Abstract
Haemonchus contortus is one of the most pathogenic and economically important parasites of sheep. Different H. contortus antigens; crude somatic antigen (CSA), excretory/secretory antigen (ESA), crude larval antigen (CLA), glutathione-S-transferase antigen (GST) and recombinant protein (rhcp 26/23) were prepared and characterized using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot. The antigens were immunologically evaluated through indirect enzyme linked immunosorbent assay (ELISA) for diagnosis of haemonchosis in experimentally and naturally infected sheep. Analysis of the resultant bands of SDS-PAGE demonstrated that 13, 6, 11, 2 and 1 protein bands from CSA, ESA, CLA, GST and rhcp 26/23, respectively and analysis of the resultant bands of western blot showed that 13, 6, 4 and 1 reactive bands detected from CSA, ESA, CLA and GST, respectively. The results of ELISA of different antigens revealed that sero-prevelance of CSA, ESA, CLA, GST and rhcp 26/23 were 78.51, 82.34, 85.319, 45.319 and 90.8% respectively, sensitivity were 100, 90, 100, 96.66 and 90%, respectively and specificity were 0, 70, 10, 70 and 6.66%, respectively with diagnostic potency were 50, 80, 55, 83.33 and 48.33%, respectively. Statistical analysis using Chi square test found that GST is the best one that can be used. The cross reactivity of GST antigen, crude Fasciola antigen and crude Moniezia antigen tested versus their homologous hyper immune sera at different dilutions using ELISA. The current study reported that GST antigen could be considered as a promising antigen for diagnosis of haemonchosis.
Keywords: Haemonchus contortus, GST, Electrophoresis, Western blot, ELISA, Diagnosis
Introduction
Sheep haemonchosis is a parasitic disease caused by Haemonchus contortus (H. contortus); a nematode species that during its adult stage lives in the host’s abomasa. Clinical cases of the infection manifested anemia, growth retard and death usually occur in lambs. The severity of disease depends on variety of factors including; intensity of the infection, isolated-related parasite virulence, host age, animal breed, and nutritional and immunological status of the infected sheep. All these factors can be aggravated by local environmental conditions (Angulo-Cubillán et al. 2007).
The detection of infection is usually based upon estimation of clinical signs and fecal examinations. But, clinical signs usually become apparent when the infection is heavy. Eggs are found in the feces after the prepatent period of approximately 3–4 weeks at which time the infection is firmly established and damage is already done. In order to circumvent these limitations the improvement of a dependable serological assay as enzyme linked immunosorbent assay (ELISA) enables the revealing of subclinical infections and/or the early detection of infections. Furthermore, sero-epidemiological studies, in which large groups of animals must be examined, might also benefit from a reliable ELISA. Usually such a test is, in contrast to fecal examinations, accurate and less time consuming (Schallig et al. 1995a).
In the present study various antigen sources such as crude somatic antigen (CSA), excretory/secretory antigen (ESA), crude larval antigen (CLA), glutathione-S-transferase antigen (GST) and recombinant protein (rhcp 26/23) have been employed in immune-enzymatic assay with promising results and characterized by SDS-PAGE and western blot. However, these serological methods suffer from some drawbacks such as the lack of detection of primary infections Gill (1991) and the extensive cross-antigenicity between most of trichostrongylids (Anderson et al. 1989 and Cuquerella et al. 1994). So, the aim of the current study was to determine the most specific antigen for diagnosis of haemonchosis by ELISA and characterize prepared antigens by SDS-PAGE and western blot for good decision in diagnosis.
Materials and methods
Antigen preparation
Adult worms of H. contortus, M. expansa and F. gigantica were collected from slaughtered sheep at El-Moneib abattoir in Egypt from January 2013 to August 2015. Worm recovery was carried out according to standard procedures of MAFF (1986). Crude somatic antigens; CSA CFA and CMA were prepared from collected worms according to Johnson et al. (2004). ESA and CLA of adult H. contortus were prepared as described by Prasad et al. (2008) and Tariq et al. (2008), respectively.
The purified GST of adult H. contortus was obtained according to Wijffels et al. (1992). Glutathione sepharose 4B column was used for purification of glutathione-S-transferase, obtained from GE Healthcare., UK. Briefly, affinity chromatography was carried out as method described by the instructions in the kit of column. The glutathione sepharose 4B packed column (2 cm bed height) was equilibrated with equilibration buffer (Tris–Hcl pH 8). Adult H. contortus crude extract (11.72 mg) was applied onto a column. The unbound fractions were eluted by washing the column with 10–20 bed volumes of equilibration buffer till the optical density (OD) value at 280 nm returned to a steady base line. Then, the glutathione bound fractions were eluted in elution buffer (50 Mm Tris–Hcl, 10 mM Glutathione reduced PH 9.6) in 1 ml fractions. Based on the OD value at 280 nm, the eluted fractions were pooled and concentrated by dialysis against polyethylene glycol, MW 8000. The purified GST specific activity was determined according to the method of Habig and Jakoby (1981) with 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. Briefly, the enzyme assay was carried out in a final volume of 1 ml containing Hc-GST, 0.1 M phosphate buffer (PH 6.5), 1 mM CDNB, and 1 mM reduced glutathione (GSH). The reaction was initiated by the addition of the aromatic substrate. The change in absorbance due to the formation of the glutathione conjugate of CDNB was recorded at 340 nm once every minute at 25 °C. The molar extinction of CDNB is 0.0096 µM−1/cm the enzyme activity was expressed as nmol/min/mg protein. The purified GST was stored at −20 °C till further use.
However, recombinant protein of adult H. contortus (rhcp 26/23) was kindly obtained from Prof. Dr. Omnia Kandil, parasitology and animal diseases department, NRC, Egypt.
The protein content of different prepared antigens was determined according to Lowry et al. (1951).
Serum samples
Six native male lambs of about 6 months old were kept for 4 weeks in-door, before beginning of the experiment, to acclimate together and were subjected to parasitological examination for any parasitic infections according to Schallig et al. (1995a). Three lambs were kept as negative control non-infected lambs. The other 3 lambs were experimentally mono-infected orally with 20,000 third larval stages L3 of H. contortus. Blood samples were collected after slaughtering of sheep.
Five hundred thirty-six sheep blood samples including; 470 samples were randomly collected, 30 samples were naturally infected with H. contortus and another 30 samples were non-infected during their post mortem examination at El-Moneib abattoir in Egypt. In addition to, 3 positive control samples and 3 negative control samples were collected from experimentally infected and non-infected sheep. After clotting of blood, serum samples were collected after centrifugation at 2000 rpm for 15 min. All samples were stored at −20 °C in small aliquots.
Twenty-one healthy white New Zealand male rabbits weighing 1.5–2 kg body weight each were obtained for preparation of different hyper immune sera. The rabbits were divided into seven groups; each group had three rabbits, were housed in isolated ventilated boxes and provided with dried balanced ration and water throughout the whole experimental period. One group is kept as negative control and the other six group were immunized with different antigens; CSA, ESA, CLA and GST of adult H. contortus, CFA and CMA in order to prepare their specific hyper immune sera according to Fagbemi et al. (1995). Briefly, each rabbit was subcutaneously injected with 200 μg of each prepared antigens/kg B. Wt. emulsified with an equal volume of complete Freund`s adjuvant. Two weeks later, two booster doses of antigens emulsified with incomplete Freund`s adjuvant were injected subcutaneously within week interval. The rabbits were slaughtered and hyper immune sera of immunized rabbits with each antigen type were individually collected, divided in aliquots and stored as rabbit anti-adult H. contortus CSA (HIS-CSA), rabbit anti-adult H. contortus ESA (HIS-ESA), rabbit anti-larval H. contortus (HIS-CLA), rabbit anti-adult H. contortus GST (HIS-GST), rabbit anti-adult F. gigantica CFA (HIS-CFA) and rabbit anti-adult M. expansa CMA (HIS-CMA), at −20 °C till used.
Antigen characterization
Seventy μg of CSA, ESA, CLA, GST and rhcp 26/23 were electrophoresed using SDS-PAGE, 10% under reducing condition according to the method of Laemmli (1970). After SDS-PAGE, protein bands were electro-blotted on nitrocellulose paper as described by Towbin et al. (1979). After blocking, the blotted nitrocellulose strips were incubated overnight against their hyper immune sera; HIS-CSA, HIS-ESA, HIS-CLA and HIS-GST at dilution 1:200. After washing, anti-rabbit IgG (whole molecule) peroxidase antibody produced in goat conjugate (Sigma-Aldrich, USA) diluted at 1:1000 in diluting buffer was added and incubated for 1 h. Then, the reaction was developed by incubation of the strips in the substrate solution containing 1-chloronaphthol, 30 mg (Sigma-Aldrich, USA) for 3–5 min.
Serological analysis
Enzyme linked immunosorbent assay was conducted to evaluate the diagnostic value of different H. contortus antigens in detection of anti-H. contortus IgG with experimentally, randomly collected and post mortem examined sheep sera according to the method of Qamar and Maqbool (2012) and identify the most sensitive and specific antigen for diagnosis of haemonchosis. In addition, cross reactivity of chosen antigen; GST of adult H. contortus, CFA and CMA was evaluated against their hyper immune sera; HIS-GST, HIS-CFA and HIS-CMA according to the method of Voller et al. (1976).
The optimal concentration of antigen, antibody and conjugate dilutions were chosen after preliminary checker-board titrations following Hudson and Hay (1989). The wells were coated with 100 μl of each diluted antigen at the concentration of 0.2 μg/well in carbonate-bicarbonate buffer, pH 9.6 and incubated for 1 h at 37 °C then incubated overnight at 4 °C. After three washing of plates with washing buffer PBS-Tween, the plates were blocked with 200 µl/well of blocking buffer (2% dry skimmed milk in PBS-T) and incubated for 2 h at room temperature. The plates were then washed several times with washing buffer. One hundred µl/well of diluted sheep serum samples at 1:200 in diluting buffer and double fold serial dilution of hyper immune sera; HIS-GST, HIS-CFA and HIS-CMA (1:50–1:3200) were added as duplicate and the plates were incubated for 1.5 h at 37 °C. After incubation, the plates were then washed three times with washing buffer. A 100 µl/well of conjugate; anti-sheep IgG (whole molecule) peroxidase antibody produced in donkey (Sigma-Aldrich, USA) and anti-rabbit IgG (whole molecule) peroxidase antibody produced in goat (Sigma-Aldrich, USA) diluted at 1:1000 in diluting buffer was added and incubated for 1 h at 37 °C. Then, the plates were washed extensively with washing buffer. One hundred µl/well of substrate solution (O, phenylenediamine, one tablet (20 mg/ml) dissolved in 50 ml substrate buffer, pH 5 and 25 μl 30% H2O2) was added to all the wells and the plates were incubated 10–20 min at 37 °C. The optimum color development was stopped by addition of 100 µl of stopping buffer (5% sodium dodecyl sulphate [SDS]) to each well. OD was read at wave length of 450 nm with an ELISA reader (BIO-TEK, INC., ELx, 800UV).
The sera were considered to be positive when the absorbance values were more than the cut off value. The cut off value was calculated according to Schallig et al. (1995a) from mean value plus three times the standard deviation of OD value of negative control sera.
Data analysis
OD data were expressed as arithmetic mean with standard deviation. The data were analyzed by the statistical computer package for social science (SPSS) using Chi square test.
Diagnostic analysis parameters were calculated for CSA, ESA, CLA, GST and rhcp 26/23 according to Thrusfield (1997).
Results
Gel electrophoresis
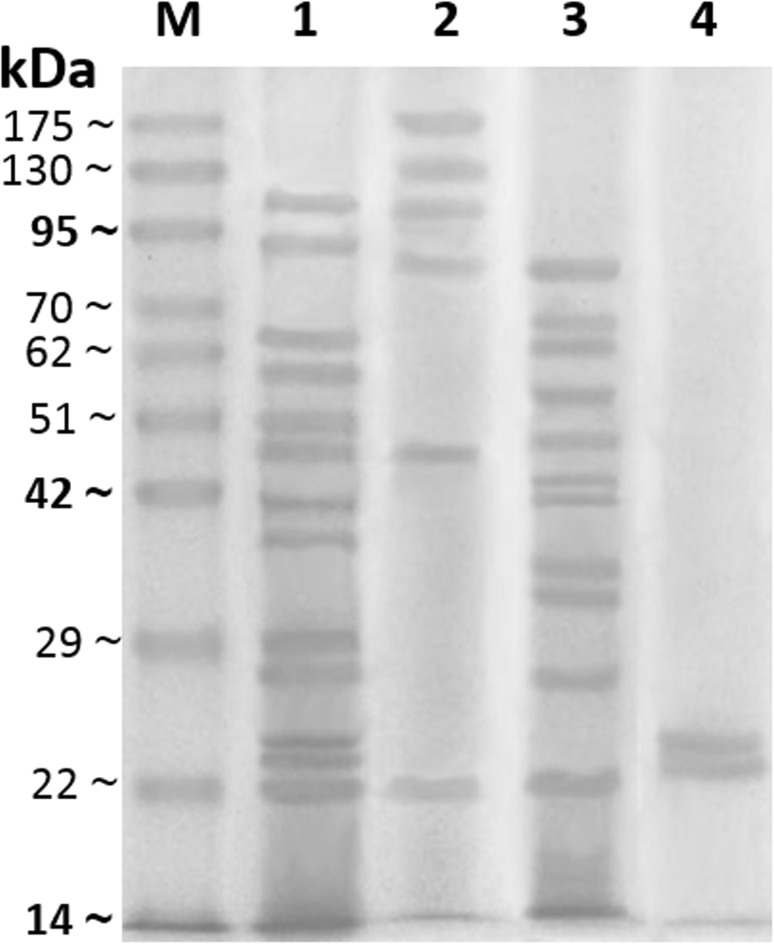
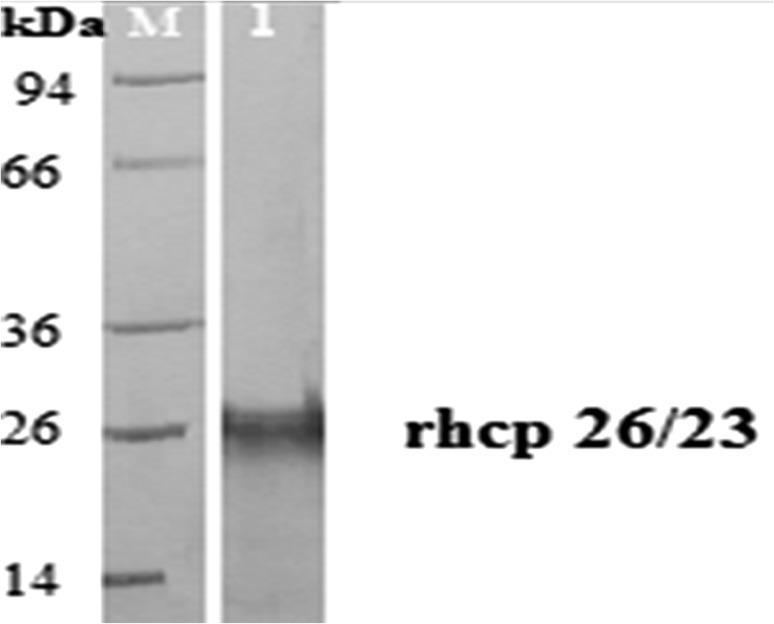
The results indicated that there were some variations in numbers and molecular weights (M.W.) of protein bands between various prepared H. contortus antigens. Electrophoretic profile of different antigens revealed that 13 individual protein bands with M.W. 22, 23.5, 24.5, 27.5, 29.7, 37.6, 41.5, 47.5, 50.9, 57, 64, 93.9 and 115 kDa were resolved from CSA, while ESA showed 6 protein bands 22, 47.5, 86.8, 105.5, 132.5 and 175 kDa, as well CLA exhibited 11 protein bands 22, 26.6, 32, 34.8, 41.5, 42.8, 47.5, 54.5, 62.5, 67 and 82.5 kDa. GST has two protein bands at M.W. 23.5, 24.5 kDa (Fig. 1). In addition, rhcp 26/23 possess one band at 26 kDa (Fig. 2). The current analysis demonstrated that 2 protein bands 47.5 and 22 kDa are common between CSA, CLA and ESA. Also, other 2 protein bands 23.5 and 24.5 kDa are dominant between CSA and GST. While one protein band 41.5 kDa is shared between CSA and CLA (Fig. 1).
Fig. 1.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, 10%) of crude somatic antigen (CSA, lane 1), excretory secretory antigen (ESA, lane 2), crude larval antigen (CLA, lane 3) and glutathione-s-transferase antigen (GST, lane 4) of H. contortus and pre-stained molecular weights protein marker (lane M, 14–175 kDa)
Fig. 2.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, 10%) of adult H. contortus recombinant protein (rhcp 26/23, lane 1) and pre-stained molecular weight protein marker (lane M, 14–94 kDa)
Western blot analysis
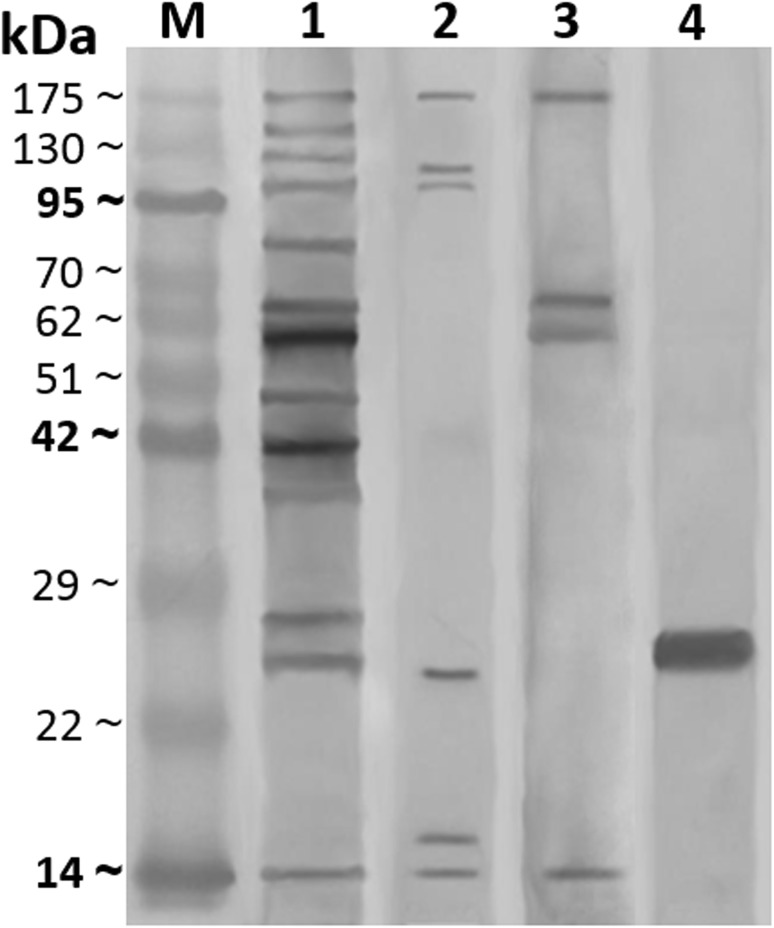
Western blot analysis of such antigens showed that CSA antigen presented 13 immunogenic bands at M.W. 14.6, 25.5, 27.5, 36.9, 41.5, 47.5, 58.7, 63.5, 79.9, 98.5, 126.5, 141.5 and 175 kDa. However, ESA antigen revealed 6 bands at M.W. 14.6, 15.7, 24.5, 98.5, 115 and 175 kDa, also CLA antigen exhibited 4 protein reactive bands at M.W. 14.6, 58.7, 62.5 and 175 kDa and GST antigen showed one immunogenic band at M.W. 25.5 kDa. The resultant common bands were demonstrated with CSA, ESA and CLA at M.W. 14.6 and 175 kDa. As well as, a band at M.W. 25.5 kDa was immune dominant between GST and CSA antigens. In addition to the reactive bands at M.W. 58.7 and 98.5 kDa were common between CSA and CLA & CSA and ESA, respectively (Fig. 3).
Fig. 3.
Western blot analysis of different prepared H. contortus antigens against their rabbit hyper immune sera; crude somatic antigen (CSA, HIS-CSA, lane 1), excretory secretory antigen (ESA, HIS-ESA, lane 2), crude larval antigen (CLA, HIS-CLA, lane 3) and glutathione-s-transferase antigen (GST, HIS-GST, lane 4) and pre-stained molecular weight protein marker (lane M, 14–175 kDa)
Serological analysis
Different H. contortus prepared antigens; CSA, ESA, CLA, GST and rhcp 26/23 were evaluated in detection of anti-H. contortus IgG in different collected sera using ELISA (Table 1). As judged by post mortem examination of 60 slaughtered sheep at El-Moneib abattoir, ELISAs scored that most of naturally infected sheep were true positive (Table 1). However, both ELISA of ESA and GST recorded that 21 and 9 out of 30 naturally non infected sheep were true negative and false positive, respectively (Table 1). Therefore, the results of ELISA showed that rhcp 26/23 detected the highest prevalence (90.8%) and GST noticed the lowest prevalence (45.319%) (Table 2). Moreover, GST scored the highest diagnostic efficacy (83.33%) followed by ESA (80%). In addition, GST and ESA antigens recorded the highest specificity (70%) followed by CLA (10%) (Table 2). Furthermore, GST (76.315%) and ESA (75%) antigens showed the highest positive predictive value while CLA revealed the highest negative predictive value (100%) followed by GST (95.45%) and ESA (78.5%) antigens (Table 2). Interestingly, GST is the better antigen for diagnosis of haemonchosis where the statistical analysis using Chi square test found that GST versus CSA, CLA, ESA and rhcp 26/23 possessed significance difference <0.05 and CSA, CLA, ESA and rhcp 26/23 versus each other insignificance difference.
Table 1.
Detection of crude somatic antigen (CSA), excretory/secretory antigen (ESA), crude larval antigen (CLA), glutathione-s-transferase antigen (GST) and recombinant protein (rchp26/23) diagnostic value against randomly collected sera, experimentally seropositive sera and post mortem parasitologically examined sera; true positive (TP), false positive (FP), true negative (TN) and false negative (FN)
| Antigens | Sera examined by ELISA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 470 Randomly collected examined sera | Experimental seropositive absorbance reactivity | 60 Post mortem parasitologically examined sera | |||||||
| Negative number | Positive number | ||||||||
| Absorbance reactivity | 30 Naturally infected | 30 Non infected | |||||||
| Low | Moderate | High | TP | FN | TN | FP | |||
| CSA | 101 | 238 | 75 | 56 | Moderate | 30 | 0 | 0 | 30 |
| ESA | 83 | 186 | 150 | 51 | High | 27 | 3 | 21 | 9 |
| CLA | 69 | 147 | 170 | 84 | Moderate | 30 | 0 | 3 | 27 |
| GST | 257 | 103 | 53 | 57 | High | 29 | 1 | 21 | 9 |
| rhcp 26/23 | 43 | 182 | 162 | 83 | Moderate | 27 | 3 | 2 | 28 |
Table 2.
Antigenicity (%) of crude somatic antigen (CSA), excretory/secretory antigen (ESA), crude larval antigen (CLA), glutathione-s-transferase antigen (GST) and recombinant protein (rchp26/23) of Haemonchus contortus
| Antigenicity (%) | Antigens | ||||
|---|---|---|---|---|---|
| CSA | ESA | CLA | GST | rhcp 26/23 | |
| Apparent prevalence | 78.51 | 82.34 | 85.319 | 45.319 | 90.8 |
| Sensitivity | 100 | 90 | 100 | 96.66 | 90 |
| Specificity | 0 | 70 | 10 | 70 | 6.66 |
| Positive predictive value | 50 | 75 | 52.63 | 76.315 | 49.09 |
| Negative predictive value | 0 | 78.5 | 100 | 95.45 | 40 |
| Diagnostic efficacy | 50 | 80 | 55 | 83.33 | 48.33 |
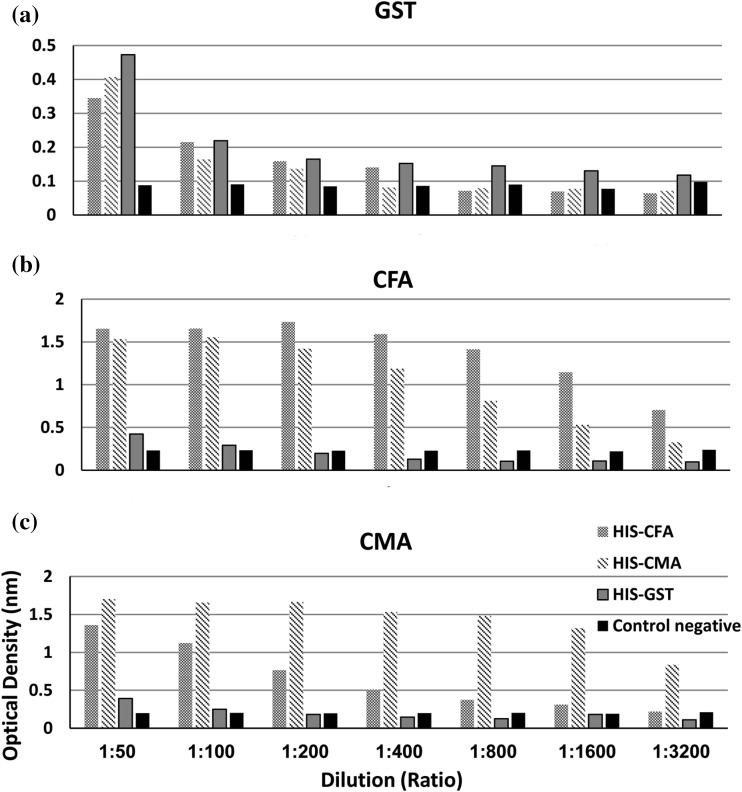
The cross reactivity of GST, CMA and CFA antigens were assessed through reaction against HIS-GST, HIS-CMA, HIS-CFA and negative control rabbit sera at different dilution from 1:50 to 1:3200. GST antigen still cross-reacted with HIS-GST, HIS-CFA and HIS-CMA till serum dilution 1:3200, 1:400 and 1:200 dilutions, respectively. CFA antigen cross reacted with HIS-GST till 1:100 dilution and with HIS-CMA till higher dilution 1:3200. As well as, CMA antigen cross reacted with HIS-GST till 1:100 dilutions and completely cross reacted with HIS-CFA till higher dilution 1:3200 (Fig. 4). Thus, the optimum serum dilution that ensure over coming this phenomena is 1:800 when using GST antigen for authentic diagnosis of haemonchosis.
Fig. 4.
Evaluation of cross reactivity between a glutathione-s-transferase antigen of H. contortus (GST), b crude F. gigantica antigen (CFA) and c crude M. expansa antigen (CMA) against their rabbit hyper immune sera; HIS-GST, HIS-CFA, HIS-CMA and negative control rabbit serum
Discussion
The current study aimed to characterize different prepared H. contortus antigens and revise the better specific antigen for diagnosis of sheep haemonchosis by ELISA with regard to its sensitivity, specificity, positive predictive value, negative predictive value and diagnostic efficacy.
The present ELISAs results showed that all different H. contortus prepared antigens recorded highest sensitivities (90–100%) when tested sera from naturally infected sheep. This suggests that ELISA assay is a reliable diagnostic technique that can be used in detection of IgG of such infection (Gowda 2014; Kandil et al. 2015). Apparent prevalences scored by all antigens were high (78.51–90.8%), except GST antigen which recorded marked difference percentage (45.319%) nonetheless, statistically GST antigen versus the other prepared antigen possessed significance difference <0.05 so, GST considered the best one. This finding could be returned to the resultant purified antigen from affinity chromatography has led to better result in diagnosis (Schallig et al. 1995b). So, we evaluated GST antigen cross reactivity against HIS-CFA and HIS-CMA and concluded that the optimum serum dilution that ensure over coming this phenomena is 1:800 at using GST antigen. Interestingly, GST antigen showed the highest diagnostic efficacy (83.33%) and specificity (70%). This result could be confirmed by GST antigen has been successful in early detection of antibody titer post-infection and has revealed to be an immunogenic protein with high sensitivity to detect anti-H. contortus antibodies as early as two weeks post-infection (Salama 2017). The false positive reactivity appeared with this antigen may be due to the persistence of antibodies of past infection or worms might be eliminated by anthelminthic medications or presence of immature worms in abomasa or recent infection (Molina et al. 1999 and Kandil et al. 2016). In addition, the band at 25.5 kDa is the immunogenic band that may be responsible for the species specificity of this antigen, although on the gel it appear at 24.5 kDa (Schallig et al. 1995a; Pour et al. 2014). This finding may be attributed to this purified antigen success in stimulation of IgG production against it and it appeared with intense binding reaction that it measured at 25.5 kDa on blotting (McKerrow et al. 1990; Richer et al. 1992).
Likewise for ESA antigen can show the same specificity of GST antigen, this finding could be accredited to the presence of a 24.5 kDa reactive band on blotting. But, ESA recorded lower percentages in other parameter except apparent prevalence (82.34%) was higher than GST antigen and this result may be attributed to the presence of a common reactive band at 98.7 kDa which appear with ESA and CSA on blotting and this band may be responsible for highest true positive number in infected examined sheep sera which might be resultant from cross reactivity with other helminthes (Kandil et al. 2015). In this study, the SDS-PAGE profile of ESA (Fig. 1, lane 2) was differed than blotting profile (Fig. 3, lane 2). This result could be returned to ESA antigen is a component of multiple antigenic macromolecules of importance in the immunological and physiological balance between the host and parasite, thus it differed in its constituents according to methods that used in its preparation. In addition, coomassie brilliant blue R-250 wasn’t enough sensitive to stain all antigenic macromolecules of such extract. However, those molecules can successively induce IgG response during generation of HIS-ESA so it appears on blotting (Maizels et al. 1991).
Although the rhcp 26/23 antigen is a recombinant antigen of adult H. contortus that scores the highest prevalence percentage (90.8%) and sensitivity (90%) but, it showed a markedly high false positive numbers in non-infected examined sheep sera that responsible for the low specificity (6%). This could be returned to the nature of this protein that successes in immunization of sheep against haemonchosis (García-Coiradas et al. 2010 and Fawzi et al. 2015) rather than that could be used in specific diagnosis and detecting IgG against H. contortus. This confirmed by SDS-gel electrophoresis which appear a protein band at 26 kDa (Fig. 2).
On the other hand, CSA and CLA also recorded high false positive number that cause (0 and 10%) specificity, respectively but the highest sensitivity (100%) each achieved due to the highest true positive number in infected examined sheep sera. This may be returned to the cross reactivity with other helminthes (Schallig et al. 1995a and Kandil et al. 2016) and the shared immunogenic band at 58.7 kDa which appear on blotting rather than the SDS-PAGE profile. This may be occur due to this protein band not abundantly present in the extract of CSA and CLA antigen so, they didn’t stained by coomassie and didn’t appear on gel in in addition to electro-blotting was performed in good condition that let this band transferred well and the IgG antibodies of HIS-CSA and HIS-CLA was incubated overnight so IgG induced against this protein band reactivity was appeared.
Therefore, this study concluded that the GST purified antigen could be considered the most promising antigen that can be used as bio marker for diagnosis of sheep haemonchosis.
Acknowledgements
This work was supported by the science and technology development fund (STDF) in Egypt (Grant number: 3825).
Compliance with ethical standards
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.
Conflict of interest
The authors declare that they have none known conflicts of interest.
References
- Anderson DV, Dixon SC, Graham RB, Smith WD, Tucker EM. Ovine monoclonal antibody to Ostertagia circumcincta. Biochem Soc Trans. 1989;17:736. doi: 10.1042/bst0170736. [DOI] [Google Scholar]
- Angulo-Cubillán FJ, García-Coiradas L, Cuquerella M, Fuente CDL, Alunda YJM. Haemonchus contortus sheep relationship: a review. Rev Cient. 2007;11:577–587. [Google Scholar]
- Cuquerella M, Gomez-Munoz MT, Carrera L, de la Fuente C. Cross antigenicity among ovine trichostrongyloidea, preliminary report. Vet Parasitol. 1994;53:243–251. doi: 10.1016/0304-4017(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Fagbemi BO, Obarisiagbon IO, Mbuh JV. Detection of circulating antigen in sera of Fasciola gigantica infected cattle with antibodies reactive with a Fasciola specific 88 kDa antigen. Vet Parasitol. 1995;58:235–246. doi: 10.1016/0304-4017(94)00718-R. [DOI] [PubMed] [Google Scholar]
- Fawzi EM, González-Sánchez ME, Corral MJ, Cuquerella M, Alunda JM. Vaccination of lambs against Haemonchus contortus infection with a somatic protein (Hc23) from adult helminthes. Int J Parasitol. 2015;44:429–436. doi: 10.1016/j.ijpara.2014.02.009. [DOI] [PubMed] [Google Scholar]
- García-Coiradas L, Angulo-Cubillán F, Valladares B, Martínez E, de la Fuente C, Alunda JM, Cuquerella M. Immunization against lamb haemonchosis with a recombinant somatic antigen of Haemonchus contortus (rHcp26/23) Vet Med Int. 2010;2010:852146. doi: 10.4061/2010/852146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS. Genetic control of acquired resistance to haemonchosis in Merino lambs. Parasite Immunol. 1991;13:617–628. doi: 10.1111/j.1365-3024.1991.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Gowda AK. Sero-prevalence of Haemonchus contortus infection in sheep by indirect ELISA using somatic antigen. J Parasit Dis. 2014;40:464–468. doi: 10.1007/s12639-014-0527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig WH, Jakoby WB. Assay for differentiation of glutathione s-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/S0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hudson L, Hay F. Pract Immunol. 3. Oxford: Blackwell; 1989. [Google Scholar]
- Johnson DA, Behnke JM, Coles GC. Copro-antigen capture EL1SA for the detection of Teladorsagia (Ostertagia) circumcincta in sheep: improvement of specificity by heat treatment. Parasitol. 2004;129:115–126. doi: 10.1017/S0031182004005256. [DOI] [PubMed] [Google Scholar]
- Kandil OM, Eid NA, Elakabawy LM, Abdelrahman KA, Helal MA. Immunodiagnostic potency of different Haemonchus contortus antigens for diagnosis of experimentally and naturally haemonchosis in Egyptian sheep. APG. 2015;6:238–247. [Google Scholar]
- Kandil OM, Hendawy SHM, El Namaky AH, Gabrashanska MP, Nanev VN. Evaluation of different Haemonchus contortus antigens for diagnosis of sheep haemonchosis by ELISA and their cross reactivity with other helminthes. J Parasit Dis. 2016 doi: 10.1007/s12639-016-0865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAFF (Ministry of Agriculture, Fisheries and Food) (1986) Manual of veterinary parasitological laboratory techniques, 3rd edn. HSMO Publications, London, UK, 160 pp
- Maizels RM, Blaxter ML, Robertson BD, Selkirk ME. Parasite antigens, parasite genes: a laboratory manual for molecular parasitology. 1. London: Cambridge University Press; 1991. [Google Scholar]
- McKerrow JH, Brindley P, Brown M, Gam AA, Staunton C, Neva FA. Strongyloidesstercoralis: identification of a protease that facilitates penetration of skin by the infective larvae. Exp Parasitol. 1990;70:134–143. doi: 10.1016/0014-4894(90)90094-S. [DOI] [PubMed] [Google Scholar]
- Molina JM, Ruiz A, Rodríguez-Ponce E, Gutiérrez AC, González J, Hernández S. Cross reactive antigens of Haemonchus contortus adult worms in Teladorsagia circumcincta infected goats. Vet Res. 1999;30:393–399. [PubMed] [Google Scholar]
- Pour LM, Farahnak A, Rad MM, Golmohamadi T, Eshraghian M. Activity assay of glutathione s-transferase (GSTs) enzyme as a diagnostic biomarker for liver hydatid cyst in vitro. Iran J Public Health. 2014;43:994–999. [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Nasir A, Singh N. Detection of anti-Haemonchus contortus antibodies in sheep by dot-ELISA with immune-affinity purified fraction of ES antigen during prepatency. Indian J Exp Biol. 2008;46:94–99. [PubMed] [Google Scholar]
- Qamar MF, Maqbool A. Biochemical studies and serodiagnosis of haemonchosis in sheep and goats. J Anim Plant Sci. 2012;22:32–38. [Google Scholar]
- Richer JK, Sakanari JA, Frank GR, Grieve RB. Dirofilaria immitis: proteases produced by third- and fourth-stage larvae. Exp Parasitol. 1992;75:213–222. doi: 10.1016/0014-4894(92)90181-9. [DOI] [PubMed] [Google Scholar]
- Salama DB (2017) Immunization of sheep against Haemonchus contortus worms. M.V.Sc. Thesis, Faculty of Veterinary Medicine, Beni-Suef University, Egypt
- Schallig HDFH, Hornok S, Cornelissen JBWJ. Comparison of two enzyme immunoassays for the detection of Haemonchus contortus infections in sheep. Vet Parasitol. 1995;57:329–338. doi: 10.1016/0304-4017(94)00693-7. [DOI] [PubMed] [Google Scholar]
- Schallig HD, van Leeuwen MA, Hendrikx WM. Isotype-specific serum antibody responses of sheep to Haemonchus contortus antigens. Vet Parasitol. 1995;56:149–162. doi: 10.1016/0304-4017(94)00675-3. [DOI] [PubMed] [Google Scholar]
- Tariq KA, Chishti MZ, Fayaz A, Shawl AS. Epidemiology of gastrointestinal nematodes of sheep managed under traditional husbandry system in Kashmir valley. Vet Parasitol. 2008;158:138–143. doi: 10.1016/j.vetpar.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Thrusfield M. Veterinary epidemiology. 2. Oxford: Blackwell Science Ltd.; 1997. pp. 134–135. [Google Scholar]
- Towbin H, Staeheline T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A, Bidwell DE, Bartleltt A. Enzyme immunoassay in diagnostic medicine. Bull World Health Organ. 1976;53:55–65. [PMC free article] [PubMed] [Google Scholar]
- Wijffels GL, Sexton JL, Salvatore L, Pettitt JM, Humphris DC, Panaccio M, Spithill TW. Primary sequence heterogeneity and tissue expression of glutathione S-transferases of Fasciola hepatica. Exp Parasitol. 1992;74:87–99. doi: 10.1016/0014-4894(92)90142-W. [DOI] [PubMed] [Google Scholar]