killer group 2 member D (NKG2D) binds a number of cellular cell surface ligands induced by stress signals including MICA/B and Rae‐1. Other activating receptors include DNAX accessory molecule‐1 (DNAM‐1), FcγRIII (CD16) (Watzl, 2014) and NKp80 (Welte et al., 2006). Engagement of a single activating receptor is not sufficient to stimulate cytotoxicity or cytokine secretion; instead, at least two different activating receptors need to be simultaneously engaged to initiate responses, with most effective responses initiated when receptors utilize different signalling pathways (Marcus et al., 2014). Acquisition of cytotoxicity also requires IL‐15 (Fehniger et al., 2007; Lucas et al., 2007). NK cells express multiple cytokine receptors and are activated by inflammatory cytokines such as IL‐2, IL‐12, IL‐15 and IL‐18. Cytokine ‘pre‐activated’ NK cells can be further activated by a single activating receptor, greatly increasing cytokine secretion or cytotoxicity (Tang et al., 2013). Activated NK cells produce multiple cytotoxins including TRAIL (Ochi et al., 2004), FasL (Chua et al., 2004), granzyme B and perforin. They also produce pro‐inflammatory cytokines IFN‐γ, TNF‐α, IL‐2 and IL‐8 (De Sanctis et al., 1997) and secrete chemokines MIP‐1α (CCL3), MIP‐1β (CCL4) and RANTES (CCL5) (Fauriat et al., 2010). NK cells facilitate the differentiation of naïve CD4+ T cells into IFN‐γ secreting Th1 T cells, by providing an early source of IFN‐γ within lymph nodes, which is required for Th1 polarization (Martin‐Fontecha et al., 2004). They also promote cross‐presentation of antigens to CD8+ T cells (Deauvieau et al., 2015). Like iNKT cells, NK cells are highly migratory, expressing a large number of chemokine receptors including CXCR1, CXCR3, CXCR4, CCR7 and CCR9 enabling them to migrate to sites of tissue inflammation, including atherosclerotic lesions (Berahovich et al., 2006; Peng and Tian, 2014).
γδ‐T cells
γδ‐T cells are T cells that develop in the thymus and express unique T‐cell receptors composed of one γ‐chain and one δ‐chain. They predominantly reside in epithelial and mucosa layers of the skin, intestine, lung and tongue where they serve as a first line of defence against infections. Activation, largely but not exclusively by innate mechanisms, initiates or propagates immune responses via cytokine‐ or cytolytic‐dependent mechanisms (Born et al., 2006; Poggi and Zocchi, 2014). Mouse and human γδ‐T cells possess many common characteristics that include innate receptor expression, antigen presentation capabilities, cytotoxicity and cytokine production (Holderness et al., 2013; Vantourout and Hayday, 2013). γδ‐T cells are composed of a number of subsets. In the mouse, they are broadly subdivided into CD27+ and CD27− γδ‐T cells and then further subdivided on the basis of different Vγ chains (Pang et al., 2012). They are highly effective at killing stressed and tumour cells and produce large amounts of pro‐inflammatory cytokines (Silva‐Santos et al., 2015). They are activated via their γδ‐T cell and NK cell receptors, but unlike αβ‐T cells, antigen recognition by their T cell receptors (TCRs) does not require MHC molecules or CD1 (Chien and Konigshofer, 2007). They express multiple NK cell receptors including NKG2D, DNAM‐1, NKp44 and FcγRIII (CD16) and are activated by stressed and/or infected cells expressing MHC I molecules such as Rae‐1, nectin and/or NKp44L (Groh et al., 1998; de Andrade et al., 2014). Activated γδ‐T cells kill via FasL, TRAIL and granzyme B/perforin (Bonneville et al., 2010). They are also activated by cytokines IL‐1, IL18 and IL‐23 and secrete large amounts of IFN‐γ, TNF‐α and IL‐17 as well as Th2 cytokines (Bonneville et al., 2010). They express chemokine receptors CCR7, CCR10 and CXCR5 and respond to multiple chemokines (Kabelitz and Wesch, 2003). Activated γδ‐T cells also influence other immune cells, enhancing NK cell‐mediated cytotoxicity (Maniar et al., 2010). They stimulate monocytes to differentiate into inflammatory dendritic cells (Eberl et al., 2009) and promote dendritic cell maturation (Leslie et al., 2002).
iNKT cells
iNKT cells are innate‐adaptive hybrid cells expressing NK receptors as well as highly restricted TCRs that recognize lipid antigens presented by the transmembrane MHC class I‐like CD1d glycoprotein. iNKT cells arise from the thymus, complete maturation in the periphery and are mainly found in the liver and spleen. Their TCRs recognize both bacterial and self‐lipid antigen‐CD1d complexes presented by antigen‐presenting cells such as dendritic cells (Godfrey et al., 2010). Mouse iNKT cells express the semi invariant TCRα Vα14Jα18 whilst human iNKT cells express Vα24Jα18 (Lantz and Bendelac, 1994). iNKT cells are classified into three subtypes depending on expression of co‐receptors CD4 or CD8 (Seino and Taniguchi, 2005). Despite an inability to definitively identify/characterize self‐lipid antigens that activate NKT cells (Fox et al., 2009), there is strong evidence for such antigens in atherosclerosis and other inflammatory disorders (Li et al., 2016; Lombardi et al., 2010). iNKT cells can also be activated by non‐TCR signals. iNKT cells constitutively express TIM‐1 (T cell Ig‐like mucin‐like‐1), a receptor for phosphatidylserine on apoptotic cells, which stimulates cell proliferation and cytokine secretion (Lee et al., 2010). These cells express the cell stress ligand receptor NKG2D, which directly activates or co‐stimulates iNKT cells together with TCRs (Kuylenstierna et al., 2011). Engagement of the Fc γ receptor (FcγRIII/CD16) also leads to activation, resulting in antibody‐mediated inflammation (Kim et al., 2006). iNKT cells express a number of activating or inhibitory killer immunoglobulin‐like (Ig) receptors (Patterson et al., 2008), including Ly49 receptors (Sköld et al., 2000) as well as natural cytotoxicity receptors NKp30 and NKp46 (Nguyen et al., 2008). Cytokines also activate iNKT cells either alone or in conjunction with TCRs (Kitamura et al., 1999). iNKT cells express receptors for IL‐12 (Kitamura et al., 1999), IL‐18 (Leite‐De‐Moraes et al., 1999), IL‐21 (Coquet et al., 2007), IL‐23 (Rachitskaya et al., 2008) and IL‐25 (Terashima et al., 2008). iNKT cells are migratory lymphocytes expressing multiple chemokine receptors (Ho et al., 2008). Chemokine receptors expressed by these cells include CCR5, CCR6, CXCR3 and CXCR4; CCR4 is predominately expressed by CD4+ iNKT cells (Kim et al., 2002; Thomas et al., 2003).
Activated NKT cells produce Th1 and Th2 cytokines including IFN‐γ, TNF‐α, IL‐2 as well as IL‐17 and IL‐4, IL‐10 and IL‐13. Factors that pre‐determine cytokine secretion include CD4 expression and tissue location (Coquet et al., 2008). The pattern of cytokine expression is more dependent on the nature of the CD1d+ antigen presenting cell than on the lipid antigen (Bai et al., 2012). Activated iNKT cells are potent killer cells expressing the cytotoxins perforin and granzyme B (Nguyen et al., 2008), FasL (CD178) (Wingender et al., 2010) and TRAIL (Huang et al., 2014). Their cytotoxic actions are greatly enhanced by IL‐4 (Kaneko et al., 2000) and IL‐15 (Liu et al., 2012).
Cytotoxic CD8+ T lymphocytes
CD8+ T cells are lymphocytes that express the CD8 coreceptor and recognize antigen peptide‐MHC class I complexes presented by antigen‐presenting cells such as dendritic cells. CD8+ T cells develop in the thymus and reside in secondary lymphoid organs. They play key roles in many inflammatory diseases (Walter and Santamaria, 2005; Kyaw et al., 2013; Carvalheiro et al., 2015) as well as in cancers and infections including cytomegalovirus (CMV) infection and Epstein–Barr virus (EBV) infections, which can be associated with atherosclerotic lesions (Khanna and Burrows, 2000; Brincks et al., 2008; Ahmadzadeh et al., 2009; Klenerman and Oxenius, 2016). They exist as a number of subsets that include short‐lived effectors (with high migratory ability and high capacity to produce cytokines and cytotoxins), effector memory cells (which accumulate in peripheral organs and become effectors upon re‐encounter with antigens), central memory cells (which rapidly proliferate and produce abundant cytokines but few cytotoxic molecules upon antigen encounter), tissue resident memory cells (that have very limited migratory capacity, hence permanently reside in peripheral tissue, producing cytokines and cytotoxic molecules upon antigen encounter) (Bisikirska et al., 2005; Gupta and Gollapudi, 2007; Marzo et al., 2007; Carvalheiro et al., 2013; Mackay et al., 2013) and regulatory cells (Bisikirska et al., 2005; Akane et al., 2016). Naïve circulating CD8+ T cells are activated by antigen presenting cells such as CD8α+ dendritic cells presenting peptide antigens on MHC class I molecules through a process called cross‐presentation (Joffre et al., 2012). CMV and EBV antigens activate, reactivate and differentiate CD8+ T cells in antigen‐specific cytotoxic T cell‐mediated responses (Khanna and Burrows, 2000; Klenerman and Oxenius, 2016). Activation can be enhanced by cytokines such as IL‐1β (Ben‐Sasson et al., 2013), IL‐2, IL‐12, IL‐15 and IL‐21 (Moroz et al., 2004; Henry et al., 2008). Activation can also be initiated in a TCR‐independent manner (Freeman et al., 2012). Like other killer cells, CD8+ T cells express killer‐like receptors including NKG2D (Verneris et al., 2004), Ly49 receptors (McMahon and Raulet, 2001) and activating and inhibitory KIRs (Bjorkstrom et al., 2012) with inhibitory KIRs mostly confined to effector CD8+ T cells (Arlettaz et al., 2004). However, responses of CD8+ T cells following activation of these receptors are only apparent after activation via TCRs (Arlettaz et al., 2004; Marzo et al., 2007). Other cell surface CD8+ T cell molecules important in regulating activity include programmed cell death‐1 (PD‐1), cytotoxic T lymphocyte antigen‐4 (CTLA‐4), T cell immunoglobulin and mucin domain‐3 (TIM‐3) and lymphocyte activity gene‐3 (LAG‐3) (Gros et al., 2014). Activated effector CD8+ cells can be subdivided based on killer cell lectin‐like receptor G1 (KLRG‐1) expression with KLRG‐1hi expression marking short‐lived effector cells and KLRGlo marking memory precursor cells (Ye et al., 2012). They can express a variety of selectins, chemokine receptors and integrins including PSGL‐1 and CD44, CCR4, CCR5, CCR7, CCR9, CCR10, CXCR3, VLA‐1 (integrin, α 1 subunit) and LFA‐1 (integrin αLβ2) enabling them to traffic and localize in different regions of the body (Nolz et al., 2011). Effector CD8+ T cells secrete pro‐inflammatory cytokines IFN‐γ and TNF‐α, IL‐17A, IL‐17F, IL‐21 and IL‐22 (Yu et al., 2013) and may also secrete IL‐14, IL‐5 and IL‐10. Like the other killer cells, they express perforin and granzyme (Janas et al., 2005), FasL (Kilinc et al., 2009) and TRAIL (Brincks et al., 2008). Highly activated cytotoxic CD8+ T cells also secrete IL‐10 to dampen inflammatory responses whilst still exerting potent cytotoxic effects (Noble et al., 2006; Trandem et al., 2011). In contrast to effector CD8+ T cells, regulatory CD8+ T cells attenuate inflammation by directly killing activated T cells (Akane et al., 2016).
CD4 + CD28− T cells
CD4 + CD28− T cells are highly differentiated human effector memory CD4+ T cells that have down‐regulated the costimulatory molecule CD28 due to loss of a CD28‐specific initiator complex (Vallejo et al., 1998; Vallejo et al., 2002). Their development and maturation process are similar to CD8 T cells. They are most abundant in elderly humans over 60 years of age (Vallejo et al., 1998) but can also be found in younger adults with chronic inflammatory disorders. Their numbers are increased in humans with rheumatoid arthritis (Bryl et al., 2001), type 2 diabetes (Shi et al., 2013; Warrington et al., 2001) and following CMV infection (van Leeuwen et al., 2004). Unlike other cytotoxic cells, these cells are not expressed in rodents. Despite the loss of CD28, these cells are not anergic and proliferate in response to stimulation. They are autoreactive to ubiquitously distributed autoantigens and exhibit a restricted TCR diversity (Schmidt et al., 1996). Surprisingly, they are resistant to the suppressive actions of CD4 + CD25 + Foxp3+ regulatory T cells (Thewissen et al., 2007) and also are resistant to activation‐induced apoptosis (Vallejo et al., 2000) due to high expression of the anti‐apoptosis factor Bcl‐2 (Schirmer et al., 1998).
CD4 + CD28− T cells express multiple chemokine receptors including CCR5, CCR7, CXCR4 and CX3CR1 enabling them to home to lymphoid organs and sites of tissue inflammation including atherosclerotic lesions (Zhang et al., 2005; Maly and Schirmer, 2015). Cytokines such as IL‐12 regulate their pattern of chemokine receptor expression (Zhang et al., 2005). CD4 + CD28− T cells are pro‐inflammatory and cytotoxic, expressing IFN‐γ and TNF‐α (Pieper et al., 2014) as well as perforin and granzyme B (Namekawa et al., 1998; Betjes et al., 2008). They respond to IL‐15 by up‐regulating granzyme B and perforin expression, increasing their cytotoxicity (Alonso‐Arias et al., 2011). In many ways, these cells mimic the effects of other cytotoxic lymphocytes, expressing cell surface markers CD11b and CD57 found on NK cells (Chapman et al., 1996; Schmidt et al., 1996). They also express NK cell‐activating receptors, which markedly increase their activity when T cell activation is suboptimal; receptors expressed include DNAM‐1 and CRACC (Fasth et al., 2010), NKG2D (Groh et al., 2003) and the KIR KIR2DS2 (Yen et al., 2001). Detailed studies of their significance in inflammatory disorders including atherosclerosis have been greatly hampered by the lack of such cells in mice.
Together, these basic immunology studies on the different cytotoxic lymphocytes indicate that they are highly migratory and their accumulation in lesions during development of atherosclerosis is most likely dependent on chemokines. Their ability to influence vulnerable lesions is largely but not exclusively dependent on their presence in lesions, where they have the potential to influence development of vulnerable atherosclerotic lesion by a number of common mechanisms involving cytotoxins. In lesions, cytotoxic lymphocytes are also very likely activated or co‐activated by a number common killer cell receptor‐dependent mechanisms. However, knowledge of the relative importance of precise mechanisms in atherosclerosis is still rather limited (see Cytotoxic Lymphocytes and Development of Atherosclerosis), and further studies are warranted to more precisely define the best therapeutic targets to effectively prevent their deleterious actions.
Cytotoxic lymphocytes and development of atherosclerosis
In the very early stages of the development of atherosclerosis, circulating leukocytes including lymphocytes migrate into intimal layers via vascular adhesion molecules up‐regulated as a result of endothelial dysfunction. Subsequent chemokine up‐regulation in atherosclerotic lesions may also contribute to lymphocyte recruitment. With progression, tertiary lymphocyte organs that develop in adventitial layers may also contribute to lymphocyte recruitment and activation. Antigens implicated in atherosclerosis are thought to be multiple in origin, but current understanding on antigens involved in atherosclerosis is limited, with the exception of modified LDL and heat shock protein60. Necrotic materials are thought to be important, yet their role in atherosclerosis remains to be elucidated.
Human atherosclerotic lesions are histologically divided into six categories; type I, presence of foam cells in the intimal layer; type II, fatty streak formation; type III, pre‐atheroma; type IV, atheroma; type V, fibrous cap formation with or without calcification; and type VI, rupture with thrombus formation. Mechanistic insights as to how cytotoxic lymphocytes influence development and progression of established atherosclerotic lesions require animal models. Several genetically modified mouse models have been developed including ApoE−/− mice and LDLR−/− mice, transgenic ApoE3‐Leiden mice and HuBTg+/+ LDLR−/− mice (Kapourchali et al., 2014). Among these genetically modified mouse models, ApoE−/− and LDLR−/− atherogenic mouse models are the most widely used as the lesions that develop in both mouse models are morphologically similar to human atherosclerotic lesions. Both stage IV and V lesions will take 14–20 weeks of high‐fat diet feeding to generate in mouse models and stage. Stage VI lesions are only seen in the innominate artery; however, mouse lesions, unlike human lesions, appear to be more resistant to rupture. Therefore recently, a model of plaque rupture has been developed using these mice (Chen et al., 2013). LDLR−/− mice have an advantage over ApoE−/− mice in that it is much easier to generate mixed bone marrow chimeric mouse models with specific gene deletions in immune cells.
Cytotoxic lymphocytes accumulate in both mouse and human atherosclerotic lesions and many appear to be involved in nearly all stages of atherosclerosis – development, progression of established lesions and vulnerable plaque development; their roles in plaque rupture are yet to be elucidated. It is also important to investigate where and how these immune cells are activated and their site of action during development/progression of advanced atherosclerosis as this information is not available currently. This knowledge will provide important insights as to how best to therapeutic target these cells. Too frequently preclinical studies have focused only on early development of atherosclerosis whilst clinical studies based on results of preclinical studies have focused on progression of vulnerable lesions and plaque rupture‐MIs and/or strokes. Cytotoxic lymphocytes including NK cells, iNKT cells and CD8+ T cells have the potential to not only influence early development of atherosclerotic lesions but also advanced atherosclerotic lesions, particularly vulnerable lesions and plaque rupture, frequently acting locally within lesions or within lymph nodes and producing pro‐inflammatory cytokines, chemokines and/or cytotoxins.
NK cells
NK cells have been strongly associated with atherosclerosis development atherosclerosis in humans and genetically modified mice. They are present in human and mouse atherosclerotic lesions (Whitman et al., 2004; Bobryshev and Lord, 2005b) and are recruited to developing lesions by chemoattractants such as monocyte chemoattractant protein‐1 (MCP‐1 also known as CCL2) and fractalkine (CX3CL1) (Allavena et al., 1994; Yoneda et al., 2000) to promote atherosclerosis development (Aiello et al., 1999; Lesnik et al., 2003). In humans with atherosclerosis, expression of the activating cell receptor CD160, which triggers cytotoxicity and cytokine secretion, is increased on circulating NK cells and suggested to contribute to atherosclerosis (Le Bouteiller et al., 2011; Zuo et al., 2015). Also, NK cells expressing the activating receptor NKG2C are increased in seropositive patients for human CMV and associate with high‐risk carotid atherosclerotic plaques (Martinez‐Rodriguez et al., 2013). Other studies indicate that patients with severe atherosclerosis have greater numbers of circulating NK cells (Clerc and Rouz, 1997); elderly patients with peripheral artery disease also have greater numbers of circulating NK cells but with reduced cytotoxic capability (Bruunsgaard et al., 2001). Immediately after non‐STEMI MI NK cell numbers are low and then increase over the ensuing 12 months possibly contributing to MI‐accelerated atherosclerosis; their failure to increase in some patients is associated with persistent low‐grade inflammation (Backteman et al., 2014). In other studies, circulating but not lymph node CD56+ NK cells are reduced in patients with acute coronary syndrome compared with patients with stable angina (Backteman et al., 2012). Given that NK cells are activated in periodontitis (Kramer et al., 2013; Wang et al., 2016) and periodontitis has been associated with cardiovascular disease (Tonetti, Van Dyke, and Working group 1 of the joint EFPAAPw, 2013), it is surprising that the role of NK cells in periodontitis‐accelerated atherosclerosis has not been investigated. Similarly, whether NK cells contribute to CMV aggravated atherosclerosis has not been investigated (Vliegen et al., 2004; Beziat et al., 2013).
In contrast to these association studies in humans, mechanistic studies defining the precise role of NK cells in atherosclerosis are more limited. Early studies in mice with a beige mutation indicated that NK cells might be atheroprotective (Schiller et al., 2002). However, these mice have a complex phenotype with defects in cell function not only restricted to NK cells but also affecting neutrophils and other cells and, this could have affected the outcome (Getz, 2002). Subsequently, Ly49A transgenic mice were used. These mice express the Ly49A inhibitory receptor under the control of the granzyme A promoter, and whilst the authors concluded that NK cells contribute to the development of atherosclerosis, the possibility that Ly49A affected other proatherogenic cells such as cytotoxic T lymphocytes cells was not excluded (Whitman et al., 2004); Ly49A is known not only to inhibit NK cells but also to prevent CD8+ T cell activation (Oberg et al., 2000). More recent studies using anti‐Asialo‐GM1 antibodies to deplete NK cells in hyperlipidaemic ApoE−/− mice also indicate that NK cells promote the development of atherosclerosis, studies supported by gain of function experiments (Selathurai et al., 2014). As anti‐Asialo‐GM1 antibodies might deplete other immune cells, we carried out a gain of function experiment where adoptive transfers involving transfer of wild type NK cells and NK cells deficient in IFN‐γ, granzyme B and perforin into triple knockout mice (i.e. T, B and NK cell‐deficient ApoE−/− mice) indicated that cytotoxic effects of NK cells are pro‐atherogenic and promote necrotic core development. However, given that lymphocyte deficient mice were used, a pro‐atherogenic role for NK cells involving secretion of IFN‐γ could not be excluded. In immune competent mice, NK cell‐derived IFN‐γ promotes CD4+ Th1 priming (Martin‐Fontecha et al., 2004). Thus in immune competent mice, NK cells might also promote atherosclerosis via a CD4+ T cell‐dependent mechanism. How NK cells are activated during the development of atherosclerosis is unknown, but given that macrophage foam cells express ligands for NKG2D receptors (Ikeshita et al., 2014), activation within lesions via NKG2D receptors is highly likely.
γδ‐T cells
To date, few studies have addressed the role of γδ‐T cells in atherosclerosis despite their identification in human atherosclerotic lesions more than 20 years ago (Kleindienst et al., 1993). In ApoE−/− mice, hyperlipidaemia increases γδ‐T cells, but aortic lipid accumulation is unaffected, suggesting no role in early lipid lesion/fatty streak development (Cheng et al., 2014). Others have shown that γδ‐T cells are the most abundant T cell within atherosclerotic lesions despite being a very minor T cell population and their deletion reduces atherosclerotic lesion size (Vu et al., 2014). It has been suggested that γδ‐T cell‐derived IL‐17 contributes to atherosclerosis. Their role in progression of established lesions and plaque rupture has not been investigated.
iNKT cells
iNKT cells migrate to developing atherosclerotic lesions and are present as a minor cell population in mouse atherosclerotic lesions (To et al., 2009). In human atherosclerotic lesions, iNKT cells are also a minor population and originally identified as CD161+ T cells (Bobryshev and Lord, 2005a). This however does not distinguish iNKT cells from CD161+ Foxp3+ T cells or other CD161+ T cell subtypes (Pesenacker et al., 2013; Gonzalez et al., 2015), but more recent studies using anti‐TCR Vα24 antibodies have definitively demonstrated their presence in human lesions (Kyriakakis et al., 2010). Early studies using loss and gain of function provide strong evidence that iNKT cells are important for development of atherosclerosis. Loss of function studies involving hyperlipidaemic NKT cell‐deficient CD1d−/− chimeric LDLR−/− mice as well as CD1d−/−ApoE−/− mice demonstrated smaller lesion development in the absence of iNKT cells (Nakai et al., 2004; Tupin et al., 2004); mice deficient in invariant Vα14 NKT cells also exhibit reduced atherosclerosis (Rogers et al., 2008). Increasing atherosclerosis by administering pharmacological doses of α‐GalCer to activate NKT cells to provide evidence that iNKT cells promote atherosclerosis (Tupin et al., 2004) is complicated by extensive bystander activation of T, B, NK and γδ‐T cells (Kitamura et al., 2000; Tupin et al., 2004; Smyth et al., 2005; Paget et al., 2012); these lymphocytes also exert iNKT cell‐independent pro‐atherogenic effects (Perry and McNamara, 2012; Tse et al., 2013; Selathurai et al., 2014; Vu et al., 2014). More recent studies indicate that iNKT cells promote atherosclerosis largely independently of bystander T, B or NK cell activation (Li et al., 2015). CD4+ iNKT cells have been identified as the proatherogenic subtype in mice. This subtype expresses lower concentrations of Ly49 inhibitory receptors‐Ly49A, Ly49C/I and Ly49G2 compared with other subtypes, possibly explaining their greater pro‐atherogenic activity (To et al., 2009). In contrast, human CD4+ iNKT cells exhibit a somewhat different pattern of killer receptors with increased expression of activating receptors NKp30 and NKp46. These cells are also highly cytotoxic, killing CD4 + CD25hiCD27lo/− regulatory T cells to promote inflammation (Nguyen et al., 2008). Although early studies suggested that pro‐inflammatory cytokines such as IFN‐γ promote iNKT cell mediated atherosclerosis (Tupin et al., 2004), more recent studies indicate a major role for cytotoxins (Li et al., 2015). CD4+ iNKT cells promote atherosclerosis and the development of large necrotic cores via mechanisms dependent on perforin and granzyme B rather than cytokines (Li et al., 2015). The cytotoxic actions of the iNKT cell increase lesion apoptotic cell numbers and necrotic cores, which in turn augment inflammation and atherosclerosis development via a sterile inflammatory response (Li et al., 2016). iNKT cell activation during the development of atherosclerosis is at least in part dependent on lipid antigens activating TCRs, indicated by findings that a CD1d‐dependent lipid antagonist to iNKT cells attenuates both the development and progression of established atherosclerosis (Li et al., 2016). Although the lipid antigens have not been identified, some appear to be carried by lipoproteins in the circulation and may also reside within atherosclerotic plaques (VanderLaan et al., 2007). iNKT cells are also important in LPS‐accelerated atherosclerosis (Ostos et al., 2002), a model resembling infection‐associated atherosclerosis. Bacterial infections involving Chlamydia pneumoniae, Porphyromonas gingivalis and Helicobacter pylori have been associated with accelerated atherosclerosis in humans (Ameriso et al., 2001; Campbell and Rosenfeld, 2014; Hussain et al., 2015). iNKT cells constitutively express TLR4 on their cell surface, and direct engagement of TLR4 on iNKT cells promotes inflammatory disorders (Kim et al., 2012). Recently iNKT‐derived IFN‐γ has been shown to induce apoptosis of marginal zone B cells, suggesting a regulatory iNKT subset. The authors implicate expansion of marginal zone B cells in relation to loss of iNKT‐derived IFN‐γ in increased atherosclerosis in long‐term high‐fat feeding (Soh et al., 2016).
Cytotoxic CD8+ T lymphocytes
Multiple lines of evidence indicate that CD8+ T cells contribute to atherosclerosis and vulnerable plaque development. Correlative studies in humans with coronary artery disease imply important roles for cytokine and cytotoxin producing CD8+ T cells in advanced coronary artery atherosclerosis (Bergstrom et al., 2012; Kolbus et al., 2013; Longenecker et al., 2013; Hwang et al., 2016). In advanced human lesions, CD8+ T cells predominate over CD4+ T cells (Gewaltig et al., 2008; Rossmann et al., 2008; Paul et al., 2016) and concentrate around shoulder regions and fibrous caps (Paul et al., 2016). They are also abundant in mouse atherosclerotic lesions (Kyaw et al., 2013). Oxidized LDL and heat shock protein peptides have been implicated in their activation (Wu et al., 1996; Rossmann et al., 2008; Kolbus et al., 2010). Activation does not appear to involve antigen presentation by CD8α + dendritic cells (Legein et al., 2015), but may involve other antigen presenting cells such as γδ‐T cells, which are present in lesions. Despite such associations, early studies in mice led to conflicting results on the significance of CD8+ T cells (Fyfe et al., 1994; Elhage et al., 2004), with conclusions largely based on poorly understood complex mouse models (Araujo et al., 1995; Schaible et al., 2002). An atheroprotective role was suggested by increased atherosclerosis in β2m‐deficient mice. But β2m‐deficient mice disrupt CD8α/α, not CD8α/β T cell development, and develop iron overload aggravating atherosclerosis (Araujo et al., 1995). While genetic knockouts of CD8 and tap1 showed no change in lesions (Elhage et al., 2004), it is likely that CD4 T cell expansion during development compensated for the CD8 T cell deficiency. More recent independent studies using specific CD8+ T cell depleting antibodies indicate pro‐atherogenic roles for CD8+ T cells (Kyaw et al., 2013; Cochain et al., 2015). Activated CD8+ T cells promote atherosclerosis and vulnerable plaque development by cytotoxic mechanisms involving perforin and granzyme B as supported by adoptive transfer studies with CD8 T cells deficient in perforin and granzyme B that failed to promote atherosclerosis development (Kyaw et al., 2013). These adoptive transfer studies suggest that CD8+ T lymphocytes promote the development of vulnerable atherosclerotic plaques by perforin and granzyme B‐mediated apoptosis of macrophages, smooth muscle cells and endothelial cells that in turn leads to secondary necrosis and necrotic core formation. These studies also suggest that CD8 T cell‐mediated cell death initiates a sterile inflammatory response (Chen and Nunez, 2010), as the transfer of CD8 T cells deficient in perforin and granzyme B led to a reduction in inflammatory MCP‐1, IL‐1β, IFN‐γ and VCAM‐1. A role for TNF‐α produced by CD8 T cells is also supported by adoptive transfer studies with CD8 T cells deficient in TNF‐α that failed to promote atherosclerosis development (Kyaw et al., 2013). While adoptive transfer of CD8 T cells deficient in IFN‐γ suggest that CD8 T cell‐derived IFN‐γ has no role in atherosclerosis (Kyaw et al., 2013), other studies indicate a role for CD8+ T cell‐derived IFN‐γ in atherosclerosis development, regulating monopoiesis and circulating inflammatory Ly6Chi monocytes (Cochain et al., 2015). A role for CD8+ T cells has been suggested in C. pneumoniae‐accelerated atherosclerosis (Zafiratos et al., 2015). It is also possible that CMV and EBV antigen‐specific CD8+ T cells may contribute to pathogen‐enhanced atherosclerosis as such viral DNAs have been detected in atherosclerotic lesions (Ibrahim et al., 2005); limited data are available linking CMV and EBV infections to atherosclerosis. Recently, PD‐1 and TIM‐3 have been implicated in regulating CD8+ T cell function in atherosclerosis in humans, by affecting TNF‐α and IFN‐γ production (Qiu et al., 2015). In contrast to these pro‐atherogenic effects of CD8+ T cells, CD8 T cell cytotoxicity increased by ApoB‐100 targeted immunisation modulates the functions of dendritic cells, monocytes and macrophages (Chyu et al., 2012; Honjo et al., 2015; Cochain and Zernecke, 2016), suggesting a possible favourable effect in atherosclerosis, but their relative relevance in vivo is uncertain.
Hypertension, hypercholesterolaemia and diabetes mellitus are major risk factors for plaque development and rupture (Bentzon et al., 2014). Hypertension elevates activated CD8+ T cell numbers in human subjects (Youn et al., 2013; Itani et al., 2016) and increases CD8+ T cell accumulation in mouse aortas, increasing augmented perivascular inflammation and augmented endothelial dysfunction (Itani et al., 2016; Mikolajczyk et al., 2016). Together with early CD8+ T cell activation in hypercholesterolaemic mice (Kolbus et al., 2010) and CD8+ T cell‐induced macrophage accumulation in metabolic diseases (Nishimura et al., 2009), cytotoxic CD8+ T cells may contribute, at least in part, to the mechanisms by which these risk factors promote plaque development and rupture.
CD4 + CD28−T cells
Association studies suggest a role for CD4 + CD28−T cells in human atherosclerosis (Liuzzo et al., 1999, 2000; Nakajima et al., 2002). These cells express multiple cytotoxins including granzymes A and B, perforin and granulysin as well as pro‐inflammatory cytokines IFN‐γ and TNF‐α (Teo et al., 2013). They are highly resistant to apoptosis (Kovalcsik et al., 2015) and appear to accumulate in vulnerable coronary atherosclerotic plaques (Nakajima et al., 2003). Activation appears to be triggered by heat shock protein 60 antigens (Zal et al., 2008; Zal et al., 2004) and by the co‐stimulatory molecules Ox40 (CD134) and 41BB (CD137) present on CD4 + CD28−T cells in acute coronary syndromes (Dumitriu et al., 2012). Cytotoxic CD4+ T cell responses have been reported in latent and chronic viral infections (Walton et al., 2013), but whether there is any role for virus‐specific CD4+ CD28−T cells in atherosclerosis is not known. CD4+ CD28−T cells are also activated by IL‐12 (Zhang et al., 2006). Cytotoxic CD4 T cells have been reported to be stimulated by plasmacytoid dendritic cell‐derived IFN‐α to induce expression of TRAIL and kill vascular smooth muscle cells in carotid atheromas (Niessner et al., 2006). Despite these associations, their role in atherosclerosis and vulnerable plaque development remains to be defined.
Collectively cytotoxic cells can effectively target and kill lesion cells by inducing apoptosis and necrosis via three mechanisms, that is, (1) cytotoxins such as perforin‐ and granzymeB‐mediated, (2) Fas–FasL or TRAIL‐mediated and (3) cytokine‐induced mechanisms (Figure 1). Macrophages, major constituents of lesion cellular contents, are major target cells killed by cytolytic mechanisms, suggesting an important role for cytotoxic cells in generating the necrotic core and vulnerable plaques. As vascular smooth muscle cells and endothelial cells can also be targeted by cytotoxic cells, cytotoxic cells are also important in destabilising plaque and inducing plaque rupture leading to MIs or strokes. Thus, targeting cytotoxic cells may be therapeutically beneficial in preventing premature atherosclerosis‐related deaths.
Figure 1.
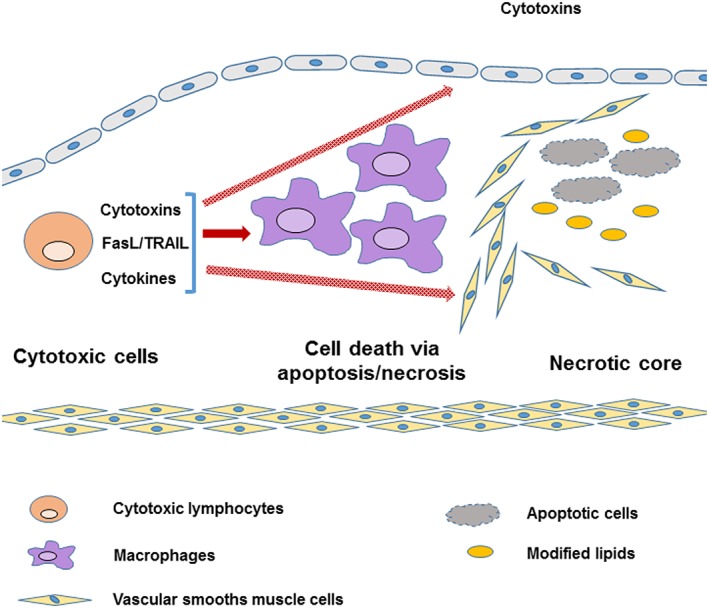
Cytotoxic lymphocytes promote lesion apoptosis and necrosis via cytotoxin‐, FasL/TRAIL‐ or cytokine‐mediated mechanisms. Lesion macrophages are major apoptotic or necrotic cells in lesions, and increased lesion apoptosis and necrosis generated larger necrotic cores, a predominant feature of vulnerable atherosclerotic plaques. Cytotoxic lymphocytes also induce apoptosis and necrosis in vascular endothelial or smooth muscle cells that may contribute to rupture of vulnerable plaques.
Pharmacologically targeting cytotoxic lymphocytes in atherosclerosis
Specific cytotoxic lymphocyte depletion could theoretically be considered as one therapeutic approach to limit their pro‐atherogenic actions during atheroma and vulnerable plaque development. However, such an approach is difficult to justify in essentially healthy immune competent subjects as it would make individuals highly susceptible to life‐threatening viral and bacterial infections. Instead, more specific approaches that target specific receptors on individual cell types or even unique cell types may be more appropriate to attenuate atherosclerosis and vulnerable plaque development. Towards this aim, pharmacological targeting could involve the use of either small molecules or long‐acting biologicals (e.g. antibodies), which are becoming increasingly accepted in atherosclerosis therapy (Stein et al., 2012). Targeting iNKT cell and CD8+ T cell activation may be an effective therapeutic strategy (Figure 2A). Recently, a CD1d lipid antagonist was shown to prevent iNKT cell activation in atherosclerotic mice and to reduce lesion inflammation and necrosis; the antagonist was also highly effective in preventing not only lesion development but also progression of established lesions (Li et al., 2016). Targeting antigen presentation with biologicals such as anti‐CD1d antibodies may also be an effective therapeutic strategy to prevent iNKT activation in atherosclerosis (Duthie et al., 2005); an anti‐human CD1d inhibitory antibody has recently been developed (Nambiar et al., 2015). Such approaches to limit activation of killer cells seem to impact on immune defence against infectious agents, but killer cells are able to respond against pathogens microbes via various innate receptors without utilizing TCR‐ or CD1d‐dependent activation. Therefore, targeting against activation of iNKT and CD8+ T cells will not be expected to compromise host defence systems. β2‐adrenoceptor s have recently been shown to be elevated on human CD8+ effector memory T cells, and β2‐adrenoceptor activation decreases IFN‐γ and TNF‐α secretion as well as cytotoxic activity of human and murine CD8+ T cells (Figure 2A). Also, long‐acting β2‐agonists such as salmeterol are effective in vivo in suppressing cytokine secretion by CD8+ T cells (Estrada et al., 2016). Whether treatment with β2‐agonists is effective in preventing CD8 + T cell activation and its consequences in atherosclerosis remains to be determined. Necrotic cells are abundant in advanced lesions and very likely contribute to the cytotoxic actions of CD8+ T cells with lesion dendritic cells utilizing C‐type lectin domain family 9 member A (Clec9A) to cross‐present necrotic cell remnant antigens to CD8+ T cells. It is tempting to speculate that preventing necrotic cell sensing by dendritic cells expressing Clec9A may also be an effective strategy to prevent CD8+ T cell activation in advanced lesions (Figure 2A); Clec9A favours antigen cross presentation to cytotoxic CD8+ T cells (Zelenay et al., 2012). Preventing migration of cytotoxic lymphocytes to atherosclerotic lesions could also be an effective therapeutic strategy to attenuate atherosclerosis (Figure 2B) but will require definition of the chemotactic factors that are responsible for migration of cytotoxic lymphocytes to lesions. A large number of receptor antagonists to G‐protein‐coupled chemokine receptors have been developed including CCR2, CCR5, CXCR3, CXCR4, CCR1 and CCR3 but have not been assessed in atherosclerosis (Suzaki et al., 2008; O'Boyle et al., 2012; Zweemer et al., 2013). The findings that NKG2D ligands are up‐regulated in human plasma and in human and mouse atherosclerotic lesions together with the findings of NKG2D deletion studies in mice indicate that NKG2D receptors are a viable therapeutic target (Figure 2C) (Xia et al., 2011). Anti‐NKG2D inhibitory antibodies are available (Kjellev et al., 2007; Steigerwald et al., 2009), but their effects on development and progression of established atherosclerosis and on vulnerable plaque development have not been assessed. One potential limitation of targeting NKG2D is that receptor expression may not be restricted to a single cell type but rather expressed on multiple cytotoxic lymphocytes in the periphery. Similarly, KIR activating and inhibitory receptors could be targeted to limit proatherogenic effects (Figure 2C). Such receptors have been targeted to increase the cytotoxicity of lymphocytes in cancer (Benson et al., 2011); antibodies could be developed to activate inhibitory receptors or inhibit activating receptors suppressing cytotoxic lymphocyte activity and attenuating atherosclerosis and vulnerable plaque development.
Figure 2.
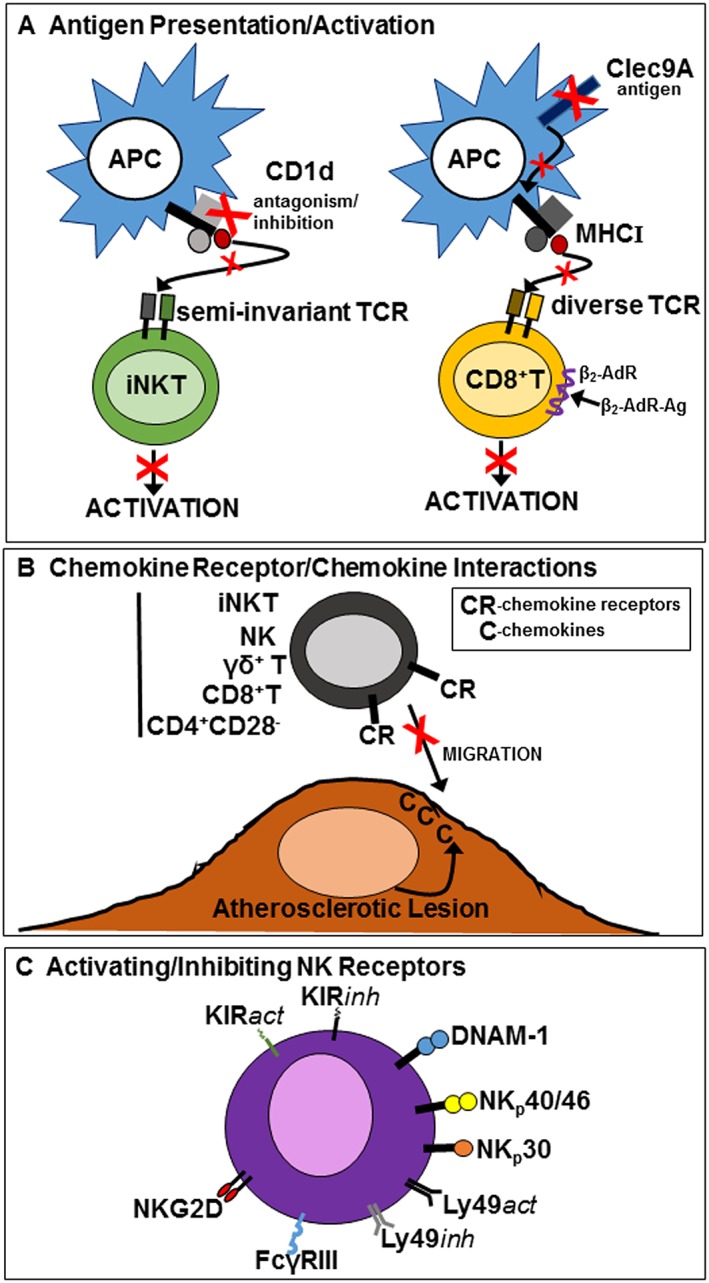
Molecules expressed by cytotoxic lymphocytes that may be targeted to attenuate atherosclerosis and vulnerable plaque development. (A) CD1d on antigen presenting cells, for example, dendritic cells to prevent TCR activation of iNKT cells and Clec9A on dendritic cells to prevent uptake of necrotic cell remnants and presentation on MHC I to activate CD8+ T cells. Also, activation of β2‐adrenoceptors (β2‐AdR) by β2‐adrenoceptor agonists (β2‐AdR‐Ag) to inhibit activated CD8+ T cells. (B) Inhibiting chemokine receptors expressed by cytotoxic lymphocytes to prevent their migration to developing/developed atherosclerotic lesions. (C) Targeting NK activating and inhibitory receptors/co‐receptors to inhibit/attenuate activation of cytotoxic lymphocytes to attenuate atherosclerosis and vulnerable plaque development with activating receptors inhibited and inhibitory receptors activated.
Given that cytotoxic lymphocytes accumulate within atherosclerotic lesions, more specific targeting of cytotoxic lymphocytes residing within lesions might also be considered as such an approach would not affect cytotoxic lymphocyte activity in other tissues or in the circulation. There is now a strong body of evidence for tissue resident memory CD8+ T cells and NK cells with unique gene expression patterns and receptor profiles characteristic of a particular tissue (Wakim et al., 2012; Sojka et al., 2014; Park and Kupper, 2015; Melsen et al., 2016). Clearly, additional studies will be required to determine whether such cytotoxic lymphocytes with unique protein expression profiles are present in atherosclerotic lesions and developing vulnerable plaques. Such an approach offers unique pharmacological opportunities to suppress atherosclerosis and vulnerable plaque development without significantly affecting other components of the immune system, minimizing the possibility of any unwanted immune suppressive effects such as increased susceptibility to infections.
Summary and conclusions
Vulnerable atherosclerotic plaques characterized by large necrotic cores and increased lesion apoptosis are an important concern in atherosclerosis management because their rupture initiates thrombotic occlusion of vital arteries causing heart attacks and strokes. Cytotoxic lymphocytes in human and mouse atherosclerotic lesions are of interest because of their ability to induce apoptosis that leads to secondary necrosis. Further research is warranted to precisely and definitively define the roles of each cytotoxic lymphocyte in development, progression and rupture of vulnerable atherosclerotic plaques. Clearly, global depletion of a cytotoxic lymphocyte is not an option, suggesting instead a targeted therapeutic strategy that specifically affects their activation or trafficking pathways. While approaches to target lipid‐antigens such as CD1d antagonists will impact on NKT cell effector functions, this will not completely abolish effector functions of other cytotoxic cells against infections that recognize pathogenic antigens presented by MHC molecules. In conclusion, it is more beneficial and clinically feasible to target cytotoxic lymphocytes through either their activation/trafficking pathways or targeting resident cytotoxic lymphocytes within lesions. More studies are needed to better understand the roles of the different cytotoxic lymphocytes in atherosclerosis, particularly in vulnerable plaque formation and rupture so that new therapeutic targets can be defined for controlling activated cytotoxic lymphocytes and their effector functions.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c).
Author contributions
T.K. and A.B drafted the manuscript. All authors have revised and approved the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This study is supported by National Health and Medical Research Council of Australia project grant nos 1068500 and 1030515.
Kyaw, T. , Peter, K. , Li, Y. , Tipping, P. , Toh, B.‐H. , and Bobik, A. (2017) Cytotoxic lymphocytes and atherosclerosis: significance, mechanisms and therapeutic challenges. British Journal of Pharmacology, 174: 3956–3972. doi: 10.1111/bph.13845.
References
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE et al. (2009). Tumor antigen‐specific CD8 T cells infiltrating the tumor express high levels of PD‐1 and are functionally impaired. Blood 114: 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ et al. (1999). Monocyte chemoattractant protein‐1 accelerates atherosclerosis in apolipoprotein E‐deficient mice. Arterioscler Thromb Vasc Biol 19: 1518–1525. [DOI] [PubMed] [Google Scholar]
- Akane K, Kojima S, Mak TW, Shiku H, Suzuki H (2016). CD8+CD122+CD49dlow regulatory T cells maintain T‐cell homeostasis by killing activated T cells via Fas/FasL‐mediated cytotoxicity. Proc Natl Acad Sci U S A 113: 2460–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The concise guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The concise guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allavena P, Bianchi G, Zhou D, van Damme J, Jilek P, Sozzani S et al. (1994). Induction of natural killer cell migration by monocyte chemotactic protein‐1, −2 and −3. Eur J Immunol 24: 3233–3236. [DOI] [PubMed] [Google Scholar]
- Alonso‐Arias R, Moro‐Garcia MA, Vidal‐Castineira JR, Solano‐Jaurrieta JJ, Suarez‐Garcia FM, Coto E et al. (2011). IL‐15 preferentially enhances functional properties and antigen‐specific responses of CD4+CD28(null) compared to CD4+CD28+ T cells. Aging Cell 10: 844–852. [DOI] [PubMed] [Google Scholar]
- Ameriso SF, Fridman EA, Leiguarda RC, Sevlever GE (2001). Detection of Helicobacter pylori in human carotid atherosclerotic plaques. Stroke 32: 385–391. [DOI] [PubMed] [Google Scholar]
- de Andrade LF, Smyth MJ, Martinet L (2014). DNAM‐1 control of natural killer cells functions through nectin and nectin‐like proteins. Immunol Cell Biol 92: 237–244. [DOI] [PubMed] [Google Scholar]
- Araujo JA, Romano EL, Brito BE, Parthe V, Romano M, Bracho M et al. (1995). Iron overload augments the development of atherosclerotic lesions in rabbits. Arterioscler Thromb Vasc Biol 15: 1172–1180. [DOI] [PubMed] [Google Scholar]
- Arlettaz L, Degermann S, De Rham C, Roosnek E, Huard B (2004). Expression of inhibitory KIR is confined to CD8+ effector T cells and limits their proliferative capacity. Eur J Immunol 34: 3413–3422. [DOI] [PubMed] [Google Scholar]
- Backteman K, Andersson C, Dahlin LG, Ernerudh J, Jonasson L (2012). Lymphocyte subpopulations in lymph nodes and peripheral blood: a comparison between patients with stable angina and acute coronary syndrome. PLoS One 7: e32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backteman K, Ernerudh J, Jonasson L (2014). Natural killer (NK) cell deficit in coronary artery disease: no aberrations in phenotype but sustained reduction of NK cells is associated with low‐grade inflammation. Clin Exp Immunol 175: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Constantinides MG, Thomas SY, Reboulet R, Meng F, Koentgen F et al. (2012). Distinct APCs explain the cytokine bias of alpha‐galactosylceramide variants in vivo. J Immunol 188: 3053–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquera S, Pedroza‐Tobias A, Medina C, Hernandez‐Barrera L, Bibbins‐Domingo K, Lozano R et al. (2015). Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res 46: 328–338. [DOI] [PubMed] [Google Scholar]
- Ben‐Sasson SZ, Wang K, Cohen J, Paul WE (2013). IL‐1beta strikingly enhances antigen‐driven CD4 and CD8 T‐cell responses. Cold Spring Harb Symp Quant Biol 78: 117–124. [DOI] [PubMed] [Google Scholar]
- Benson DM Jr, Bakan CE, Zhang S, Collins SM, Liang J, Srivastava S et al. (2011). IPH2101, a novel anti‐inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood 118: 6387–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzon JF, Otsuka F, Virmani R, Falk E (2014). Mechanisms of plaque formation and rupture. Circ Res 114: 1852–1866. [DOI] [PubMed] [Google Scholar]
- Berahovich RD, Lai NL, Wei Z, Lanier LL, Schall TJ (2006). Evidence for NK cell subsets based on chemokine receptor expression. J Immunol 177: 7833–7840. [DOI] [PubMed] [Google Scholar]
- Bergstrom I, Backteman K, Lundberg A, Ernerudh J, Jonasson L (2012). Persistent accumulation of interferon‐gamma‐producing CD8+CD56+ T cells in blood from patients with coronary artery disease. Atherosclerosis 224: 515–520. [DOI] [PubMed] [Google Scholar]
- Betjes MG, Huisman M, Weimar W, Litjens NH (2008). Expansion of cytolytic CD4+CD28‐ T cells in end‐stage renal disease. Kidney Int 74: 760–767. [DOI] [PubMed] [Google Scholar]
- Beziat V, Liu LL, Malmberg JA, Ivarsson MA, Sohlberg E, Bjorklund AT et al. (2013). NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood 121: 2678–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC (2005). TCR stimulation with modified anti‐CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest 115: 2904–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkstrom NK, Beziat V, Cichocki F, Liu LL, Levine J, Larsson S et al. (2012). CD8 T cells express randomly selected KIRs with distinct specificities compared with NK cells. Blood 120: 3455–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobryshev YV, Lord RS (2005a). Co‐accumulation of dendritic cells and natural killer T cells within rupture‐prone regions in human atherosclerotic plaques. J Histochem Cytochem 53: 781–785. [DOI] [PubMed] [Google Scholar]
- Bobryshev YV, Lord RS (2005b). Identification of natural killer cells in human atherosclerotic plaque. Atherosclerosis 180: 423–427. [DOI] [PubMed] [Google Scholar]
- Bonneville M, O'Brien RL, Born WK (2010). Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 10: 467–478. [DOI] [PubMed] [Google Scholar]
- Born WK, Reardon CL, O'Brien RL (2006). The function of gammadelta T cells in innate immunity. Curr Opin Immunol 18: 31–38. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB (2013). Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13: 101–117. [DOI] [PubMed] [Google Scholar]
- Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL (2008). CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol 181: 4918–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK (2001). Decreased natural killer cell activity is associated with atherosclerosis in elderly humans. Exp Gerontol 37: 127–136. [DOI] [PubMed] [Google Scholar]
- Bryl E, Vallejo AN, Weyand CM, Goronzy JJ (2001). Down‐regulation of CD28 expression by TNF‐alpha. J Immunol 167: 3231–3238. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Rosenfeld ME (2014). Persistent C. pneumoniae infection in atherosclerotic lesions: rethinking the clinical trials. Front Cell Infect Microbiol 4: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalheiro H, da Silva JA, Souto‐Carneiro MM (2013). Potential roles for CD8(+) T cells in rheumatoid arthritis. Autoimmun Rev 12: 401–409. [DOI] [PubMed] [Google Scholar]
- Carvalheiro H, Duarte C, Silva‐Cardoso S, da Silva JA, Souto‐Carneiro MM (2015). CD8+ T cell profiles in patients with rheumatoid arthritis and their relationship to disease activity. Arthritis Rheumatol 67: 363–371. [DOI] [PubMed] [Google Scholar]
- Chapman A, Stewart SJ, Nepom GT, Green WF, Crowe D, Thomas JW et al. (1996). CD11b+CD28‐CD4+ human T cells: activation requirements and association with HLA‐DR alleles. J Immunol 157: 4771–4780. [PubMed] [Google Scholar]
- Chen GY, Nunez G (2010). Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Bui AV, Diesch J, Manasseh R, Hausding C, Rivera J et al. (2013). A novel mouse model of atherosclerotic plaque instability for drug testing and mechanistic/therapeutic discoveries using gene and microRNA expression profiling. Circ Res 113: 252–265. [DOI] [PubMed] [Google Scholar]
- Chen YC, Huang AL, Kyaw TS, Bobik A, Peter K (2016). Atherosclerotic plaque rupture: identifying the straw that breaks the camel's back. Arterioscler Thromb Vasc Biol 36: e63–e72. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Wu R, Hedrick CC (2014). Gammadelta (gammadelta) T lymphocytes do not impact the development of early atherosclerosis. Atherosclerosis 234: 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien YH, Konigshofer Y (2007). Antigen recognition by gammadelta T cells. Immunol Rev 215: 46–58. [DOI] [PubMed] [Google Scholar]
- Chua HL, Serov Y, Brahmi Z (2004). Regulation of FasL expression in natural killer cells. Hum Immunol 65: 317–327. [DOI] [PubMed] [Google Scholar]
- Chyu KY, Zhao X, Dimayuga PC, Zhou J, Li X, Yano J et al. (2012). CD8+ T cells mediate the athero‐protective effect of immunization with an ApoB‐100 peptide. PLoS One 7: e30780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc G, Rouz PM (1997). Lymphocyte subsets in severe atherosclerosis before revascularization. Ann Intern Med 126: 1004–1005. [DOI] [PubMed] [Google Scholar]
- Cochain C, Koch M, Chaudhari SM, Busch M, Pelisek J, Boon L et al. (2015). CD8+ T cells regulate monopoiesis and circulating Ly6C‐high monocyte levels in atherosclerosis in mice. Circ Res 117: 244–253. [DOI] [PubMed] [Google Scholar]
- Cochain C, Zernecke A (2016). Protective and pathogenic roles of CD8+ T cells in atherosclerosis. Basic Res Cardiol 111: 71. [DOI] [PubMed] [Google Scholar]
- Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS et al. (2008). Diverse cytokine production by NKT cell subsets and identification of an IL‐17‐producing CD4‐NK1.1‐ NKT cell population. Proc Natl Acad Sci U S A 105: 11287–11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ et al. (2007). IL‐21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol 178: 2827–2834. [DOI] [PubMed] [Google Scholar]
- De Sanctis JB, Blanca I, Bianco NE (1997). Secretion of cytokines by natural killer cells primed with interleukin‐2 and stimulated with different lipoproteins. Immunology 90: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deauvieau F, Ollion V, Doffin AC, Achard C, Fonteneau JF, Verronese E et al. (2015). Human natural killer cells promote cross‐presentation of tumor cell‐derived antigens by dendritic cells. Int J Cancer 136: 1085–1094. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Finlayson CJ, Loftus IM, Antunes RF, Lim P et al. (2012). High levels of costimulatory receptors OX40 and 4‐1BB characterize CD4+CD28null T cells in patients with acute coronary syndrome. Circ Res 110: 857–869. [DOI] [PubMed] [Google Scholar]
- Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ (2005). Both CD1d antigen presentation and interleukin‐12 are required to activate natural killer T cells during Trypanosoma cruzi infection. Infect Immun 73: 1890–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton GR, Lewis CE (2015). The look AHEAD trial: implications for lifestyle intervention in type 2 diabetes mellitus. Prog Cardiovasc Dis 58: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser B (2009). A rapid crosstalk of human gammadelta T cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathog 5: e1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhage R, Gourdy P, Brouchet L, Jawien J, Fouque MJ, Fievet C et al. (2004). Deleting TCR alpha beta+ or CD4+ T lymphocytes leads to opposite effects on site‐specific atherosclerosis in female apolipoprotein E‐deficient mice. Am J Pathol 165: 2013–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada LD, Agac D, Farrar JD (2016). Sympathetic neural signaling via the beta2‐adrenergic receptor suppresses T‐cell receptor‐mediated human and mouse CD8(+) T‐cell effector function. Eur J Immunol 46: 1948–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasth AE, Bjorkstrom NK, Anthoni M, Malmberg KJ, Malmstrom V (2010). Activating NK‐cell receptors co‐stimulate CD4(+)CD28(−) T cells in patients with rheumatoid arthritis. Eur J Immunol 40: 378–387. [DOI] [PubMed] [Google Scholar]
- Fauriat C, Long EO, Ljunggren HG, Bryceson YT (2010). Regulation of human NK‐cell cytokine and chemokine production by target cell recognition. Blood 115: 2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR et al. (2007). Acquisition of murine NK cell cytotoxicity requires the translation of a pre‐existing pool of granzyme B and perforin mRNAs. Immunity 26: 798–811. [DOI] [PubMed] [Google Scholar]
- Fox LM, Cox DG, Lockridge JL, Wang X, Chen X, Scharf L et al. (2009). Recognition of lyso‐phospholipids by human natural killer T lymphocytes. PLoS Biol 7: e1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BE, Hammarlund E, Raue HP, Slifka MK (2012). Regulation of innate CD8+ T‐cell activation mediated by cytokines. Proc Natl Acad Sci U S A 109: 9971–9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froelich CJ, Metkar SS, Raja SM (2004). Granzyme B‐mediated apoptosis – the elephant and the blind men? Cell Death Differ 11: 369–371. [DOI] [PubMed] [Google Scholar]
- Fyfe AI, Qiao JH, Lusis AJ (1994). Immune‐deficient mice develop typical atherosclerotic fatty streaks when fed an atherogenic diet. J Clin Invest 94: 2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz GS (2002). Do natural killer cells participate in a killer vascular disease? Arterioscler Thromb Vasc Biol 22: 1251–1253. [DOI] [PubMed] [Google Scholar]
- Gewaltig J, Kummer M, Koella C, Cathomas G, Biedermann BC (2008). Requirements for CD8 T‐cell migration into the human arterial wall. Hum Pathol 39: 1756–1762. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Stankovic S, Baxter AG (2010). Raising the NKT cell family. Nat Immunol 11: 197–206. [DOI] [PubMed] [Google Scholar]
- Gonzalez Y, Herrera MT, Juarez E, Salazar‐Lezama MA, Bobadilla K, Torres M (2015). CD161 expression defines a Th1/Th17 polyfunctional subset of resident memory T lymphocytes in bronchoalveolar cells. PLoS One 10: e0123591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Bruhl A, El‐Gabalawy H, Nelson JL, Spies T (2003). Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A 100: 9452–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Steinle A, Bauer S, Spies T (1998). Recognition of stress‐induced MHC molecules by intestinal epithelial gammadelta T cells. Science 279: 1737–1740. [DOI] [PubMed] [Google Scholar]
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E et al. (2014). PD‐1 identifies the patient‐specific CD8(+) tumor‐reactive repertoire infiltrating human tumors. J Clin Invest 124: 2246–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Gollapudi S (2007). Effector memory CD8+ T cells are resistant to apoptosis. Ann N Y Acad Sci 1109: 145–150. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Ornelles DA, Mitchell LM, Brzoza‐Lewis KL, Hiltbold EM (2008). IL‐12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J Immunol 181: 8576–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LP, Denney L, Luhn K, Teoh D, Clelland C, McMichael AJ (2008). Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur J Immunol 38: 1913–1922. [DOI] [PubMed] [Google Scholar]
- Holderness J, Hedges JF, Ramstead A, Jutila MA (2013). Comparative biology of gammadelta T cell function in humans, mice, and domestic animals. Annu Rev Anim Biosci 1: 99–124. [DOI] [PubMed] [Google Scholar]
- Honjo T, Chyu KY, Dimayuga PC, Yano J, Lio WM, Trinidad P et al. (2015). ApoB‐100‐related peptide vaccine protects against angiotensin II‐induced aortic aneurysm formation and rupture. J Am Coll Cardiol 65: 546–556. [DOI] [PubMed] [Google Scholar]
- Huang JR, Tsai YC, Chang YJ, Wu JC, Hung JT, Lin KH et al. (2014). alpha‐Galactosylceramide but not phenyl‐glycolipids induced NKT cell anergy and IL‐33‐mediated myeloid‐derived suppressor cell accumulation via upregulation of egr2/3. J Immunol 192: 1972–1981. [DOI] [PubMed] [Google Scholar]
- Hussain M, Stover CM, Dupont A (2015). P. gingivalis in periodontal disease and atherosclerosis – scenes of action for antimicrobial peptides and complement. Front Immunol 6: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y, Yu HT, Kim DH, Jang J, Kim HY, Kang I et al. (2016). Expansion of CD8(+) T cells lacking the IL‐6 receptor alpha chain in patients with coronary artery diseases (CAD). Atherosclerosis 249: 44–51. [DOI] [PubMed] [Google Scholar]
- Ibrahim AI, Obeid MT, Jouma MJ, Moasis GA, Al‐Richane WL, Kindermann I et al. (2005). Detection of herpes simplex virus, cytomegalovirus and Epstein‐Barr virus DNA in atherosclerotic plaques and in unaffected bypass grafts. J Clin Virol 32: 29–32. [DOI] [PubMed] [Google Scholar]
- Ikeshita S, Miyatake Y, Otsuka N, Kasahara M (2014). MICA/B expression in macrophage foam cells infiltrating atherosclerotic plaques. Exp Mol Pathol 97: 171–175. [DOI] [PubMed] [Google Scholar]
- Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM et al. (2016). Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas ML, Groves P, Kienzle N, Kelso A (2005). IL‐2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol 175: 8003–8010. [DOI] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, Amigorena S (2012). Cross‐presentation by dendritic cells. Nat Rev Immunol 12: 557–569. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D (2003). Features and functions of gamma delta T lymphocytes: focus on chemokines and their receptors. Crit Rev Immunol 23: 339–370. [DOI] [PubMed] [Google Scholar]
- Kalofoutis C, Piperi C, Kalofoutis A, Harris F, Phoenix D, Singh J (2007). Type II diabetes mellitus and cardiovascular risk factors: current therapeutic approaches. Exp Clin Cardiol 12: 17–28. [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F et al. (2000). Augmentation of Valpha14 NKT cell‐mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A‐induced hepatitis. J Exp Med 191: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapourchali FR, Surendiran G, Chen L, Uitz E, Bahadori B, Moghadasian MH (2014). Animal models of atherosclerosis. World J Clin Cases 2: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan WN, Inzucchi SE, Sawan C, Macko RF, Furie KL (2013). Obesity: a stubbornly obvious target for stroke prevention. Stroke 44: 278–286. [DOI] [PubMed] [Google Scholar]
- Khanna R, Burrows SR (2000). Role of cytotoxic T lymphocytes in Epstein–Barr virus‐associated diseases. Annu Rev Microbiol 54: 19–48. [DOI] [PubMed] [Google Scholar]
- Kilinc MO, Rowswell‐Turner RB, Gu T, Virtuoso LP, Egilmez NK (2009). Activated CD8+ T‐effector/memory cells eliminate CD4+ CD25+ Foxp3+ T‐suppressor cells from tumors via FasL mediated apoptosis. J Immunol 183: 7656–7660. [DOI] [PubMed] [Google Scholar]
- Kim CH, Johnston B, Butcher EC (2002). Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT cell subsets with distinct cytokine‐producing capacity. Blood 100: 11–16. [DOI] [PubMed] [Google Scholar]
- Kim HY, Kim S, Chung DH (2006). FcgammaRIII engagement provides activating signals to NKT cells in antibody‐induced joint inflammation. J Clin Invest 116: 2484–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim HS, Kim HY, Oh SJ, Chung DH (2012). Direct engagement of TLR4 in invariant NKT cells regulates immune diseases by differential IL‐4 and IFN‐gamma production in mice. PLoS One 7: e45348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y et al. (1999). The natural killer T (NKT) cell ligand alpha‐galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)‐12 production by dendritic cells and IL‐12 receptor expression on NKT cells. J Exp Med 189: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H, Ohta A, Sekimoto M, Sato M, Iwakabe K, Nakui M et al. (2000). alpha‐galactosylceramide induces early B‐cell activation through IL‐4 production by NKT cells. Cell Immunol 199: 37–42. [DOI] [PubMed] [Google Scholar]
- Kjellev S, Haase C, Lundsgaard D, Urso B, Tornehave D, Markholst H (2007). Inhibition of NKG2D receptor function by antibody therapy attenuates transfer‐induced colitis in SCID mice. Eur J Immunol 37: 1397–1406. [DOI] [PubMed] [Google Scholar]
- Kleindienst R, Xu Q, Willeit J, Waldenberger FR, Weimann S, Wick G (1993). Immunology of atherosclerosis. Demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am J Pathol 142: 1927–1937. [PMC free article] [PubMed] [Google Scholar]
- Klenerman P, Oxenius A (2016). T cell responses to cytomegalovirus. Nat Rev Immunol 16: 367–377. [DOI] [PubMed] [Google Scholar]
- Kolbus D, Ljungcrantz I, Andersson L, Hedblad B, Fredrikson GN, Bjorkbacka H et al. (2013). Association between CD8+ T‐cell subsets and cardiovascular disease. J Intern Med 274: 41–51. [DOI] [PubMed] [Google Scholar]
- Kolbus D, Ramos OH, Berg KE, Persson J, Wigren M, Bjorkbacka H et al. (2010). CD8+ T cell activation predominate early immune responses to hypercholesterolemia in Apoe(−)(/)(−) mice. BMC Immunol 11: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalcsik E, Antunes RF, Baruah P, Kaski JC, Dumitriu IE (2015). Proteasome‐mediated reduction in proapoptotic molecule Bim renders CD4(+)CD28null T cells resistant to apoptosis in acute coronary syndrome. Circulation 131: 709–720. [DOI] [PubMed] [Google Scholar]
- Kramer B, Kebschull M, Nowak M, Demmer RT, Haupt M, Korner C et al. (2013). Role of the NK cell‐activating receptor CRACC in periodontitis. Infect Immun 81: 690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuylenstierna C, Bjorkstrom NK, Andersson SK, Sahlstrom P, Bosnjak L, Paquin‐Proulx D et al. (2011). NKG2D performs two functions in invariant NKT cells: direct TCR‐independent activation of NK‐like cytolysis and co‐stimulation of activation by CD1d. Eur J Immunol 41: 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A et al. (2013). Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE‐deficient mice. Circulation 127: 1028–1039. [DOI] [PubMed] [Google Scholar]
- Kyriakakis E, Cavallari M, Andert J, Philippova M, Koella C, Bochkov V et al. (2010). Invariant natural killer T cells: linking inflammation and neovascularization in human atherosclerosis. Eur J Immunol 40: 3268–3279. [DOI] [PubMed] [Google Scholar]
- Lantz O, Bendelac A (1994). An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I‐specific CD4+ and CD4‐8‐ T cells in mice and humans. J Exp Med 180: 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouteiller P, Tabiasco J, Polgar B, Kozma N, Giustiniani J, Siewiera J et al. (2011). CD160: a unique activating NK cell receptor. Immunol Lett 138: 93–96. [DOI] [PubMed] [Google Scholar]
- Lee HH, Meyer EH, Goya S, Pichavant M, Kim HY, Bu X et al. (2010). Apoptotic cells activate NKT cells through T cell Ig‐like mucin‐like‐1 resulting in airway hyperreactivity. J Immunol 185: 5225–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen EM, Remmerswaal EB, Vossen MT, Rowshani AT, Wertheim‐van Dillen PM, van Lier RA et al. (2004). Emergence of a CD4+CD28‐ granzyme B+, cytomegalovirus‐specific T cell subset after recovery of primary cytomegalovirus infection. J Immunol 173: 1834–1841. [DOI] [PubMed] [Google Scholar]
- Legein B, Janssen EM, Theelen TL, Gijbels MJ, Walraven J, Klarquist JS et al. (2015). Ablation of CD8alpha(+) dendritic cell mediated cross‐presentation does not impact atherosclerosis in hyperlipidemic mice. Sci Rep 5: 15414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite‐De‐Moraes MC, Hameg A, Arnould A, Machavoine F, Koezuka Y, Schneider E et al. (1999). A distinct IL‐18‐induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J Immunol 163: 5871–5876. [PubMed] [Google Scholar]
- Leslie DS, Vincent MS, Spada FM, Das H, Sugita M, Morita CT et al. (2002). CD1‐mediated gamma/delta T cell maturation of dendritic cells. J Exp Med 196: 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnik P, Haskell CA, Charo IF (2003). Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J Clin Invest 111: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kanellakis P, Hosseini H, Cao A, Deswaerte V, Tipping P et al. (2016). A CD1d‐dependent lipid antagonist to NKT cells ameliorates atherosclerosis in ApoE−/− mice by reducing lesion necrosis and inflammation. Cardiovasc Res 109: 305–317. [DOI] [PubMed] [Google Scholar]
- Li Y, To K, Kanellakis P, Hosseini H, Deswaerte V, Tipping P et al. (2015). CD4+ natural killer T cells potently augment aortic root atherosclerosis by perforin‐ and granzyme B‐dependent cytotoxicity. Circ Res 116: 245–254. [DOI] [PubMed] [Google Scholar]
- Liu D, Song L, Wei J, Courtney AN, Gao X, Marinova E et al. (2012). IL‐15 protects NKT cells from inhibition by tumor‐associated macrophages and enhances antimetastatic activity. J Clin Invest 122: 2221–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL et al. (2000). Monoclonal T‐cell proliferation and plaque instability in acute coronary syndromes. Circulation 101: 2883–2888. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Kopecky SL, Frye RL, O'Fallon WM, Maseri A, Goronzy JJ et al. (1999). Perturbation of the T‐cell repertoire in patients with unstable angina. Circulation 100: 2135–2139. [DOI] [PubMed] [Google Scholar]
- Lombardi V, Stock P, Singh AK, Kerzerho J, Yang W, Sullivan BA et al. (2010). A CD1d‐dependent antagonist inhibits the activation of invariant NKT cells and prevents development of allergen‐induced airway hyperreactivity. J Immunol 184: 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE et al. (2013). Markers of inflammation and CD8 T‐cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV‐infected individuals. HIV Med 14: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A (2007). Dendritic cells prime natural killer cells by trans‐presenting interleukin 15. Immunity 26: 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML et al. (2013). The developmental pathway for CD103(+)CD8+ tissue‐resident memory T cells of skin. Nat Immunol 14: 1294–1301. [DOI] [PubMed] [Google Scholar]
- Maly K, Schirmer M (2015). The story of CD4+ CD28‐ T cells revisited: solved or still ongoing? J Immunol Res 2015: 348746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE et al. (2010). Human gammadelta T lymphocytes induce robust NK cell‐mediated antitumor cytotoxicity through CD137 engagement. Blood 116: 1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W et al. (2014). Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol 122: 91–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NB, Swain SL (2011). Cytotoxic CD4 T cells in antiviral immunity. J Biomed Biotechnol 2011: 954602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Rodriguez JE, Munne‐Collado J, Rasal R, Cuadrado E, Roig L, Ois A et al. (2013). Expansion of the NKG2C+ natural killer‐cell subset is associated with high‐risk carotid atherosclerotic plaques in seropositive patients for human cytomegalovirus. Arterioscler Thromb Vasc Biol 33: 2653–2659. [DOI] [PubMed] [Google Scholar]
- Martin‐Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A et al. (2004). Induced recruitment of NK cells to lymph nodes provides IFN‐gamma for T(H)1 priming. Nat Immunol 5: 1260–1265. [DOI] [PubMed] [Google Scholar]
- Marzo AL, Yagita H, Lefrancois L (2007). Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J Immunol 179: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CW, Raulet DH (2001). Expression and function of NK cell receptors in CD8+ T cells. Curr Opin Immunol 13: 465–470. [DOI] [PubMed] [Google Scholar]
- Melsen JE, Lugthart G, Lankester AC, Schilham MW (2016). human circulating and tissue‐resident CD56(bright) natural killer cell populations. Front Immunol 7: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk TP, Nosalski R, Szczepaniak P, Budzyn K, Osmenda G, Skiba D et al. (2016). Role of chemokine RANTES in the regulation of perivascular inflammation, T‐cell accumulation, and vascular dysfunction in hypertension. FASEB J 30: 1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA (2004). IL‐21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL‐2, IL‐15, and IL‐21. J Immunol 173: 900–909. [DOI] [PubMed] [Google Scholar]
- Mortality GBD, Causes of Death C (2015). Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385: 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnee K, Bundhun PK, Quan H, Tang Z (2016). Comparing the clinical outcomes between insulin‐treated and non‐insulin‐treated patients with type 2 diabetes mellitus after coronary artery bypass surgery: a systematic review and meta‐analysis. Medicine (Baltimore) 95: e3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y, Iwabuchi K, Fujii S, Ishimori N, Dashtsoodol N, Watano K et al. (2004). Natural killer T cells accelerate atherogenesis in mice. Blood 104: 2051–2059. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Goek O, Zhang X, Kopecky SL, Frye RL, Goronzy JJ et al. (2003). De novo expression of killer immunoglobulin‐like receptors and signaling proteins regulates the cytotoxic function of CD4 T cells in acute coronary syndromes. Circ Res 93: 106–113. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ et al. (2002). T‐cell‐mediated lysis of endothelial cells in acute coronary syndromes. Circulation 105: 570–575. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Wight TN, Sueishi K (2008). Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res 79: 14–23. [DOI] [PubMed] [Google Scholar]
- Nambiar J, Clarke AW, Shim D, Mabon D, Tian C, Windloch K et al. (2015). Potent neutralizing anti‐CD1d antibody reduces lung cytokine release in primate asthma model. MAbs 7: 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namekawa T, Wagner UG, Goronzy JJ, Weyand CM (1998). Functional subsets of CD4 T cells in rheumatoid synovitis. Arthritis Rheum 41: 2108–2116. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Vanichsarn C, Nadeau KC (2008). Increased cytotoxicity of CD4+ invariant NKT cells against CD4+CD25hiCD127lo/− regulatory T cells in allergic asthma. Eur J Immunol 38: 2034–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM (2006). Pathogen‐sensing plasmacytoid dendritic cells stimulate cytotoxic T‐cell function in the atherosclerotic plaque through interferon‐alpha. Circulation 114: 2482–2489. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M et al. (2009). CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920. [DOI] [PubMed] [Google Scholar]
- Noble A, Giorgini A, Leggat JA (2006). Cytokine‐induced IL‐10‐secreting CD8 T cells represent a phenotypically distinct suppressor T‐cell lineage. Blood 107: 4475–4483. [DOI] [PubMed] [Google Scholar]
- Nolz JC, Starbeck‐Miller GR, Harty JT (2011). Naive, effector and memory CD8 T‐cell trafficking: parallels and distinctions. Immunotherapy 3: 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg L, Eriksson M, Fahlen L, Sentman CL (2000). Expression of Ly49A on T cells alters the threshold for T cell responses. Eur J Immunol 30: 2849–2856. [DOI] [PubMed] [Google Scholar]
- O'Boyle G, Fox CR, Walden HR, Willet JD, Mavin ER, Hine DW et al. (2012). Chemokine receptor CXCR3 agonist prevents human T‐cell migration in a humanized model of arthritic inflammation. Proc Natl Acad Sci U S A 109: 4598–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi M, Ohdan H, Mitsuta H, Onoe T, Tokita D, Hara H et al. (2004). Liver NK cells expressing TRAIL are toxic against self hepatocytes in mice. Hepatology 39: 1321–1331. [DOI] [PubMed] [Google Scholar]
- Ostos MA, Recalde D, Zakin MM, Scott‐Algara D (2002). Implication of natural killer T cells in atherosclerosis development during a LPS‐induced chronic inflammation. FEBS Lett 519: 23–29. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Kramer MC, Woudstra P, Yahagi K, Ladich E, Finn AV et al. (2015). Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: a pathology study. Atherosclerosis 241: 772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget C, Chow MT, Duret H, Mattarollo SR, Smyth MJ (2012). Role of gammadelta T cells in alpha‐galactosylceramide‐mediated immunity. J Immunol 188: 3928–3939. [DOI] [PubMed] [Google Scholar]
- Pang DJ, Neves JF, Sumaria N, Pennington DJ (2012). Understanding the complexity of gammadelta T‐cell subsets in mouse and human. Immunology 136: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CO, Kupper TS (2015). The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 21: 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S, Chaidos A, Neville DC, Poggi A, Butters TD, Roberts IA et al. (2008). Human invariant NKT cells display alloreactivity instructed by invariant TCR‐CD1d interaction and killer Ig receptors. J Immunol 181: 3268–3276. [DOI] [PubMed] [Google Scholar]
- Paul VS, Paul CM, Kuruvilla S (2016). Quantification of various inflammatory cells in advanced atherosclerotic plaques. J Clin Diagn Res 10: EC35–EC38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH (2011). Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 89: 216–224. [DOI] [PubMed] [Google Scholar]
- Peng H, Tian Z (2014). NK cell trafficking in health and autoimmunity:a comprehensive review. Clin Rev Allergy Immunol 47: 119–127. [DOI] [PubMed] [Google Scholar]
- Perry HM, McNamara CA (2012). Refining the role of B cells in atherosclerosis. Arterioscler Thromb Vasc Biol 32: 1548–1549. [DOI] [PubMed] [Google Scholar]
- Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR (2013). CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood 121: 2647–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper J, Johansson S, Snir O, Linton L, Rieck M, Buckner JH et al. (2014). Peripheral and site‐specific CD4(+) CD28(null) T cells from rheumatoid arthritis patients show distinct characteristics. Scand J Immunol 79: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi A, Zocchi MR (2014). NK cell autoreactivity and autoimmune diseases. Front Immunol 5: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MK, Wang SC, Dai YX, Wang SQ, Ou JM, Quan ZW (2015). PD‐1 and Tim‐3 pathways regulate CD8+ T cells function in atherosclerosis. PLoS One 10: e0128523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D et al. (2008). Cutting edge: NKT cells constitutively express IL‐23 receptor and RORgammat and rapidly produce IL‐17 upon receptor ligation in an IL‐6‐independent fashion. J Immunol 180: 5167–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Fernandes‐Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES (2017). Cleavage of DFNA5 by caspase‐3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 8: 14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L, Burchat S, Gage J, Hasu M, Thabet M, Willcox L et al. (2008). Deficiency of invariant V alpha 14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc Res 78: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann A, Henderson B, Heidecker B, Seiler R, Fraedrich G, Singh M et al. (2008). T‐cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T‐cell receptor repertoire. Exp Gerontol 43: 229–237. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Collins HL, Priem F, Kaufmann SH (2002). Correction of the iron overload defect in beta‐2‐microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J Exp Med 196: 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller NK, Boisvert WA, Curtiss LK (2002). Inflammation in atherosclerosis: lesion formation in LDL receptor‐deficient mice with perforin and Lyst(beige) mutations. Arterioscler Thromb Vasc Biol 22: 1341–1346. [DOI] [PubMed] [Google Scholar]
- Schirmer M, Vallejo AN, Weyand CM, Goronzy JJ (1998). Resistance to apoptosis and elevated expression of Bcl‐2 in clonally expanded CD4+CD28‐ T cells from rheumatoid arthritis patients. J Immunol 161: 1018–1025. [PubMed] [Google Scholar]
- Schmidt D, Goronzy JJ, Weyand CM (1996). CD4+ CD7‐ CD28‐ T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest 97: 2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino K, Taniguchi M (2005). Functionally distinct NKT cell subsets and subtypes. J Exp Med 202: 1623–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selathurai A, Deswaerte V, Kanellakis P, Tipping P, Toh BH, Bobik A et al. (2014). Natural killer (NK) cells augment atherosclerosis by cytotoxic‐dependent mechanisms. Cardiovasc Res 102: 128–137. [DOI] [PubMed] [Google Scholar]
- Shi B, Du X, Wang Q, Chen Y, Zhang X (2013). Increased PD‐1 on CD4(+)CD28(−) T cell and soluble PD‐1 ligand‐1 in patients with T2DM: association with atherosclerotic macrovascular diseases. Metab Clin Exp 62: 778–785. [DOI] [PubMed] [Google Scholar]
- Silva‐Santos B, Serre K, Norell H (2015). gammadelta T cells in cancer. Nat Rev Immunol 15: 683–691. [DOI] [PubMed] [Google Scholar]
- Sköld M, Cardell S (2000). Differential regulation of Ly49 expression on CD4+ and CD4-CD8- (double negative) NK1.1+ T cells. Eur J Immunol 30: 2488–2496. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y (2005). Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med 201: 1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh SY, Faveeuw C, Thiam CH, Khoo LH, Yeo KP, Lim SY et al. (2016). NKT Cell hyporesponsiveness leads to unrestrained accumulation of marginal zone B cells in hypercholesterolemic apolipoprotein E‐deficient mice. J Immunol 197: 3894–3904. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Plougastel‐Douglas B, Yang L, Pak‐Wittel MA, Artyomov MN, Ivanova Y et al. (2014). Tissue‐resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife 3: e01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald J, Raum T, Pflanz S, Cierpka R, Mangold S, Rau D et al. (2009). Human IgG1 antibodies antagonizing activating receptor NKG2D on natural killer cells. MAbs 1: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB et al. (2012). Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med 366: 1108–1118. [DOI] [PubMed] [Google Scholar]
- Suzaki Y, Hamada K, Nomi T, Ito T, Sho M, Kai Y et al. (2008). A small‐molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur Respir J 31: 783–789. [DOI] [PubMed] [Google Scholar]
- Tang F, Sally B, Ciszewski C, Abadie V, Curran SA, Groh V et al. (2013). Interleukin 15 primes natural killer cells to kill via NKG2D and cPLA2 and this pathway is active in psoriatic arthritis. PLoS One 8: e76292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay C, Liu YH, Hosseini H, Kanellakis P, Cao A, Peter K et al. (2016). B‐cell‐specific depletion of tumour necrosis factor alpha inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation. Cardiovasc Res 111: 385–397. [DOI] [PubMed] [Google Scholar]
- Teo FH, de Oliveira RT, Mamoni RL, Ferreira MC, Nadruz W Jr, Coelho OR et al. (2013). Characterization of CD4+CD28null T cells in patients with coronary artery disease and individuals with risk factors for atherosclerosis. Cell Immunol 281: 11–19. [DOI] [PubMed] [Google Scholar]
- Terashima A, Watarai H, Inoue S, Sekine E, Nakagawa R, Hase K et al. (2008). A novel subset of mouse NKT cells bearing the IL‐17 receptor B responds to IL‐25 and contributes to airway hyperreactivity. J Exp Med 205: 2727–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewissen M, Somers V, Hellings N, Fraussen J, Damoiseaux J, Stinissen P (2007). CD4+CD28null T cells in autoimmune disease: pathogenic features and decreased susceptibility to immunoregulation. J Immunol 179: 6514–6523. [DOI] [PubMed] [Google Scholar]
- Thomas SY, Hou R, Boyson JE, Means TK, Hess C, Olson DP et al. (2003). CD1d‐restricted NKT cells express a chemokine receptor profile indicative of Th1‐type inflammatory homing cells. J Immunol 171: 2571–2580. [DOI] [PubMed] [Google Scholar]
- To K, Agrotis A, Besra G, Bobik A, Toh BH (2009). NKT cell subsets mediate differential proatherogenic effects in ApoE−/− mice. Arterioscler Thromb Vasc Biol 29: 671–677. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Van Dyke TE, Working group 1 of the joint EFPAAPw (2013). Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol 40 (Suppl 14): S24–S29. [DOI] [PubMed] [Google Scholar]
- Trandem K, Zhao J, Fleming E, Perlman S (2011). Highly activated cytotoxic CD8 T cells express protective IL‐10 at the peak of coronavirus‐induced encephalitis. J Immunol 186: 3642–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse K, Tse H, Sidney J, Sette A, Ley K (2013). T cells in atherosclerosis. Int Immunol 25: 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK et al. (2004). CD1d‐dependent activation of NKT cells aggravates atherosclerosis. J Exp Med 199: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AN, Bryl E, Klarskov K, Naylor S, Weyand CM, Goronzy JJ (2002). Molecular basis for the loss of CD28 expression in senescent T cells. J Biol Chem 277: 46940–46949. [DOI] [PubMed] [Google Scholar]
- Vallejo AN, Nestel AR, Schirmer M, Weyand CM, Goronzy JJ (1998). Aging‐related deficiency of CD28 expression in CD4+ T cells is associated with the loss of gene‐specific nuclear factor binding activity. J Biol Chem 273: 8119–8129. [DOI] [PubMed] [Google Scholar]
- Vallejo AN, Schirmer M, Weyand CM, Goronzy JJ (2000). Clonality and longevity of CD4+CD28null T cells are associated with defects in apoptotic pathways. J Immunol 165: 6301–6307. [DOI] [PubMed] [Google Scholar]
- VanderLaan PA, Reardon CA, Sagiv Y, Blachowicz L, Lukens J, Nissenbaum M et al. (2007). Characterization of the natural killer T‐cell response in an adoptive transfer model of atherosclerosis. Am J Pathol 170: 1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantourout P, Hayday A (2013). Six‐of‐the‐best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol 13: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verneris MR, Karimi M, Baker J, Jayaswal A, Negrin RS (2004). Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood 103: 3065–3072. [DOI] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S (2008). Functions of natural killer cells. Nat Immunol 9: 503–510. [DOI] [PubMed] [Google Scholar]
- Vliegen I, Duijvestijn A, Grauls G, Herngreen S, Bruggeman C, Stassen F (2004). Cytomegalovirus infection aggravates atherogenesis in apoE knockout mice by both local and systemic immune activation. Microbes Infect / Institut Pasteur 6: 17–24. [DOI] [PubMed] [Google Scholar]
- Vu DM, Tai A, Tatro JB, Karas RH, Huber BT, Beasley D (2014). Gammadelta T cells are prevalent in the proximal aorta and drive nascent atherosclerotic lesion progression and neutrophilia in hypercholesterolemic mice. PLoS One 9: e109416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Woodward‐Davis A, Liu R, Hu Y, Villadangos J, Smyth G et al. (2012). The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol 189: 3462–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter U, Santamaria P (2005). CD8+ T cells in autoimmunity. Curr Opin Immunol 17: 624–631. [DOI] [PubMed] [Google Scholar]
- Walton S, Mandaric S, Oxenius A (2013). CD4 T cell responses in latent and chronic viral infections. Front Immunol 4: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang W, Xu L, Jin JO (2016). Porphyromonas gingivalis lipopolysaccharide induced proliferation and activation of natural killer cells in vivo. Molecules 21: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington KJ, Takemura S, Goronzy JJ, Weyand CM (2001). CD4+,CD28‐ T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum 44: 13–20. [DOI] [PubMed] [Google Scholar]
- Watzl C (2014). How to trigger a killer: modulation of natural killer cell reactivity on many levels. Adv Immunol 124: 137–170. [DOI] [PubMed] [Google Scholar]
- Welte S, Kuttruff S, Waldhauer I, Steinle A (2006). Mutual activation of natural killer cells and monocytes mediated by NKp80‐AICL interaction. Nat Immunol 7: 1334–1342. [DOI] [PubMed] [Google Scholar]
- Whitman SC, Rateri DL, Szilvassy SJ, Yokoyama W, Daugherty A (2004). Depletion of natural killer cell function decreases atherosclerosis in low‐density lipoprotein receptor null mice. Arterioscler Thromb Vasc Biol 24: 1049–1054. [DOI] [PubMed] [Google Scholar]
- Wingender G, Krebs P, Beutler B, Kronenberg M (2010). Antigen‐specific cytotoxicity by invariant NKT cells in vivo is CD95/CD178‐dependent and is correlated with antigenic potency. J Immunol 185: 2721–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Giscombe R, Holm G, Lefvert AK (1996). Induction of human cytotoxic T lymphocytes by oxidized low density lipoproteins. Scand J Immunol 43: 381–384. [DOI] [PubMed] [Google Scholar]
- Xia M, Guerra N, Sukhova GK, Yang K, Miller CK, Shi GP et al. (2011). Immune activation resulting from NKG2D/ligand interaction promotes atherosclerosis. Circulation 124: 2933–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M et al. (2016). Pathophysiology of native coronary, vein graft, and in‐stent atherosclerosis. Nat Rev Cardiol 13: 79–98. [DOI] [PubMed] [Google Scholar]
- Ye F, Turner J, Flano E (2012). Contribution of pulmonary KLRG1(high) and KLRG1(low) CD8 T cells to effector and memory responses during influenza virus infection. J Immunol 189: 5206–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen JH, Moore BE, Nakajima T, Scholl D, Schaid DJ, Weyand CM et al. (2001). Major histocompatibility complex class I‐recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med 193: 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda O, Imai T, Goda S, Inoue H, Yamauchi A, Okazaki T et al. (2000). Fractalkine‐mediated endothelial cell injury by NK cells. J Immunol 164: 4055–4062. [DOI] [PubMed] [Google Scholar]
- Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY et al. (2013). Immunosenescent CD8+ T cells and C‐X‐C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62: 126–133. [DOI] [PubMed] [Google Scholar]
- Yu Y, Cho HI, Wang D, Kaosaard K, Anasetti C, Celis E et al. (2013). Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol 190: 1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafiratos MT, Manam S, Henderson KK, Ramsey KH, Murthy AK (2015). CD8+ T cells mediate Chlamydia pneumoniae‐induced atherosclerosis in mice. Pathog Dis 73 pii: ftv052. https://doi.org/10.1093/femspd/ftv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zal B, Kaski JC, Akiyu JP, Cole D, Arno G, Poloniecki J et al. (2008). Differential pathways govern CD4+ CD28‐ T cell proinflammatory and effector responses in patients with coronary artery disease. J Immunol 181: 5233–5241. [DOI] [PubMed] [Google Scholar]
- Zal B, Kaski JC, Arno G, Akiyu JP, Xu Q, Cole D et al. (2004). Heat‐shock protein 60‐reactive CD4+CD28null T cells in patients with acute coronary syndromes. Circulation 109: 1230–1235. [DOI] [PubMed] [Google Scholar]
- Zelenay S, Keller AM, Whitney PG, Schraml BU, Deddouche S, Rogers NC et al. (2012). The dendritic cell receptor DNGR‐1 controls endocytic handling of necrotic cell antigens to favor cross‐priming of CTLs in virus‐infected mice. J Clin Invest 122: 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ (2011). CD8(+) T cells: foot soldiers of the immune system. Immunity 35: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Nakajima T, Goronzy JJ, Weyand CM (2005). Tissue trafficking patterns of effector memory CD4+ T cells in rheumatoid arthritis. Arthritis Rheum 52: 3839–3849. [DOI] [PubMed] [Google Scholar]
- Zhang X, Niessner A, Nakajima T, Ma‐Krupa W, Kopecky SL, Frye RL et al. (2006). Interleukin 12 induces T‐cell recruitment into the atherosclerotic plaque. Circ Res 98: 524–531. [DOI] [PubMed] [Google Scholar]
- Zuo J, Shan Z, Zhou L, Yu J, Liu X, Gao Y (2015). Increased CD160 expression on circulating natural killer cells in atherogenesis. J Transl Med 13: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweemer AJ, Nederpelt I, Vrieling H, Hafith S, Doornbos ML, de Vries H et al. (2013). Multiple binding sites for small‐molecule antagonists at the CC chemokine receptor 2. Mol Pharmacol 84: 551–561. [DOI] [PubMed] [Google Scholar]


