Abstract
A survey was conducted to isolate indigenous EPN, specifically from the northeastern part of India, a biodiversity hotspot region, to record the occurrence and their further use as biological control agent. The morphological and molecular analysis (ITS rDNA for Steinernema and 16S rRNA for Xenorhabdus) revealed that the entomopathogenic nematodes isolated from four different habitats and its symbiotic bacteria are conspecific with Steinernema sangi and Xenorhabdus vietnamensis respectively. The phylogenetic analysis based on maximum parsimony (MP) revealed that Steinernema sangi belongs to feltiae-kraussei-oregonense group. The study constitutes the first report of Steinernema sangi and its symbiotic bacteria Xenorhabdus vietnamensis outside the type locality, Vietnam, and in particular from India.
Keywords: 16S rRNA, Biodiversity hotspot, ITS rDNA, Northeastern India, Steinernema sangi, Xenorhabdus vietnamensis
Introduction
Due to a growing concern over the resistance developed by certain insect pests to chemical pesticides and serious threats pose to public health, a search for alternative biological control agents is necessitated. Entomopathogenic nematodes (EPNS) are a good candidate to be integrated in insect pest management due to their potential in killing a wide range of insects. EPNs, belonging to the families Steinernematidae and Heterorhabditae in particular, are parasites of various insects, and have been employed for biological control of many insect pests (Kaya et al. 2006). The unique characteristic of EPN is that they are symbiotically associated with bacteria, viz. Xenorhabdus spp. in steinernematids and Photorhabdus spp. in heterorhabditids, in their digestive tract (Boemare 2002). The nematodes after entering the insects’ body release the associated bacteria, which rapidly multiply and kill the host by inducing septicaemia within 24–72 h. The nematodes initiate its development, then feed on the bacterial cells and the host tissues until the food resources in the host cadaver are depleted. Finally, they emerge as a new generation of infective juveniles (IJs) in the soil in search of new hosts (Hazir et al. 2003).
For EPN to be an effective biological pesticide, it is essential to identify naturally-adapted species in a specific area (Stock et al. 1999). Surveys revealed that these nematodes have a world-wide distribution with the exception of Antarctica (Campos-Herrera et al. 2012). Currently, there are 95 and 16 valid species of Steinernema and Heterorhabditis respectively (Hunt and Subbotin 2016), and the work on species description is still continuing.
In India, the works on entomopathogenic nematodes was initiated using exotic species/strains of Steinernema carpocapsae, S. glaseri, S. feltiae and Heterorhabditis bacteriophora. But the results were highly inconsistent due to poor adaptability of the nematodes in an alien environment (Kaya et al. 2006). Since then, surveys have been focussed to indigenous species resulting in the recovery of several species and strains. Currently, ten species of valid entomopathogenic nematodes viz. Heterorhabditis indica, H. bacteriophora, S. abbasi (=S. thermophilum), S. bicornutum, S. carpocapsae (=S. meghalayense), S. glaseri, S. hermaphroditum (=S. dharanai), S. riobrave, S. siamkayai and S. surkhetense have been documented from different parts of the country (Lalramliana and Yadav 2010; Hussaini et al. 2001; Ganguly and Singh 2000; Ganguly et al. 2002, 2011; Kulkarni et al. 2012; Bhat et al. 2017). The objective of this study is to isolate indigenous EPN from the northeastern part of India, a biodiversity hotspot region, to record the occurrence and their further use as biological control agent.
Materials and methods
Nematode isolation and identification
Soil samples were collected at a depth of 10–15 cm covering an area about 1 sq. m from different spots in Mizoram, northeastern India during 2014–2016 (Fig. 1). It was pooled and made up to 400–500 grams in polyethylene bags and transported to laboratory. All the sampling information relating to date, location and soil type were recorded. Isolation of the nematodes was done using baiting techniques (Bedding and Akhurst 1975). The samples were baited with ten numbers of the baiting agent, larvae of Galleria mellonella, in a plastic container (ca 500 ml). The containers were sealed with a lid, turned upside down and kept at room temperature. Larval mortality was observed daily for ten days. The observed dead or infected larvae were transferred to modified White traps (Kaya and Stock 1997). The extracted nematodes were re-infected to the last instar larvae of G. mellonella at 23 ± 3 °C, and the infective juveniles of the nematodes produced were stored with aerated water in an incubator at 12–15 °C.
Fig. 1.
Map of Mizoram, northeastern India showing collection locality (filled circle) of Steinernema sangi
For morphological and morphometric analysis of the nematodes, thirty each of first-generation females, males and IJs were randomly selected. The nematodes were transferred to Ringer’s solution and killed at 60 °C. It was then placed in tri-ethanolamine–formalin (TAF) fixative (Kaya and Stock 1997) and processed to anhydrous glycerine following Seinhorst (1959). Observations were made using an Olympus CX41 microscope.
For molecular characterization of the nematodes, DNA was extracted from a single adult female nematodes (5 replicates) using QIAamp DNA mini kit (Qiagen). The primers used for ITS amplification were primer TW81 (5′-GTTTCCGTAGGTGAACCTGC-3′) forward and AB28 (5′-ATATGCTTAAGTTCAGCGGGT-3′) reverse as described by Joyce et al. (1994). The amplification was done using a ProFlex™ 3 × 32-Well PCR System (Applied Biosystems) and the conditions were: 1 cycle of 94 °C for 1 min followed by 35 cycles at 94 °C for 60 s; 55 °C for 60 s; and 72 °C for 2 min. The last step was 1 cycle at 72 °C for 10 min. The PCR products were directly sequenced in both directions at Scigenom, Kochi, Kerala, India.
Bacteria isolation and identification
The bacteria associated with the EPN were isolated following Emelianoff et al. (2008). The genomic DNA was extracted using QIAamp DNA mini kit (Qiagen) and the 16S rRNA gene was amplified following Tailliez et al. (2006) using primers 16SP1 (5′-GAAGAGTTTGATCATGGCTC-3′) forward and 16SP2 (5′-AAGGAGGTGATCCAGCCGCA-3′) reverse. The conditions applied for the amplification of the gene were: 94 °C for 2 min followed by 35 cycles of 30 s at 95 °C, 30 s at 63 °C and 1 min at 72 °C, followed by 7 min at 72 °C. The PCR products were directly sequenced at Scigenom Kochi, Kerala, India.
Sequence alignment and analysis
The resulting sequences were edited using FinchTV 1.4.0 software packages (Geospiza, Inc.; Seattle, WA, USA; http://www.geospiza.com) and aligned using Clustal X 1.64 (Thompson et al. 1997). The phylogenetic relationships were established using Maximum Parsimony (MP), in MEGA 5 (Tamura et al. 2011) with the selected species sequences of the genera Steinernema and Xenorhabdus retrieved from GenBank. Bootstrap analysis was carried out with 1000 datasets. Heterorhabditis indica (AY321483) and Photorhabdus luminescens (AY594267) obtained from the GenBank were used as the outgroup taxon for Steinernema spp. and Xenorhabdus spp. respectively.
Results
Nematode Identification
Morphological Identification
The nematodes were recovered from 4 localities out of the 76 villages surveyed. The analysis reveals that the morphometric and morphology of the IJs, females, and males of the isolates (Table 1) fall within the range of the original description of Steinernema sangi (Phan et al. 2001) from Vietnam. All the measurements including the D % (distance of excretory pore from anterior end/distance of oesophagous length × 100), E % (distance of excretory pore from anterior end/tail length × 100), ratios (A, B, C) and the morphological characters viz. the shape and length of spicule and gubernaculum in male, the absence of epiptygma and the presence of mucron in female undoubtedly indicate that the isolated nematodes is conspecific to Steinernema sangi.
Table 1.
Comparative morphometrics of Steinernema sangi. Measurements are in μm, and data are expressed in the form of mean ± SD (range)
| Character | Infective juvenile | After Phan et al. (2001) | 1st generation female | After Phan et al. (2001) | 1st generation male | After Phan et al. (2001) |
|---|---|---|---|---|---|---|
| Body length | 748.8 ± 6.2 (697.5–797.5) |
753 ± 18 (704–784) |
5959.6 ± 147.6 (4580–7120) |
6030 ± 679.8 (4830–7200) |
1585 ± 51.0 (1322.5–2192.5) |
1674 ± 220.5 (1440–2325) |
| Body width | 32.5 ± 0.3 (27.5–35) |
35 ± 2.3 (30–40) |
321 ± 4.02 (270–360) |
336 ± 22.9 (270–360) |
156.3 ± 4.2 (127.5–202.5) |
159 ± 27.8 (120–225) |
| EP | 48.8 ± 0.6 (42.5–52.5) |
51 ± 1.8 (46–54) |
98.4 ± 1.6 (77.5–115) |
101 ± 10.4 (80–121) |
76.3 ± 1.2 (67.5–92.5) |
82 ± 9.2 (67–99) |
| NR | 95 ± 0.7 (87.5–100) |
91 ± 3 (78–97) |
154.4 ± 1.0 (140–165) |
158 ± 8.2 (140–170) |
127.5 ± 3.5 (105–167.5) |
126 ± 13 (109–166) |
| Oesophagus length | 125 ± 1.3 (107.5–140) |
127 ± 3.9 (120–138) |
221.5 ± 2.9 (200–250) |
229 ± 7.8 (216–240) |
165 ± 1.5 (152.5–182.5) |
166 ± 15 (150–221 |
| Tail length | 77.5 ± 0.7 (72.5–87.5) |
81 ± 3 (76–89) |
48.8 ± 1.0 (37.5–60) |
49 ± 7.3 (36–62) |
33.8 ± 0.8 (27.5–43.8) |
32 ± 3.9 (27–42) |
| Anal body width | 17.5 ± 0.3 (15–20) |
18 ± 0.6 (17–19) |
107.8 ± 2.2 (87.5–137.5) |
111 ± 13.6 (84–140) |
41.3 ± 0.7 (35–47.5) |
43 ± 2.6 (40–50) |
| Mucron length | – | – | 5.7 ± 0.3 (5–7.5) |
6 ± 1.2 (5–8) |
– | Opaque |
| Spicule Length | – | – | – | 60 ± 0.7 (55–72.5) |
63 ± 4.4 (58–80 |
|
| Spicule width | – | – | – | 12.5 ± 0.3 (10–15) |
12 ± 1.1 (10–14) |
|
| Gubernaculum length | – | – | – | 37.5 ± 0.8 (30–45) | 40 ± 3.1 (34–46) |
|
| Gubernaculum width | – | – | – | 5 ± 12.2 (43–53) |
7.5 ± 0.2 (5–10) |
7 ± 0.8 (5–9) |
| V % = V/BL ×100 | – | – | 48.8 ± 0.6 (38.6–53.7) |
– | ||
| A | 23.3 ± 0.2 (21.5–25.8) |
22 ± 1.3 (19–25) |
18.5 ± 0.3 (15–21.7) |
18 ± 2 (14–21) |
10.2 ± 0.1 (8.9–13.3) |
10.6 ± 1.2 (8.7–12.5) |
| B | 5.9 ± 0.1 (5.4–6.7) |
5.9 ± 0.2 (5.6–6.3) |
26.6 ± 0.6 (20.5–31.6) |
26 ± 2.9 (20–31) |
9.9 ± 0.2 (8.2–12) |
10.1 ± 1 (8.8–12.0) |
| C | 9.6 ± 0.1 (8.4–10.4) |
9.3 ± 0.3 (8.7–10.2) |
123.2 ± 3.1 (95.6–165.1) |
125 ± 18.9 (99–170) |
49.5 ± 1.1 (39.1–70.1) |
52 ± 7.7 (40–75) |
| D % = EP/ES × 100 | 38.8 ± 0.6 (32.1–44.2) |
40 ± 1.7 (36–44) |
44 ± 0.5 (36.9–48.9) |
44 ± 4.7 (35–51) |
45.6 ± 0.6 (40–52.3) |
49 ± 5.8 (42–63) |
| E % = EP/TL × 100 | 62.9 ± 0.9 (54.3–70) |
62 ± 3.1 (56–70) |
203.7 ± 4.3 (154.1–258.8) |
209 ± 23.9 (162–249) |
232.2 ± 5.2 (187.5–318.2) |
255 ± 31.5 (209–341) |
| SW = SPL/ABW | – | – | – | 1.5 ± 0.0 (1.3–1.7) |
1.5 ± 0.1 (1.2–1.6) |
|
| GS = GL/SPL | – | – | – | 0.6 ± 0.0 (0.5–0.7) |
0.6 ± 0.04 (0.5–0.7) |
EP distance of excretory pore from anterior end; NR distance of nerve ring position from anterior end; V distance of vulva position from anterior end; A body length/body width; B Body length/oesophagus length; C Body length/tail length
Nucleotide Analysis and Molecular identification
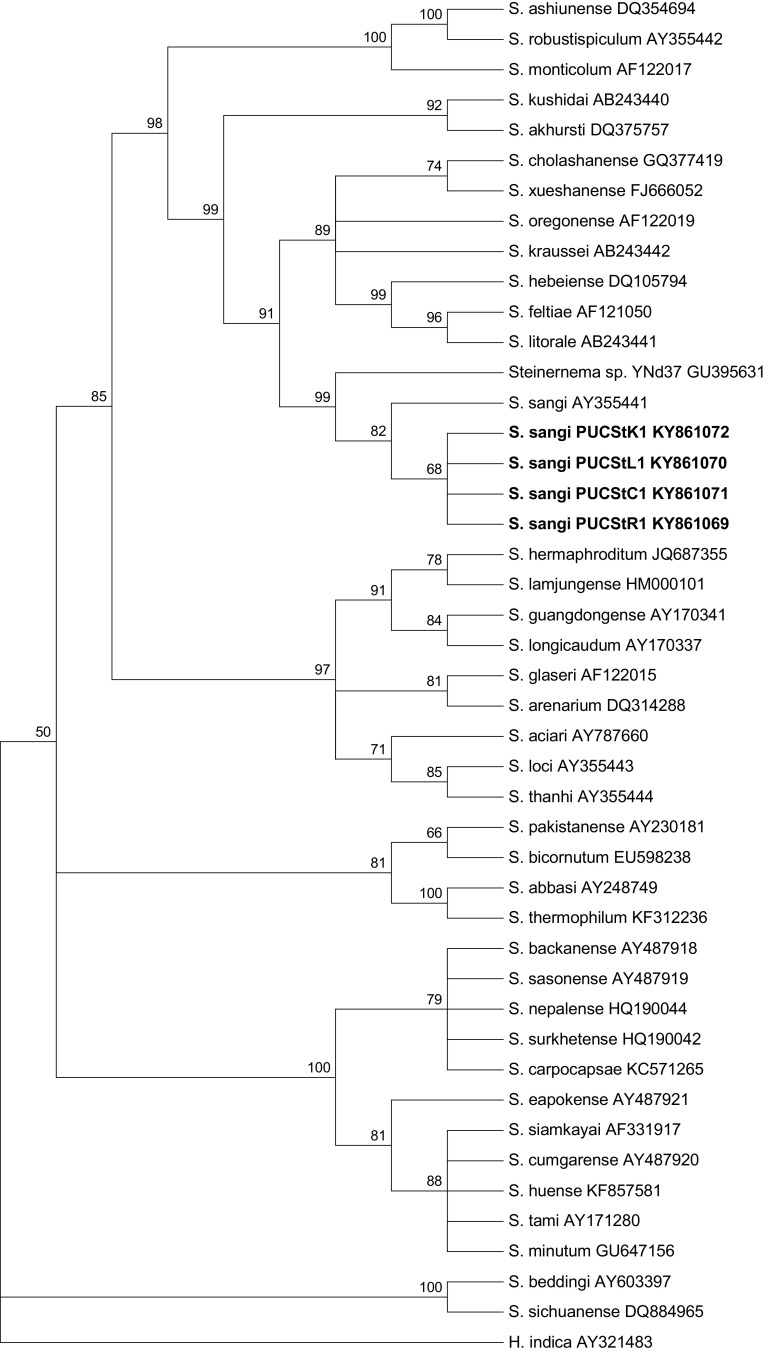
The generated sequence of ITS1 + 5.8S + ITS2 + 28S partial sequence region of the nematode isolate is 759 bp long, with ITS1 = 255 bp, 5.8S = 157 bp, ITS2 = 308 bp and 28S = 38 bp. The developed sequences have been deposited in GenBank (under the accession number KY861069–KY861072). The average base composition [Thymine (T); Cytosine (C); Adenine (A) and Guanine (G)] were T = 34.0%, C = 19.4%, A = 23.6% G = 23.0%, which showed ITS gene of developed sequences of Steinernema sangi were A + T rich (57.0%). The BLAST search of GenBank showed that our nematode has a high similarity (99%) with that sequence available for S. sangi (AY355441). Other closely related species includes Steinernema sp. (GU395631) with 98% similarity, S. cholashanense (GQ377419) with 90% similarity, S. kraussei (KM016394) and S. oregonense with 88% similarity, S. xueshanense, S. texanum and S. akhursti with 87% and S. weiseri, S. litorale and S. feltiae with 86%. The sequences of all other identified Steinernema species available in GenBank showed ≤86% similarity. The K2P distance reveals that the four developed sequences and a single database sequence (AY355441) of Steinernema sangi exhibit an average intraspecies distance of 0.1 ± 0.1% (0.0–0.3%) and an average interspecies distance of 23.7 ± 2.7% (1.0–49.0%) with respect to all other species analysed. The closest species is Steinernema sp. YNd37 (GU395631) with a distance of 1.0–1.3%. The phylogenetic relationships between Steinernema sangi from India and another 39 species of Steinernema acquired from the GenBank are presented in Fig. 2 (tree length = 1082, CI 0.5186, RI 0.7996). The present study constitutes the first report of Steinernema sangi in India.
Fig. 2.
Phylogenetic relationship of Steinernema sangi with other Steinernema spp. based on analysis of the ITS rDNA regions. Numbers indicated at the nodes represents bootstrap proportion values (50% or more, 1000 replicates). Numbers after each species and isolate indicate the GenBank Accession numbers
Bacterial Identification
The symbiotic bacteria isolate is identified through 1192 bp long sequence of 16S rRNA gene. The developed sequences have been deposited in GenBank under the accession number KY859768–KY859770. The BLAST search of GenBank showed that our bacterial isolate has a high similarity (99% with only 1 bp difference) with the two data base sequences of Xenorhabdus vietnamensis (DQ205447 and NR_115713). Other closely related species exhibiting higher similarity were Xenorhabdus japonica (DQ202310) (99% with 16 bp difference); X. khoisanae (JX623972) and X. koppenhoeferi (NR_043637) (98%); X. doucetiae (NR_043642), X. ehlersii (DQ202312), X. hominickii (DQ205449) and X. kozodoii (EU190977) (97%); X. indica (GU480966), X. bovienii (DQ205455) and X. budapestensis (DQ211714) (96%). The sequences of other Xenorhabdus species available in GenBank showed ≤96% similarity. The K2P distance reveals that the distance between the developed sequences and the two database sequences of Xenorhabdus vietnamensis is 0.00%, ascertaining the similarity, whereas the interspecies distance between its closest species X. japonica is only 0.7%. Moreover, the distance between the developed sequence and data base sequence of Xenorhabdus vietnamensis (GU480991) is 3.4% indicating that they are not conspecific, and furthermore, the distance between the data base sequence of X. vietnamensis (GU480991) and X. ishibashi is only 0.4%. The phylogenetic relationships between Xenorhabdus vietnamensis from India and another 19 species of Xenorhabdus acquired from the GenBank are presented in Fig. 3 (tree length = 288, CI 0.4550, RI 0.6171).
Fig. 3.
Phylogenetic relationship of Xenorhabdus vietnamensis with other Xenorhabdus spp. based on analysis of the 16S rRNA regions. Numbers indicated at the nodes represents bootstrap proportion values (50% or more, 1000 replicates). Numbers after each species and isolate indicate the GenBank Accession numbers
Discussion
Since isolates of EPNs from different geographical area exhibit differences in their behavioural and physiological adaptations, identification and documentation of locally-adapted species of a particular region is required for the successful use of EPN as a biological control agent (Stock et al. 1999). Furthermore, accurate identification is very important in understanding the geographical distribution and biodiversity of steinernematids (Kerry and Hominick 2002). However, the identification of Steinernema to the species level based solely on morphological analysis might be risky (Spiridonov et al. 2004). The aim of the study, therefore, was to isolate locally-adapted species and identify to a species level through a combination of morphometric and molecular analysis for future use as an effective biological control agent.
The morphometric measurements falls within the ranges of Steinernema sangi described by Phan et al. (2001) with a slight, but negligible, variation in morphometric measurement. These differences may be attributed to both intraspecific genetic variations and environmental (Rolston et al. 2009; Bolnick et al. 2011). Steinernema sangi have been described originally from the forest of Xuanmy, Thuongxuan, Thanhhoa province, Vietnam (Phan et al. 2001) and never been reported elsewhere. The Indian isolate of Steinernema sangi was also collected from the forest soil of Mizoram, northeast India. The relationship between habitat and abundance of entomopathogenic nematodes had been reported by a number of workers. The high rate of occurrence is reported in disturbed habitat with high human impacts like agricultural fields (Campos-Herrera et al. 2007; de Brida et al. 2017), whereas, it is higher in natural habitats (Stock et al. 2008; Valadas et al. 2014). It is thus obvious that the occurrence of EPNs relative to the habitat type varied among the species, and also depending on the geographical areas. However, we strongly agreed to the remarks of Stock et al. (1999) and Stock and Gress (2006) that the natural habitats offered a high chance of finding native EPN species compare to human modified areas because they are more likely uncontaminated by introduced nematodes.
The phylogenetic analysis inferred from ITS rDNA region indicated that Steinernema sangi is a species belonging to the feltiae-kraussei-oregonense (Clade III) group of Spiridonov et al. (2004) forming a monophyletic group with S. feltiae, S. litorale, S. hebeiense, S. kraussei, S. oregonense, S. xueshanense, S. cholashanense and Steinernema sp. YNd37. Within this group, the isolated Steinernema sangi formed a cluster with Steinernema sp. YNd37 and did not cluster with other species supporting the report of Nguyen et al. (2007, 2008). Among the group, Steinernema kushidai and S. akhursti form a monophyletic group; and similarly S. monticolum, S. ashiunense and S. robustipiculum form another monophyletic group. The tree inferred from the present study highly support monophyletic clades which are in agreement with other phylogenetic studies of the genus Steinernema (Nguyen et al. 2007, 2008; Qiu et al. 2011; Spiridonov et al. 2004; Stock et al. 2001).
A mutualistic relationship between steinernematids and their symbiotic bacteria, with the exception of few Xenorhabdus spp., is species specific (Adams et al. 2006). The symbiotic bacteria associated with Steinernema sangi is Xenorhabdus vietnamensis (Tailliez et al. 2006). For identification and phylogenetic analysis, the sequence of 16S rRNA gene is commonly employed. However, due to the stringent functional constraints, the phylogenetic information in the sequence of 16S rRNA is insufficient (Tailliez et al. 2010). The insufficiency is also observed in this study where the developed and data base sequences of X. vietnamensis exhibit 99% nucleotide identity (K2P distance of only 0.7%) with database sequence of X. japonica. In spite of limitations in its application, 16S rRNA still represents the most important target of study in bacterial ecology (Větrovský and Baldrian 2013). The phylogenetic analysis inferred from the 16S rRNA sequence of the Indian Xenorhabdus vietnamensis reveals that it forms a distinct clade with two data base sequences of X. vietnamensis (K2P distance of 0.00% ascertaining the conspecificity) and X. japonica (K2P distance of 0.7%). However, the wide K2P distance (3.4%) between the Indian Xenorhabdus vietnamensis (alongwith two database sequences of X. vietnamensis) and X. vietnamensis (GU480991) (Lee and Stock 2010) retrieved from Genbank is not acceptable and needs to be resolved. It is apparent that Xenorhabdus vietnamensis (GU480991) is a result of misidentification of the bacteria and its host.
In India, a number of indigenous steinernematids and heterorhabditids have been documented. However, works on the EPNs in northeast India is very limited, and moreover, less is known about their occurrence and potential in the region. So far, five species of EPNs viz. Heterorhabditis indica, H. bacteriophora, Steinernema glaseri, S. abbasi (=S. thermophilum) and S. carpocapsae (=S. meghalayense), have been documented from northeastern India where the bioefficacy and ecological characters of only some species have been studied (Lalramliana et al. 2005; Lalramliana and Yadav 2010, 2016; Ganguly et al. 2011; Yadav and Lalramliana 2012a, b, c; Devi et al. 2016). Among the isolated EPN species from India, several species were claimed as species new to science, viz. Heterorhabditis indica (Poinar et al. 1992), Steinernema thermophilum (Ganguly and Singh 2000), S. masoodi and S. seemae (Ali et al. 2005 ), S. mushtaqi (Pervez et al. 2009), S. qazii (Ali et al. 2009), S. sayeedae (Ali and Shaheen 2011), S. meghalayense (Ganguly et al. 2011) and S. dharanai (Kulkarni et al. 2012). However, further studies treated Steinernema thermophilum as a junior synonym of S. abbasi (Hunt 2007); S. meghalayense and S. dharanai as a junior synonym of S. carpocapsae and S. hermaphroditum respectively; S. masoodi, S. seemae, S. qazii and S. sayeedae under species inquirendae and S. mushtaqi as nomen nudum (Hunt and Subbotin 2016). Therefore, with the isolation of Steinernema sangi from the northeastern region, eleven valid EPN species are presently known from India.
In conclusion, the present study enriched the diversity of indigenous EPNs and provides information on the extended distribution of Steinernema sangi and its symbiotic bacteria in India. Moreover, the occurrence of this species in Mizoram reveals the possibility of further distributional range extension in other parts of northeastern India, including Bangladesh and the eastern part of India. Further, it opens up the prospect of investigating this particular species for biocontrol capabilities against local insect pests.
Acknowledgements
We are grateful to DST-SERB, Government of India for financial support (No. BT/388/NE/TBP/2012); and Dr Tawnenga, Principal, Pachhunga University College for providing laboratory facilities. VRL acknowledge the DBT, Government of India for Institutional Biotech Hub.
Author’s contribution
HCL - Isolation of bacteria, molecular analysis of the nematodes and bacteria and edited the manuscript; VLH - Collect soil samples, nematode extraction, morphological analysis of nematode and edited the manuscript; VRL - Data analysis and interpretation, edited the manuscript; LRL - Data analysis and interpretation, drafted the manuscript.
Compliance with ethical standards
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- Adams BJ, Fodor A, Koppenhöfer HS, Stackebrandt E, Stock SP, Klein MG. Biodiversity and systematics of nematode-bacterium entomopathogens. Biol Contrl. 2006;37:32–49. doi: 10.1016/j.biocontrol.2005.11.008. [DOI] [Google Scholar]
- Ali SS, Shaheen A. Steinernema sayeedae sp. n. a heat tolerant EPN from banana Rhizosphere of Koshambhi District, U.P, India. Trends Biosci. 2011;4:123–125. [Google Scholar]
- Ali SS, Shaheen A, Pervez R, Hussain MA. Steinernema masoodi sp. n. and Steinernema seemae sp. n. (Rhabditida: Steinernematidae) Int J Nematol. 2005;15:89–99. [Google Scholar]
- Ali SS, Shaheen A, Asif M, Akhtar MH. Steinernema qazii sp. n. (Nematoda: Rhabditidae: Steinernematidae) from Kanpur, India. Trends Biosci. 2009;2:59–64. [Google Scholar]
- Bedding RA, Akhurst RJ. A simple technique for the determination of insect parasitic Rhabditid nematodes in soil. Nematologica. 1975;21:109–110. doi: 10.1163/187529275X00419. [DOI] [Google Scholar]
- Bhat AH, Istkhar Chaubey AK, Půža V, San-Blas E. First report and comparative study of Steinernema surkhetense (Rhabditida: Steinernematidae) and its symbiont bacteria from subcontinental India. J Nematol. 2017;49:92–102. doi: 10.21307/jofnem-2017-049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boemare NE. Biology, taxonomy and systematics of Photorhabdus and Xenorhabdus. In: Gaugler R, editor. Entomopathogenic Nematology. UK: CABI Publishing, Wallingford; 2002. pp. 35–36. [Google Scholar]
- Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM, Novak M, Rudolf VHW, Schreiber SJ, Urban MC, Vasseur D. Why intraspecific trait variation matters in community ecology. Trends Ecol Evol. 2011;26:183–192. doi: 10.1016/j.tree.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Herrera R, Escuer M, Labrador S, Robertson L, Barrios L, Gutiérrez C. Distribution of the entomopathogenic nematodes from La Rioja (Northern Spain) J Invertebr Pathol. 2007;95:125–139. doi: 10.1016/j.jip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Campos-Herrera R, Barbercheck M, Hoy CW, Stock SP. Entomopathogenic nematodes as a model system for advancing the frontiers of ecology. J Nematol. 2012;44:162–176. [PMC free article] [PubMed] [Google Scholar]
- de Brida AL, Rosa JM, Oliveira CM, Castro BM, Serrão JE, Zanuncio JC, Leite LG, Wilcken SRS. Entomopathogenic nematodes in agricultural areas in Brazil. Sci Rep. 2017;7:45254. doi: 10.1038/srep45254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi G, Mishra H, Bhattacharyya B, Nath DJ. Occurrence of entomopathogenic nematode (Rhabditida: Heterorhabditidae, Steinernematidae) in white grub infested areas of Majuli, Assam, India. J Biopest. 2016;9:148–156. [Google Scholar]
- Emelianoff V, Chapuis E, Le Brun N, Chiral M, Moulia C, Ferdy JB. A survival-reproduction trade-off in entomopathogenic nematodes mediated by their bacterial symbionts. Evolution. 2008;62:932–942. doi: 10.1111/j.1558-5646.2008.00319.x. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Singh LK. Steinernema thermophilum sp. n. (Rhabditida: Steinernematidae) from India. Int J Nematol. 2000;10:183–191. [Google Scholar]
- Ganguly S, Singh M, Lal M, Singh LK, Vyas RV, Patel DJ. New record of an entomopathogenic nematode, Steinernema riobrave Cabanillas, Poinar and Raulston, 1994 from Gujarat, India. Indian J Nematol. 2002;32:223. [Google Scholar]
- Ganguly S, Rathore KS, Sushil K, Singh M. Steinernema meghalayensis sp. n. (Rhabditida:Steinernematidae) from northeastern hilly region of India. Indian J Nematol. 2011;41:83–97. [Google Scholar]
- Hazir S, Kaya HK, Stock SP, Keskin N. Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biological control of soil pests. Turk J Biol. 2003;27:181–202. [Google Scholar]
- Hunt DJ. Overview of taxonomy and systematics. In: Nguyen KB, Hunt DJ, editors. Entomopathogenic nematodes: systematics, phylogeny and bacterial symbionts. Nematology Monographs and Perspectives 5. Leiden: Brill Publishing; 2007. pp. 27–57. [Google Scholar]
- Hunt DJ, Subbotin SA. Taxonomy and systematics. In: Hunt DJ, Nguyen KB, editors. Advances in entomopathogenic nematode taxonomy and phylogeny. Nematology monographs and perspectives 12. Leiden: Brill Publishing; 2016. pp. 13–58. [Google Scholar]
- Hussaini SS, Ansari MA, Ahmad W, Subbotin SA. Identification of some Indian populations of Steinernema species (Nematoda) by RFLP analysis of ITS region of rDNA. Int J Nematol. 2001;11:73–76. [Google Scholar]
- Joyce SA, Reid A, Driver F, Curran J (1994) Application of polymerase chain reaction (PCR) methods to the identification of entomopathogenic nematodes. In: Burnell AM, Ehlers RU, Masson JP (eds) Proceeding of symposium and workshop, St. Patrick’s College, Maynooth, Co. Kildare, Ireland. European Commission, DGXII, Luxembourg, pp 178–187
- Kaya HK, Stock SP. Techniques in insect nematology. In: Lacey LA, editor. Manual of techniques in insect pathology. San Diego: Academic Press; 1997. pp. 281–324. [Google Scholar]
- Kaya HK, Aguillera MM, Alumai A, Choo HY, de la Torre M, Fodor A, Ganguly S, Hazir S, Lakatos T, Pye A, Wilson M, Yamanaka S, Yang H, Ehlers RU. Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biol Contr. 2006;38:134–155. doi: 10.1016/j.biocontrol.2005.11.004. [DOI] [Google Scholar]
- Kerry BR, Hominick WM. Biological control. In: Lee DL, editor. The biology of nematodes. London: Taylor & Francis; 2002. pp. 483–510. [Google Scholar]
- Kulkarni N, Rizvi AN, Kumar V, Paunikar S, Mishra VK. Morphological and molecular characterization of Steinernema dharanaii sp. n.: a new entomopathogenic nematode from India. Indian J Trop Biodiv. 2012;20:107–116. [Google Scholar]
- Lalramliana, Yadav AK. Occurrence of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Meghalaya, NE India. Sci Vis. 2010;10:89–100. [Google Scholar]
- Lalramliana, Yadav AK. Effects of storage temperature on survival and infectivity of three indigenous entomopathogenic nematodes strains (Steinernematidae and Heterorhabditidae) from Meghalaya, India. J Parasit Dis. 2016;40:1150–1154. doi: 10.1007/s12639-014-0639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalramliana, Shylesha AN, Yadav AK. Effects of temperature and relative humidity on the emergence of infective juveniles of Heterorhabditis indica from Meghalaya, India. Indian J Nematol. 2005;35:157–159. [Google Scholar]
- Lee MM, Stock SP. A multilocus approach to assessing co-evolutionary relationships between Steinernema spp. (Nematoda: Steinernematidae) and their bacterial symbionts Xenorhabdus spp. (γ-Proteobacteria: Enterobacteriaceae) Syst Parasitol. 2010;77:1–12. doi: 10.1007/s11230-010-9256-9. [DOI] [PubMed] [Google Scholar]
- Nguyen KB, Stuart RJ, Andalo V, Gozel U, Rogers ME. Steinernema texanum n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from Texas, USA. Nematology. 2007;9:379–396. doi: 10.1163/156854107781352025. [DOI] [Google Scholar]
- Nguyen KB, Půža V, Mracek Z. Steinernema cholashanense n. sp. (Rhabditida, Steinernematidae) a new species of entomopathogenic nematode from the province of Sichuan, Chola Shan Mountains, China. J Invertebr Pathol. 2008;97:251–264. doi: 10.1016/j.jip.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Pervez R, Ali SS, Asif M (2009) A new species of entomopathogenic nematodes Steinernema mushtaqi sp. n. (Nematoda: Rhabditidae : Steinernematidae) from chickpea rhizosphere, In: Proceedings of international conference on legumes (ICGL), Indian Institute of Pulses Research, Kanpur, February 2009, pp 14–16
- Phan KL, Nguyen NC, Moens M. Steinernema sangi sp. n. (Rhabditida: Steinernematidae) from Vietnam. Russ J Nematol. 2001;9:1–7. [Google Scholar]
- Poinar GO, Jr, Karunakar GK, David H. Heterorhabditis indicus n sp. (Rhabditida: Nematoda) from India: separation of Heterorhabditis spp. by infective juveniles. Fundam Appl Nematol. 1992;15:467–472. [Google Scholar]
- Qiu L, Zhao J, Wu Z, Lv Z, Pang Y. Steinernema pui sp. n. (Rhabditida, Steinernematidae), a new entomopathogenic nematode from Yunnan, China. Zootaxa. 2011;2767:1–13. [Google Scholar]
- Rolston A, Meade C, Boyle S, Kakouli-Duarte T, Downes M. Intraspecific variation among isolates of the entomopathogenic nematode Steinernema feltiae from Bull Island, Ireland. Nematology. 2009;11:439–451. doi: 10.1163/156854109X447015. [DOI] [Google Scholar]
- Seinhorst JW. A rapid method for transfer of nematodes from fixative to anhydrous glycerine. Nematologica. 1959;4:67–69. doi: 10.1163/187529259X00381. [DOI] [Google Scholar]
- Spiridonov SE, Reid AP, Podrucka K, Subbotin SA, Moens M. Phylogenetic relationships within the genus Steinernema (Nematoda: Rhabditida) as inferred from analyses of sequences of the ITS1-5.8S-ITS2 region of rDNA and morphological features. Nematology. 2004;6:547–566. doi: 10.1163/1568541042665304. [DOI] [Google Scholar]
- Stock SP, Gress JC. Diversity and phylogenetic relationships of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) from the Sky Islands of southern Arizona. J Invertebr Pathol. 2006;92:66–72. doi: 10.1016/j.jip.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Stock SP, Pryor BM, Kaya HK. Distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in natural habitats in California. Biodiv Conserv. 1999;8:535–549. doi: 10.1023/A:1008827422372. [DOI] [Google Scholar]
- Stock SP, Campbell JF, Nadler SA (2001) Phylogeny of Steinernema Travassos, 1927 (Cephalobina: Steinernematidae) inferred from ribosomal DNA sequences and morphological characters. J Parasitol 87:877–889. doi:10.1645/0022-3395(2001)087[0877:POSTCS]2.0.CO;2 [DOI] [PubMed]
- Stock SP, Al-Banna L, Darwish R, Katbeh A. Diversity and distribution of entomopathogenic nematodes (Nematoda: Steinernematidae, Heterorhabditidae) and their bacterial symbionts (γ-Proteobacteria: Enterobacteriaceae) in Jordan. J Invertebr Pathol. 2008;98:228–234. doi: 10.1016/j.jip.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Tailliez P, Pagès S, Ginibre N, Boemare N. New insight into diversity in the genus Xenorhabdus, including the description of ten novel species. Int J Syst Evol Microbiol. 2006;56:2805–2818. doi: 10.1099/ijs.0.64287-0. [DOI] [PubMed] [Google Scholar]
- Tailliez P, Laroui C, Ginibre N, Paule A, Pages S, Boemare N. Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa: X. vietnamensis sp. nov., P. luminescens subsp. caribbeanensis subsp. nov., P. luminescens subsp. hainanensis subsp. nov., P. temperata subsp. khanii subsp. nov., P. temperata subsp. tasmaniensis subsp. nov., and the reclassification of P. luminescens subsp. thracensis as P. temperata subsp. thracensis comb. nov. Int J Syst Evol Microbiol. 2010;60:1921–1937. doi: 10.1099/ijs.0.014308-0. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Toby J, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadas V, Laranjo M, Mota M, Oliveira S. A survey of entomopathogenic nematode species in continental Portugal. J Helminthol. 2014;88:327–341. doi: 10.1017/S0022149X13000217. [DOI] [PubMed] [Google Scholar]
- Větrovský T, Baldrian P. The variability of the 16S rRNA gene in bacterial genomes and its consequences for bacterial community analyses. PLoS ONE. 2013;8:e57923. doi: 10.1371/journal.pone.0057923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav AK, Lalramliana Evaluation of the efficacy of three indigenous strains of entomopathogenic nematodes from Meghalaya, India against mustard sawfly, Athalia lugens proxima Klug (Hymenoptera: Tenthredinidae) J Parasit Dis. 2012;36:175–180. doi: 10.1007/s12639-012-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav AK, Lalramliana Efficacy of indigenous entomopathogenic nematodes from Meghalaya, India against the larvae of taro leaf beetle, Aplosonyx chalybaeus (Hope) J Parasit Dis. 2012;36:149–154. doi: 10.1007/s12639-012-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav AK, Lalramliana Soil moisture effects on the activity of three entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) isolated from Meghalaya, India. J Parasit Dis. 2012;36:94–98. doi: 10.1007/s12639-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]