Abstract
Heavy drinkers show an attentional bias towards alcohol-related visual cues. A recent study in our laboratory (Weafer & Fillmore, 2013) showed that alcohol decreases attentional bias among heavy drinkers, suggesting that alcohol satiates motivation to drink in heavy drinkers. Little is known, however, about how this satiety effect might change across the time course of the blood alcohol concentration (BAC) curve. It is possible that attentional bias may return later in the time course if the satiety effect begins to diminish. The current study tested this hypothesis in a group of high-risk binge drinkers (n = 20). Participants completed a visual-probe task to measure their attentional bias and a self-report measure of their desire for alcohol after receiving 0.64 g/kg and 0.0 g/kg alcohol (placebo) during separate dose challenge sessions. The measures were obtained during the ascending limb of the BAC curve under alcohol (test 1) and again during the descending limb (test 2) at a comparable BAC. The measures also were obtained at the same times following placebo. Under alcohol, no attentional bias was observed during test 1, but drinkers reported increased desire to drink. During test 2, attentional bias was evident, but participants reported less desire to drink. Attentional bias was not correlated with desire to drink at any time point. Following placebo, attentional bias was evident during both tests. These findings show that alcohol causes a temporary reduction of attentional bias among heavy drinkers. These changes do not correspond with their self-reported motivation to drink.
Keywords: Attentional bias, binge drinkers, alcohol effects, acute tolerance, implicit cognition
Introduction
Alcohol users often show an attentional bias towards alcohol-related cues in the environment. This attentional bias is thought to result from a conditioning history wherein these cues become associated with the pleasurable effects of alcohol (Franken, 2003). As the associative pairing between the alcohol effects and alcohol cues develops, these cues begin to acquire incentive salience, increasing the likelihood that drinkers will attend to these cues and initiate or continue a drinking episode in their presence (Wiers, Rinck, Kordts, Houben, & Strack, 2010). As such, recent cognitive models of substance use consider attentional bias to be a causal factor in dysregulated drinking (Field & Cox, 2008; Field, Wiers, Christiansen, Fillmore, & Verster, 2010; Franken, 2003; Robbins & Ehrman, 2004).
Attentional bias towards alcohol-related cues can be modeled in the laboratory using a visual-probe task. When completing this task, participants view a pair of images presented side by side on a computer screen: one of these is an alcohol-related image and the other is a neutral image. This display of visual cues models a natural environment where different objects compete for the viewer’s attention. Participants are instructed to look at both of these images. More time spent fixated on alcohol-related images relative to neutral images is indicative of attentional bias towards these cues.
The majority of research in this area has focused on finding differences in attentional bias between low- and high-risk drinkers. For example, Townshend and Duka (2001) found that heavy drinkers showed more attentional bias than light drinkers using the visual-probe task. Likewise, Johnsen et al. (1994) found that alcohol-dependent inpatients showed more attentional bias towards alcohol cues than did light drinkers. Overall, these studies and others (e.g., Cox, Blount, & Rozak, 2000; Field, 2005; Ryan, 2002) show that high-risk substance users typically show more attentional bias towards substance-related cues relative to their low-risk counterparts. However, these studies have only examined attentional bias when drinkers are in a sober state. It is equally important to understand what happens to drinkers’ attentional bias when they become intoxicated. If drinkers continue to show attentional bias towards alcohol cues as they become intoxicated, these cues may continue to exert stimulus control over the drinker. This could result in excessive alcohol intake even when the drinker is already intoxicated, leading to binge alcohol use.
There are a few studies that have examined how drinkers’ attentional bias changes following acute doses of alcohol. Although the findings of these studies have conflicted with one another in some cases (Duka & Townshend, 2004; Miller & Fillmore, 2011; Schoenmakers, Wiers, & Field, 2008), most have supported the notion that attentional bias persists following a priming dose of alcohol. Our group (Weafer & Fillmore, 2013) measured attentional bias in heavy and moderate drinkers following three doses of alcohol (i.e., 0.64 g/kg, 0.45 g/kg, and placebo). This study showed that while sober (i.e., under placebo), heavy drinkers had higher attentional bias compared to the moderate social drinkers. Under the active alcohol doses, however, the heavy drinkers showed a reduction in attentional bias so that their level of attentional bias was no different than that of the moderate drinkers. The conclusions of this laboratory investigation are supported by similar results from a field study examining attentional bias in young adults who were leaving a bar after drinking (Schoenmakers & Wiers, 2010). The field study found that participants who had reportedly consumed the most alcohol that evening also showed the least attentional bias. Taken together, these findings suggest that alcohol consumption can result in a reduction of attentional bias towards alcohol cues, possibly because alcohol consumption satiates the motivation to drink.
Evidence that reduced attentional bias following alcohol consumption could indicate a satiety effect raises questions about the utility of attentional bias to measure transient changes in the motivational state of the drinker during the time-course of a drinking episode, as BAC initially ascends to peak and then declines. For example, after initial alcohol consumption, it is not known how long drinkers remain satiated as indicated by their reduced attentional bias. The duration of the satiation period is important to understand because people may become more likely to reinitiate drinking once attentional bias returns and the alcohol-related cues re-assert stimulus control over their behavior. Although Weafer and Fillmore (2013) found an acute reduction in attentional bias after drinking, they measured attentional bias only once after participants consumed alcohol as their BACs ascended. It has long been known that the effects of alcohol change over the time course of the BAC curve (Goldberg, 1943; Mellanby, 1919). Furthermore, the effects of alcohol on motor control and cognitive functioning tend to diminish during the latter phase of the BAC time course (Fillmore, Marczinski, & Bowman, 2005; Schweizer & Vogel-Sprott, 2008). Drinkers show less alcohol-induced behavioral and cognitive impairment during the descending limb, even at BACs that produced severe impairment during the ascending limb. Similarly, drinkers report less subjective intoxication during the descending limb relative to comparable BACs on the ascending limb (Roberts, Milich, & Fillmore, 2013). Given the well-characterized changes in the acute effects of alcohol that occur across the time-course of a single dose, it is also conceivable that attentional bias could differ across ascending and descending limbs of the blood alcohol curve, possibly reflecting changes in the individual’s motivation to drink.
The purpose of the present study was to examine how changes in attentional bias over the time course of a single alcohol dose might correspond to changes in the drinker’s motivation to drink. Based on prior research (Weafer & Fillmore, 2013; Schoenmakers & Wiers, 2010), we expected that attentional bias would be diminished early after drinking as BAC ascended. However, it is possible that attentional bias could quickly return when the drinker’s perceived level intoxication begins to diminish soon after BAC has peaked and begins to decline. If attentional bias returns during the descending limb, it could indicate that alcohol cues have reasserted stimulus control over the drinker possibly contributing to a resumption of drinking, despite still having an elevated BAC from the initial dose.
The current study tested this hypothesis by measuring attentional bias in a group of drinkers under two doses of alcohol (i.e., placebo, 0.64 g/kg). Binge drinkers were identified based on their drinking habits according to a Timeline Follow-Back (Sobell & Sobell, 1992) as well as their scores on the Alcohol Use Disorder Identification Test (Saunders, Aasland, Babor, Delafuente, & Grant, 1993). Using these measures, we identified a group of heavy drinkers that reported frequent binge drinking episodes (NIAAA, 2004) and experienced adverse psychosocial consequences as a result of their drinking. Their attentional bias was measured using a visual-probe task. To assess how attentional bias changed across the time-course of the alcohol doses, participants completed the task twice during each dose: once as BAC ascended (i.e., test 1) and again during the descending limb of the BAC curve (i.e., test 2). We predicted that following alcohol participants would show reduced attentional bias during the ascending limb of the BAC curve, followed by an increase in their attentional bias during the descending limb despite their elevated BACs at this time.
We also measured participants’ motivation to drink over the time course using a conventional visual analogue scale that measured desire to drink (de Wit, 1991; Fillmore, 2001). We compared the changes in drinkers’ attentional bias with their changes self-reported motivation to drink as BAC ascended and declined.
Method
Participants
Participants were 20 adult heavy drinkers (11 men and 9 women) between the ages of 21 and 35 years. The sample included participants who identified as White (n = 16), African American (n = 2), and Asian American (n = 2). Volunteers were recruited through advertisements (i.e., newspaper ads and posters) seeking adults for studies on the effects of alcohol on cognitive functions. Volunteers with past or current severe psychiatric diagnoses (e.g., bipolar disorder, schizophrenia) were not eligible for participation, nor were volunteers who reported infrequent drinking or potential risk for alcohol dependence. Dependence risk was determined by a score of 5 or higher on the Short Michigan Alcoholism Screening Test (Selzer, Vinokur, & Van Rooijen, 1975). Individuals who reported other high-risk indicators of dependence (e.g., prior treatment for an alcohol use disorder, prior driving under the influence conviction) were not invited to participate. Other participant characteristics are described in Table 1.
Table 1.
Participants’ Demographic Characteristics and Drinking Habits
| Mean
|
SD
|
|
|---|---|---|
| Demographic | ||
| Age | 23.8 | 4.1 |
| Weight (kg) | 74.0 | 12.3 |
| Drinking habits | ||
| TLFB | ||
| Total Days | 40.1 | 14.5 |
| Total Drinks | 328.7 | 299.8 |
| Binge Days | 23.2 | 9.3 |
| Drunk Days | 22.8 | 16.1 |
| AUDIT | 12.4 | 3.4 |
Note. TLFB is the Timeline Follow-Back. AUDIT is the Alcohol Use Disorders Identification Test. TLFB variables describe alcohol use during the past 90 days.
Assessment of Drinking Habits and Inclusion Criteria
The Timeline Follow-back calendar (TLFB; Sobell & Sobell, 1992) was used to assess daily patterns of alcohol use over the past 12 weeks. This measure uses a structured calendar anchored with holidays and other notable dates to facilitate recall of past drinking episodes. For each day, participants estimated the number of standard drinks they consumed and the number of hours they spend drinking. This information, along with gender and body weight, was used to estimate the resultant BAC obtained for each drinking day using anthropometric-based BAC estimation formulae that assume an average clearance rate of 15 mg/100 ml per hour (Watson, Watson, & Batt, 1981). These formulae have been used in previous studies and have been shown to yield high correlations with actual BACs obtained under laboratory conditions (Fillmore, 2001). Binge days were identified as days in which the reported alcohol use of a participant was estimated to yield a BAC of 80 mg/100 ml or higher (NIAAA, 2004). The TLFB provided four measures of drinking habits: (a) binge days (total number of binge episodes); (b) drunk days (total number of days that participants reported feeling drunk); (c) drinking days (total number of days that alcohol was consumed); (d) total drinks (total number of drinks consumed).
Participants also completed the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993). The AUDIT is a screening instrument that is used to identify at-risk problem drinkers. The 10-item self-report questionnaire consists of 10 items about drinking patterns, negative psychosocial outcomes, and other indicators of alcohol use disorder. Scores on this measure can range from 0 (no alcohol-related problems) to 40 (severe alcohol-related problems). A cutoff score of 6 or higher for women and 8 or higher for men provides the greatest degree of accuracy for identifying problem drinkers (Reinert & Allen, 2002).
Both the TLFB and the AUDIT were used to identify a sample of high-risk binge drinkers. Volunteers were invited to participate only if they reported 10 or more binge days on the TLFB and had a score of 6 or higher for women and 8 or higher for men on the AUDIT.
Materials
Visual-probe task
A visual-probe task was used to measure attentional bias towards alcohol-related images. During this task, participants viewed a neutral and alcohol-related image presented side-by-side on a computer monitor. Upon offset of the images, a visual-probe replaced either the left or right image. Participants were instructed to respond to the visual-probe by pressing one of the two response keys on a keyboard to indicate the side where the target appeared. They were instructed to look at both images, although they were not told about the content of the pictures prior to viewing. The pictures consisted of 10 alcohol-related images that were matched with 10 neutral images (i.e., non-alcohol images). The alcohol images depicted an alcohol beverage, and these images were matched with neutral images of nonalcohol drinks (e.g., a bottle of soda matched with a bottle of beer). All images were photographed against a plain background.
The 10 image pairs were presented four times each, once for each of the four possible picture/target combinations (i.e., left and right picture locations and left and right visual-probe locations) for a total of 40 test trials. The task also included 40 “filler” trials that consisted of 10 pairs of neutral images (e.g., a shoe) presented 4 times each. These filler trials were included to reduce the likelihood of habituation to the alcohol stimuli that could occur if all trials contained alcohol-related images. The 40 filler trials were intermixed with the 40 test trials, so the task included 80 trials in total.
Each trial occurred in the following order. First, a fixation point (+) was presented at the center of the screen for 500 ms. Second, a pair of images was displayed for 1,000 ms. By selecting this stimulus presentation length, our task likely assessed bias in the early-phase orienting response (see Field and Cox [2008] for a discussion on the relation between stimulus presentation length and attentional processes in the visual probe task). Third, a visual-probe (an “X”) appeared on the left or right side of the screen in the position where one of the pictures was previously displayed. Participants then pressed one of two keys (“>” or “/”) on a standard keyboard to indicate the location of the target.
Fixations were measured using a Tobii T120 Eye Tracking Monitor (Tobii Technology, Sweden). Stimuli were presented on the Tobii Monitor and dual embedded cameras tracked participants’ gaze locations. Participants were seated with their heads approximated 60 cm in front of the computer with a free range of head and neck motion. Gaze locations were sampled at 120 Hz and fixations were defined as gazes with standard deviations less than 0.5 degrees of visual angle for durations of 50 ms or longer. For each trial, we calculated the total duration of all fixations directed towards each image type (i.e., alcohol and neutral images). These values were averaged across trials to produce a mean fixation time for each image type.
Self-reported Motivation and Intoxication
Participants provided ratings of subjective states using a visual analogue scale (VAS). They rated their current motivation to drink and their current level of perceived intoxication by clicking a vertical line on a scale ranging from one (not at all) to nine (very much). Previous research has shown that these scales are sensitive to changes in subjective effects that occur over the time course of an alcohol dose (e.g., Fillmore, 2001).
Procedure
Familiarization Session
Participants completed a familiarization session during which they became acquainted with laboratory procedures, completed questionnaires, and provided informed consent for participation. Volunteers who did not meet criteria for participation in the study were paid $10 and discontinued.
Test Sessions
Participants completed the assessment battery under two doses of alcohol, 0.0 (placebo) and 0.64 g/kg. Participants were blinded to dose order and doses were administered on separate days in a counterbalanced order. Sessions were separated by a minimum of 1 day and a maximum of 1 week. Alcohol doses were calculated on the basis of body weight and administered as absolute alcohol mixed with three parts carbonated soda. The 0.64 g/kg alcohol dose produces an average peak BAC of 80 mg/100 ml approximately 65 min post drinking and begins to decline 75 min post drinking (Fillmore, Marczinski, & Bowman, 2005; Roberts et al., 2013). The placebo dose (0.0 g/kg) consisted of a volume of carbonated mix that matched the total volume of the 0.64 g/kg alcohol drink. A small amount (3 ml) of alcohol was floated on the surface of the beverage. It was sprayed with an alcohol mist that resembled condensation and provided a strong alcoholic scent as the beverage was consumed.
All drinks were consumed in 6 min. Participants performed the 10-min test battery (visual-probe task, visual analogue scale) twice after each dose. Test 1 occurred 40–50 min post administration. In the active dose condition, this time interval corresponded to the middle of the ascending limb of the BAC curve. Test 2 occurred 100–110 min post administration. This time the interval corresponded to the early phase of the descending limb. We selected these testing times because they occurred at different limbs on the BAC curve that are separated by 1 hour where BACs are approximately similar.
BACs were determined from expired air samples measured by an Intoxilyzer, Model 400 (CMI, Inc., Owensboro, KY). BACs were measured 30, 40, 70, 90, 100, 130, and 150 min following beverage administration. Breath samples also were obtained at these times during the placebo session, ostensibly to measure BACs. Once the testing was finished, participants remained at leisure in a lounge area until their BACs, which were monitored at 20-min intervals, reached 20 mg/100 ml or below. Participants received a meal during this leisure time and were allowed to watch movies and read magazines. Transportation home was provided as needed. Upon completing the final session, participants were paid $85 and debriefed.
Criterion Measures and Data Analyses
The visual-probe task measured participants’ attentional bias towards alcohol cues as the mean fixation time on each type of picture. An attentional bias score was calculated by subtracting the mean fixation time on neutral images from the mean fixation time on alcohol images. A positive score indicated an attentional bias to the alcohol cues and larger scores indicated greater attentional bias.
Attentional bias scores were analyzed using a 2 dose (placebo versus 0.64 g/kg alcohol) × 2 test (test 1—ascending limb versus test 2—descending limb) repeated measures analysis of variables (ANOVA). A priori one sample t tests were used to test whether attentional bias was present during each test. Attentional bias was indicated if attentional bias scores were significantly greater than zero. A select set of a priori t tests tested for the hypothesized reduction of attentional under alcohol at test 1 and subsequent increase in attentional bias at test 2.
Self-reported effects on the visual analogue scale were analyzed using a 2 dose (placebo versus 0.64 g/kg alcohol) × 2 test (test 1 versus test 2) repeated measures ANOVA. If the dose × time interaction was significant, then a priori t tests were used to explore how subjective ratings changed within each dose.
Finally, we examined the relations among attentional bias and self-reported motivation to drink using zero-order correlation analyses. These analyses tested whether self-reported motivation for alcohol was associated with attentional bias under each dose.
Results
Drinking and Other Substance Use
Participants’ self-reported drinking habits are presented in Table 1. The sample reported heavy alcohol use, including frequent days characterized by binge drinking and self-reported drunkenness. Their elevated scores on the AUDIT further confirmed that participants were high-risk drinkers. In addition to frequently using alcohol, some participants reported past month use of nicotine (n = 4) and marijuana (n = 8).
Blood Alcohol Concentrations
BACs at each time point are presented in Table 2. This table shows that following 0.64 g/kg alcohol, participants’ BACs increased steadily during the first 70 mins and then began to decline. Thus, test 1 occurred during the ascending limb (mean BAC = 79.9 mg/100 ml) and test 2 occurred during the descending limb (mean BAC = 65.5 mg/100 ml). Gender differences in BACs were tested by a 2 (gender × 8 time (30, 40, 70, 90, 100, 130, and 150 min post-administration) mixed-design ANOVA. Neither the main effect of gender, F (1, 18) = 0.1, p = .716, η2 = .01, nor the time × gender interaction, F (7, 126) = 0.3, p = .951, η2 = .01, was significant, suggesting that men and women achieved comparable BACs. No detectable BAC was observed in the placebo condition.
Table 2.
Mean Blood Alcohol Concentrations (mg/100 ml)
| Min past Dose | 30 | 40 | 70 | 80 | 90 | 110 | 130 | 150 |
|---|---|---|---|---|---|---|---|---|
| BAC | ||||||||
| M | 65.4 | 79.9 | 73.7 | 70.0 | 66.9 | 65.5 | 53.1 | 45.3 |
| SD | 15.4 | 16.1 | 11.0 | 9.9 | 9.9 | 8.0 | 8.1 | 6.7 |
Attentional Bias
Attentional bias scores are plotted in Figure 1 and fixation times are listed by picture type in Table 3. As predicted, the 2 (dose) × 2 (test) ANOVA of attentional bias scores found a significant interaction, F (1, 19) = 5.3, p = .033, η2 = .22. Figure 1 illustrates the interaction. Under alcohol no attentional bias was observed during test 1. A one-sample t test confirmed that attentional bias scores did not differ significantly from zero at this time, t (19) = 0.3, p = .739, d = 0.07. However, under alcohol during test 2, participants’ attentional bias level was significantly higher than zero, t (19) = 3.2, p = .005, d = 0.86. This increase in attentional bias from test 1 to test 2 was significant, t (19) = 2.7, p = .015, d = 0.60. Following placebo, attentional bias was observed during each test and with each test showing a similar magnitude of bias. One-sample t tests confirmed the observation of attentional bias for test 1, t (19) = 2.8, p = .011, d = 0.62, and test 2, t (19) = 2.4, p = .025, d = 0.55, and a t test comparison between test 1 and test 2 revealed no significant difference in the magnitude of attentional bias over time, t (19) < 0.01, p = .993, d < 0.01. The level of attentional bias under alcohol was also directly compared to placebo at each test using a priori t tests. The t tests confirmed that during test 1, attentional bias was lower under alcohol compared with placebo, t (19) = 1.9, p = .037, d = 0.42, but during test 2 no difference in the magnitude of attentional bias was observed between alcohol and placebo t (19) = −0.8, p = .783, d = −0.18.
Figure 1.
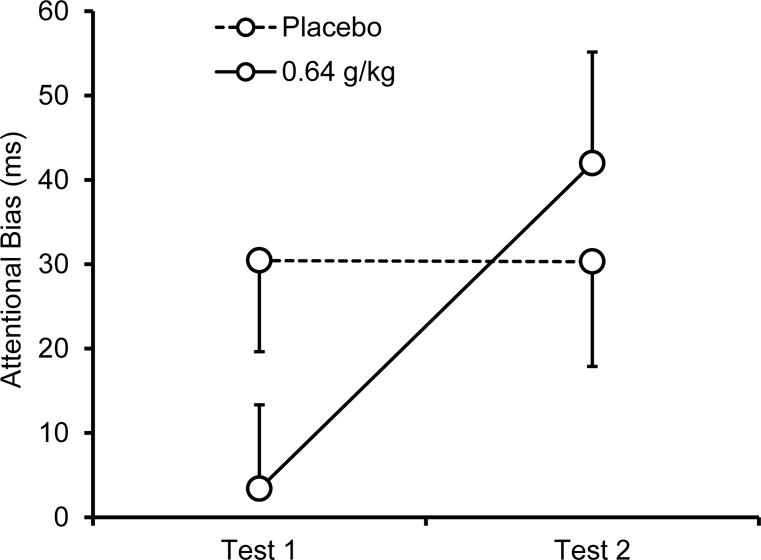
Mean (+ SE) attentional bias score following 0.64 g/kg alcohol and placebo during test 1 (ascending limb) and test 2 (descending limb).
Table 3.
Fixation times separated by picture type
| Test 1 | Test 2 | |||
|---|---|---|---|---|
|
| ||||
| Dose | Alcohol | Neutral | Alcohol | Neutral |
| Placebo | 370.5 (54.2) | 340.1 (61.2) | 359.7 (62.8) | 329.4 (70.9) |
| 0.64 g/kg | 333.9 (71.3) | 330.5 (72.7) | 346.8 (74.7) | 304.9 (74.7) |
Note. Reported values are mean (SD) fixation time for each picture type. Test 1 and Test 2 occurred during the ascending and descending limb of the BAC curve, respectively.
Self-reported Motivation and Intoxication
Subjective alcohol effects are plotted in Figure 2. The figure shows that compared with placebo, alcohol increased motivation to drink during test 1, but less so during test 2. The 2 (dose) × 2 (test) ANOVA revealed a significant main effect of dose, F (1, 19) = 9.6, p = .006, η2 = .34, showing that self-reported motivation to drink increased following alcohol. There also was a significant main effect of test, F (1, 19) = 16.1, p = .001, η2 = .56, indicating that self-reported motivation to drink decreased during test 2 following both doses. The dose × test interaction was not significant, F (1, 19) = 3.0, p = .101, η2 = .14.
Figure 2.
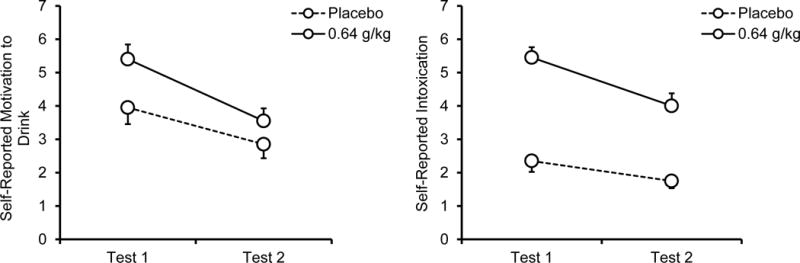
Mean (+ SE) self-reported motivation to drink and intoxication following 0.64 g/kg alcohol and placebo during test 1 (ascending limb) and test 2 (descending limb).
Self-reported intoxication showed a similar pattern. The 2 (dose) × 2 (test) ANOVA showed a significant main effect of dose, F (1, 19) = 63.8, p < .001, η2 = .77, resulting from an increase following the alcohol relative to placebo. There also was a significant main effect of test, F (1, 19) = 23.9, p < .001, η2 = .56, because participants reported less intoxication during test 2 following both doses. The dose × test interaction trended towards significance, F (1, 19) = 4.3, p = .053, η2 = .22. An a priori t test found a significant reduction in subjective intoxication from test 1 to test 2 following alcohol, t (19) = 4.4, p < .001, d = 0.99, and placebo, t (19) = 2.3, p = .030, d = 0.53, however, the reduction was larger following alcohol.
Relations of Attention Bias with Self-reported Motivation to Drink
Correlation analyses were used to determine whether participants’ attentional bias was associated with their self-reported desire for alcohol during the ascending and descending limb of the blood alcohol curve. Attentional bias and motivation to drink were not significantly correlated following placebo at test 1, r (18) = .03, p = .910, or at test 2, r (18) = −.17, p = .469. Similarly, following alcohol, the correlation between attentional bias and motivation to drink at test 1 was nonsignificant despite a moderate effect size of the correlation coefficient, r (18) = .37, p = .109. The correlation between these two variables during test 2 was not significant, r (18) = −.02, p = .940. Taken together, these findings suggest that attentional bias was not reliably correlated with self-reported motivation to drink.
Discussion
This study examined the acute effects of alcohol on attentional bias in binge drinking adults at two different time points of the BAC curve. We hypothesized that alcohol would satiate participants’ motivation to drink, as evidenced by a reduction in attentional bias following 0.64 g/kg alcohol. However, we expected this satiation effect to be brief such that attentional bias would return after the BAC peaked and began to decline. Results of the study confirmed this hypothesis. In the alcohol-free state (i.e., following placebo), participants displayed a significant attentional bias effect that was remarkably consistent in magnitude across tests. Following 0.64 g/kg alcohol, drinkers showed an immediate reduction of attentional bias during test 1 as BAC ascended. However, their attentional bias returned to a magnitude comparable to their sober state during test 2 when BAC began to decline under the active dose. Self-reported motivation to drink showed a different pattern of changes under alcohol. Compared with placebo, drinkers reported an increase in motivation to drink during test 1 following alcohol, and this increase persisted throughout test 2 as BAC peaked and began to decline. The discrepancy between changes in attentional bias and self-reported motivation to drink were also observed at the level of individual differences among participants. Correlation analyses found that attentional bias and self-reported motivation to drink were not correlated during any test, replicating previous findings that attentional bias is only weakly related self-reported indications of motivation to drink (Field et al., 2009).
The finding on the resumption of attentional bias during the descending limb builds on our previous research by showing that the acute alcohol-induced reduction in attentional bias reported by Weafer and Fillmore (2013) abates before drinkers reach sobriety. We suggested that the initial loss of attentional bias was due to an acute reduction in the drinkers’ motivation to drink after consumed a bolus dose as alcohol (i.e., a satiation effect). Others have also suggested that acute administration of alcohol could diminish attentional bias, perhaps reflecting reductions in drinkers’ motivation to drink (Duka & Townshend, 2004; Schoenmakers & Wiers, 2010). However, the present research shows that such a drop in attentional bias after drinking is a relatively short–lived occurrence. The rapid recovery of attentional bias was particularly striking given that participants’ BACs were still elevated when attentional bias reemerged. Indeed, during test 2, at the time when participants showed full recovery of attentional bias, their BACs were elevated almost to a level that would constitute a binge episode (i.e., 66 mg/100 ml; NIAAA, 2010). As BACs begin to descend and attentional bias returns, these drinkers may fall back under the stimulus control of alcohol cues and reinitiate the drinking episode. As such, attentional bias could continue to play a role in promoting drinking, particularly later in a drinking episode.
The study also examined the concordance between drinkers’ attentional bias and their self-reported motivation to drink. We found no correlation between attentional bias and self-reported motivation in either changes over time under alcohol or in the individual differences in each measure at a given time point under the dose. The discrepancies between these two measures are of considerable theoretical interest for understanding the cognitive and motivational processes that lead people to drink to excess. Motivation to drink is most often assessed using self-report measures, typically by asking participants to rate their level of subjective craving or desire to drink on a Likert-type scale such as that used in the current study. However, a limitation of these self-report measures is that in some cases they show little or no correlation with actual substance use behavior (Geier, Mucha, & Pauli, 2000; Miller & Gold, 1994; Tiffany & Conklin, 2000). That is, oftentimes how much people say they want to drink and if/how much they do drink have very little to do with one another.
To explain this apparent discrepancy between self-reported desire for alcohol and actual consumption of alcohol, dual-process models of motivation recognize that explicit processes, such as those assessed by self-report measures, provide an incomplete picture of an individual’s motivational state. Indeed, such models assert that both implicit and explicit motivational process can lead to drinking (Ames & Stacey, 1998; Moss & Albery, 2009; Wiers & Stacy, 2006). Explicit processes occur at the level of conscious awareness, and people are able to reflect on and verbally describe these motivational states. Implicit motivational processes, on the other hand, occur automatically and may influence drinkers’ behavior without their conscious awareness (Field, Munafo, & Franken, 2009). Attentional bias is believed to be an implicit process that influences drinking behavior (Rook, Hine, & Thorsteinsson, 2008; Wiers & Stacy, 2006). Although explicit and implicit processes can both contribute to behavioral outcomes (i.e., drinking), they are not always consistent and at times are at odds with one another (Wiers & Stacy, 2006). The current study found differences in how alcohol affects self-reported motivation to drink and attentional bias at different time points on the BAC curve, suggesting a level of divergence in these two motivational processes that has not been described previously. This divergence is further supported by the lack of significant correlation between attentional bias and subjective desire at any point.
The dissociation between changes in self-reported motivation to drink and attentional bias with respect to time course effects is a new finding and more research will be required before firm conclusions can be drawn. Most importantly it will be necessary to understand whether the increase in attentional bias during the latter phase of the BAC curve is associated with higher risk for drinking. Experimental studies have shown that manipulations of attentional bias (i.e., attentional retraining) cause corresponding changes in drinking behavior (Wiers et al., 2010); these findings support the notion that the alcohol-induced changes in attentional bias reported here should correspond to actual changes in drinking behavior outside of the laboratory. Nonetheless, a more direct test of this hypothesis could involve measuring acute changes in attentional bias after drinking to determine if these changes correspond to differences in drinking behavior when participants are given ad libitum access to alcohol or complete an operant response task to earn alcohol.
Results of this study could provide a better understanding how certain behavioral reactions to alcohol can increase risk for alcohol abuse. The idea that aberrant responses to alcohol may characterize high-risk drinkers is well recognized and has much empirical support (Fillmore, 2003). Most of this work has focused on how alcohol affects subjective states (e.g., stimulation, sedation) and mechanisms of cognitive control (e.g., motor coordination, inhibitory control) in high-risk drinkers (Newlin & Thomson, 1990; Roberts et al., 2013; Schuckit, 1994; Weafer, Fillmore, & Milich, 2009). Given that attentional bias could represent implicit motivation to drink, it appears that drinkers show a satiety effect after drinking, and as satiety fades, they may again be compelled to drink. An interesting possibility is that a reduced duration of satiety after drinking acts as a risk factor for problematic alcohol use, such that drinkers who show briefer satiety effects are more likely to drink heavily than their peers who remain satiated longer. Individuals who show only a brief period of satiety may begin to drink again sooner after their peers with longer periods of satiety, resulting in greater overall rates of consumption. Because we only measured attentional bias at two time points, we cannot test this hypothesis using data from the current study. However, our group has shown that adults with attention-deficit/hyperactivity disorder, a group characterized by high rates of problem drinking, show a rapid increase in attentional bias during the ascending limb after consuming an initial dose of alcohol (Roberts, Fillmore, & Milich, 2012). It is also possible that these individuals experience a very brief period of satiety followed by a rapid increase in attentional bias above what was observe while they were sober. Examining individual differences in satiety duration following alcohol and its relation to drinking habits will be an important question for future research.
Some other limitations also should be considered. First, our study did not include a comparison group of light social drinkers, so it is unclear whether the reported effects would also be seen outside of high-risk binge drinkers. We did not include a comparison group because prior work in our laboratory has found that moderate social drinkers do not show changes in attentional bias after receiving alcohol (Miller & Fillmore, 2011; Weafer & Fillmore, 2013). Examining time course effects in this group would be unlikely to yield additional information beyond these prior studies. Second, it is possible that consuming the placebo beverage itself might increase attentional bias due to the olfactory and gustatory cues that participants likely associated with alcohol consumption. It will be important to determine whether attentional bias following placebo provides an accurate estimate of attentional bias that would be obtained in a no beverage control condition. Third, although our sample size provided ample power to detect responses to alcohol and changes in those responses over time, the power to detect correlations among the study variables was limited.
To our knowledge, this is the first study to consider time course effects on attentional bias following an acute dose of alcohol, and our findings highlight the importance of considering time past dosing when conducting such studies. Although a large amount of research has documented how the effects of alcohol on mechanisms of behavioral control can change as a function of limb of the BAC curve (Schweizer & Vogel-Sprott, 2008; Schweizer et al., 2006), less is known about how the acute effects of alcohol on cognitive processes involved in appetitive responses to alcohol, such as attentional bias, can change over the time course. Indeed, many of the studies in this area have reported conflicting results (Duka & Townshend, 2004; Miller & Fillmore, 2011; Schoenmakers et al., 2008), and this may have to do with differences among the studies in the timing of the task in relation to alcohol dosing procedures. Researchers examining how acute alcohol doses affect appetitive responses to alcohol should be mindful of how these time course effects might influence their findings.
Acknowledgments
This research was supported by National Institute of Alcohol Abuse and Alcoholism grant R01 AA018274 and National Institute of Drug Abuse grant T32 DA035200.
References
- Ames SL, Stacy AW. Implicit cognition in the prediction of substance use among drug offenders. Psychology of Addictive Behaviors. 1998;12:272–281. doi: 10.1037/0893-164x.12.4.272. [DOI] [Google Scholar]
- Chutuape MAD, Mitchell SH, de Wit H. Ethanol preloads increase ethanol preference under concurrent random-ratio schedules in social drinkers. Experimental and Clinical Psychopharmacology. 1994;2:310–318. doi: 10.1037/1064-1297.2.4.310. [DOI] [Google Scholar]
- Cox WM, Blount JP, Rozak AM. Alcohol abusers’ and nonabusers’ distraction by alcohol and concern-related stimuli. American Journal of Drug and Alcohol Abuse. 2000;26:489–495. doi: 10.1081/ada-100100258. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology. 2004;176:353–356. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Field M. Cannabis ‘dependence’ and attentional bias for cannabis-related words. Behavioural Pharmacology. 2005;16:473–476. doi: 10.1097/00008877-200509000-00021. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IHA. A Meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological Bulletin. 2009;135:589–607. doi: 10.1037/A0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC. Acute alcohol effects on inhibitory control and implicit cognition: Implications for loss of control over drinking. Alcoholism: Clinical and Experimental Research. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT. Cognitive preoccupation with alcohol and binge drinking in college students: Alcohol-induced priming of the motivation to drink. Psychology of Addictive Behaviors. 2001;15:325–332. doi: 10.1037/0893-164X.15.4.325. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: Current approaches and findings. Behavioral and Cognitive Neuroscience Reviews. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. Journal of Studies on Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Franken IHA. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Goldberg L. Quantitative studies on alcohol tolerance in man. Acta Psychologica Scandanavia. 1943;5:7–128. [Google Scholar]
- Holloway FA. Low-dose alcohol effects on human behavior and performance. Alcohol, Drugs, and Driving. 1995;11:39–56. [Google Scholar]
- Johnsen BH, Laberg JC, Cox WM, Vaksdal A, Hugdahl K. Alcohol subjects’ attentional bias in the proccessing of alcohol-related words. Psychology of Addictive Behaviors. 1994;20:171–177. doi: 10.1037/0893-164X.20.2.171. [DOI] [Google Scholar]
- Mellanby E. Alcohol: Its absorption into and disappearance from the blood under different conditions. London: Medical Research Committee; 1919. [Google Scholar]
- Miller MA, Fillmore MT. Persistence of attentional bias toward alcohol-related stimuli in intoxicated social drinkers. Drug and Alcohol Dependence. 2011;117:184–189. doi: 10.1016/j.drugalcdep.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Miller NS, Gold MS. Dissociation of “conscious desire” (craving) from and relapse in alcohol and cocaine dependence. Annals of Clinical Psychiatry. 1994;6:99–106. doi: 10.3109/10401239409148988. [DOI] [PubMed] [Google Scholar]
- Moss AC, Albery IP. A dual-process model of the alcohol-behavior link for social drinking. Psychological Bulletin. 2009;135:516–530. doi: 10.1037/A0015991. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA council approves definition of binge drinking. NIAAA Newsletter. 2004 Winter;3 2004. [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychological Bulletin. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): A review of recent research. Alcoholism: Clinical and Experimental Research. 2002;26:272–279. doi: 10.1111/j.1530-0277.2002.tb02534.x. [DOI] [PubMed] [Google Scholar]
- Robbins SJR, Ehrman RN. The role of attentional bias in substance abuse. Behavioral and Cognitive Neuroscience Reviews. 2004;3:243–260. doi: 10.1177/1534582305275423. [DOI] [PubMed] [Google Scholar]
- Roberts W, Fillmore MT, Milich R. Drinking to distraction: Does alcohol increase attentional bias in adults with ADHD? Experimental and Clinical Psychopharmacology. 2012;20:107–117. doi: 10.1037/a0026379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W, Milich R, Fillmore MT. Reduced acute recovery from alcohol impairment in adults with ADHD. Psychopharmacology (Berl) 2013;228:65–74. doi: 10.1007/s00213-013-3016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook SE, Hine DW, Thorsteinsson EB. Implicit cognition and substance use: A meta-analysis. Addictive Behaviors. 2008;33:1314–1328. doi: 10.1016/j.addbeh.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Rose AK, Duka T. Effects of dose and time on the ability of alcohol to prime social drinkers. Behavioural Pharmacology. 2006;17:61–70. doi: 10.1097/01.fbp.0000189814.61802.92. [DOI] [PubMed] [Google Scholar]
- Ryan F. Attentional bias and alcohol dependence: A controlled study using the modified Stroop paradigm. Addictive Behaviors. 2002;27:471–482. doi: 10.1016/s0306-4603(01)00183-6. doi: S0306-4603(01)00183-6. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, Delafuente JR, Grant M. Development of the Alcohol-Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schoenmakers T, Wiers RW, Field M. Effects of a low dose of alcohol on cognitive biases and craving in heavy drinkers. Psychopharmacology. 2008;197:169–178. doi: 10.1007/s00213-007-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers TM, Wiers RW. Craving and attentional bias respond differently to alcohol priming: A field study in the pub. European Addiction Research. 2010;16:9–16. doi: 10.1159/000253859. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low-level of response to alcohol as a predictor of future alcoholism. American Journal of Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: A review of acute tolerance and recovery of cognitive performance. Experimental and Clinical Psychopharmacology. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology. 2006;31:1301–1309. doi: 10.1038/sj.npp.1300941. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, Van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) Journal of Studies on Alcohol. 1975;36:117. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Personality and Social Psychology Review. 2004;8:220–247. doi: 10.1207/s15327957pspr0803_1. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95:S145–S153. doi: 10.1046/j.1360-0443.95.8s2.3.x. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology. 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Prediction of blood alcohol concentrations in human subjects: Updating the Widmark Equation. Journal of Studies on Alcohol. 1981;42:547–556. doi: 10.15288/jsa.1981.42.547. [DOI] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Acute alcohol effects on attentional bias in heavy and moderate drinkers. Psychology of Addictive Behaviors. 2013;27:32–41. doi: 10.1037/A0028991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT, Milich R. Increased sensitivity to the disinhibiting effects of alcohol in adults with ADHD. Experimental and Clinical Psychopharmacology. 2009;17:113–121. doi: 10.1037/A0015418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Kordts R, Houben K, Strack F. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction. 2010;105:279–287. doi: 10.1111/j.1360-0443.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Stacy AW. Implicit cognition and addiction. Current Directions in Psychological Science. 2006;15:292–296. doi: 10.1111/j.1467-8721.2006.00455.x. [DOI] [Google Scholar]


