Abstract
Trichomonas vaginalis is a flagellate parasite living in the genital tract and it is accounted as a sexually transmitted disease. The clinical symptoms vary from the asymptomatic to the severe form which is usually associated with the irritation, itching and infertility in some severe cases. Many drugs have been applied to treat this disease and Metronidazole is the gold standard for treatment; however, it has also detected that this medicine has many side-effects which it has been motivated the researchers to find an appropriate alternative for this medicine. One of the treatment options is the use of the herbal medicines and natural compounds. Thus, the aim of this study was to compare the in vitro anti-T. vaginalis activity of nano-emulsion of Micana cordifolia and Metronidazole. In this study, T. vaginalis was isolated from the clinical samples and were cultured on a modified Dorsate medium. The nano-emulsion of M. cordifolia was prepared by heating technique. The effect of nano-emulsion of M. cordifolia was separately investigated on the T. vaginalis at the times of 12, 24 and 72 h and the obtained data were analyzed by the Spss 20 using the ANOVA test. The results indicated that the concentration of 100 ppm of nano-emulsion of M. cordifolia at the times of 12, 24 and 72 h has the anti-T. vaginalis activity of 44 ± 1.66, 37 ± 1 and 25 ± 2, respectively. It is also observed that the concentration of 500 ppm of the extract has the best effect and was able to eliminate the 85% of T. vaginalis. Furthermore, the anti-T. vaginalis activity of nano M. cordifolia was observed to be 100% in the concentrations of 1000 ppm. It can be concluded, based on the results, that the nano M. cordifolia has acceptable efficacy on the elimination of T. vaginalis and it can be a suitable alternative for Metronidazole after implementation of complementary tests on laboratory animals and human cells.
Keywords: Micana cordifolia, Trichomonas vaginalis, Extract, Metronidazole
Introduction
Trichomonas vaginalis is a flagellate parasite which is living in the genital tract. Several studies have shown that about a third of the world population is annually affected with a sexually transmitted disease (STD) (Edwards et al. 2016). T. vaginalis has known as one of important causative agents of these diseases so that approximately 180 million women are annually infected by this parasite in the entire of the world (Lin et al. 2015; Workowski and Bolan 2015). In developing countries, more than 50% of the patients who is admitted to the STD clinics are suffering from the trichomoniasis (Workowski and Bolan 2015). This parasite can cause the vaginal and cervical infections in the women and also can cause the urinary tract infection in both men and women (Doxtader and Elsheikh 2016). Vaginal discharge is of most common clinical symptom of this disease which it can be associated with the abundant secretion, irritation, itching, frequent urination and lower abdominal pain (Kissinger 2015). This infection is often clinically asymptomatic in the men, but it can affect the prostate, seminal vesicle and the upper parts of the urogenital system in severe cases (Doxtader and Elsheikh 2016).
The disease is often transmitted through sexual intercourse and it can easily transmit between sexual partners; it has a significant prevalence between the female prisoners and prostitutes and is considered as a major health problem among these populations. This disease has been reported from all regions of the country and its prevalence varies from 3 to 70%. The people without clinical symptoms has key role in the transmission of this disease and can be extremely important as a carrier from an epidemiological point of view (Poole and McClelland 2013). The presence of clinical symptoms is the diagnosis basis for the physicians to determine the infection; but, in some cases, the trichomoniasis cannot be certainly diagnosed by only its clinical symptoms such as irritation, itching and smelly discharge and the definite diagnosis of this disease should be performed by vitro methods (Mehlhorn 2009).
The selective drug and gold standard used to treat the trichomoniasis is the Metronidazole. The results of the previous studies have indicated that Metronidazole has carcinogenic effects on the human cells and the drug resistance has established against to this drug which can be considered as the reasons to conduct the studies for new cost-effective and available drug (Workowski and Bolan 2015). So far, various drugs have examined for the in vivo and in vitro treatment of trichomoniasis infection. Of the drugs used, the herbal medicines, as natural products, have found more popularity than other drugs for researchers (Abdali et al. 2015; Ziaei Hezarjaribi et al. 2016).
Micana cordifolia belongs to the order of Asterales. The antimicrobial and antifungal effects of this plant have been proven in various studies and an acceptable effect of this plant has been reported (Muelas-Serrano et al. 2000; Laurella et al. 2012; Kar et al. 2013). Given the importance of trichomoniasis in our country and around the world, this study was conducted to detect the efficacy of the nano M. cordifolia in elimination of T. vaginalis. On the other hand, this study was aimed to investigate the in vitro anti-T. vaginalis activity of Nano M. cordifolia.
Materials and methods
Preparation of Micana cordifolia extracts
In this study, M. cordifolia was provided from Mountainous area of Mazandaran province, Iran. The leaves of M. cordifolia, after plant herbarium confirmation, were separated and were dried in the shade and crushed into powder. This powder was maintained in the dark containers and the extracts of the plant was prepared by distillation method. The extracts of M. cordifolia (1% w/w) in span (0.5% w/w) as oil phase were combined with the Tween 80 (1% w/w) in deionized water as the aqueous phase. The nano-emulsion was prepared by following method: Tween 80 was dispersed in deionized water and the Span 60 as mineral oil was added. The water and oil phases were heated at 40° C and Oil phase was added to the water phase by homogenization and were mixed for 5 min at 22,000 rpm (D-91126 Schwabach, Heidolph, Germany). The mixture was sonicated for 40 min using an Ultrasonicator (Bandelinsonopuls Berlin, Germany) and then, the obtained nano-emulsion of oil in water was cooled in the ice bath. The concentrations of 100, 500 and 1000 ppm of the extracts were applied in this study (Rahimi et al. 2015).
Gas chromatography
Gas chromatography–mass spectrometry (GC–MS) was carried out by Hewlett Packard 6890 series and the DB-5 capillary column (30 m × 0.25 mm, film thickness 0.25 µm) which it was initially performed for 5 min at 60 °C and then, it was continued with increasing the temperature of the device up to 220 °C (4°C per each min). The Helium was used as the carrier with the speed of 2 ml/min.
Cultivation of T. vaginalis
Parasites isolated from patients, after confirmation of species, were cultured in the Dorsate medium and subsequently, the parasite was counted by Neubauer slide. The number of 500,000 parasites per ml was used for this study.
The effect of nano M. cordifolia on T. vaginalis
In the present study, the different parasite concentrations with Metronidazole were used as a positive control and also the medium with DMSO and Tween 80 were used as the negative control. The tubes containing 500,000 parasites were incubated at 37 °C and the herbal nano-emulsion were consequently added. Finally, the number of the living Trophozoite in positive and negative controls and in different concentration was counted by the microscopy technique and methylene blue 1%. The percentage growth inhibition was calculated by the following Eq. 1:
| 1 |
Statistical analysis
The data obtained from this study were analyzed by Spss 20 software using the ANOVA test.
Cytotoxicity assay
The 2,5-diphenyl tetrazolium bromide (MTT) colorimetric technique established by Rahimi (Rahimi et al. 2015) with revision was utilized to display the cytotoxic activity of nano M. cordifolia. Summery, the macrophage cells (1 × 104 cells/ml) were cultured for 24 h on cell culture plates, and were then exposed to 3 different concentrations (100, 500 and 1000 ppm) of nano M. cordifolia. Additionally; metronidazole and DMSO were used as positive and negative controls, respectively. For analysis of the viability, 10 μl MTT reagents in 5.0 mg/ml PBS was added to each well and then kept at 37 °C for 3 h after incubation time, centrifuged at 1300 rpm for 5 min.( Sariego et al. (2014) DMSO was added to the Sediment and after 20 min. The optical density (OD) of each well was measured at 550 nm. All experiences were done in triplicate.
Results
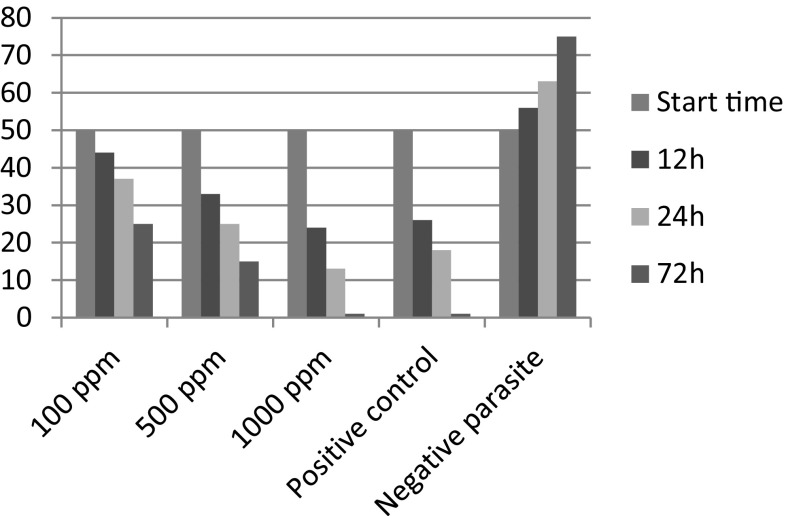
The nano M. cordifolia extracts were used in this study. The nano-emulsion of the M. cordifolia in the concentrations of 100, 500 and 1000 ppm has an acceptable effect against T. vaginalis, which it has detailed in the Table 1 and Fig. 1. The best results are observed in the concentration of 1000 ppm.
Table 1.
Effect of the concentrations of nano-emulsion of M. cordifolia (100, 500 and 1000 ppm) on the T. vaginalis after 12, 24 and 72 h compared with the positive controls (Metronidazole) and negative control (culture medium with DMSO and Tween 80)
| Parasite count | First time 10,000 parasite/ml |
12 h 10,000 parasite/ml |
24 h 10,000 parasite/ml |
72 h 10,000 parasite/ml |
P value | |
|---|---|---|---|---|---|---|
| Nano plant | 100 ppm | 50 | 42 | 37 | 23 | <0.05 |
| 100 ppm | 50 | 46 | 39 | 28 | <0.05 | |
| 100 ppm | 50 | 45 | 36 | 24 | <0.05 | |
| Mean (±) | 44 ± 1.66 | 37 ± 1 | 25 ± 2 | |||
| 500 ppm | 50 | 36 | 24 | 18 | <0.05 | |
| 500 ppm | 50 | 31 | 29 | 14 | <0.05 | |
| 500 ppm | 50 | 33 | 23 | 14 | <0.05 | |
| Mean (±) | 33 ± 1.66 | 25 ± 2.33 | 15 ± 1.66 | |||
| 1000 ppm | 50 | 26 | 17 | 3 | <0.05 | |
| 1000 ppm | 50 | 19 | 11 | 0 | <0.05 | |
| 1000 ppm | 50 | 28 | 13 | 1 | <0.05 | |
| Mean (±) | 24 ± 3.6 | 13 ± 2 | 1 ± 0.3 | |||
| Negative control Positive control Metronidazole |
50 | 56 | 63 | 75 | <0.05 | |
| 50 | 31 | 19 | 0 | <0.05 | ||
| 50 | 26 | 21 | 1 | <0.05 | ||
| 50 | 21 | 16 | 2 | <0.05 | ||
| 26 ± 3.33 | 18 ± 2 | 1 ± 0.33 | ||||
Fig. 1.
Effect of the concentrations of nano-emulsion of M. cordifolia (100, 500 and 1000 ppm) on the T. vaginalis after 12, 24 and 72 h compared with the positive controls (Metronidazole) and negative control (culture medium with DMSO and Tween 80)
According to Table 1, there is a significance difference between the effects of different concentrations of nano-emulsion at different times (P < 0.05). The results of this study have shown that the concentration of 1000 ppm of nano-emulsion has an appropriate ability equal with the Metronidazole, the gold standard drug for the treatment of trichomoniasis, against the T. vaginalis.
Discussion
In present study, the anti-T. vaginalis activity of nano-emulsion of M. cordifolia were compared to Metronidazole ability in the elimination of this parasite which it indicates the a considerable efficacy of the nano-emulsion in this case. The use of herbal medicines to treat a variety of diseases has achieved a large attention recently; so that approximately 20,000 plants are used for this purpose in the world. Many well-known and effective drugs have the plant origin. For example, the quinine, an anti-malarial drug, is provided from cinchona bark which it also has the synthetic derivatives such as chloroquine, amodiaquine and (Swartzwelder et al. 1955; Sapp 1964; Genc 1976).
There are various studies towards the use of the herbal medicines for the elimination of T. vaginalis parasite. Artemisia, Zataria multiflora, Myrtus (Simbar et al. 2008), Lavandula (Moon et al. 2006), Allium sativum, Asafoetida (Ahmed 2010), Artemisia absinthium, Achillea (Rafieian et al. 2011), Eucalyptus (Hassani et al. 2013) and Lavandulifolia Stachys (Youse et al. 2012) are of the plants which has been used to destroy the T. vaginalis in Iran. Furthermore, other plants such as Polygala decumbens, Maytenus imbricate, Harungana madagascariensis, Hypericum polyanthemum and etc. have been used in the various researches towards the elimination of the T. vaginalis in other parts of the world (Iwalewa et al. 2008; Cornejo and Janovec 2010; Frasson et al. 2012; Cargnin et al. 2013). Azadbakht and Ziaei has conducted two separate studies to investigate the effects of the Artemisia, Z. multiflora and Myrtus in elimination of the T. vaginalis (Azadbakht et al. 2003). In these studies, the concentration of 0.001 mg/ml of Artemisia was able to destroy the all of parasites at the beginning of the cultivation; however, the Z. multiflora and Myrtus has completely destroyed the parasites in the concentration of 0.004 ppm. In addition, the most efficiency of the methanolic extracts of plants tested was observed in concentrations of 0.01 (Azadbakht et al. 2003). Kazemian et al. has examined the inhibition activity of Eucalyptus on the T. vaginalis and has shown that the hydroalcoholic extracts of this plant in concentrations of 60 and 90 mg/l can reach to 100% of efficacy against this parasite (Kazemian et al. 2012). Mehdi et al. in the In vitro studies has demonstrated that the Eucalyptus in pH = 4.65 and Myrtus in pH = 5.35 after 24 h are able to eliminate all parasites in an environment (Mahdi et al. 2006).
M. cordifolia is orally used as a diuretic and carminative medicine and is topically used as an anti-inflammatory and analgesic medicine. Recent studies have suggested that the M. cordifolia due to its effective components can successfully eliminate the infectious agents such as bacteria, fungi and parasites (Sen and Batra 2012).
In addition, the anti-parasitic effects of M. cordifolia have been surveyed in various studies and it has detected that this plant has also an anti-parasitic potential (Muschietti et al. 2013).
In this study, the nano-emulsion of M. cordifolia was used in concentrations of 100, 500 and 1000 ppm and it was observed that the concentration of 1000 ppm of this nano-emulsion has outstanding effect against the T. vaginalis so that its effect is equal to the ability of Metronidazole as a Gold standard drug in elimination of these parasites. Recently, the nano-science has gained a variety of applications in various fields which application of this science in medicine and pharmacy industries is considered as a most important application of the nano-science (Koushik et al. 2016). For example, nano-silver compounds have effectively utilized in the treatment of Giardiasis and Leishmaniasis and the results of these studies has been totally satisfactory (Shahcheraghi et al. 2016). Medical Nano-science has provided a new path for treatment, diagnosis, monitoring and biological control of the diseases. This material can be involved alone or have been involved in drug delivery into cells. This material can target the specific cell or intracellular organs and these capabilities are extensively used in medical and pharmaceutical sciences. Given the role of nanoparticles, the drug can bind to their target within the cell or on its surface and are able to have good efficacy. In addition, it is led to avoid the non-specific binding of the drug and to reduce the side-effects of the drug (Want et al. 2016).
Typically, the target of the pharmaceutical compounds is the monocytes, macrophages, dendritic cells, endothelial cells and cancer cells. Parasitic infections such as malaria, Leishmaniasis, Toxoplasmosis and trypanosomiasis can be suitable targets for this family of drugs. However, the application of nano-science has not largely applied in medical Parasitology which it is due to lack of a comprehensive study in this area. Leishmania is an obligate intracellular parasite that it causes the cutaneous leishmaniasis, mucocutaneous leishmaniasis and visceral infection in the human and the Pentavalent compounds are the selective drug for the treatment Leishmania but it has enormous side effects due to its high toxicity. Several studies have revealed that the liposomes compound along with nanoparticles, due to its small size and specific shape, can easily enter into the cells and can play an acceptable anti-Leishmania activity (Want et al. 2016).
Furthermore, the nano-materials have been applied to eliminate intracellular tachyzoites of Toxoplasma and have demonstrated superior effects. In different studies, the researchers has been injected the nano-gold with specific antibody of the cell surface antigens into the body; these compounds were bonded to the cell surface and then were entered into the cell and were led to destroy the intracellular tachyzoite. This feature can be employed to eliminate other intracellular parasites which there are in the blood for long time such as malaria (Fernandes-Cunha et al. 2016).
Bavand et al. (2014) has studied the effect of gold nanoparticles in the three concentrations of 0.05, 0.1 and 1.3 ppm on the human isolates of Giardia lamblia cyst. In that study, the researchers have compared the effects of gold nanoparticles with Metronidazole (positive control group) (Golami et al. 2016). The results of this study have shown that gold nanoparticles in concentration of 0.3 were able to destroy 96% of this G. lamblia within 180 min while the Metronidazole destroyed 99% of this parasite which there is no statistically significant difference. In another study, Jaffari et al. has used the nano silver for the treatment of cutaneous leishmaniasis caused by L. major and has observed the same effect with the positive control group (Jaffary et al. 2016).
In conclusion, given the importance of the trichomoniasis and its considerable prevalence in the world, the finding of the effective treatment with minimal side effects is accounted as important health care goals. The results of this study have shown that M. cordifolia as a natural compound has a suitable potential for elimination of the T. vaginalis and it can be an appropriate alternative to Metronidazole and other synthetic drugs. It is also proposed that the side-effects of M. cordifolia on the human cells and also it be used in vivo.
References
- Abdali K, Jahed L, et al. Comparison of the effect of vaginal Zataria multiflora cream and oral metronidazole pill on results of treatments for vaginal infections including trichomoniasis and bacterial vaginosis in women of reproductive age. Biomed Res Int. 2015;2015:683640. doi: 10.1155/2015/683640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SA. In vitro effects of aqueous extracts of garlic (Allium sativum) and onion (Allium cepa) on Trichomonas vaginalis. Parasitol United J. 2010;3:45–54. [Google Scholar]
- Azadbakht M, Ziai H, et al. Effect of essential oils of Artemisia. Zataria and Myrtus on Trichomonas vaginalis. J Med Plants. 2003;4(8):35–40. [Google Scholar]
- Bavand Z, Gholami SH, Honary S, Rahimi Esboei B, Torbi N, Barabadi H. In vitro evaluation of the effect of gold nanoparticles on Giardia lamblia cyst. Arak Med Univ J. 2014;16(10):27–37. [Google Scholar]
- Cargnin ST, de Brum Vieira P, et al. Anti-Trichomonas vaginalis activity of Hypericum polyanthemum extract obtained by supercritical fluid extraction and isolated compounds. Parasitol Int. 2013;62(2):112–117. doi: 10.1016/j.parint.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Cornejo F, Janovec J. Seeds of Amazonian plants. Princeton: Princeton University Press; 2010. [Google Scholar]
- Doxtader EE, Elsheikh TM. Diagnosis of trichomoniasis in men by urine cytology. Cancer Cytopathol. 2016;125:55–59. doi: 10.1002/cncy.21778. [DOI] [PubMed] [Google Scholar]
- Edwards T, Burke P, et al. Trichomonas vaginalis: clinical relevance, pathogenicity and diagnosis. Crit Rev Microbiol. 2016;42(3):406–417. doi: 10.3109/1040841X.2014.958050. [DOI] [PubMed] [Google Scholar]
- Fernandes-Cunha GM, Rezende CM, et al. Anti-Toxoplasma activity and impact evaluation of lyophilization, hot molding process, and gamma-irradiation techniques on CLH-PLGA intravitreal implants. J Mater Sci Mater Med. 2016;27(1):1–12. doi: 10.1007/s10856-015-5621-1. [DOI] [PubMed] [Google Scholar]
- Frasson AP, dos Santos O, et al. First report of anti-Trichomonas vaginalis activity of the medicinal plant Polygala decumbens from the Brazilian semi-arid region, Caatinga. Parasitol Res. 2012;110(6):2581–2587. doi: 10.1007/s00436-011-2787-4. [DOI] [PubMed] [Google Scholar]
- Genc S. The treatment of giardiasis and trichomoniasis with resochin and metronidazole. Mikrobiyol Bül. 1976;10(3):303. [PubMed] [Google Scholar]
- Golami S, Rahimi-Esboei B, et al. Survey on efficacy of chloroformic extract of Artemisia annua against Giardia lamblia trophozoite and cyst in vitro. J Parasit Dis. 2016;40(1):88–92. doi: 10.1007/s12639-014-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani S, Asghari G, et al. Effects of different extracts of Eucalyptus camaldulensis on Trichomonas vaginalis parasite in culture medium. Adv Biomed Res. 2013;2(1):47. doi: 10.4103/2277-9175.114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwalewa EO, Omisore N, et al. Anti-protozoan activities of Harungana madagascariensis stem bark extract on trichomonads and malaria. J Ethnopharmacol. 2008;117(3):507–511. doi: 10.1016/j.jep.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Jaffary F, Nilforoushzadeh MA, et al. A comparison between the effects of glucantime, topical trichloroacetic acid 50% plus glucantime, and fractional carbon dioxide laser plus glucantime on cutaneous leishmaniasis lesions. Dermatol Res Pract. 2016;11(3):16–20. doi: 10.1155/2016/6462804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar A, Mohammad N, et al. Phytochemical screening of medicinal plant-mikania cordifolia and determination of its charactaristics. Innov J Life Sci. 2013;1(3):24–27. [Google Scholar]
- Kazemian A, Darani HY, Zebardast N, Sereshti M, Banaeian Sh, Safdari F, et al. Evaluation of effect of hydro-alcoholic exteract of Eucalyptuson Trichomonas vaginalis. J Med Plant. 2012;2(22):9. [Google Scholar]
- Kissinger P. Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infect Dis. 2015;15(1):307. doi: 10.1186/s12879-015-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koushik O, Rao Y, et al. Nano drug delivery systems to overcome cancer drug resistance—a review. J Nanomed Nanotechnol. 2016;7(378):2. [Google Scholar]
- Laurella LC, Frank FM, et al. In vitro evaluation of antiprotozoal and antiviral activities of extracts from Argentinean Mikania species. Sci World J. 2012;3(2):96–101. doi: 10.1100/2012/121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W-C, Chang W-T, et al. The pathogenesis of human cervical epithelium cells induced by interacting with Trichomonas vaginalis. PLoS ONE. 2015;10(4):e0124087. doi: 10.1371/journal.pone.0124087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi N, Gany Z, et al. Alternative drugs against Trichomonas vaginalis. East Mediterr Health J. 2006;12:679–684. [PubMed] [Google Scholar]
- Mehlhorn H, Al-Quraishy S, Aziza A, et al. Fine structure of the bird parasites Trichomonas gallinae and Tetratrichomonas gallinarum from cultures. Parasitol Res. 2009;105(3):751–756. doi: 10.1007/s00436-009-1451-8. [DOI] [PubMed] [Google Scholar]
- Moon T, Wilkinson JM, et al. Antiparasitic activity of two Lavandula essential oils against Giardia duodenalis, Trichomonas vaginalis and Hexamita inflata. Parasitol Res. 2006;99(6):722–728. doi: 10.1007/s00436-006-0234-8. [DOI] [PubMed] [Google Scholar]
- Muelas-Serrano S, Nogal J, et al. In vitro screening of American plant extracts on Trypanosoma cruzi and Trichomonas vaginalis. J Ethnopharmacol. 2000;71(1):101–107. doi: 10.1016/S0378-8741(99)00185-3. [DOI] [PubMed] [Google Scholar]
- Muschietti LV, Sulsen VP, et al. Bioprospection of Potential Trypanocidal Drugs: a Scientific Literature Survey over the Period 2000–2010. Stud Nat Prod Chem. 2013;39:297–336. doi: 10.1016/B978-0-444-62615-8.00009-6. [DOI] [Google Scholar]
- Poole DN, McClelland RS. Global epidemiology of Trichomonas vaginalis. Sex Transm Infect. 2013;89(6):418–422. doi: 10.1136/sextrans-2013-051075. [DOI] [PubMed] [Google Scholar]
- Rafieian M, Hejazi SH, et al. Effect of Achillea millefolium, Artemisia absinthium and Juglans regia leaves extracts on Trichomonas vaginalis, in vitra. J Shahrekord Univ Med Sci. 2011;12(4):62–69. [Google Scholar]
- Rahimi MT, Ahmadpour E, et al. Scolicidal activity of biosynthesized silver nanoparticles against Echinococcus granulosus protoscolices. Int J Surg. 2015;19:128–133. doi: 10.1016/j.ijsu.2015.05.043. [DOI] [PubMed] [Google Scholar]
- Sapp OL. Toxic psychosis due to quinacrine and chloroquine. JAMA. 1964;187(5):373–375. doi: 10.1001/jama.1964.03060180059026. [DOI] [PubMed] [Google Scholar]
- Sariego I, Monzote L, et al. Setting a colorimetric assay with MTT for assessment of trichomonicidal activity. Curr Clin Pharmacol. 2014;9(3):283–287. doi: 10.2174/157488470903140806120218. [DOI] [PubMed] [Google Scholar]
- Sen A, Batra A. Evaluation of antimicrobial activity of different solvent extracts of medicinal plant: Melia azedarach L. Int J Curr Pharm Res. 2012;4(2):67–73. [Google Scholar]
- Shahcheraghi SH, Ayatollahi J, et al. Application of nano drugs in treatment of leishmaniasis. Glob J Infect Dis Clin Res. 2016;2(1):018–020. [Google Scholar]
- Simbar M, Azarbad Z, et al. A comparative study of the therapeutic effects of the Zataria multiflora vaginal cream and metronidazole vaginal gel on bacterial vaginosis. Phytomedicine. 2008;15(12):1025–1031. doi: 10.1016/j.phymed.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Swartzwelder J, Mule J, et al. Trichomonas vaginalis infection; evaluation of the susceptibility to systemic medication. Med Times. 1955;83(7):704. [PubMed] [Google Scholar]
- Want MY, Yadav P, et al. Nanomedicines for therapy of visceral leishmaniasis. J Nanosci Nanotechnol. 2016;16(3):2143–2151. doi: 10.1166/jnn.2016.10935. [DOI] [PubMed] [Google Scholar]
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines. Reprod Endocrinol. 2015;24:51–56. [Google Scholar]
- Youse HA, Kazemian A, et al. Effect of Echinophora platyloba, Stachys lavandulifolia, and Eucalyptus camaldulensis plants on Trichomonas vaginalis growth in vitro. Adv Biomed Res. 2012;1(1):79. doi: 10.4103/2277-9175.102987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei Hezarjaribi H, Momeni Z, et al. Effects of hydroalcoholic extract of Saponaria officinalis leaf on growth of Trichomonas vaginalis in vitro. J Mazandaran Univ Med Sci. 2016;25(134):52–59. [Google Scholar]