ABSTRACT
Human cytomegalovirus (HCMV) persistently infects 40% to 100% of the human population worldwide. Experimental and clinical evidence indicates that humoral immunity to HCMV plays an important role in restricting virus dissemination and protecting the infected host from disease. Specific immunoglobulin preparations from pooled plasma of adults selected for high titers of HCMV antibodies have been used for the prevention of CMV disease in transplant recipients and pregnant women. Even though incubation of HCMV particles with these preparations leads to the neutralization of viral infectivity, it is still unclear whether the antibody-treated HCMV particles (referred to here as HCMV-Ab) enter the cells and modulate antiviral immune responses. Here we demonstrate that HCMV-Ab did enter macrophages. HCMV-Ab did not initiate the expression of immediate early antigens (IEAs) in macrophages, but they induced an antiviral state and rendered the cells less susceptible to HCMV infection upon challenge. Resistance to HCMV infection seemed to be due to the activation of intrinsic restriction factors and was independent of interferons. In contrast to actively infected cells, autologous NK cells did not degranulate against HCMV-Ab-treated macrophages, suggesting that these cells may not be eliminated by innate effector cells. Interestingly, HCMV-Ab-treated macrophages stimulated the proliferation of autologous adaptive CD4+ and CD8+ T cells. Our findings not only expand the current knowledge on virus-antibody immunity but may also be relevant for future vaccination strategies.
IMPORTANCE Human cytomegalovirus (HCMV), a common herpesvirus, establishes benign but persistent infections in immunocompetent hosts. However, in subjects with an immature or dysfunctional immune system, HCMV is a major cause of morbidity and mortality. Passive immunization has been used in different clinical settings with variable clinical results. Intravenous hyperimmune globulin preparations (IVIg) are obtained from pooled adult human plasma selected for high anti-CMV antibody titers. While HCMV neutralization can be shown in vitro using different systems, data are lacking regarding the cross-influence of IVIg administration on the cellular immune responses. The aim of this study was to evaluate the effects of IVIg on distinct components of the immune response against HCMV, including antigen presentation by macrophages, degranulation of innate natural killer cells, and proliferation of adaptive CD4+ and CD8+ T cells.
KEYWORDS: adaptive immunity, cytomegalovirus, innate immunity, intrinsic defenses, macrophages, neutralizing antibodies
INTRODUCTION
Human cytomegalovirus (HCMV) is an enveloped virus that belongs to the family Herpesviridae. This virus infects the majority of humans worldwide and establishes a persistent infection. Postnatally, HCMV rarely causes severe complications in healthy individuals. However, it is a significant cause of morbidity and mortality in immunologically immature and severely immunocompromised individuals (1). These features reveal the importance of constant immune surveillance for control of HCMV. The adaptive immune response against HCMV engages both humoral and cellular immunity, is long-lasting, and is among the strongest ever documented in humans (2). Healthy HCMV carriers possess neutralizing antibodies targeting virus envelope glycoproteins (3) as well as nonneutralizing antibodies targeting tegument and nonstructural proteins. HCMV neutralizing antibodies are directed mostly against glycoprotein B (gB) or the glycoprotein complex gH-gL-UL128-UL130-UL131A and are able to block the infectivity of cell-free virus in fibroblasts and in endothelial, epithelial, and myeloid cells (4, 5). Even though the exact neutralization mechanism is not fully understood, it is believed that through binding at virion proteins that are important for virus entry into target cells, these antibodies can block virion attachment, entry, and uncoating (6). Whether these noninfectious complexes formed by antibody-treated HCMV particles (referred to here as HCMV-Ab) can initiate immune responses, such as antibody-dependent cellular phagocytosis, antibody-dependent cellular cytotoxicity, and activation of innate and adaptive effector cells, such as NK and T cells, respectively, is still not known. Macrophages express various Fc receptors and play important roles in mediating the clearance of IgG-opsonized foreign materials, such as particle-antibody complexes, from the human body (7). At the same time, HCMV can establish a productive infection in macrophages (8), inducing substantial changes in morphology, immunophenotype, and secretory properties (9). Since we and others have reported previously that HCMV-infected macrophages can stimulate NK cell activity and present antigens to T cells (9–11), in this study we investigated whether HCMV-Ab can trigger macrophage immune functions. HCMV particles were incubated with IgG preparations for intravenous use (IVIg), which are frequently used for the prevention of CMV disease in transplant recipients and pregnant women, and we investigated whether HCMV-Ab were taken up by macrophages and whether they exerted immunomodulatory effects on intrinsic, innate, and adaptive antiviral responses.
Taken together, our findings demonstrate that HCMV-Ab enter macrophages but are not able to initiate the replication cycle. However, HCMV-Ab induce an antiviral state in macrophages, rendering them less susceptible to challenge with infectious HCMV. Importantly, after HCMV-Ab uptake, macrophages can present input viral antigens to adaptive T cells without initiating interferon and NK cell responses.
RESULTS
HCMV-Ab enter macrophages but do not initiate virus replication.
We prepared antibody-treated HCMV particles (HCMV-Ab) by incubating cell-free virus with polyclonal HCMV antibodies for 30 min at 37°C. To standardize the preparation, we used HCMV strain TB40/E and a commercially available intravenous preparation of pooled immune globulin. Primary M2 macrophages either were treated with cell-free virus or with HCMV-Ab for 1 h. Then the cells were washed and were incubated with fresh media for an additional 23 h. Twenty-four hours after infection (24 hpi), macrophages were fixed and were stained for immediate early antigens (IEAs) to identify infected cells. In line with previous studies (4, 11), HCMV pooled antibodies completely neutralized the infectivity of cell-free virus in macrophages (Fig. 1A). A comparable neutralizing effect was observed when cell-free HCMV was incubated with human serum obtained from HCMV-seropositive donors but not with serum from HCMV-seronegative donors (11).
FIG 1.
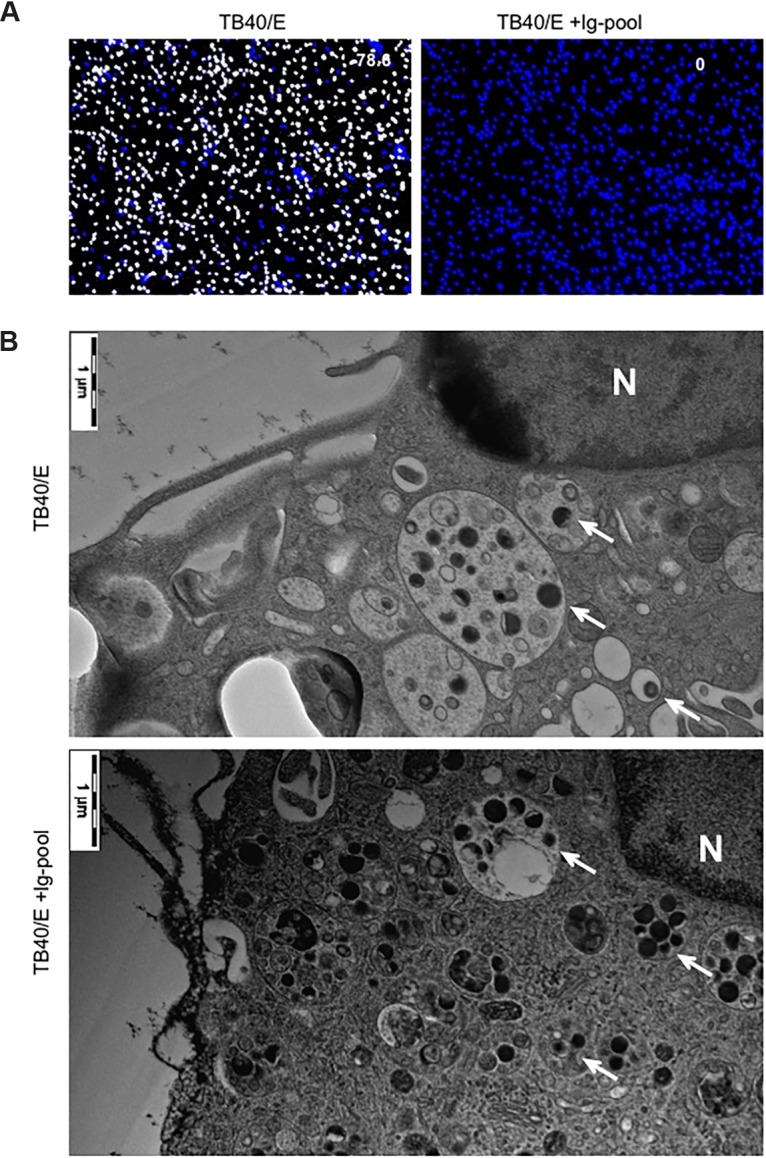
HCMV-Ab enter macrophages. A medium containing cell-free TB40/E or a combination of cell-free TB40/E and an Ig pool (TB40/E + IgG-pool) was incubated at 37°C for 30 min. The amount of TB40/E was calculated to reach an MOI of 5. Afterward, the two types of preparations were added to macrophages (1 × 105) in a volume of 100 μl for 1 h. Cells were washed twice and were replenished with new medium. (A) At 24 h posttreatment, macrophages were stained for quantitative evaluation of IEA positive cells (number in the top right corner of the images). The presence of HCMV IEA (white fluorescence) indicates infected cells, and cell nuclei are stained with DAPI (blue fluorescence). Results of one experiment representative of three are shown. (B) At 3 h after treatment, macrophages were washed, fixed by high-pressure freezing, freeze-substituted, plastic embedded, and analyzed by electron microscopy. Round black dots indicate HCMV particles (arrows), and N indicates the nucleus.
First, we analyzed whether HCMV-Ab could enter macrophages. To facilitate the detection of input viral particles by electron microscopy, we applied large amounts of cell-free virus (multiplicity of infection [MOI], 5), which, without antibodies, resulted in a >75% infection rate in macrophages at 24 hpi. After 3 h, large numbers of input viral particles could be found in intracellular vesicles in both TB40/E- and HCMV-Ab-treated macrophages (Fig. 1B). Since HCMV-Ab enter macrophages without leading to IEA expression and productive infection, we assume that HCMV-Ab are processed differently from infectious viral particles inside the cell. In order to analyze this in more detail, we set out to investigate whether intracellular HCMV-Ab are capable of inducing intrinsic immune responses (e.g., perturbation of viral restriction factors), interferon secretion, NK cell responses, and T cell responses.
HCMV-Ab-treated macrophages upregulate the antiviral intrinsic restriction factor IFI16.
Since IFN-γ inducible protein 16 (IFI16), promyelocytic leukemia (PML) protein, and Sp100 have been described previously as restriction factors of HCMV infection (12–14), we investigated whether the expression of these factors was affected by HCMV-Ab treatment. Macrophages were either left untreated or treated with pooled immune globulins (Ig-pool), cell-free TB40/E, or HCMV-Ab for 1 h, after which they were incubated with fresh medium for an additional 23 h. After 24 h of treatment, cells were collected for Western blot analysis. HCMV-Ab-treated macrophages upregulated IFI16 relative to its expression in mock-treated cells (Fig. 2A), but not PML or Sp100 (Fig. 2B). Macrophages treated with antibodies alone also upregulated the expression of IFI16, thus suggesting that the IFI16 upregulation in HCMV-Ab-treated macrophages was due to the presence of antibodies. Of note, macrophages treated with cell-free TB40/E presented an opposite pattern for these restriction factors, exhibiting downregulation of IFI16 and upregulation of PML and Sp100 (Fig. 2).
FIG 2.
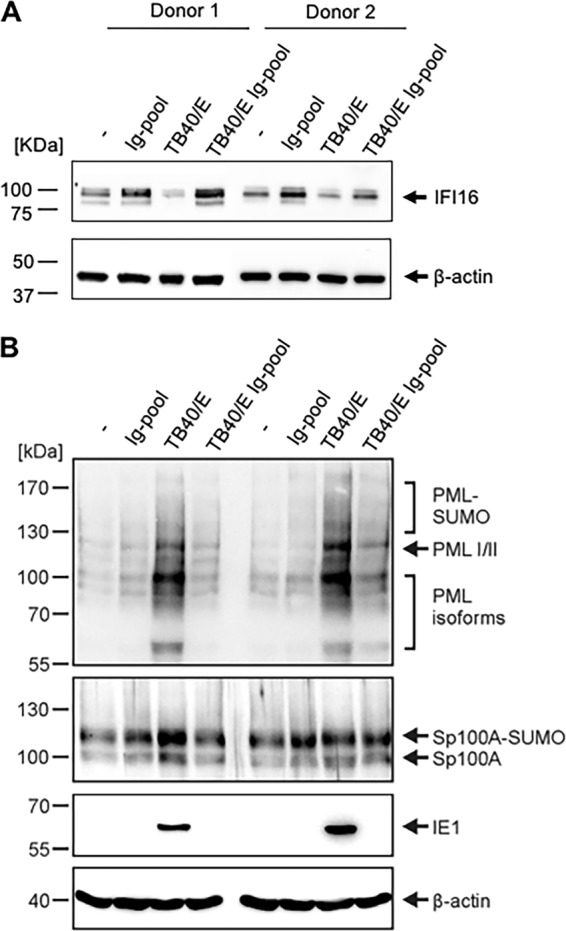
IFI16, PML, and Sp100 expression in HCMV-Ab-treated macrophages. Medium alone and medium containing either an Ig pool, cell-free TB40/E, or the combination of cell-free TB40/E and an Ig pool were incubated at 37°C for 30 min. The amount of TB40/E was calculated to reach an MOI of 3. The four media were then added to macrophages (6 × 105) in a volume of 600 μl for 1 h in 24-well plates. Cells were washed twice and were replenished with RMPI 1640 medium. At 24 h posttreatment, the total levels of IFI16 (A), PML, and Sp100 (B) were evaluated by Western blot analysis in cell lysates.
HCMV-Ab-treated macrophages do not induce natural killer cell or interferon responses.
The hallmarks of antiviral innate immunity are NK cell and interferon responses. NK cells recognize HCMV-infected cells, and it has been shown previously that NK cells can release their cytotoxic granules in response to HCMV-infected autologous macrophages (10, 11). We therefore analyzed whether NK cells could degranulate in response to noninfected, HCMV-Ab-treated macrophages. Purified autologous NK cells were added to macrophages that were either left untreated or treated with pooled immune globulins (Ig-pool), cell-free TB40/E, or HCMV-Ab-treated macrophages (Fig. 3A). As shown in Fig. 3B, NK cells degranulated when cocultivated with infected macrophages but not when coincubated with macrophages treated with Ig-pool alone or HCMV-Ab.
FIG 3.
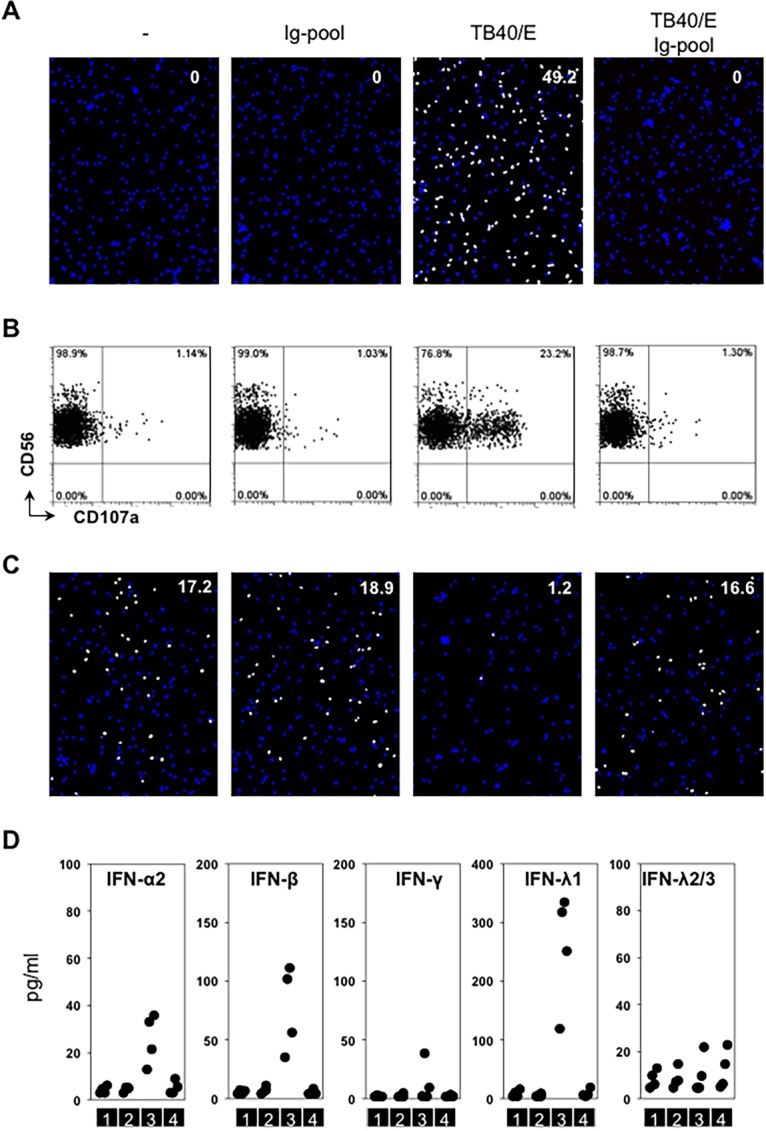
HCMV-Ab do not induce innate immune responses. (A and B) The four types of media described in the legend to Fig. 2 were added to 1 × 105 macrophages seeded in 96-well plates in a volume of 100 μl for 60 min (TB40/E MOI, 3). Cells were washed twice and were incubated in RPMI 1640 medium. After 24 h, treated cells were either stained for quantitative evaluation of IEA positive cells (number in the top right corner of the images) (A) or incubated with autologous purified NK cells (effectors) at an effector-to-target cell ratio of 1 in the presence of an anti-CD107a antibody. NK degranulation was evaluated after 5 h of coculture (B). (C) The supernatants from each condition described for panel A were collected at 24 h posttreatment. They were filtered with a 0.1-μm filter and were transferred to uninfected autologous macrophages (1 × 105) for 24 h. Then the supernatants were discarded, and cells were first challenged with cell-free TB40/E in a volume of 100 μl for 60 min (MOI, 0.3) and then washed twice and incubated in new media. At 24 h postchallenge, IEA expression was evaluated. Results of one experiment representative of four are shown. (D) The supernatants from each condition described for panel A were collected at 24 h posttreatment. The concentrations of interferons in supernatants were tested by bead-based ELISA. Sections 1, 2, 3, and 4 indicate four sequential treatment groups: medium alone, medium containing an Ig pool, medium containing cell-free TB40/E, and medium containing a combination of cell-free TB40/E and an Ig pool.
Next, we asked whether these differently treated macrophages could secrete interferons and/or induce resistance to cell-free HCMV infection. Conditioned media obtained from macrophages treated for 24 h as described for Fig. 3A were collected, filtered to remove residual viral particles, and added to uninfected autologous macrophages for an additional 24 h. Then these treated macrophages were further challenged with a low dose (MOI, 0.3) of cell-free virus. As shown in Fig. 3C, susceptibility to HCMV challenge was comparable for all treated macrophages except for those treated with the supernatants obtained from actively infected macrophages. Macrophages exposed for 24 h to supernatants obtained from actively infected macrophages were significantly less susceptible to cell-free virus (Fig. 3C). Since interferons are capable of inducing antiviral effects in target cells (15, 16), we measured the amounts of type I, II and III interferons in the macrophage-conditioned supernatants used for the transfer experiments. As shown in Fig. 3D, active HCMV infection of macrophages (condition 3) was accompanied by secretion of interferon alpha, beta, and lambda 1 in all donors tested and secretion of interferon gamma in one donor out of four. In contrast, macrophages treated with the Ig pool alone or with HCMV-Ab (Fig. 3D, conditions 2 and 4, respectively) did not secrete any type of interferon and resembled untreated macrophages (Fig. 3D, condition 1). Taken together, these results reveal that HCMV-Ab-treated macrophages do not induce NK cell or interferon responses.
HCMV-Ab-treated macrophages induce the proliferation of adaptive T cells.
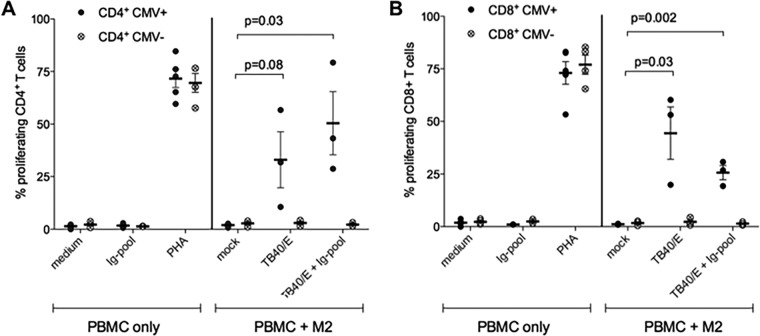
Macrophages are professional antigen-presenting cells, and in our previous study, we demonstrated that HCMV-infected macrophages can stimulate the proliferation of autologous T cells obtained from HCMV-seropositive subjects (9). Since HCMV-Ab enter macrophages, we hypothesized that HCMV-Ab-treated macrophages could present antigens from input virus to T cells and induce their proliferation. By using the autologous T cell proliferation assay described previously (9), we tested whether HCMV-Ab-treated macrophages induce T cell proliferation. As shown in Fig. 4, cell-free HCMV and HCMV-Ab-treated macrophages stimulated the proliferation of autologous CD4- and CD8-positive T cells obtained from HCMV-seropositive (CMV+) blood donors but not that of T cells from HCMV-seronegative (CMV−) blood donors. Mock-treated macrophages did not stimulate T cell proliferation independently of the HCMV serostatus of the cell donor. These results indicate that HCMV-Ab-treated macrophages can present input viral antigens and stimulate the proliferation of adaptive T cells.
FIG 4.
HCMV-Ab induce adaptive immune responses. Macrophages derived from HCMV-seropositive or -seronegative blood donors (CMV+ and CMV−, respectively) were either left untreated (mock), infected with TB40/E at an MOI of 3, or stimulated with TB40/E and an Ig pool. After 1 day, macrophages were harvested, irradiated (30 cGy), and added as stimulators at a ratio of 1:8 to autologous CFSE-labeled PBMC. As controls, PBMC alone were either left untreated (medium) or stimulated with an Ig pool alone or 5 μg/ml phytohemagglutinin (PHA). Six days later, PBMC were harvested and were stained with fluorescently labeled antibodies directed against CD4 and CD8, and the percentage of proliferating CFSElow PBMC was assessed by flow cytometry. Mean values ± standard errors of the means from at least 3 CMV-seronegative and 3 CMV-seropositive blood donors are shown. Each dot represents cells obtained from one blood donor.
HCMV-Ab induce an antiviral status in primary macrophages.
While HCMV-infected macrophages secreted type I and III interferons, which can induce an anti-HCMV state in uninfected macrophages, HCMV-Ab-treated macrophages did not produce interferons (Fig. 3D). We decided to assess whether the uptake of noninfectious HCMV-Ab could alter macrophage susceptibility to HCMV infection via an interferon-independent mechanism.
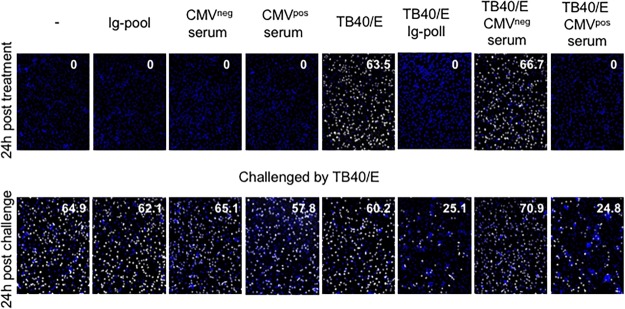
To test this hypothesis, macrophages were either left untreated or treated with Ig pool antibodies, cell-free TB40/E, or HCMV-Ab for 1 h; then they were incubated with fresh medium for an additional 23 h. After 24 h of treatment, while a portion of the cells was fixed and stained to quantify IEA-positive infected cells (Fig. 5A), the other portion of cells was challenged with cell-free infectious virus (Fig. 5B). As shown in Fig. 5B (and Fig. 5D, condition 4), HCMV-Ab-treated macrophages were less susceptible to infection by cell-free HCMV than macrophages that were left untreated or stimulated with Ig pool antibody alone (Fig. 5B and D, conditions 1 and 2, respectively). In addition to the Ig pool, we also tested sera obtained from HCMV-seronegative and -seropositive individuals, and we observed that HCMV resistance could be elicited only when seropositive human sera containing specific HCMV antibodies were used (Fig. 6). To investigate whether genetically pure and stable HCMV laboratory strains can also induce this antiviral status, we used BAC4, the bacterial artificial clones derived from TB40/E, and we could confirm that BAC4-Ab-treated macrophages were also less susceptible to cell-free virus challenge (data not shown).
FIG 5.
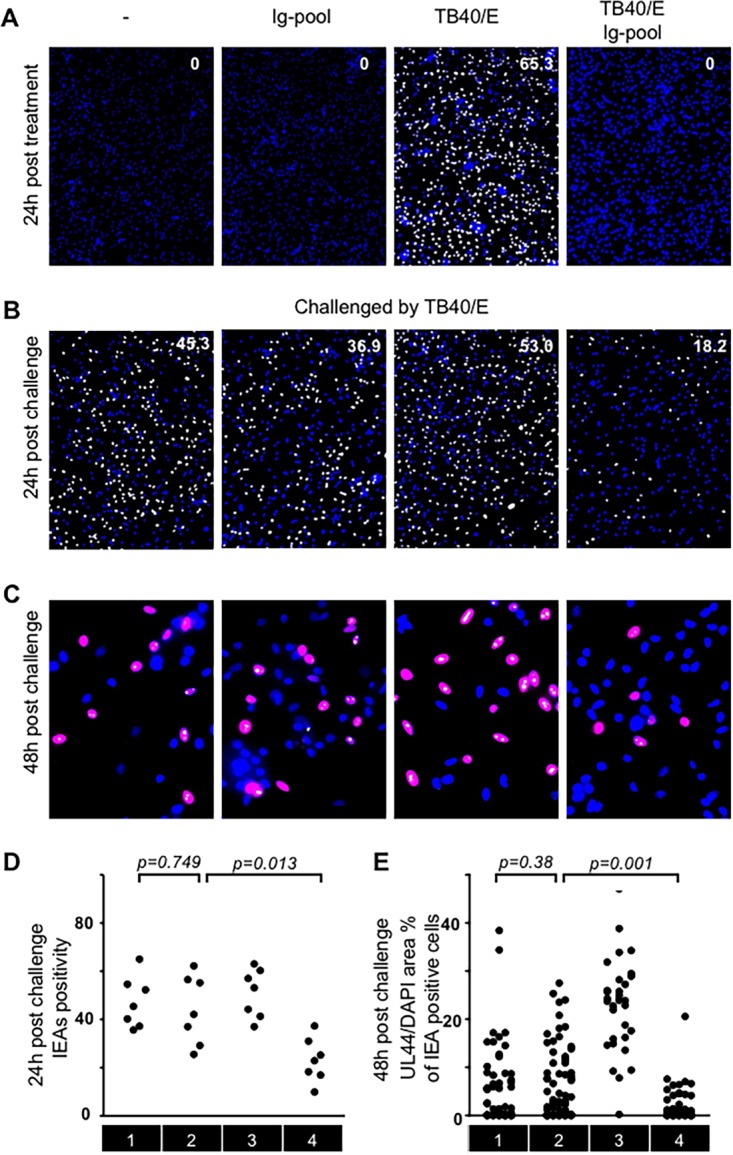
HCMV-Ab-treated macrophages are less susceptible to HCMV infection. (A, B, and D) The four types of media described in the legend to Fig. 2 were added to macrophages (1 × 105) in a volume of 100 μl for 60 min (MOI, 3). Cells were washed twice and were incubated in RPMI 1640 medium. After 24 h, treated cells were either stained for quantitative evaluation of IEA positive cells (number in the top right corner of the images) (A) or challenged by cell-free TB40/E in a volume of 100 μl for 60 min at an MOI of 3. Then the cells were washed twice and were replenished with new media. Twenty-four hours later, IEA expression was evaluated (B), and cumulative results from different donors were summarized (D). (C and E) At 48 h postchallenge, the expression of IEA and pUL44 was evaluated. The presence of HCMV IEA (pseudopink fluorescence) indicates infected cells, pUL44 signals are indicated by pseudowhite fluorescence, and cell nuclei are stained blue (DAPI). Ratios of pUL44 fluorescence to nuclear area were analyzed in IEA-positive cells (C), and cumulative results from different donors were summarized (E). Sections 1, 2, 3, and 4 indicate four sequential treatment groups: medium alone, medium containing an Ig pool, medium containing cell-free TB40/E, and medium containing a combination of cell-free TB40/E and an Ig pool.
FIG 6.
Serum from CMV-seropositive but not CMV-seronegative subjects is comparable to the IgG pool. Medium alone and media containing either an Ig pool, CMV-seronegative serum, CMV-seropositive serum, cell-free TB40/E, or a combination of cell-free TB40/E with an Ig pool or sera were incubated at 37°C for 30 min prior to inoculation with macrophages. Media were added to macrophages (1 × 105) in a volume of 100 μl for 60 min (MOI, 3). Cells were washed twice and were incubated in RPMI 1640 medium. After 24 h, treated cells were either stained for quantitative evaluation of IEA positive cells (number in the top right corner of the images) or challenged by cell-free TB40/E in a volume of 100 μl for 60 min at an MOI of 3. Then cells were washed twice and were replenished with new medium. Twenty-four hours later, IEA expression was evaluated.
HCMV genes are known to be expressed in a temporally ordered cascade: first immediate early (IE), then early (E) and early-late (E-L), and finally late (L) proteins (17). Since 20% of HCMV-Ab-treated macrophages could still be infected by cell-free virus challenge, we further evaluated the effect of HCMV-Ab treatment on the HCMV protein expression cascade. For this purpose, we established a new approach to quantify the kinetic accumulation of HCMV proteins and to follow the progression of the viral replication cycle. In contrast to the classical investigations performed by Western blotting, this method is based on the measurement of the ratio between the total nuclear area and the area occupied by fluorescently labeled viral proteins (Fig. 5C) and allows a quantitative analysis irrespective of the numbers of infected cells present in the whole cell culture. Since the HCMV early protein pUL44 accumulates predominantly in infected nuclei (8), we analyzed pUL44 expression at 48 hpi in IEA-positive macrophages present in cultures that were either left untreated or treated with the Ig pool, cell-free TB40/E, or HCMV-Ab. The ratio of the pUL44 signal to the DAPI signal was greater in infected macrophages (Fig. 5C and E, condition 3), because these cells were infected for 72 h and accumulated the largest amount of the viral DNA polymerase accessory protein pUL44. The pUL44/DAPI ratio was significantly lower in HCMV-Ab-treated IEA-positive macrophages (Fig. 5C and E, condition 4) than in macrophages treated with antibody alone or untreated cells (Fig. 5E, conditions 2 and 1, respectively). These data indicate not only that HCMV-Ab-treated macrophages are less susceptible to infection, as evidenced by the expression of IEAs at the beginning of the viral replication cycle, but that also the HCMV protein expression cascade is delayed in macrophages upon HCMV-Ab treatment. Interestingly, when epithelial cells were treated with HCMV-Ab, we did not observe the induction of the antiviral state described for macrophages (Fig. 7), suggesting that the antiviral molecular mechanisms elicited by HCMV-Ab might be cell type specific.
FIG 7.
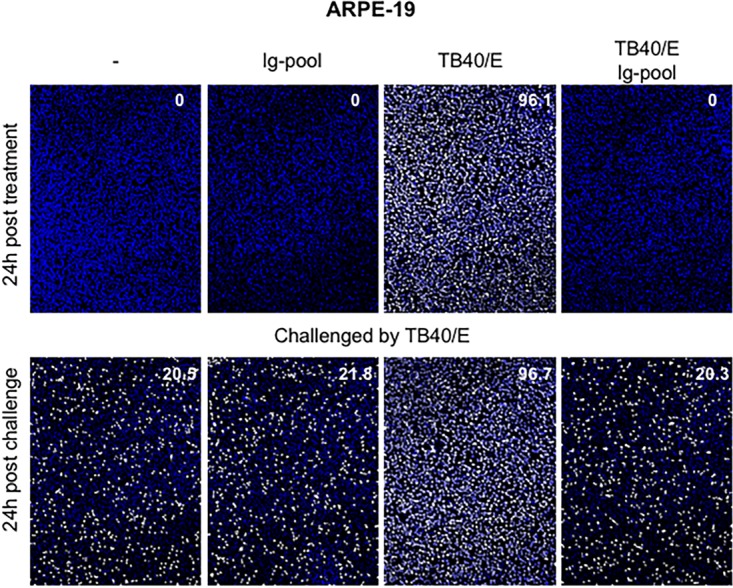
HCMV-Ab-treated epithelial cells are not resistant to HCMV challenge. The four types of media described in the legend to Fig. 2 were added to ARPE-19 retinal pigment epithelial cells (1 × 105) in a volume of 100 μl for 60 min (MOI, 3). Cells were washed twice and were incubated in their respective culture media. After 24 h, treated cells were either stained for quantitative evaluation of IEA positive cells (number in the top right corner of the images) or challenged by cell-free TB40/E in a volume of 100 μl for 60 min at an MOI of 3. Cells were then washed twice and were replenished with new medium. Twenty-four hours later, IEA expression was evaluated by indirect immunofluorescence.
DISCUSSION
The binding of viral particles to neutralizing antibodies is supposed to change the ability of the virus to interact with its cognate cellular receptors and to result in defective or abortive viral entry, increased intracellular degradation, modulated receptor signaling, and eventually inflammatory responses. Beyond their role in preventing infection, antibody-Fc receptor interactions have also been implicated in the regulation of different immune responses, such as antibody-dependent cellular phagocytosis/cytotoxicity, as well as activation of innate and adaptive effector cells.
Since human macrophages are susceptible to HCMV infection in vivo as well as in vitro (18, 19) and are equipped with a broad range of Fc receptors, we investigated whether immune complexes formed by HCMV particles and IVIg are taken up by macrophages and processed for presentation to innate and adaptive effector cells, such as NK cells and T lymphocytes. Our data reveal that HCMV particles that were preincubated with antibodies are able to enter macrophages. Furthermore, they induce an interferon-independent antiviral state in these cells and stimulate the proliferation of autologous T cells. To the best of our knowledge, our study is the first to assess interferon production from HCMV-infected macrophages at the protein level. Notably, we first revealed that HCMV-infected cells produce interferon lambda 1, which can exert potent antiviral activities mainly on epithelial cells (20).
Recently, our understanding of antibody-mediated immunity has been enlarged by the discovery that viruses can also be neutralized in the cell cytoplasm by a mechanism called “antibody-dependent intracellular neutralization.” Mallery, McEwan, and colleagues reported that mammalian cells express a cytosolic IgG receptor, tripartite motif-containing 21 (TRIM21), which binds antibody-bound nonenveloped viruses and bacteria with very high affinity and targets them for proteasome-dependent intracellular degradation (21, 22). Of note, uptake of antibody-coated nonenveloped viruses was reported to result in the production of proinflammatory cytokines and interferon as well as in the modulation of potential NK cell ligands and induction of an antiviral status (22). Here we provide evidence that HCMV-Ab induce neither interferons nor NK cell responses. Moreover, while in the previous studies it was observed that antibody-coated nonenveloped viruses could still infect target cells and that the infection rates could be increased in TRIM21 knockout cells (21), in our study, HCMV-Ab did enter macrophages but did not initiate the virus replication cycle. Taken together, our data suggest that the HCMV-Ab-induced antiviral status in macrophages might be TRIM21 independent.
The molecular mechanisms leading to macrophage resistance to infection by cell-free virus upon HCMV-Ab uptake are still not fully elucidated, and further experiments are necessary to understand whether virion attachment, entry, genome delivery, and genome expression are impeded. Previous reports on superinfection exclusion (23), a phenomenon by which the first virus infecting a cell can prevent subsequent viruses from further infecting the same cell, would suggest two possibilities. On the one hand, the uptake of antibody-coated HCMV particles could induce changes in the amount and/or availability of the receptor(s) used by HCMV to enter macrophages, thus reducing viral entry during challenge with infectious cell-free virus (mechanism of receptor interference). Alternatively, intrinsic restriction factors could be activated after HCMV-Ab uptake and thus would restrict not the entry step but, instead, the expression of IEAs. Both mechanisms have already been reported upon herpes simplex virus 1 (HSV-1) infection (24, 25), but in contrast to our setting, in which HCMV-Ab entry is sufficient to prevent subsequent HCMV infection, HSV-1 superinfection exclusion was dependent on viral gene expression. Since IFI16, PML, and Sp100 are not involved in the induction of the antiviral state, and moreover, HCMV-Ab-treated macrophages exhibited delayed HCMV protein expression, it is likely that additional and still unidentified restriction factors account for the resistance. HCMV-Ab-treated macrophages did not secrete any type of interferons. Interferon regulatory protein 3 (IRF3), previously recognized as capable of eliciting anti-HSV-1 responses via IFN-independent mechanisms (26–28), did not undergo phosphorylation or nuclear translocation in our experimental settings (data not shown), suggesting that IRF3 may not be involved in the antiviral state elicited by HCMV-Ab. In further attempts to identify the underlying mechanisms, it will be important to take into account the fact that HCMV-Ab-treated epithelial cells did not present the antiviral state described in macrophages, suggesting that either cell type-specific restriction factors or cell type-specific uptake mechanisms could explain this phenomenon.
While previous studies revealed that HCMV enters dendritic cells (DCs) via a macropinocytosis-like pathway (29) and type 2 macrophages via a megapinocytosis endocytic pathway (30), the pathway used by HCMV-Ab to enter macrophages is still unclear. Since macrophages express various Fc receptors both on the plasma membrane and in the cytoplasm (CD64/FcγRI, CD32/FcγRII, CD16/FcγRIII, and TRIM21), Fc receptor-mediated HCMV-Ab entry into macrophages cannot be excluded. HCMV transmission between cells is highly cell associated in vivo (31–33). This gives HCMV an advantage in transmission by shielding the virus from neutralizing antibodies. Current HCMV therapeutic approaches are focused on the generation of neutralizing antibodies (3, 34–37), some of which are potent for neutralizing cell-free virus in vitro but may have no effect on cell-associated transmission. Our observation offers a new approach to developing therapeutic interventions by using viral particles and neutralizing antibodies to trigger antiviral responses without introducing infections. Future experiments utilizing anti-HCMV monoclonal antibodies (MAbs) with defined epitope specificities may be helpful for implementing the HCMV-Ab-induced immune responses.
In summary, previous studies have demonstrated that immune complexes can be degraded in phagocytes and processed for antigen presentation, thus allowing the induction of antiviral immune responses. Our study confirms this knowledge for antibody-treated HCMV particles and additionally reveals that no NK cell response was triggered, while at the same time, HCMV-Ab-treated macrophages were able to stimulate both CD4+ and CD8+ T-cell proliferation. We have shown previously that macrophages treated with UV-inactivated and nonreplicating virus elicit the proliferation of autologous T cells (9), indicating that cross-presentation is active in macrophages and that major histocompatibility complex class I (MHC-I) epitopes can be processed in the absence of viral gene expression. Since the abilities of macrophages to cross-present antigens from UV-inactivated HCMV or HCMV-Ab are comparable, it is possible that UV-inactivated viral particles as well as HCMV-Ab escape the endosomes, reach the cytoplasm, and are processed by the immunoproteasome. Alternatively, since we observed abundant multilamellar vesicular structures in macrophages treated with HCMV-Ab, which were not present in infected macrophages, we hypothesize that an MHC-I-rich compartment (corresponding to the multilamellar vesicular structures) fuses with the endosomes containing viral antigens and in this way leads to TAP-independent loading of MHC molecules. Our study might be relevant to efforts to optimize vaccine strategies and enhance antiviral immune responses.
MATERIALS AND METHODS
Study subjects and cells.
Buffy coats obtained from healthy blood donors as well as human AB serum obtained from HCMV-seronegative donors were purchased from the Transfusion Center of the Ulm University Hospital (Institut für Klinische Transfusionsmedizin und Immungenetik Ulm GmbH, Ulm, Germany). Ex vivo monocytes, macrophages, peripheral blood mononuclear cells (PBMC), and purified NK cells were cultured in RPMI 1640 medium (GIBCO/Invitrogen) containing 10% fetal calf serum (FCS; GIBCO/Invitrogen). Macrophage colony-stimulating factor (M-CSF; R&D Systems)-polarized M2 macrophages were obtained from human monocytes as described previously (9). NK cells were enriched by negative selection from PBMC (Miltenyi). Retinal pigment epithelial cells (ARPE-19 cells) were cultured in minimal essential medium (MEM; GIBCO/Invitrogen) containing 10% fetal bovine serum (FBS).
Preparation of viral stocks and infection of macrophages.
HCMV strain TB40/E was propagated in human foreskin fibroblasts (HFFs). For the preparation of virus stocks, supernatants containing cell-free virus were harvested at 5 to 7 days postinfection. Cellular debris was removed by centrifugation at 2,800 × g for 10 min, and virus particles were pelleted from the supernatants by ultracentrifugation (70,000 × g for 70 min at 10°C). Virus pellets were resuspended in MEM containing the cryopreservant sucrose buffer, aliquoted, and stored at −80°C. The infectious titers of TB40/E preparations were determined as described previously (38). Macrophages were infected using the indicated MOI for 1 h, after which they were washed and replenished with RPMI 1640 medium.
HCMV antibodies and cytokine detection.
Pooled intravenous immune globulin (Gamunex; Talecris Biotherapeutics) was commercially purchased and was used at a 1:20 dilution. All sera/plasma were heated to 56°C for 30 min, stored at −20°C, and used at a 1:10 dilution. HCMV IgG serology was determined with an enzyme-linked fluorescence assay (Vidas CMV IgG assay; bioMérieux). Interferons were quantified by a bead-based immunoassay (LEGENDplex; Biolegend) according to the manufacturer's recommendations.
Fluorescence staining and nuclear-area-based quantitative analysis.
To determine the infection rates, macrophages were fixed at 24 h postinfection with 80% acetone and were incubated with monoclonal antibodies against HCMV immediate early antigens (IEAs) (Argene Biosoft), followed by staining with Alexa Fluor 555 (AF555)- or AF488-conjugated goat anti-mouse immunoglobulins (Molecular Probes/Invitrogen). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Roche). The numbers of IEA and DAPI signals were determined in three frames per well using Photoshop (Adobe). The infection rate was calculated as the number of IEA-positive nuclei per hundred DAPI-positive cells.
To costain pUL44 and IEAs, p52 (pUL44, BS510; kindly provided by Bodo Plachter) was used as the primary antibody, followed by staining with AF555-conjugated goat anti-mouse immunoglobulins (Molecular Probes/Invitrogen). AF488-conjugated IEA was used to evaluate IEA expression (8B1.2; Millipore). To determine the ratio of the pUL44 area to the nuclear area, five frames were randomly collected in both fluorescence and DAPI channels with a 40× objective. Area analysis was performed by using ImageJ (NIH). We applied a semiautomated image analysis protocol for this purpose (39). Briefly, fluorescence or DAPI signals in a single frame were converted to grayscale and were adjusted manually by threshold settings. Then the size of the analyzed area was automatically calculated in square inches.
Western blotting.
Cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were blotted onto polyvinylidene difluoride membranes. Protein bands on the blots were visualized by chemiluminescence (Millipore). The following primary antibodies were used for Western blotting: a monoclonal antibody against immediate early antigen 1 (IEA1; antibody 63-27; kindly provided by William Britt), a β-actin antibody (AC-15; Sigma-Aldrich), and an IFI16 antibody (IG7; Santa Cruz). PML and Sp100 were detected as described previously (40).
Electron microscopy.
Macrophages were seeded overnight on carbon-coated sapphire discs with a finder grid pattern (diameter, 3 mm; Engineering Office, M. Wohlwend GmbH, Sennwald, Switzerland) prior to treatments. Treated macrophages were cultivated for an additional 3 h and were then high-pressure frozen, freeze-substituted, and finally embedded in epoxy resin as described previously (9). The samples were imaged with a Zeiss transmission electron microscope at an acceleration voltage of 80 kV.
NK cell degranulation assay.
During the coculture of purified NK cells and treated macrophages, monensin (GolgiStop; 2 μM; BD), brefeldin A (5 mg/ml; Sigma), interleukin 2 (IL-2) (Proleukin; 20 IU/ml), and a phycoerythrin (PE)-conjugated anti-CD107 antibody (H4A3; BD) were added for 5 h. After gating on NK cells, the surface expression of CD107a on CD3− CD56+ cells was analyzed by flow cytometry. MAbs against CD3 (UCHT1; BD) and CD56 (HCD56; Biolegend) were used to identify NK cells.
T cell proliferation assay.
Macrophages were either left untreated, treated with HCMV-Ab (TB40/E Ig-pool), or incubated with TB40/E at an MOI of 5. After 1 day, cells were harvested, resuspended in RPMI medium containing 5% human AB serum (HS), and irradiated (3,000 cGy) in order to prevent cell replication. Autologous PBMC were thawed, labeled with carboxyfluorescein succinimidyl ester (CFSE; Biolegend) according to the manufacturer's instructions, and resuspended in RPMI medium with 5% HS. Macrophages were distributed into 96-well U-bottom microplates prior to the addition of CFSE-labeled PBMC at a macrophage/PBMC ratio of 1:8. As controls, PBMC alone were left untreated or were stimulated either with 5 μg/ml phytohemagglutinin-L (PHA-L; eBioscience) or with immunoglobulin alone (Ig pool; 1:20). After 6 days, cultures were harvested for the evaluation of T cell proliferation by using MAbs against CD4 (RPA-T4; BD) and CD8 (BW135/80; Miltenyi).
Statistical analysis.
The nonparametric Kruskal-Wallis test was performed for multigroup comparison. Results were considered significant at the two-sided P level of 0.05.
ACKNOWLEDGMENTS
We thank Christian Sinzger for providing HCMV strains, Paul Walther for making available the electron microscope, and Marlies Just for testing the HCMV serostatus of all blood donors.
This study was financially supported by the Deutsche Forschungsgemeinschaft (DFG) (SPP 1175 Me 1740/1-2), the “Sonderlinie Medizin, Kompetenznetzwerk Zytomegalie” Baden-Württemberg, Ministerium für Wissenschaft, Forschung und Kunst Baden-Württemberg (to T.M.), and the Carl Zeiss Stiftung project “Infektionsbiologie humaner Makrophagen” (to G.F.). The funders were not involved in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Z.W. conceived, designed, and performed research, analyzed data, and cowrote the paper. R.Q. and L.W. performed research and analyzed data. M.B., M.S., and D.H. performed experiments. T.S. and T.M. designed research, analyzed data, and drafted and revised the manuscript. G.F. designed and performed research, analyzed data, and cowrote the paper.
We declare no financial or commercial conflict of interest.
REFERENCES
- 1.Pass RF. 2001. Cytomegalovirus, p 2675–2705. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 4th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.La Rosa C, Diamond DJ. 2012. The immune response to human CMV. Future Virol 7:279–293. doi: 10.2217/fvl.12.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. 2010. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol 84:1005–1013. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. 2012. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J Virol 86:7444–7447. doi: 10.1128/JVI.00467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerna G, Sarasini A, Patrone M, Percivalle E, Fiorina L, Campanini G, Gallina A, Baldanti F, Revello MG. 2008. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol 89:853–865. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
- 6.Klasse PJ. 2014. Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv Biol 2014:157895. doi: 10.1155/2014/157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. 2014. The function of Fcγ receptors in dendritic cells and macrophages. Nat Rev Immunol 14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 8.Sinzger C, Eberhardt K, Cavignac Y, Weinstock C, Kessler T, Jahn G, Davignon JL. 2006. Macrophage cultures are susceptible to lytic productive infection by endothelial-cell-propagated human cytomegalovirus strains and present viral IE1 protein to CD4+ T cells despite late downregulation of MHC class II molecules. J Gen Virol 87:1853–1862. doi: 10.1099/vir.0.81595-0. [DOI] [PubMed] [Google Scholar]
- 9.Bayer C, Varani S, Wang L, Walther P, Zhou S, Straschewski S, Bachem M, Soderberg-Naucler C, Mertens T, Frascaroli G. 2013. Human cytomegalovirus infection of M1 and M2 macrophages triggers inflammation and autologous T-cell proliferation. J Virol 87:67–79. doi: 10.1128/JVI.01585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romo N, Magri G, Muntasell A, Heredia G, Baia D, Angulo A, Guma M, Lopez-Botet M. 2011. Natural killer cell-mediated response to human cytomegalovirus-infected macrophages is modulated by their functional polarization. J Leukoc Biol 90:717–726. doi: 10.1189/jlb.0311171. [DOI] [PubMed] [Google Scholar]
- 11.Wu Z, Sinzger C, Frascaroli G, Reichel J, Bayer C, Wang L, Schirmbeck R, Mertens T. 2013. Human cytomegalovirus-induced NKG2Chi CD57hi natural killer cells are effectors dependent on humoral antiviral immunity. J Virol 87:7717–7725. doi: 10.1128/JVI.01096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gariano GR, Dell'Oste V, Bronzini M, Gatti D, Luganini A, De Andrea M, Gribaudo G, Gariglio M, Landolfo S. 2012. The intracellular DNA sensor IFI16 gene acts as restriction factor for human cytomegalovirus replication. PLoS Pathog 8:e1002498. doi: 10.1371/journal.ppat.1002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavalai N, Papior P, Rechter S, Leis M, Stamminger T. 2006. Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections. J Virol 80:8006–8018. doi: 10.1128/JVI.00743-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavalai N, Adler M, Scherer M, Riedl Y, Stamminger T. 2011. Evidence for a dual antiviral role of the major nuclear domain 10 component Sp100 during the immediate-early and late phases of the human cytomegalovirus replication cycle. J Virol 85:9447–9458. doi: 10.1128/JVI.00870-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann A, Hauka S, Maywald M, Le VT, Schmidt SK, Daubener W, Hengel H. 2014. Checks and balances between human cytomegalovirus replication and indoleamine-2,3-dioxygenase. J Gen Virol 95:659–670. doi: 10.1099/vir.0.061994-0. [DOI] [PubMed] [Google Scholar]
- 16.Sainz B Jr, LaMarca HL, Garry RF, Morris CA. 2005. Synergistic inhibition of human cytomegalovirus replication by interferon-alpha/beta and interferon-gamma. Virol J 2:14. doi: 10.1186/1743-422X-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers J, Angulo A, Amaratunga D, Guo H, Jiang Y, Wan JS, Bittner A, Frueh K, Jackson MR, Peterson PA, Erlander MG, Ghazal P. 1999. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J Virol 73:5757–5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinzger C, Plachter B, Grefte A, The TH, Jahn G. 1996. Tissue macrophages are infected by human cytomegalovirus in vivo. J Infect Dis 173:240–245. doi: 10.1093/infdis/173.1.240. [DOI] [PubMed] [Google Scholar]
- 19.Poole E, Juss JK, Krishna B, Herre J, Chilvers ER, Sinclair J. 2015. Alveolar macrophages isolated directly from human cytomegalovirus (HCMV)-seropositive individuals are sites of HCMV reactivation in vivo. J Infect Dis 211:1936–1942. doi: 10.1093/infdis/jiu837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnelly RP, Kotenko SV. 2010. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res 30:555–564. doi: 10.1089/jir.2010.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. 2010. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A 107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwan WA, Tam JC, Watkinson RE, Bidgood SR, Mallery DL, James LC. 2013. Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol 14:327–336. doi: 10.1038/ni.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cumby N, Edwards AM, Davidson AR, Maxwell KL. 2012. The bacteriophage HK97 gp15 moron element encodes a novel superinfection exclusion protein. J Bacteriol 194:5012–5019. doi: 10.1128/JB.00843-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloutskin A, Yee MB, Kinchington PR, Goldstein RS. 2014. Varicella-zoster virus and herpes simplex virus 1 can infect and replicate in the same neurons whether co- or superinfected. J Virol 88:5079–5086. doi: 10.1128/JVI.00252-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Criddle A, Thornburg T, Kochetkova I, DePartee M, Taylor MP. 2016. gD-independent superinfection exclusion of alphaherpesviruses. J Virol 90:4049–4058. doi: 10.1128/JVI.00089-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins SE, Noyce RS, Mossman KL. 2004. Innate cellular response to virus particle entry requires IRF3 but not virus replication. J Virol 78:1706–1717. doi: 10.1128/JVI.78.4.1706-1717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noyce RS, Collins SE, Mossman KL. 2006. Identification of a novel pathway essential for the immediate-early, interferon-independent antiviral response to enveloped virions. J Virol 80:226–235. doi: 10.1128/JVI.80.1.226-235.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noyce RS, Taylor K, Ciechonska M, Collins SE, Duncan R, Mossman KL. 2011. Membrane perturbation elicits an IRF3-dependent, interferon-independent antiviral response. J Virol 85:10926–10931. doi: 10.1128/JVI.00862-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haspot F, Lavault A, Sinzger C, Laib Sampaio K, Stierhof YD, Pilet P, Bressolette-Bodin C, Halary F. 2012. Human cytomegalovirus entry into dendritic cells occurs via a macropinocytosis-like pathway in a pH-independent and cholesterol-dependent manner. PLoS One 7:e34795. doi: 10.1371/journal.pone.0034795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer A, Subramanian N, Villinger C, Frascaroli G, Mertens T, Walther P. 2016. Megapinocytosis: a novel endocytic pathway. Histochem Cell Biol 145:617–627. doi: 10.1007/s00418-015-1395-2. [DOI] [PubMed] [Google Scholar]
- 31.Ziemann M, Hennig H. 2014. Prevention of transfusion-transmitted cytomegalovirus infections: which is the optimal strategy? Transfus Med Hemother 41:40–44. doi: 10.1159/000357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murrell I, Bedford C, Ladell K, Miners KL, Price DA, Tomasec P, Wilkinson GWG, Stanton RJ. 2017. The pentameric complex drives immunologically covert cell-cell transmission of wild-type human cytomegalovirus. Proc Natl Acad Sci U S A 114:6104–6109. doi: 10.1073/pnas.1704809114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinzger C, Mangin M, Weinstock C, Topp MS, Hebart H, Einsele H, Jahn G. 2007. Effect of serum and CTL on focal growth of human cytomegalovirus. J Clin Virol 38:112–119. doi: 10.1016/j.jcv.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Freed DC, Tang Q, Tang A, Li F, He X, Huang Z, Meng W, Xia L, Finnefrock AC, Durr E, Espeseth AS, Casimiro DR, Zhang N, Shiver JW, Wang D, An Z, Fu TM. 2013. Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine. Proc Natl Acad Sci U S A 110:E4997–E5005. doi: 10.1073/pnas.1316517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wussow F, Chiuppesi F, Martinez J, Campo J, Johnson E, Flechsig C, Newell M, Tran E, Ortiz J, La Rosa C, Herrmann A, Longmate J, Chakraborty R, Barry PA, Diamond DJ. 2014. Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex. PLoS Pathog 10:e1004524. doi: 10.1371/journal.ppat.1004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiuppesi F, Kaltcheva T, Meng Z, Barry PA, Diamond DJ, Wussow F. 2017. Identification of a continuous neutralizing epitope within UL128 of human cytomegalovirus. J Virol 91:e01857-16. doi: 10.1128/JVI.01857-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha S, Li F, Troutman MC, Freed DC, Tang A, Loughney JW, Wang D, Wang IM, Vlasak J, Nickle DC, Rustandi RR, Hamm M, DePhillips PA, Zhang N, McLellan JS, Zhu H, Adler SP, McVoy MA, An Z, Fu TM. 2017. Neutralization of diverse human cytomegalovirus strains conferred by antibodies targeting viral gH/gL/pUL128-131 pentameric complex. J Virol 91:e02033-16. doi: 10.1128/JVI.02033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, Frascaroli G, Mertens T. 2013. Assessment of natural killer cell responses to human cytomegalovirus-infected macrophages. Methods Mol Biol 1064:289–298. doi: 10.1007/978-1-62703-601-6_21. [DOI] [PubMed] [Google Scholar]
- 39.Agley CC, Velloso CP, Lazarus NR, Harridge SD. 2012. An image analysis method for the precise selection and quantitation of fluorescently labeled cellular constituents: application to the measurement of human muscle cells in culture. J Histochem Cytochem 60:428–438. doi: 10.1369/0022155412442897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagenknecht N, Reuter N, Scherer M, Reichel A, Muller R, Stamminger T. 2015. Contribution of the major ND10 proteins PML, hDaxx and Sp100 to the regulation of human cytomegalovirus latency and lytic replication in the monocytic cell line THP-1. Viruses 7:2884–2907. doi: 10.3390/v7062751. [DOI] [PMC free article] [PubMed] [Google Scholar]