Abstract
The prevalence of bovine Sarcocystosis is high in the most regions of the world. It can be a human health problem due to consumption of raw or under cooked hamburgers or other bovine meat products. This study was carried out to investigate the prevalence and species identification of Sarcocystis among of hamburgers, using PCR–RFLP methods in Kashan, central Iran. Overall 200 raw industrial hamburgers samples with at least 60% meat were randomly collected from nine different brands in Kashan, central Iran. The genomic DNA was extracted and a PCR–RFLP method was used to amplify an approximately 900 bp fragment at the 18S rRNA(SSU) gene, restriction enzyme BclI was used for species identification. The results showed that 58 (29%) of 200 tested hamburger samples were infected to Sarcocystis spp. The prevalence rate was 31.25 and 26.9% in the hamburgers with 90 and 60–75% meat, respectively. According to PCR–RFLP analysis, 43 (74.1%) of the 58 isolates were Sarcocystis cruzi, 12 (20.7%) showed co-infection to S. cruzi and Sarcocystis hirsuta, 2 (3.5%) was mixed infected to S. cruzi and Sarcocystis hominis, 1 (1.7%) showed the pattern of mix infection to three species. This study revealed one-third of industrial hamburger were infected to S. cruzi or mixed infection of S. cruzi with other bovine sarcocytosis. To prevent cattle infection, the possible ingestion of the disposal sporocyst stage from dogs must be eliminated. Although in this study, the prevalence of S. hominis was low and cannot be considered as a major zoonosis, it should be recommended avoiding eating under cooked hamburger and other bovine meat products to prevent human infection.
Keywords: Sarcocystis, Cattle, Hamburger, Fast food, Iran
Introduction
Sarcocystis species are obligated intracellular protozoan parasites that infect human and a wide range of domestic and wild animals. This parasite has two hosts in life cycle consist of a definitive and some or an intermediate host (Singla and Juyal 2014). The definitive host is usually a predator (man and carnivorous animals) and the intermediate host its respective prey or herbivorous animals (Dubey 2015). The sexual and asexual stages of life cycle occurs in carnivore final hosts and herbivore intermediate hosts, respectively. A final host become infected by consumption meat of intermediate host containing encysted stage (sarcocyst) and oocysts are expelled in the feces of final hosts. Intermediate host can infected through ingestion of environmentally resistant parasite oocysts. After several developmental stages, sarcocysts are formed in skeletal and cardiac muscles of intermediate host (Bucca et al. 2011; Fayer et al. 2015). Although most livestock are intermediate hosts for more than one species of Sarcocystis but species of this parasite usually infects only one species of intermediate and final host (Dubey 2015). Nowadays 200 species of Sarcocystis are recognized, however the number of Sarcocystis species has continuously increased (Fayer et al. 2015). Sarcocystosis with its significant economic, medical and veterinary impact is an important public health problem in many countries (Daryani et al. 2006). Symptoms of human intestinal sarcocystosis included abdominal discomfort, nausea, stomach ache, loose stool or diarrhea (Singla and Juyal 2014). Infection with some Sarcocystis spp. in animal can result in loss of weight, anemia, abortion and death in very heavy infection (Fayer 2014).
At least three species of Sarcocystis are known to infect cattle as the intermediate host, namely Sarcocystis cruzi, Sarcocystis hirsuta and Sarcocystis hominis which canids, felids and humans are the final hosts of them, respectively (Tenter 1995; Fayer et al. 2015). Recently, two new species were identified in cattle and named S. rommeli and S. heydorni. Human is the definitive host for S. heydorni, but for S. rommeli the definitive host is still unknown (Dubey et al. 2015, 2016). These two species can be distinguished from others using transmission electron microscopic for structures of cyst walls.
Humans afford intestinal sarcocystosis from eating under-cooked or raw beef or pork infected to S. hominis or S. suihominis, respectively. It has been well established that a considerable rate of the cattle populations are infected to Sarcocystis sp in many regions of the world. The prevalence of bovine sarcocystosis is more than 90% in most regions of the world (Vangeel et al. 2007; Obijiaku et al. 2013; Meistro et al. 2015; Nourollahi-Fard et al. 2015). Several investigations have indicated that the infection rate of sarcocystosis in slaughtered animals has been ranged between 3.5% up to 100% throughout the Iran using different methods (Daryani et al. 2006; Nourollahi Fard et al. 2009; Nourollahi-Fard et al. 2015).
Some histological and molecular study showed that a relatively high prevalence of S. hominis in cattle (Fayer 2014; Vangeel et al. 2007; Obijiaku et al. 2013; Meistro et al. 2015) Since the rate of Sarcocystis infection is high in cattle tissues, it can be a human health problem in regions that consumption of raw or under cooked hamburgers or other bovine meat product is common. Hamburger as a popular type of fast foods is produced and consumed in Iran and all over the world so that in USA about 5 billion hamburgers are consumed annually. Hamburger in Iran, is mainly formed of raw beef meat (Hajimohammadi et al. 2014a; Prayson et al. 2008). The purpose of this study was to investigate the prevalence and identification of species of Sarcocystis among of fast food hamburgers, using PCR–RFLP methods in Kashan, central Iran.
Materials and methods
Sample collection
This cross-sectional study was carried out from March 2015 to April 2016 in Kashan, central Iran. Overall 200 raw industrial hamburgers samples with at least 60% meat were randomly collected from 9 different brands in Kashan central Iran. The weight of each sample, the name of brand, product and expiration date, percentage of meat content in the hamburgers were recorded. A 15 g of each hamburger sample was selected, squashed and mixed well and then stored in −20 °C until DNA extraction.
DNA extraction
The genomic DNA extraction was carried out from 10 to 50 mg of each sample using DNP™ kit (Cinnagen, Iran), according to the instructions of the manufacturer protocol. The extracted DNA was stored at −20 °C for PCR amplification.
PCR amplification
A fragment of the 18S rRNA(SSU) gene was amplified by a single PCR using the forward primer sarF 5′-CGT GGT AAT TCT ATG GCT AAT ACA-3′ and reverse sarR 5′-TTT ATG GTT AAG ACT ACG ACG GTA-3′ based on the study of Yang et al. (2002). This primer combination is specific for Sarcocystis genus and does not amplify other and host DNA as described before (Hajimohammadi et al. 2014a, b). The amplified fragment size was approximately 900 bp (922, 953 and 961 bp for S. hominis, S. hirsuta and S. cruzi, respectively).
DNA amplification was achieved in a total volume of 20 μL. The PCR reaction mixture comprised of (final concentration) 10 mM Tris–HCl (pH = 8.9) (final concentration), 50 mM KCl, 1.5 mM MgCl2, 200 nM each of deoxynucleotide triphosphate (dNTP), 20 pmol each of primers and 0.25 µL of Taq DNA polymerase (Pishgam, Iran). Then 1–3 µL of DNA, depending on DNA concentration was added to the PCR reaction mixture and amplified in an automated PCR machine (Flexcycler 2, Germany).
The PCR was performed under the following conditions: an initial denaturation step at 94 °C for 5 min and 35 cycles at 94 °C for 45 s (denaturation), 57.5 °C for 45 s (annealing), 72 °C for 60 s (extension) with a final extension step for 5 min at 72 °C. Distilled water used as a negative control. Five µL of each PCR products were separated by electrophoresis on 1% agarose gel, stained by ethidium bromide and then visualized under ultraviolet light to evaluate success of the reaction.
Restriction fragment length polymorphism (RFLP)
PCR products were digested with the restriction endonucleases BclI (Fermentas, Lithuania) to distinguish between species. Based on the databases, specific fragment sizes of RFLP digestion with this enzyme results in 358 and 595 bp for S. hirsuta and 782 and 140 bp for S. homins. S. cruzi remains uncut with BclI enzyme. RFLP analysis was carried out directly on PCR products in a 15 µL reaction volume, including: 8 µL of PCR product was added to 1X reaction buffer and 1 µL (10 U/μL) BclI and 5 µL distilled water. Digestion mixture took place at 55 °C for 3 h. The restricted fragments were separated and visualized by electrophoresis on 2% high resolution grade agarose gel, stained with ethidium bromide and visualized under ultraviolet light. A 100 bp DNA ladder (Yektatajhiz, Iran) was used as a size marker.
Statistical analysis
The statistical significance among prevalence of Sarcocystis from hamburger samples was determined using Chi square test (SPSS-16 software; Chicago, IL, USA). The p < 0.05 level were considered for statistical analysis.
Results
Out of 200 hamburger samples examined, 58 (29%) (CI 22.7–35.2%) were infected by at least one Sarcocystis species and showed approximately a 900 bp fragment (Fig. 1). However, no macroscopic Sarcocystis were found in any of the hamburger samples.
Fig. 1.
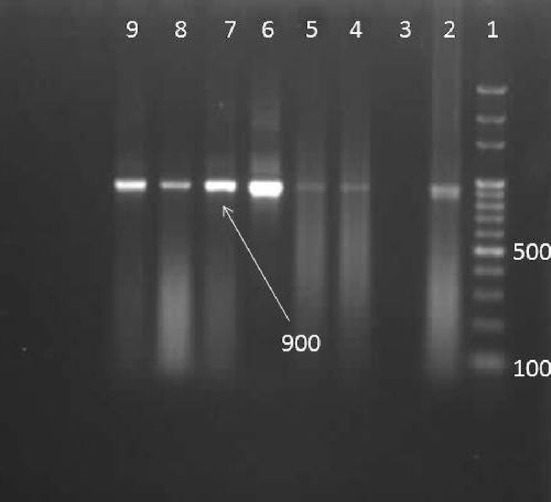
1% Agarose gel electrophoresis of the PCR product of Sarcocystis sp. 1: 100-bpDNA ladder. Lane 2: positive control, lane 3: negative, lane 4–9 samples
The positivity rate was 31.25% (30/96 cases) in the hamburgers with meat 90 and 26.9% (28/104 cases) in the hamburgers with 60–75% meat. No significant difference was found between the infection rate of industrial hamburgers and the percentage of meat content (p > 0.05).
According to PCR–RFLP analysis of the species identification of Sarcocystis, the digested 900 bp amplified fragments using BclI revealed that 43 (74.1%) of the 58 isolates were S. cruzi, 12 (20.7%) showed co-infection to S. cruzi and S. hirsuta, 2 (3.5%) was mixed infected to S. cruzi and S. hominis, 1 (1.7%) showed the pattern of mix infection to three species (Fig. 2). The occurrence of more than one species infection in the hamburgers was 25.9%.
Fig. 2.
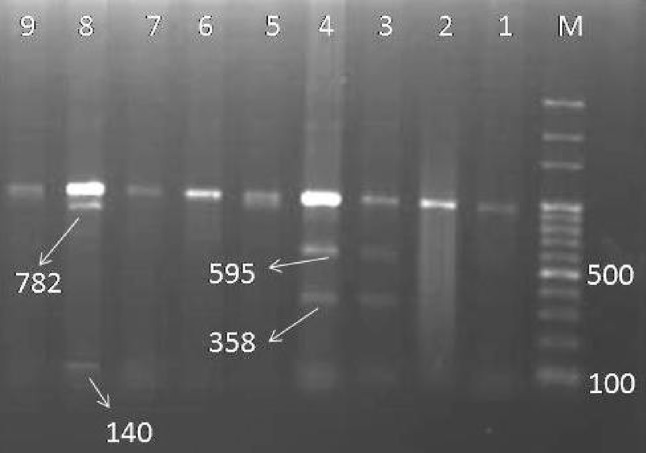
2% Agarose gel electrophoresis of the BclI digested PCR product of Sarcocystis sp. M: 100-bp DNA ladder. Lane 1, 2, 5–7, 9: S. cruzi, lane 3–4: S. cruzi mixed with S. hirsuta, lane 8: S. cruzi mixed with S. hominis
Discussion
Although only S. hominis from beef and S. suihominis from pork, are known to infect humans as definitive hosts, due to this fact that consumption of pork is forbidden in Islamic countries, The only source of intestinal human sarcocystosis in Iran is ingestion of under cooked or raw bovine meat products containing mature cysts of S. hominis.
The results of our study revealed that 29% of industrial hamburgers prepared for sale in Kashan, central Iran are infected at least with one species of Sarcocystis using PCR method. However, another similar study, using other methods such as digestion method, showed an infection rate of 56.0% of Sarcocystis infection in hamburgers, 20% of hot dogs and 8% for sausage in Ahvaz, southern Iran (Rahdar and Salehi 2011). Nematollahi et al. (2015) reported that the prevalence rate of Sarcocystis spp in both traditional and industrial hamburger of Tabriz, northwest of Iran, was 56.25%, using both impression smear and digestion methods (Nematollahi et al. 2015). However, Jahed-Khaniki and Kia (2006) reported only 6.25% infection from meat supplied for hamburger in Iran by histopathologic method (Jahed-Khaniki and Kia 2006).
Prayson et al. (2008) reported that two out of eight (25%) of hamburgers were infected with Sarcocystis spp using histopathologic method (Prayson et al. 2008). It seems this difference in prevalence is related to methods of studies. The most sensitive method to detect light Sarcocystis infection in meat is digestion of host tissues (Dubey et al. 1989), while in histology and molecular methods, only a small pace (10–50 mg) or a thin section used for examination, which may not contain any infection.
There are only a few reports on the prevalence and species identification of Sarcocystis in humbargers using molecular methods. Hajimohammadi et al. (2014c) reported among 25 commercial hamburger samples, 17 (68%) samples were infected to Sarcocystis spp in Yazd province, central Iran using PCR–RFLP methods. (Hajimohammadi et al. 2014c). A similar previous study in Yazd Previnous showed that (87%) of the traditional and (67.8%) industrial hamburger were infected to Sarcocystis sp. (Hajimohammadi et al. 2014a). This inconvenience with our results could be due to using different brands and difference in studies regions.
In this study, species identification of Sarcocystis by PCR–RFLP analysis showed that the prevalence rate of S. cruzi, (74.1%), in the industrial hamburgers was higher than other species or mixed infection and S. cruzi was predominant species. This finding is agreement with most of the previous studies. Hajimohammadi et al. (2014a) reported that among 190 hamburger samples in Yazd province, central Iran, 39 and 67.8%, of the traditional and industrial hamburgers were infected to S. cruzi, respectively (Hajimohammadi et al. 2014a). In a recent study, molecular differentiation of bovine Sarcocystis using PCR–RFLP in tissue samples were obtained from diaphragmatic muscle of 101 cattle slaughtered in Shiraz, Fars Province, Iran showed that 98.9% of positive samples were infected with S. cruzi (Akhlaghi et al. 2016). There are many data on prevalence of Sarcocystis spp., that reported high occurrence of S. cruzi in cattle slaughtered in Iran and various areas of the world (Nourollahi Fard et al. 2009; Nourani et al. 2010; Bucca et al. 2011; Latif et al. 2013; Meistro et al. 2015; Akhlaghi et al. 2016).
These data point out that the environment is heavily contaminated with sporocyst of S. cruzi disposal of dogs as the definitive host. These sporocysts are infectious for cattle as susceptible intermediate host. Some studies confirm that the prevalence of intestinal Sarcocystis in stray and domestic dogs in most localities worldwide is high (Arbabi et al. 2001; Beiromvand et al. 2013; Traub et al. 2014). For example Traub et al. (2014) evaluated the prevalence of gastrointestinal parasites of 411 stray and refuge dogs in four locations in India in 2014 and reported that 44.2% of dogs were infected with Sarcocystis sp.
According to the results of the study, the macroscopic infection to Sarcocystis was not seen in hamburgers and it is agreement with low prevalence of S. hirsuta in this study. This may be due to the fact that macroscopic infection may be eliminated in meat investigation during official inspection in the factory or slaughterhouses. In this study only three cases of infection to S. hominis or mix infection of S. hominis and other species were seen. This findings have also been observed in most of previous studies on human and cattle in Iran. Human intestinal Sarcocytosis is not a common disease in humans in Iran and reported as cases (Rezaian and Ghorbani 1985; Hooshyar and Rezaian 1994). Molecular identification of S. hominis in native cattle of Iran reported as case report by Hajimohammadi et al. (2014b) and Akhlaghi et al. (2016).
The first case identification of S. hominis in hamburgers in Iran was reported by Moghaddam Ahmadi et al. (2015). However, a pervious study on industrial and traditional raw hamburgers in Yazd, Iran showed that a high prevalence of infection to S. hominis (Hajimohammadi et al. 2014a). This rate of infection in such meat hamburgers in Iran is surprising and also questionable. Due to low incidence of S. hominis in human and native cattle in Iran. This may be related to the imported beef from other countries for production of hamburgers and other bovine meat products.
It is concluded that this study revealed one-third of industrial hamburgers were infected to S. cruzi or mixed infection of S. cruzi with other bovine Sarcocystis. To prevent infection of cattle, the possible ingestion of the disposal sporocyst stage from dogs must be eliminated. Dogs should be kept away from grazing cattle with some techniques such as fencing. These measures will prevent contamination of water, feed, and bedding with sporocyst stage of Sarcocystis.
Although in this study, the prevalence rate of S. hominis was low and cannot be considered as a major zoonosis, it should be strongly recommended avoiding eating raw or under-cooked hamburger and other bovine meat products to prevent human infection.
Acknowledgements
We would like to thank Dr. Mahdi Delavari for her advice and assistance throughout the study. This study was financially supported by Vice-Chancellor of Research, Kashan University of Medical Sciences, Kashan, Iran (Grant No. 93141).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The manuscript does not contain clinical studies or patient data.
References
- Akhlaghi M, Razavi M, Hosseini A. Molecular differentiation of bovine Sarcocystis. Parasitol Res. 2016;115:2721–2728. doi: 10.1007/s00436-016-5020-7. [DOI] [PubMed] [Google Scholar]
- Arbabi M, Dorudgar A, Hooshyar H, Asadi MA. Evaluation of Giardia and Sarcocystis contamination in dog-related animal in Kashan region during the years 1999–2001. Feyz. 2001;5(19):83–89. [Google Scholar]
- Beiromvand M, Akhlaghi L, Fattahi Massom SH, Meamar AR, Motevalian A, Oormazdi H, Razmjou E. Prevalence of zoonotic intestinal parasites in domestic and stray dogs in a rural area of Iran. Prev Vet Med. 2013;109(1–2):162–167. doi: 10.1016/j.prevetmed.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Bucca M, Brianti E, Giuffrida A, Ziino G, Cicciari S, Panebianco A. Prevalence and distribution of Sarcocystis spp. cysts in several muscles of cattle slaughtered in Sicily, Southern Italy. Food Control. 2011;22:105–108. doi: 10.1016/j.foodcont.2010.05.015. [DOI] [Google Scholar]
- Daryani A, Alaei R, Dehghan MH, Arab R, Sharif M, Ziaei H. Survey of Sarcocystis infection in slaughtered sheep and buffaloes in Ardabil, Iran. J Anim Vet Adv. 2006;1:60–62. doi: 10.3923/ajava.2006.60.64. [DOI] [Google Scholar]
- Dubey JP. Foodborne and waterborne zoonotic sarcocystosis. Food Water Parasitol. 2015;1:2–11. doi: 10.1016/j.fawpar.2015.09.001. [DOI] [Google Scholar]
- Dubey JP, Speer CA, Fayer R. Sarcocystis of animals and man. Boca Raton: CRC Press; 1989. p. 215. [Google Scholar]
- Dubey JP, Wilpe EV, Bernal RC, Verma SK, Fayer R. Sarcocystis heydorni, n. sp. (Apicomplexa: Sarcocystidae) with cattle (Bos taurus) and human (Homo sapiens) cycle. Parasitol Res. 2015;114(11):4143–4147. doi: 10.1007/s00436-015-4645-2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Moré G, Wilpe EV, Bernal RC, Verma SK, Schares G. Sarcocystis rommeli, n. sp. (Apicomplexa: Sarcocystidae) from cattle (Bos taurus) and its differentiation from Sarcocystis hominis. J Eukaryot Microbiol. 2016;63(1):62–68. doi: 10.1111/jeu.12248. [DOI] [PubMed] [Google Scholar]
- Fayer R. Sarcocystis spp. in human infections. Clin Microbiol Rev. 2014;17(4):894–902. doi: 10.1128/CMR.17.4.894-902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R, Esposito DH, Dubey JP. Human infections with Sarcocystis species. Clin Microbiol Rev. 2015;28(2):295–311. doi: 10.1128/CMR.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajimohammadi B, Dehghani A, Moghadam Ahmadi M, Eslami G, Oryan A, Khamesipour A. Prevalence and species identification of Sarcocystis in raw hamburgers distributed in Yazd, Iran using PCR–RFLP. J Food Qual Hazards Control. 2014;1:15–20. [Google Scholar]
- Hajimohammadi B, Eslami G, Oryan A, Zohourtabar A, Pourmirzaei Tafti H, Moghadam Ahmadi M. Molecular identification of Sarcocystis hominis in native cattle of central Iran: a case report. Trop Biomed. 2014;31(1):183–186. [PubMed] [Google Scholar]
- Hajimohammadi B, Moghadam Ahmadi M, Eslami G, Oryan A, Dehghani A, Zohourtabar A. Molecular method development to identify foodborne Sarcocystis hominis in raw beef commercial hamburger. Int J Enteric Pathog. 2014;2(4):e21139. doi: 10.17795/ijep21139. [DOI] [Google Scholar]
- Hooshyar H, Rezaian M. Human infection with Sarcosystis hominis in northern of Iran: a report of two cases. Res Recon J. 1994;7(24):98–99. [Google Scholar]
- Jahed-Khaniki GR, Kia EB. Detection of the Sarcocystis cysts from meat supplied for hamburger in Iran by histological method. J Med Sci. 2006;6:18–21. doi: 10.3923/jms.2006.18.21. [DOI] [Google Scholar]
- Latif B, Vellayan S, Heo CC, Kannan Kutty M, Omar E, Abdullah S, Tappe D. High prevalence of muscular Sarcocystosis in cattle and water buffaloes from Selangor, Malaysia. Trop Biomed. 2013;30(4):699–705. [PubMed] [Google Scholar]
- Meistro S, Peletto S, Pezzolato M, Varello K, Botta M, Richelmi G, Biglia C, Baioni E, Modesto P, Acutis P, Bozzetta E. Sarcocystis spp. prevalence in bovine minced meat: a histological and molecular study. Ital J Food Saf. 2015;4:85–87. doi: 10.4081/ijfs.2015.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam Ahmadi M, Hajimohammadi B, Eslami G, Oryan A, Yasini S, Ardakani A, Zohourtabar A, Zare S. First identification of Sarcocystis hominis in Iranian traditional hamburger. J Parasit Dis. 2015;39(4):770–772. doi: 10.1007/s12639-014-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nematollahi A, Khoshkerdar A, Ashrafi Helan J, Shahbazi P, Hassanzadeh P. A study on rate of infestation to Sarcocystis cysts in supplied raw hamburgers. J Parasit Dis. 2015;39(2):276–279. doi: 10.1007/s12639-013-0339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourani H, Matin S, Nouri A, Azizi H. Prevalence of thin-walled Sarcocystis cruzi and thick-walled Sarcocystis hirsuta or Sarcocystis hominis from cattle in Iran. Trop Anim Health Prod. 2010;42:1225–1227. doi: 10.1007/s11250-010-9552-z. [DOI] [PubMed] [Google Scholar]
- Nourollahi Fard SR, Asghari M, Nouri F. Survey of Sarcocystis infection in slaughtered cattle in Kerman, Iran. Tropl Anim Health Prod. 2009;41:1633–1636. doi: 10.1007/s11250-009-9358-z. [DOI] [PubMed] [Google Scholar]
- Nourollahi-Fard SR, Kheirandish R, Sattari S. Prevalence and histopathological finding of thin-walled and thick-walled Sarcocysts in slaughtered cattle of Karaj abattoir, Iran. J Parasit Dis. 2015;39(2):272–275. doi: 10.1007/s12639-013-0341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijiaku IN, Ajog I, Umoh JU, Lawal IA, Atu BO. Sarcocystis infection in slaughtered cattle in Zango abattoir, Zaria, Nigeria. Vet World. 2013;6(6):346–349. doi: 10.5455/vetworld.2013.346-349. [DOI] [Google Scholar]
- Prayson B, McMahon JT, Prayson RA. Fast food hamburgers: What are we really eating? Ann Diag Pathol. 2008;12:406–409. doi: 10.1016/j.anndiagpath.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Rahdar M, Salehi M. The prevalence of Sarcocystis infection in meat-production by using digestion method in Ahvaz, Iran. Jundishapur J Microbiol. 2011;4(4):295–299. [Google Scholar]
- Rezaian M, Ghorbani M. Human infection with Isospora hominis: a case report. Iran J Publ Health. 1985;14(1–4):9–15. [Google Scholar]
- Singla LD, Juyal PD. Sarcocystosis. In: Garg SR, editor. Zoonosis: parasitic and mycotic diseases. New Delhi: Daya Publishing House; 2014. pp. 235–250. [Google Scholar]
- Tenter AM. Current research on Sarcocystis species of domestic animals. Int J Parasitol. 1995;25(11):1311–1330. doi: 10.1016/0020-7519(95)00068-D. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Pednekar RP, Cuttell L, Porter RB, Abd Megat Rani PA, Gatne ML. The prevalence and distribution of gastrointestinal parasites of stray and refuge dogs in four locations in India. Vet Parasitol. 2014;205(1–2):233–238. doi: 10.1016/j.vetpar.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Vangeel L, Houf K, Chiers K, Vercruysse J, D’Herde K, Ducatelle R. Molecular-based identification of Sarcocystis hominis in Belgian minced beef. J Food Prot. 2007;70(6):1523–1526. doi: 10.4315/0362-028X-70.6.1523. [DOI] [PubMed] [Google Scholar]
- Yang ZQ, Li QQ, Zuo YX, Chen XW, Chen YJ, Nie L, Wei CG, Zen JS, Attwood SW, Zhang XZ, Zhang YP. Characterization of Sarcocystis species in domestic animals using a PCR-RFLP analysis of variation in the 18S rRNA gene: a cost-effective and simple technique for routine species identification. Exp Parasitol. 2002;102:212–217. doi: 10.1016/S0014-4894(03)00033-X. [DOI] [PubMed] [Google Scholar]


