Abstract
Faecal worm egg counts (FEC) are very important indicators in assessing the intensity of helminth infections in animal hosts and inform decisions taken in parasite control programmes. FEC are however affected by many factors which include the dose level of larval challenge, species composition of the worms, worm burden, female worm fecundity and concurrent infections to name but these few. The relevance of time of collection of faecal sample for FEC is not fully known and in most cases faeces for analysis is collected whenever feasible for the researcher on sample collection days. The significance of the time of collection of faeces on FEC was therefore investigated at two different periods of the day, morning and afternoon. Faecal samples were collected at 7–8 a.m. (morning) and at 2–3 p.m. (afternoon) on same sampling days from 6 mice and 10 West African Dwarf goat kids experimentally infected with Heligmosomoides bakeri and Haemonchus contortus respectively. FEC were conducted using the flotation and modified McMaster techniques. Overall, the 2–3 p.m. FEC tended to be higher than the 7–8 a.m. FEC in both animal species but the difference was not significant (P > 0.05). The time of collection of faecal samples for faecal worm egg counts is therefore not a crucial factor that may significantly affect FEC in H. bakeri and H. contortus infected mice and goats respectively although it would be advisable to maintain particular periods for collection of faeces in particular experiments for best results of FEC to be obtained.
Keywords: Variation in faecal egg counts, Time, H. bakeri, H. contortus, Mice and goats
Introduction
Mice and goats are largely used as experimental animals for the study of the impact of parasitic infections in animal hosts both in single and concurrent infections. Goats have been used to study some of the considerable variation in response to gastrointestinal (GI) nematode infections which have been shown to be largely due to variation in genetic resistance of the animals (Stear and Wakelin 1998; Baker et al. 1999; Fakae et al. 2003; Chiejina et al. 2005). Recently too, Ngongeh (2011) developed an Heligmosomoides bakeri-mouse model for the preliminary study of some responses of GI nematode infections in small ruminants. In a review by Wakelin (2000), H. polygurus (currently called H. bakeri) is used as a model for studying chronic nematode infections in domestic animals where long-lasting and persistent infections are common unlike in some model infections in rodent hosts where powerful immune and inflammatory responses eliminate the worms after a short time following infection. H. polygyrus is also a valuable model for monitoring the effects of strong selection for resistance to infection as reviewed by Wakelin (2000).
One of the most important parameters often taken antemortem in all the aforementioned studies is faecal egg counts (FEC) for the estimation of the level of parasite burdens in host animals. FEC give an estimate of the worm burden of the host animal and has been shown to correlate well with the worm burdens in small ruminants and mice (Chiejina et al. 2005; Ngongeh 2011). The significance of FEC/worm burdens in predicting the pathogenic effects inflicted by the parasites to their host and in taking decisions on whether to treat or not cannot be over emphasized. FEC are however known to be dependent on both endogenous and exogenous factors some of which include the level of larval challenge, species composition, worm burden together with the degree with which worm establishment, and adult worm fecundity is affected by host’s immune response (Douch et al. 1996; Ngongeh 2011).
The influence of sample collection time on FEC is not fully known and therefore not often emphasized although the faecal worm egg counts of Dicrocoelium dendriticum infected sheep were found to be higher in the faeces of the animals collected in the afternoon than in the faeces collected in the morning time (Bayram et al. 2006). Campo et al. (2007) had also reported that more D. dendriticum eggs were excreted by lambs infected with the parasite in the afternoon than in the morning. Infections of the red-legged partridge with the nematode, Aonchoteca caudinflata and the protozoa, Eimeria have also been used to show that faecal egg and coccidian oocyst counts increased from dawn to dusk (Villanua et al. 2006).
The rare knowledge of the importance of time of collection of faeces from hosts for faecal egg counts may be the reason why faeces for FEC can be collected in the morning or in the afternoon on sampling days as situations permit. The current study therefore conducted the present study in order to find out if FEC conducted on faeces collected from the same set of experimentally infected mice and goats at 7–8 a.m. (morning) and that collected at 2–3 p.m. (afternoon) on the same day would differ significantly. The a priori hypothesis is that FEC conducted on faeces collected in the morning and the afternoon will be similar.
Materials and methods
Experimental animals and their management
Six 10 week old male albino mice and 4–6 months old male West African Dwarf (WAD) goat kids were used for the experiment. The mice were maintained in plastic cages with wood shavings. The goats were tagged and placed two per pen. The floors of the dwarf walled pens were concreted and litter were cleared every morning before introducing fresh feed. The goats were fed cut and carry grass twice a day. The grass meal was supplemented daily with a mixture of concentrate feed (Grower’s marsh, Vital Feed®, Grand Cereals Ltd., Jos, Plateau State, Nigeria) and palm kernel cake at a 1:2 ratio, i.e. one part of concentrate and two parts of kernel cake.
Ethical consideration
The ethical conditions governing the use and conduct of experiments with life animals were strictly observed as stipulated by Ward and Elsea (1997), and the experimental protocol was approved by Michael Okpara University of Agriculture, Umudike Animal Ethics Committee.
Infective larvae
The infective larvae (L3) of Haemonchus contortus and H. bakeri were obtained from faecal cultures set from faeces collected from donor WAD goats and outbred albino mice (maintained at the college of Veterinary Medicine farm at Michael Okpara University of Agriculture, Umudike) infected with H. contortus and H. bakeri respectively.
The mice were orally infected with 600 infective larvae (L3) of H. bakeri while the goats were each infected with 4500 L3 of H. contortus on day zero of the experiment.
Infection of experimental animals
The goats were each infected with 4500 L3 of H. contortus orally using a stomach tube on day zero of the experiment while the mice were each infected with 400 L3 orally as described by Fakae (2001).
Faecal collection
The tagged goats were tethered with cotton ropes to defecate in clean bare concrete floors for periods ranging from 5 to 30 min far apart from each other to avoid mixing of their faeces. Once a goat defecated, the faecal pellets were collected and the goat returned to its pen. Faeces were collected from any goat that did not defecate within the tethered period per rectum. Mice faeces were collected as described by Ngongeh (2011), whereby each mouse was placed in a plastic bowl and allowed to defecate within minutes and the mice returned to their cage.
Faecal egg counts
Following patency of all the infected mice and goats tested by the flotation technique, FEC were conducted on faeces collected every three days in the mornings and afternoons respectively till the end of the experiment. Faecal egg counts were conducted using both flotation and modified McMaster techniques (Fakae et al. 1999) for low and high FEC respectively. The FEC was done at 7–8 a.m. (morning) and at 2–3 p.m. (afternoon) on every sampling day. The mean number of eggs for each period was determined by simple arithmetic means, the egg per gramme of faeces (EPG) for individual mice were summed and the total divided by the number of mice to give the mean EPG for each period of sampling. The same was done for the goats to obtain their mean EPG for both morning and afternoon on each sampling day.
Statistical analysis
The mean FEC in the morning and afternoon periods were compared using t test. Probabilities (P) of 0.05 or less were considered significant.
Results
Mice
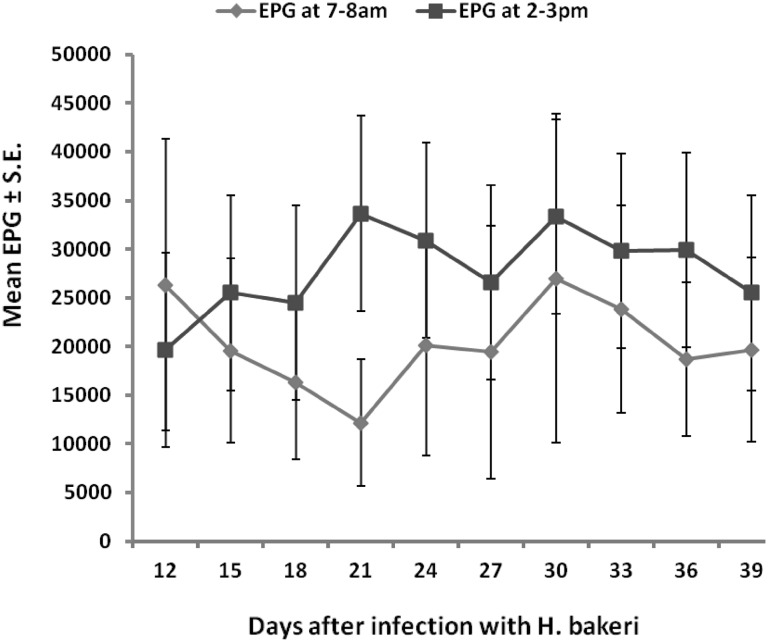
Faecal worm egg counts of the mice fluctuated in the course of the study both in the morning and afternoon periods but apart from the first sampling day (D12) when the FEC was higher in the morning than in the afternoon, the FEC tended to be higher in the afternoon than in the morning throughout the rest of the experiment (Fig. 1) although the difference was not statistically significant (P > 0.05).
Fig. 1.
Mean FEC of outbred albino mice infected with 400 L3 H. bakeri
Goat
The EPG of the goats obtained at both the morning and afternoon periods also fluctuated tending to be higher in the afternoon than in the morning period but the difference was not statistically significant (P > 0.05) (Fig. 2).
Fig. 2.
Mean FEC of WAD goats infected with 4500 L3 of H. contortus
Discussion
There was no significant difference in the FEC obtained from faeces collected in the morning and that collected in the afternoon periods in both mice and goats experimentally infected with H. bakeri and H. contortus respectively although the FEC tended to be higher in the afterenoons in both species of animals. The time of collection of faecal sample is therefore not likely to be a crucial factor that affects FEC. However, it would be advisable to maintain faecal sample collection within particular periods of the day for a given study as much as possible to avoid minor variations in FEC due to changes in the time of collection of faeces since FEC tended to be higher in the afternoon.
The results obtained in this study contrast with the findings of Campo et al. (2007) and Bayram et al. (2006) who indepently reported significant differences in FEC of sheep infected with Dicrocoelium dendriticm. However, the studies of Campo et al. (2007) and Bayram et al. (2006) involved both different parasite and host species. The findings of Villanua et al. (2006) also contrast with the results obtained in this study in that both FEC and Eimeria oocyst counts of red-legged partridge infected with the capllarid nematode, Aonchoteca caudinflata and Eimeria respectively were significantly dependent on the sampling time and increased as the day progressed. Variation in faecal nematode worm egg counts or other developmental stages of parasites passed in host faeces may therefore depend either on the host or parasite species involved or both of them.
The non significant variation of worm FEC of mice and goats infected with H. bakeri and H. contortus respectively is an important finding as it denotes that the time of collection of faeces for egg counts in the models used may not be considered critical. Faeces for FEC can therefore be collected at any period of the day and acceptable results would still be obtained as FEC do not varied significantly in infections of the parasite in these host species. The results could be extrapolated to similar farm animals or host-parasite models.
Compliance with ethical standards
Conflict of interest
The author declare that we do not have any conflict of interest.
References
- Baker RL, Mwamachi DM, Audho JO, Aduda EO, Thorpe W. Genetic resistance to gastrointestinal nematode parasites in Red Maasai, Dorfer and Maasai X Dorfer ewes in the sub-humid tropics. Anim Sci. 1999;69:335–344. doi: 10.1017/S1357729800050906. [DOI] [Google Scholar]
- Bayram S, Veli C, Mustafa M, Recep T. Changes in faecal egg counts at different hours of the day and relationship between faecal egg count and parasite burden in sheep naturally infected with Dicrocoelium dendriticum. Turk J Vet Anim Sci. 2006;30:107–111. [Google Scholar]
- Campo R, Manga-Gonzlez MY, Gonzlez-Lanza C. Relationship between egg output and parasitic burden in lambs experimentally infected different doses of Dicrocoelium dendriticum (Digenea) Vet Parasitol. 2007;87:139–149. doi: 10.1016/S0304-4017(99)00165-X. [DOI] [PubMed] [Google Scholar]
- Chiejina SN, Musongong GA, Fakae BB, Behnke JM, Ngongeh LA, Wakelin D. The modulatory influence of Trypanosoma brucei on Haemonchus contortus in Nigerian West African Dwarf goats segregated into weak and strong responders to the nematode. Vet Parasitol. 2005;128:29–40. doi: 10.1016/j.vetpar.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Douch PGC, Green RS, Morris CA, Mcewan JC, Windon RG. Phenotypic markers for selection of nematode-resistant sheep. Int J Parasitol. 1996;62:199–206. doi: 10.1016/s0020-7519(96)80062-2. [DOI] [PubMed] [Google Scholar]
- Fakae BB. Primary infection of TO outbred Mice with differing intensities of H. polygyrus infective larvae. Int J Agric Sci Biol Sci. 2001;1:1–4. [Google Scholar]
- Fakae BB, Chiejina SN, Behnke JM, Ezeokonkwo RC, Nnadi PA, Onyenwe WI, Gilbert FS, Wakelin D. The response of West African Dwarf goat to experimental infections with Haemonchus contortus. Res Vet Sci. 1999;66:147–158. doi: 10.1053/rvsc.1998.0262. [DOI] [PubMed] [Google Scholar]
- Fakae BB, Chiejina SN, Musongong GA, Ngongeh LA, Behnke JM, Wakelin D (2003) Prospects for genetic selection for resistance to gastrointestinal nematodes in the West African Dwarf goats. In: Proceedings of the 28th annual conference of the Nigerian society for animal production, vol 28
- Ngongeh LA (2011) A laboratory model for the preliminary study of some aspects of gastrointestinal nematode infections of small ruminants. Ph.D Thesis. University of Nigeria, Nsukka, p 116
- Stear MJ, Wakelin D. Genetic resistance to parasitic infection. Revue des sciences et techniques. Office International des Epizooties. 1998;17:143–155. doi: 10.20506/rst.17.1.1089. [DOI] [PubMed] [Google Scholar]
- Villanua D, Perez-Rodriguez L, Gortazar C, Hofle U, Vinuela J. Avoiding bias in parasite excretion estimates: the effect of sampling time and type of faeces. Parasitilogy. 2006;133:251–259. doi: 10.1017/S003118200600031X. [DOI] [PubMed] [Google Scholar]
- Wakelin D. Rodent models of genetic resistance to parasitic infections. In: Axford RFE, Bishop SC, Nicholas FW, Owen JB, editors. Breeding for resistance in farm animals. 2. Wallingford: CABI Publishing; 2000. [Google Scholar]
- Ward JW, Elsea JR (1997) Animal case and use in drug fate and metabolism. In: Edward RJ, Jean LH (eds) Methods and techniques, 1st edn. Marcel Deker, New York