Abstract
Electronic cigarettes (e-cigarettes) are promoted as low-risk alternatives to combustible cigarettes. However, the effects of chronic inhalation of potential toxicants emitted by ecigarettes remain largely unexamined. It is conceivable that smoking-induced chronic diseases result in cellular injury, in the absence of effective repair by stem cells. This study evaluates the effect of cigarette and e-cigarette aerosol extracts on the survival and differentiation of bone marrow-derived mesenchymal stem cells (MSCs). MSC growth and osteogenic differentiation were examined after exposure to smoke extracts. Data revealed detrimental effects of both cigarette and e-cigarette extracts on MSC morphology and growth. Levels and activity of alkaline phosphatase, an osteogenic marker, decreased and induction of osteoblastic differentiation was impaired. Both smoke extracts prevented osteogenic differentiation from progressing, evident by decreased expression of terminal osteogenic markers and mineralization. Elevated levels of reactive oxygen species (ROS) were detected in cells exposed to smoke extracts. Moreover, decreased differentiation potential was concomitant with severe down-regulation of Connexin 43 expression, leading to the loss of gap junction-mediated communication, which together with elevated ROS levels, could explain decreased proliferation and loss of differentiation potential. Hence, e-cigarettes present similar risk as combustible cigarettes with respect to tissue repair impairment.
Introduction
The detrimental impact of cigarette smoking on health is amply documented and ranges from oral diseases1, to systemic malfunction, inflammation2, infertility3,4, cancer and abnormal cell differentiation and tissue repair1. Awareness has been raised among smokers and policy-makers, and has resulted in proactive measures aiming at curbing cigarette smoking. Controversially, waterpipe smoking is gaining popularity worldwide, alongside another globally spreading phenomenon, the use of electronic cigarette (e-cigarette) or “vaping”5.
E-cigarettes are often claimed to be a safer alternative to conventional tobacco products and are sometimes marketed as a smoking cessation tool. Some research has suggested a decrease in the disease burden of e-cigarette vaping, compared to combustible cigarette smoking6. However, e-cigarette liquids have been reported to be cytotoxic7,8, and e-cigarette aerosol emissions have been shown to exert negative effects in animal models9–14. Nevertheless, partly due to the recent emergence of the e-cigarette, there is a lack of information on its long-term effects on health and studies on e-cigarette safety are not yet conclusive. Combustible cigarette smoke compromises cell growth and tissue repair1,15,16; however, the impact of e-cigarette aerosols on cell differentiation and tissue repair has not been studied.
A stable epithelial layer with a relatively slow cell turnover rate lines the respiratory tract17. Upon injury, progenitor and stem cells are recruited to repair damaged tissues. However, smokers develop chronic conditions, from long-term exposure to smoke, suggesting impaired tissue healing and remodelling. Previously, we explored the effect of waterpipe smoke on alveolar type II-derived cells18 and on endothelial cells19, detailing cytotoxic, mutagenic, inflammatory and anti-proliferative effects. The onset of systemic inflammation and the compromised ability of local cells to heal the damaged tissues were proposed as a plausible mechanism underlying tobacco smoke-induced diseases such as chronic obstructive pulmonary disease (COPD) and vascular diseases18,19. These conditions remain with no cure and a rather modest clinical management20,21.
Stem cells are at the core of tissue repair and remodelling. Bone marrow-derived mesenchymal stem cells (MSCs) are frequently recruited to the site of injury22 and are extensively studied for the treatment and repair of tissues such as in cardiac injury23,24. Among the documented hazards associated with smoking, generation of reactive oxygen species (ROS) and alteration of gap junctional complexes are tightly associated with modulation of repair mechanisms. Indeed, multiple studies have highlighted the importance of gap junctions in protecting cells against oxidative stress-induced cell death25 and in modulation of cell proliferation and survival25,26, tumorigenesis27, and differentiation28,29. Nicotine was shown to down-regulate the expression levels of Connexin 43 (Cx43) in human endothelial cells30,31, which affects viability, proliferation, and angiogenesis32. In addition, low Cx43 expression is strongly associated with the metastatic phenotype of cancer cells33,34, while up-regulation of Cx43 expression restores the sensitivity of lung carcinoma cells to chemotherapy in vitro 35. Cx43 is reported to be highly expressed in MSCs36. MSCs undergoing osteoblastic differentiation express Cx43; Cx43 plays a major role in osteoblast progenitor migration and homing to bone in vivo 28,37–39.
The present study investigates the effect of combustible cigarette and e-cigarette smoke extracts on the ability of stem cells to differentiate, as impairment of stem cell differentiation leads to tissue repair impairment, causing chronic diseases.
Results
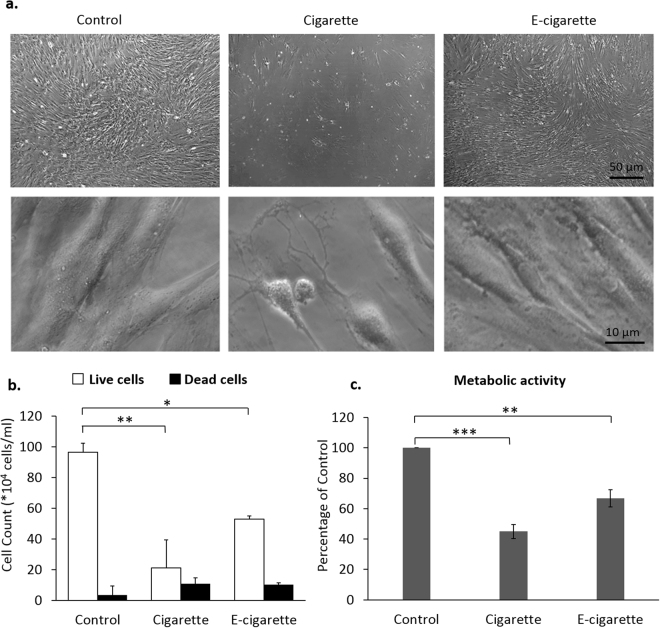
Smoke extracts inhibit MSC proliferation and induces morphological changes
The concentration of smoke extract to be used throughout the study was determined by exposing MSCs to increasing concentrations of smoke extracts. Media were replenished every third day over 12 days. Treated MSCs showed poor viability at concentrations higher than 0.6 mg/ml and 6 mg/ml for cigarette and e-cigarette extracts, respectively. At higher concentrations, cells stopped proliferating, acquired spindle-like morphology with long processes and, eventually, sloughed off. Therefore, for the aims of this study, the concentrations of 0.4 mg/ml and 4 mg/ml were chosen for the cigarette and e-cigarette sets, respectively, applied every third day, for 3 weeks. We note that the tenfold difference in concentration between e-cigarette and cigarette extracts is consistent with the approximately tenfold greater amount of aerosol particulate matter inhaled by an e-cigarette user for the same nicotine delivery. By day 21 of the experiment, in response to repeated exposure, cells underwent morphological alterations that were more pronounced in the cigarette- than in e-cigarette samples (Fig. 1a). At high magnification, microscopic images of MSCs treated with smoke extracts display altered morphology with variable response to the insult ranging from mild changes to severe shrinkage and collapsing of cytoplasm. Morphological alteration were observed, albeit to a lesser extent, in cells treated with e-cigarette smoke extracts with overall smaller cells, compared to control MSCs (Fig. 1a; lower panel). Trypan blue exclusion assay showed an 80% (p < 0.005) and 50% (p < 0.01) decrease in the number of cigarette- and e-cigarette-treated cells, respectively (Fig. 1b). The extent of cell death was also measured in cigarette- and e-cigarette-treated wells, where the number of dead cells was threefold higher in both groups, as compared to control. This trend in cell viability was paralleled and confirmed by a comparable decrease in the metabolic activity of the cells. Indeed, the MTT assay showed a 50% (p < 0.001) and 30% (p < 0.005) decrease in the metabolic activity of cigarette- and e-cigarette-treated cells, respectively (Fig. 1c). These data indicate that both cigarette and e-cigarette particles compromise MSCs proliferation.
Figure 1.
Cigarette and e-cigarette smoke extracts alter MSC morphology and growth. MSCs were repeatedly (once every 3 days) exposed to cigarette and e-cigarette smoke extracts for 21 days. (a) MSC morphology was examined by light microscopy (upper panel). High magnification DIC images are shown in the lower panel. (b) On day 21, cell viability was assessed using the trypan blue dye exclusion assay. Histograms display averages ± SD of 3 independent experiments in duplicates. (c) On day 21, the metabolic activity of MSCs was assessed by MTT. Histograms display averages ± SD of 2 independent experiments in triplicates. *p < 0.01, **p < 0.005 and ***p < 0.001.
Smoke extracts impair MSCs differentiation
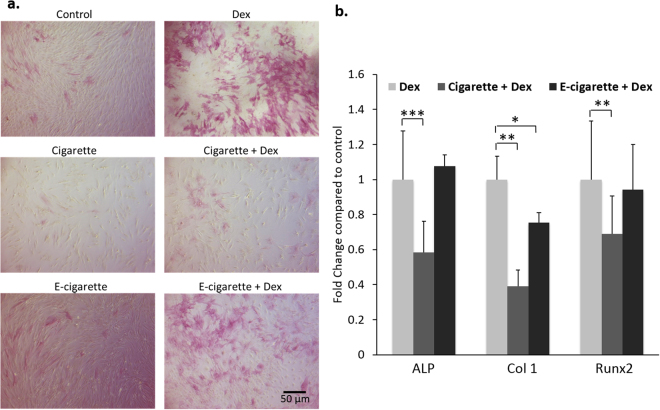
The potential of MSCs to differentiate was evaluated by inducing osteoblastic differentiation using 50 nM Dex. The effect of repeated exposure to smoke extract on the induction of MSCs differentiation, as monitored by the expression of different osteogenic differentiation markers, ALP, Col 1, and Runx2 was assessed. Dex induced ALP activity (Fig. 2a) and ALP gene expression in MSCs (Fig. 2b). Both cigarette and e-cigarette reduced basal ALP activity (Fig. 2a). Only cigarette smoke extracts significantly decreased ALP mRNA levels upon treatment with Dex (p < 0.0001) (Fig. 2b). Given the role of ALP as an early osteogenic marker and that Dex drives MSCs to early osteogenic differentiation, these data indicate that early osteogenic differentiation is less affected by e-cigarette than by cigarette smoke extracts. In addition, the expression of Col 1 and Runx2 decreased in smoke extracts and Dex-treated MSCs (p < 0.01) (Fig. 2b). Only Col 1 mRNA levels decreased significantly upon e-cigarette smoke extract treatment (p < 0.05).
Figure 2.
Cigarette and e-cigarette smoke extracts attenuate MSC differentiation. (a) MSCs were induced into osteogenic differentiation by treatment with 50 nM Dex for 14 days. ALP activity was then assessed using a chromogenic ALP assay where purple staining indicates ALP activity. The light microscopy images are representative of 3 independent experiments. (b) MSCs were simultaneously treated with 50 nM Dex for osteogenic differentiation and cigarette or e-cigarette smoke extracts for 14 days. Expression of osteogenic markers (alkaline phosphatase [ALP], collagen 1 [Col 1] and Runt-related transcription factor 2 [Runx2]) was then assessed using qRT-PCR. Histograms display data normalized against GAPDH and show fold changes relative to control. Results are displayed as averages ± SD of 3 independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001.
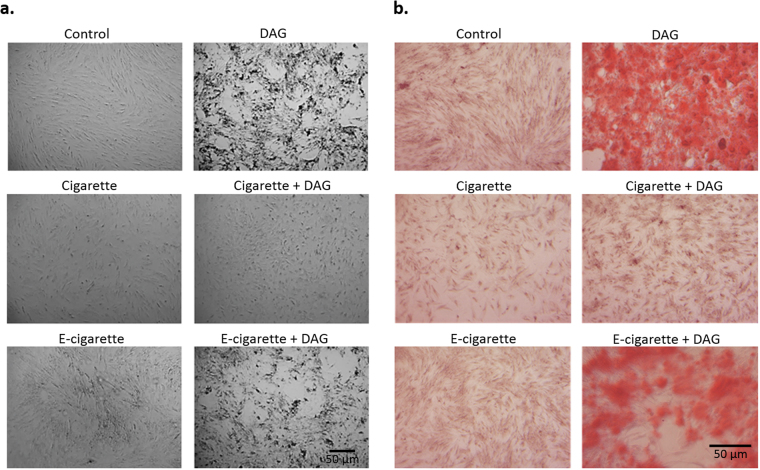
DAG treatment drives MSCs beyond the early differentiation induced by Dex alone. DAG induces extensive morphology change in MSCs, which became rounded with deposits visible in the background; however, smoke impeded DAG-induced morphological changes (Fig. 3a). Alizarin Red S staining confirmed osteogenic differentiation in DAG-treated cells that showed dense red-orange patches, attesting to mineralization (Fig. 3b). A much less pronounced Alizarin Red S staining was observed in smoke-treated MSCs. This suggests that not only cigarette, but also e-cigarette smoke extracts largely impair the progress of differentiation beyond ALP expression.
Figure 3.
Cigarette and e-cigarette smoke extracts block progress towards terminal osteogenic differentiation. MSCs were treated with cigarette and e-cigarette smoke extracts in the presence or absence of DAG for 21 days. (a) Cell morphology was observed under the light microscope. Mineral deposition in the plates is evident as black spots. (b) MSCs were stained with Alizarin Red S stain and observed under the light microscope. Orange staining indicates mineralized calcium deposits. Images are representative of 3 independent experiments.
E-cigarette smoke extracts lead to overproduction of ROS
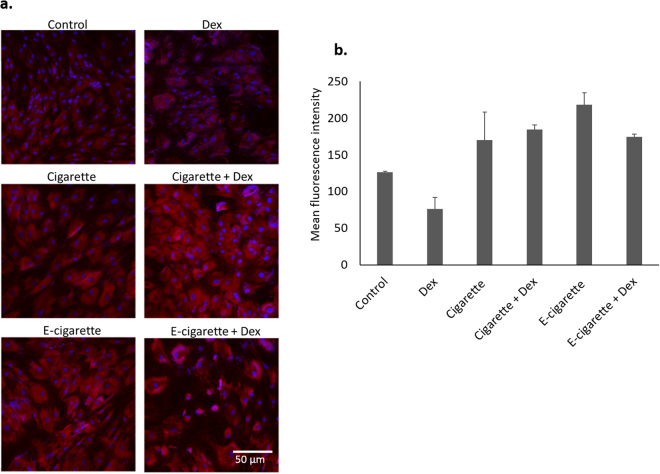
The impaired differentiation of MSCs upon cigarette and e-cigarette challenge could be due to several factors. ROS are important upstream osteogenic differentiation mediators in MSCs. ROS levels were visualized in control and smoke-exposed MSCs to determine whether e-cigarette extracts induce ROS generation and whether high levels of ROS may be involved in hindering osteogenesis. Figure 4a shows that MSCs exhibit high levels of ROS in response to smoke extract, as compared to control. Control cells had a mean fluorescence intensity (MFI) of 126 ± 1.4, while cigarette-treated cells had MFI of 170 ± 38.2 (Fig. 4b) and ROS levels were highest in e-cigarette-treated MSCs (MFI = 218 ± 16.2). Furthermore, differentiated cells exhibited low levels of ROS (Fig. 4a, left lower panel), with MFI = 76 ± 15.5, while smoke-exposed cells also treated with Dex maintained elevated ROS levels, which could, in part, explain smoke-induced inhibition of osteogenic differentiation.
Figure 4.
Cigarette and e-cigarette smoke extracts enhance ROS production. (a) MSCs were treated with cigarette and e-cigarette smoke extracts for 14 days. ROS generation was evaluated using the DHE assay, counterstained with DAPI and observed by fluorescence microscopy. Red indicates presence of ROS. (b) Quantification of ROS generation. DHE mean fluorescence intensities of 5 fields from 2 images per condition were measured using the Zen 2011 software. Histograms display averages ± SD of mean fluorescence intensities.
Cigarette and e-cigarette smoke extracts inhibit cell-cell communication
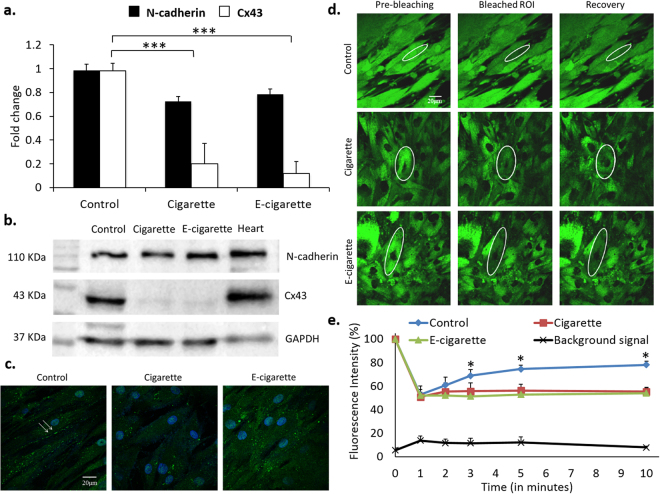
In order to further explore the mechanisms behind MSCs growth and differentiation impairment, the integrity of cell-cell communication was assessed upon exposure to smoke extracts. Figure 5a shows a marked decrease in Cx43 mRNA levels. Western blot and immunofluorescence (Fig. 5b,c) show a sharp downregulation of Cx43 protein levels in both cigarette- and e-cigarette-treated cells. N-cadherin, a component of junctional complexes involved in cell-cell contact, was also examined and its expression was maintained both at the mRNA and protein levels (Fig. 5a,b). This suggests that the integrity of the cell layer is preserved and that downregulation of Cx43 is a specific effect of smoke exposure and not secondary to the disruption of cell membrane integrity and cell-cell contact. Downregulation of Cx43 was further investigated at the functional level. FRAP studies show that smoke-treated cells do not recover fluorescence after photo-bleaching to at least 50% of their initial fluorescence intensity (Fig. 5d,e). The background fluorescence intensity is maintained throughout the experimental duration (black curve, Fig. 5e). Cigarette- and e-cigarette-challenged cells did not recover fluorescence, compared to a 30-40% recovery in control cells (blue curve). Indeed, fluorescence recovery between minute 1 and minute 10 was not significant in the cigarette (p = 0.32) or in the e-cigarette (p = 0.52) treated cells, while in the untreated cells, fluorescence recovery was significant at 10 minutes (p < 0.005). Similarly, at the end of recovery period (10 minutes), fluorescence difference between treated and control cells was significant (p < 0.05), while the difference of fluorescence intensity between cigarette and e-cigarette treated cells was not significant (p = 0.25). Both cigarette and e-cigarette smoke extracts compromised gap junction-mediated intercellular communication.
Figure 5.
Cigarette and e-cigarette smoke extracts inhibit cell-cell communication in MSCs. MSCs were treated with cigarette and e-cigarette smoke extracts for 14 days. (a) qRT-PCR of Cx43 and N-cadherin was performed on total RNA obtained from control and treated cells. Data were normalized against GAPDH and show fold changes (averages ± SEM of 3 independent experiments) relative to the control. (b) Western blots of Cx43 and N-cadherin were performed on total protein extracts obtained from control and treated cells. GAPDH was used as a loading control and total heart lysates as a technical positive control. Blots are representative of 3 independent experiments. (c) Immunofluorescence staining of Cx43 was performed on control and treated MSCs. Arrows indicate gap junction plaques between adjacent cells. Images are representative of at least 10 fields from 2 independent experiments. (d) MSCs from the control and treated wells were stained with calcein-AM and then subjected to 50% photo-bleaching by the 488-nm laser. Fluorescence recovery after photo-bleaching (FRAP) was monitored for 10 minutes under the 63X objective. White ellipses indicate regions of interest (ROI). Images are representative of at least 5 ROIs per condition, from 2 independent experiments. (e) Quantification of fluorescence intensity of ROIs relative to reference cells of control and treated cells. Values represent the fluorescence intensity (averages ± SD) of each ROI based on several measurements calculated by the Zeiss Zen 2011 software. *p < 0.05 and ***p < 0.001.
Discussion
Currently, there is no consensus on the use of e-cigarettes as nicotine surrogates or as smoking cessation tools and the concept remains controversial due to the relatively recent emergence of “vaping” trends40 and a paucity of data on its net health effects in the population. Understandably, much attention to date has focused on e-cigarette aerosol toxicant content41–51, and particularly those toxicants thought to be causative agents in cigarette smoke-related disease – the so-called Hoffmann analytes52. E-cigarette proponents have pointed out that under most conditions, e-cigarettes emit fewer and less Hoffmann analytes than do combustible cigarettes. While toxicant analysis is a powerful tool for studying the potential risk of product exposure, it is not generally known a priori which toxicants to scan; indeed, due to their different sources, e-cigarette aerosol toxicity may be due to constituents not included in the Hoffmann list. Recent literature on the effects of e-cigarette on animals and cells10–14,50,53,54 suggests that the relatively low amounts of Hoffmann analytes in e-cigarette aerosols may not provide an adequate picture of the possible effects of long-term use. This study is one of the earliest works that examined the potential effects of e-cigarette aerosol extracts on human stem cells, suggesting that e-cigarette smoke particles may adversely impact human health.
The role of stem cells and their capability to differentiate and repair organs damaged by smoking is crucial in diseases associated with tobacco use like COPD. This study compared the effect of exposure to combustible cigarette and e-cigarette smoke extracts on the survival of stem cells and their differentiation potential. As a proof of concept, and due to the relative ease of induction and assessment of differentiation in MSCs, the difficulty in obtaining organ-specific stem cells, and the fact that MSCs are recruited to sites of injury for tissue repair, the well-established model of MSC differentiation into osteoblast-like cells was used to assess the effects of smoke extracts on the differentiation potential of MSCs. Our results demonstrated that both cigarette and e-cigarette smoke extracts significantly affect the proliferation of MSCs. This finding was in agreement with a previous study that demonstrated the attenuation of A549 alveolar cellular growth secondary to cigarette exposure9. This loss of proliferative potential was accompanied by morphological changes, also indicative of alterations in cell behaviour. Addition of Dex to induce MSCs into osteoblastic differentiation did not salvage the cells and failed to induce MSC differentiation in the cigarette-treated samples. E-cigarette-treated MSCs, however, seemed to retain differentiation potential in the presence of Dex, though to a lesser extent than control cells. However, attempts to progress MSC differentiation by the addition of DAG failed in the treated cells. Exposure to either cigarette or e-cigarette smoke extracts compromised response of MSCs to DAG, as evidenced by the absence of differentiation features (mineralization and calcium deposition).
MSC differentiation potential is modulated by the type and level of ROS55–57; indeed, MSC osteogenesis occurs under low ROS levels58–60. Cigarette smoke contains high concentration of ROS-inducing agents61, which are known to decrease the differentiation potential of MSCs into osteoblasts62. MSCs exposed to both cigarette and e-cigarette smoke extracts generated high levels of ROS, as defined by increased superoxide radical levels54,63,64, which can explain attenuated differentiation of smoke-exposed cells65.
Gap junction-mediated intercellular communication is pivotal to the regulation of several cellular processes including proliferation, differentiation, apoptosis and tumorigenesis66. Cx43 is the main connexin in bone marrow-derived MSCs36 and appears to be directly involved in osteogenic differentiation. Our data suggest that the loss of Cx43, upon exposure to cigarette and e-cigarette smoke extracts, and the subsequent loss of cell-cell communication, is associated with the decrease in the differentiation potential and hence the attenuated potential of MSCs to undergo osteogenic differentiation. Cx43 also protects cells from oxidative stress25, and the down-regulation of Cx43 can explain impaired growth and differentiation of MSCs upon exposure to the combination of smoke and Dex. This observation may suggest that e-cigarette can be a risk factor for osteoporosis, a chronic condition tightly linked to cigarette smoking67 and comorbidity with COPD68. Our results suggest that smoke extracts might be inhibiting the cytoplasmic exchange of survival and differentiation signals, by down-regulating Cx43 expression in MSCs. In addition to the fact that nicotine might be a player in the impairment of differentiation69, smoke contains other ingredients that are toxic and could impair differentiation as well70,71. Subsequently, tissue repair might be compromised upon exposure to both cigarette and e-cigarette.
Therefore, while e-cigarette aerosols are commonly understood to contain fewer and less of the toxicants that have been linked to diseases in combustible cigarette smokers, this study adds to the growing evidence that suggests that a cautionary approach to e-cigarette proliferation is necessary.
Material and Methods
Smoke extract preparation
Combustible and electronic cigarette aerosols were generated using the Oro-nasal Respiratory Exposure System (ONARES, CH Technologies, USA), as previously described9. Briefly, combustible cigarette particulate matter was generated from reference 3R4F cigarettes (University of Kentucky, Lexington, KY) with 9.4 mg tar, and 0.726 mg nicotine per cigarette, following a puffing protocol of one 2-second puff every minute and a volume of 35 ml/puff (ISO standard). E-cigarette particulate matter was obtained from pre-filled V4L CoolCart (strawberry flavor, 3.5 Ohm, 18 mg/ml labelled nicotine concentration) cartomizer cartridges; by 4-second puff duration, 1.2 l/minute flow rate, and 14-second inter-puff interval72.
Aerosol particles were trapped onto 47-mm glass fiber filter disks type A/E (Pall Laboratory, USA) and then extracted in cell culture medium using a syringe and sterilized through a 0.22-μm filter (Costar, Corning Inc., New York, USA), to the final concentrations to be applied onto cells.
Cells and cell culture
Bone marrow-derived mesenchymal stem cells (MSCs), isolated from healthy volunteers, were purchased from Lonza (Cologne, Germany). These MSCs retain the ability to self-renew and differentiate into multiple mesodermal lineages (osteogenic, adipogenic, chondrogenic and bone marrow stroma). MSCs were cultured in complete Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with glucose (1 g/l), fetal bovine serum (10%) and penicillin/streptomycin (100 units of potassium penicillin and 100 µg of streptomycin sulphate per 1 ml of DMEM).
Smoke extracts were freshly prepared prior to addition to cells. Ascending concentrations of combustible and e-cigarette total smoke extracts were applied onto the cells for 2 weeks. Cells were monitored daily by microscopy to determine the concentration to be used to enable cellular and molecular observations.
Dexamethasone (Dex) (DEXAMED, Medochemie, Limassol, Cyprus) was used at a concentration of 50 nM to induce osteogenic differentiation73,74, added onto the cells either alone or simultaneously with the smoke extract. To further differentiate MSCs into osteoblast-like cells, an osteogenic differentiation combination was used (50 nM Dex, 50 μM ascorbic acid and 10 mM beta-glycerophosphate, abbreviated as DAG).
MSCs were plated at a density of 7,000 cells/cm2. Smoke extract exposure and differentiation started when cells reached 70% confluence; treatment was applied every third day, for a total of 14 or 21 days.
Morphological and histochemical assessment
MSCs were seeded onto 6-well plates (Corning, New York, USA) and treated with smoke extracts +/− Dex (14 days) or +/−DAG (21 days).
Morphological changes
Cells were monitored daily by light microscopy and images were captured to document changes in cell confluence and morphology.
Cell viability
Cell growth was assessed using the trypan blue exclusion assay. Control and treated cells were plated in duplicates in 24-well plates. At pre-determined time points, MSCs were released from the plate using 0.05% Trypsin-EDTA (R-001-100, Life Technologies, California, USA), then equal volumes of cells and trypan blue dye and counted using a haemocytometer.
In parallel, the MTT assay was performed to evaluate the metabolic activity of MSCs. Control and treated cells were seeded in duplicates onto flat-bottom 96-well plates. At each time point, cells in 90 µl media were incubated with 10 µl of a 5 mg/ml MTT substrate solution, thiazolyl blue tetrazolium bromide salt (Sigma-Aldrich Co, Missouri, USA) for 4 hours. The reaction was stopped by the addition of 100 µl of solubilizing solution (12 mM HCl, 346 mM SDS and 5% isobutanol); formazan dye was then quantified at a wavelength of 595 nm using a scanning multi-well spectrophotometer (Thermo Scientific Multiskan EX, Thermo Scientific, USA). Proliferation results were reported as percentages of control.
Alkaline phosphatase activity
Alkaline phosphatase (ALP) is an enzyme highly active in bone tissue. ALP activity was semi-quantitatively evaluated using the Leukocyte Alkaline Phosphatase Kit (R86, Sigma-Aldrich, Missouri, USA). On day 14, cells were washed in phosphate-buffered saline (PBS), fixed and stained for ALP activity, according to the manufacturer’s instructions. Purple dye deposits indicate cells with ALP activity.
Alizarin red staining
On day 21, MSCs were washed in PBS, fixed in a solution of equal volumes of acetone and xylene for 30 minutes, and rinsed twice in distilled water. Cells were stained with Alizarin Red S (40 mM) for 20 minutes at room temperature, with gentle rocking then washed in distilled water to remove excess dye and left to air dry. Dark orange-red patches indicate areas of osteogenic mineralization. Images were captured using a Zeiss Axiovert microscope (Primovert HDcam, Carl Zeiss, Germany).
Reactive oxygen species generation
MSCs were treated with cigarette and e-cigarette extracts +/− Dex for 14 days (4 repeated applications of the experimental media). Cells were washed in PBS and incubated for 30 minutes at 37 °C with 10 μM dihydroethidium (DHE). DHE reacts with ROS, to produce red fluorescent 2-hydroxyethidium63. Cells were counterstained with DAPI. Images were captured and analyzed using a laser-scanning confocal microscope (LSM 710, Carl Zeiss, Germany), operated by the Zen 2011 software. DHE mean fluorescence intensities of 5 fields from 2 separate images per condition (obtained using fixed acquisition settings for comparison purposes) were measured using the Zen 2011 software. Histograms display averages ± standard deviations (SD) of mean fluorescence intensities.
Molecular analysis
Gene expression by quantitative PCR
Total RNA was extracted from smoke extract-treated and control MSCs 21 days after culture using the NucleoSpin® RNA II extraction kit (Macherey-Nagel, Düren, Germany) as per manufacturer’s instructions. Total extracted RNA was then reverse-transcribed with the RevertAid® first strand cDNA synthesis kit (Thermo Scientific), using random primers, following the manufacturer’s protocol. cDNA was amplified using an iQ SYBR Green Supermix (BioRad Laboratories, Hercules, California, USA) on a BioRad CFX96 real-time PCR system. Cycling conditions were as follows: 95 °C for 3 minutes followed by 40 amplification cycles (95 °C denaturation for 3 seconds, annealing for 30 seconds at the primers’ melting temperature, and extension at 72 °C for 30 seconds) and a final extension cycle at 72 °C for 5 minutes. Relative expression of target genes was performed according to the comparative ∆∆Ct method using human glyceraldehyde 3-phosphate dehydrogenase (gapdh) as a reference gene for normalization. Gene expression levels were assessed for ALP, Collagen 1A1 (Col 1) and runt-related transcription factor 2 (Runx2). The primers used were as follows: ALP 5′-acaagcactcccacttcatctgga-3′ and 5′-tcacgttgttcctgttcagctcgt-3′; Col 1 5′-ttttgtattcaatcactgtcttgcc-3′ and 5′-cagccgcttcacctacagc-3′ and Runx2 5′-tccggaatgcctctgctgttatga-3′ and 5′-aaggtgaaactcttgcctcgtcca-3′.
Protein expression by western blot
Total cell lysates were obtained by scraping cells in 2 µl/cm2 of sample buffer (126 mM Tris/HCl, 20% glycerol (v/v), 40 mg/ml of sodium dodecyl sulphate [SDS]). Collected cell lysates were quantified using the BioRad DC quantification kit according to the manufacturer’s instructions. After heating at 95 °C for 5 minutes and addition of 0.7 M β-mercaptoethanol and 5 µl of bromophenol blue, 50 µg of proteins were loaded on a 10% SDS-PAGE gel and transferred onto a methanol-activated PVDF membrane. Membranes were blocked in fat-free milk (5% w/v) and probed with the following antibodies: mouse monoclonal antibody against human N-cadherin (33–3900, Invitrogen, California, USA); Rabbit polyclonal antibody against human Cx43 (71-0700, Life Technologies, California, USA). Membranes were then incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody and proteins were detected by chemoluminescence (Santa Cruz technologies, California, USA). Equal loading was assessed using mouse monoclonal HRP-conjugated antibody against the housekeeping enzyme GAPDH (Abnova Corporation, Taipei, Taiwan). Images were captured using the BioRad Chemidoc MP system.
Protein localization by immunohistochemistry
MSCs were seeded onto sterile coverslips in 24-well plates and treated with cigarette or e-cigarette extracts for 14 days. At the end of the experimental duration, cells were washed in PBS, fixed and permeabilized in ice-cold absolute ethanol. Cells were blocked with 3% (v/v) non-immune goat serum (NGS) in PBS for one hour at room temperature. Cx43 antibody, diluted in 1% (v/v) NGS in PBS to the concentration of 1 μg/ml, was added onto the cells, overnight at 4 °C in a humidified box. Labelling was achieved by the addition of Alexa 488-labelled fluorescent goat-anti-rabbit secondary antibody. Cell nuclei were stained with 1 μg/ml Hoechst 33324 solution (H3570, Molecular Probes, New York, USA). Finally, ProLong Antifade reagent (P36930, Molecular Probes, New York, USA) was used to cover the cells. Images were acquired using the LSM 710.
Intercellular communication by fluorescence recovery after photo-bleaching (FRAP)
Functionality of gap junction-mediated intercellular communication upon smoke exposure was assessed using the FRAP assay. MSCs were seeded onto confocal dishes (MatTek Corporation, Massachusetts, USA) and treated with cigarette or e-cigarette extracts. On day 14, cells were labelled with calcein-AM (C3100, Molecular Probes, New York, USA), by incubation in 1 μM calcein-AM reconstituted in DMSO, for one hour at 37 °C. Experiments were performed by live imaging at 37 °C using the LSM 710 (63x/1.46 Oil Plan-Apochromatic objective). Experimental cells or regions of interest (ROI) were chosen based on cell confluence, cell-cell contact (allowing gap junction communication) and comparable fluorescence intensity of neighbouring cells. Selected cells were photo-bleached using the 408 nm laser at 10% laser power. Fifteen iterations at 10-second intervals achieved a 50% decrease of ROI fluorescence intensity. Fluorescence recovery was assessed under the 488 nm laser. Images were captured at 10-second intervals and fluorescence intensity of the ROI was quantified over time and normalized to that of control, unbleached, calcein-loaded cells.
Statistical analysis
Data were reported as mean ± standard deviation and statistical analyses, using Student’s t-test, were performed using Microsoft Excel of the Microsoft Office Suite. A p-value of less than 0.05 was considered significant.
Electronic supplementary material
Acknowledgements
This research was partly funded by the, the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and the Centre for Tobacco Products of the U.S. Food and Drug Administration, the Mikati Research Fund and Medical Practice Plan. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
Author Contributions
M.S. conceived and designed the study. A.S. and J.S. supervised experiments and wrote the manuscript. A.S., J.S., M.H., H.C. and Y.H. performed and or contributed to the experiments, data acquisition and analysis. A.H. and A. Shihadeh provided logistical and intellectual support. All authors reviewed the manuscript and approved its submission.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
A. Shaito and J. Saliba contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14634-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ng TK, et al. Cigarette smoking hinders human periodontal ligament-derived stem cell proliferation, migration and differentiation potentials. Scientific reports. 2015;5:7828. doi: 10.1038/srep07828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marinucci L, Bodo M, Balloni S, Locci P, Baroni T. Sub-toxic nicotine concentrations affect extracellular matrix and growth factor signaling gene expressions in human osteoblasts. Journal of cellular physiology. 2014;229:2038–2048. doi: 10.1002/jcp.24661. [DOI] [PubMed] [Google Scholar]
- 3.Camlin NJ, McLaughlin EA, Holt JE. Through the smoke: use of in vivo and in vitro cigarette smoking models to elucidate its effect on female fertility. Toxicology and applied pharmacology. 2014;281:266–275. doi: 10.1016/j.taap.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Ghani R, Qazzaz M, Dabdoub N, Muhammad R, Abdul-Ghani AS. Studies on cigarette smoke induced oxidative DNA damage and reduced spermatogenesis in rats. Journal of environmental biology/Academy of Environmental Biology, India. 2014;35:943–947. [PubMed] [Google Scholar]
- 5.Caponnetto P, Campagna D, Papale G, Russo C, Polosa R. The emerging phenomenon of electronic cigarettes. Expert review of respiratory medicine. 2012;6:63–74. doi: 10.1586/ers.11.92. [DOI] [PubMed] [Google Scholar]
- 6.Oh AY, Kacker A. Do electronic cigarettes impart a lower potential disease burden than conventional tobacco cigarettes? Review on E-cigarette vapor versus tobacco smoke. The Laryngoscope. 2014;124:2702–2706. doi: 10.1002/lary.24750. [DOI] [PubMed] [Google Scholar]
- 7.Bahl V, et al. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reproductive toxicology (Elmsford, N.Y.) 2012;34:529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Behar, R. Z. et al. Identification of Toxicants in Cinnamon-Flavored Electronic Cigarette Refill Fluids. Toxicology in vitro: an international journal published in association with BIBRA, 10.1016/j.tiv.2013.10.006 (2013). [DOI] [PubMed]
- 9.Husari, A. et al. Acute Exposure to Electronic and Combustible Cigarette Aerosols: Effects in an Animal Model and in Human Alveolar Cells. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco, 10.1093/ntr/ntv169 (2015). [DOI] [PMC free article] [PubMed]
- 10.Anderson C, Majeste A, Hanus J, Wang S. E-Cigarette Aerosol Exposure Induces Reactive Oxygen Species, DNA Damage, and Cell Death in Vascular Endothelial Cells. Toxicological sciences: an official journal of the Society of Toxicology. 2016;154:332–340. doi: 10.1093/toxsci/kfw166. [DOI] [PubMed] [Google Scholar]
- 11.Higham A, et al. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respiratory research. 2016;17:56. doi: 10.1186/s12931-016-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauterstein DE, et al. Frontal Cortex Transcriptome Analysis of Mice Exposed to Electronic Cigarettes During Early Life Stages. International journal of environmental research and public health. 2016;13:417. doi: 10.3390/ijerph13040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGrath-Morrow SA, et al. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PloS one. 2015;10:e0118344. doi: 10.1371/journal.pone.0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubenstein DA, Hom S, Ghebrehiwet B, Yin W. Tobacco and e-cigarette products initiate Kupffer cell inflammatory responses. Molecular immunology. 2015;67:652–660. doi: 10.1016/j.molimm.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Izzotti A, Pulliero A. Molecular damage and lung tumors in cigarette smoke-exposed mice. Annals of the New York Academy of Sciences. 2015;1340:75–83. doi: 10.1111/nyas.12697. [DOI] [PubMed] [Google Scholar]
- 16.Deeb, R. S. et al. Smoking-associated Disordering of the Airway Basal Stem/Progenitor Cell Metabotype. American journal of respiratory cell and molecular biology, 10.1165/rcmb.2015-0055OC (2015). [DOI] [PMC free article] [PubMed]
- 17.Boers JE, den Brok JL, Koudstaal J, Arends JW, Thunnissen FB. Number and proliferation of neuroendocrine cells in normal human airway epithelium. American journal of respiratory and critical care medicine. 1996;154:758–763. doi: 10.1164/ajrccm.154.3.8810616. [DOI] [PubMed] [Google Scholar]
- 18.Rammah M, Dandachi F, Salman R, Shihadeh A, El-Sabban M. In vitro cytotoxicity and mutagenicity of mainstream waterpipe smoke and its functional consequences on alveolar type II derived cells. Toxicology letters. 2012;211:220–231. doi: 10.1016/j.toxlet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rammah M, Dandachi F, Salman R, Shihadeh A, El-Sabban M. In vitro effects of waterpipe smoke condensate on endothelial cell function: a potential risk factor for vascular disease. Toxicology letters. 2013;219:133–142. doi: 10.1016/j.toxlet.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minai OA, Benditt J, Martinez FJ. Natural history of emphysema. Proceedings of the American Thoracic Society. 2008;5:468–474. doi: 10.1513/pats.200802-018ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi, S. M. et al. Prevalence and Global Initiative for Chronic Obstructive Lung Disease Group Distribution of Chronic Obstructive Pulmonary Disease Detected by Preoperative Pulmonary Function Test. Plos One10, doi:UNSP e0115787 10.1371/journal.pone.0115787 (2015). [DOI] [PMC free article] [PubMed]
- 22.Hannoush EJ, et al. Impact of enhanced mobilization of bone marrow derived cells to site of injury. The Journal of trauma. 2011;71:283–289. doi: 10.1097/TA.0b013e318222f380. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Mignone J, MacLellan WR. Cardiac Regeneration and Stem Cells. Physiological reviews. 2015;95:1189–1204. doi: 10.1152/physrev.00021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathiasen AB, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) European heart journal. 2015;36:1744–1753. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- 25.Kar R, Riquelme MA, Werner S, Jiang JX. Connexin 43 channels protect osteocytes against oxidative stress-induced cell death. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28:1611–1621. doi: 10.1002/jbmr.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Sinovas A, et al. The modulatory effects of connexin 43 on cell death/survival beyond cell coupling. Progress in biophysics and molecular biology. 2007;94:219–232. doi: 10.1016/j.pbiomolbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Huang RP, et al. Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43) Cancer research. 1998;58:5089–5096. [PubMed] [Google Scholar]
- 28.Gramsch B, et al. Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast-like cell line. Experimental cell research. 2001;264:397–407. doi: 10.1006/excr.2000.5145. [DOI] [PubMed] [Google Scholar]
- 29.Donahue HJ. Gap junctions and biophysical regulation of bone cell differentiation. Bone. 2000;26:417–422. doi: 10.1016/S8756-3282(00)00245-3. [DOI] [PubMed] [Google Scholar]
- 30.Haussig S, Schubert A, Mohr FW, Dhein S. Sub-chronic nicotine exposure induces intercellular communication failure and differential down-regulation of connexins in cultured human endothelial cells. Atherosclerosis. 2008;196:210–218. doi: 10.1016/j.atherosclerosis.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Tsai CH, et al. Down-regulating effect of nicotine on connexin43 gap junctions in human umbilical vein endothelial cells is attenuated by statins. European journal of cell biology. 2004;82:589–595. doi: 10.1078/0171-9335-00348. [DOI] [PubMed] [Google Scholar]
- 32.Wang HH, et al. Activation of endothelial cells to pathological status by down-regulation of connexin43. Cardiovascular research. 2008;79:509–518. doi: 10.1093/cvr/cvn112. [DOI] [PubMed] [Google Scholar]
- 33.Spath C, Schlegel F, Leontyev S, Mohr FW, Dhein S. Inverse Relationship between Tumor Proliferation Markers and Connexin Expression in a Malignant Cardiac Tumor Originating from Mesenchymal Stem Cell Engineered Tissue in a Rat in vivo Model. Frontiers in pharmacology. 2013;4:42. doi: 10.3389/fphar.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zibara K, et al. Anti-angiogenesis therapy and gap junction inhibition reduce MDA-MB-231 breast cancer cell invasion and metastasis in vitro and in vivo. Scientific reports. 2015;5:12598. doi: 10.1038/srep12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu M, et al. Cx43 reverses the resistance of A549 lung adenocarcinoma cells to cisplatin by inhibiting EMT. Oncology reports. 2014;31:2751–2758. doi: 10.3892/or.2014.3163. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, et al. Up-regulation of connexin-43 expression in bone marrow mesenchymal stem cells plays a crucial role in adhesion and migration of multiple myeloma cells. Leukemia & lymphoma. 2015;56:211–218. doi: 10.3109/10428194.2014.913289. [DOI] [PubMed] [Google Scholar]
- 37.Lim KT, et al. Synergistic effects of orbital shear stress on in vitro growth and osteogenic differentiation of human alveolar bone-derived mesenchymal stem cells. BioMed research international. 2014;2014:316803. doi: 10.1155/2014/316803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Nieto D, et al. Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood. 2012;119:5144–5154. doi: 10.1182/blood-2011-07-368506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossello RA, Kohn DH. Gap junction intercellular communication: a review of a potential platform to modulate craniofacial tissue engineering. Journal of biomedical materials research. Part B, Applied biomaterials. 2009;88:509–518. doi: 10.1002/jbm.b.31127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagener TL, Floyd EL, Stepanov I. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. 2017;26:e23–e28. doi: 10.1136/tobaccocontrol-2016-053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bekki K, et al. Carbonyl compounds generated from electronic cigarettes. International journal of environmental research and public health. 2014;11:11192–11200. doi: 10.3390/ijerph111111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Hellani, A. et al. Nicotine and Carbonyl Emissions From Popular Electronic Cigarette Products: Correlation to Liquid Composition and Design Characteristics. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco, 10.1093/ntr/ntw280 (2016). [DOI] [PMC free article] [PubMed]
- 43.Farsalinos KE, Kistler KA, Gillman G, Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2015;17:168–174. doi: 10.1093/ntr/ntu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farsalinos KE, Voudris V, Poulas K. E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction (Abingdon, England) 2015;110:1352–1356. doi: 10.1111/add.12942. [DOI] [PubMed] [Google Scholar]
- 45.Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. International journal of hygiene and environmental health. 2016;219:268–277. doi: 10.1016/j.ijheh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Goel R, et al. Highly reactive free radicals in electronic cigarette aerosols. Chemical research in toxicology. 2015;28:1675–1677. doi: 10.1021/acs.chemrestox.5b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goniewicz ML, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogunwale MA, et al. Aldehyde Detection in Electronic Cigarette Aerosols. ACS omega. 2017;2:1207–1214. doi: 10.1021/acsomega.6b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pankow JF, et al. Benzene formation in electronic cigarettes. PloS one. 2017;12:e0173055. doi: 10.1371/journal.pone.0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soussy S, et al. Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tobacco control. 2016;25:ii88–ii93. doi: 10.1136/tobaccocontrol-2016-053220. [DOI] [PubMed] [Google Scholar]
- 51.Tayyarah R, Long GA. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regulatory toxicology and pharmacology: RTP. 2014;70:704–710. doi: 10.1016/j.yrtph.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder. Chemical research in toxicology. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- 53.Hwang JH, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. Journal of molecular medicine (Berlin, Germany) 2016;94:667–679. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- 54.Schweitzer KS, et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. American journal of physiology. Lung cellular and molecular physiology. 2015;309:L175–187. doi: 10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang DY, et al. Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Molecular and cellular biochemistry. 2013;374:13–20. doi: 10.1007/s11010-012-1498-1. [DOI] [PubMed] [Google Scholar]
- 56.Shao JS, et al. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Annals of the New York Academy of Sciences. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- 57.Imhoff BR, Hansen JM. Differential redox potential profiles during adipogenesis and osteogenesis. Cellular & molecular biology letters. 2011;16:149–161. doi: 10.2478/s11658-010-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun N, et al. Effect of advanced oxidation protein products on the proliferation and osteogenic differentiation of rat mesenchymal stem cells. International journal of molecular medicine. 2013;32:485–491. doi: 10.3892/ijmm.2013.1402. [DOI] [PubMed] [Google Scholar]
- 59.Bai XC, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochemical and biophysical research communications. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 60.Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem cells (Dayton, Ohio) 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 61.Halliwell B, Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environmental health perspectives. 1994;102(Suppl 10):5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tormos KV, et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell metabolism. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao H, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free radical biology & medicine. 2003;34:1359–1368. doi: 10.1016/S0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, et al. Imaging ROS signaling in cells and animals. Journal of Molecular Medicine (Berlin, Germany) 2013;91:917–927. doi: 10.1007/s00109-013-1067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He C, Hart PC, Germain D, Bonini MG. SOD2 and the Mitochondrial UPR: Partners Regulating Cellular Phenotypic Transitions. Trends in biochemical sciences. 2016;41:568–577. doi: 10.1016/j.tibs.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mroue RM, El-Sabban ME, Talhouk RS. Connexins and the gap in context. Integrative biology: quantitative biosciences from nano to macro. 2011;3:255–266. doi: 10.1039/c0ib00158a. [DOI] [PubMed] [Google Scholar]
- 67.Cusano, N. E. Skeletal Effects of Smoking. Current osteoporosis reports, 10.1007/s11914-015-0278-8 (2015). [DOI] [PubMed]
- 68.Sarkar M, Bhardwaj R, Madabhavi I, Khatana J. Osteoporosis in chronic obstructive pulmonary disease. Clinical medicine insights. Circulatory, respiratory and pulmonary medicine. 2015;9:5–21. doi: 10.4137/CCRPM.S22803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ben-Yehudah A, et al. Nicotine exposure during differentiation causes inhibition of N-myc expression. Respiratory research. 2013;14:119. doi: 10.1186/1465-9921-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laugesen, J. L. a. M. in Society of Toxicology Conference.
- 71.Welz C, et al. Cytotoxic and Genotoxic Effects of Electronic Cigarette Liquids on Human Mucosal Tissue Cultures of the Oropharynx. Journal of environmental pathology, toxicology and oncology: official organ of the International Society for Environmental Toxicology and Cancer. 2016;35:343–354. doi: 10.1615/JEnvironPatholToxicolOncol.2016016652. [DOI] [PubMed] [Google Scholar]
- 72.Talih S, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2015;17:150–157. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamanouchi K, Gotoh Y, Nagayama M. Dexamethasone enhances differentiation of human osteoblastic cells in vitro. J Bone Miner Metab. 1997;15:23–29. doi: 10.1007/BF02439451. [DOI] [Google Scholar]
- 74.Liu YC, et al. CCL5/RANTES is important for inducing osteogenesis of human mesenchymal stem cells and is regulated by dexamethasone. Bioscience trends. 2014;8:138–143. doi: 10.5582/bst.2014.01047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.