Abstract
Cardiovascular disease (CVD) is a major cause of morbidity and mortality worldwide. Inflammatory processes arising from metabolic abnormalities are known to precipitate the development of CVD. Several metabolic and inflammatory markers have been proposed for predicting the progression of CVD, including high density lipoprotein cholesterol (HDL‐C). For ~50 years, HDL‐C has been considered as the atheroprotective ‘good’ cholesterol because of its strong inverse association with the progression of CVD. Thus, interventions to increase the concentration of HDL‐C have been successfully tested in animals; however, clinical trials were unable to confirm the cardiovascular benefits of pharmaceutical interventions aimed at increasing HDL‐C levels. Based on these data, the significance of HDL‐C in the prevention of CVD has been called into question. Fundamental in vitro and animal studies suggest that HDL‐C functionality, rather than HDL‐C concentration, is important for the CVD‐preventive qualities of HDL‐C. Our current review of the literature positively demonstrates the negative impact of systemic and tissue (i.e. adipose tissue) inflammation in the healthy metabolism and function of HDL‐C. Our survey indicates that HDL‐C may be a good marker of adipose tissue health, independently of its atheroprotective associations. We summarize the current findings on the use of anti‐inflammatory drugs to either prevent HDL‐C clearance or improve the function and production of HDL‐C particles. It is evident that the therapeutic agents currently available may not provide the optimal strategy for altering HDL‐C metabolism and function, and thus, further research is required to supplement this mechanistic approach for preventing the progression of CVD.
Linked Articles
This article is part of a themed section on Targeting Inflammation to Reduce Cardiovascular Disease Risk. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.22/issuetoc and http://onlinelibrary.wiley.com/doi/10.1111/bcp.v82.4/issuetoc
Abbreviations
- Apo‐A1
apolipoprotein A1
- Apo‐A2
apolipoprotein A2
- AT
adipose tissue
- CETP
cholesterol ester transfer protein
- CRP
C‐reactive protein
- CVD
cardiovascular disease
- FA
fatty acid
- LCAT
lecithin cholesterol acyltransferase
- LPL
lipoprotein lipase
- PLTP
phospholipid transfer protein
- RA
Rheumatoid arthritis
- RCT
reverse cholesterol transport
- sC5b‐9
serum complement membrane attack complex generated by the assembly of C5 through C9 complements
- SR‐B1
scavenger receptor class B type 1
- SREBP‐1c
sterol regulatory element binding protein‐1c
- TG
triglyceride
- VLDL‐TG
very LDL triglyceride
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Enzymes e |
| IL‐1β | 5‐LOX |
| TNF‐α | Caspase 1 |
| Nuclear hormone receptors b | Cathepsin B |
| PPAR‐α | COX‐1 |
| PPAR‐β/δ | COX‐2 |
| PPAR‐γ | JNK |
| LXR‐α | MMP3 |
| LXR‐β | MMP7 |
| RAR‐α | MMP9 |
| Catalytic receptors c | MMP12 |
| NLRP3 | MPO |
| Transporters d | P38 MAPK |
| ABCA1 | PCSK9 |
| ABCG1 | sPLA2 |
| ABCB11 | tPA |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,eAlexander et al., 2015a,b,c,d,e).
Introduction
There have been several sharp turns in the evidence trail marking the atheroprotective role of high density lipoproteins (HDLs). Very early studies on dyslipidaemia highlighted the positive correlation between total triglycerides (TGs) with the risk of developing cardiovascular disease (CVD). Since the mid‐1970s, numerous epidemiological and animal studies, including the Framingham Study (Gordon et al., 1977), reported that HDL cholesterol (HDL‐C) has the strongest inverse relationship with the development of CVD of the known serum lipid factors (Badimon et al., 1990; Rubin et al., 1991; Liu et al., 1994; Plump et al., 1994). This association is underpinned by reverse cholesterol transport (RCT) – a process by which HDL‐C transfers the cholesterol from peripheral cells, for example, lipid‐laden foam cells, to the liver for secretion into bile and faeces. The promotion of RCT is considered a major anti‐atherogenic function of HDL‐C (Gofman et al., 1966; Miller and Miller, 1975; Rhoads et al., 1976). Based on these findings, interventions to increase the levels of HDL‐C were developed but found to be ineffective for preventing cardiovascular outcomes in several large clinical trials (Brousseau et al., 2004; McKenney et al., 2006; Barter et al., 2007; Boden et al., 2011; Lüscher et al., 2012; Schwartz et al., 2012). Given these results, the importance of HDL‐C for preventing CVD has been questioned and revisited. An expert panel from the National Lipid Association concluded that although ‘HDL‐C is not a therapeutic target at the present time’, ‘rigorous research into the biology and clinical significance of low HDL‐C should continue’ and that ‘the development of novel drugs designed to modulate the serum levels and functionality of HDL particles should also continue’ (Toth et al., 2013), a recommendation which has been echoed by other experts in the field (Brown et al., 2014; Toth et al., 2014). In our opinion, the key issue in this matter is the functionality of HDL‐C, which can be affected by adipose tissue (AT) and low‐grade systemic inflammation (Brewer, 2007; Rader and Daugherty, 2008; Rosenson, 2010; Zhang et al., 2010; Chung et al., 2011). Here, we discuss the data available illustrating the effect of inflammation on the functionality of HDL particles and potential therapeutic interventions that can help reverse these effects and, thus, prevent the development of metabolic abnormalities leading to the progression of CVD and associated co‐morbidities.
Mechanism of the synthesis of mature or functional HDL‐C
Currently, the most important known function of HDL‐C is to provide the successful transfer of cholesterol from peripheral tissues to the liver for extraction (i.e. RCT). The HDL‐C particles that can effectively accomplish this task are the functionally mature ones, which are rich in apolipoprotein A1 (Apo‐A1) and cholesterol (Rader and Daugherty, 2008). Several apolipoproteins, enzymes and transfer proteins participate in formation and function of these mature HDL‐C particles. The first step in the formation of HDL‐C requires Apo‐A1 and ATP‐binding cassette transporter A1 (ABCA1) (Lee and Parks, 2005; Zannis et al., 2006). ABCA1 mediates the efflux of phospholipids and free cholesterol from AT to Apo‐A1, a step that is necessary for the initial lipidation of Apo‐A1 and formation of nascent HDL‐C particles (Verghese et al., 2007; Phillips, 2014). The next step is the maturation of HDL‐C particles, which involves several enzymes – lipoprotein lipase (LPL), phospholipid transfer protein (PLTP) and lecithin cholesterol acyltransferase (LCAT). LPL hydrolyzes circulating very LDL triglyceride (VLDL‐TGs), while PLTP transfers phospholipids and free cholesterol from the surface of VLDL‐TG to HDL‐C (Tall et al., 1985; Rinninger et al., 1998; 2001; Ji et al., 2014). Thereafter, LCAT esterifies cholesterol, rendering it more hydrophobic and amenable for efficient packaging and transport by HDL‐C to the liver (Rader, 2009; Dobiásová and Frohlich, 1999; Asztalos et al., 2007). Finally, the transporter ABCG1 mediates cholesterol efflux from the surface of cells and macrophages to mature HDL‐C particles (Kennedy et al., 2005). The mature particles are subject to cholesterol ester transfer protein (CETP)‐mediated exchange of cholesteryl esters with TGs from VLDL or LDL, which subsequently binds to LDL receptors in the liver (Bruce et al., 1998). Successful completion of this exchange process and binding of HDL‐C to scavenger receptor class B type I (SR‐B1) receptors in the liver allows for elimination of cholesterol in the liver, thereby preventing the deposition of cholesterol in the endothelium and the development of atherosclerosis.
In addition to playing a major role in RCT, the HDL‐C particles have been shown to (a) have anti‐inflammatory, anti‐oxidative and anti‐apoptotic properties; (b) contribute to innate immunity, the modulation of glucose metabolism and platelet function; and (c) influence stem cells and embryogenesis (Gordon et al., 2011). The changes in the functionality of HDL‐C particles are discussed throughout this review article; however, our review is mainly focused on the effect of inflammatory processes on the functionality and atheroprotective properties of HDL‐C particles. The functional diversity of HDL‐C particles is related to their compositional complexity and heterogeneity. As an example, mature cholesterol and Apo‐A1‐rich HDL‐C particles have been shown to be successful at RCT, while the smaller, cholesterol‐poor, TG‐ and Apo‐A2‐rich HDL‐C particles degrade easily and are unable to contribute to RCT. The published data suggest that the differences in HDL‐C functionality depend on the composition of the HDL‐C particles (Asztalos et al., 2011).
Several assays have been proposed to assess the functionality of HDL‐C. Some assays are designed to measure the anti‐inflammatory and anti‐oxidative properties of HDL‐C, while others evaluate HDL‐C RCT efflux (Navab et al., 1991; 2001; Zhang et al., 2003; Annema et al., 2010; Suzuki et al., 2010; Khera et al., 2011). Furthermore, electrophoretic and NMR methods have been developed to estimate HDL‐C particle size and composition. Thus far, there has been no consensus regarding the superiority of one method versus another for HDL‐C characterization, and attempts to standardize the various nomenclature systems of HDL‐C are a work in progress (Asztalos et al., 2011). Further research is needed to elucidate the relationship between HDL‐C particle heterogeneity and function (Gordon et al., 2011).
Effect of inflammatory processes on HDL‐C metabolism
Several factors and conditions, including genetic (i.e. familial disorders) and acquired (e.g. decreased cholesterol efflux, inflammation, hypertriglyceridaemia and AT dysfunction), affect the concentration and functionality of HDL‐C. Here, we will focus on mechanisms related to inflammation, which have been depicted in detail in Figure 1.
Figure 1.
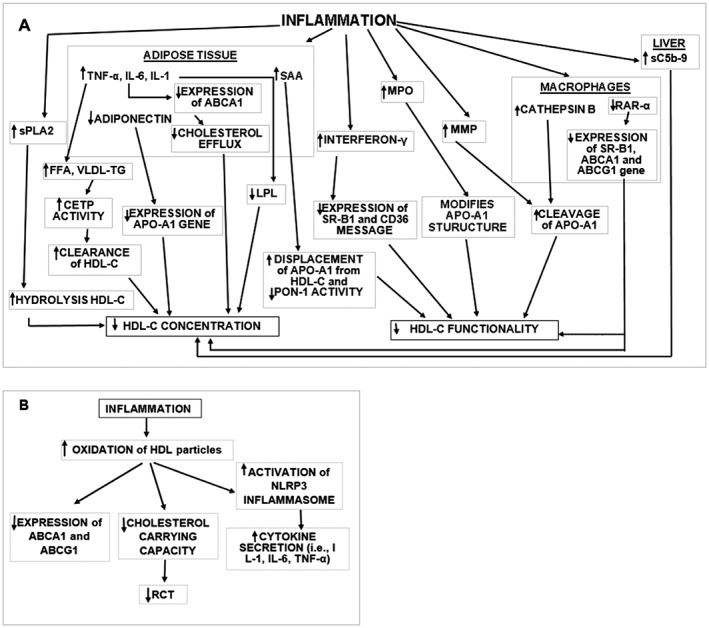
Schematic presentation of the mechanisms causing decreased HDL‐C concentration or function due to inflammation. (A) The majority of mechanisms that affect HDL‐C metabolism are associated with AT inflammation and function. Increased levels of FFA and VLDL‐TG can enhance the activity of CETP resulting in TG‐enriched HDL‐C particles. These particles are prone to higher liver clearance rates. Decreased adiponectin levels affect the expression of the Apo‐A1 gene. Inflammatory markers have been shown to affect LPL and ABCA1 gene expression impeding maturation of HDL‐C. Other mechanisms which affect HDL‐C concentration include (a) enhanced sPLA2 activity causing increased hydrolysis of HDL‐C; (b) increased secretion of complement sC5b‐9 from liver; and (c) decreased expression of RAR‐α in macrophages. The later mechanism also affects HDL‐C function and results in (a) increased production of SAA in AT which displaces Apo‐A1 from HDL‐C and decreases PON‐1 activity; (b) interferon‐γ secretion which decreases the expression of SR‐B1 and CD36; (c) MPO expression which modifies Apo‐A1 structure; and (d) MMP and macrophage cathepsin B‐induced cleavage of Apo‐A1. (B) Mechanisms associated with the modification of HDL‐C particles. Inflammatory environments induce HDL oxidation activating NLRP3 inflammasome pathways and the secretion of cytokines while decreasing ABCA1 and ABCG1 expression and the cholesterol carrying capacity of HDL‐C via RCT. FFA, free fatty acid; RAR‐α, retinoic acid receptor‐α.
Adipose tissue lipid kinetics
Cholesterol efflux occurs in several tissues, including the liver, intestine and AT (Basso et al., 2003; Sahoo et al., 2004; Lee and Parks, 2005; Timmins et al., 2005; Singaraja et al., 2006; Zannis et al., 2006; Verghese et al., 2007; Chung et al., 2011; Ji et al., 2012; Phillips, 2014). Regarding the role of AT, in vitro and animal studies demonstrated that cholesterol efflux from adipocytes plays a significant role in the initial lipidation of Apo‐A1 and the formation of mature and functional HDL‐C particles (Zhang et al., 2010; Chung et al., 2011). These results are supported by studies demonstrating that hepatic cholesterol efflux was essential but insufficient to correct HDL‐C deficiency in hepatic ABCA1−/− knockout mice, illustrating that extrahepatic ABCA1 expression and cholesterol metabolism are critical for the formation of mature HDL‐C particles (Singaraja et al., 2006). Others showed that individuals with compensated liver cirrhosis have higher levels of IL‐6 and NF‐κB but lower levels of HDL‐C and Apo‐A1 (Trieb et al., 2016). Thus, it appears that although the liver plays a significant role, it is not isolated in its contribution to the formation of mature HDL‐C particles.
With the development of AT inflammation, several pathways are activated leading to the impairment of HDL‐C metabolism. AT inflammation has been shown to suppress the expression and function of cholesterol transfer proteins (e.g. ABCA1) leading to decreased efflux of cholesterol from AT (De Haan et al., 2014; Figure 1). This results in the formation of immature rather than mature HDL‐C particles, which fail to successfully transfer cholesterol to the liver (Rashid and Genest, 2007). In our recent clinical study, we used a deuterium labelling approach to estimate the fractional synthesis of triglycerides (f TG) in AT in humans with differing degrees of obesity. Our results demonstrated that f TG is inversely associated with the markers of insulin sensitivity (Tuvdendorj et al., 2013). Furthermore, f TG is associated with the total concentration of HDL‐C and the fractional contribution of large HDL‐C particles (Tuvdendorj et al., 2016). Based on the principles of the stable isotope tracer labelling approach (synthesis‐breakdown/lipolysis = net balance; Turner et al., 2003; Wolfe and Chinkes, 2005; Tuvdendorj et al., 2013, 2016) and the reports that AT TG efflux directly correlates with the efflux of cholesterol (Le Lay et al., 2003; Verghese et al., 2007), we assumed that the f TG represented AT cholesterol efflux in these individuals. Taken together, these data suggest that inflammation in AT is one of the principal factors affecting HDL‐C functionality (Figure 1A). Thus, these data suggest that metabolically healthier people have high cholesterol efflux and higher levels of circulating total and functional HDL‐C particles that are able to fulfil their atheroprotective role. Notably, our data describing the association between AT lipid flux and HDL‐C metabolism were true for women but not for men, supporting the sex‐dependent nature of lipid metabolism (Hazzard and Applebaum‐Bowden, 1990; Williams, 1997).
An additional factor in reduced HDL‐C functionality arises from AT dysfunction and chronic inflammation hindering the ability of adipocytes to take up excess dietary calories. As a result, the concentration of circulating fatty acids (FAs) is increased. Because FAs are used in the liver for the synthesis of triglycerides and VLDL‐TG, a concomitant increase in the overall concentration of circulating lipids occurs (Figure 1A). As a result, CETP modulates the increased exchange of TGs for cholesteryl esters in HDL‐C particles. These TG‐enriched HDL‐C particles are vulnerable to clearance resulting in a decreased concentration of HDL‐C (Rashid et al., 2002; Figure 1A).
Inflammatory markers
As depicted in Figure 1A, from a cell signalling perspective, AT inflammation is associated with increased activation of pro‐inflammatory pathways resulting in enhanced secretion of inflammatory cytokines [i.e. TNF‐α, IL‐6, IL‐1β, C‐reactive protein (CRP) and serum amyloid A (SAA; Mortensen, 2001; Ryden et al., 2002; Berg and Scherer, 2005; Stienstra et al., 2012; Rodríguez‐Hernández et al., 2013)]. These cytokines increase the activity of downstream factors (e.g. transcription factor activator protein 1, NF‐κB and INF regulatory factor), thus up‐regulating the gene expression of inflammatory mediators (Figure 1A). Notably, TNF‐α plays a central role in this inflammatory process. It promotes the secretion of other pro‐inflammatory cytokines and decreases the production of the anti‐inflammatory cytokine adiponectin (Hotamisligil et al., 1993; Kern et al., 2001; Ryden et al., 2002; Xu et al., 2003). Animal, but not human, studies have shown that decreased production of adiponectin results in decreased expression of mRNA for Apo‐A1 and ABCA1, resulting in decreased levels of Apo‐A1 and HDL‐C (Arita et al., 1999; Hotta et al., 2000, 2001; Kondo et al., 2002; Oku et al., 2007). Additionally, all these cytokines suppress the activity of LPL, which also leads to decreased levels of both the Apo‐A1 and HDL‐C (Hotamisligil et al., 1993; Kern et al., 2001; Ryden et al., 2002; Dusanov et al., 2016; Jung et al., 2016; O'Reilly et al., 2016; Ottobelli et al., 2016) (Figure 1A).
Impaired HDL‐C metabolism is also associated with inflammation‐induced macrophage migration to AT (Figure 1A). Macrophages cause cell‐mediated modifications of Apo‐A1, such as chlorination, nitration, oxidation and proteolysis. In vitro studies demonstrated that macrophages limit the ability of Apo‐A1 to solubilize lipids and promote ABCA1‐dependent cholesterol efflux. The proteolytic mechanism identified is C‐terminal cleavage of Apo‐A1 at Ser 228 by cathepsin B, which diminishes the functionality of Apo‐A1 and HDL‐C (Figure 1A). Cathepsins are proteases that are secreted by inflammatory macrophages (Brehm et al., 2014; Abd‐Elrahman et al., 2016; Yan et al., 2016). In point of fact, this cathepsin B‐promoted cleavage process is inhibited by the lipidation of Apo‐A1, which causes the C‐terminal region of Apo‐A1 to become more α‐helical, thereby providing cleavage protection (Dinnes et al., 2016).
In chronic inflammation, adipocytes secrete SAA (Poitou et al., 2005; Sjöholm et al., 2005), which has a high affinity for HDL‐C and is known to displace apolipoproteins from HDL‐C (Figure 1A). When SAA is attached to HDL‐C, LCAT‐mediated esterification of HDL‐C is reduced affecting the formation of mature HDL‐C particles. SAA also inhibits the activity of HDL‐associated antioxidant enzyme paraoxonase (PON‐1) resulting in HDL‐C being unable to prevent the oxidation of LDL. Thus, SAA affects both the synthesis of mature HDL‐C and its anti‐oxidative properties (Clifton et al., 1985; Malle et al., 1993; Kappelle et al., 2011). Interestingly, patients with rheumatoid arthritis (RA) have been shown to have increased levels of SAA and low levels of HDL‐C (van Eijk et al., 2009) along with higher rates of cardiovascular complications (Lehtinen, 1993; Lautermann and Braun, 2002; Peters et al., 2004). Interventions to ameliorate inflammation in patients with RA have been shown to decrease SAA (van Eijk et al., 2009; McInnes et al., 2013) and improve HDL‐C metabolism (van Eijk et al., 2009; Charles‐Schoeman et al., 2016), which is discussed below (see subsection ‘Methotrexate and Etanercept’).
Other factors affecting HDL‐C metabolism
Complement systems 3 and 4 (C3 and C4 respectively) play a significant role in inflammation, dyslipidaemia and metabolic syndrome. They have been shown to increase the levels of CRP, TG and fibrinogen. Increased plasma levels of circulating sC5b‐9 complex have been shown to be inversely correlated with the concentration of HDL‐C (Pasqui et al., 2000; 2002; Onat et al., 2005; Liu et al., 2016); Figure 1A). Inflammation also results in increased secretion of secretory PLA2 (sPLA2) from various tissues. sPLA2 increases the hydrolysis of HDL‐C particles, breaking down the phospholipids from HDL‐C and thus decreasing the HDL‐C concentration (Tietge et al., 1999; Rye and Duong, 2000). The above factors affect the concentration and, consequently, the functionality of HDL‐C (Figure 1A).
Several other agents have also been shown to directly affect the functionality of HDL‐C. Low‐grade systemic inflammation causes a down‐regulation in the expression of retinoic acid receptor‐α and alters its binding to the promoters of SR‐B1 and ABCA1 (Maitra and Li, 2013). This leads to decreased expression of SR‐B1, ABCA1 and ABCG1 in macrophages and thus decreased RCT (Figure 1A). Matrix metalloproteinases (MMPs) are expressed during inflammation and modulate the function of inflammatory cytokines. MMP3, MMP7 and MMP12 have been shown to cleave Apo‐A1 at its carboxyl terminus (Lindstedt et al., 1999). Furthermore, INF‐γ, another inflammatory cytokine, has been shown to induce activation of macrophages and to decrease the expression of SR‐B1 and messaging associated with CD36 (Zuckerman et al., 2000; Imachi et al., 2001; Bujold et al., 2009). Additionally, the conditional acute phase reactant myeloperoxidase (MPO) uses chloride ions and cell‐generated hydrogen peroxide to create hypochlorous acid that damages Apo‐A1 (Smith, 2010; Figure 1A), thus altering the structure and function of HDL‐C. Plasma Apo‐A1 is a selective target for MPO‐mediated protein modification which results in high levels of covalent modifications. All of these mechanisms have been shown to reduce the capacity of HDL‐C to take up cholesterol and thus inhibit HDL‐C function (i.e. RCT). HDL‐C particles become oxidized during acute inflammation and present an additional source of impaired transport (Figure 1B). The oxidized HDL‐C particles exacerbate inflammation by activating the NLRP3 inflammasome and thereby impacting HDL‐C and the inflammatory environment by (1) activating downstream cytokines and caspase 1; (2) inducing the secretion of IL‐18 and IL‐1β; and (3) decreasing ABCA1 and ABCG1 activity (Figure 1B), thus further impairing HDL‐C (van Lenten et al., 1995; Nakajima et al., 2000; He et al., 2013; Li et al., 2016).
Anti‐inflammatory interventions and HDL‐C metabolism
The essential question at this time is whether interventions to suppress inflammation and inflammatory markers can influence HDL‐C levels and improve HDL‐C functionality. The CETP inhibitors and other established pharmaceuticals are known to affect HDL‐C metabolism; however, these drugs do not affect inflammatory processes; therefore, we will not be discussing them in this review. In this section, we will discuss interventions that alter HDL‐C metabolism via inflammatory pathways, including several pharmaceutical and non‐pharmaceutical interventions that have been shown to ameliorate inflammation and potentially improve HDL‐C metabolism, as well as others which are currently being evaluated. A summary of these interventions, including positive and negative treatment outcomes, is presented in Table 1.
Table 1.
Effects of pharmaceutical agents on inflammation and HDL‐C metabolism
| Pharmaceutical agents | Effect on | References | |
|---|---|---|---|
| Inflammation | HDL‐C metabolism | ||
| Aspirin | Inhibits NF‐κB and MAPK | Increases expression of ABCB11 in the liver leading to increased bile acid excretion and RCT |
Amann and Peskar, 2002
Demetz et al., 2014 |
| Decreases inflammatory cytokines | Increases SR‐B1 expression leading to increased cholesterol transport. | Tancevski et al., 2006 Herová et al., 2014 | |
| – | Increases LCAT and PON‐1 activity | Jafarnejad et al., 2008 | |
| Salsalates | Inhibits NF‐κB | Increases adiponectin and expression of ABCA1 and AMPK leading to increased cholesterol efflux to Apo‐A1 and HDL‐C | Fullerton et al., 2015 Fakhri et al., 2014 Goldfine et al., 2013a Goldfine et al., 2013b |
| Metformin | Decreases NF‐κB, CRP and IL‐6 | Increases PON‐1 enzyme activity and the anti‐oxidative function of HDL‐C | Camps et al., 2016 Yoshifumi, 2016 Goldberg et al., 2016 |
| Statins | Decreases NF‐κB(at high dose). Also decrease MMP9 and CRP |
Increases the expression of Apo‐A1 gene by activating PPAR‐α Decreases VLDL‐TG, TGs and CETP activity and raises HDL‐C concentration |
Kim et al., 2007; Singh et al., 2008; Bonnet et al., 2008; van de Ree et al., 2003 Martin et al., 2001 Schaefer and Asztalos, 2006 |
| Extended release niacin | Decreases NF‐κB, TNF‐α and IL‐6 | Decreases clearance of HDL‐C by decreasing CETP activity. Increases Apo‐A1 and adiponectin mRNA expression raising HDL‐C concentration | Si et al., 2014 Yadav et al., 2015 Digby et al., 2010 |
| – | – | May increase anti‐ApoA1 antibody formation that reduces HDL‐C anti‐oxidative function | Batuca et al., 2016 |
| Methotrexate | Decreases MMP‐1, TNF‐α, IL‐6, IL‐1 and IFN‐γ | Increases expression of ABCA1 leading to enhanced RCT | Coomes et al., 2011 Cutolo et al., 2001 Reiss et al., 2008 Chan and Cronstein, 2010 |
| PPAR‐α agonists | Decrease NF‐κB, CRP and IL‐6 | Increase Apo‐A1 and adiponectin leading to increase HDL‐C concentration. Increase ABCA1, ABCG1 and SR‐B1 activity in macrophages leading to enhanced RCT | Ogata et al., 2009 Mahdy et al., 2012 Colin et al., 2015 Wagner et al., 2010 |
| – | PPAR‐α agonists did not decrease inflammation in rodents with renal crystal formation. Fenofibrate did not decrease inflammatory markers in one study | – | Taguchi et al., 2016 Hogue et al., 2008 |
| PPAR‐γ agonists | Suppresses IFN‐γ and increases M2 macrophages |
Increase adiponectin and ABCA1 leading to higher HDL‐C concentration. Enhance cholesterol efflux and increase expression of SR‐B1 |
Colin et al., 2015
Zuckerman et al., 2000 Bujold et al., 2009 |
| Biological agents (IL‐6 inhibitor, JAK inhibitor and TNF‐α inhibitor) |
Modulate immune response Decrease inflammatory cytokines |
Improve HDL‐C concentration and metabolism |
Souto et al., 2015; Genovese et al., 2008 Mathieu et al., 2010 Kawashiri et al., 2011 Ghoreschi et al., 2011 van Eijk et al., 2009 McInnes et al., 2013 Charles‐Schoeman et al., 2016 |
| – | – | Etanercept may cause hypertriglyceridaemia | Haroon and Devlin, 2009 |
| LXR agonists | Decrease TNF‐α, IL‐6, IL‐1β, MMP‐9 and IFN‐γ | Increase HDL concentration and function by increasing ABCA1, ABCG1 and RCT |
Joseph et al., 2003
Wang et al., 2006 Jamroz‐Wisniewska et al., 2007 Jiang et al., 2003 Miao et al., 2004 Repa et al., 2000b Naik et al., 2006 |
| – | – | May cause increase in TGs, VLDL and LDL by inducing SREBP‐1c |
Repa et al., 2000a
Schultz et al., 2000 Grefhorst et al., 2002 |
| Sildenafil | Decrease NF‐κB, TNF‐α and IL‐1 | Increase HDL‐C function by unknown mechanism | Nunes et al., 2015 |
|
HDL‐C reconstituted therapy Apo‐A1 Milano |
Decrease MMP‐9 | Increase HDL‐C function by increasing ABCA1 activity |
Uehara et al., 2015
Nasr et al., 2015 |
| 4F mimetic peptide | Decrease inflammatory cytokines | Increase RCT |
Bloedon et al., 2008
Smythies et al., 2010 |
| 5‐Lipoxygenase inhibitors | Decrease adipose tissue inflammation, SAA, CRP and MPO levels | Improve HDL‐C metabolism |
Horrillo et al., 2010
Bäck et al., 2007 Allayee et al., 2007 |
Pharmaceutical approaches
Current therapies
Aspirin and salsalates
Aspirin is one of the oldest drugs in use and its effects in treating inflammation are widely known. It inhibits COX‐1 and COX‐2 and thus the synthesis of prostaglandins and thromboxane (Spite and Serhan, 2010). In terms of CVD treatment, aspirin has been shown to reduce the risk of first myocardial infarction in men in direct correlation with initial CRP levels ( Ridker et al., 1997). Aspirin does not, however, appear to reduce inflammation via pathways which impact CRP levels, as aspirin has been shown to be minimally effective in reducing CRP levels in at‐risk diabetic populations (Hovens et al., 2008) and aspirin‐treated healthy volunteers (Feng et al., 2000; Feldman et al., 2001). Currently, meta‐analyses indicate that aspirin therapy should only be recommended for the most at‐risk patients for the prevention of cardiovascular events in diabetics due to its pro‐haemorrhagic effects (Pignone et al., 2010). Mechanistically, in addition to its effects on COX‐1 and COX‐2, aspirin inhibits the activation of NF‐κB, activator protein 1 and MAPK (Amann and Peskar, 2002). In terms of its direct impact on HDL‐C metabolism, studies in isolated human macrophages showed that aspirin increased SR‐B1 expression and labelled HDL‐associated cholesteryl oleate uptake, as well as enhancing SR‐B1 expression in mice in vivo (Tancevski et al., 2006). Additional studies in M1 macrophages showed reduced inflammatory cytokine secretion upon exposure to aspirin, which was associated with a reduction in chemerin secretion by adipose tissue (Herová et al., 2014). Increased levels of circulating chemerin have been shown to inversely associate with HDL‐C levels (Herová et al., 2014). Rodent studies demonstrated that aspirin increases the expression of ATP binding cassette subfamily B member 11 (ABCB11) in the liver leading to increased bile acid excretion and enhanced RCT, indicating an improvement in cholesterol transfer to HDL‐C (Demetz et al., 2014). Further studies in diabetic rats showed that long‐term aspirin therapy reduces HbA1c and advanced glycated end product formation while improving HDL functionality (Jafarnejad et al., 2008). Additionally, HDL‐C may play a role in increasing the oxidative capacity of aspirin via the HDL‐associated enzyme PON‐1, which actively hydrolyzes aspirin to salicylate, whose free radical scavenging properties may protect against atherosclerosis (Santanam and Parthasarathy, 2007). Interestingly, aspirin‐resistant patients were revealed to have reduced HDL‐C levels, indicating that aspirin efficacy may in some way be associated with HDL‐C function (Azmin et al., 2013).
Salsalate, which is chemically related to aspirin, has been shown to increase the levels of adiponectin, HDL‐C and Apo‐A1 (Goldfine et al., 2013a,b; Fakhri et al., 2014; Fullerton et al., 2015) while also suppressing the activation of NF‐κB. Salsalate increases the expression of ABCA1 gene and activates AMPK, leading to increased cholesterol efflux to HDL‐C and Apo‐A1 (Goldfine et al., 2013a,b; Fakhri et al., 2014; Fullerton et al., 2015). Additionally, salsalate decreases the levels of HbA1c, fasting blood sugar and C‐peptide.
Metformin
Metformin is an anti‐diabetic drug, which also suppresses inflammation, decreases the levels of LDL‐C and leptin and reduces body weight (Camps et al., 2016; Yoshifumi, 2016). It activates AMPK, which suppresses pathways associated with NF‐κB. It also reduces the levels of other biomarkers of inflammation, including CRP, IL‐6, E‐selectins, intracellular adhesion molecule 1 (ICAM‐1), fibrinogen and tissue plasminogen activator, but with less effect than lifestyle modifications (Goldberg et al., 2016). Furthermore, metformin up‐regulates the activity of PON‐1 and thus increases the anti‐oxidative capacity of HDL‐C to prevent LDL oxidation. Goldberg et al. (2016) report that metformin increases HDL‐C independently of changes in adiponectin. In spite of these effects, recent reports suggest that metformin‐sensitive AMPK could be a key player in the development of Alzheimer's disease pathology (Domise et al., 2016). Thus, careful consideration in using metformin, as well as salsalate (Goldfine et al., 2013a,b; Fakhri et al., 2014; Fullerton et al., 2015), may be required. Metformin has been reported to cause metabolic acidosis and renal failure in some patients, which also needs to be taken into consideration.
Statins
Statins have been shown to have anti‐inflammatory effects, which are most evident at higher dosages. High‐dose atorvastatin (80 mg) suppresses NF‐κB‐associated inflammation, reduces the levels of MMP9 (Kim et al., 2007; Singh et al., 2008) and markedly decreases the concentration of CRP (van de Ree et al., 2003; Bonnet et al., 2008). Statin therapy is known for its ability to not only decrease LDL‐C levels but also concurrently increase HDL‐C. Multiple pathways are involved in the elevation of HDL‐C levels. Firstly, statins inhibit Rho factor, which results in increased activation of PPAR‐α and thus in an increased expression of the Apo‐A1 gene (Martin et al., 2001; Schaefer and Asztalos, 2006). Secondly, statins decrease VLDL‐TG and TG levels as well as CETP activity. The synergetic effect is an increased concentration of HDL‐C (Martin et al., 2001; Schaefer and Asztalos, 2006).
Extended release niacin
Niacin exerts its atheroprotective effects by acting on AT through GPCRs, thereby influencing both pro‐ and anti‐inflammatory markers. In vivo studies show that niacin decreases the levels of inflammatory markers TNF‐α and IL‐6 and suppresses the activation of the NF‐κB pathway (Si et al., 2014). The use of extended release niacin increases Apo‐A1 and HDL‐C levels while decreasing levels of total cholesterol, TG, LDL, monocyte chemoattractant protein (MCP‐1) and SAA (Si et al., 2014; Yadav et al., 2015). While some studies showed that niacin administration had no effect on the anti‐oxidative capacity of HDL, others demonstrated a decrease in oxidized‐LDL‐induced cell apoptosis and a reduction in blood vessel wall inflammation (Si et al., 2014; Yadav et al., 2015). Niacin also up‐regulates the expression of factors involved in RCT (Si et al., 2014), decreases CETP activity and reduces HDL‐C clearance. Additionally, niacin increases adiponectin mRNA expression (Digby et al., 2010).
Methotrexate and etanercept
Methotrexate is a disease modifying anti‐rheumatic drug, which is used as a first line treatment for RA. It exerts its anti‐inflammatory effect by promoting the accumulation of adenosine, which subsequently binds to the A2A receptor. As a result, the expression of ABCA1 and 27‐hydroxylase is promoted and RCT is increased. This action reverses the atherosclerotic effect of other COX‐2 inhibitors used to treat RA, as has been demonstrated in RA patients (Reiss et al., 2008; Chan and Cronstein, 2010). Additionally, methotrexate decreases MMP1, LTB4, inflammatory cytokine (e.g. TNF‐α, IL‐6 and IL‐1β) and INF‐γ expression while increasing the expression of anti‐inflammatory IL‐10 (Coomes et al., 2011; Cutolo et al., 2001). The ongoing cardiovascular inflammation reduction trial will probe the efficacy of methotrexate therapy on patients who have suffered from prior myocardial infarction combined with diabetes or metabolic syndrome (Everett et al., 2013). Although a negative impact on the ratio of total cholesterol to HDL‐C has been observed following methotrexate administration, the impact of methotrexate on HDL‐C function is unknown (Navarro‐Millán et al., 2013). The impact of targeting methotrexate‐sensitive pathways on CVD progression will provide useful insights into the inflammation processes at work in atherosclerosis.
Etanercept is a TNF‐α inhibitor that is used to treat RA. Several clinical trials have investigated its effect on inflammation and HDL‐C parameters in patients with RA (van Eijk et al., 2009; Charles‐Schoeman et al., 2016). These and other studies have demonstrated improvements in HDL‐C metabolism. Interestingly, the majority, but not all (Rodriguez‐Jimenez et al., 2014), reports showed that treatment with etanercept also decreased the concentration of TNF‐α (van Eijk et al., 2009; Charles‐Schoeman et al., 2016). It is possible that the effect of etanercept on HDL‐C metabolism was exerted via the SAA mechanism (van Eijk et al., 2009).
Peroxisome proliferator‐activated receptors agonists
PPARs are a nuclear receptor subfamily with three members, PPAR‐α, PPAR‐γ and PPAR‐β/δ. PPAR agonists have been shown to have anti‐inflammatory properties. PPAR‐γ agonists increase the expression of adiponectin and display anti‐inflammatory activity by promoting the polarization of monocytes towards alternative M2 macrophages (Colin et al., 2015). Rosiglitazone decreases the levels of inducible NO synthase (iNOS), ICAM‐1 and COX‐2 (Cuzzocrea et al., 2004). The PPAR‐γ agonist 15‐deoxy‐delta12, 4‐PGJ2 (15d–PGJ2) reverses the INF‐γ‐related effects on HDL‐C metabolism (Zuckerman et al., 2000; Bujold et al., 2009). This agonist enhances CD36 messaging, leading to increased cholesterol efflux to HDL‐C, and increases the expression of SR‐B1 and its binding to HDL‐C (Zuckerman et al., 2000; Bujold et al., 2009). PPAR‐α agonists, for example, fibrates, ameliorate inflammation by decreasing the levels of CRP and IL‐6 while inhibiting the activation of the NF‐κB pathway (Ogata et al., 2009; Mahdy et al., 2012). PPAR‐α agonists additionally decrease VLDL‐TG levels by increasing β‐oxidation of free FAs in the liver, thus decreasing the availability of FAs for VLDL‐TG synthesis (Mahdy et al., 2012). Reduced VLDL‐TG levels are also facilitated by PPAR‐γ agonists, which increase the expression of ABCA1. However, the reports on the effect of PPAR‐γ agonists on the concentration of HDL‐C are not consistent. Carreón‐Torres et al. (2009) demonstrated that in rabbits, rosiglitazone increases the production rate of Apo‐A1, resulting in increased concentration of HDL‐C and increased activity of PON‐1. Mizoguchi et al. (2011), who studied insulin tolerant and diabetic patients, reported that treatment with pioglitazone increased HDL‐C levels while decreasing CRP and the size of atherosclerotic plaques. In this particular study, patients receiving aspirin, renin angiotensin system inhibitors and statins were included, which may have biased the final results. In contrast, Millar et al. (2010) demonstrated that in subjects with metabolic syndrome, rosiglitazone increased the production rate of Apo‐A2 with no effect on Apo‐A1 metabolism. PPAR‐α agonists have been shown to stimulate the synthesis of both Apo‐A1 and Apo‐A2 and thus increase plasma HDL‐C levels (Colin et al., 2015). They have also been shown to stimulate the activity of ABCA1, ABCG1 and SR‐B1 in macrophages and thus increase RCT (Mahdy et al., 2012; Colin et al., 2015).
PPAR agonists are already being used in clinical practice; however, they are primarily used to treat dyslipidaemia and insulin resistance. PPAR‐γ agonists are primarily used to treat type 2 diabetes and they play a significant role in enhancing FA oxidation in the liver (Colin et al., 2015). PPAR‐β/δ agonists are used to improve lipid metabolism, as they by reduce TG and LDL‐C and increase HDL‐C levels. Moreover, PPAR‐β/δ activation increases the expression of genes promoting insulin sensitivity (Colin et al., 2015). Unfortunately, many of the currently available medications are either ineffective or have adverse effects that may outweigh their benefits for treating inflammation‐related impairments in HDL‐C function. For example, fibrates have a weak impact on PPAR‐α activity, although a newer agent, K‐877, binds strongly to PPAR‐α and is in phase III clinical trials for atherosclerotic dyslipidaemia in Japan (Colin et al., 2015). Wagner et al. (2010) demonstrated that in monkeys, the new PPAR‐α agonist CP‐900691 increases the levels of adiponectin and HDL‐C while decreasing the levels of CRP, TG, VLDL and LDL‐C. Other selective PPAR agonists, for example, CER‐002 (PPAR‐δ), DSP‐8658 (PPAR‐α/ϒ), INT131 (PPAR‐α/ϒ) and GFT505 (PPAR‐α/δ), are undergoing clinical trials and exhibit promise for treating the cardiovascular risks associated with metabolic syndrome and type 2 diabetes (Colin et al., 2015). To date, there is no information on the effect of these new agonists on inflammation‐related abnormalities.
Future therapies
HDL‐C reconstituted therapy
The use of artificial components of HDL‐C, as a reconstituted therapy (i.e. rHDL), has also been investigated. Apo‐A1 Milano and Fukuoka Apo‐A1 mimetic peptides have proved effective in animal models (Uehara et al., 2015). This approach enhanced the biological function of HDL‐C without elevating its concentration. Both therapeutics act as anti‐atherosclerotic agents and remove cholesterol via the ABCA1 transporter. Notably, when these agents were used in patients with symptomatic carotid plaque, no significant differences were noted in expression of genes involved in formation of thrombus (Nasr et al., 2015). However, the use of reconstituted peptides prevented the significant postoperative surge in plasma IL‐6, which was seen in the placebo group. Surgical intervention reduced systemic levels of tissue factor, MMP9 and MCP‐1 in the rHDL group, although the effects on MMP9 and MCP‐1 were abolished in the immediate postoperative period (Nasr et al., 2015). The 4F mimetic peptide was studied and found to have anti‐inflammatory properties in vitro and in humans (Bloedon et al., 2008; Smythies et al., 2010). The mimetic peptide decreased the levels of pro‐inflammatory cytokines and the adhesion of monocytes to human endothelial cells while increasing RCT by enhancing cholesterol efflux in macrophages (Smythies et al., 2010).
Liver X receptors agonists
The results from studies in mice have demonstrated that agonists of liver X receptors ‐α and ‐β (LXR‐α and LXR‐β respectively) increase the total concentration and the size of HDL‐C particles by up‐regulating the expression of ABCA1 and ABCG1 (Repa et al., 2000b; Jiang et al., 2003; Miao et al., 2004). Furthermore, LXR agonists have been shown to promote RCT resulting in the reduced deposition of cholesterol in atherosclerotic plaques (Naik et al., 2006). Conflictingly, LXR agonists have also been shown to up‐regulate the expression of CETP in CETP‐expressing transgenic mice, which completely abolished the beneficial effect of LXR on HDL‐C metabolism and increased the levels of LDL‐C and VLDL‐TGs (Jiang et al., 2003; Masson et al., 2004; Beltowski, 2008). LXR agonists also induced the sterol regulatory element binding protein‐1c in the liver, which has been shown to associate with the increased concentration of TGs (Repa et al., 2000a; Schultz et al., 2000; Grefhorst et al., 2002). In contrast, it has been hypothesized that LXR‐β selective agonists would decrease the levels of TG and LDL‐C. Several LXR‐β selective pyrazole and imidazole biaryl sulfones have been prepared. In particular, imidazole 18 (EXEL‐04286652, BMS‐779788) is an LXR‐β partial agonist that induces ABCA1, making it a reagent of interest for further study (Kick et al., 2015; Matsuda et al., 2015). Additionally, a novel synthetic, steroidal LXR ligand, ATI‐111, has been developed. This molecule exhibits a strong effect on LXR‐α with a modest effect on LXR‐β. Encouragingly, animal and in vitro studies indicate that ATI‐111 has beneficial anti‐atherosclerotic and anti‐inflammatory effects ranging from reduced hypertriglyceridaemia to decreased atherosclerotic lesions. To assess the full potential of ATI‐111, clinical trials will be necessary. Presently, a phase I clinical trial with XL‐652 (XL‐014), a novel LXR ligand, is underway (Colin et al., 2015). Furthermore, the LXR agonists have been shown to have anti‐inflammatory properties. Mouse studies with LXR agonists T0901317 and GW3965 demonstrated decreased levels of inflammatory cytokines TNF‐α, IL‐6, IL‐1β, IFN‐γ, MMP‐9 and ICAM‐1 (Joseph et al., 2003; Wang et al., 2006; Jamroz‐Wisniewska et al., 2007), indicating another beneficial side of LXR agonists warranting further investigation.
Phosphodiesterase‐5 inhibitors
Sildenafil inhibits cGMP‐specific PDE5, an enzyme that promotes degradation of cGMP. Inhibition of PDE5 results in smooth muscle relaxation, which alleviates erectile dysfunction and pulmonary arterial hypertension (Balhara et al., 2015; Igarashi et al., 2016). Sildenafil therapy increased HDL‐C and decreased P‐selectin and LDL‐C levels through mechanisms which are not yet understood (Mandosi et al., 2015). Nunes et al. (2015) reported that administration of sildenafil reduces the expression of pro‐inflammatory cytokines IL‐1β and TNF‐α while increasing the levels of anti‐inflammatory cytokine IL‐10. In addition, sildenafil has been shown to reduce the expression of GFAP, NF‐κB, inactive AMPK and iNOS and to increase IKβα levels (Nunes et al., 2015). Thus, sildenafil may potentially be used to treat inflammation and the associated decrease in HDL‐C function.
Monoclonal antibodies
A monoclonal antibody, canakinumab, is an IL‐1β inhibitor which has been shown to be effective in treating juvenile RA (Gencer et al., 2015). The canakinumab anti‐inflammatory thrombosis outcomes study trial is currently underway to determine the effects of canakinumab on stable CVD patients who exhibit high levels of inflammation (hsCRP >2 mg. L‐1; Ridker et al., 2011). While existing data on canakinumab would indicate that overall HDL‐C levels will not be affected (Ridker et al., 2012), the impact of targeting inflammation via the IL‐1β pathway for reducing cardiovascular events will provide insight into the role of inflammation in CVD and the potential benefits of a more thorough investigation of the effect of canakinumab on HDL‐C. A similar but more direct approach for treating dyslipidaemia may reside in the use of monoclonal antibody inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9), such as alirocumab and evolocumab, which were approved by the FDA in 2015. PCSK9, which contributes to the development of atherosclerosis, is believed to be expressed in macrophages, smooth muscles and endothelium. Functionally, PCSK9 down‐regulates the expression of the stress response genes and reduces inflammation in liver cells, indicating that PCSK9 affects metabolic pathways beyond cholesterol metabolism. Inhibition of PCSK9 improves the removal of LDL‐C from blood by the liver (Lan et al., 2010). Furthermore, Walley et al. (2014) demonstrated that reducing PCSK9 function increases pathogen lipid clearance via the LDL receptor, thereby decreasing the inflammatory response and improving sepsis outcomes in both mice and humans. This finding is in contrast to that of Sahebkar et al. (2016), who conducted a meta‐analysis evaluating the effects of several PCSK9 inhibitors on the levels of high‐sensitivity CRP (hs‐CRP) and demonstrated that PCSK9 inhibitors do not affect the hs‐CRP levels. The PCKS9 story is relatively new and will be revisited in coming years. Nevertheless, monoclonal antibodies show promise for ameliorating inflammation and related dyslipidaemia.
Biological agents
According to several recent studies and meta‐analyses, biological agents for the treatment of inflammatory arthritis induced changes in several lipid profiles. Specifically, tocilizumab, an IL‐6 inhibitor, and tofacitinib, a JAK inhibitor, are recombinant proteins, which have been shown to decrease the levels of inflammatory cytokines and increase HDL‐C concentration. The mechanisms whereby these agents exert their beneficial effect involve modulation of the immune response. JAK inhibitors block intracellular signalling of several cytokines. This effect was confounded by the inability of the TNF‐α antagonists to show any marked improvement in HDL‐C (Dahlqvist et al., 2006; Genovese et al., 2008; Soubrier et al., 2008; Mathieu et al., 2010; Ghoreschi et al., 2011; Kawashiri et al., 2011; van Vollenhoven et al., 2012; McInnes et al., 2013; Souto et al., 2015).
5‐LOX inhibitors (theophylline and montelukast)
Theophylline and montelukast have been investigated for the treatment of inflammation‐related asthma (Allayee et al., 2007). Both of these drugs inhibit the pathway controlled by 5‐lipoxygenase (5‐LOX), a critical agent in the leukotriene pathway, which is expressed in AT and plays a significant role in obesity‐related AT inflammation (Horrillo et al., 2010). Modulation of the leukotriene pathway, using 5‐LOX activated protein (i.e. FLAP) inhibitors, has been shown to decrease the levels of systemic pro‐inflammatory cytokines, AT macrophage content and systemic insulin resistance (Bäck et al., 2007; Horrillo et al., 2010). Hakonarson et al. (2005) demonstrated that interventions using 5‐LOX inhibitors decreased the levels of inflammatory markers such as SAA, CRP and MPO. Similarly, Allayee et al. (2007) showed that treatment of asthmatics with theophylline and montelukast decreased the levels of CRP, IL‐6, VLDL‐TG and LDL‐C. Unfortunately, in this report, the HDL‐C levels were reduced in the treatment group compared with placebo, indicating a potential detrimental effect of montelukast and theophylline therapy on HDL‐C metabolism. Nevertheless, because of the reported significance of 5‐LOX‐associated pathways in obesity‐related inflammation, further studies that target this strategy for reducing inflammation are of interest.
Non‐pharmaceutical approaches
Diet and dietary components
A hypocaloric, high‐fat, low‐carb diet has been found to decrease CRP and increase adiponectin. This regime has also been shown to lower hepatic VLDL‐TG and TG secretion and decrease their hydrolysis by hepatic lipase, thereby increasing HDL‐C (Ruth et al., 2013). Increased intake of tree nuts causes a decrease in total cholesterol, LDL‐C, TG and Apo‐B, while exerting no effect on HDL‐C, Apo‐A1, CRP and hypertension (Ros et al., 2004; Demonty et al., 2009; Sabate and Wien, 2013; Del Gobbo et al., 2015). Although numerous isolated reports show the beneficial effect of red wine and resveratrol supplementation on inflammatory markers, the meta‐analyses demonstrated no positive impact from resveratrol supplementation on cardiovascular risk factors (Sahebkar et al., 2015). In fact, a slight decrease in HDL‐C levels has been reported (Sahebkar et al., 2015). Krill oil consumption, which has an anti‐oxidative effect, is associated with increased HDL‐C and Apo‐A1 and decreased TG and inflammation levels in healthy young adults (Berge et al., 2015; Cicero et al., 2016). Caffeic acid is a naturally occurring phenolic compound found in many fruits, vegetables and herbs (Moon et al., 2009). It decreases TNF‐α‐induced induction of adhesion molecules including ICAM‐1, vascular adhesion molecule (VCAM‐1) and P selectin. It also decreases TNF‐α‐induced activation of IL‐8 and NF‐κB (Moon et al., 2009). Aloe vera exhibits several beneficial effects. It decreases the activation of inflammasome NLRP3, IL‐8, IL‐6, IL‐1β and TNF‐α, as well as the activation of inflammatory NF‐κB, p38 and JNK pathways, and thus decreases inflammation and raises HDL‐C levels (Budai et al., 2013; Kumar et al., 2013).
Exercise and life style interventions; bariatric surgery; and electro acupuncture therapy
Exercise and life style interventions show promising effects on inflammatory markers and lipid profiles. These strategies increase the levels of adiponectin and HDL‐C and decrease inflammatory cytokines such as INF‐γ (Nishida et al., 2015; Davidson et al., 2017, Wefers et al., 2016). The improvement in HDL‐C metabolism with weight loss can occur via improvements in several of the mechanisms discussed above. For example, increased levels of adiponectin activate ceramidase and the formation of sphingosine‐1‐phosphate, thus altering HDL‐C sphingolipid content, thereby improving HDL‐C function (Belalcazar et al., 2012). Goldberg et al. (2016) showed that lifestyle intervention increases the levels of adiponectin and HDL‐C while decreasing the levels of inflammatory markers CRP, IL‐6, E selectin, ICAM‐1 and fibrinogen. Moreover, weight loss results in decreased levels of TG, which diminishes the activity of CETP and thus results in higher levels of functional HDL‐C. Animal studies demonstrated that weight loss results in increased expression of ABCG1 protein in AT leading to increased cholesterol efflux (Edgel et al., 2012), which is associated with enhanced levels of functional HDL‐C particles (Wesnigk et al., 2016). Taken together, these and other reports suggest that weight loss beneficially affects HDL‐C metabolism.
Roux en‐Y gastric bypass surgery boosts HDL‐C levels and endothelial function. This results in decreased apoptosis of endothelial cells and increased production of nitric oxide and enhanced PON‐1 activity. Additionally, there is an increase in macrophage‐induced cholesterol efflux. Furthermore, anti‐inflammatory and anti‐oxidative effects are enhanced because of a decrease in TNF‐α‐mediated VCAM‐1 expression and NADPH oxidase activity (Adams et al., 2012; Osto et al., 2015).
Electro‐acupuncture shows promise in treating obesity and controlling inflammation. It decreases BMI and the concentrations of IL‐6, TNF‐α, TG and LDL‐C, while increasing the levels of adiponectin and HDL‐C (Firouzjaei et al., 2016).
Conclusions
While the association of HDL‐C metabolism with the progression of CVD is still being investigated, the evidence supporting a link between tissue and systemic inflammation, lipid kinetics and CVD progression continues to grow. Our current review details the manner in which tissue and systemic inflammation modulates HDL‐C metabolism via several pathways, for example, cholesterol efflux, hyperlipidaemia and apolipoprotein modification. The data available suggest that regardless of its correlation with the progression of CVD, HDL‐C metabolism may provide a window into systemic or tissue (i.e. adipose) health. Our survey of available anti‐inflammatory interventions indicates that increasing Apo‐A1, ABCA1, SR‐B1 and adiponectin levels may improve the production and functioning of HDL‐C particles. The potential health benefits and indirect improvements in HDL‐C metabolism resulting from anti‐inflammatory interventions must, however, be balanced with their potential side effects (Table 2). Additionally, it should be noted that not all the agents discussed are purely anti‐inflammatory and thus may affect HDL‐C metabolism via other factors and mechanisms. Although a healthy lifestyle is the best approach to prevent the development of inflammation, the challenges of implementing lifestyle modification in the general population will require consistent social and medical support. Thus, pharmaceutical interventions to ameliorate inflammation and improve the functionality of HDL‐C and dyslipidaemia are of significant interest. A summary of the common anti‐inflammatory agents of interest for enhancing HDL‐C function, with a list of associated side effects, is presented in Table 2. The question of whether the side effects outweigh the benefits of these agents for ameliorating inflammation and HDL‐C metabolism will need to be addressed by future clinical trials. In conclusion, further research is needed to elucidate and target the mechanisms linking HDL‐C metabolism to both inflammation and the progression of CVD. Some of the studies should address the key mechanisms underlying the complexity and heterogeneity of HDL‐C particles, which should provide a more detailed understanding of the specific functions of these particles.
Table 2.
Side effects of pharmaceutical agents that have anti‐inflammatory properties and can contribute to improving the HDL‐C function
| Pharmaceutical agents | Side effects | References |
|---|---|---|
| Aspirin | Drug resistance can occur in some patients. | Azmin et al., 2013; Zhang et al., 2017 |
| Polyp formation and exacerbation of respiratory disease has been observed. | Eskandarian et al., 2012; Cook and Stevenson, 2016 | |
| Chronic salicylate intoxication can cause SIRS. | Chalasani et al., 1996 | |
| Aspirin can cause gastritis and increased risk of gastrointestinal bleeding. | Gartner, 1976 | |
| Salsalates | Poor tolerance in HIV patients. | Hileman et al., 2010 |
| Activate AMPK and may cause Alzheimer's disease. | Domise et al., 2016 | |
| Metformin | Effect may depend on race and ethnicity. | Zhang et al., 2015 |
| It may cause lactic acidosis especially if given in renal diseases. | Lalau, 2010 | |
| It causes hepatotoxicity in PON‐1‐ deficient mice. | García‐Heredia et al., 2016 | |
| Activates AMPK and may cause Alzheimer's disease. | Domise et al., 2016 | |
| Statins | Down‐regulate ABCA1 and ABCG1 activity in macrophages. | Sone et al., 2004; Wang et al., 2013; Wong et al., 2008 |
| Cause myopathy in some patients. | Lahaye et al., 2014; Brinton et al., 2016; Jacobson, 2009; Rosenson, 2004 | |
| Rosuvastatin did not reduce inflammation in sepsis associated acute respiratory distress syndrome. | Truwit et al., 2014 | |
| Unsafe in pregnancy. | Hosokawa et al., 2003 | |
| They may cause hepatotoxicity. | Russo et al., 2014 | |
| Niacin | There is increased risk of flushing with niacin use. | Maccubbin et al., 2009 |
| May cause macular oedema. | Domanico et al., 2015 | |
| May lead to the development of hepatitis. | Etchason et al., 1991 | |
| PPAR‐α agonists | Did not decrease inflammation in rodents with renal crystal formation. | Taguchi et al., 2016 |
| Fenofibrate did not decrease inflammatory markers in one study. | Hogue et al., 2008 | |
| Fibrates may cause increased risk of renal problems. | Zhao et al., 2012 | |
| PPAR‐γ agonists | Thiazolidinediones may increase risk of myocardial infarction and heart failure especially rosiglitazone. |
Singh et al., 2007
Nissen and Wolski, 2007 |
| Increased risks of fractures in women. | Loke et al., 2009 | |
| There is increased risk of bladder cancer with pioglitazone. | Ferwana et al., 2013 | |
| Biological agents (IL‐6 inhibitor, JAK inhibitor and TNF‐α inhibitor) | Several toxic side effects. | Pichler, 2006 |
| Increase chance of fungal infections with anti‐TNF‐α fusion inhibitors. | Tragiannidis et al., 2016 | |
| LXR agonists | May cause increase in TGs, VLDL and LDL by inducing SREBP‐1c | Repa et al., 2000a; Schultz et al., 2000; Grefhorst et al., 2002 |
| Sildenafil | May cause hypotension if given with nitrates. | Webb et al., 1999 |
| Monoclonal antibodies | Adverse effects including acute anaphylaxis, serum sickness, cardiotoxicity etc. | Hansel et al., 2010; Kizhedath et al., 2016 |
| Increase chance of fungal infections with anti‐TNFα monoclonal antibodies. | Tragiannidis et al., 2016 | |
| 5‐Lipoxygenase inhibitors | ||
| Theophylline | Can cause adverse effects due to theophylline toxicity. | Eason and Markowe, 1989 |
| Montelukast | Increased risk of ecchymosis. | Aypak et al., 2013 |
| Methotrexate | May cause elevation of liver enzymes and hepatotoxicity. | Curtis et al., 2010 |
| May cause bone marrow suppression. | Sosin and Handa, 2003 |
Author contributions
F.I., W.S.B. and D.T. did the literature search; F.I., W.S.B. and D.T. wrote the manuscript; and F.I., W.S.B., M.I.K., S.T., K.H.M., N.A. and D.T discussed and edited the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported in part by a 1KL2TR001441 NIH Training grant, the Institute for Translational Sciences at the UTMB, a Clinical and Translational Science Award (#UL1 TR001439) from the National Center for Advancing Translational Sciences, and the Claude D. Pepper OAIC grant (#P30‐AG024832), National Institutes of Health.
Iqbal, F. , Baker, W. S. , Khan, M. I. , Thukuntla, S. , McKinney, K. H. , Abate, N. , and Tuvdendorj, D. (2017) Current and future therapies for addressing the effects of inflammation on HDL cholesterol metabolism. British Journal of Pharmacology, 174: 3986–4006. doi: 10.1111/bph.13743.
References
- Abd‐Elrahman I, Meir K, Kosuge H, Ben‐Nun Y, Weiss Sadan T, Rubinstein C et al. (2016). Characterizing cathepsin activity and macrophage subtypes in excised human carotid plaques. Stroke 47: 1101–1108. [DOI] [PubMed] [Google Scholar]
- Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC (2012). Health benefits of gastric bypass surgery after 6 years. JAMA 308: 1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allayee H, Hartiala J, Lee W, Mehrabian M, Irvin CG, Conti DV et al. (2007). The effect of montelukast and low‐dose theophylline on cardiovascular disease risk factors in asthmatics. Chest 132: 868–874. [DOI] [PubMed] [Google Scholar]
- Amann R, Peskar BA (2002). Anti‐inflammatory effects of aspirin and sodium salicylate. Eur J Pharmacol 447: 1–9. [DOI] [PubMed] [Google Scholar]
- Annema W, Nijstad N, Tölle M, De Boer JF, Buijs RV, Heeringa P et al. (2010). Myeloperoxidase and serum amyloid A contribute to impaired in vivo reverse cholesterol transport during the acute phase response but not group IIA secretory phospholipase A(2). J Lipid Res 51: 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J et al. (1999). Paradoxical decrease of an adipose‐specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83. [DOI] [PubMed] [Google Scholar]
- Asztalos BF, Schaefer EJ, Horvath KV, Yamashita S, Miller M, Franceschini G et al. (2007). Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J Lipid Res 48: 592–599. [DOI] [PubMed] [Google Scholar]
- Asztalos BF, Tani M, Schaefer EJ (2011). Metabolic and functional relevance of HDL subspecies. Curr Opin Lipidol 22: 176–185. [DOI] [PubMed] [Google Scholar]
- Aypak C, Türedi Ö, Solmaz N, Yıkılkan H, Görpelioğlu S (2013). A rare adverse effect of montelukast treatment: ecchymosis. Respir Care 58: e104‐e106. [DOI] [PubMed] [Google Scholar]
- Azmin S, Sahathevan R, Rabani R, Nafisah WY, Tan HJ, Raymond AA et al. (2013). Biochemical aspirin resistance in stroke patients – a cross‐sectional single centre study. EXCLI J 12: 907. [PMC free article] [PubMed] [Google Scholar]
- Bäck M, Sultan A, Ovchinnikova O, Hansson GK (2007). 5‐Lipoxygenase‐activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circ Res 100: 946–949. [DOI] [PubMed] [Google Scholar]
- Badimon JJ, Badimon L, Fuster V (1990). Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol fed rabbit. J Clin Invest 85: 1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balhara YP, Sarkar S, Gupta R (2015). Phosphodiesterase‐5 inhibitors for erectile dysfunction in patients with diabetes mellitus: a systematic review and meta‐analysis of randomized controlled trials. Indian J Endocrinol Metab 19: 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M et al. (2007). Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- Basso F, Freeman L, Knapper CL, Remaley A, Stonik J, Neufeld EB et al. (2003). Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J Lipid Res 44: 296–302. [DOI] [PubMed] [Google Scholar]
- Batuca JR, Amaral MC, Favas C, Paula FS, Ames PR, Papoila AL et al. (2016). Extended release‐niacin increases anti‐ApoA‐I antibodies that block the anti‐oxidant effect of HDL‐C: the EXPLORE clinical trial. Br J Clin Pharmacol. doi:10.1111/bcp.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belalcazar LM, Lang W, Haffner SM, Hoogeveen RC, Pi‐Sunyer FX, Schwenke DC (2012). Adiponectin and the mediation of HDL‐cholesterol change with improved lifestyle: the Look AHEAD Study. J Lipid Res 53: 2726–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltowski J (2008). Liver X receptors (LXR) as therapeutic targets in dyslipidemia. Cardiovasc Ther 26: 297–316. [DOI] [PubMed] [Google Scholar]
- Berge RK, Ramsvik MS, Bohov P, Svardal A, Nordrehaug JE, Rostrup E et al. (2015). Krill oil reduces plasma triacylglycerol level and improves related lipoprotein particle concentration, fatty acid composition and redox status in healthy young adults – a pilot study. Lipids Health Dis 4: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Scherer PE (2005). Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96: 939–949. [DOI] [PubMed] [Google Scholar]
- Bloedon LT, Dunbar R, Duffy D, Pinell‐Salles P, Norris R, DeGroot BJ et al. (2008). Safety, pharmacokinetics, and pharmacodynamics of oral apoA‐I mimetic peptide D‐4F in high‐risk cardiovascular patients. J Lipid Res 49: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes‐Nickens P, Koprowicz K et al. (2011). Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
- Bonnet J, McPherson R, Tedgui A, Simoneau D, Nozza A, Martineau P (2008). Effects of l0‐mg versus 80‐mg atorvastatin on high‐sensitivity C‐reactive protein in patients with stable coronary artery disease: results of the CAP (comparative atorvastatin pleiotropic effects) Study. Clin Ther 30: 2298–2313. [DOI] [PubMed] [Google Scholar]
- Brehm A, Geraghty P, Campos M, Garcia‐Arcos I, Dabo AJ, Gaffney A et al. (2014). Cathepsin G degradation of phospholipid transfer protein (PLTP) augments pulmonary inflammation. FASEB J 28: 2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer HB (2007). HDL metabolism and the role of HDL in the treatment of high‐risk patients with cardiovascular disease. Curr Cardiol Rep 9: 486–492. [DOI] [PubMed] [Google Scholar]
- Brinton EA, Maki KC, Jacobson TA, Sponseller CA, Cohen JD (2016). Metabolic syndrome is associated with muscle symptoms among statin users. J Clin Lipidol 10: 1022–1029. [DOI] [PubMed] [Google Scholar]
- Brown WV, Ansell BJ, Mackey RH, Toth PP (2014). JCL roundtable: HDL in the primary care setting. J Clin Lipidol 8: 364–372. [DOI] [PubMed] [Google Scholar]
- Brousseau ME, Schaefer EJ, Wolfe ML, Bloedon LT, Digenio AG, Clark RW et al. (2004). Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med 350: 1505–1515. [DOI] [PubMed] [Google Scholar]
- Bruce C, Chouinard RA Jr, Tall AR (1998). Plasma lipid transfer proteins, high‐density lipoproteins, and reverse cholesterol transport. Annu Rev Nutr 18: 297–330. [DOI] [PubMed] [Google Scholar]
- Budai MM, Varga A, Milesz S, Tőzsér J, Benkő S (2013). Aloe vera downregulates LPS‐induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol Immunol 56: 471–479. [DOI] [PubMed] [Google Scholar]
- Bujold K, Rhainds D, Jossart C, Febbraio M, Marleau S, Ong H (2009). CD36‐mediated cholesterol efflux is associated with PPARgamma activation via a MAPK‐dependent COX‐2 pathway in macrophages. Cardiovasc Res 83: 457–464. [DOI] [PubMed] [Google Scholar]
- Camps J, Hernández‐Aguilera A, García‐Heredia A, Cabré N, Luciano‐Mateo F, Arenas M et al. (2016). Relationships between metformin, paraoxonase‐1 and the chemokine (C‐C motif) ligand 2. Curr Clin Pharmacol 11. doi:10.2174/1574884711666160915152941. [DOI] [PubMed] [Google Scholar]
- Carreón‐Torres E, Rendón‐Sauer K, Monter‐Garrido M, Toledo‐Ibelles P, Gamboa R, Menjivar M et al. (2009). Rosiglitazone modifies HDL structure and increases HDL‐Apo AI synthesis and catabolic rates. Clin Chim Acta 401: 37–41. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Roman J, Jurado RL (1996). Systemic inflammatory response syndrome caused by chronic salicylate intoxication. South Med J 89: 479–482. [DOI] [PubMed] [Google Scholar]
- Chan ES, Cronstein BN (2010). Methotrexate – how does it really work? Nat Rev Rheumatol 6: 175–178. [DOI] [PubMed] [Google Scholar]
- Charles‐Schoeman C, Lee YY, Shahbazian A, Wang X, Elashoff D, Curtis JR et al. (2016). Improvement in HDL function in early rheumatoid arthritis patients treated with methotrexate monotherapy or combination therapy in the TEAR trial. Arthritis Rheumatol . doi:10.1002/art.39833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Sawyer JK, Gebre AK, Maeda N, Parks JS (2011). Adipose tissue ATP binding cassette transporter A1 contributes to high‐density lipoprotein biogenesis in vivo. Circulation 124: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero AF, Rosticci M, Morbini M, Cagnati M, Grandi E, Parini A et al. (2016). Lipid‐lowering and anti‐inflammatory effects of omega 3 ethyl esters and krill oil: a randomized, cross‐over, clinical trial. Arch Med Sci 12: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton PM, Mackinnon AM, Barter PJ (1985). Effects of serum amyloid A protein (SAA) on composition, size, and density of high density lipoproteins in subjects with myocardial infarction. J Lipid Res 26: 1389–1398. [PubMed] [Google Scholar]
- Colin S, Chinetti‐Gbaguidi G, Kuivenhoven JA, Staels B (2015). Emerging small molecule drugs. Handb Exp Pharmacol 224: 617–630. [DOI] [PubMed] [Google Scholar]
- Cook KA, Stevenson DD (2016). Current complications treatment of aspirin‐exacerbated respiratory disease. Expert Rev Respir Med 10: 1305–1316. [DOI] [PubMed] [Google Scholar]
- Coomes E, Chan ESL, Reiss AB (2011). Methotrexate in atherogenesis and cholesterol metabolism. Cholesterol 2011, Article ID 503028: 8. doi:10.1155/2011/503028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JR, Beukelman T, Onofrei A, Cassell S, Greenberg JD, Kavanaugh A et al (2010). Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann Rheum Dis 69: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Sulli A, Pizzorni C, Seriolo B (2001). Anti‐inflammatory mechanisms of methotrexate in rheumatoid arthritis. Leader Ann Rheum Dis 60: 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NS (2004). Rosiglitazone, a ligand of the peroxisome proliferator‐activated receptor‐gamma, reduces acute inflammation. Eur J Pharmacol 483: 79–93. [DOI] [PubMed] [Google Scholar]
- Dahlqvist SR, Engstrand S, Berglin E, Johnson O (2006). Conversion towards an atherogenic lipid profile in rheumatoid arthritis patients during long‐term infliximab therapy. Scand J Rheumatol 35: 107–111. [DOI] [PubMed] [Google Scholar]
- Davidson WS, Inge TH, Sexmith H, Heink A, Elder D, Hui DY et al. (2017). Weight loss surgery in adolescents corrects high‐density lipoprotein subspecies and their function. Int J Obes (Lond). 41: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan W, Bhattacharjee A, Ruddle P, Kang MH, Hayden MR (2014). ABCA1 in adipocytes regulates adipose tissue lipid content, glucose tolerance, and insulin sensitivity. J Lipid Res 55: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D (2015). Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta‐analysis, and dose–response of 61 controlled intervention trials. Am J Clin Nutr 102: 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetz E, Schroll A, Auer K, Heim C, Patsch JR, Eller P et al. (2014). The arachidonic acid metabolome serves as a conserved regulator of cholesterol metabolism. Cell Metab 20: 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonty I, Ras RT, van der Knaap HC, Duchateau GS, Meijer L, Zock PL et al. (2009). Continuous dose–response relationship of the LDL‐cholesterol‐lowering effect of phytosterol intake. J Nutr 139: 271–284. [DOI] [PubMed] [Google Scholar]
- Digby JE, McNeill E, Dyar OJ, Lam V, Greaves DR, Choudhury RP (2010). Anti‐inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, RANTES, and MCP‐1 and upregulation of adiponectin. Atherosclerosis 209: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnes DL, White MY, Kockx M, Traini M, Hsieh V, Kim MJ et al. (2016). Human macrophage cathepsin B‐mediated C‐terminal cleavage of apolipoprotein A‐I at Ser228 severely impairs antiatherogenic capacity. FASEB J. pii: fj.201600508R. doi:10.1096/fj.201600508R. [DOI] [PubMed] [Google Scholar]
- Dobiásová M, Frohlich JJ. Clin Chim Acta(1999). Advances in understanding of the role of lecithin cholesterol acyltransferase (LCAT) in cholesterol transport. Clin Chim Acta 286: 257–271. [DOI] [PubMed] [Google Scholar]
- Domanico D, Verboschi F, Altimari S, Zompatori L, Vingolo EM (2015). Ocular effects of niacin: a review of the literature. Med Hypothesis Discov Innov Ophthalmol 4: 64–71. [PMC free article] [PubMed] [Google Scholar]
- Domise M, Didier S, Marinangeli C, Zhao H, Chandakkar P, Buée L et al. (2016). AMP‐activated protein kinase modulates tau phosphorylation and tau pathology in vivo. Sci Rep 6: 26758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusanov S, Heggen E, Tonstad S (2016). Characteristics of metabolic syndrome in morbidly obese subjects. Metab Syndr Relat Disord. doi:10.1089/met.2016.0062. [DOI] [PubMed] [Google Scholar]
- Eason J, Markowe HL (1989). Aminophylline toxicity–how many hospital asthma deaths does it cause? Respir Med 83: 219–226. [DOI] [PubMed] [Google Scholar]
- Edgel KA, McMillen TS, Wei H, Pamir N, Houston BA, Caldwell MT et al. (2012). Obesity and weight loss result in increased adipose tissue ABCG1 expression in db/db mice. Biochim Biophys Acta 1821: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandarian R, Darabian M, Heshmatnia J, Ghorbani R (2012). Acetyl salicylic acid resistance in patients with chronic stable angina and the correlation with coronary risk factors. Saudi Med J 33: 39–43. [PubMed] [Google Scholar]
- Etchason JA, Miller TD, Squires RW, Allison TG, Gau GT, Marttila JK et al. (1991). Niacin‐induced hepatitis: a potential side effect with low‐dose time‐release niacin. Mayo Clin Proc 66: 23. [DOI] [PubMed] [Google Scholar]
- Everett BM, Pradhan AD, Solomon DH, Paynter N, MacFadyen J, Zaharris E et al. (2013). Rationale and design of the cardiovascular inflammation reduction trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 166: 199–207.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhri M, Imani EF, Khalili N (2014). The effect of salsalate on biochemical factors and endothelial dysfunction of prediabetic patients: a randomized clinical trial. J Res Med Sci 19: 287–292. [PMC free article] [PubMed] [Google Scholar]
- Feldman M, Jialal I, Devaraj S, Cryer B (2001). Effects of low‐dose aspirin on serum C‐reactive protein and thromboxane B2 concentrations: a placebo‐controlled study using a highly sensitive C‐reactive protein assay. J Am Coll Cardiol 37: 2036–2041. [DOI] [PubMed] [Google Scholar]
- Feng D, Tracy RP, Lipinska I, Murillo J, McKenna C, Tofler GH (2000). Effect of short‐term aspirin use on C‐reactive protein. J Thromb Thrombolysis 9: 37–41. [DOI] [PubMed] [Google Scholar]
- Ferwana M, Firwana B, Hasan R, Al‐Mallah MH, Kim S, Montori VM et al. (2013). Pioglitazone and risk of bladder cancer: a meta‐analysis of controlled studies. Diabetes Med 30: 1026–1032. [DOI] [PubMed] [Google Scholar]
- Firouzjaei A, Li GC, Wang N, Liu WX, Zhu BM (2016). Comparative evaluation of the therapeutic effect of metformin monotherapy with metformin and acupuncture combined therapy on weight loss and insulin sensitivity in diabetic patients. Nutr Diabetes 6: e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton MD, Ford RJ, McGregor CP, LeBlond ND, Snider SA, Stypa S et al. (2015). Salicylate improves macrophage cholesterol homeostasis via activation of AMPK. J Lipid Res 56: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Heredia A, Riera‐Borrull M, Fort‐Gallifa I, Luciano‐Mateo F, Cabré N, Hernández‐Aguilera A et al. (2016). Metformin administration induces hepatotoxic effects in paraoxonase‐1‐deficient mice. Chem Biol Interact 249: 56–63. [DOI] [PubMed] [Google Scholar]
- Gartner AH (1976). Aspirin‐induced gastritis and gastrointestinal bleeding. J Am Dent Assoc 93: 111–117. [DOI] [PubMed] [Google Scholar]
- Gencer B, Laaksonen R, Buhayer A, Mach F (2015). Use and role of monoclonal antibodies and other biologics in preventive cardiology. Swiss Med Wkly 145: w14179. [DOI] [PubMed] [Google Scholar]
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E et al. (2008). Interleukin‐6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease‐modifying antirheumatic drugs: the tocilizumab in combination with traditional disease modifying antirheumatic drug therapy study. Arthritis Rheum 58: 2968–2980. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW et al. (2011). Modulation of innate and adaptive immune responses by tofacitinib (CP‐690,550). J Immunol 186: 4234–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofman JW, Young W, Tandy R (1966). Ischemic heart disease, atherosclerosis, and longevity. Circulation 34: 679–697. [DOI] [PubMed] [Google Scholar]
- Goldfine AB, Buck JS, Desouza C, Fonseca V, Chen YD, Shoelson SE et al. (2013a). Targeting inflammation using salsalate in patients with type 2 diabetes: effects on flow‐mediated dilation (TINSAL‐FMD). Diabetes Care 36: 4132–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine AB, Conlin PR, Halperin F, Koska J, Permana P, Schwenke D et al. (2013b). A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia 56: 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Temprosa M, Mele L, Orchard T, Mather K, Bray G et al. (2016). Change in adiponectin explains most of the change in HDL particles induced by lifestyle intervention but not metformin treatment in the Diabetes Prevention Program. Metabolism 65: 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SM, Hofmann S, Askew DS, Davidson WS (2011). High density lipoprotein: it's not just about lipid transport anymore. Trends Endocrinol Metab 22: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR (1977). The Framingham Study. High density lipoprotein as a protective factor against coronary heart disease. Am J Med 62: 707–714. [DOI] [PubMed] [Google Scholar]
- Grefhorst A, Elzinga BM, Voshol PJ, Plösch T, Kok T, Bloks VW et al. (2002). Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride‐rich very low density lipoprotein particles. J Biol Chem 277: 34182–34190. [DOI] [PubMed] [Google Scholar]
- Hakonarson H, Thorvaldsson S, Helgadottir A, Gudbjartsson D, Zink F, Andresdottir M (2005). Effects of a 5‐lipoxygenase‐activating protein inhibitor on biomarkers associated with risk of myocardial infarction a randomized trial. JAMA 293: 2245–2256. [DOI] [PubMed] [Google Scholar]
- Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT (2010). The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 9: 325–338. [DOI] [PubMed] [Google Scholar]
- Haroon M, Devlin J (2009). Marked hypertriglyceridemia upon treatment with etanercept. Joint Bone Spine 76: 570–571. [DOI] [PubMed] [Google Scholar]
- Hazzard WR, Applebaum‐Bowden D (1990). Why women live longer than men: the biologic mechanism of the sex differential in longevity. Trans Am Clin Climatol Assoc 101: 168–188. [PMC free article] [PubMed] [Google Scholar]
- He BM, Zhao SP, Peng ZY (2013). Effects of cigarette smoking on HDL quantity and function: implications for atherosclerosis. J Cell Biochem 114: 2431–2436. [DOI] [PubMed] [Google Scholar]
- Herová M, Schmid M, Gemperle C, Loretz C, Hersberger M (2014). Low dose aspirin is associated with plasma chemerin levels and may reduce adipose tissue inflammation. Atherosclerosis 235: 256–262. [DOI] [PubMed] [Google Scholar]
- Hileman CO, Carman TL, Gripshover BM, O'Riordan M, Storer NJ, Harrill DE et al. (2010). Salsalate is poorly tolerated and fails to improve endothelial function in virologically suppressed HIV‐infected adults. AIDS 24: 1958–1961. [DOI] [PubMed] [Google Scholar]
- Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagné C, Couture P (2008). Differential effect of atorvastatin and fenofibrate on plasma oxidized low‐density lipoprotein, inflammation markers, and cell adhesion molecules in patients with type 2 diabetes mellitus. Metabolism 57: 380–386. [DOI] [PubMed] [Google Scholar]
- Horrillo R, González‐Périz A, Martínez‐Clemente M, López‐Parra M, Ferré N, Titos E et al. (2010). Lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol 184: 3978–3987. [DOI] [PubMed] [Google Scholar]
- Hosokawa A, Bar‐Oz B, Ito S (2003). Use of lipid‐lowering agents (statins) during pregnancy. Can Fam Physician 49: 747. [PMC free article] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y et al. (2000). Plasma concentrations of a novel, adipose‐specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599. [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC et al. (2001). Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50: 1126–1133. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM (1993). Adipose expression of tumor necrosis factor‐alpha: direct role in obesity‐linked insulin resistance. Science 259: 87–91. [DOI] [PubMed] [Google Scholar]
- Hovens MMC, Snoe JD, Groeneveld Y, Frlich M, Tamsma JT, Huisman MV (2008). Effects of aspirin on serum C‐reactive protein and interleukin‐6 levels in patients with type 2 diabetes without cardiovascular disease: a randomized placebo‐controlled crossover trial. Diabetes Obes Metab 10: 668–674. [DOI] [PubMed] [Google Scholar]
- Igarashi A, Inoue S, Ishii T, Tsutani K, Watanabe H (2016). Comparative effectiveness of oral medications for pulmonary arterial hypertension. Int Heart J 57: 466–472. [DOI] [PubMed] [Google Scholar]
- Imachi H, Murao K, Cao WM, Ohyama T, Sato M, Sasaguri Y et al. (2001). Expression of HDL receptor, CLA‐1 in human smooth‐muscle cells and effect of interferon‐gamma on its regulation. Horm Metab Res 33: 389–393. [DOI] [PubMed] [Google Scholar]
- Jacobson TA (2009). Myopathy with statin‐fibrate combination therapy: clinical considerations. Nat Rev Endocrinol 5: 507–518. [DOI] [PubMed] [Google Scholar]
- Jafarnejad A, Bathaie SZ, Nakhjavani M, Hassan MZ (2008). Investigation of the mechanisms involved in the high‐dose and long‐term acetyl salicylic acid therapy of type I diabetic rats. J Pharmacol Exp Ther 324: 850–857. [DOI] [PubMed] [Google Scholar]
- Jamroz‐Wisniewska A, Wojcicka G, Horoszewicz K, Beltowski J (2007). Liver X receptors (LXRs). Part II: non‐lipid effects, role in pathology, and therapeutic implications. Postepy Hig Med Dosw (Online) 61: 760–785. [PubMed] [Google Scholar]
- Ji A, Wroblewski JM, Cai L, de Beer MC, Webb NR, van der Westhuyzen DR (2012). Nascent HDL formation in hepatocytes and role of ABCA1, ABCG1, and SR‐BI. J Lipid Res 53 (3): 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji A, Wroblewski JM, Webb NR, van der Westhuyzen DR (2014). The impact of phospholipid transfer protein on nascent HDL formation and remodeling. Arterioscler Thromb Vasc Biol 34: 1910–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XC, Beyer TP, Li Z, Liu J, Quan W, Schmidt RJ et al. (2003). Enlargement of high density lipoprotein in mice via liver X receptor activation requires apolipoprotein E and is abolished by cholesteryl ester transfer protein expression. J Biol Chem 278: 49072–49078. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Castrillo A, Bryan A, Laffitte BA, Mangelsdorf DJ, Tontonoz P (2003). Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med : 213–219. [DOI] [PubMed] [Google Scholar]
- Jung UJ, Seo YR, Ryu R, Choi MS (2016). Differences in metabolic biomarkers in the blood and gene expression profiles of peripheral blood mononuclear cells among normal weight, mildly obese and moderately obese subjects. Br J Nutr 116: 1022–1032. [DOI] [PubMed] [Google Scholar]
- Kappelle PJ, Bijzet J, Hazenberg BP, Dullaart RP (2011). Lower serum paraoxonase‐1 activity is related to higher serum amyloid a levels in metabolic syndrome. Arch Med Res 42: 219–225. [DOI] [PubMed] [Google Scholar]
- Kawashiri SY, Kawakami A, Yamasaki S, Imazato T, Iwamoto N, Fujikawa K et al. (2011). Effects of the anti‐interleukin‐6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol Int 31: 451–456. [DOI] [PubMed] [Google Scholar]
- Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC et al. (2005). ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab 1: 121–131. [DOI] [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G (2001). Adipose tissue tumor necrosis factor and interleukin‐6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280: E745–E751. [DOI] [PubMed] [Google Scholar]
- Khera AV, Cuchel M, De la Llera‐Moya M, Rodrigues A, Burke MF, Jafri K et al. (2011). Cholesterol efflux capacity, high‐density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kick E, Martin R, Xie Y, Flatt B, Schweiger E, Wang TL et al. (2015). Liver X receptor (LXR) partial agonists: biaryl pyrazoles and imidazoles displaying a preference for LXRβ. Bioorg Med Chem Lett 25: 372–377. [DOI] [PubMed] [Google Scholar]
- Kim YS, Ahn Y, Hong MH, Kim KH, Park HW, Hong YJ et al. (2007). Rosuvastatin suppresses the inflammatory responses through inhibition of c‐Jun N‐terminal kinase and nuclear factor‐kappaB in endothelial cells. J Cardiovasc Pharmacol 49: 376–383. [DOI] [PubMed] [Google Scholar]
- Kizhedath A, Wilkinson S, Glassey J (2016). Applicability of predictive toxicology methods for monoclonal antibody therapeutics: status quo and scope. Arch Toxicol. doi:10.1007/s00204‐016‐1876‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Shimomura I, Matsukawa Y, Kumada M, Takahashi M, Matsuda M et al. (2002). Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome diabetes. Diabetes 51: 2325–2328. [DOI] [PubMed] [Google Scholar]
- Kumar M, Rakesh S, Nagpal R, Hemalatha R, Ramakrishna A, Sudarshan V et al. (2013). Probiotic lactobacillus rhamnosus GG and Aloe vera gel improve lipid profiles in hypercholesterolemic rats. Nutrition 29: 574–579. [DOI] [PubMed] [Google Scholar]
- Lahaye C, Beaufrére AM, Boyer O, Drouot L, Soubrier M, Tournadre A (2014). Immune‐mediated myopathy related to anti 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase antibodies as an emerging cause of necrotizing myopathy induced by statins. Joint Bone Spine 81: 79–82. [DOI] [PubMed] [Google Scholar]
- Lalau JD (2010). Lactic acidosis induced by metformin: incidence, management and prevention. Drug Saf 33: 727–740. [DOI] [PubMed] [Google Scholar]
- Lan H, Pang L, Smith MM, Levitan D, Ding W, Liu L et al. (2010). Proprotein convertase subtilisin/kexin type 9 (PCSK9) affects gene expression pathways beyond cholesterol metabolism in liver cells. J Cell Physiol 224: 273–281. [DOI] [PubMed] [Google Scholar]
- Lautermann D, Braun J (2002). Ankylosing spondylitis – cardiac manifestations. Clin Exp Rheumatol 20 (6 Suppl 28): S11–S15. [PubMed] [Google Scholar]
- Le Lay S, Robichon C, Le Liepvre X, Dagher G, Ferre P, Dugail I (2003). Regulation of ABCA1 expression and cholesterol during adipose differentiation of 3 T3‐L1 cells. J Lipid Res 44: 1499–1507. [DOI] [PubMed] [Google Scholar]
- Lee JY, Parks JS (2005). ATP‐binding cassette transporter AI and its role in HDL formation. Curr Opin Lipidol 16: 19–25. [DOI] [PubMed] [Google Scholar]
- Lehtinen K (1993). Mortality and causes of death in 398 patients admitted to hospital with ankylosing spondylitis. Ann Rheum Dis 52: 174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WL, Hua LG, Qu P, Yan WH, Ming C, Jun YD et al. (2016). NLRP3 inflammasome: a novel link between lipoproteins and atherosclerosis. Arch Med Sci 12: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt L, Saarinen J, Kalkkinen N, Welgus H, Kovanen PT (1999). Matrix metalloproteinases‐3, ‐7, and ‐12, but not ‐9, reduce high density lipoprotein‐induced cholesterol efflux from human macrophage foam cells by truncation of the carboxyl terminus of apolipoprotein A‐I: PARALLEL losses of pre‐{beta} particles and the high affinity component of efflux. J Biol Chem 274: 22627–22634. [DOI] [PubMed] [Google Scholar]
- Liu AC, Lawn RM, Verstuyft JG, Rubin EM (1994). Human apolipoprotein A‐I prevents atherosclerosis associated with apolipoprotein[a] in transgenic mice. J Lipid Res 35: 2263–2267. [PubMed] [Google Scholar]
- Liu Z, Tang Q, Wen J, Tang Y, Huang D, Huang Y et al. (2016). Elevated serum complement factors 3 and 4 are strong inflammatory markers of the metabolic syndrome development: a longitudinal cohort study. Sci Rep 6: 18713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke YK, Singh S, Furberg CD (2009). Long‐term use of thiazolidinediones and fractures in type 2 diabetes: a meta‐analysis. CMAJ 180: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher TF, Taddei S, Kaski JC, Jukema JW, Kallend D, Münzel T et al. (2012). Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal‐VESSEL randomized clinical trial. Eur Heart J 33: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccubbin D, Koren MJ, Davidson M, Gavish D, Pasternak RC, Macdonell G et al. (2009). Flushing profile of extended‐release niacin/laropiprant versus gradually titrated niacin extended‐release in patients with dyslipidemia with and without ischemic cardiovascular disease. Am J Cardiol 104: 74. [DOI] [PubMed] [Google Scholar]
- Mahdy AK, Wonnerth A, Huber K, Wojta J (2012). Cardiovascular disease risk reduction by raising HDL cholesterol – current therapies and future opportunities. Br J Pharmacol 167: 1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U, Li L (2013). Molecular mechanisms responsible for the reduced expression of cholesterol transporters from macrophages by low‐dose endotoxin. Arterioscler Thromb Vasc Biol 33: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malle E, Stienmetz A, Raynes JG (1993). Serum amyloid A (SAA): an acute phase protein and apolipoprotein. Atherosclerosis 102: 131–146. [DOI] [PubMed] [Google Scholar]
- Mandosi E, Giannetta E, Filardi T, Lococo M, Bertolini C, Fallarino M et al. (2015). Endothelial dysfunction markers as a therapeutic target for sildenafil treatment and effects on metabolic control in type 2 diabetes. Expert Opin Ther Targets 19: 1617–1622. [DOI] [PubMed] [Google Scholar]
- Martin G, Duez H, Blanquart C, Berezowski V, Poulain P, Fruchart JC et al. (2001). Statin‐induced inhibition of the Rho‐signaling pathway activates PPAR‐a and induces HDL apoA‐I. J Clin Invest 107: 1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D, Staels B, Gautier T, Desrumaux C, Athias A, Le Guern N et al. (2004). Cholesteryl ester transfer protein modulates the effect of liver X receptor agonists on cholesterol transport and excretion in the mouse. J Lipid Res 45: 543–550. [DOI] [PubMed] [Google Scholar]
- Mathieu S, Dubost JJ, Tournadre A, Malochet‐Guinamand S, Ristori JM, Soubrier M (2010). Effects of 14 weeks of TNF alpha blockade treatment on lipid profile in ankylosing spondylitis. Joint Bone Spine 77: 50–52. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Okuda A, Watanabe Y, Miura T, Ozawa H, Tosaka A et al. (2015). Design and discovery of 2‐oxochromene derivatives as liver X receptor β‐selective agonists. Bioorg Med Chem Lett 25: 1274–1278. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD et al. (2013). Effect of interleukin‐6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo‐controlled study. Ann Rheum Dis 74: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney JM, Davidson MH, Shear CL, Revkin JH (2006). Efficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below‐average high‐density lipoprotein cholesterol levels on a background of atorvastatin. J Am Coll Cardiol 48: 1782–1790. [DOI] [PubMed] [Google Scholar]
- Miao B, Zondlo S, Gibbs S, Cromley D, Hosagrahara VP, Kirchgessner TG et al. (2004). Raising HDL cholesterol without inducing hepatic steatosis and hypertriglyceridemia by a selective LXR modulator. J Lipid Res 45: 1410–1417. [DOI] [PubMed] [Google Scholar]
- Miller GJ, Miller NE (1975). Plasma‐high‐density‐lipoprotein concentration and development of ischaemic heart‐disease. Lancet 1: 16–19. [DOI] [PubMed] [Google Scholar]
- Millar JS, Ikewaki K, Bloedon LT (2010). The effect of rosiglitazone on HDL metabolism in subjects with metabolic syndrome and low HDL. J Lipid Res 52: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T et al. (2011). Pioglitazone attenuates atherosclerotic, plaque inflammation in patients with impaired glucose tolerance or diabetes, a prospective, randomized, comparator‐controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging 4: 1110–1118. [DOI] [PubMed] [Google Scholar]
- Moon MK, Lee YJ, Kim JS, Kang DG, Lee HS (2009). Effect of caffeic acid on tumor necrosis factor‐alpha‐induced vascular inflammation in human umbilical vein endothelial cells. Biol Pharm Bull 32: 1371–1377. [DOI] [PubMed] [Google Scholar]
- Mortensen R (2001). C‐reactive protein, inflammation, and innate immunity. Immunol Res 24: 163–176. [DOI] [PubMed] [Google Scholar]
- Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP et al. (2006). Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation 113: 90–97. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Origuchi N, Matsunaga T, Kawai S, Hokari S, Nakamura H et al. (2000). Localization of oxidized HDL in atheromatous plaques and oxidized HDL binding sites on human aortic endothelial cells. Ann Clin Biochem 37: 179–186. [DOI] [PubMed] [Google Scholar]
- Nasr H, Torsney E, Poston RN, Hayes L, Gaze DC, Basser R et al. (2015). Investigating the effect of a single infusion of reconstituted high‐density lipoprotein in patients with symptomatic carotid plaques. Ann Vasc Surg 29: 1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW et al. (1991). Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest 88: 2039–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM (2001). A cell‐free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res 42: 1308–1317. [PubMed] [Google Scholar]
- Navarro‐Millán I, Charles‐Schoeman C, Yang S, Bathon JM, Bridges SL, Chen L et al. (2013). Changes in lipoproteins associated with methotrexate or combination therapy in early rheumatoid arthritis: results from the treatment of early rheumatoid arthritis trial. Arthritis Rheum 65: 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y, Tanaka K, Hara M, Hirao N, Tanaka H, Tobina T et al. (2015). Effects of home‐based bench step exercise on inflammatory cytokines and lipid profiles in elderly Japanese females: a randomized controlled trial. Arch Gerontol Geriatr 61: 443–451. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K (2007). Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471. [DOI] [PubMed] [Google Scholar]
- Nunes AK, Rapôso C, Rocha SW, Barbosa KP, Luna RL, da Cruz‐Höfling MA et al. (2015). Involvement of AMPK, IKβα‐NFκB, and eNOS in the sildenafil anti‐inflammatory mechanism in a demyelination model. Brain Res 1627: 119–133. [DOI] [PubMed] [Google Scholar]
- Ogata M, Tsujita M, Hossain MA, Akita N, Gonzalez FJ, Staels B et al. (2009). On the mechanism for PPAR agonists to enhance ABCA1 gene expression. Atherosclerosis 205: 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku H, Matsuura F, Koseki M, Sandoval JC, Yuasa‐Kawase M, Tsubakio‐Yamamoto K et al. (2007). Adiponectin deficiency suppresses ABCA1 expression and ApoA‐I synthesis in the liver. FEBS Lett 581: 5029–5533. [DOI] [PubMed] [Google Scholar]
- Onat A, Uzunlar B, Hergenç G, Yazici M, Sari I, Uyarel H et al. (2005). Cross‐sectional study of complement C3 as a coronary risk factor among men and women. Clin Sci (Lond) 108: 129–135. [DOI] [PubMed] [Google Scholar]
- O'Reilly M, Dillon E, Guo W, Finucane O, McMorrow A, Murphy A et al. (2016). High‐density lipoprotein proteomic composition, and not efflux capacity, reflects differential modulation of reverse cholesterol transport by saturated and monounsaturated fat diets. Circulation 133: 1838–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osto E, Doytcheva P, Corteville C, Bueter M, Dörig C, Stivala S et al. (2015). Rapid and body weight‐independent improvement of endothelial and high‐density lipoprotein function after Roux‐en‐Y gastric bypass: role of glucagon‐like peptide‐1. Circulation 131: 871–881. [DOI] [PubMed] [Google Scholar]
- Ottobelli CE, De Souza WM, Da Silva TP, Moresco RN, Moretto MB (2016). Adipocytokines, inflammatory and oxidative stress markers of clinical relevance altered in young overweight/obese subjects. Clin Biochem 49: 548–553. [DOI] [PubMed] [Google Scholar]
- Pasqui AL, Giovanni B, Luca P, Auteri A (2000). Complement activation in hypercholesterolemia. Nutr Metab Cardiovasc Dis 10: 137–142. [PubMed] [Google Scholar]
- Pasqui AL, Puccetti G, Bova M, Di Renzo F, Bruni M, Pastorelli A et al. (2002). Relationship between serum complement and different lipid disorders. Clin Exp Med 2: 33–38. [DOI] [PubMed] [Google Scholar]
- Peters MJ, van der Horst‐Bruinsma IE, Dijkmans BA, Nurmohamed MT (2004). Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin Arthritis Rheum 34: 585–592. [DOI] [PubMed] [Google Scholar]
- Phillips MC (2014). Molecular mechanisms of cellular cholesterol efflux. J Biol Chem 289: 24020–24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler WJ (2006). Adverse side‐effects to biological agents. Allergy 61: 912–920. [DOI] [PubMed] [Google Scholar]
- Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D et al. (2010). Aspirin for primary prevention of cardiovascular events in people with diabetes. J Am Coll Cardiol 55: 2878–2886. [DOI] [PubMed] [Google Scholar]
- Plump AS, Scott CJ, Breslow JL (1994). Human apolipoprotein A‐I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E‐deficient mouse. Proc Natl Acad Sci U S A 91: 9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitou C, Viguerie N, Cancello R, De Matteis R, Cinti S et al. (2005). Serum amyloid A: production by human white adipocyte and regulation by obesity and nutrition. Diabetologia 48: 519–528. [DOI] [PubMed] [Google Scholar]
- Rader DJ (2009). Lecithin: cholesterol acyltransferase and atherosclerosis, another high‐density lipoprotein story that doesn't quite follow the script. Circulation 120: 549–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader DJ, Daugherty A (2008). Translating molecular discoveries into new therapies for atherosclerosis. Nature 451: 904–913. [DOI] [PubMed] [Google Scholar]
- Rashid S, Barrett PH, Uffelman KD, Watanabe T, Adeli K, Lewis GF (2002). Lipolytically modified triglyceride‐enriched HDLs are rapidly cleared from the circulation. Arterioscler Thromb Vasc Biol 22: 483–487. [DOI] [PubMed] [Google Scholar]
- Rashid S, Genest J (2007). Effect of obesity on high‐density lipoprotein metabolism. Obesity (Silver Spring) 15: 2875–2888. [DOI] [PubMed] [Google Scholar]
- Reiss AB, Carsons SE, Anwar K, Rao S, Edelman SD, Zhang H et al. (2008). Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP‐1 monocyte/macrophages. Arthritis Rheum 58: 3675–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I et al. (2000a). Regulation of mouse sterol regulatory element‐binding protein‐1c gene (SREBP‐1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev 14: 2819–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K et al. (2000b). Regulation of absorption and ABC1‐mediated efflux of cholesterol by RXR heterodimers. Science 289: 1524–1529. [DOI] [PubMed] [Google Scholar]
- Rhoads GG, Gulbrandsen CL, Kagan A (1976). Serum lipoproteins and coronary heart disease in a population study of Hawaii Japanese men. N Engl J Med 294: 293–298. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH (1997). Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336: 973–979. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Thuren T, Zalewski A, Libby P (2011). Interleukin‐1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the canakinumab anti‐inflammatory thrombosis outcomes study (CANTOS). Am Heart J 162: 597–605. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J et al. (2012). Effects of interleukin‐1β inhibition with canakinumab on hemoglobin A1c, lipids, C‐reactive protein, interleukin‐6, and fibrinogen: a phase IIb randomized, placebo‐controlled trial. Circulation 126: 2739–2748. [DOI] [PubMed] [Google Scholar]
- Rinninger F, Kaiser T, Mann WA, Meyer N, Greten H, Beisiegel U (1998). Lipoprotein lipase mediates an increase in the selective uptake of high density lipoprotein‐associated cholesteryl esters by hepatic cells in culture. J Lipid Res 39: 1335–1348. [PubMed] [Google Scholar]
- Rinninger F, Brundert M, Brosch I, Donarski N, Budzinski RM, Greten H (2001). Lipoprotein lipase mediates an increase in selective uptake of HDL‐associated cholesteryl esters by cells in culture independent of scavenger receptor BI. J Lipid Res 42: 1740–1751. [PubMed] [Google Scholar]
- Rodríguez‐Hernández H, Simental‐Mendía LE, Rodríguez‐Ramírez G, Reyes‐Romero MA (2013). Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol 2013: 678159. doi:10.1155/2013/678159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Jimenez NA, Garcia‐Gonzalez CE, Ayala‐Lopez KP, Trujillo‐Hernandez B, Aguilar‐Chavez EA, Rocha‐Muñoz AD et al. (2014). Modifications in lipid levels are independent of serum TNF‐α in rheumatoid arthritis: results of an observational 24‐week cohort study comparing patients receiving etanercept plus methotrexate or methotrexate as monotherapy. Biomed Res Int 2014: 510305. doi:10.1155/2014/510305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros E, Nuñez I, Pérez‐Heras A, Serra M, Gilabert R, Casals E et al. (2004). A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation 109: 1609–1614. [DOI] [PubMed] [Google Scholar]
- Rosenson RS (2004). Current overview of statin‐induced myopathy. Am J Med 116: 408. [DOI] [PubMed] [Google Scholar]
- Rosenson RS (2010). Functional assessment of HDL: moving beyond static measures for risk assessment. Cardiovasc Drug Ther 24: 71–75. [DOI] [PubMed] [Google Scholar]
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM (1991). Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 353: 265–267. [DOI] [PubMed] [Google Scholar]
- Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE et al. (2014). Spectrum of statin hepatotoxicity: experience of the drug‐induced liver injury network. Hepatology 60: 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth MR, Port AM, Shah M, Bourland AC, Istfan NW, Nelson KP et al. (2013). Consuming a hypocaloric high fat low carbohydrate diet for 12 weeks lowers C‐reactive protein, and raises serum adiponectin and high density lipoprotein‐cholesterol in obese subjects. Metabolism 62: 1779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden M, Dicker A, van Harmelen V, Hauner H, Brunnberg M, Perbeck L et al. (2002). Mapping of early signaling events in tumor necrosis factor‐alpha‐mediated lipolysis in human fat cells. J Biol Chem 277: 1085–1091. [DOI] [PubMed] [Google Scholar]
- Rye KA, Duong MN (2000). The influence of phospholipid depletion on the size, structure and remodeling of reconstituted high density lipoproteins. J Lipid Res 41: 1640–1650. [PubMed] [Google Scholar]
- Sabate J, Wien M (2013). Consumption of nuts in the prevention of cardiovascular disease. Curr Nutr Rep 2: 258–266. [Google Scholar]
- Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM et al. (2015). Lack of efficacy of resveratrol on C‐reactive protein and selected cardiovascular risk factors ‐results from a systematic review and meta‐analysis of randomized controlled trials. Int J Cardiol 189: 47–55. [DOI] [PubMed] [Google Scholar]
- Sahebkar A, Di Giosia P, Stamerra CA, Grassi D, Pedone C, Ferretti G et al (2016). Effect of monoclonal antibodies to PCSK9 on high‐sensitivity C‐reactive protein levels: a meta‐analysis of 16 randomized controlled treatment arms. Br J Clin Pharmacol 81: 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo D, Trischuk TC, Chan T, Drover VA, Ho S, Chimini G et al. (2004). ABCA1‐dependent lipid efflux to apolipoprotein A‐I mediates HDL particle formation and decreases VLDL secretion from murine hepatocytes. J Lipid Res 45: 1122–1131. [DOI] [PubMed] [Google Scholar]
- Santanam N, Parthasarathy S (2007). Aspirin is a substrate for paraoxonase‐like activity: implications in atherosclerosis. Atherosclerosis 191: 272–275. [DOI] [PubMed] [Google Scholar]
- Schaefer EJ, Asztalos BF (2006). The effects of statins on high‐density lipoproteins. Curr Atheroscler Rep 8: 41–49. [DOI] [PubMed] [Google Scholar]
- Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L et al. (2000). Role of LXRs in control of lipogenesis. Genes Dev 14: 2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J et al. (2012). Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- Si Y, Zhang Y, Zhao J, Guo S, Zhai L, Yao S et al. (2014). Niacin inhibits vascular inflammation via downregulating nuclear transcription factor‐κB signaling pathway. Mediators Inflamm 2014, Article ID 263786, 12 pages. doi:10.1155/2014/263786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaraja RR, Van Eck M, Bissada N, Zimetti F, Collins HL, Hildebrand RB et al. (2006). Both hepatic and extrahepatic ABCA1 have discrete and essential functions in the maintenance of plasma high‐density lipoprotein cholesterol levels in vivo. Circulation 114: 1301–1309. [DOI] [PubMed] [Google Scholar]
- Singh S, Loke YK, Furberg CD (2007). Thiazolidinediones and heart failure: a teleo‐analysis. Diabetes Care 30: 2148–2153. [DOI] [PubMed] [Google Scholar]
- Singh U, Devaraj S, Jialal I, Siegel D (2008). Comparison effect of atorvastatin (10 versus 80 mg) on biomarkers of inflammation and oxidative stress in subjects with metabolic syndrome. Cardiol 102: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöholm K, Palming J, Olofsson LE, Gummesson A, Svensson PA, Lystig TC et al. (2005). A microarray search for genes predominantly expressed in human omental adipocytes: adipose tissue as a major production site of serum amyloid A. J Clin Endocrinol Metab 90: 2233–2239. [DOI] [PubMed] [Google Scholar]
- Smith JD (2010). Myeloperoxidase, inflammation, and dysfunctional high‐density lipoprotein. J Clin Lipidol 4: 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies LE, White CR, Maheshwari A, Palgunachari MN, Anantharamaiah GM, Chaddha M et al. (2010). Apolipoprotein A‐I mimetic 4F alters the function of human monocyte‐derived macrophages. Am J Physiol Cell Physiol 298: C1538–C1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone H, Shimano H, Shu M, Nakakuki M, Takahashi A, Sakai M et al. (2004). Statins downregulate ATP‐binding‐cassette transporter A1 gene expression in macrophages. Biochem Biophys Res Commun 316: 790–794. [DOI] [PubMed] [Google Scholar]
- Sosin M, Handa S (2003). Low dose methotrexate and bone marrow suppression. BMJ 326: 266–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrier M, Jouanel P, Mathieu S, Poujol D, Claus D, Dubost JJ et al. (2008). Effects of anti‐tumor necrosis factor therapy on lipid profile in patients with rheumatoid arthritis. Joint Bone Spine 75: 22–24. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto A, Salgado E, Maneiro JR, Mera A, Carmona L, Gómez‐Reino JJ (2015). Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta‐analysis. Arthritis Rheumatol 67: 117–127. [DOI] [PubMed] [Google Scholar]
- Spite M, Serhan CN (2010). Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res 107: 1170–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG (2012). The inflammasome puts obesity in the danger zone. Cell Metab 15: 10–18. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Pritchard DK, Becker L, Hoofnagle AN, Tanimura N, Bammler TK et al. (2010). High‐density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation 122: 1919–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K, Okada A, Hamamoto S, Unno R, Kobayashi T, Ando R et al. (2016). Differential roles of peroxisome proliferator‐activated receptor‐α and receptor‐γ on renal crystal formation in hyperoxaluric rodents. PPAR Res 2016: 9605890. doi:10.1155/2016/9605890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall AR, Krumholz S, Olivecrona T, Deckelbaum RJ (1985). Plasma phospholipid transfer protein enhances transfer and exchange of phospholipids between very low density lipoproteins and high density lipoproteins during lipolysis. J Lipid Res 26: 842–851. [PubMed] [Google Scholar]
- Tancevski I, Wehubgerm A, Schgoer W, Eller P, Cuzzocrea S, Foeger B et al. (2006). Aspirin regulates expression and function of scavenger receptor‐BI in macrophages: studies in primary human macrophages and in mice. FASEB J 20: 1328–1335. [DOI] [PubMed] [Google Scholar]
- Tietge UJF, Maugeais C, Cain W, Grass D, Glick JM, deBeer FC et al. (1999). Overexpression of secretory phospholipase A2 causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl esters and apolipoprotein A‐I. Arterioscler Thromb Vasc Biol 19: 1284–1290.10323781 [Google Scholar]
- Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A et al. (2005). Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA‐I. J Clin Invest 115: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M et al. (2013). High‐density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol 7: 484–525. [DOI] [PubMed] [Google Scholar]
- Toth PP, Barylski M, Nikolic D, Rizzo M, Montalto G, Banach M (2014). Should low high‐density lipoprotein cholesterol (HDL‐C) be treated? Best Pract Res Clin Endocrinol Metab 28: 353–368. [DOI] [PubMed] [Google Scholar]
- Tragiannidis A, Kyriakidis I, Zündorf I, Groll AH (2016). Invasive fungal infections in pediatric patients treated with tumor necrosis alpha (TNF‐α) inhibitors. Mycoses . doi:10.1111/myc.12576. [DOI] [PubMed] [Google Scholar]
- Trieb M, Horvath A, Birner‐Gruenberger R, Spindelboeck W, Stadlbauer V, Taschler U et al. (2016). Liver disease alters high‐density lipoprotein composition, metabolism and function. Biochim Biophys Acta 861: 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE et al. (2014). Rosuvastatin for sepsis‐associated acute respiratory distress syndrome. N Engl J Med 370: 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SM, Murphy EJ, Neese RA, Antelo F, Thomas T, Agarwal A et al. (2003). Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am J Physiol Endocrinol Metab 285: E790–E803. [DOI] [PubMed] [Google Scholar]
- Tuvdendorj D, Chandalia M, Batbayar T, Saraf M, Beysen C, Murphy EJ et al. (2013). Altered subcutaneous abdominal adipose tissue lipid synthesis in obese, insulin‐resistant humans. Am J Physiol Endocrinol Metab 305: E999–E1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvdendorj D, Munoz AO, Ruiz‐Barros V, Schwarz JM, Montalto G, Chandalia M et al. (2016). In vivo triglyceride synthesis in subcutaneous adipose tissue of humans correlates with plasma HDL parameters. Atherosclerosis 251: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Chiesa G, Saku K (2015). High‐Density Lipoprotein‐Targeted Therapy and Apolipoprotein A‐I Mimetic Peptides. Circ J 79: 2523–2528. [DOI] [PubMed] [Google Scholar]
- van de Ree MA, Huisman MV, Princen HM, Meinders AE, Kluft C, DALI‐Study Group (2003). Strong decrease of high sensitivity C‐reactive protein with high‐dose atorvastatin in patients with type 2 diabetes mellitus. Atherosclerosis 166: 129–135. [DOI] [PubMed] [Google Scholar]
- van Eijk IC, de Vries MK, Levels JH, Peters MJ, Huizer EE, Dijkmans BA et al. (2009). Improvement of lipid profile is accompanied by atheroprotective alterations in high‐density lipoprotein composition upon tumor necrosis factor blockade: a prospective cohort study in ankylosing spondylitis. Arthritis Rheum 60: 1324–1330. [DOI] [PubMed] [Google Scholar]
- van Lenten BJ, Hama SY, De Beer FC, Stafforini DM, McIntyre TM, Prescott SM et al. (1995). Anti‐inflammatory HDL becomes pro‐inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 96: 2758–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S et al. (2012). Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 367: 508–519. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Arrese EL, Soulages JL (2007). Stimulation of lipolysis enhances the rate of cholesterol efflux to HDL in adipocytes. Mol Cell Biochem 302: 241–248. [DOI] [PubMed] [Google Scholar]
- Wagner JD, Shadoan MK, Zhang L, Ward GM, Royer LJ, Kavanagh K et al. (2010). A selective peroxisome proliferator‐activated receptor alpha agonist, CP‐900691, improves plasma lipids, lipoproteins, and glycemic control in diabetic monkeys. J Pharmacol Exp Ther 333: 844–853. [DOI] [PubMed] [Google Scholar]
- Walley KR, Thain KR, Russell JA, Reilly MP, Meyer NJ, Ferguson JF et al. (2014). PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med 6: 258ra143. doi:10.1126/scitranslmed.3008782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Dahle MK, Agren J, Myhre AE, Reinholt FP, Foster SJ et al. (2006). Activation of the liver X receptor protects against hepatic injury in endotoxemia by suppressing Kupffer cell activation. Shock 25: 141–146. [DOI] [PubMed] [Google Scholar]
- Wang W, Song W, Wang Y, Chen L, Yan X (2013). HMG‐CoA reductase inhibitors, simvastatin and atorvastatin, downregulate ABCG1‐mediated cholesterol efflux in human macrophages. J Cardiovasc Pharmacol 62: 90–98. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Freestone S, Allen MJ, Muirhead GJ (1999). Sildenafil citrate and blood‐pressure‐lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 83: 21C–28C. [DOI] [PubMed] [Google Scholar]
- Wefers JF, Woodlief TL, Carnero EA, Helbling NL, Anthony SJ, Dubis GS et al. (2016). Relationship among physical activity, sedentary behaviors, and cardiometabolic risk factors during gastric bypass surgery‐induced weight loss. Surg Obes Relat Dis S1550‐7289(16)30675‐X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesnigk J, Bruyndonckx L, Hoymans VY, De Guchtenaere A, Fischer T, Schuler G et al. (2016). Impact of lifestyle intervention on HDL‐induced eNOS activation and cholesterol efflux capacity in obese adolescent. Cardiol Res Pract 2016: 2820432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM (1997). Cardiovascular risk factors in women. Proc Nutr Soc 56: 383–391. [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Chinkes DL (2005). Isotope tracers in metabolic research: principles and practice of kinetic analysis, 2nd edn. Wiley‐Liss: New York, New York, USA. [Google Scholar]
- Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ (2008). The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 196: 180–189. [DOI] [PubMed] [Google Scholar]
- Xu H, Burnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. (2003). Chronic inflammation in fat plays a crucial role in the development of obesity‐related insulin resistance. J Clin Invest 112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Liu Y, Kwok S, Hama S, France M, Eatough R et al. (2015). Effect of extended‐release niacin on high‐density lipoprotein (HDL) functionality, lipoprotein metabolism, and mediators of vascular inflammation in statin‐treated patients. J Am Heart Assoc 4: e001508. doi:10.1161/JAHA.114.001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wang HW, Bowman RL, Joyce JA (2016). STAT3 and STAT6 signaling pathways synergize to promote cathepsin secretion from macrophages via IRE1α activation. Cell Rep 16: 2914–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshifumi S (2016). Metformin and inflammation: its potential beyond glucose‐lowering effect. Endocr Metab Immune Disord Drug Targets 15: 196–205. [DOI] [PubMed] [Google Scholar]
- Zannis VI, Chroni A, Krieger M (2006). Role of apoA‐I, ABCA1, LCAT, and SR‐BI in the biogenesis of HDL. J Mol Med (Berl) 84: 276–294. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ (2003). Overexpression of apolipoprotein A‐I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation 108: 661–663. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, McGillicuddy FC, Hinkie CC, O'Neill S, Glick JM, Rothblat GH et al. (2010). Adipocyte modulation of high‐density lipoprotein cholesterol. Circulation 121: 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Gao F, Luo H, Zhang CT, Zhang R (2015). Differential response in levels of high‐density lipoprotein cholesterol to one‐year metformin treatment in prediabetic patients by race/ethnicity. Cardiovasc Diabetol 14: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xie H, Zheng X, Chai Y, Tang Z, Chen H et al. (2017). Salicylic acid retention impairs aspirin reactivity in type 2 diabetes. Eur J Pharmacol 794: 234–245. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Weir MA, Manno M, Cordy P, Gomes T, Hackam DG et al. (2012). New fibrate use and acute renal outcomes in elderly adults: a population‐based study. Ann Intern Med 156: 560. [DOI] [PubMed] [Google Scholar]
- Zuckerman SH, Panousis C, Mizrahi J, Evans G (2000). The effect of gamma‐interferon to inhibit macrophage‐high density lipoprotein interactions is reversed by 15‐deoxy‐delta12,14‐prostaglandin J2. Lipids 35: 1239–1247. [DOI] [PubMed] [Google Scholar]


