Abstract
Free-living amoebae (FLA) are a group of protozoa with the capabilities of growth in the environment and invasion to the human body which have been isolated from different water sources. Acanthamoeba, Naegleria, and Balamuthia are the most important FLA. These cause a variety of severe complications of eye and central nervous system. Despite the fact that various studies have demonstrated the prevalence of FLA in different parts of Iran, there is no comprehensive evaluation and conclusion regarding the pollution of various water sources in Iran. This review was carried out to achieve the prevalence pattern of FLA in water resources across Iran to design appropriate health strategies. For this purpose, 8 online databases in English and Persian and also graduate thesis and national parasitology congresses were studied. The key words such as “free living amoebae”, “Acanthamoeba”, “Naegleria”, “Hartmannella”, “Balamuthia”, “Sappinia”, “Vermamoebae”, “Valkampfia”, “water resources”, “water” and “Iran” were used to search articles between 1990 to 2017. From a total of 236 articles found, 38 reliable articles were included in the study. From the total number of investigated studies, the estimated prevalence was obtained as 36% among 2430 samples. Although Acanthamoeba prevalence has been considered as a priority, most kinds of free-living amoebae were found in all kinds of water resources. Due to the lack of free-living amoebae prevalence in more than a quarter of the Iranian provinces, more studies are recommended to achieve a better perspective to make comprehensive decisions to improve the hygiene of water resources.
Keywords: Free living Amoeba, Water sources, Iran, Systematic review
Introduction
Free-living amoebae (FLA) as amphizoic amoebae are a group of single cell organisms with the abilities of growth in natural environments such as water, soil, and dust. In appropriate conditions, they are pathogenic in humans and animals (Khan 2006). Although the FLA have been spread from pole to equator (Retana-Moreira et al. 2015; Lorenzo-Morales et al. 2007a; Yousuf et al. 2013; Winck et al. 2011; Onichandran et al. 2014; Kong 2009); their pathogenic cases are rarely reported which may be caused by lack of understanding from the part of the medical staff and misdiagnosis (Visvesvara et al. 2007). The FLA are generally found in two forms: trophozoite and cyst (as resistant form to physicochemical changes and disinfectants). Although their classification is still changing, we can say Acanthamoeba, Naegleria, Balamuthia, Sappinia, Vermamoebae and Valkampfia are the most important genera of FLA (Lorenzo-Morales et al. 2010).
Acanthamoeba genus is more prevalent compared to others and its infection have been reported frequently (Latifi et al. 2014; Niyyati et al. 2014; Ondriska et al. 2004; Stapleton et al. 2009; De Jonckheere 2003). Acanthamoeba in cases with impaired immune systems cause complications as skin ulcers, upper respiratory tract infection and granulomatous amoebic encephalitis (GAE) (Khan 2003). Nevertheless, there are many reports regarding the various cases of vision loss and blindness caused by amoebic keratitis (AK) in healthy people (Stapleton et al. 2009; Lorenzo-Morales et al. 2015). Of course, the same cases of AK caused by Vermamoebae and Valkampfia were reported (Abedkhojasteh et al. 2013). Based on the sequencing of stem 29-1 region of 18srRNA gene, Acanthamoeba are divided into 17 genotypes including T1–T17 and the T4, T3, and T5 genotypes are more prevalent and pathogenic (Kong 2009). A total of more than 30 known species of Naegleria genus, N. fawleri and N. australiensis are considered to be pathogenic by causing meningoencephalitis (Khan 2006). Usually parasites entrance via the central olfactory nervous systems is mediated by diving in contaminated water which causes poor prognosis of meningoencephalitis (Niyyati et al. 2010). Vermamoeba vermiformis genus which was previously called Hartmannella vermiformis or Limax amoeba, is considered to be the pathogen responsible for AK, currently (Niyyati et al. 2010; Lorenzo-Morales et al. 2007b). Vannellidae family as V. persistens have been isolated from various sources of water, but there is uncertainty about its pathogenicity (Nazar et al. 2012).
However, FLA have been isolated from different water sources such as water (Yousuf et al. 2013; Winck et al. 2011), water tanks (Lorenzo-Morales et al. 2006; Mosayebi et al. 2014), swimming pools (Dabirzadeh et al. 2015), water ponds and facades (Lorenzo-Morales et al. 2005a), rivers and lakes (Lorenzo-Morales et al. 2005b), fountains and aqueducts, wells and water channels (Armand et al. 2015), and even bottles of mineral water (Trabelsi et al. 2012). Soil, dust (Karamati et al. 2016; Niyyati et al. 2009a), air conditioning devices, hospital equipment (Lasjerdi et al. 2011a), dental units and ophthalmology equipment (Retana-Moreira et al. 2015; Lasjerdi et al. 2015), respiratory tract tube (Memari et al. 2015), animal waste (Lorenzo-Morales et al. 2007a), etc are the other sources for isolating FLA. The hygiene of water resources has always been of particular importance due to the different uses and constant exposure to humans. Moreover, today symbiosis of FLA has been proven with other microorganisms such as Legionella, Toxoplasma, Campylobacter, Pseudomonas and Helicobacter (Huang and Hsu 2010; Bonilla-Lemus et al. 2010). As mentioned above, the modes of entrance and the host immune system determined the severity and prognosis. Continuous or accidental exposure with contaminated water even in healthy people may lead to the disease (Lorenzo-Morales et al. 2005a, 2015). Therefore, due to strategic planning of water hygiene and the lack of a comprehensive study, this study was carried out to delineate the prevalence of FLA contamination in water sources.
Analysis method
This review has been conducted by searching English scientific databases such as PubMed, Science Direct, Web of Science, Scopus, Google Scholar and three national Persian databases (Magiran, SID, IranMedex) between 1990 and 2017. Search keywords included “free-living amoebae”, “Acanthamoeba”, “Naegleria”, “Hartmannella”, “Balamuthia”, “Sappinia”, “Vermamoebae”, “Valkampfia”, “water resources”, “water” and “Iran”. Full papers and abstracts of the cross-sectional studies were selected in both Persian and English. Articles have survey the prevalence of FLA in water resources of Iran as well as studies of FLA species genotyping. Studies with other samples than water such as soil, equipment, humans or animals derivation were excluded. Articles were selected by inclusion and exclusion criteria, reliability of study design and validity of data and by two researchers separately.
Results
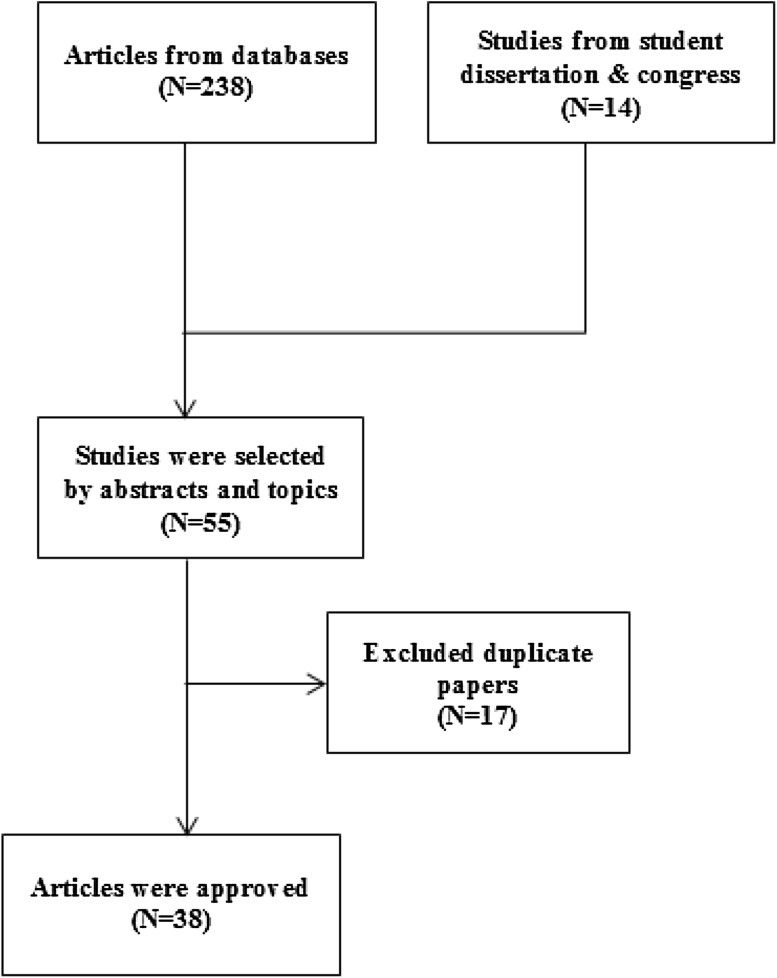
Of the 252 papers obtained from all of the databases, 38 articles were reliable and were scrutinized (Fig. 1). In the selected articles, 28 articles were scrutinized to assess the prevalence of merely Acanthamoeba. Amoeba culture was used as detection method in 12 studies while other studies used molecular methods (Fig. 2; Table 1).
Fig. 1.
Study diagram
Fig. 2.
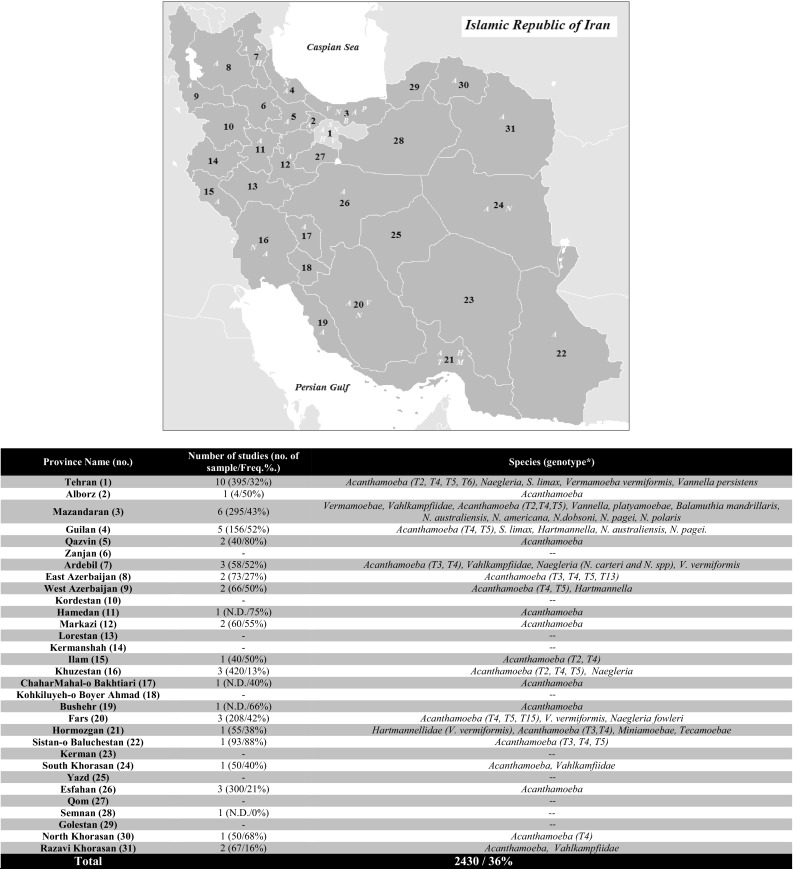
FLA distribution in Iran by province. (A): Acanthamoeba sp., (N): Naegleria sp., (B): Balamuthia sp., (V): Vannella sp., (H): Hartmannella or V. vermiformis sp., (P): Platyamoebae sp
Table 1.
FLA prevalence in water source of Iran by article (M; multi city, C.M.; Culture medium)
| Author(s)/Year (ref.) | Provenience (city) | Method | Sample type (Freq.%) | Genus (genotype) |
|---|---|---|---|---|
| Latifi et al. (2017) | Mazandaran (M.) | C.M. & PCR | Hot spring (54%) | Vahlkamfiids (N. australiensis, N. dobsoni, N. americana, N. pagei, N. polaris and N. fultoni) |
| Mafi et al. (2017) | Tehran (Tehran) | C.M. | Pool and park ponds (24%) | Acanthamoeba, Hartmannella, Vahlkampfiids (Naegleria) |
| Khezri et al. (2016) | West Azerbaijan (M.) | C.M. & PCR | River and tap water (45%) | Acanthamoeba (T4, T2) |
| Aghajani et al. (2016) | Sistan-o Baluchestan (M.) | C.M. & PCR | Pool and pond (41%) | Acanthamoeba (T4, T5, T3) |
| Aghajani et al. (2016) | Isfahan (Isfahan) | C.M. | Tap water and environmental water (45%) | Acanthamoeba |
| Shokri et al. (2016) | Mazandaran (Sari & suburbs) | C.M. & PCR | Lake, river, waterscape, sea, tap water, pool, waterhole, rice field and fishpond (55%) | Acanthamoeba (T4, T2) |
| Niyyati et al. (2016b) | Ilam (Dehloran) | C.M. & PCR | Recreational geothermal water; swimming pool, spring, river (50%) | Acanthamoeba (T2, T4) |
| Latifi et al. (2016) | Mazandaran (M.) | C.M. & PCR | Hot spring (3%) | Balamuthia mandrillaris |
| Niyyati et al. (2015c) | Guilan (Rasht) | C.M. & PCR | Recreational water; pond, pool, river (15%) | Naegleria spp. (N. australiensis, N. pagei) |
| Niyyati et al. (2015a) | Hormozgam (Kish) | C.M. & PCR | Tap water; household water resources (38%) | Acanthamoeba (T3; A. griffini, T4; V. vermiformis, T5; A. lenticulata, T11), Hartmannella Vermiformis |
| Thecamoeba, miniamoebae | ||||
| Mahmoudi et al. (2015a) | Guilan (M.) | C.M. | River, dam, lagoon (88%) | Acanthamoeba |
| Armand et al. (2015) | Fars (Shiraz) | C.M. & PCR | Pool, pond park and tap water (59%) | Acanthamoeba (T4, T7, T5; A. lenticulata, T15; A. jacobsi), Vermamoebae |
| Behniafar et al. (2015) | East Azerbaijan | C.M. & PCR | Tap water, river, hot/cold water springs, well (25%) | Acanthamoeba (T4, T3, T13) |
| Mahmoudi et al. (2015c) | Razavi Khorasan (Mashhad) | C.M. | Square fountain and ponds (10%) | Acanthamoeba |
| Mahmoudi et al. (2015b) | Guilan, Mazandaran, Alborz, Tehran. | C.M. & PCR | Tap water, hot spring, lake dam, river, sea, waterfall and pond (71%) | Acanthamoeba (T4, T5), V. vermiformis, Saccamoeba limax |
| Niyyati et al. (2015b) | Tehran (Tehran) | C.M. & PCR | Surface water (19%) | Acanthamoeba (T4, T5) |
| Dabirzadeh et al. (2015) | Sistan-o Baluchestan (Zahedan) | C.M. & PCR | Square fountain, ponds (88%) | Acanthamoeba |
| Behravan et al. (2015) | South Khorasan (Birjand) | C.M. & PCR | Surface water; square fountain and ponds water distribution stations (42%) | Acanthamoeba, Vahlkamfiidae |
| Salehi (2014) | North Khorasan (Bojnurd) | C.M. & PCR | Surface water; tap water, river, swimming pool, square fountain and creek (68%) | Acanthamoeba (T4) |
| Latifi et al. (2014) | Mazandaran (M.) | C.M. | Hot spring (26%) | Acanthamoeba, Vahlkamfiidae, Vermamoebae, Vannella, Platyamoeba |
| Mosayebi et al. (2014) | Markazi (M.) | C.M. | Drinking water; tap water, spring, aqueduct, well (61%) | – |
| Hooshyar et al. (2013) | Qazvin (Qazvin) | C.M. & PCR | Surface water (80%) | Acanthamoeba (T2; A. palestinensis, T4; A. polyphaga) |
| Ghadar-ghadr et al. (2012) | Fars (Shiraz) | C.M. | Tap water, well, water tank (35%) | Acanthamoeba, Naegleria |
| Hosseinbigi et al. (2012) | Qazvin (Qazvin) | C.M. & PCR | Square fountain and ponds (80%) | Acanthamoeba |
| Rahdar et al. (2012) | Khuzestan (ahvaz) | C.M. & PCR | Pool, river, tap water, pond, aqueduct (72%) | Acanthamoeba (T4, T2) |
| Mahmoudi et al. (2012) | Guilan (M.) | C.M. | Tap water, river, sea, waterfall and pond (70%) | Acanthamoeba |
| Nazar et al. (2012) | Tehran (Tehran) | C.M. & PCR | Recreational water environments; fountain and pond (54%) | V. vermiformis, Vannella persistens |
| Solgi et al. (2012a) | Ardebil (M.) | C.M. & PCR | Hot spring (27%) | Hartmannella Vermiformis |
| Naegleria | ||||
| Niyyati et al. (2012) | Tehran (suburbs of Tehran) | C.M. & PCR | Recreational surface water; river and pond (27%) | Acanthamoeba (T4, T15; A. jacobsi) |
| Naegleria (N. Fultoni, N. pagei, N. Clarki) | ||||
| Solgi et al. (2012a) | Ardebil (M.) | C.M. & PCR | Hot spring (33%) | Thermotolerant Acanthamoeba (T4, T3;A. griffinii) |
| Badirzadeh et al. (2011) | Ardebil (M.) | C.M. & PCR | Hot spring (78%) | Acanthamoeba castellanii |
| Vahlkamfiids | ||||
| Rasti et al. (2011) | Isfahan (Kashan) | C.M. | Swimming pool (0%) | – |
| Nazar et al. (2011) | Tehran (Tehran) | C.M. & PCR | Park pond and pool (32%) | Acanthamoeba (T4, T5) |
| Bagheri et al. (2010) | Iran (14 cap. of provincial) | C.M. | Tap water (48%) | Acanthamoeba spp. |
| Niyyati et al. (2009b) | Tehran (Tehran) | C.M. & PCR | Pool (1 case reported) | Acanthamoeba (T2, T6) |
| Eftekhar et al. (2009) | Tehran (Tehran) | C.M. & PCR | Square fountain and ponds (59%) | Acanthamoeba |
| Rezaeian et al. (2008) | Tehran (Tehran) | C.M. | Tap water, swimming pool and pond (33%) | Acanthamoeba spp. |
| Rezaian et al. (2003) | Khuzestan (Kazeroun) | C.M. | Surface water; spring, river, creek and pond (4%) | Acanthamoeba, Naegleria |
FLA in drinking water (tank and tap water)
Despite all controversies surrounding the prevalence of FLA, they were seen in most studies on samples of drinking water (particularly Acanthamoeba). As an apparent contradiction, no contamination was found in Shiraz in 2015 (Armand et al. 2015) while 10% prevalence have been reported in previous studies with same sample (Ghadar-ghadr et al. 2012). This conflict was reported in Arak between 2010 and 2014 (Mosayebi et al. 2014; Bagheri et al. 2010). Importantly, even one positive case has been reported in Ahvaz, Birjand and Semnan. Of course, in a comprehensive study on the tap water of 14 hospitals in the provincial capital, 12 positive cases were found (Bagheri et al. 2010). Although except Acanthamoeba, other FLA have been neglected in most studies, there are many reports of Hartmannella, Naegleria, Thecamoeba, and Miniamoebae (Niyyati et al. 2015a). Even though in many studies, genotype determination has not been conducted; in genotyping study of Acanthamoeba, T4 was considered as dominant genotype and T3, T5, T11 & T13 genotypes were the most common, respectively (Behniafar et al. 2015).
FLA in surface and groundwater
River water as the most popular surface water have been examined frequently. Rivers in Mazandaran and Gilan providences (northern Iran) as the rivers of Tehran province, have been studied more (Mahmoudi et al. 2012, 2015a, b). In other regions, more studies are required, even though few studies have been conducted in Bojnurd, Arak, Karaj, Kazeroun, Ahvaz, East and West Azarbaijan (Behniafar et al. 2015; Niyyati et al. 2012; Rahdar et al. 2012; Salehi 2014). The infestation rate of FLA was about 47%, among the total samples taken from various rivers. The most species were seen and Acanthamoeba, Naegleria, V. vermiformis and Saccamoeba had the highest prevalence, respectively. However, Acanthamoeba has still attracted the most attention, and this may be ignoring the other FLA (Mahmoudi et al. 2012; Rahdar et al. 2012; Salehi 2014). Other resources such as canals of agricultural water, wells, lakes and lagoons, aqueducts, dams and waterfalls have been studied to a limited extent. However, in some studies, the exact number of samples has not been mentioned, but through the mentioned cases, lakes (61.1%), wells (48.2%) and agricultural water channels (24.6%) were found as the most contaminated, respectively (Mahmoudi et al. 2012; Rahdar et al. 2012; Rezaian et al. 2003). Due to insignificant number of samples collected from aqueducts, dams and waterfalls, there is no reliable estimation of the prevalence (Mosayebi et al. 2014; Mahmoudi et al. 2015b). Similarly, Acanthamoeba was the main purpose of most studies, while other FLA such as V. vermiformis, Saccamoeba, Naegleria have also been detected (Mahmoudi et al. 2015a, b; Niyyati et al. 2012). Although in most cases, genotyping of Acanthamoeba have not been done, T4 genotype (89.4%) was considered as the most frequent and also T2, T3, T5 and T15 have been isolated (Behniafar et al. 2015; Mahmoudi et al. 2015b; Niyyati et al. 2012; Rahdar et al. 2012).
FLA in Recreational Water (pools, springs and sea)
Although there are limited studies on the prevalence of FLA in the Caspian Sea (North of Iran), most studies in recreational water have been done on springs and pools (Latifi et al. 2014; Behniafar et al. 2015; Badirzadeh et al. 2011; Solgi et al. 2012a, b). However, Acanthamoeba seen in most samples (80%) were collected from the Caspian Sea (Mahmoudi et al. 2015a, b). Moreover, various studies have indicated a prevalence of 27.2% on hot/cold springs in Ardebil, Mazandaran, Gilan, East Azerbaijan and Arak (Mosayebi et al. 2014; Behniafar et al. 2015; Badirzadeh et al. 2011; Solgi et al. 2012a, b). In addition to rare amoebae (such as Vannella and Platyamoeba), other FLA such as Acanthamoeba, Vahlkamfidae, Naegleria, Hartmannella and Vermamoebae have been isolated in springs (Mosayebi et al. 2014; Behniafar et al. 2015; Badirzadeh et al. 2011; Solgi et al. 2012a, b). Importantly, pathogenic amoebae Balamuthia menderiallis were isolated from hot springs (Latifi et al. 2016). Among the studies that performed sequencing, T4 and T3 are known as the most common genotypes of Acanthamoeba (Behniafar et al. 2015; Badirzadeh et al. 2011; Solgi et al. 2012a, b).
Surprisingly, only 5% of indoor swimming pool samples present FLA. The pools of Tehran, Kashan, Shiraz, Sari, Dehloran and Bojnoord (Rahdar et al. 2012; Salehi 2014; Rasti et al. 2011; Rezaeian et al. 2008) have been analyzed and Acanthamoeba on T4 genotype have been the predominant and T5, T3, and T6 genotype have also been reported(Armand et al. 2015; Niyyati et al. 2009b). Although there are reports of other FLA such as Naegleria and Vermamoebae, due to the lack of sufficient attention toward them, it’s not reliable to report the decisive prevalence of FLA on Iran’s pools (Armand et al. 2015).
FLA in stagnant water (ponds, fountains and streams)
Most studies have been conducted on fountains and ponds water in city squares and parks in Tehran, Shiraz, Qazvin, Mashhad, Birjand, Bojnoord, Sistan (Nazar et al. 2011, 2012; Dabirzadeh et al. 2015; Salehi 2014; Rezaeian et al. 2008; Behravan et al. 2015; Hosseinbigi et al. 2012; Niyyati et al. 2015b). Acanthamoeba, Naegleria, Valkampfia, Hartmannella, and Vanella were seen in 55% of samples, approximately. Due to the attention given to Acanthamoeba in many cases, other types of FLA have been neglected (Nazar et al. 2011, 2012; Dabirzadeh et al. 2015; Armand et al. 2015; Salehi 2014; Mahmoudi et al. 2015c; Rezaeian et al. 2008; Behravan et al. 2015; Hosseinbigi et al. 2012; Niyyati et al. 2015b). In few cases, genotyping were done and T4 genotype was identified as the dominant genotype, again. Other genotype such as T2, T3, T5 and T15 have been reported (Armand et al. 2015; Hooshyar et al. 2013).
Discussion and conclusion
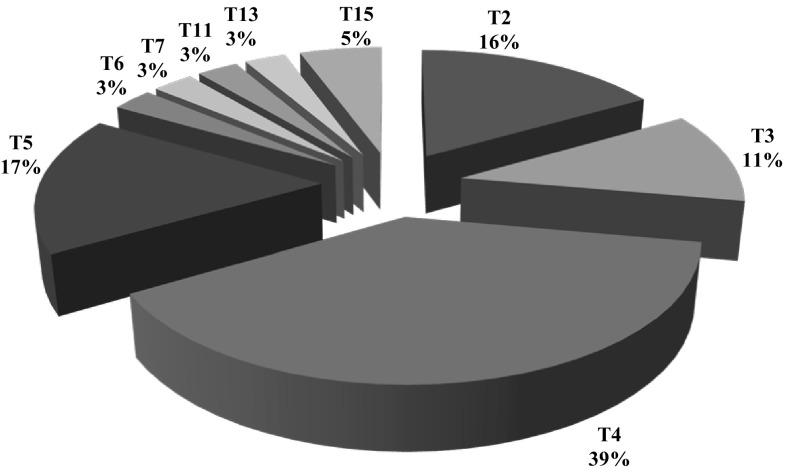
In recent years, due to the importance of FLA, studies on FLA prevalence in Iranian water sources have increased. Although more than half of the studies have been conducted within the three provinces; Tehran, Mazandaran and Gilan, there is no epidemiological study in almost 30% of the provinces of Iran (Fig. 2). Due to suspicious reports of the contaminated cases in other countries and doubtful production process, the bottles of drinking mineral water are other points being neglected for investigation (Maschio et al. 2015; Rivera et al. 1981). Unfortunately, in many studies, Acanthamoeba were evaluated and other FLA were ignored (Fig. 2). Also, Iranian researchers have been concerned with genotyping, that’s why today we cannot state dominant genotype, accurately (Fig. 3).
Fig. 3.
Acanthamoeba genotype report by article (Freq.%)
However, due to the multiple uses of tap water, such as washing and bathing in addition to drinking in Iran, accurate and constant exploration is required. Overall, based on studies conducted in Iran on different water resources, FLA contamination is about 36%. It also seems that Acanthamoeba is the dominant species and T4 were estimated as the predominant genotypes of Acanthamoeba on Iran’s water resources (Fig. 3). Although there are few comprehensive studies of epidemiological prevalence of FLA on water throughout the world, the prevalence of 36% seems close to reality. The prevalence of Acanthamoeba and Naegleria in drinking water on Pakistan (southeastern neighbor of Iran) have been estimated as 30 and 8%, respectively (Yousuf et al. 2013). In addition, antibodies against Acanthamoeba and Naegleria were found on the sera of whole local people. In addition, the lower seroprevalence of antibody against Balamuthia demonstrates the significance of this rare and pathogenic amoeba. The presence of FLA in water resources in South Korea and US (Florida State) has been reported as 30-15% and 8.2%, respectively (Seal et al. 2006). These findings, confirmed the variety of effectiveness of water purification and distribution systems in different countries. It seems important to consider that Acanthamoeba was detected in tap water in one third of AK patients, in UK (Kilvington et al. 2004). This indicates the inefficiency of filtration methods of drinking water (i.e. ozone and activated carbon filtration) in replacing efficient methods (Thomas et al. 2008).
As expected, direct contact with the soil has been confirmed as the highest prevalence of FLA on aqueducts, wells, rivers, springs and agricultural canals. It should be noted that water resources are mentioned to have drinking, washing and entertainment usage in tourist areas, particularly (Mosayebi et al. 2014; Ghadar-ghadr et al. 2012; Salehi 2014; Rezaian et al. 2003). Due to constant population exposure, ponds, fountains and pools of squares and parks are considered as important resources of hand-made standing non-drinkable water. In addition to people who are directly in contact with them, they may be considered as a predisposing factor to people who inhale artificial water droplets containing FLA (especially Naegleria) produce by fountain (Nazar et al. 2011, 2012). Due to the patient’s use of swimming pools and springs, appropriate notification should be given to patients with immune system disorders (Behniafar et al. 2015; Badirzadeh et al. 2011; Solgi et al. 2012a, b).
However, in most studies, it can be seen that FLA detection has been improved by observing cyst or trophozoite in non-nutrient agar (NNA) medium and have ignored verification by molecular methods. The morphological diagnostic methods as objective method depend on the technician skills and are accompanied by ignoring or misdiagnosis. However, PAGE as FLA diagnosis key can be reliable when condensing methods and special staining (i.e. Giemsa and Trichrome) are used by experts, accurately (Garcia 2006; Page 1988). Another requirement to achieve reliable results is the accuracy of compounds present in Page’s Amoeba saline solution. Negligence in any of the foregoing, may lead to some suspicious results (Rezaian et al. 2003; Rasti et al. 2011; Manesh et al. 2016).
Importantly, symbiosis with other microorganisms in FLA (particularly Acanthamoeba), except for a few cases is a neglected subject in Iranian scientific studies (Mahmoudi et al. 2015a; Niyyati et al. 2015b). Nevertheless, there are numerous evidences which admit the coexistence of a variety of microorganisms in FLA, i.e. Legionella, Pseudomonas, Pasteurella, Aeromonas, mycobacteria and chlamydia (Okude et al. 2012; Delafont et al. 2014; Zeybek and Binay 2014).
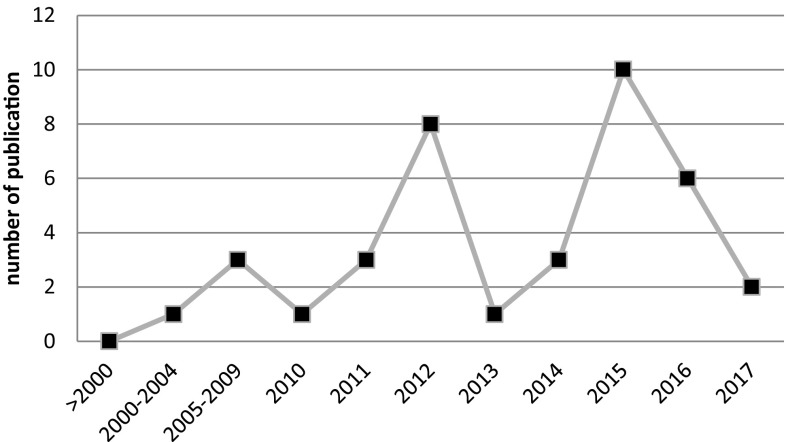
Recently, the reports of AK have increased in Iran (Hajialilo et al. 2015, 2016) (Fig. 4). On the other hand, comprehensive investigation have shown that FLA isolation is possible due to various sources in Iran such as; mouth or nose of human (Memari et al. 2016), soil (Niyyati et al. 2016a), animal feces (Niyyati et al. 2009b), hospital equipment (Lasjerdi et al. 2011b; Niyyati et al. 2015c). It could be helpful to make decisions for comprehensive health policy, detailed morphological and molecular examination of all resources, including water and soil to access the accurate prevalence of all species of FLA particularly Acanthamoeba, Balamuthia, Naegleria, Hartmannella, Vanella, Vermamoebae, Saccamoeba, Miniamoebae, Sappinia (and also less known amoebae i.e. Mayorella, Korotnevella and Echinamoeba) (Ramirez et al. 2014).
Fig. 4.
Number of publications of FLA by Iranian scientist
Acknowledgements
We would like to thank Dr. Ali Reza Esmaeli for helpful comments on the manuscript.
Abbreviation
- AK
Amoebic keratitis
- PCR
Polymerase chain reaction
- FLA
Free living amoeba
- GAE
Granulomatous amoebic encephalitis
- NNA
Non-nutrient agar
Compliance with ethical standards
Conflict to interest
All the authors declare that they have no conflict of interest.
References
- Abedkhojasteh H, Niyyati M, Rahimi F, Heidari M, Farnia S, Rezaeian M. First report of Hartmannella keratitis in a cosmetic soft contact lens wearer in Iran. Iran J Parasitol. 2013;8(3):481–485. [PMC free article] [PubMed] [Google Scholar]
- Aghajani A, Dabirzadeh M, Maroufi Y, Hooshyar H. Identification of acanthamoeba genotypes in pools and stagnant water in ponds in Sistan region in Southeast Iran. Turkiye Parazitol Derg. 2016;40(3):132–136. doi: 10.5152/tpd.2016.4428. [DOI] [PubMed] [Google Scholar]
- Armand B, Motazedian MH, Asgari Q. Isolation and identification of pathogenic free-living amoeba from surface and tap water of Shiraz City using morphological and molecular methods. Parasitol Res. 2015;9:1–9. doi: 10.1007/s00436-015-4721-7. [DOI] [PubMed] [Google Scholar]
- Badirzadeh A, Niyyati M, Babaei Z, Amini H, Badirzadeh H, Rezaeian M. Isolation of free-living amoebae from Sarein hot springs in Ardebil province, Iran. Iran J Parasitol. 2011;6(2):1–8. [PMC free article] [PubMed] [Google Scholar]
- Bagheri H, Shafiei R, Shafiei F, Sajjadi S. Isolation of Acanthamoeba spp. From drinking waters in several hospitals of Iran. Iran J Parasitol. 2010;5(2):19–25. [PMC free article] [PubMed] [Google Scholar]
- Behniafar H, Niyyati M, Lasjerdi Z. Molecular characterization of pathogenic acanthamoeba isolated from drinking and recreational water in East Azerbaijan, Northwest Iran. Environ Health Insights. 2015;9:7–12. doi: 10.4137/EHI.S27811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behravan M, Malekaneh M, Mesbahzadeh B, Sharifzadeh G, Namaei MH, Behniafar H, et al. Microscopic isolation and characterization of free living amoebae (FLA) from surface water sources in Birjand, the capital city of the South Khorasan. J Birjand Univ Med Sci. 2015;22(2):161–168. [Google Scholar]
- Bonilla-Lemus P, Ramirez-Bautista GA, Zamora-Munoz C, Ibarra-Montes Mdel R, Ramirez-Flores E, Hernandez-Martinez MD. Acanthamoeba spp. in domestic tap water in houses of contact lens wearers in the metropolitan area of Mexico City. Exp Parasitol. 2010;126(1):54–58. doi: 10.1016/j.exppara.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Dabirzadeh M, Daghajani A, Hooshyar H. Isolation and identification of Acanthamoeba from pools water and ponds in Sistan area, Sistan Baluchestan province. J Zabol Univ Med Sci Health Serv. 2015;7(1):e3391. [Google Scholar]
- De Jonckheere JF. Epidemiological typing of Acanthamoeba strains isolated from keratitis cases in Belgium. Bull Soc Belge Ophtalmol. 2003;287:27–33. [PubMed] [Google Scholar]
- Delafont V, Mougari F, Cambau E, Joyeux M, Bouchon D, Hechard Y, et al. First evidence of amoebae-mycobacteria association in drinking water network. Environ Sci Technol. 2014;48(20):11872–11882. doi: 10.1021/es5036255. [DOI] [PubMed] [Google Scholar]
- Eftekhar M, Nazemalhosseini Mojarad E, Haghighi A, Sharifi Sarasiabi K, Nochi Z, Athari A. Detection of Acanthamoeba from fresh water using polymerase chain reaction. Res Med. 2009;33(1):43–46. [Google Scholar]
- Garcia LS. Diagnostic medical parasitology. Washington: American Society for Microbiology Press; 2006. [Google Scholar]
- Ghadar-ghadr S, Solhjoo K, Norouz-nejad M, Rohi R, Zia-Jahromi S. Isolation and identification of free living amoeba (Naegleria and Acanthamoeba) in Shiraz water resources by morphological criteria. Pars Jahrom Univ Med Sci. 2012;10(3):33–42. [Google Scholar]
- Hajialilo E, Niyyati M, Solaymani M, Rezaeian M. Pathogenic free-living amoebae isolated from contact lenses of keratitis patients. Iran J Parasitol. 2015;10(4):541–546. [PMC free article] [PubMed] [Google Scholar]
- Hajialilo E, Behnia M, Tarighi F, Niyyati M, Rezaeian M. Isolation and genotyping of Acanthamoeba strains (T4, T9, and T11) from amoebic keratitis patients in Iran. Parasitol Res. 2016;115(8):3147–3151. doi: 10.1007/s00436-016-5072-8. [DOI] [PubMed] [Google Scholar]
- Hooshyar H, Hosseinbigi B, Saraei M, Alizadeh S, Eftakhar M, Rasti S, et al. Genotyping of acanthamoeba isolated from surface and stagnant waters of Qazvin, central Iran. Iran Red Crescent Med J. 2013;15(6):536–538. doi: 10.5812/ircmj.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinbigi B, Sarie SahnehSaraie M, Alizadeh S, Rasti S, Eftakhar M, Khosro-Shahi N, et al. Isolation and molecular identification of Acanthamoeba in surface stagnant waters of Qazvin. J Qazvin Univ Med Sci. 2012;16(3):26–32. [Google Scholar]
- Huang SW, Hsu BM. Isolation and identification of Acanthamoeba from Taiwan spring recreation areas using culture enrichment combined with PCR. Acta Trop. 2010;115(3):282–287. doi: 10.1016/j.actatropica.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Karamati SA, Niyyati M, Lorenzo-Morales J, Lasjerdi Z. Isolation and molecular characterization of Acanthamoeba genotypes isolated from soil sources of public and recreational areas in Iran. Acta Parasitol. 2016;61(4):784–789. doi: 10.1515/ap-2016-0108. [DOI] [PubMed] [Google Scholar]
- Khan NA. Pathogenesis of Acanthamoeba infections. Microb Pathog. 2003;34(6):277–285. doi: 10.1016/S0882-4010(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006;30(4):564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- Khezri A, Fallah E, Mostafazadeh M, Spotin A, Shahbazi A, Mahami-Oskouei M, et al. Molecular and morphometric characterization of Acanthamoeba spp. from different water sources of Northwest Iran as a neglected focus, co-bordered with the country of Iraq. Jundishapur J Microbiol. 2016;9(11):e38481. doi: 10.5812/jjm.38481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilvington S, Gray T, Dart J, Morlet N, Beeching JR, Frazer DG, et al. Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest Ophthalmol Vis Sci. 2004;45(1):165–169. doi: 10.1167/iovs.03-0559. [DOI] [PubMed] [Google Scholar]
- Kong HH. Molecular phylogeny of Acanthamoeba. Korean J Parasitol. 2009;47(Suppl):S21–S28. doi: 10.3347/kjp.2009.47.S.S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasjerdi Z, Niyyati M, Haghighi A, Zaeri F, Nazemalhosseini Mojarad E. First report of Vannellidae amoebae (Vannella spp.) isolated from biofilm source. Iran J Parasitol. 2011;6(4):84–89. [PMC free article] [PubMed] [Google Scholar]
- Lasjerdi Z, Niyyati M, Haghighi A, Shahabi S, Biderouni FT, Taghipour N, et al. Potentially pathogenic free-living amoebae isolated from hospital wards with immunodeficient patients in Tehran, Iran. Parasitol Res. 2011;109(3):575–580. doi: 10.1007/s00436-011-2288-5. [DOI] [PubMed] [Google Scholar]
- Lasjerdi Z, Niyyati M, Lorenzo-Morales J, Haghighi A, Taghipour N. Ophthalmology hospital wards contamination to pathogenic free living Amoebae in Iran. Acta Parasitol. 2015;60(3):417–422. doi: 10.1515/ap-2015-0057. [DOI] [PubMed] [Google Scholar]
- Latifi A, Niyyati M, Valayi N, Lasjerdi Z. Frequency survey of free-living amoebae isolated from improved hot springs of Mazandaran Province, 2014. Res Med. 2014;38(4):214–220. [Google Scholar]
- Latifi AR, Niyyati M, Lorenzo-Morales J, Haghighi A, Seyyed Tabaei SJ, Lasjerdi Z. Presence of Balamuthia mandrillaris in hot springs from Mazandaran province, northern Iran. Epidemiol Infect. 2016;144(11):2456–2461. doi: 10.1017/S095026881600073X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifi AR, Niyyati M, Lorenzo-Morales J, Haghighi A, Tabaei SJ, Lasjerdi Z, et al. Occurrence of Naegleria species in therapeutic geothermal water sources, Northern Iran. Acta Parasitol. 2017;62(1):104–109. doi: 10.1515/ap-2017-0012. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Ortega-Rivas A, Foronda P, Martinez E, Valladares B. Isolation and identification of pathogenic Acanthamoeba strains in Tenerife, Canary Islands. Spain from water sources. Parasitol Res. 2005;95(4):273–277. doi: 10.1007/s00436-005-1301-2. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Monteverde-Miranda CA, Jimenez C, Tejedor ML, Valladares B, Ortega-Rivas A. Evaluation of Acanthamoeba isolates from environmental sources in Tenerife, Canary Islands, Spain. Ann Agric Environ Med. 2005;12(2):233–236. [PubMed] [Google Scholar]
- Lorenzo-Morales J, Ortega-Rivas A, Martinez E, Khoubbane M, Artigas P, Periago MV, et al. Acanthamoeba isolates belonging to T1, T2, T3, T4 and T7 genotypes from environmental freshwater samples in the Nile Delta region, Egypt. Acta Trop. 2006;100(1–2):63–69. doi: 10.1016/j.actatropica.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Lopez-Darias M, Martinez-Carretero E, Valladares B. Isolation of potentially pathogenic strains of Acanthamoeba in wild squirrels from the Canary Islands and Morocco. Exp Parasitol. 2007;117(1):74–79. doi: 10.1016/j.exppara.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Martínez-Carretero E, Batista N, Álvarez-Marín J, Bahaya Y, Walochnik J, Valladares B. Early diagnosis of amoebic keratitis due to a mixed infection with Acanthamoeba and Hartmannella. Parasitol Res. 2007;102(1):167–169. doi: 10.1007/s00436-007-0754-x. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Marciano-Cabral F, Lindo JF, Visvesvara GS, Maciver SK. Pathogenicity of amoebae. Exp Parasitol. 2010;126(1):2–3. doi: 10.1016/j.exppara.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafi M, Niyyati M, Haghighi A, Lasjerdi Z. Contamination of swimming pools and park ponds with free living amoebae in Tehran. MJTUOMS. 2017;38(6):60–67. [Google Scholar]
- Mahmoudi MR, Taghipour N, Eftekhar M, Haghighi A, Karanis P. Isolation of Acanthamoeba species in surface waters of Gilan province-north of Iran. Parasitol Res. 2012;110(1):473–477. doi: 10.1007/s00436-011-2530-1. [DOI] [PubMed] [Google Scholar]
- Mahmoudi MR, Kazemi B, Haghighi A, Karanis P. Detection of acanthamoeba and toxoplasma in river water samples by molecular methods in Iran. Iran J Parasitol. 2015;10(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi MR, Rahmati B, Seyedpour SH, Karanis P. Occurrence and molecular characterization of free-living amoeba species (Acanthamoeba, Hartmannella, and Saccamoeba limax) in various surface water resources of Iran. Parasitol Res. 2015;114(12):4669–4674. doi: 10.1007/s00436-015-4712-8. [DOI] [PubMed] [Google Scholar]
- Mahmoudi MR, Berenji F, Fata A, Najafzadeh MJ, Asadian A, Salehi M. Morphological characterization of potentially pathogenic thermophilic amoebae isolated from surface water in Mashhad, Iran. Jundishapur J Microbiol. 2015;8(4):e25944. doi: 10.5812/jjm.8(4)2015.25944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manesh RM, Niyyati M, Yousefi HA, Eskandarian AA. Isolation of Acanthamoeba spp. from different water sources in Isfahan, central Iran, 2014. J Parasit Dis. 2016;40(4):1483–1486. doi: 10.1007/s12639-015-0716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschio VJ, Chies F, Carlesso AM, Carvalho A, Rosa SP, Van Der Sand ST, et al. Acanthamoeba T4, T5 and T11 isolated from mineral water bottles in southern Brazil. Curr Microbiol. 2015;70(1):6–9. doi: 10.1007/s00284-014-0676-7. [DOI] [PubMed] [Google Scholar]
- Memari F, Niyyati M, Haghighi A, Seyyed Tabaei SJ, Lasjerdi Z. Occurrence of pathogenic Acanthamoeba genotypes in nasal swabs of cancer patients in Iran. Parasitol Res. 2015;114(5):1907–1912. doi: 10.1007/s00436-015-4378-2. [DOI] [PubMed] [Google Scholar]
- Memari F, Niyyati M, Lorenzo-Morales J, Jonaydi Z. Isolation and molecular characterization of Acanthamoeba strains isolated from the oral cavity of immunosuppressed individuals in Tehran, Iran. Acta Parasitol. 2016;61(3):451–455. doi: 10.1515/ap-2016-0060. [DOI] [PubMed] [Google Scholar]
- Mosayebi M, Ghorbanzadeh B, Eslamirad Z, Ejtehadifar M, Rastad B. The isolation and detection of acanthamoeba keratitis in rural water sources of Arak, Iran. Med Lab J. 2014;7(4):66–71. [Google Scholar]
- Nazar M, Haghighi A, Niyyati M, Eftekhar M, Tahvildar-Biderouni F, Taghipour N, et al. Genotyping of Acanthamoeba isolated from water in recreational areas of Tehran, Iran. J Water Health. 2011;9(3):603–608. doi: 10.2166/wh.2011.152. [DOI] [PubMed] [Google Scholar]
- Nazar M, Haghighi A, Taghipour N, Ortega-Rivas A, Tahvildar-Biderouni F, Nazemalhosseini Mojarad E, et al. Molecular identification of Hartmannella vermiformis and Vannella persistens from man-made recreational water environments, Tehran, Iran. Parasitol Res. 2012;111(2):835–839. doi: 10.1007/s00436-012-2906-x. [DOI] [PubMed] [Google Scholar]
- Niyyati M, Lorenzo-Morales J, Rahimi F, Motevalli-Haghi A, Martin-Navarro CM, Farnia S, et al. Isolation and genotyping of potentially pathogenic Acanthamoeba strains from dust sources in Iran. Trans R Soc Trop Med Hyg. 2009;103(4):425–427. doi: 10.1016/j.trstmh.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Mohebali M, Maghsood AH, et al. Genotyping of Acanthamoeba isolates from clinical and environmental specimens in Iran. Exp Parasitol. 2009;121(3):242–245. doi: 10.1016/j.exppara.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Martin-Navarro CM, Mohebali M, et al. First report of a mixed infection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp Parasitol. 2010;126(1):89–90. doi: 10.1016/j.exppara.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Niyyati M, Lasjerdi Z, Nazar M, Haghighi A, Nazemalhosseini Mojarad E. Screening of recreational areas of rivers for potentially pathogenic free-living amoebae in the suburbs of Tehran, Iran. J Water Health. 2012;10(1):140–146. doi: 10.2166/wh.2011.068. [DOI] [PubMed] [Google Scholar]
- Niyyati M, Rahimi F, Lasejerdi Z, Rezaeian M. Potentially pathogenic free-living amoebae in contact lenses of the asymptomatic contact lens wearers. Iran J Parasitol. 2014;9(1):14–19. [PMC free article] [PubMed] [Google Scholar]
- Niyyati M, Lasgerdi Z, Lorenzo-Morales J. Detection and molecular characterization of potentially pathogenic free-living amoebae from water sources in Kish Island, Southern Iran. Microbiol Insights. 2015;8(Suppl 1):1–6. doi: 10.4137/MBI.S24099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyyati M, Mafi M, Haghighi A, Hakemi Vala M. Occurrence of potentially pathogenic bacterial-endosymbionts in Acanthamoeba Spp. Iran J Parasitol. 2015;10(2):181–188. [PMC free article] [PubMed] [Google Scholar]
- Niyyati M, Lasjerdi Z, Zarein-Dolab S, Nazar M, Behniafar H, Mahmoudi MR, et al. Morphological and molecular survey of naegleria spp. in water bodies used for recreational purposes in Rasht city, Northern Iran. Iran J Parasitol. 2015;10(4):523–529. [PMC free article] [PubMed] [Google Scholar]
- Niyyati M, Karamati SA, Lorenzo Morales J, Lasjerdi Z. Isolation of Balamuthia mandrillaris from soil samples in North-Western Iran. Parasitol Res. 2016;115(2):541–545. doi: 10.1007/s00436-015-4770-y. [DOI] [PubMed] [Google Scholar]
- Niyyati M, Saberi R, Latifi A, Lasjerdi Z. Distribution of acanthamoeba genotypes isolated from recreational and therapeutic geothermal water sources in Southwestern Iran. Environ Health Insights. 2016;10:69–74. doi: 10.4137/EHI.S38349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okude M, Matsuo J, Nakamura S, Kawaguchi K, Hayashi Y, Sakai H, et al. Environmental chlamydiae alter the growth speed and motility of host acanthamoebae. Microbes Environ. 2012;27(4):423–429. doi: 10.1264/jsme2.ME11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondriska F, Mrva M, Lichvar M, Ziak P, Murgasova Z, Nohynkova E. First cases of Acanthamoeba keratitis in Slovakia. Ann Agric Environ Med. 2004;11(2):335–341. [PubMed] [Google Scholar]
- Onichandran S, Kumar T, Salibay CC, Dungca JZ, Tabo HA, Tabo N, et al. Waterborne parasites: a current status from the Philippines. Parasit Vectors. 2014;7:244. doi: 10.1186/1756-3305-7-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page FC. A new key to freshwater and soil gymnamoebae: with instructions for culture. Ambleside: Freshwater Biological Association; 1988. pp. 95–96. [Google Scholar]
- Rahdar M, Niyyati M, Salehi M, Feghhi M, Makvandi M, Pourmehdi M, et al. Isolation and genotyping of acanthamoeba strains from environmental sources in Ahvaz city, Khuzestan province, southern Iran. Iran J Parasitol. 2012;7(4):22–26. [PMC free article] [PubMed] [Google Scholar]
- Ramirez E, Robles E, Martinez B, Ayala R, Sainz G, Martinez ME, et al. Distribution of free-living amoebae in a treatment system of textile industrial wastewater. Exp Parasitol. 2014;145(Suppl):S34–S38. doi: 10.1016/j.exppara.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Rasti S, Asadi MA, Iranshahi L, Hooshyar H, Gilasi HR, Zahiri A. Evaluation of parasitic and fungal contamination and physicochemical parameters of indoor public swimming pools in Kashan during 2008-9. KAUMS J (FEYZ) 2011;15(1):74–80. [Google Scholar]
- Retana-Moreira L, Abrahams-Sandi E, Castro-Artavia E, Fernandez-Sanchez A, Castro-Castillo A, Reyes-Batlle M, et al. Isolation and molecular characterization of acanthamoeba strains from dental units in Costa Rica. J Eukaryot Microbiol. 2015;62(6):733–736. doi: 10.1111/jeu.12229. [DOI] [PubMed] [Google Scholar]
- Rezaeian M, Niyyati M, Farnia S, Motevalli Haghi A. Isolation of Acanthamoeba spp. from different environmental sources. Iran J Parasitol. 2008;3(1):44–47. [Google Scholar]
- Rezaian M, Bagheri F, Farnia S, Babai Z. Isolation of pathogenic amoeba (naegleria and acanthameoba) from water sources and margin soils of rivers and lakes in Kazerun. J School Public Health Inst Public Health Res. 2003;1(3):41–48. [Google Scholar]
- Rivera F, Galvan M, Robles E, Leal P, Gonzalez L, Lacy AM. Bottled mineral waters polluted by protozoa in Mexico. J Protozool. 1981;28(1):54–56. doi: 10.1111/j.1550-7408.1981.tb02803.x. [DOI] [PubMed] [Google Scholar]
- Salehi M. Acanthamoeba Strains genotypes prevalence in water Sources in Bojnurd City: Short Communication. J Birjand Univ Med Sci. 2014;21(2):260–266. [Google Scholar]
- Seal DV, II, Shoff M, Rogerson A, Kessler K, Schatz S. Prevalence of acanthamoeba and other naked amoebae in South Florida tap water. Investig Ophthalmol Vis Sci. 2006;47(13):2409. [Google Scholar]
- Shokri A, Sarvi S, Daryani A, Sharif M. Isolation and Genotyping of Acanthamoeba spp. as Neglected Parasites in North of Iran. Korean J Parasitol. 2016;54(4):447–453. doi: 10.3347/kjp.2016.54.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solgi R, Niyyati M, Haghighi A, Mojarad EN. Occurrence of thermotolerant Hartmannella vermiformis and Naegleria spp. in hot springs of Ardebil Province, Northwest Iran. Iran J Parasitol. 2012;7(2):47–52. [PMC free article] [PubMed] [Google Scholar]
- Solgi R, Niyyati M, Haghighi A, Taghipour N, Tabaei SJ, Eftekhar M, et al. Thermotolerant Acanthamoeba spp. isolated from therapeutic hot springs in Northwestern Iran. J Water Health. 2012;10(4):650–656. doi: 10.2166/wh.2012.032. [DOI] [PubMed] [Google Scholar]
- Stapleton F, Ozkan J, Jalbert I, Holden BA, Petsoglou C, McClellan K. Contact lens-related acanthamoeba keratitis. Optom Vis Sci. 2009;86(10):E1196–E1201. doi: 10.1097/OPX.0b013e3181baae11. [DOI] [PubMed] [Google Scholar]
- Thomas V, Loret JF, Jousset M, Greub G. Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ Microbiol. 2008;10(10):2728–2745. doi: 10.1111/j.1462-2920.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- Trabelsi H, Dendana F, Sellami A, Sellami H, Cheikhrouhou F, Neji S, et al. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol (Paris) 2012;60(6):399–405. doi: 10.1016/j.patbio.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50(1):1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- Winck MA, Caumo K, Rott MB. Prevalence of acanthamoeba from tap water in Rio Grande do Sul, Brazil. Curr Microbiol. 2011;63(5):464–469. doi: 10.1007/s00284-011-0003-5. [DOI] [PubMed] [Google Scholar]
- Yousuf FA, Siddiqui R, Subhani F, Khan NA. Status of free-living amoebae (Acanthamoeba spp., Naegleria fowleri, Balamuthia mandrillaris) in drinking water supplies in Karachi, Pakistan. J Water Health. 2013;11(2):371–375. doi: 10.2166/wh.2013.112. [DOI] [PubMed] [Google Scholar]
- Zeybek Z, Binay AR. Growth ability of Gram negative bacteria in free-living amoebae. Exp Parasitol. 2014;145(Suppl):S121–S126. doi: 10.1016/j.exppara.2014.06.009. [DOI] [PubMed] [Google Scholar]