Abstract
Toxocariasis is a zoonotic disease usually caused by dog and cat roundworms, Toxocara canis and T. cati. Detection and diagnosis is difficult in paratenic and accidental hosts, including humans, as they cannot be detected through conventional methods such as fecal examination. Diagnosis therefore relies on immunological methods and molecular methods such as enzyme-linked immunosorbent assay (ELISA) and Western Blot, which are both time-consuming and requires sophisticated equipment. In the Philippines, only a few studies are available on Toxocara seroprevalence. Therefore, there is a need to adapt methods for serodiagnosis of Toxocara infection in humans for the Philippine setting. A dot enzyme linked immunosorbent assay (dot-ELISA) was standardized using T. canis excretory-secretory antigens. Test sera were collected from laboratory rats (Sprague–Dawley strain) experimentally infected with embryonated eggs of T. canis and Ascaris suum as well as rice field rats naturally infected with Taenia taeniaeformis and Nippostrongylus sp. Optimum conditions used were 20 µg/ml antigen concentration and 1:10 serum dilution. The sensitivity, specificity, positive, and negative predictive values were 90% (95% CI 55.5–99.7%), 100% (95% CI 69.2–100.0%), 100% (95% CI 66.4–100%), and 90.9% (95% CI 58.7–99.8%), respectively. Dot-ELISA has the potential to be developed as a cheaper, simpler, and more practical method for detection of anti-Toxocara antibodies on accidental hosts. This is a preliminary study conducted on experimental animals before optimization and standardization for human serum samples.
Keywords: Toxocara canis, Rattus norvegicus, Toxocariasis, Dot ELISA, Zoonosis, Paratenic hosts, Accidental host
Introduction
Toxocara canis (dog roundworm) and Toxocara cati (cat roundworm) are nematodes found to infect dogs and cats, respectively. These are soil-transmitted helminths that may also affect humans, causing toxocariasis (Woodhall et al. 2014). This disease is underdiagnosed and is considered as a Neglected Parasitic Infection by the Centers for Disease Control and Prevention (2013). Humans become infected with Toxocara through accidental ingestion of viable embryonated eggs that are present in the soil or contaminated hands and fomites. This disease can also be acquired, although rarely, from ingestion of undercooked paratenic hosts, like chicken and rabbit, that are contaminated with the larvae (Moreira et al. 2014). Children are at a higher risk of infection due to geophagy as well as playing with soil that may be contaminated with eggs (Woodhall et al. 2014). The clinical manifestations of this disease are non-specific and may vary widely ranging from asymptomatic cases to systemic infections which may be attributed to the size of inoculum and the host response against migrating larvae (Pawlowski 2001). In many cases, infected hosts were asymptomatic; however, it can also be clinically manifested in four forms based on organs and tissues affected: visceral larva migrans, ocular larva migrans, neurological toxocariasis, and covert toxocariasis (Despommier 2003; Magnaval et al. 2001; Roldan et al. 2010).
Toxocara eggs cannot be detected through fecal examination since the adult worms do not develop in humans. Definitive diagnosis is challenging as it requires direct observation of the larva in tissues. Hence, immunological and molecular methods, such as polymerase chain reaction, have been developed for diagnosing the disease (Watthanakulpanich 2010; Rai et al. 1997; Wu et al. 1997; Van De et al. 2013). The most commonly used immunological tests are the enzyme-linked immunosorbent assay (ELISA) and Western blot. However, these tests are time-consuming and require sophisticated equipment (Watthanakulpanich 2010).
The seroprevalence of toxocariasis worldwide is high, especially among tropical and developing countries. Low socioeconomic status is also correlated with high prevalence of this disease (Rubinsky-Elefant et al. 2010). In the Philippines, there are limited studies available on Toxocara. A study done in Los Baños, Laguna, found a high helminth egg contamination rate (43%) in soil (Fajutag and Paller 2013). The same study also revealed a high serological prevalence (49%) in primary school children by the detection of anti-Toxocara antibodies in serum. Another study conducted by Paller and de Chavez (2014) reported that 31% of the soil samples collected in rural towns of Laguna, Philippines were positive for eggs of soil-transmitted helminths; seventy-seven percent of these were Toxocara eggs. Because of this, there is a need to efficiently diagnose neglected parasitic infections that undermine the health of the public, especially the younger age groups. Furthermore, it is not a far off notion that toxocariasis is already present and highly prevalent in the Philippines because of the presence of stray cats and dogs in the country and the close relations of humans with cats and dogs.
As of the moment, there is no local method for the detection of the said disease. For this reason, there is a need to create a sensitive and highly specific local method for the immunological diagnosis of toxocariasis in human serum. This study aimed to develop a dot ELISA for the immunological diagnosis of toxocariasis using a rat model.
Materials and methods
Ethical considerations
Rats were maintained and infected in accordance with institutional guidelines. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of the Philippines Los Baños with assigned protocol number 2015-0051.
Preparation of Toxocara canis egg culture
Female T. canis adults were acquired from dogs dewormed with pyrantel embonate. The female worms were dissected at the anterior non-bifurcated and bifurcated portions of the uterus. To prevent fungal growth, the eggs obtained were placed in a 2% formalin solution for 35 days until embryonation (Alcantara-Neves et al. 2008). This suspension was stirred daily for aeration. Some of these eggs were used for the artificial infection of Sprague–Dawley rats, while others were hatched into larvae for obtaining excretory-secretory antigens.
Artificial infection of rats
A total of 54 male Sprague–Dawley rats, approximately 5–8 weeks old, were used. Thirty-three of these were orally infected with 500 T. canis embryonated eggs. Ten of these rats were used as positive control, another ten rats were orally infected with embryonated Ascaris suum eggs for cross-reactivity tests while two rats were left uninfected and used to optimize the dot ELISA. Three rats were sacrificed each observation day (3, 5, 8, 10, 15, 30, and 60 days post infection). This was done to determine when the anti-T. canis antibodies can be detected in sera.
The ten rats used as positive control were dissected. Brains were divided into small portions and viewed under a microscope using squash method to observe presence of larvae. The lungs, liver, heart, kidneys, eyes, masseters, and diaphragm were also checked for the presence of larvae. These organs were subjected to artificial tissue digestion in a digestive solution (pepsin, 5 g; 37% HCl, 10 ml in 1000 ml distilled water) at 37 °C with constant stirring using a magnetic stirrer (Janecek et al. 2014; Zibaei et al. 2010). The sedimental liquid was centrifuged at 1500 rpm for 2 min. The resulting supernatant was discarded and the sediments were viewed under a light microscope at 200x and 400x magnifications. All of the positive controls were found to be infected with the larvae and were included in the study.
Collection of sera from rats with natural helminth infection
A total of 19 rice field rats were dissected and examined for intestinal parasites. Among these rats, only those found to have single infection were used. Three of these were infected with Taenia taeniaeformis while six were infected with Nippostrongylus sp. Sera from rats infected with Ascaris suum, T. taeniaeformis, and Nippostrongylus sp. were used to test for cross-reactions.
Collection of sera from the artificially infected rats
Five ml of blood from each rat were collected via cardiac puncture. They were then placed in serum-separating tubes. The collected blood was then centrifuged at 3300 rpm for 10 min.
Preparation of Toxocara canis excretory-secretory (ES) antigens
Decortication
The method of Thomas et al. (2014) was used, with some modifications. Egg cultures were transferred to centrifuge tubes and were washed in distilled water by centrifugation for four times at 2000 rpm for 5 min to remove the formalin in the solution. The eggs were then soaked in an equal volume of 4% sodium hypochlorite (NaOCl) in order to remove the outer mamillated membrane of the eggs. After 2 h, PBS was added to the solution and was centrifuged at 1400 rpm for 2 min to remove the chlorine. The egg suspension was then transferred to RPMI 1640 containing 100 iu/ml penicillin and 100 ug/ml streptomycin, and was gently shaken in order to induce hatching. Afterwards, the suspension was incubated overnight at 37 °C in 5% CO2 to further induce hatching.
The suspension containing hatched larvae was centrifuged at 2000 rpm for 5 min. Larvae were collected and then gently layered on 5 ml of Histopaque (density 1.077 g/ml) for density gradient centrifugation. The suspension was then centrifuged for 30 min at 1600 rpm. The viable larvae at the bottom of the tube were collected.
Larval culture
Viable larvae were placed in culture flasks containing RPMI 1640 with 100 iu/ml penicillin and 100 ug/ml streptomycin to prevent bacterial growth. The larval concentration was then adjusted to 1000 larvae/ml. Larval cultures were maintained at 37 °C in 5% CO2. The culture media where the larvae release their excretory-secretory (ES) antigens were collected and replaced every week.
ES antigen collection and concentration
Collected media were placed in Amicon columns (10 kDa cutoff) and centrifuged at 3000 rpm for 30 min at 4 °C to concentrate the antigens. The ES antigens were reconcentrated three times using PBS to a final volume of 200 µl (Maizels et al. 1984). Protein concentration was measured using Bradford assay and its absorbance was measured under 595 nm.
Dot ELISA test
The dot ELISA was performed using the method of Bojanich et al. (2012) with some modifications. The optimal concentration of the ES antigen and the serum dilution were determined via checkerboard titration. ES antigen concentrations were tested on different dilutions of sera. Two µl of varying concentrations of T. canis ES antigen in PBS (10, 20, and 30 µg/ml) diluted in carbonate-bicarbonate buffer were dotted onto nitrocellulose membranes (0.45 µm pore size). After air drying for 30 min, the membranes were incubated with the blocking solution (PBS with 1% skim milk) for 1 h to block nonspecific binding sites.
The membranes were then washed with PBS with 0.05% Tween 20 (PBS-T) for 5 min. Positive T. canis sera was diluted in PBS with 1% skim milk. Twelve µl of the diluted sera (1:10, 1:20, and 1:30) was spotted. After 30 min, the strips were washed again with PBS-T three times to remove unbound primary antibodies. Twelve µl of goat anti-rat IgG conjugated with horseradish peroxidase (1:1000 dilution in PBS) was spotted. After 30 min, the membranes were washed again with PBS-T to remove unbound secondary antibody. The membranes were then placed in a Petri dish containing freshly prepared substrate (4-chloro-1-naphthol and methanol in PBS containing 30% H2O2). After an hour, the reaction was stopped by washing the membranes several times with distilled water. Appearance of color indicates a positive result, while the absence of color was considered a negative result.
The antigen concentration used in the succeeding assays was 20 µg/ml, while the serum dilution used was 1:10. This antigen concentration was preferred over 30 µg/ml because the resulting color reactions had the same intensity. The 1:10 serum dilution was chosen because it produced the most distinct color reaction.
Statistical analysis
The dot ELISA developed was not compared with the reference standard because the number of infected and uninfected individuals were known. The sensitivity, specificity, positive predictive values, negative predictive values of the dot ELISA were determined. The 95% confidence intervals of the resulting values were computed.
Sensitivity was calculated as the percentage of serum samples of rats artificially infected with Toxocara that had a positive result in dot ELISA. On the other hand, specificity was computed as the percent of sera of uninfected rats showing negative results.
The positive predictive value was computed as the proportion of rats infected with T. canis showing positive results over the total number of rats presenting positive results. This describes the chances of having the disease when the test presents a positive result. On the other hand, the negative predictive value was computed as the proportion of uninfected rats showing negative results over the total number of rats showing negative results. This describes the chances of being uninfected when the test shows a negative result.
Results and discussion
Diagnosis of toxocariasis is very challenging as the parasite cannot be detected through fecal examination. Infection may manifest as a variety of syndromes. Furthermore, asymptomatic cases of the disease may also occur. Thus, the signs and symptoms of this disease are nonspecific and may be similar to those of other diseases. There is lack of surveillance of toxocariasis in the Philippines despite the close proximity of humans with dogs and cats, as well as an abundance of stray animals.
A study was conducted to develop a dot enzyme-linked immunosorbent assay (ELISA) for the diagnosis of T. canis infection using the rat as a model.
Dot-ELISA test
Toxocara canis eggs were used either for infecting Sprague–Dawley rats, or hatched into larvae and cultured in RPMI to obtain excretory-secretory antigens. The optimum antigen concentration used in the assay was 20 µg/ml, while the best serum dilution was 1:10. The sensitivity, specificity, positive, and negative predictive values of the test were detected as 90, 100, 100, and 90.9%, respectively.
Different sera were tested in the dot ELISA. The presence of anti-T. canis antibodies from T. canis-infected sera were determined using the assay. Nine of ten T. canis-infected sera exhibited a blue-violet color in the assay (Fig. 1). Hence, the dot ELISA has a 90% sensitivity (95% CI 55.5–99.7%). The light color of the positive results in the assay may have been due to a low concentration of antibodies present in the sera of the T. canis-infected rats. Moreover, it is also possible that the negative result obtained from the T. canis-infected sera may have been due to extremely low, undetectable levels of antibody present.
Fig. 1.
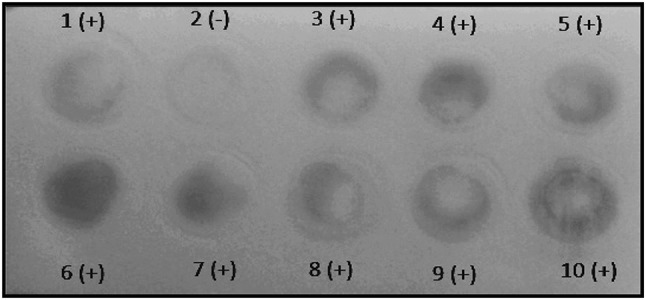
Dot ELISA results of T. canis-infected sera showing positive results except dot no. 2
These results can be explained by the dosage of eggs administered to the rats. Furthermore, the larvae may have also migrated to immunologically privileged sites such as the brain, where immune reactions are infrequently observed (Holland and Hamilton 2013). In these sites, they are not exposed to cellular responses and cannot be encapsulated as they cannot be detected by the host’s lymphoid system because of the protection offered by the blood–brain barrier (Dunsmore et al. 1983).
None of the ten uninfected laboratory rats showed positive results, resulting to 100% (95% CI 69.2–100%) specificity. The high specificity of the assay developed may have been due to the use of excretory-secretory (ES) antigens. Camargo et al. (1992) compared the use ES and somatic antigens in dot ELISA for detecting T. canis infection in humans. Both ES and somatic antigens displayed 100% sensitivity. However, the assay employing somatic antigens resulted to a specificity of only 85.7%, while the assay that contained ES antigens resulted to a specificity of 95.3%. This was because somatic antigens contain various nonspecific antigens, making cross-reactions more frequent (de Savigny and Tizard 1977).
On the other hand, ten out of the ten T. canis-infected rats had shown positive results, giving the test a positive predictive value of 100%. Meanwhile, one out of ten uninfected rats showed a positive result, thus giving the test a negative predictive value of 90.9%.
Determination of cross-reactions using sera of rats infected with other helminths
Sera from animals artificially and naturally infected with other helminths such as Ascaris suum, T. taeniaeformis, and Nippostrongylus sp. were used to test for cross-reactivity. None of the nine Ascaris suum-infected, three T. taeniaeformis-infected, and six Nippostrongylus sp.-infected rats showed positive reactions. Thus, no cross-reactions occurred. Results of the sera used to determine sensitivity, specificity, and cross-reactions are summarized in Table 1.
Table 1.
Summary of dot ELISA results using Toxocara canis-infected and uninfected, Ascaris suum-infected Taenia taeniaeformis-infected, and Nippostronglyus sp.-infected rat sera for the detection of Toxocara canis infection
| Sera tested | Total number of sera | Number of positive results | Number of negative results |
|---|---|---|---|
| Uninfected sera (negative control) | 10 | 0 | 10 |
| Toxocara canis-infected sera (positive control) | 10 | 9 | 1 |
| Ascaris suum-infected sera | 9 | 0 | 9 |
| Taenia taeniaeformis-infected sera | 3 | 0 | 3 |
| Nippostrongylus sp.-infected sera | 6 | 0 | 6 |
However, authors have reported cross-reactions in ELISA which used ES antigens for the detection of toxocariasis using human sera. Jacquier et al. (1991) reported cross-reactions with Trichinella, Stongyloides, and Fasciola species. Another author also reported cross-reactions with sera from ascariasis, trichinellosis, strongyloidiasis, filariasis, and anisakiasis patients. Patients infected with Strongyloides stercoralis, Fasciola hepatica, and Taenia sp. also exhibited positive results in a study by Roldan et al. (2006).
Camargo et al. (1992) preabsorbed human sera in Ascaris suum female extract to increase the sensitivity of both ELISA and dot ELISA which used somatic and ES antigens. This was done to remove ES antigens that cross-react with Ascaris antigens. The ELISA and dot ELISA which used somatic antigens and unabsorbed sera had 100% sensitivity, and 57.1 and 85.7% specificity, respectively. Upon absorbing the sera with A. suum female extract, the sensitivity of the ELISA with somatic antigen decreased to 95.4%, while the sensitivity of the dot ELISA with somatic antigen remained at 100%. However, the specificities increased to 83.7 and 95.3%, respectively.
On the other hand, the ELISA and dot ELISA which used ES antigens and unabsorbed sera showed 100 and 95.4% sensitivity, respectively, while the specificities were 90.5 and 95.3%, respectively. However, the specificity of the dot ELISA increased to 97.6%, while that of the ELISA was retained. The sensitivity of the ELISA decreased to 95.4%, while that of the dot ELISA was retained.
Taenia taeniaeformis is a member of the Phylum Platyhelminthes, Class Cestoda, Order Cyclophyllidea, Family Taeniidae (Girdhar 2003). On the other hand, T. canis, A. suum, and Nippostrongylus sp. are all members of Phylum Nematoda. However, Nippostrongylus sp. belongs to Class Chromadorea, Order Rhabditida. On the other hand, both T. canis and A. suum belong to Class Secernentea, Order Ascaridida (Myers et al. 2016a, b). Despite these differences, they exhibit similarities in larval migration with T. canis. T. taeniaeformis, A. suum, and T. canis larvae migrate to the liver in the rat host. T. taeniaeformis eggs hatch into oncospheres in the small intestine of the rat then reach the liver after penetrating the small intestine. In the liver, they develop into strobilocerci (Widmer et al. 2012). In contrast, A. suum exhibits migration to both the liver and lungs in the rat (Bradbury et al. 1974). Nippostrongylus sp., on the other hand, exhibit larval migration to the lungs in rats, just like A. suum and T. canis (Ferens et al. 1990).
Studies have shown that there is at least one band weighing around 55–66 kDa responsible for the cross-reactivity of A. suum and T. canis (Nunes et al. 1997). Another study identified a 14 kDa protein similar to both A. suum and T. canis excretory-secretory antigens (Kennedy et al. 1989). Romasanta et al. (2003) on the other hand, identified 190, 160, and 33 kDa antigens that may cause cross-reactivity between T. canis and A. suum which may be due to similar proteins found on their teguments.
Hence, it is possible that the antigen concentration used was low enough to not cause any cross-reactions among T. canis and the three helminths.
Determination of the appearance of antibodies using sera obtained from T. canis-infected rats at different days post infection
Sera of rats sacrificed at three, five, eight, ten, fifteen, thirty, and sixty DPI were also tested in the assay to determine when the levels of antibody would be detectable in sera. All sera from rats sacrificed at three, five, eight, and ten DPI showed negative results. Positive results appeared from 15 DPI. All sera from 15 and 30 DPI showed positive results. Also, there is an increasing intensity of color from 15 DPI to 60 DPI. However, at 60 DPI, only one of two sera showed positive results.
During the early stages of infection, the host may have only been starting to mount an immune response such that antibody levels may have only increased to sufficient levels at 15 DPI. Afterwards, there is an increasing concentration of antibodies, as seen in the increasing intensity of the color, such that at 60 DPI, a very intense color was observed in one of the infected sera. This may be because the host is starting to produce more antibodies so that the pathogen can successfully be eliminated. However, one of the infected sera showed a negative result at 60 DPI.
In BALB/c mice that were infected with three doses of 50 T. canis eggs in the 1st, 14th, and 28th day, and mice infected with three doses of 50 eggs in the first, fifth, and eighth day, antibodies have only been observed at 6 months after the infection. However, mice that were given 200 eggs in a single dose exhibited positive results at 30 days post-infection (Chieffi et al. 1995). Another study using BALB/c mice found that the concentration of total IgE in serum samples of BALB/c mice experimentally infected with different T. canis inoculum size (100 and 1000 eggs) has resulted to an increase of total IgE in serum at 14 dpi, persisting up to 60 dpi. Similarly, the same inoculum size has elicited a significant increase to Toxocara-specific IgG1 that also persisted up to 60 dpi. However, IgG2a levels were very low or absent (Pinelli et al. 2007).
Kayes et al. (1985) indicated that in dosages of 5, 25, or 125 T. canis eggs, antibody production in weanling female CBA/J mice increases from the second to the fourth week after infection. However, when 250 eggs were inoculated, antibody levels peaked 2 weeks after infection and have remained at the same level. It has also been observed that antibody levels increased with increasing dosage of infective eggs.
A study by Bowman et al. (1987), revealed that in 7 week-old male BALB/c BYJ mice that have been infected with 500 eggs, initial detection of IgM occurred during the first week of infection, and the highest levels have been observed at 3–6 weeks after infection. On the other hand, IgG response was detected at the second week of infection, with the peak at 6–8 weeks after infection. In the same study, levels of circulating antigen were highest during the first week of infection, while the lowest detectable levels were observed at 3 months post-infection.
In an experimental infection of 1000 T. canis eggs in 4 month old New Zealand White rabbits, 16 out of 17 animals exhibited detectable amounts of IgG in sera at 14 days post-infection. All animals exhibited high titers at 60 days post-infection (Bin et al. 2016).
Thus, the variations observed in the time of detection and the concentration of antibodies in sera may have been due to factors such as the species of the experimental host, strain used, age of the experimental host, sex of the animal, and dosage and frequency of infection of embryonated eggs/inoculum size.
Toxocariasis can manifest as a variety of syndromes, namely visceral larva migrans (VLM), ocular larva migrans (OLM), neurological toxocariasis, and common or covert toxocariasis (Macpherson 2013). Furthermore, asymptomatic cases may also develop (Mazur-Melewska et al. 2012). Because of the variety of syndromes that the infection causes, nonspecific syndromes are presented, leading to misdiagnosis and underdiagnosis of the infection. Definitive diagnosis is very challenging, as it can only be made through direct observation of the larva, either through the aid of ophthalmic and medical imaging techniques, biopsy, or autopsy (Pawlowski 2001).
Unlike the standard ELISA, the dot ELISA does not require a spectrophotometer to be able to read the results since only color change is observed. Furthermore, it is better than the standard ELISA in terms of rapidity and simplicity which makes it more practical for field use and more adaptable to the Philippine setting. This study may initiate the development of diagnostic kits for the detection of parasitic diseases in the Philippines, thus increasing public awareness on these infections and improving surveys on the prevalence of these infections in the country. Furthermore, the assay should be optimized on human sera to evaluate its performance both in laboratory and field conditions.
Acknowledgements
The researchers would like to thank the National Immunological Testing Laboratory (NITL), the National Institute of Molecular Biology and Biotechnology (BIOTECH) for the assistance and facilities provided. This research was supported by the University of the Philippines Enhanced Work and Research Grant (UP ECWRG) and the Department of Science and Technology – Accelerated Science and Technology Human Resource and Development Program (DOST-ASTHRDP).
Author contributions
Conceived and designed the experiments: VGVP, IKMV; Performed the experiments: IKMV, CMB; Data analysis: IKMV; Contributed reagents/materials/analysis tools: VGVP, IKMV, CMB; All authors participated in writing the final paper.
Compliance with ethical standards
Conflict of interest
None.
References
- Alcantara-Neves NM, dos Santos AB, Mendonca LR, Figuereido CAV, Pontes-de-Carvalho L. An improved method to obtain antigen-excreting Toxocara canis larvae. Exp Parasitol. 2008;119(3):349–351. doi: 10.1016/j.exppara.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Bin LLC, Santarem VA, Laposy CB, Rubinsky-Elefant G, Roldan WH, Giuffrida R. Kinetics and avidity of anti-Toxocara antibodies (IgG) in rabbits experimentally infected with Toxocara canis. Rev Bras Parasitol Vet. 2016;25(1):99–104. doi: 10.1590/S1984-29612015067. [DOI] [PubMed] [Google Scholar]
- Bojanich MV, Marino GL, Lopez MA, Alonzo JM. An evaluation of the dot-ELISA procedure as a diagnostic test in an area with a high prevalence of human Toxocara canis infection. Mem Inst Oswaldo Cruz. 2012;107(2):194–197. doi: 10.1590/S0074-02762012000200007. [DOI] [PubMed] [Google Scholar]
- Bradbury SM, Percy DH, Strejan GH. Immunology of Ascaris suum infection. I. Production of reaginic antibodies to worm components in rats. Int Arch Allergy Appl Immunol. 1974;46(4):498–511. doi: 10.1159/000231153. [DOI] [PubMed] [Google Scholar]
- Bowman DD, Mika-Grieve M, Grieve RB. Circulating excretory-secretory antigen levels and specific antibody responses in mice infected with Toxocara canis. Am J Trop Med Hyg. 1987;36(1):75–82. doi: 10.4269/ajtmh.1987.36.75. [DOI] [PubMed] [Google Scholar]
- Camargo ED, Nakamura PM, Vaz AJ, da Silva MV, Chieffi PP, de Melo EO. Standardization of dot-ELISA for the serological diagnosis of toxocariasis and comparison of the assay with ELISA. Rev Inst Med Trop Sao Paulo. 1992;34(1):55–60. doi: 10.1590/S0036-46651992000100010. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2013) Parasites-Toxocariasis (also known as roundworm infection). http://www.cdc.gov/parasites/toxocariasis/index.html. Accessed 15 June 2015
- Chieffi PP, Peres BA, de Mello EO, Kanamura H, Brandao MM. Persistence of specific antibody response in different experimental infections of mice with Toxocara canis larvae. Rev Inst Med Trop Sao Paulo. 1995;37(3):187–190. doi: 10.1590/S0036-46651995000300001. [DOI] [PubMed] [Google Scholar]
- De Savigny DH, Tizard IR. Toxocara larva migrans: the use of larval secretory antigens in haemagglutination and soluble antigen fluorescent antibody tests. Trans R Soc Trop Med Hyg. 1977;71(6):501–507. doi: 10.1016/0035-9203(77)90144-4. [DOI] [PubMed] [Google Scholar]
- Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003;16(2):256–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmore JD, Thompson RCA, Bates IA. The accumulation of Toxocara canis larvae in the brains of mice. Int J Parasitol. 1983;33(5):517–521. doi: 10.1016/S0020-7519(83)80017-4. [DOI] [PubMed] [Google Scholar]
- Fajutag AJM, Paller VGV. Toxocara egg soil contamination and its seroprevalence among public school children in Los Baños, Laguna, Philippines. Southeast Asian J Trop Med Public Health. 2013;44(4):552–560. [PubMed] [Google Scholar]
- Ferens WA, Arai HP, Befus AD. Trickle infections with Nippostrongylus brasiliensis in rats: larval migration through the lungs. J Parasitol. 1990;76(5):685–689. doi: 10.2307/3282983. [DOI] [PubMed] [Google Scholar]
- Girdhar M (2003) Taenia taeniaeformis. Animal Diversity Web. http://animaldiversity.org/accounts/Taenia_taeniaeformis. Accessed 17 May 2016
- Holland CV, Hamilton CM. The significance of cerebral toxocariasis: a model system for exploring the link between brain involvement, behavior and the immune response. J Exp Biol. 2013;216:78–83. doi: 10.1242/jeb.074120. [DOI] [PubMed] [Google Scholar]
- Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocariasis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991;29(9):1831–1835. doi: 10.1128/jcm.29.9.1831-1835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecek E, Beineke A, Schnieder T, Strube C. Neurotoxocarosis: marked preference of Toxocara canis for the cerebrum and T. cati for the cerebellum in the paratenic model host mouse. Parasit Vectors. 2014;7:194. doi: 10.1186/1756-3305-7-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayes SG, Omholt PE, Grieve RB. Immune responses of CBA/J mice to graded infections with Toxocara canis. Infect Immun. 1985;48(3):697–703. doi: 10.1128/iai.48.3.697-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MW, Qureshi F, Fraser EM, Haswell-Elkins MR, Elkins DB, Smith HV. Antigenic relationships between the surface-exposed, secreted and somatic materials of the nematode parasites Ascaris lumbricoides, Ascaris suum, and Toxocara canis. Clin Exp Immunol. 1989;75(3):493–500. [PMC free article] [PubMed] [Google Scholar]
- Macpherson CNL. The epidemiology and public health importance of toxocariasis: a zoonosis of global importance. Int J Parasitol. 2013;43(12):999–1008. doi: 10.1016/j.ijpara.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. 2001;39(1):1–11. doi: 10.3347/kjp.2001.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels RM, de Savigny D, Ogilvie BM. Characterization of surface and excretory-secretory antigens of Toxocara canis infective larvae. Parasite Immunol. 1984;6(1):23–27. doi: 10.1111/j.1365-3024.1984.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Mazur-Melewska K, Mania A, Figlerowicz M, Kemnitz P, Sluzewski W, Michalak M. The influence of age on a clinical presentation of Toxocara spp. infection in children. Ann Agric Environ Med. 2012;19(2):233–236. [PubMed] [Google Scholar]
- Moreira GMSG, de Lima Telmo P, Mendonça M, Moreira AN, McBride AJA, Scaini CJ, Conceição FR. Human toxocariasis: current advances in diagnostics, treatment, and interventions. Trends Parasitol. 2014;30(9):456–464. doi: 10.1016/j.pt.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey TA (2016a) Ascaris suum. The Animal Diversity Web. http://animaldiversity.org. Accessed 17 May 2016
- Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey TA (2016b) Toxocara canis. The Animal Diversity Web. http://animaldiversity.org. Accessed 17 May 2016
- Nunes CM, Tundisi RN, Garcia JF, Heinemann MB, Ogassawara S, Richtzenhain LJ. Cross-reactions between Toxocara canis and Ascaris suum in the diagnosis of visceral larva migrans by Western blotting technique. Rev Inst Med Trop Sao Paulo. 1997;39(5):253–256. doi: 10.1590/S0036-46651997000500002. [DOI] [PubMed] [Google Scholar]
- Paller VGV, De Chavez ERC. Toxocara (Nematoda:Ascaridida) and other soil-transmitted helminth eggs contaminating soils in selected urban and rural areas in the Philippines. Sci World J. 2014;2014:38622. doi: 10.1155/2014/386232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski Z. Toxocariasis in humans: clinical expression and treatment dilemma. J Helminthol. 2001;75(4):299–305. doi: 10.1017/S0022149X01000464. [DOI] [PubMed] [Google Scholar]
- Pinelli E, Brandes S, Dormans J, Gremmer E, van Loveren H. Infection with the roundworm Toxocara canis leads to exacerbation of experimental allergic airway inflammation. Clin Exp Allergy. 2007;38(4):649–658. doi: 10.1111/j.1365-2222.2007.02908.x. [DOI] [PubMed] [Google Scholar]
- Rai SK, Uga S, Wu Z, Takahashi Y, Matsumura T. Use of polymerase chain reaction in the diagnosis of toxocariasis: an experimental study. Southeast Asian J Trop Med Public Health. 1997;28(3):541–544. [PubMed] [Google Scholar]
- Roldan W, Cornejo W, Espinoza Y. Evaluation of the dot enzyme-linked immunosorbent assay in comparison with standard ELISA for the immunodiagnosis of human toxocariasis. Mem Inst Oswaldo Cruz. 2006;101(1):71–74. doi: 10.1590/S0074-02762006000100013. [DOI] [PubMed] [Google Scholar]
- Roldan WH, Cavero YA, Espinoza YA, Jimenez S, Gutierrez CA. Human toxocariasis: a seroepidemiological survey in the Amazonian city of Yurimaguas, Peru. Rev Inst Med Trop Sao Paulo. 2010;52(1):37–42. doi: 10.1590/S0036-46652010000100006. [DOI] [PubMed] [Google Scholar]
- Romasanta A, Romero JL, Arias M, Sánchez-Andrade R, López C, Suárez JL, Díaz P, Díez-Baños P, Morrondo P, Paz-Silva A. Diagnosis of parasitic zoonoses by immunoenzymatic assays—analysis of cross-reactivity among the excretory/secretory antigens of Fasciola hepatica, Toxocara canis, and Ascaris suum. Immunol Invest. 2003;32(3):131–142. doi: 10.1081/IMM-120022974. [DOI] [PubMed] [Google Scholar]
- Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010;104(1):3–23. doi: 10.1179/136485910X12607012373957. [DOI] [PubMed] [Google Scholar]
- Thomas D, Jeyathilakan N, Basith SA, Senthilkumar TMA. In vitro production of Toxocara canis excretory-secretory (TES) antigen. J Parasit Dis. 2014;40(3):1038–1043. doi: 10.1007/s12639-014-0630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de N, Vu Trung N, Duyet LV, Chai JY. Molecular diagnosis of an ocular toxocariasis patient in Vietnam. Korean J Parasitol. 2013;51(5):563–567. doi: 10.3347/kjp.2013.51.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watthanakulpanich D. Diagnostic trends of human toxocariasis. J Trop Med Parasitol. 2010;33(1):44–52. [Google Scholar]
- Widmer D, Jurczynski K. Infection with the strobilocercus of Taenia taeniaeformis in a Malagasy Giant Jumping Rat (Hypogeomys antimena) J Zoo Wildl Med. 2012;43(4):914–921. doi: 10.1638/2012-0116R1.1. [DOI] [PubMed] [Google Scholar]
- Woodhall DM, Eberhard ML, Parise ME. Neglected parasitic infections in the United States: toxocariasis. Am J Trop Med Hyg. 2014;90(5):810–813. doi: 10.4269/ajtmh.13-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Nagano I, Xu D, Takahashi Y. Primers for polymerase chain reaction to detect genomic DNA of Toxocara canis and T. cati. J Helminthol. 1997;71(1):77–78. doi: 10.1017/S0022149X00000845. [DOI] [PubMed] [Google Scholar]
- Zibaei M, Sadjjadi SM, Uga S. Experimental Toxocara cati infection in gerbils and rats. Korean J Parasitol. 2010;48(4):331–333. doi: 10.3347/kjp.2010.48.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]


