Abstract
An important goal of vaccination against viruses and virus-driven cancers is to elicit cytotoxic CD8+ T cells specific for virus-derived peptides. CD8+ T cell responses can be enhanced by engaging help from natural killer T (NKT) cells. We have produced synthetic vaccines that induce strong peptide-specific CD8+ T cell responses in vivo by incorporating an NKT cell-activating glycolipid. Here we examine the effect of a glycolipid-peptide conjugate vaccine incorporating an NKT cell-activating glycolipid linked to an MHC class I-restricted peptide from a viral antigen in human peripheral blood mononuclear cells. The vaccine induces CD1d-dependent activation of human NKT cells following enzymatic cleavage, activates human dendritic cells in an NKT-cell dependent manner, and generates a pool of activated antigen-specific CD8+ T cells with cytotoxic potential. Compared to unconjugated peptide, the vaccine upregulates expression of genes encoding interferon-γ, CD137 and granzyme B. A similar vaccine incorporating a peptide from the clinically-relevant human papilloma virus (HPV) 16 E7 oncoprotein induces cytotoxicity against peptide-expressing targets in vivo, and elicits a better antitumor response in a model of E7-expressing lung cancer than its unconjugated components. Glycolipid-peptide conjugate vaccines may prove useful for the prevention or treatment of viral infections and tumors that express viral antigens.
Introduction
Eliciting proliferation and activation of cytotoxic CD8+ T cells that can destroy cells expressing pathogen-derived or tumor-associated peptides is an important goal in vaccine development1,2. However, unadjuvanted peptides fail to elicit strong cytotoxic T cell responses and, while live attenuated pathogens can generate CD8+ T cell-mediated immunity3–5, safety and manufacturing considerations favor synthetic adjuvants. Most adjuvants in clinical use, such as alum, oil-in-water emulsions, and Toll-like receptor (TLR) ligands6–8, have been approved based on their capacity to elicit antibody-mediated immune responses, not cytotoxic T cell responses. There is an urgent need for synthetic adjuvants capable of eliciting strong cytotoxic T cell responses.
While most existing adjuvants engage pattern recognition receptors (PRRs) within or on antigen-presenting cells (APCs), an alternative method of enhancing an immune response is to exploit the capacity of CD4+ T cells to provide ‘help’ to APCs9. For example, the keyhole limpet hemocyanin (KLH) carrier protein can activate CD4+ T cells to shape the cytotoxic T cell response against co-administered peptides10,11. However, such ‘non-cognate’ CD4+ T cell help generates weaker memory CD8+ T cell responses than does help from CD4+ T cells recognizing the same antigen12,13, while the latter approach may be constrained by difficulties identifying shared CD4+ and CD8+ epitopes given the extreme polymorphism of the major histocompatibility complex (MHC) in humans14.
Natural killer T (NKT) cells are a class of T cells expressing canonical T cell receptors that recognize glycolipid antigens presented by the non-classical MHC molecule, CD1d. NKT cells exist in a semi-activated state capable of responding rapidly to antigenic stimulation15. APCs express CD1d and are capable of processing and presenting glycolipids, such as the potent synthetic antigen α-galactosylceramide (α-GalCer), to NKT cells16. Similar to classical CD4+ T cell help, NKT cell help promotes dendritic cell (DC) licensing, maturation and the production of pro-inflammatory cytokines, e.g., IFN-γ and IL-12, which enhance CD8+ T cell responses against co-presented peptide antigens17. This help depends on peptide and glycolipid presentation by the same APC, and in vivo requires an interaction between CD40 and CD40L18,19. Potential advantages of exploiting NKT rather than conventional CD4+ T cell help in a clinical context include avoiding the need to select adjuvants according to MHC class II expression20, and eliciting a CD8+ T cell response with a distinct chemokine receptor profile21,22. In mouse models, NKT cell activation at the time of vaccination or infection promotes virus-specific CD8+ T cell memory23,24. Although there is abundant evidence of NKT cell adjuvant activity in murine models in vivo, evidence for an adjuvant effect in humans, who have much lower NKT cell frequencies, is much more limited.
We have previously synthesized glycolipid-peptide conjugate vaccines comprising an α-GalCer prodrug covalently linked to an antigenic peptide via a self-immolating para-aminobenzyl (PAB) linker. These vaccines were designed to co-deliver CD8+ T cell peptide epitopes and α-GalCer to the same APC and elicit functional peptide-specific CD8+ T cell responses in mouse models of allergic airway inflammation and B.16 melanoma25,26. We have also shown that a glycolipid-peptide conjugate vaccine incorporating α-GalCer with an immunodominant HLA-A*02-restricted peptide from the cytomegalovirus (CMV) pp65 protein can stimulate human NKT cells and peptide-specific CD8+ T cells25.
Here we investigate the activity and mechanism of a glycolipid-peptide conjugate vaccine that elicits virus-specific CD8+ T cell responses in human PBMCs. We show that this vaccine activates human DCs and CD8+ T cells in a manner dependent on NKT cells, and that the CD8+ T cells elicited by this vaccine are peptide-specific and have cytotoxic potential. Furthermore, we show that a vaccine of the same design but incorporating a human papilloma virus (HPV) E7 peptide is capable of delaying tumor growth in an in vivo mouse model of E6/E7-expressing lung cancer.
Results
Glycolipid-peptide conjugate vaccine requires cathepsin cleavage and induces CD1d-dependent NKT cell proliferation
The glycolipid-peptide conjugate vaccine α-GalCer-pp65495-503 (Fig. 1A) consists of a pro-drug form of the glycolipid α-galactosylceramide (α-GalCer), which readily reverts to its more stable N-acyl form under physiological conditions25, linked via a cathepsin-B-cleavable linker to the peptide sequence FFRK-NLVPMVATV (here termed pp65495-503), which contains a HLA-A*02-restricted epitope from cytomegalovirus (CMV) pp65 protein. CD8+ T-cells specific for NLVPMVATV can be readily detected in PBMCs from HLA-A*02+ CMV-seropositive healthy donors using loaded MHC class I multimers27. The peptide sequence incorporates the cleavage sequence FFRK at the N-terminus to promote proteolytic generation of the NLVPMVATV epitope within APCs28.
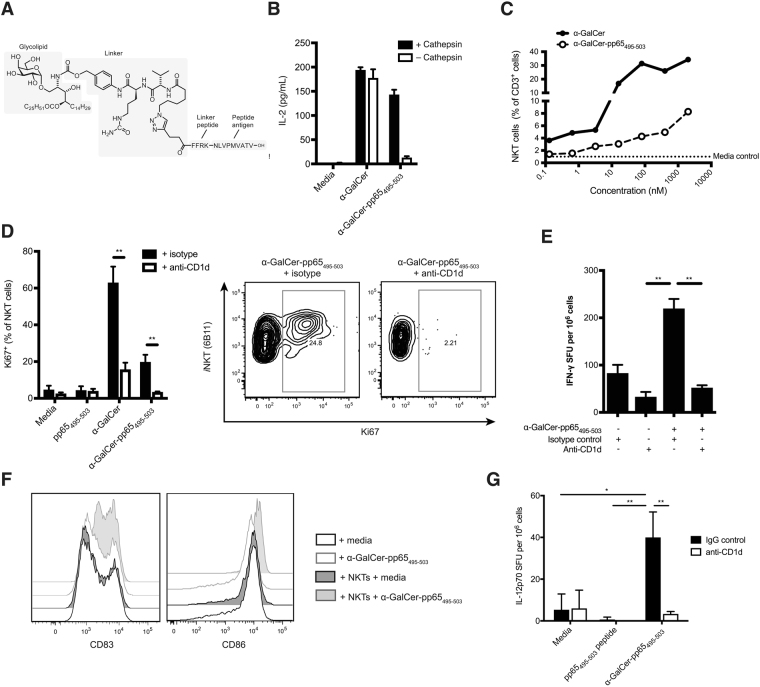
Figure 1.
α-GalCer-pp65495-503 conjugate vaccine activates human NKT cells and DCs (A) Chemical structure of the conjugate vaccine, α-GalCer-pp65495-503, containing the HLA-A*02-restricted ‘NLV’ peptide from cytomegalovirus pp65 protein linked via an enzymatically cleavable linker to a pro-α-GalCer (B) IL-2 production by mouse NKT hybridoma cells was measured by enzyme-linked immunosorbent assay (ELISA) 18 h after addition of equimolar concentrations of α-GalCer or α-GalCer-pp65495-503 pre-treated with cathepsin-B or PBS **p < 0.01; Bonferroni multiple comparison test. (C) The number of NKT cells (% of total CD3+ cells) was quantified by flow cytometry in PBMCs from a HLA-A*02 negative donor 72 h after addition of varying concentrations of α-GalCer or α-GalCer-pp65495-503; representative of two independent experiments. (D) Proliferation of NKT cells was measured by flow cytometry using anti-Ki67 72 h after treatment of PBMCs from a HLA-A*02 negative donor with equimolar concentrations of pp65495-503 peptide, α-GalCer, or α-GalCer-pp65495-503 with anti-CD1d or matched isotype control antibody **p < 0.01; Bonferroni multiple comparison test. Data representative of two independent experiments. (E) IFN-γ production was measured by ELISpot 72 h after treatment of PBMCs from a HLA-A*02 negative donor with α-GalCer-pp65495-503+/− anti-CD1d or matched isotype control antibody **p < 0.01; Student’s T test; SFU, spot-forming units. (F) Expression of the activation markers CD83 and CD86 on monocyte-derived (mo)DCs derived from a HLA-A*02 negative donor 48 h after treatment with α-GalCer-pp65495-503 or media control, in the presence or absence of autologous NKT cells. Result representative of three independent experiments.
To show that conjugate vaccine must first be cleaved into its active components in order to stimulate NKT cells, α-GalCer-pp65495-503 and free α-GalCer were pre-treated with cathepsin-B or PBS control, and loaded onto plate-bound mouse CD1d monomers. Unlike free α-GalCer, α-GalCer-pp65495-503 required pre-treatment with cathepsin-B in order to stimulate IL-2 production by the mouse hybridoma NKT cell line DN32.3, indicating that the α-GalCer-pp65495-503 vaccine requires proteolytic processing to produce free α-GalCer capable of activating NKT cells (Fig. 1B).
We have previously shown that α-GalCer-pp65495-503 is able to induce IFN-γ production and CD137 up-regulation on human NKT cells25. To determine whether α-GalCer-pp65495-503 can also induce proliferation of NKT cells, PBMCs derived from an HLA-A*02-negative donor were cultured in the presence of equimolar concentrations of α-GalCer or α-GalCer-pp65495-503 conjugate. Quantification of NKT cells (as a % of total CD3+ cells) showed that addition of α-GalCer-pp65495-503 induced NKT cell expansion in a dose-dependent manner, although overall expansion was lower with the vaccine than with free α-GalCer (Fig. 1C). Similarly, intracellular staining using anti-Ki67 showed proliferation of NKT cells in response to both α-GalCer-pp65495-503 and to free α-GalCer, which could be abolished by addition of an anti-CD1d antibody (Fig. 1D). As expected, the peptide alone did not trigger NKT cell proliferation above the level of the media-only control. Finally, interferon (IFN)-γ ELISpot demonstrated that α-GalCer-pp65495-503 induced IFN-γ production, and that this was blocked by anti-CD1d (Fig. 1E). Taken together, these data demonstrate that the glycolipid-peptide conjugate vaccine α-GalCer-pp65495-503 induces proliferation and activation of human NKT cells in a CD1d-dependent manner.
In mice, the presentation of α-GalCer by DCs on CD1d activates NKT cells to ‘license’ DCs, in a manner analogous to traditional CD4+ T cell help17. This leads to up-regulation of DC co-stimulatory markers and increased IL-12 production, which further activates NKT cells, as well as augmenting peptide-specific CD8+ T cell responses29–31. To assess the ability of α-GalCer-pp65495-503 to activate human DCs, expression of the maturation marker CD83 and co-stimulatory molecule CD86 was assessed by flow cytometry 48 h after addition of α-GalCer-pp65495-503 to a co-culture of human moDCs and autologous NKT cells expanded ex vivo (Fig. 1F). α-GalCer-pp65495-503 did not induce DC activation in the absence of NKT cells indicating that it does not directly engage PRRs. Instead, only moDCs treated with α-GalCer-pp65495-503 and co-cultured with NKT cells showed elevated expression of CD83 and CD86 above levels seen on unsupplemented moDCs. Similar results were seen using unconjugated α-GalCer (data not shown). Upon presentation of α-GalCer to NKT cells, murine dendritic cells produce IL-1229. To determine whether the α-GalCer-pp65495-503 conjugate can induce IL-12 production by human APCs, PBMCs from an HLA-A*02-negative donor were cultured with pp65 peptide, α-GalCer, α-GalCer-pp65495-503 conjugate or media control in the presence of anti-CD1d antibody or IgG control; IL-12p70 production was determined by ELISpot (Fig. 1G ). Both α-GalCer and α-GalCer-pp65495-503 led to CD1d-dependent IL-12p70 production, consistent with DC activation. Thus, α-GalCer-pp65495-503 activates human DCs in an NKT cell- and CD1d-dependent manner.
Glycolipid-peptide conjugate vaccine leads to peptide-specific CD8+ T cell activation
We have previously shown that the α-GalCer-pp65495-503 conjugate vaccine can induce activated peptide-responsive CD8+ T cells25. To verify the peptide specificity of CD8+ T cell activation, we made an alternative conjugate vaccine incorporating the immunodominant HLA-A*02-restricted peptide from influenza A virus (IAV) matrix-1 (M1) protein, here termed α-GalCer-M158-66. Dual dextramer staining of PBMCs treated with α-GalCer-pp65495-503 or α-GalCer-M158-66 showed that the α-GalCer-pp65495-503 conjugate vaccine induced expansion of pp65495-503-specific but not of M158-66-specific CD8+ T cells, and vice versa for α-GalCer-M158-66 (Fig. 2A,B), confirming that the activation of CD8+ T cells by glycolipid-peptide conjugate vaccines is peptide-specific.
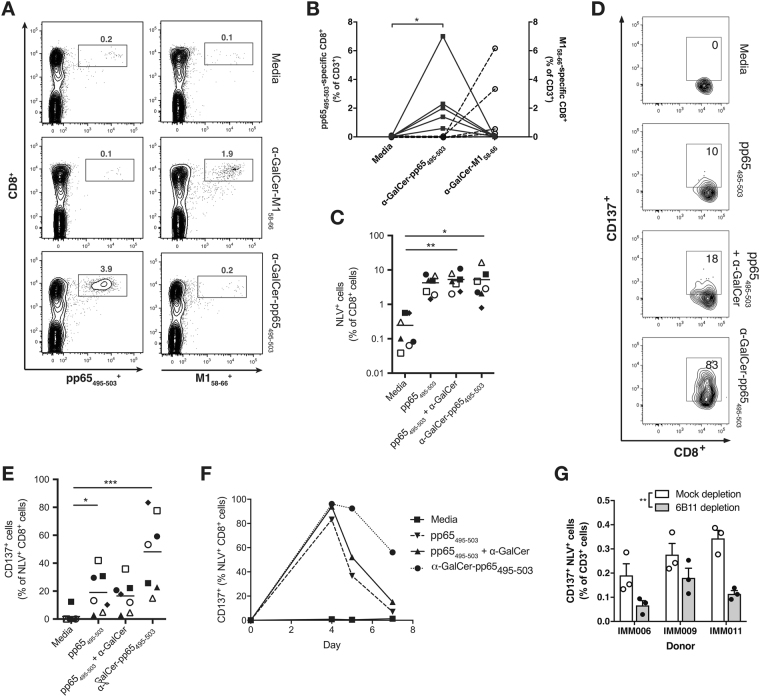
Figure 2.
α-GalCer-pp65495-503 activates NLV-specific CD8+ T cells in an NKT cell-dependent manner (A, B) Peptide specificity of the CD8+ T cell pool expanded with α-GalCer-pp65495-503, or with a similar conjugate vaccine containing an influenza matrix protein-derived peptide (α-GalCer-M158-66) was demonstrated measured by dual dextramer staining; representative flow cytometry plots (B) and summary of results of five HLA-A*02+ CMV-responsive donors tested (B) are shown. Square symbols and solid lines represent pp65495-503-loaded tetramer+ cells; black open circles and dashed lines represent M158-66-loaded tetramer+ cells; *p < 0.01; Friedman test with Dunn’s multiple comparison tests. Frequency (C) and CD137 expression (D,E) of NLV-specific CD8+ T cells was quantified by flow cytometry 7 days after adding equimolar concentrations of pp65495-503 peptide alone, peptide admixed with α-GalCer or α-GalCer-pp65495-503 to PBMCs from HLA-A*02+ donors. *p < 0.05, **p < 0.01, ***p < 0.001; Friedman test with Dunn’s multiple comparison tests. Representative flow cytometry plots are shown in (D). (F) Time-course showing CD137 status of NLV-specific CD8+ T cells 4–7 days after exposure to pp65495-503 peptide, peptide admixed with α-GalCer or α-GalCer-pp65495-503; result representative of two independent experiments. (G) PBMCs were depleted of NKT cells by immunomagnetic beads, or mock depleted, and CD137+ status of NLV-specific CD8+ T cells was quantified by flow cytometry 7 days after addition of α-GalCer-pp65495-503, in triplicate. The results from three separate donors in three separate experiments are shown. ***p < 0.001 for an effect of NKT cell depletion by two-way ANOVA; **p < 0.01, Bonferroni’s post tests.
We assessed the capacity of the α-GalCer-pp65495-503 conjugate vaccine to stimulate peptide-specific CD8+ T cell responses using PBMCs from HLA-A2+ CMV seropositive donors known to harbor a pre-existing population of NLVPMVATV- (NLV; pp65495-503) specific CD8+ T cells in three donors previously reported25 and four additional donors. Among these donors, a median of 0.08% of CD3+ T cells were NKT cells (range 0.02–0.35%), as assessed by expression of the Vα24 Jα18 TCR. After seven days of culture, the proportion of peptide-specific CD8+ T cells (as a % of total CD8+ cells) detectable with fluorescent NLV-loaded HLA-A2 dextramers was significantly increased in cultures containing α-GalCer-pp65495-503 compared to unsupplemented (media-only) controls (Fig. 2C). In this in vitro system, the total number of NLV-specific CD8+ T cells did not differ between cultures treated with α-GalCer-pp65495-503, its admixed components or the pp65495-503 peptide alone.
After seven days in culture NLV-specific CD8+ T cells expanded with α-GalCer-pp65495-503 showed higher expression of the T cell activation marker CD137 (4-1BB), a surface glycoprotein belonging to the tumor-necrosis factor receptor superfamily (TNFRSF)32,33, compared to cells treated with either peptide alone or admixed peptide and α-GalCer (Fig. 2D,E). Binding of CD137 to its ligand 4-1BBL promotes increased T cell proliferation, cytokine production, functional maturation, and prolonged survival of CD8+ T cells34–36. Interestingly, at earlier time-points CD137 expression was also elevated in response to admixed peptide and α-GalCer as well as peptide alone, however, this elevated expression was only sustained on NLV-specific CD8+ T cells expanded with α-GalCer-pp65495-503, suggesting that conjugation modifies the T cell response to peptide or affects the kinetics of peptide presentation (Fig. 2F).
To determine whether NKT cells are required for the CD8+ T cell response to α-GalCer-pp65495-503, NKT cells were depleted from whole PBMCs derived from three separate donors. Compared to mock-depleted controls, NKT depletion led to a significant reduction in the proportion of CD137+ NLV-specific T cells in response to α-GalCer-pp65495-503 (Fig. 2G), indicating that full vaccine-induced activation of peptide-specific CD8+ T cells requires NKT cells.
Glycolipid-peptide conjugate elicits peptide-specific CD8+ T cells with cytotoxic potential
To assess the functional outcome of α-GalCer-pp65495-503 treatment, intracellular staining for LAMP-1 (CD107a), a marker of degranulation of cytotoxic molecules, and the pro-inflammatory cytokine IFN-γ was performed on NLV-specific CD8+ T cells. Both LAMP-1 and IFN-γ were expressed to a higher degree on NLV-specific CD8+ T cells expanded with α-GalCer-pp65495-503, compared to those exposed to peptide alone, or to admixed peptide and α-GalCer (Fig. 3A,B). In contrast, α-GalCer-pp65495-503 did not induce expression of LAMP-1 or IFN-γ on the dextramer negative CD8+ T cell population within the cultures (data not shown), indicating that the induction of these molecules is restricted to antigen-specific T cells.
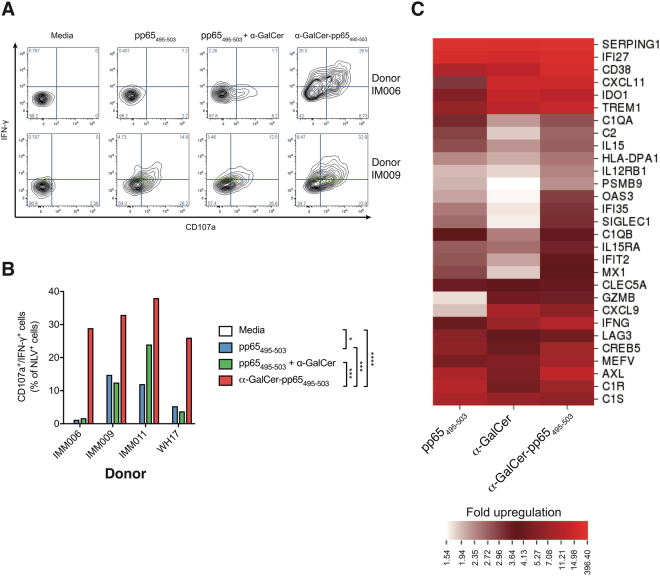
Figure 3.
α-GalCer-pp65495-503 elicits a cytotoxic CD8+ T cell phenotype and expression of interferon-inducible genes. (A) Representative flow plots and (B) summary graph from four donors showing expression of CD107a and IFN-γ by NLV-specific CD8+ T cells 7 days after addition of equimolar concentrations of pp65495-503 peptide alone, pp65495-503 admixed with α-GalCer or the α-GalCer-pp65495-503 conjugate. ****p < 0.0001; ***p < 0.001; *p < 0.05, two way ANOVA with Tukey’s multiple comparisons test. (C) Nanostring RNA analysis was performed on whole PBMCs from human donors (n = 4) treated with α-GalCer-pp65495-503, pp65495-503 peptide, or α-GalCer for 7 days. Heatmap shows the genes at least two-fold up-regulated in response to α-GalCer-pp65495-503, compared with media alone, in all four donors.
To further explore the effect of α-GalCer-pp65495-503 treatment on human PBMCs, Nanostring RNA analysis was performed on RNA derived from whole PBMCs after seven days of culture with α-GalCer-pp65495-503 conjugate, its components (pp65495-503 peptide or α-GalCer), and media control (Fig. 3C). α-GalCer-pp65495-503 led to elevated expression of the interferon-inducible genes IFI35, OAS3, IFIT2, and MX1, compared with levels seen in response to either peptide alone, α-GalCer alone or media control. Compared to pp65495-503 peptide, the α-GalCer-pp65495-503 conjugate led to higher expression of the genes encoding interferon gamma-induced protein-10 (IP-10; CXCL10), IFN-γ (IFNG) and CD137 (TNFRSF9), consistent with the phenotypic findings of increased IFN-γ and CD137 expression (Supplementary Table 1). Although upregulation of LAMP1 was not observed at the RNA level (mean 1.1 fold expression in α-GalCer-pp65495-503- compared to pp65495-503-treated cells), expression of the cytotoxin granzyme B (GZMB) was enhanced by the conjugate vaccine.
Taken together, these data indicate that the α-GalCer-pp65495-503 glycolipid-peptide conjugate vaccine can elicit peptide-specific T cells with cytotoxic potential in human PBMCs.
Glycolipid-peptide conjugate elicits cytotoxic responses to a viral antigen in vivo
To assess whether conjugate vaccines elicit peptide-specific cytotoxicity against viral antigens in vivo, conjugate vaccine α-GalCer-E749-57 (Fig. 4A ) was synthesized using the H-2Db-restricted peptide RAHYNIVTF from HPV16-E7 protein, an oncoprotein associated with HPV-driven malignancies including cervical and head and neck cancers. Again, FFRK was added to the N-terminus of the viral sequence to promote proteolytic release of the minimal MHC-binding peptide.
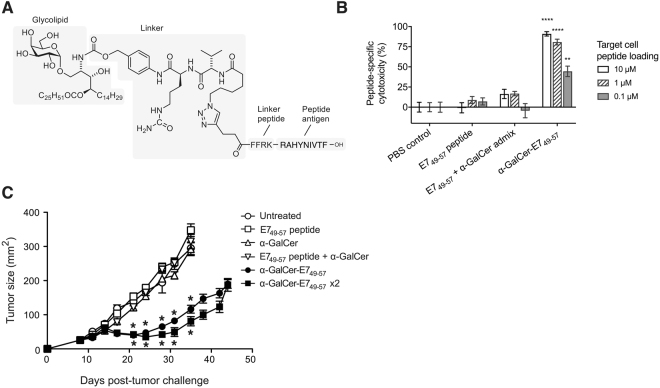
Figure 4.
α-GalCer-E749-57 conjugate vaccine delays growth of an established HPV16-E7-expressing tumor in vivo. (A) Chemical structure of the conjugate vaccine, α-GalCer-E749-57, incorporating the RAHYNIVTF peptide from HPV16-E7 protein (B) C57BL/6 mice (5 per group) were vaccinated intravenously with α-GalCer-E749-57, α-GalCer and peptide admix, E749-57 peptide alone or PBS control. Specific lysis of E749-57 peptide-loaded splenocytes was assessed in vivo 10 days after vaccination. ****p < 0.0001; **p < 0.01, one-way ANOVA with Tukey’s multiple comparisons (C) 2.5 × 105 HPV16-E6 and -E7 protein-expressing TC-1 cells were implanted subcutaneously into C57/BL6 mice (n = 5 per group). On day 8, E749-57 peptide, α-GalCer, admix or α-GalCer-E749-57 were administered intravenously; one group was left untreated, and one group received a second dose of α-GalCer-E749-57 after a further seven days. Tumor size was determined as the product of the two diameters. *p < 0.0001 (difference between treatment group, and each of the four non-conjugate vaccine groups) by one-way ANOVA.
To assess capacity of this vaccine to induce peptide-specific responses, mice were vaccinated with α-GalCer-E749-57, admixed α-GalCer and E749-57 peptide or saline control. Splenocytes were restimulated ex vivo with the E749-57 peptide, and IFN-γ production assessed by ELISpot. Compared to its admixed components or saline control, α-GalCer-E749-57 led to increased peptide-induced IFN-γ production (Supplementary Fig. S1). To determine whether the vaccine could induce cytotoxicity against peptide-expressing targets in vivo, animals were vaccinated with α-GalCer-E749-57, α-GalCer and peptide admix, E749-57 peptide alone, or saline control, then E749-57 peptide-pulsed or unpulsed target splenocytes were administered, and their frequency assessed by flow cytometry. The results indicate that the α-GalCer-E749-57 vaccine induces in vivo cytotoxicity against cells expressing a virus-derived peptide (Fig. 4B).
To assess functional activity, α-GalCer-E749-57 vaccine was administered to animals implanted with cells from the TC-1 tumor cell line, which expresses HPV16-E6 and -E7 proteins. A single dose of α-GalCer-E749-57 vaccine eight days after tumor inoculation significantly delayed tumor growth relative to saline- or unconjugated component-treated control animals. This activity was improved further with a second dose of vaccine 7 days after the first. The antitumor activity was dependent on the vaccine design, with antigen and adjuvant covalently linked together, as injection of unconjugated components alone or as an admix did not provide antitumor activity (Fig. 4C ).
Discussion
We have previously shown that synthetic vaccines that combine an NKT-activating glycolipid with an immunodominant antigenic peptide are effective against a model antigen (ovalbumin) in vivo, and that a similar vaccine can activate human CMV-specific CD8+ T cells in vitro 25,26. He we report that the activation of human virus-specific CD8+ T cells induced by these vaccines is NKT cell-dependent and peptide-specific, and that the vaccines activate human antigen-presenting cells in the presence of NKT cells, elicit peptide-specific CD8+ T cells with cytotoxic potential, and induce cytotoxicity against a clinically-relevant viral oncoprotein in vivo.
To-date, most pre-clinical studies using NKT cell agonists to drive peptide-specific T cell or antibody responses have been performed in mice, which have much higher circulating NKT cell frequencies than most humans37. Although human NKT cell frequencies typically exceed those of a CD4+ T cell of a given peptide specificity38,39, the reduced NKT cell niche has been raised as a barrier to generating effective immunotherapies utilizing these cells40. The current study provides “proof of principle” that human NKT cells can be successfully recruited to augment cytotoxic CD8+ T cell responses, despite their low frequency.
An important component of the α-GalCer-pp65495-503 vaccine is the cathepsin-B-sensitive linker, the removal of which permits an O→N acyl migration to form α-GalCer. As the glycolipid component of the vaccine must reach the late endosomal or lysosomal compartment for loading onto CD1d41, the requirement for this extra processing step may explain why α-GalCer-pp65495-503 was a less effective NKT cell agonist than free α-GalCer in vitro. However, given that repeated systemic α-GalCer injections have been shown to drive long-term NKT cell functional anergy arising from the development of an IL-10-secreting NKT cell subset (NKT10)42,43, this reduced activity may be advantageous in vivo at preventing NKT cell over-activation from stymying the downstream CD8+ T cell response or inducing tissue damage in the liver and other tissues where NKT cells are prevalent.
Crucially, this study uses ‘real-life’ viral antigens, rather than a model antigen, such as ovalbumin. The CMV epitope was selected based on the ability to detect CD8+ T cells specific for this virus-derived peptide at a high frequency in a large proportion of human donors27. Due to the persistent nature of CMV infection, CMV-reactive T cells have an altered phenotype compared with other virally-reactive or naïve tumor-associated antigen (TAA)-reactive T cells44. For example, CMV-specific CD8+ T cells are chronically activated and express the TH1-associated transcription factors T-bet (TBX21) and eomesodermin (EOMES), as well as IFNG mRNA and IFN-γ–regulated genes45. We cannot therefore be certain that the responses generated against the immunodominant CMV antigen will extend to other viral- or tumor-associated peptides, although it is reassuring that we observe CD8+ T cell responses to an immunodominant influenza matrix protein epitope using a similar vaccine.
The human cell in vitro culture system we used showed no difference in the frequency of NLV-specific CD8+ T cells between cultures treated with α-GalCer-pp65495-503, its admixed components or peptide alone, and while we demonstrate upregulation of molecules associated with cytotoxic function in response to the conjugate vaccine, in vitro killing of peptide-loaded targets is not demonstrated here. In vitro immunological studies are limited by the lack of a tertiary lymphoid structure that facilitates juxtaposition of the various immune cells involved46. Additional challenges to working with primary human cells in vitro, include: (1) the restricted number of professional, cross-presenting APCs and NKT cells present in each well; (2) the enforced proximity inherent in cell culture conditions, which all but ensures co-delivery of antigen and adjuvant to the same APC, regardless of conjugation; and (3) the substantial degree of heterogeneity between humans, which makes it difficult to detect differences within a small donor pool. Differences between peptide and conjugated vaccines may also be understated in in vitro studies due to the role of biodistribution in determining the nature and magnitude of the downstream immune response. For example, α-GalCer is known to bind apolipoprotein E (ApoE), enabling it to be efficiently acquired by DCs, via low-density lipoprotein (LDL) receptors47. Notably our data do show that a similar conjugate vaccine can induce cytotoxicity and delay tumour growth in vivo.
Oncogenic viral antigens are likely to be excellent targets for immunotherapeutic vaccine strategies aimed at reinforcing tumor-specific T-cell responses48. A recent clinical study in patients with HPV16+ high-grade vulvar intraepithelial neoplasia showed that vaccination with peptides in montanide, an oil emulsion that improves antigen uptake, could induce partial or complete histological regression of lesions in more than 50% of patients treated49. Importantly, this trial reported that clinical efficacy was related to the strength of vaccine-induced immune responses. It is conceivable that the outcome could be improved further with a vaccine that is designed to improve the function of APCs, as has been described here. The antitumor response reported here in mice against a tumor expressing the HPV16 E7 protein is notable both for the low dose of vaccine need for activity, and the fact that activity was reliant on conjugation of the glycolipid and peptide components. Studies of this vaccine design with other tumor-associated antigens, and in models of viral infection, are ongoing.
In conclusion, we show that in human PBMCs a glycolipid-peptide conjugate vaccine can harness NKT cells as cellular adjuvants to enhance cytotoxic CD8+ T cell responses against virus-derived peptides. We have demonstrated that a similar conjugate vaccine directed against an oncogenic viral protein is capable of generating a significantly better anti-tumor response than its admixed components in a model of HPV-associated cancer in vivo. Human trials will be necessary to determine whether glycolipid-peptide conjugates can safely enhance peptide-specific CD8+ T cell responses with clinical benefit.
Methods
Conjugate vaccine synthesis
Conjugate vaccines were synthesized per previously reported protocols25. Briefly, α-GalCer was treated with hydrochloric acid in dioxane and heated to 65 °C to reveal the phytosphingosine amine which was subsequently capped with the linker group N-(6-azidohexanoyl)-valine-citruline-4-aminobenzyl 4-nitrophenyl carbonate. The azido group was subsequently conjugated to N-terminally modified MHC class I peptides (i.e. 4-pentynoyl-FFRK-NLVPMVATV for α-GalCer-pp65495-503, 4-pentynoyl-FFRK-RAHYNIVTF for α-GalCer-E749-57 and 4-pentynoyl-PLTK-GILGFVFTL for α-GalCer-M158-66)25 utilizing copper catalyzed alkyne-azide cycloaddition methodology50. The vaccines were purified by the use of preparative HPLC (Phenomenex Luna C18(2), 5 µm, 250 × 30 mm, 30 °C, 40 mL/min) with a TFA modified water-MeOH mobile phase. Analytical data: α-GalCer-E749-57, HRMS-ESI m/z calculated for C162H260N32O33 [M + 2H]2+ 1590.9826, found 1590.9823; α-GalCer-M158-66, HRMS-ESI m/z calculated for C150H252N23O32 [M + H]+ 2887.8799, found 2887.8804. The peptides 4-pentynoyl-FFRK-RAHYNIVTF (E749-57), 4-pentynoyl-PLTKGILGFVFTL (M158-66) and 4-pentynoyl-FFRK-NLVPMVATV (pp65495-503) were purchased from Peptides & Elephants GmbH (Potsdam, Germany).
Solubilization and administration of compounds for biological studies
Solubilization of α-GalCer and vaccines was achieved by freeze-drying the samples in the presence of sucrose, L-histidine, and Tween 20 as previously described51. All solubilized compounds were diluted in sterile water before use in vitro.
Isolation of human PBMCs
Venous blood was drawn into heparin-containing tubes, diluted 1:1 in phosphate buffered saline (PBS; Gibco), and PBMCs isolated by density centrifugation (Lymphoprep™; Axis-Shield, Oslo, Norway). PBMCs were washed twice and either used fresh or cryopreserved in 10% dimethyl sulfoxide (Sigma-Aldrich) and 90% fetal bovine serum (FBS; Gibco) and thawed immediately before use. All donors gave written informed consent; this study was approved by the Human Ethics Committee of Victoria University, and all experiments were performed in accordance with relevant guidelines and regulations.
Cell-free CD1d presentation assay
Dependence of NKT cell activation on proteolytic cleavage of α-GalCer-pp65495-503 was tested in a cell-free assay. Flat-bottomed 96-well tissue culture plates were coated with 5 μg/mL mouse CD1d monomers (NIH Tetramer Core Facility) overnight at 4 °C. Equimolar concentrations of the vaccine α-GalCer-pp65495-503 and free α-GalCer were pre-treated with cathepsin-B (Sigma-Aldrich) or mock-treated with PBS for 24 h, at 37 °C, before the equivalent of 50 ng/digest was added to the coated plate for 3 h, at 37 °C. Plates were then washed with Iscove’s Minimum Essential Medium (IMDM; Gibco). DN32.D3 NKT hybridoma cells52 were seeded at a density of 3 × 104 cells/well and the plate was incubated at 37 °C in IMDM supplemented with 5% fetal bovine serum (FBS) (Sigma-Aldrich), 2 mM glutamax, 100 U/mL penicillin, 100 mg/mL streptomycin and 50 mM 2-mercaptoethanol (all Invitrogen). The following day, supernatants were collected and IL-2 concentration determined by ELISA (BioLegend), according to the manufacturer’s instructions.
Generation of monocyte-derived (mo)DC
Immature DC were generated from human PBMCs over 6 days from adherent monocytes using RPMI (Gibco) supplemented with 2% autologous human serum containing 1000 U/mL GM-CSF and 1000 U/mL IL-4. Cytokines were re-added after 3 days in culture. For co-culture experiments to assess DC activation by flow cytometry, 5 × 104 DCs/well were cultured in low adherence 96-well plates (Nunc) with 5 × 103 flow-sorted human NKT cells/well in the presence of 1 µM of α-GalCer-pp65495-503 or α-GalCer.
Flow cytometric analysis
The following antibodies (Ab) were used: anti-CD209 (DCN46; BD), anti-CD83 (HB1SE; Biolegend), anti-CD86 (IT2.2; BioLegend), anti-CD3 (UCHT1; BioLegend), anti-CD19 (HIB19; BioLegend), anti-CD8 (RPA-T8; BioLegend), anti-Vα24 Jα18 TCR (6B11; Biolegend), anti-CD137 (4B4-1; BioLegend), anti-Ki67 (Ki67; BD Pharmingen), PE-HLA-A*0201 NLVPMVATV and APC-HLA-A*0201 GILGFVFTL (both Immudex). Cells were stained for 15 min at room temperature, washed 2 × in flow buffer (PBS + 5% FBS) and fixed in 1:1 4% formalin:PBS before analysis. In some experiments (Fig. 3A–F), MHC class I dextramers PE-HLA-A*0201 NLVPMVATV or PE-HLA-A*0201 GILGFVFTL (Immudex) were added after live/dead staining, for 10 min prior to surface Ab staining. All flow cytometry was performed on a BD Biosciences LSRFortessa or LSRII SORP flow cytometer. Cells were stained with Fixable Live/Dead NIR (Invitrogen). Data analysis was performed using FlowJo software (Tree Star, Inc.). For all analyses, dead cells and doublets were first excluded by gating.
Intracellular cytokine staining
For intracellular cytokine staining of peptide-specific CD8+ T cells (Fig. 4E), anti-CD29/49d (BD Biosciences) and anti-CD107a (H4A3; BioLegend) were added to the cells 1 h before addition of 300 ng/mL monensin and 500 ng/mL brefeldin-A (both Sigma-Aldrich). After 18 h in culture, live/dead and surface Ab staining was performed before cells were fixed in Cytofix/Cytoperm™ solution (BD Biosciences) for 20 min. Intracellular cytokine staining was then performed using anti-IFN-γ (clone 45.B3; BioLegend) for 30 min at room temperature. Cells were washed 2× in PermWash™ buffer (BD Biosciences) and PBS before analysis. For intranuclear staining of NKT cells with anti-Ki67 (Fig. 1D), following live/dead and surface Ab staining, cells were permeabilized and fixed in FoxP3 Perm/Fix buffer (BioLegend) at room temp for 20 min. Cells were washed and re-suspended in FoxP3 Perm buffer (BioLegend) for anti-Ki67 staining for 30 min at room temperature, then washed 2× in flow buffer before analysis.
NKT cell expansion ex vivo
For co-culture experiments, NKT cells were sorted from PBMCs isolated from a healthy human donor with a resting NKT cell frequency of 1% of CD3+ T cells. Briefly, 10 × 106 PBMCs/mL were incubated with anti-CD3, anti-CD19 and anti-Vα24 Jα18 TCR (6B11) for 30 min on ice. Cells were washed 2× in sterile flow buffer and re-suspended in 2 mL sterile flow buffer containing 2.5 µg/mL DAPI. The DAPIlow CD3+ CD19− 6B11+ cells were then collected using a BD Biosciences Influx Cell Sorter. Cells were seeded into a 96-well plate at 5 × 103 cells/well in IMDM supplemented with 5% human AB serum (Sigma-Aldrich), 2 mM glutamax, 100 U/ml penicillin and 100 mg/mL streptomycin and 50 mM 2-mercaptoethanol (complete IMDM; cIMDM), and containing 1 μg/mL PHA (Sigma-Aldrich) plus 100 U/mL IL-2 (Peprotech) and 105 irradiated (50 Gy) PBMC feeder cells. Fresh cIMDM containing 100 U/mL IL-2 was added every 3-4 days. Feeder cells were replaced every 10 days. Before use, dead cells were removed using the MACS Dead Cell Removal Kit (Miltenyi Biotec).
Analysis of in vitro human NKT cell activation
PBMCs from a HLA-A*02-negative healthy human donor were cultured in cIMDM at 3 × 105 cells/well with 1 μM α-GalCer or molar equivalent of α-GalCer-pp65495-503 in the presence of 50 μg/mL LEAF-pure anti-CD1d (clone 51.1; BioLegend) or matched isotype control. For 7 day cultures (Fig. 1C), 50 U/mL of IL-2 was added on day 2. IFN-γ secretion was assessed by Elispot as described below.
ELISpot assay
IFN-γ production was quantified after 24 h using a human IFN-γ ELISpot kit (Mabtech), according to manufacturer’s instructions. Briefly, Millipore MultiScreen-HA 96-well filter plates (Millipore) were coated with 5 μg/mL IFN-γ mAb in PBS overnight at 4 °C. Cells were seeded at 2 × 105 cells/well in cIMDM and incubated with the relevant compounds overnight. After washing with PBS, plates were incubated with biotin-labeled anti-IFN-γ for 1 h at room temperature. Plates were then washed and treated with 1:1000 dilution of streptavidin-alkaline phosphatase (Sigma) for 30 min at room temperature. Plates were developed with nitro-blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate substrate (Mabtech) until all spots were clearly visible. Developed plates were dried and counted on an automated ELISpot reader (Autoimmun Diagnostika, Strassberg, Germany). Cells treated with PHA were used as a positive control.
Stimulation of human CMV-specific CD8+ T cells in vitro
PBMCs from HLA-A*02 positive CMV-seropositive donors were cultured cIMDM at 3 × 105 cells/well for 5–7 days with pp65495-503 peptide, admixed peptide and α-GalCer, or the α-GalCer-pp65495-503 vaccine all at equimolar concentrations of 1 µM.
Depletion of NKT cells
PBMCs from HLA-A*02 positive CMV-seropositive donors were incubated at 4 °C for 10 min with anti-Vα24 Jα18 TCR biotinylated antibody (clone 6B11; eBioScience). Cells were then washed twice in Würzburger buffer and incubated at 4 °C, gently shaking, with twice the recommended concentration of biotin binding beads (Dynabead; Invitrogen) for 20 min. Unbound cells were isolated using a DynaMag-15 magnet (ThermoFisher). This process was repeated three times to ensure >99% of NKT cells were depleted from the PBMCs.
RNA isolation and Nanostring analysis
After seven days in culture as detailed above (“Stimulation of human CMV-specific CD8+ T cells in vitro”), RNA was extracted from PBMCs derived from four donors using the RNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions. RNA purity and integrity were assessed using a Nanodrop spectrophotometer (ThermoFisher) and gel electrophoresis. Nanostring RNA analysis of 700 immune-related genes was performed using the nCounter GX Human PanCancer Immune profiling Kit (XT) on the nCounter® Analysis System. Data were analyzed using the nSolver Analysis software package and Microsoft Excel. Default normalization settings were used for all data. The online tool CIMminer (https://discover.nci.nih.gov/cimminer/home.do) was used to generate heat maps.
In vivo cytotoxicity assay
Conjugate vaccines were diluted in sterile PBS for injection. Animals were injected intravenously with 8.5 μg of α-GalCer-E749-57 conjugate vaccine, equimolar concentrations of α-GalCer and E749-57, an equimolar concentration of E749-57 peptide alone, or PBS control. After 10 days, syngeneic splenocytes were loaded with 10 μM, 1 μM or 0.1 μM E749-57 peptide and labeled with 2.5 μM, 0.5 μM, or 0.1 μM carboxyfluorescein succinimidyl ester (Life Technologies), respectively. A control target population without antigen was labeled with 20 μM chloro-methyl-benzoyl-aminotetramethyl-rhodamine (Life Technologies). A mixture of the four target cell populations was injected intravenously into immunized mice, and specific lysis of peptide-loaded targets was assessed 18 h later by flow cytometric analysis of peripheral blood. Survival of peptide-pulsed targets was calculated relative to that of the control target, and cytotoxic activity was expressed as percent specific lysis.
Analysis of anti-tumor activity in vivo
The mouse lung cancer cell line TC-1, a mouse lung epithelial cell line transformed with HPV16-E6 and -E7 genes53, was cultured in RPMI1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin (all from Gibco), and 500 mg/ml G418 (Sigma), and resuspended in PBS for subcutaneous injection. Groups of naive C57BL/6 mice (n = 5) were implanted with 2.5 × 105 cells in the flank. On day 8, when tumors reached at least 5mm in diameter, mice were given one intravenous treatment with either 1.5 µg (0.47 nmol) of α-GalCer-E749-57 conjugate vaccine, an admix of 100 ng (0.09 nmol) HPV16 E749-57 peptide and 5 nmol of α-GalCer, HPV16 E749-57 peptide alone or α-GalCer alone; the concentrations of α-GalCer and peptide used were selected as the optimal concentrations based on in vivo titrations. Some mice received two doses of the α-GalCer-E749-57 with a week interval. A control group was injected with PBS. Tumor growth was monitored three times per week, with tumor size calculated as the product of the two bisecting diameters. Measurements were stopped for each group when the first mouse developed a tumor exceeding 400 mm2. The C57BL/6 mice used were purchased from Animal Production Colonies, Frederick Cancer Research Facility, National Cancer Institute. All animal experiments were approved by the Animal Care and Use Committee of the National Cancer Institute, and all experiments were performed in accordance with relevant guidelines and regulations.
Statistical analysis and data availability
All statistical analysis was performed using GraphPad Prism software (GraphPad Prism Inc). For Fig. 1B,D and 2F, two-way analysis of variance (ANOVA) was performed before the Bonferroni multiple comparisons test. For Fig. 1E, the Mann-Whitney U test was used. For Fig. 2B, D, the Friedman test was used, with Dunn’s multiple comparisons. In all experiments, P values ≤ 0.05 were taken to be significant. The data generated during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors wish to acknowledge the Thompson Family Foundation, Genesis Oncology Trust, Royal Arch Masonic Centennial Award, Health Research Council of New Zealand (grant 14/502), the New Zealand Ministry of Business Innovation and Employment (grant RTV1603), the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and the Gui Foundation for support of this research. We are grateful to the NIH Tetramer Core Facility for provision of the CD1d monomers, to Catherine Wood for phlebotomy and to the volunteer blood donors. Correspondence regarding the chemical synthesis should be addressed to G.F.P. (email: Gavin.Painter@vuw.ac.nz); all other correspondence should be addressed to R.W. (email: rweinkove@malaghan.org.nz).
Author Contributions
R.W., I.F.H. and G.F.P. conceived the research. G.F.P. lead the chemical team that designed and manufactured the peptide-glycolipid conjugate vaccines. B.J.C. and R.J.A. manufactured, purified and analyzed the peptide-glycolipid conjugate vaccines. M.S., A.A., C.B. and K.F. conducted the in vitro experiments. M.S. and A.H. conducted the RNA analysis. M.T. and J.A.B. designed and analysed the in vivo tumor experiments. T.L.O. and C.T. conducted the experiments for and prepared Fig. 4B and Supplementary figure 1. R.W. arranged human ethical approval and consented human donors. M.S. and R.W. drafted the manuscript. All authors reviewed and approved the manuscript.
Competing Interests
Competing financial interests: B.J.C., R.J.A., G.F.P. and I.F.H. are inventors on patents related to the glycolipid-peptide conjugate vaccines used in this study. These patents have been licensed by a company, Avalia Immunotherapies, for commercialization. I.F.H. and G.F.P. are the founding C.S.O. and C.T.O. of Avalia Immunotherapies. T.L.O. is employed by Avalia Immunotherapies. R.W. has received compensation as a scientific advisory board member for Avalia Immunotherapies. M.S., A.A., C.R.B., K.J.F., A.H., J.A.B. and M.T. declare no potential conflicts of interest.
Footnotes
M. Speir and A. Authier-Hall contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14690-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
G. F. Painter, Email: rweinkove@malaghan.org.nz
R. Weinkove, Email: Gavin.Painter@vuw.ac.nz
References
- 1.Wang RF, Wang HY. Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res. 2017;27:11–37. doi: 10.1038/cr.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altenburg AF, Rimmelzwaan GF, de Vries RD. Virus-specific T cells as correlate of (cross-)protective immunity against influenza. Vaccine. 2015;33:500–506. doi: 10.1016/j.vaccine.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 3.Akondy RS, et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J. Immunol. 2009;183:7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Querec T, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sei JJ, et al. Effector and Central Memory Poly-Functional CD4(+) and CD8(+) T Cells are Boosted upon ZOSTAVAX((R)) Vaccination. Front Immunol. 2015;6:553. doi: 10.3389/fimmu.2015.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apostolico Jde S, Lunardelli VA, Coirada FC, Boscardin SB, Rosa DS. Adjuvants: Classification, Modus Operandi, and Licensing. J Immunol Res. 2016;2016:1459394. doi: 10.1155/2016/1459394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temizoz B, Kuroda E, Ishii KJ. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016;28:329–338. doi: 10.1093/intimm/dxw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banday AH, Jeelani S, Hruby VJ. Cancer vaccine adjuvants–recent clinical progress and future perspectives. Immunopharmacol. Immunotoxicol. 2015;37:1–11. doi: 10.3109/08923973.2014.971963. [DOI] [PubMed] [Google Scholar]
- 9.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu, K., Thomas, E. K., Giedlin, M. & Mule, J. J. Enhancement of tumor lysate- and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res.61, 2618–2624 (2001). [PubMed]
- 11.Millard AL, Ittelet D, Schooneman F, Bernard J. Dendritic cell KLH loading requirements for efficient CD4+ T-cell priming and help to peptide-specific cytotoxic T-cell response, in view of potential use in cancer vaccines. Vaccine. 2003;21:869–876. doi: 10.1016/S0264-410X(02)00534-0. [DOI] [PubMed] [Google Scholar]
- 12.Ressing ME, et al. Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma. J. Immunother. 2000;23:255–266. doi: 10.1097/00002371-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Hwang ML, Lukens JR, Bullock TN. Cognate memory CD4+ T cells generated with dendritic cell priming influence the expansion, trafficking, and differentiation of secondary CD8+ T cells and enhance tumor control. J. Immunol. 2007;179:5829–5838. doi: 10.4049/jimmunol.179.9.5829. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Zhang M, Cong H. Advances in the study of HLA-restricted epitope vaccines. Hum Vaccin Immunother. 2013;9:2566–2577. doi: 10.4161/hv.26088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano, T. et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science278, 1626–1629 (1997). [DOI] [PubMed]
- 17.Gottschalk C, Mettke E, Kurts C. The Role of Invariant Natural Killer T Cells in Dendritic Cell Licensing, Cross-Priming, and Memory CD8(+) T Cell Generation. Front Immunol. 2015;6:379. doi: 10.3389/fimmu.2015.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermans IF, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 19.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, et al. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput. Biol. 2008;4:e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speir M, Hermans IF, Weinkove R. Engaging Natural Killer T Cells as ‘Universal Helpers’ for Vaccination. Drugs. 2017;77:1–15. doi: 10.1007/s40265-016-0675-z. [DOI] [PubMed] [Google Scholar]
- 22.Semmling V, et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat. Immunol. 2010;11:313–320. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- 23.Guillonneau C, et al. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc. Natl. Acad. Sci. USA. 2009;106:3330–3335. doi: 10.1073/pnas.0813309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reilly EC, et al. Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection. PLoS ONE. 2012;7:e37991. doi: 10.1371/journal.pone.0037991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson RJ, et al. NKT cell-dependent glycolipid-peptide vaccines with potent anti-tumour activity. Chem Sci. 2015;6:5120–5127. doi: 10.1039/C4SC03599B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson RJ, et al. A self-adjuvanting vaccine induces cytotoxic T lymphocytes that suppress allergy. Nat. Chem. Biol. 2014;10:943–949. doi: 10.1038/nchembio.1640. [DOI] [PubMed] [Google Scholar]
- 27.Engstrand M, et al. Characterization of CMVpp65-specific CD8+ T lymphocytes using MHC tetramers in kidney transplant patients and healthy participants. Transplantation. 2000;69:2243–2250. doi: 10.1097/00007890-200006150-00005. [DOI] [PubMed] [Google Scholar]
- 28.Flechtner JB, et al. High-affinity interactions between peptides and heat shock protein 70 augment CD8+ T lymphocyte immune responses. J. Immunol. 2006;177:1017–1027. doi: 10.4049/jimmunol.177.2.1017. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura H, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomura M, et al. A novel function of Valpha14+ CD4+ NKT cells: stimulation of IL-12 production by antigen-presenting cells in the innate immune system. J. Immunol. 1999;163:93–101. [PubMed] [Google Scholar]
- 31.Vincent MS, et al. CD1-dependent dendritic cell instruction. Nat. Immunol. 2002;3:1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- 32.Sica G, Chen L. Modulation of the immune response through 4-1BB. Adv. Exp. Med. Biol. 2000;465:355–362. doi: 10.1007/0-306-46817-4_30. [DOI] [PubMed] [Google Scholar]
- 33.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin. Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 34.Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J. Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]
- 35.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J. Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- 36.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 37.Berzins SP, et al. Systemic NKT cell deficiency in NOD mice is not detected in peripheral blood: implications for human studies. Immunol. Cell Biol. 2004;82:247–252. doi: 10.1046/j.1440-1711.2004.01238.x. [DOI] [PubMed] [Google Scholar]
- 38.Geiger R, Duhen T, Lanzavecchia A, Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J. Exp. Med. 2009;206:1525–1534. doi: 10.1084/jem.20090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwok WW, et al. Frequency of epitope-specific naive CD4(+) T cells correlates with immunodominance in the human memory repertoire. J. Immunol. 2012;188:2537–2544. doi: 10.4049/jimmunol.1102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carreno LJ, Saavedra-Avila NA, Porcelli SA. Synthetic glycolipid activators of natural killer T cells as immunotherapeutic agents. Clin Transl Immunology. 2016;5:e69. doi: 10.1038/cti.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elewaut D, et al. The adaptor protein AP-3 is required for CD1d-mediated antigen presentation of glycosphingolipids and development of Valpha14i NKT cells. J. Exp. Med. 2003;198:1133–1146. doi: 10.1084/jem.20030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parekh VV, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J. Clin. Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J. Clin. Invest. 2014;124:3725–3740. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Aalderen MC, et al. Infection history determines the differentiation state of human CD8+ T cells. J. Virol. 2015;89:5110–5123. doi: 10.1128/JVI.03478-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hertoghs KM, et al. Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. J. Clin. Invest. 2010;120:4077–4090. doi: 10.1172/JCI42758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cupedo T, Stroock A, Coles M. Application of tissue engineering to the immune system: development of artificial lymph nodes. Front Immunol. 2012;3:343. doi: 10.3389/fimmu.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Elzen P, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 48.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer. 2016;16:219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 49.van Poelgeest MI, et al. Vaccination against Oncoproteins of HPV16 for Noninvasive Vulvar/Vaginal Lesions: Lesion Clearance Is Related to the Strength of the T-Cell Response. Clin. Cancer Res. 2016;22:2342–2350. doi: 10.1158/1078-0432.CCR-15-2594. [DOI] [PubMed] [Google Scholar]
- 50.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 51.Giaccone G, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 52.Bendelac A, et al. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 53.Ji H, et al. Antigen-specific immunotherapy for murine lung metastatic tumors expressing human papillomavirus type 16 E7 oncoprotein. Int. J. Cancer. 1998;78:41–45. doi: 10.1002/(SICI)1097-0215(19980925)78:1<41::AID-IJC8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.