Abstract
We aimed to evaluate whether obesity was associated with a certain clinicopathologic characteristics of metachronous CRA. This retrospective longitudinal cohort study included 2,904 subjects who had at least one resected CRA at index colonoscopy and who subsequently underwent one or more surveillance colonoscopies within 5 years. Of the 2,904 subjects, 60.9% (n = 1,769) were normal, 35.8% (n = 1,040) were overweight, and 3.3% (n = 95) were obese. Patients with any metachronous CRA were 53.7% (n = 1,559). In multivariate analyses, higher BMI at index colonoscopy was significantly associated with any metachronous CRA (overweight, OR = 1.07; obese, OR = 1.82; p for trend = 0.049). Regarding the multiplicity, the ORs of ≥ 3, ≥ 4 and ≥ 5 metachronous CRAs significantly increased as index BMI increased (p for trend < 0.001, = 0.007 and = 0.004, respectively). In negative binomial regression regarding the incidence for total number of metachronous CRA, the higher BMI the subject has at the time of index colonoscopy, the more metachronous CRAs the subject will have at the surveillance colonoscopy (p for trend = 0.016). Higher index BMI was significantly associated with the risk of multiple metachronous CRAs on surveillance colonoscopy within 5 years.
Introduction
Epidemiologic studies have consistently reported an association between colorectal cancer (CRC) and obesity1,2. The incidence of CRC was significantly greater in obese patients of either sex3. Obesity is associated with a 30–70% increased risk of CRC and an increased risk of death from CRC1,4,5. Some studies have reported that weight loss after bariatric surgery or physical activity helped reduce the risk of CRC-related mortality6,7. In addition, several studies have suggested a close link between obesity and colorectal adenoma (CRA)8–13. Obesity increased the risk of CRA in both sexes and among ethnically diverse populations14. Furthermore, it has been reported that obesity increases the risk of metachronous CRA2,15,16. Physical activity could decrease the risk of CRA through negative feedback related to low-grade inflammation17. Although the role of obesity in CRA or CRC pathophysiology has not been fully elucidated, obesity contributes to progression from CRA to CRC. This progression could occur through adipokine secretion, chronic low-grade inflammation, insulin, and insulin-like growth factors related to obesity5.
However, there have not been any studies to evaluate which characteristics of metachronous colorectal lesions were influenced by index obesity in the longitudinal study. Therefore, we aimed to determine whether index obesity is associated with metachronous CRA in terms of prevalence, multiplicity, and advanced adenoma (AA) on surveillance colonoscopy within 5 years in the longitudinal cohort study.
Results
Baseline characteristics of subjects and index colonoscopy findings according to index body mass index
The clinical and index colonoscopy findings by body mass index (BMI) are summarized in Table 1. The mean age of the study population was 57.5 ± 9.0 years, and 60.9% (n = 1,769) were normal weight, 35.8% (n = 1,040) were overweight, and 3.3% (n = 95) were obese. Age, current smoking, and family history of CRC did not differ among BMI groups. However, sex, use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs), and index colonoscopy findings significantly differed. BMI was not associated with the prevalence of low risk adenoma (LRA) or high risk adenoma (HRA) at the index colonoscopy (p = 0.164). However, in the HRA group, subjects with higher BMI were likely to have ≥3 non-AA (NAA) (p = 0.011) but not AA (p = 0.463). Subjects with higher BMI had more any CRAs (p = 0.001). When any CRA was classified into both NAA and AA categories, subjects with higher BMI were likely to have more NAAs (p = 0.003) but not more AAs (p = 0.107). The locations of any CRAs, NAAs, and AAs were not associated with BMI.
Table 1.
Baseline characteristics of subjects and index colonoscopy findings according to index body mass index.
| Overall (n = 2,904) | Index BMI (kg/m2) | p for trend | |||
|---|---|---|---|---|---|
| <25 (n = 1,769, 60.9%) | 25–29 (n = 1,040, 35.8%) | ≥30 (n = 95, 3.3%) | |||
| Age (years), mean ± SD | 57.5 ± 9.0 | 57.8 ± 9.1 | 57.2 ± 9.1 | 56.1 ± 8.6 | 0.059† |
| Sex, n (%) | <0.001* | ||||
| Men | 2,071 (71.3) | 1,208 (68.3) | 802 (77.1) | 61 (64.2) | |
| Women | 833 (28.7) | 561 (31.7) | 238 (22.9) | 34 (35.8) | |
| Current smoking, n (%) | 541 (18.6) | 322 (18.2) | 198 (19.0) | 21 (22.1) | 0.609* |
| Family history of CRC, n (%) | 97 (3.4) | 59 (3.4) | 34 (3.4) | 4 (4.3) | 0.901* |
| Use of aspirin or NSAIDs, n (%) | 377 (13.0) | 201 (11.4) | 152 (14.6) | 24 (25.3) | <0.001* |
| Index colonoscopic findings | |||||
| Prevalence, n (%) | |||||
| LRA | 1,693 (58.3) | 1,055 (59.6) | 587 (56.4) | 51 (53.7) | 0.164* |
| HRA | 1,211 (41.7) | 714 (40.4) | 453 (43.6) | 44 (46.3) | |
| ≥3 NAA | 655 (22.6) | 368 (20.8) | 259 (24.9) | 28 (29.5) | 0.011* |
| AA | 773 (26.6) | 462 (26.1) | 289 (27.8) | 22 (23.2) | 0.463* |
| Number, mean ± SD | |||||
| Any CRA | 2.3 ± 2.1 | 2.2 ± 2.0 | 2.5 ± 2.3 | 2.4 ± 1.8 | 0.001** |
| NAA | 1.9 ± 1.9 | 1.9 ± 1.8 | 2.1 ± 2.0 | 2.1 ± 1.6 | 0.003** |
| AA | 0.3 ± 0.7 | 0.3 ± 0.6 | 0.4 ± 0.7 | 0.3 ± 0.6 | 0.107** |
| Location, n (%) | |||||
| Any CRAs | 0.158* | ||||
| Right colon | 909 (31.4) | 550 (31.2) | 333 (32.2) | 26 (27.4) | |
| Left colon | 1,193 (41.2) | 751 (42.6) | 407 (39.3) | 35 (36.8) | |
| Both | 792 (27.4) | 463 (26.2) | 295 (28.5) | 34 (35.8) | |
| NAA | 0.290* | ||||
| Right colon | 922 (32.7) | 556 (32.5) | 338 (33.4) | 28 (30.1) | |
| Left colon | 1,157 (41.1) | 724 (42.3) | 399 (39.4) | 34 (36.6) | |
| Both | 739 (26.2) | 432 (252) | 276 (27.2) | 31 (33.3) | |
| AA | 0.386* | ||||
| Right colon | 239 (32.0) | 136 (30.3) | 93 (33.9) | 10 (41.7) | |
| Left colon | 441 (59.0) | 277 (61.7) | 152 (55.5) | 12 (50.0) | |
| Both | 67 (9.0) | 36 (8.0) | 29 (10.6) | 2 (8.3) | |
BMI, body mass index; SD, standard deviation; CRC, colorectal cancer; NSAIDs, nonsteroidal anti-inflammatory drugs; LRA, low risk adenoma; HRA, high risk adenoma; NAA, non-advanced adenoma; AA, advanced adenoma; CRA, colorectal adenoma. *Values by Cochran-Armitage trend for categorical variables. **Values by linear regression. †Values by One-way ANOVA analyses of variances for continuous variables among groups.
Surveillance colonoscopy findings according to index BMI
The average follow-up period was 3.0 years, and patients underwent an average of 1.3 surveillance colonoscopies. The follow-up period (p = 0.102) and frequency of surveillance colonoscopy (p = 0.238) did not differ significantly among BMI groups. The characteristics of metachronous CRAs detected in surveillance colonoscopies were compared among BMI groups (Table 2). As index BMI increased, the proportion of subjects with any CRA increased significantly (p = 0.014). An increase across index BMI groups was positively associated with higher incidence of ≥3 NAA (p < 0.001), ≥3 any CRAs (p < 0.001), ≥4 any CRAs (p < 0.001) and ≥5 any CRAs (p < 0.001) in surveillance colonoscopy, but not with the incidence of AA (p = 0.936). In terms of CRA number, the total numbers of any CRAs and NAAs significantly increased as index BMI increased (p = 0.001 for any CRAs; p = 0.001 for NAA). However, the number of AAs (p = 0.977) and the incidence of CRC (p = 0.231) did not change significantly as index BMI increased. The locations of any CRAs (p = 0.487), NAAs (p = 0.750), and AAs (p = 0.637) did not differ among BMI categories. To summarize the univariate analyses, higher BMI was likely to have a significant association with multiple metachronous CRAs on surveillance colonoscopy.
Table 2.
Surveillance colonoscopy findings according to index body mass index.
| Total (n = 2,904) | Index BMI (kg/m2) | p for trend | |||
|---|---|---|---|---|---|
| <25 (n = 1,769, 60.9%) | 25–29 (n = 1,040, 35.8%) | ≥30 (n = 95, 3.3%) | |||
| Incidence, n (%) | |||||
| Any CRAs | 1,559 (53.7) | 920 (52.0) | 577 (55.5) | 62 (65.3) | 0.014* |
| LRA | 987 (36.4) | 606 (36.7) | 347 (35.7) | 34 (38.6) | 0.789* |
| HRA | 381 (14.0) | 197 (11.9) | 163 (16.8) | 21 (23.9) | <0.001* |
| Any AA | 191 (6.6) | 117 (6.6) | 67 (6.4) | 7 (7.4) | 0.936* |
| ≥3 NAA | 468 (16.1) | 242 (13.7) | 201 (19.3) | 25 (26.3) | <0.001* |
| ≥3 any CRAs | 514 (17.7) | 273 (15.4) | 215 (20.7) | 26 (27.4) | <0.001* |
| ≥4 any CRAs | 316 (10.9) | 165 (9.3) | 135 (13.0) | 16 (16.8) | <0.001* |
| ≥5 any CRAs | 207 (7.1) | 103 (5.8) | 92 (8.8) | 12 (12.6) | <0.001* |
| Number, mean ± SD | |||||
| Any CRAs | 1.4 ± 2.1 | 1.3 ± 2.1 | 1.5 ± 2.2 | 1.8 ± 2.1 | 0.001** |
| NAA | 1.3 ± 2.0 | 1.2 ± 2.0 | 1.4 ± 2.1 | 1.7 ± 2.0 | 0.001** |
| AA | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.977** |
| Location, n (%) | |||||
| Any CRAs | 0.487* | ||||
| Right colon | 715 (41.0) | 442 (61.8) | 250 (35.0) | 23 (3.2) | |
| Left colon | 463 (26.6) | 271 (58.5) | 172 (37.1) | 20 (4.3) | |
| Both | 565 (32.4) | 325 (57.5) | 215 (38.1) | 25 (4.4) | |
| NAA | 0.750* | ||||
| Right colon | 688 (41.6) | 421 (61.2) | 242 (35.2) | 25 (3.6) | |
| Left colon | 503 (30.4) | 293 (58.3) | 188 (37.4) | 22 (4.4) | |
| Both | 461 (27.9) | 266 (57.7) | 175 (38.0) | 20 (4.3) | |
| AA | 0.637* | ||||
| Right colon | 325 (75.2) | 211 (64.9) | 101 (31.1) | 13 (4.0) | |
| Left colon | 95 (22.0) | 59 (62.1) | 33 (34.7) | 3 (3.2) | |
| Both | 12 (2.8) | 6 (50.0) | 6 (50.0) | 0 | |
| CRC incidence, n (%) | 37 (1.3) | 27 (1.5) | 10 (1.0) | 0 | 0.231* |
| Follow-up period (years), mean ± SD | 3.0 ± 1.1 | 2.9 ± 1.1 | 3.0 ± 1.1 | 2.9 ± 1.0 | 0.102† |
| Frequency of surveillance colonoscopy, mean ± SD | 1.3 ± 0.5 | 1.2 ± 0.5 | 1.3 ± 0.5 | 1.3 ± 0.6 | 0.238† |
BMI, body mass index; CRA, colorectal adenoma; LRA, low risk adenoma; HRA, high risk adenoma; AA, advanced adenoma; NAA, non-advanced adenoma; SD, standard deviation; CRC, colorectal cancer. *Values by Cochran-Armitage trend for categorical variables. **Values by linear regression. †Values by One-way ANOVA analyses of variances for continuous variables among groups.
Association of index BMI with the characteristics of metachronous CRA
Next, to evaluate the association between index BMI and the risk of metachronous CRA, univariate analyses (Supplementary Tables 1 and 2) and subsequent logistic multivariate analyses (Table 3 and Supplementary Table 2) were performed. Obese subjects with ≥30 kg/m2 had a significant association with the presence of any metachronous CRA (adjusted OR, 1.82; 95% CI, 1.15−2.89) compared to those with <25 kg/m2. There was a statistically significant trend in increased adjusted OR of any CRA as increasing BMI (p for trend = 0.049) (Table 3). When stratified by sex, both obese men and women had higher risk of metachronous CRA (adjusted OR, 1.77; 95% CI, 1.01−3.09 for men; adjusted OR, 2.30; 95% CI, 1.08−4.90 for women) compared to those with normal BMI, but did not reach statistical significance (p for trend = 0.061 for men and 0.075 for women) (Table 4).
Table 3.
Association between clinicopathologic characteristics and metachronous colorectal adenomas.
| Logistic multivariate analyses | Negative binomial regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any CRAs (n = 1,559) (vs. Absent CRA, n = 1, 345) | ≥ 3 any CRAs (n = 514) (vs. < 3, n = 2,390) | ≥ 4 any CRAs (n = 316) (vs. < 4, n = 2,588) | ≥ 5 any CRAs (n = 207) (vs. < 5, n = 2,697) | Metachronous CRAs (n = 2,904) | ||||||
| aOR (95% CI) | p value | aOR (95% CI) | p value | aOR (95% CI) | p value | aOR (95% CI) | p value | Exp (B) (95% CI) | p value | |
| Age | 1.02 (1.01–1.03) | <0.001 | 1.03 (1.02–1.05) | <0.001 | 1.30 (1.02–1.05) | <0.001 | 1.04 (1.02–1.06) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| Men | 1.87 (1.56–2.24) | <0.001 | 2.62 (1.96–3.51) | <0.001 | 2.89 (1.97–4.24) | <0.001 | 3.20 (1.92–5.32) | <0.001 | 1.68 (1.48–1.90) | <0.001 |
| Index BMI (kg/m2) | ||||||||||
| <25 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||||
| 25–29 | 1.07 (0.91–1.26) | 0.432 | 1.34 (1.08–1.67) | 0.009 | 1.35 (1.03–1.77) | 0.031 | 1.46 (1.05–2.04) | 0.024 | 1.08 (0.97–1.20) | 0.158 |
| ≥30 | 1.82 (1.15–2.89) | 0.011 | 2.23 (1.31–3.80) | 0.003 | 1.93 (1.01–3.69) | 0.048 | 2.39 (1.13–5.06) | 0.023 | 1.40 (1.07–1.84) | 0.016 |
| p for trend | 0.049 | <0.001 | 0.007 | 0.004 | 0.016 | |||||
| Current smoking | 1.23 (0.99–1.52) | 0.058 | 1.25 (0.96–1.62) | 0.079 | 1.08 (0.78–1.49) | 0.638 | 1.30 (0.89–1.91) | 0.172 | 1.14 (1.00–1.30) | 0.048 |
| Family history of CRC | 1.27 (0.82–2.00) | 0.284 | 1.13 (0.62–2.08) | 0.681 | 1.16 (0.55–2.45) | 0.705 | 1.09 (0.42–2.81) | 0.860 | 1.04 (0.79–1.39) | 0.762 |
| Use of aspirin or NSAIDs | 1.08 (0.85–1.37) | 0.517 | 1.01 (0.75–1.37) | 0.956 | 1.38 (0.97–1.96) | 0.071 | 1.36 (0.89–2.08) | 0.158 | 1.05 (0.09–1.22) | 0.544 |
| Index colonoscopy | ||||||||||
| LRA | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 | 1.00 (reference) | |
| HRA | 1.80 (1.53–2.12) | 2.29 (1.84–2.84) | 2.71 (2.06–3.56) | 2.99 (2.12–4.23) | 1.76 (1.58–1.95) | <0.001 | ||||
| Follow-up period | 1.13 (1.05–1.22) | 0.002 | 1.16 (1.05–1.29) | 0.005 | 1.16 (1.02–1.33) | 0.026 | 1.25 (1.06–1.48) | 0.008 | 1.12 (1.06–1.18) | <0.001 |
| Frequency of surveillance | 2.58 (2.11–3.16) | <0.001 | 3.10 (2.55–3.77) | <0.001 | 3.54 (2.84–4.40) | <0.001 | 3.64 (2.85–4.64) | <0.001 | 1.87 (1.71–2.06) | <0.001 |
CRA,colorectal adenoma; aOR, adjusted odds ratio; 95% CI, 95% confidence interval; BMI, body mass index; CRC, colorectal cancer; NSAIDs, nonsteroidal anti-inflammatory drugs; LRA, low risk adenoma; HRA, high risk adenoma. Hosmer-Lemeshow Goodness-of-Fit test of each model showed p = 0.552 for any CRAs, p = 0.887 for ≥3 any CRAs, p = 0.470 for ≥4 any CRAs, and p = 0.427 for ≥5 any CRAs.
Table 4.
Association between index body mass index and metachronous colorectal adenomas stratified by sex.
| Index BMI (kg/m2) | Logistic multivariate analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| Any CRAs (n = 1,559) (vs. Absence, n = 1,345) | ≥3 any CRAs (n = 514) (vs. <3, n = 2,390) | ≥4 any CRAs (n = 316) (vs. <4, n = 2,588) | ≥5 any CRAs (n = 207) (vs. <5, n = 2,697) | |||||
| n (% of total) | aOR (95% CI) | n (% of total) | aOR (95% CI) | n (% of total) | aOR (95% CI) | n (% of total) | aOR (95% CI) | |
| Overall | ||||||||
| <25 | 920 (52.0) | 1.00 (reference) | 273 (15.4) | 1.00 (reference) | 165 (9.3) | 1.00 (reference) | 103 (5.8) | 1.00 (reference) |
| 25–29 | 577 (55.5) | 1.07 (0.91–1.26) | 215 (20.7) | 1.34 (1.08–1.67) | 135 (13.0) | 1.35 (1.03–1.77) | 92 (8.8) | 1.46 (1.05–2.04) |
| ≥30 | 62 (65.3) | 1.82 (1.15–2.89) | 26 (27.4) | 2.23 (1.31–3.80) | 16 (16.8) | 1.93 (1.01–3.69) | 12 (12.6) | 2.39 (1.13–5.06) |
| p for trend | 0.049 | <0.001 | 0.007 | 0.004 | ||||
| Men | ||||||||
| <25 | 701 (58.0) | 1.00 (reference) | 229 (19.0) | 1.00 (reference) | 143 (11.8) | 1.00 (reference) | 93 (7.7) | 1.00 (reference) |
| 25–29 | 473 (59.0) | 1.01 (0.90–1.30) | 194 (24.2) | 1.43 (1.12–1.81) | 124 (15.5) | 1.41 (1.05–1.88) | 85 (10.6) | 1.47 (1.04–2.09) |
| ≥30 | 42 (68.9) | 1.77 (1.01–3.09) | 21 (34.4) | 2.38 (1.28–4.40) | 12 (19.7) | 2.08 (0.76–3.49) | 9 (14.8) | 1.89 (0.79–4.51) |
| p for trend | 0.061 | <0.001 | 0.015 | 0.016 | ||||
| Women | ||||||||
| <25 | 219 (39.0) | 1.00 (reference) | 44 (7.8) | 1.00 (reference) | 22 (3.9) | 1.00 (reference) | 10 (1.8) | —* |
| 25–29 | 104 (43.7) | 1.12 (0.81–1.54) | 21 (8.8) | 1.00 (0.56–1.78) | 11 (4.6) | 0.97 (0.44–2.11) | 7 (2.9) | —* |
| ≥30 | 20 (58.8) | 2.30 (1.08–4.90) | 5 (14.7) | 1.86 (0.62–5.64) | 4 (11.8) | 2.72 (0.79–9.45) | 3 (8.8) | —* |
| p for trend | 0.075 | 0.504 | 0.334 | —* | ||||
CRA, colorectal adenoma; AA, advanced adenoma; BMI, body mass index; n, number; aOR, adjusted odd ratio; 95% CI, 95% confidence interval. Adjusted for age, index BMI, current smoking, family history of colorectal cancer, use of aspirin or nonsteroidal anti-inflammatory drugs, LRA or HRA at index, follow-up period, and frequency of surveillance colonoscopy in men and women analyses. Additionally adjusted by sex in overall analyses. Hosmer-Lemeshow Goodness-of-Fit test of each model showed p = 0.702 for any CRAs in men, p = 0.100 for any CRAs in women, p = 0.656 for ≥3 any CRAs in men, p = 0.143 for ≥3 any CRAs in women, p = 0.844 for ≥4 any CRAs in men, p = 0.916 for ≥4 any CRAs in women, p = 0.930 for ≥5 any CRAs in men, and p = 0.662 for ≥5 any CRAs in women. *For ≥5 any CRAs in women, we couldn’t calculate adjusted ORs and 95% CI because of <30 cases.
For multiplicity of metachronous CRAs, the adjusted ORs for ≥3 metachronous CRAs stepwise increased across increasing BMI categories (adjusted OR, 1.34; 95% CI, 1.08−1.67 for subjects with 25−30 kg/m2; adjusted OR, 2.23, 95% CI, 1.31−3.80 for subjects with >30 kg/m2; p trend <0.001) (Table 3). Likewise, the adjusted OR for ≥4 CRAs (adjusted OR, 1.35; 95% CI, 1.03−1.77 for subjects with 25−30 kg/m2; adjusted OR, 1.93; 95% CI, 1.01−3.69 for subjects with >30 kg/m2; p trend = 0.007), ≥5 CRAs (adjusted OR, 1.46; 95% CI, 1.05−2.04 for subjects with 25−30 kg/m2; adjusted OR, 2.39; 95% CI, 1.13−5.60 for subjects with >30 kg/m2; p trend = 0.004) increased stepwise across increasing BMI categories (Table 3). In negative binomial regression regarding the incidence for total number of metachronous CRA, the higher BMI the subject has at the time of index colonoscopy, the more metachronous CRAs the subject will have at the surveillance colonoscopy (p for trend = 0.016) (Table 3 ). When stratified by sex (Table 4 ), adjusted ORs increased significantly in men (p for trend <0.001, < 0.015 and 0.016 for ≥3, ≥4, and ≥5 metachronous CRAs, respectively). However, among women, BMI was not significantly associated with ≥3 and ≥4 metachronous CRAs. Too few women had ≥5 any metachronous CRAs to calculate the adjusted OR (n < 30). In addition, the incidence of metachronous AAs was not significantly associated with BMI in analyses overall (Supplementary Table 2).
Discussion
This large longitudinal cohort study demonstrated a dose-dependent association between index BMI and the multiplicity of metachronous CRAs on surveillance within 5 years after the index colonoscopy. This association between index BMI and the risk of multiple CRAs persisted in analyses of men, but not in women.
Many previous studies have reported an association between obesity and CRAs in cross-sectional design and meta-analysis2,8,9,11,12,16. In addition, some longitudinal studies have reported that obesity was related to metachronous CRAs10,13,15,18. However, all of them have focused that obesity is associated with prevalence of CRAs or metachronous CRAs, but didn’t concern about a certain specific feature having metachronous CRAs2,10,15,16,19. To date, studies on specific characteristics of metachronous CRAs related to obesity are scarce and inconsistent. One study investigated the relationship between BMI and CRA growth by following-up in situ over 3 years, which suggested that adenoma growth was associated with BMI13. However, the result of these positive associations was only from analyses of 13 cases. It didn’t seem not be enough to test statistical assumptions. Meanwhile, another study found no difference among obesity and non-obesity groups in size of metachronous CRAs in spite of large pooling study including 1,213 subjects2. With regard to tumor location, a pooled analysis of seven prospective studies found an association between obesity and metachronous CRAs in proximal colon18. However, another prospective study found no differences among obesity and non-obesity group according to CRA location2.
In the present study, we focused on the effect of increased index BMI on multiple metachronous CRAs. To our knowledge, three studies have evaluated an association between BMI and multiple metachronous CRAs. Among them, two studies have demonstrated no difference between BMI and multiple metachronous CRAs. One study with 163 Japanese autopsy subjects demonstrated that the mean number of polyps had no significant trend with increasing BMI quartile20. In another longitudinal study of 119 subjects, monotonic trends between various indices representing obesity and metachronous CRAs were inconsistent21. However, the former study was not only relatively small, but also an observational study that did not adjust for other possible risk factors for multiple metachronous CRAs. The latter study had a limited sample size for conducting stratified number of metachronous CRAs. To our knowledge, there is only one clinical study of 8,213 subjects consisting of nearly 90% Caucasian reporting an increased risk for 1> any metachronous CRAs in obese men18. This report have demonstrated that risk for 1> any metachronous CRAs were increased with OR 1.4 in obese men with BMI ≥ 30 kg/m2 compared to those of subjects with normal BMI, which finding is consistent with our results18. Even though 1,904 subjects was all Asian and proportion of obesity with ≥30 kg/m2 was lower than those of the former study, we have clearly shown that increasing index BMI in a dose-response manner is a strong predictor for developing multiple metachronous CRAs. In subgroup analyses, the OR of ≥3 metachronous CRAs significantly increased according to index BMI category (BMI <25, 25−29, ≥30 kg/m2). In addition, we demonstrated that obesity was not associated with AA at index data, corresponding to both cross-sectional and longitudinal data. Therefore, we suggest the obesity significantly affects the formation of colorectal tumor, but not metachronous AA22. Molecular studies have shown that insulin, insulin-like growth factor (IGF-1) axis, adipokines, sex-steroid hormones, and chronic low-grade inflammation are the main pathways linking obesity and colorectal neoplasia, including CRAs and CRC. Some experimental studies support the involvement of obesity in initiating colorectal tumorigenesis. An adiponectin deficiency was associated with the development of early colorectal neoplasm rather than advanced CRC23,24. Adipose tissues express higher quantities of pro-inflammatory molecules such as TNF-α, IL-6, CRP25. Elevated pro-inflammatory markers, including CRP and prostaglandin E2, are considered to be biomarkers of multiple CRAs or AA risk26.
Our results demonstrated a difference in the effect of obesity on metachronous CRAs between genders. BMI was associated with multiple metachronous CRAs in men but not in women. In a study with similar results about this issue, little heterogeneous association among gender with a stronger relation for men alike our results18. There has been similarly observed gender difference of BMI impact in the association for CRC risk or CRA prevalence1,2,13,16,27. A strong association with adenoma risk among men than women has been reported10,16,19. A cohort study stratified by age reported an association between BMI and cancer risk in premenopausal but not postmenopausal women27. We performed further analyses stratified by age (stratified at 50 years as the mean menopause age in Korean women), but did not find a significant association between BMI and multiple metachronous CRAs in women. These results are probably related to both the small number of women and the lower incidence of overall CRAs than in men. This weak association between obesity and CRC in women has been explained in part by the protective action of estrogen, as suggested by epidemiologic studies28. At least three hormonal axes, insulin-like growth factors, insulin, and estrogen, might be relevant in obesity and CRC risk in women28,29. Obesity increases the risk of CRC through insulin and the insulin-like growth factors axis29. In postmenopausal women, a major source of estrogen is adipose tissue. Insulin and insulin-like growth factors and estrogen seem to have opposite influences in postmenopausal women27,28. The difference in associations between BMI and CRAs between men and women could be influenced by the different distribution of central body fat30.
Our study is limited by several factors. First, we could not assess actual fluctuations in BMI over the follow-up period. Our study design was based on index BMI. Weight gain or loss over 5 year period might be an effect. Nevertheless, previous studies showed short-term weight change over 4 year period and weight gain and loss did not affect metachronous CRAs9–11,15. Second, this was not a population-based study, but a unique cohort study that included participants who underwent surveillance colonoscopies after index colonoscopy at academic institutions across the country. Therefore, our study was subject to selection bias, and generalization is limited. Third, our data consistently demonstrated that obesity was not related to the risk for metachronous AA. This finding contrasts with some previous reports, which found increasing BMI to be associated with the risk of metachronous AA10,16,19. Our non-significant trend might be originated from relatively short follow-up duration and lower prevalence of AA than those in West31.
Nevertheless, the main strength of our study is the large longitudinal cohort design. To date, several cross- sectional studies have shown a relationship between obesity and CRAs. These results could not demonstrate causality between obesity and CRAs due to limitation of study design. We suggested the new feature of multiplicity of metachronous CRAs related to obesity. We applied multivariate analyses, the trend for linearity to assess the dose-response relationship, and negative binomial regression between BMI and multiple metachronous CRAs.
In conclusion, this study identified that increased index BMI is associated with increased risk of multiple metachronous CRAs. This phenomenon significant remained in subgroup analyses for men but not women. Further studies are necessary to confirm our results and identify the underlying mechanisms in multiple CRAs in obese subjects. Higher index BMI may be a considerable clinical factor in determining of post-poylpectomy surveillance strategy.
Methods
Subjects
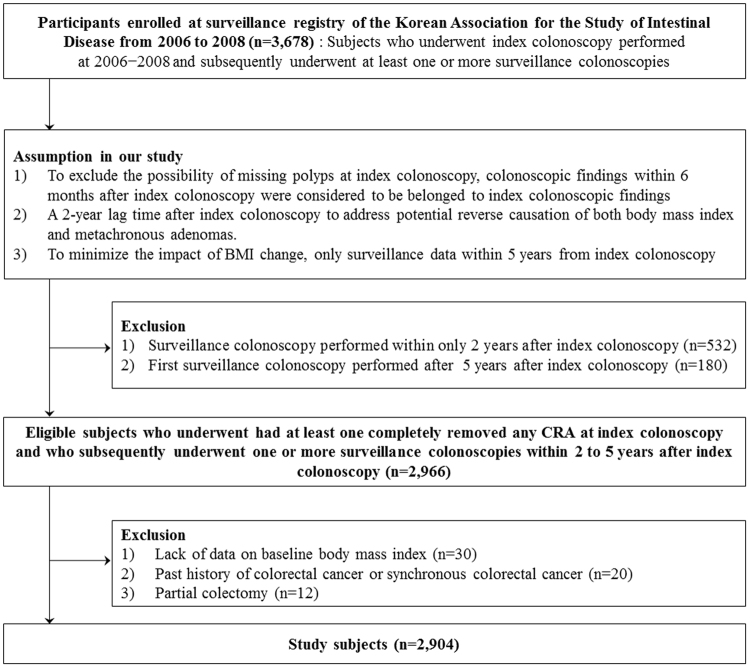
We conducted a retrospective longitudinal cohort study using a multicenter polyp surveillance registry of the Korean Association for the Study of Intestinal Disease. Data were pooled from 3,687 subjects who underwent an index colonoscopy, from 2006 to 2008, who subsequently underwent at least one or more surveillance colonoscopies. Indications for index colonoscopy were screening of CRC, anemia, rectal bleeding, constipation, diarrhea, weight loss, abdominal pain, and positive fecal occult blood test. Subjects were included if they met any of the following criteria: 1) those who underwent a qualified index colonoscopy; 2) whose polyps were removed at the index colonoscopy; 3) who had at least one CRA that was histologically confirmed; and 4) those between 30 and 85 years of age. In this study, qualified index colonoscopy was defined when cecal intubation was successful, all colorectal polyps were completely removed, and the bowel preparation was adequate. In addition, all colonoscopies were performed by experienced endoscopists who were professors or fellows at university hospitals and had performed more than 500 supervised colonoscopies.
To exclude the possibility of missing polyps at the qualified index colonoscopy, we considered an adenoma to be missed at the index colonoscopy if any adenoma was detected on a colonoscopy within 6 months after the index colonoscopy. To evaluate the effect of BMI on metachronous CRAs, subjects who underwent the first surveillance colonoscopy at least 2 years after the index colonoscopy were selected to address the potential reverse causation of both BMI and metachronous CRA. To minimize the impact of BMI change over time, subjects who underwent at least one surveillance colonoscopy within 2 to 5 years from the index colonoscopy were included.
The exclusion criteria were as follows: 1) lack of BMI data at the time of index colonoscopy (n = 30); 2) surveillance colonoscopy performed within only 2 years after index colonoscopy (n = 532); 3) first surveillance colonoscopy performed more than 5 years after index colonoscopy (n = 180); 4) past history of CRC or synchronous CRC (n = 20); and 5) previous partial colectomy (n = 12). Finally, a total of 2,904 subjects were included in this study (Fig. 1). This study was approved by the Institutional Review Board of the Ewha Womans University Mokdong Hospital (EUMC 2015-04-041). Due to the retrospective nature of the study, informed consent was waived. All the experimental procedures were conducted in accordance with institutional ethical guidelines. This study has been carried out in accordance with the Declaration of Helsinki.
Figure 1.
Flow diagram of study subjects.
Data collection and definitions
Medical history and clinical data were obtained from patient’s medical records. These data included age at index colonoscopy, sex, body weight and height at the time of index colonoscopy. BMI was calculated as weight in kilograms divided by the square of the height in meters. Subjects were categorized into 3 groups according to baseline BMI: < 25 kg/m2 (normal weight), 25–29 kg/m2 (overweight), and ≥ 30 kg/m2 (obese). Current smoking, family history of CRC, and use of aspirin or NSAIDs during the previous 12 months were also collected.
CRA characteristics at index and surveillance colonoscopies were collected from endoscopy and pathology reports. CRAs were stratified into LRA and HRA groups. LRA was defined as one or two non-AAs. HRA was further classified as any AA or 3 or more non-AAs. AA was defined as any polyp with one or more of the following features: ≥ 10 mm, villous histology, or high-grade dysplasia. Subjects simultaneously diagnosed with AA and non-AA were classified into AA group. If all CRAs were located in ascending colon, hepatic flexure, or transverse colon, the location of the lesion was defined as ‘right colon’. If all CRAs were located in splenic flexure, descending colon, sigmoid colon, or rectum, the location of the lesion was defined as ‘left colon’. If adenomas were present in both the right and left colon, the location was ‘both sided’.
Statistical analyses
Data entry and statistical analyses were performed with the Statistical Package for the Social Science (SPSS) version 20.0 (SPSS Inc., Chicago, Illinois, US). Index and surveillance colonoscopy findings were summarized as mean and standard deviation for continuous variables and frequency and percentage for categorical variables. The analysis was stratified by index BMI. Linear regression for continuous variables and Cochran-Armitage analyses for categorical variables were used to assess the linear trend. For the age, follow-up period, frequency of surveillance colonoscopy of continuous variables, we used the One-way ANOVA analyses for variance among patients.
Logistic multivariate analyses were conducted to ascertain the impact of index BMI on metachronous CRAs. Adjusted odds ratio (OR) and 95% confidence interval (CI) for BMI categories were calculated separately for subjects with vs without any CRAs, < 2 vs ≥ any CRAs, < 3 vs ≥ 3 any CRAs, < 4 vs ≥ 4 any CRAs, < 5 vs ≥ 5 CRAs, and with or without any AA. The Hosmer-Lemeshow Goodness-of-Fit test was used to assess the calibration of the each model in logistic multivariate analyses. However, good calibration ability are not sufficient for a model about ≥ 2 any CRAs to be clinically useful. Therefore, the data relevant to ≥ 2 any CRAs were excluded. In addition, we compared the incidence for total number of metachronous CRA as continuous, not binary variables. We used negative binomial regression for number of metachronous CRAs because the assumption of equality of the mean and variance in the Poisson model did not hold true. In all statistical models, we adjusted for age, sex, index BMI status, current smoking, family history of CRC, use of aspirin or NSAID, index colonoscopic findings, follow-up period, and frequency of surveillance.
Electronic supplementary material
Acknowledgements
This study was supported by intramural research promotion grants from Ewha Womans University College of Medicine (1-2016-1470-001-1, Chang Mo Moon) and Young Medical Science Researcher Grants from Ewha Womans University College of Medicine (1-2016-1951-001-1, Chung Hyun Tae).
Author Contributions
C.M. Moon Study supervised the project. S.-A. Jung, C.S. Eun, J.J. Park, G.S. Seo, J.M. Cha, S.C. Park, J. Chun, H.J. Lee, Y. Jung, J.O. Kim, Y.-E. Joo, S.-J., Boo and D.I. Park extracted the data. C.H. Tae and C.M. Moon conducted the analysis and interpreted the data. C.H. Tae drafted the manuscript. C.M. Moon critically revised the manuscript. All the authors contributed to manuscript revision.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14163-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murphy TK, Calle EE, Rodriguez C, Kahn HS, Thun MJ. Body mass index and colon cancer mortality in a large prospective study. American Journal of Epidemiology. 2000;152:847–854. doi: 10.1093/aje/152.9.847. [DOI] [PubMed] [Google Scholar]
- 2.Kitahara CM, et al. Prospective investigation of body mass index, colorectal adenoma, and colorectal cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Journal of Clinical Oncology. 2013;31:2450–2459. doi: 10.1200/JCO.2012.48.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GLOBOCAN. Estimated cancer incidence, mortality and prevalence worldwide in 2012 http://globocan.iarc.fr/Default.aspx (2012).
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New England Journal of Medicine. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55:285–291. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal disease. 2005;7:204–213. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes and Control. 1996;7:253–263. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 8.Okabayashi K. et al. Body mass index category as a risk factor for colorectal adenomas: a systematic review and meta-analysis. The American Journal of Gastroenterology107, 1175-1185; quiz 1186, 10.1038/ajg.2012.180 (2012). [DOI] [PubMed]
- 9.Ben Q, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology. 2012;142:762–772. doi: 10.1053/j.gastro.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 10.Laiyemo AO, et al. Obesity, weight change, and risk of adenoma recurrence: a prospective trial. Endoscopy. 2012;44:813–818. doi: 10.1055/s-0032-1309837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bird CL, Frankl HD, Lee ER, Haile RWO. weight gain, large weight changes, and adenomatous polyps of the left colon and rectum. American Journal of Epidemiology. 1998;147:670–680. doi: 10.1093/oxfordjournals.aje.a009508. [DOI] [PubMed] [Google Scholar]
- 12.Omata F, Deshpande GA, Ohde S, Mine T, Fukui T. The association between obesity and colorectal adenoma: systemic review and meta-analysis. Scandinavian Journal of Gastroenterology. 2013;48:136–146. doi: 10.3109/00365521.2012.737364. [DOI] [PubMed] [Google Scholar]
- 13.Davidow AL, et al. Recurrent adenomatous polyps and body mass index. Cancer Epidemiology, Biomarkers & Prevention. 1996;5:313–331. [PubMed] [Google Scholar]
- 14.Almendingen K, Hofstad B, Vatn MH. Does high body fatness increase the risk of presence and growth of colorectal adenomas followed up in situ for 3 years? The American Journal of Gastroenterology. 2001;96:2238–2246. doi: 10.1111/j.1572-0241.2001.03942.x. [DOI] [PubMed] [Google Scholar]
- 15.Sedjo RL, et al. Change in body size and the risk of colorectal adenomas. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:526–531. doi: 10.1158/1055-9965.EPI-06-0229. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs ET, et al. Association between body size and colorectal adenoma recurrence. Clinical Gastoenterology and Hepatology. 2007;5:982–990. doi: 10.1016/j.cgh.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauret KG, et al. Physical activity and reduced risk of incident sporadic colorectal adenomas: observational support for mechanisms involving energy balance and inflammation modulation. American Journal of Epidemiology. 2004;159:983–992. doi: 10.1093/aje/kwh130. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs ET, et al. Association between body mass index and colorectal neoplasia at follow-up colonoscopy: a pooling study. American Journal of Epidemiology. 2009;169:657–666. doi: 10.1093/aje/kwn401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung YS, Park JH, Park DI, Sohn CI. & Choi K. Weight Change and Obesity Are Associated with a Risk of Adenoma Recurrence. Digestive Disease and Sciences. 2016;61:2694–2703. doi: 10.1007/s10620-016-4194-2. [DOI] [PubMed] [Google Scholar]
- 20.Stemmermann GN, Heilbrun LK, Nomura AM. Association of diet and other factors with adenomatous polyps of the large bowel: a prospective autopsy study. The American Journal of Clinical Nutrition. 1998;47:312–317. doi: 10.1093/ajcn/47.2.312. [DOI] [PubMed] [Google Scholar]
- 21.Sass DA, et al. Relationship of visceral adipose tissue to recurrence of adenomatous polyps. The American Journal of Gastroenterology. 2004;99:687–693. doi: 10.1111/j.1572-0241.2004.04136.x. [DOI] [PubMed] [Google Scholar]
- 22.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 23.Tae CH, et al. Involvement of adiponectin in early stage of colorectal carcinogenesis. BMC Cancer. 2014;14:811. doi: 10.1186/1471-2407-14-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu XT, et al. Meta-analysis: circulating adiponectin levels and risk of colorectal cancer and adenoma. Journal of Digestive Diseases. 2011;12:234–244. doi: 10.1111/j.1751-2980.2011.00504.x. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. Journal of Clinical Investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davenport JR, et al. Evaluation of pro-inflammatory markers plasma C-reactive protein and urinary prostaglandin-E2 metabolite in colorectal adenoma risk. Molecular Carcinogenesis. 2016;55:1251–1261. doi: 10.1002/mc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terry P, Giovannucci E, Bergkvist L, Holmberg L, Wolk A. Body weight and colorectal cancer risk in a cohort of Swedish women: relation varies by age and cancer site. British Journal of Cancer. 2001;85:346–349. doi: 10.1054/bjoc.2001.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giovannucci E. Obesity, gender, and colon cancer. Gut. 2002;51:147. doi: 10.1136/gut.51.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaaks R, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol. Biomark. Prev., 9: 345-349, 2000. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:1103–1104. [PubMed] [Google Scholar]
- 30.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. The American Journal of Clinical Nutrition. 1992;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 31.Leshno A, et al. Prevalence of colorectal neoplasms in young, average risk individuals: A turning tide between East and West. World Journal of Gastroenterology. 2016;22:7365–7372. doi: 10.3748/wjg.v22.i32.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.