Abstract
Background
Peripheral immune system cytokines may play an integral role in underlying sensitized stress response and alcohol craving during early withdrawal. To date, the nature of these immune changes during early abstinence have not been examined.
Methods
Thirty-nine early abstinent, treatment-seeking alcohol dependent individuals and 46 socially drinking controls were exposed to three guided imageries: stress, alcohol cue and neutral. These were presented randomly across consecutive days. Plasma measures of tumor necrosis factor alpha (TNFα), tumor necrosis factor receptor 1 (TNFR1), interleukin-6 (IL-6), and interleukin-10 (IL-10), were collected at baseline, immediately after imagery and at various recovery time-points. Ratings of alcohol craving, negative mood and anxiety were also obtained at the same time-points.
Results
The alcohol group demonstrated decreased basal IL-10 compared with controls particularly following exposure to alcohol cue. They also showed a dampened TNFα and TNFR1 response to stress and cue, respectively, and a generalized suppression of IL-6. In the alcohol group, these immune system adaptations occurred alongside significant elevations in anxiety, negative mood and alcohol craving.
Conclusions
Findings demonstrate that broad immuno-suppression is still observed in alcohol dependent individuals after three weeks of abstinence and may be linked to motivation for alcohol.
Keywords: alcohol dependence, cytokines, immune system, stress, TNFα, IL-6, IL-10
INTRODUCTION
Individual response to stressors are significant factors contributing to relapse during early abstinence from alcohol. In support of this, elevated craving and relapse during the early stages of stable recovery has been characterized by robust chronic dysregulation of core stress systems of the brain and sensitized negative mood and anxiety in the face of challenge (Fox et al., 2007; Gohier et al., 2003; Sinha et al., 2011; Ericsson et al., 1994). While these bio-behavioral stress systems have proved useful targets for the development of new relapse medications (Fox et al., 2012b; Fox et al., 2012a; Fox et al., 2014; Fox et al., 2013b; Milivojevic et al., 2016; Jin, 2011; McKee et al., 2015), there remains a pressing need to continue elucidating novel therapeutic targets that may be coupled with these mechanisms and underlie compulsive drinking. In view of this, peripheral immune system signaling may promote motivation for alcohol by directly contributing to salient neurobiological changes and/ or by underlying negative mood states associated with elevated stress-related craving and relapse risk (Dantzer et al., 2008).
Motivation for alcohol and substances is characterized by compulsive drives including sensitization of the core stress systems of the brain, elevated negative affective states (Fox et al., 2007; Sinha et al., 2009; Litt and Cooney, 1999) and inhibitory dyscontrol, often in the face of stressors (Adinoff, 2004; Koob and Volkow, 2016; Fox and Sinha, 2009). Notably, there is evidence to suggest that immune system adaptations may impinge upon these processes and, as such, provide a critical system for treatment intervention. While a healthy acute response to stress or alcohol is defined by a complex series of self-limiting immune signaling cascades and HPA (hypothalamic-pituitary-adrenal) axis feed forward loops appropriate to the given stimulus (Elenkov, 2008; Elenkov and Chrousos, 1999; Dunn et al., 2005), repetitive exacerbation of immune system signaling over time, may produce maladaptive, long-term behavioral alterations, via microglial activation (Brites and Fernandes, 2015; Marshall et al., 2013). Microglia propagate inflammatory responses that originate in the periphery to elicit sickness behavior, including negative mood, decreased social interaction and increased sleep (Dantzer et al., 2008). While the precise pattern of chronic adaptations are unclear, prior clinical and preclinical evidence has linked disruptions in immuno-regulation to factors salient to stress-induced motivation for alcohol, including elevated deleterious mood and functioning of inhibitory processes.
For example, disruption of immuno-regulatory mechanisms has been observed in mood-related disorders including depression, where both immune suppression and activation have been documented (Blume et al., 2011; Irwin and Miller, 2007; Raison et al., 2006). Pathological activation of inflammatory mediators has been associated with similar deleterious mood symptoms in chronically ill patients (O'Connor et al., 2009; Maes et al., 2009; McNally et al., 2008), and the treatment of both patients (Dunn et al., 2005; Gohier et al., 2003; Valentine et al., 1998) and animals (Bonaccorso et al., 2003; Brebner et al., 2000; Salome et al., 2008; Silverman et al., 2007) with pro-inflammatory cytokines can produce negative affective moods similar to those associated with the negative reinforcing effects of withdrawal from alcohol (Cooney et al., 1997; Fox et al., 2007; Litt and Cooney, 1999; Sinha et al., 2009; Teichner et al., 2001). In certain cases, attenuated levels of pro-inflammatory markers have also been associated with high depressive symptomatology (Podlipny et al., 2010; Haack et al., 1999), and individuals with mild to moderate depression have shown reduced binding of the radiotracer [11C]PBR28, to translocator protein 18 kDa (TSPO; a marker of microglial activation) compared with controls using Positron emission tomography (PET) (Hannestad et al., 2013). In support of this, studies from our own laboratory have previously highlighted tonic immuno-suppression of both pro- and anti-inflammatory markers to predict elevated hazardous drinking in actively drinking problem drinkers (Fox et al., 2013a)
Chronic peripheral immune system adaptations may also impinge upon central neural systems that underlie regulatory function, goal-oriented behaviors and impulse control. Again, while the precise mechanisms are not well understood, acute artificially-induced peripheral inflammation has been shown not only to increase deleterious mood, but also to exert adaptations within regions of the prefrontal cortex implicated in inhibitory regulation. These include higher normalized glucose metabolism in the right anterior insula, and, lower levels in the right anterior cingulate (Harrison et al., 2009; Hannestad et al., 2012). Similarly, inflammation-induced poor mood change has been associated with reduced connectivity of the sub genular anterior cingulate to amygdala, medial prefrontal cortex, and nucleus accumbens (Harrison et al., 2009).
Compulsive alcohol seeking is a chronic stress state defined by dysregulation of HPA-SAM (sympathetic-adrenal-medullary) system function (Fox et al., 2007; Sinha et al., 2009, 2011; Adinoff et al., 1998), sensitized negative mood and poor regulatory function in the face of stress. As immuno-dysregulation underlies the pathogenesis of chronically elevated depression, negative affect and changes in fronto-striatal inhibitory systems, and may be mediated by glucocorticoid sensitivity, we propose that peripheral immune system dysregulation may also represent a salient mechanism for driving compulsive alcohol seeking during early abstinence. This is supported by preclinical studies that have shown neuro-immune receptor signaling to regulate binge drinking in rats (Marshall et al., 2016) and ethanol consumption in mice (Agrawal et al., 2011; Blednov et al., 2015; Blednov et al., 2011; Blednov et al., 2012; Franklin et al., 2015; Wen et al., 2012). Despite these findings, few human studies have focused on examining the contribution of peripheral immune system changes in the context of addiction-related behaviors such as alcohol “wanting”, or craving.
We propose to systematically examine changes in peripheral cytokines at baseline and following personalized stress and alcohol cue imagery (a variant stressor) during 3 weeks of abstinence from alcohol in dependent individuals compared with social drinkers. We predict that compared with controls, alcohol dependent individuals will demonstrate a sensitized negative mood, anxiety and craving response to both stress and alcohol cue, which will be characterized by dysregulations in peripheral cytokines both at baseline and following stress.
METHODS
Participants
Thirty-nine treatment seeking alcohol dependent individuals and 46 socially drinking controls, participated in the current study. All participants were recruited via advertisements placed either on-line or in local newspapers and magazines. Current alcohol dependence was determined using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders IV; SCID IV – (First et al., 1997). All participants were also tested for positive urine toxicology screens upon admission to the Clinical Neuroscience Research Unit (CNRU) for inpatient treatment and research of the Connecticut Mental Health Center (CMHC). Exclusion criteria for alcohol dependent patients included DSM-IV dependence for any drug other than alcohol or nicotine and any psychiatric illness requiring medication.
All controls were light social drinkers (25 drinks or less per month) as classified by the Cahalan Quantity Frequency Variability Index (Cahalan et al., 1969) and were excluded if they met current or lifetime dependence criteria for alcohol or any other illicit drug. All participants using prescribed medications or failing to meet health requirements were also ineligible. Participants underwent stringent medical assessments that included electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic and thyroid function to ensure good physical health. While 8 out of the 39 participants in the alcohol group underwent an assisted medical detoxification protocol on the CNRU inpatient unit, they were not permitted into the research component of their stay unless they were medication free and deemed to be in good health. All dependent individuals were, however, free to continue with treatment regardless of entry into the research component. All participants gave written and verbal consent and the Human Investigation Committee of the Yale University School of Medicine approved the study.
General Procedures
Alcohol dependent participants were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) for 4 to 5 weeks of inpatient life skills program as well as standard group counseling treatment for alcohol addiction (Mercer and Woody, 1992) and study participation. During the first week of inpatient stay, alcohol dependent participants were administered structured baseline assessments measuring psychiatric and substance use history.
During week 4 of inpatient stay, all participants took part in 3-day laboratory challenge experiment, run across consecutive days, where they were presented with 3 personalized 5-minute imagery conditions, one per day in a randomized and counterbalanced order. Both staff and participants were blind to the presentation order. The three personalized imagery conditions comprised: i) stress imagery; ii) alcohol cue imagery (a variant stressor), and iii) neutral imagery (an intra-individual control condition). Personalized imagery scripts were developed and scripted during week 2 of inpatient stay, from participants’ recent life events (see Sinha et al., 2003 for full details). All scripts were written by a clinician and recorded onto an audiotape to be played in the laboratory sessions.
Socially Drinking participants were admitted to the Hospital Research Unit (HRU) of the Yale Clinical Center of Investigation (YCCI) located at Yale/New Haven Hospital (YNHH) for a three night stay. During this time they took part in an identical 3-day laboratory challenge study to that conducted in the alcohol dependent group. All control participants were required to stay on the unit, within a similar controlled environment to that of the alcohol dependent participants. This included a similar diet as well as supervised and regulated smoking breaks. Baseline demographics, psychiatric and substance use assessments as well as imagery scripts were prepared prior to their admission to the HRU. Socially drinking controls were also exposed to an alcohol-related script for the alcohol cue condition.
Laboratory Sessions
All laboratory sessions were conducted approximately 21 days after admission to allow for normalization of neurobiological changes associated with acute alcohol abstinence. On each testing day, subjects abstained from breakfast and were brought into the testing room at 7:45 AM. All subjects were allowed an initial smoke break at 7:30 AM in order to reduce the impact of nicotine abstinence effects. After settling into a sitting position on a hospital bed, a heparin-treated catheter was inserted by the research nurse in the antecubital region of the subject’s non-preferred arm, in order to periodically obtain blood samples. A blood pressure cuff was placed on the subject’s preferred arm to monitor blood pressure (SBP and DBP) and a pulse sensor was placed on the subject’s forefinger to obtain a measure of heart rate. This was followed by a 45-minute adaptation period during which the participants were instructed to practice relaxation. At 9:00 AM, participants were provided with headphones and given the following instructions for the imagery procedure: “Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation”. The length of each script was approximately 5-minutes. Heart rate and blood pressure was continuously monitored during the imagery period.
Subjective ratings of alcohol craving and anxiety as well as heart rate, blood pressure and plasma were collected at two baseline time-points: one, 20 minutes prior to imagery exposure (−20 time-point) and one, 5 minutes prior to imagery exposure (−5 time-point), immediately following imagery (0 time-point) and periodically at various recovery time-points until 1 hour post imagery (+ 5, +15, +30, +45, +60). The Alcohol Urges Questionnaire (AUQ) was administered at 3 time-points only: baseline (−5), immediately following imagery (0) and 5 minutes post imagery (+5). After the final assessments, the IV line, blood pressure cuff and pulse sensor were removed and breakfast was served.
Laboratory Assessments
Plasma Cytokine Measures
Immediately following collection, tubes were placed on ice. Plasma was subsequently separated by centrifugation at 4 C for 15 minutes at 1000 × g. Plasma was then aliquoted and stored in polypropylene tubes at −70 C until the time of the assay. Cytokine concentrations were quantitatively determined by enzyme-linked immune-sorbent assays using the DuoSet ELISA Development Kit from R&D systems (Minneapolis, MN, USA) as previously described (Stowe et al., 2010). Assaying of all plasma cytokines was conducted at Microgen Laboratories, La Marque, TX, under the direction of Dr. Raymond Stowe.
Subjective Measures
Alcohol craving
The desire for using alcohol was assessed using a 10-point Visual Analog Scale (VAS) in which 1 = ‘not at all’ and 10 = ‘extremely high’. The Alcohol Urges Questionnaire - AUQ; (Bohn et al., 1995), which is a reliable and valid 8-item scale commonly used for the repeated assessments of alcohol craving (Drobes and Thomas, 1999), was also administered at 3 specific time-points.
Anxiety and Negative Mood
Anxiety was assessed using the Anxiety and Fear subscales from the Differential Emotion Scale- DES; (Izard, 1972). Negative mood was calculated using the Sadness subscale. The DES scale comprises 30 specific emotional adjectives (or items) and participants are required to rate on a five-point scale the extent to which each word describes the way s/he feels at the current time. The subscale Sadness included items such as downhearted, upset, and distressed. The sub-scales of Anxiety and Fear comprised items such as anxious, tense and nervous.
Statistical Analysis
The experimental groups were compared on demographics and alcohol use using ANOVA or chi square analysis. Linear Mixed Effect (LME) models were implemented in order to analyze all data, using SPSS software (version 21). The Between-subjects factor of Group (Alcohol Dependent (AD) Vs Social Drinkers (SDs)) and the Within-subjects factors of Imagery Condition (stress, alcohol cue and neutral) and Time-points (varying levels) were the fixed effects. Participants represented the random effect factor. A compound symmetry covariance structure was applied to all analyses. In the case of significant basal variance, baseline measures were included as covariates in the response data analyses. Race, age and smoking status (smoker/non smoker) were also included as covariates due to Group variation and potential impact on the Dependent Variables. All cytokine data were Log transformed to meet normal distribution assumptions, and any extreme outlying data points with an interquartile range greater than X3 were removed. Bonferroni tests were used as adjustments for multiple comparisons.
RESULTS
Participants
Both the AD group and socially drinking controls were matched for gender and IQ. However, the AD group were significantly older (37.6 years Vs 31.7 years) and comprised a greater number of Hispanics (19.6% Vs 0%) and regular smokers (89.7% Vs 15.6%). As such, secondary analyses were also conducted for all Dependent Variables using age, race and smoking status as covariates in all LME models.
Subjective Measures
Alcohol Craving (AUQ)
Following imagery exposure, a significant Group × Imagery Condition interaction was observed, [F2, 672 = 4.5, p=.01] showing that the AD group reported significantly higher ratings of alcohol craving in the stress condition compared with the SD group (p=.05). The AD group also reported significantly higher alcohol craving in the stress compared with the neutral imagery condition (S>N, p<.0001) and the cue compared with the neutral imagery condition (C>N, p<.0001). By contrast in the SD group, there was only a trend for higher alcohol craving in the cue compared with the neutral imagery condition (C>N, p<.07) and no significant elevation following stress exposure compared with neutral imagery (S>N, p= ns). A significant Group × Time-point interaction, [F2, 672 = 8.2, p<.0001] also showed that the AD group reported higher alcohol craving immediately following exposure to all three imagery conditions (0 time-point) compared with the control group (p=.004). The addition of covariates had no significant effect on the model.
Alcohol Craving (VAS)
(Fig. 1a) A main effect of Group, [F1, 390 = 6.9, p=.009] indicated significantly higher reported alcohol craving in the AD group compared with the social drinkers across all conditions and time-points. As anticipated, a significant main effect of Imagery Condition, [F2, 517 = 14.4, p<.0001] also showed that in both groups, alcohol craving response was significantly higher following exposure to stress compared with neutral imagery (S>N, p=.002) and following cue exposure compared with neutral imagery (C>N, p<.0001).
Figure 1.
A significant Group × Imagery Condition × Time-point interaction, [F14, 1416 = 3.5, p<.0001] indicated that higher alcohol craving was reported by the AD compared with the SD group immediately following stress exposure (0: p<.0001), 5 minutes later (+5: p<.02) and 15 minutes later (+15: p=.05). Similarly, the AD group also reported higher craving compared with the SD group immediately following exposure to the alcohol cue imagery (0 time-point: p<.0001), and 5 minutes later (+5: p<.02). In addition, while both groups demonstrated greater alcohol craving immediately after exposure to stress and cue imagery compared with neutral imagery exposure (0 time-point: p<.0001, in all cases), this persisted to the +5 time-point in the AD group (+5 time-point: S>N, p=.007, C>N, p=.001). The addition of covariates had no significant effect on the model.
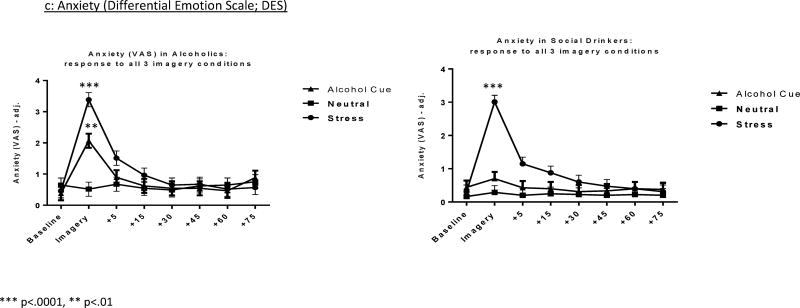
Anxiety (DES)
(Fig. 1b) A significant Group × Imagery Condition interaction, [F2, 1922 = 3.5, p=.03] from the composite Anxiety and Fear score, also showed that while greater Anxiety and Fear were reported following stress compared with neutral imagery in both groups (p<.0001 in both cases) only the AD group reported higher Anxiety and Fear following cue compared with neutral imagery (p=.001). This anxiety effect of cue-related imagery was not observed in the social drinkers. The addition of covariates had no significant effect on the model.
Negative mood (Sadness - DES)
A significant Group × Imagery Condition interaction, [F2, 1922 = 3.8, p=.02] from the Sadness score again indicated that while greater sadness was reported in both groups following exposure to stress compared with neutral imagery (p<.0001, in both cases), this was only observed following exposure to cue in the AD group (C>N, p=.03). Findings were not altered by the addition of covariates into the model.
Cytokine Measures
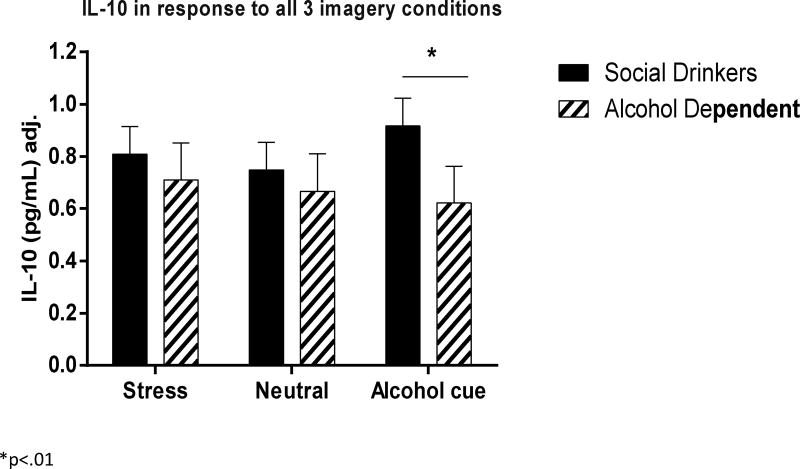
IL-10
(Fig. 2) A main effect of Group [F1, 77 = 4.8, p=.03] indicated that the AD individuals demonstrated a lower IL-10 response to all three imagery conditions compared with socially drinking controls. A Group × Imagery Condition interaction [F2, 1042 = 3.1, p<.05] further showed that IL-10 attenuation in the AD group compared with controls was greater following exposure to alcohol cue (p=.007) and stress (p=.07) imagery (trend only). In addition, while the controls responded to the alcohol cue with an increase in IL-10 compared with neutral imagery condition (p=.03), this was not observed in the alcohol dependent group.
Figure 2.
When age, race and smoking status were also entered into the model as covariates, smoking status was seen to have an impact on the overall model (p=.06), however, a Group × Imagery Condition interaction was maintained [F2, 636 = 4.0, p<.02]. Again, this indicated that the controls demonstrated an increased IL-10 response to cue relative to the neutral imagery condition (C>N, p=.002), which was not seen in the alcohol group. However, the Between Group difference was no longer observed.
Correlation analyses also indicated that IL-10 response was significantly negatively associated with alcohol craving immediately following exposure to cue-related imagery (r=−.26, p=.05). This indicated that the lower the IL-10 response to cue, the higher the reports of alcohol craving.
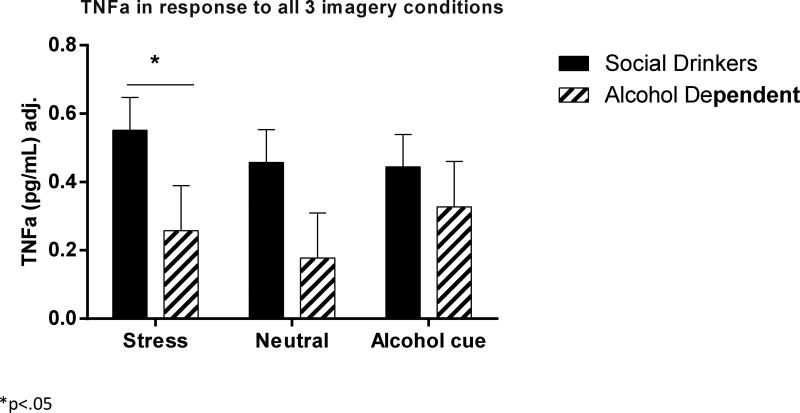
TNFα
(Fig. 3) A significant Group × Imagery Condition interaction [F2, 918 = 5.5, p=.004] indicated that an attenuation in TNFα was observed in the AD group compared with the socially drinking controls in the stress imagery condition (p<.05). In addition, while the controls showed a trend for higher levels of TNFα following stress exposure compared with cue (p<.07) and neutral (p=.1) imagery conditions, this was not observed in the alcohol group. All covariates had an insignificant impact on the overall model.
Figure 3.
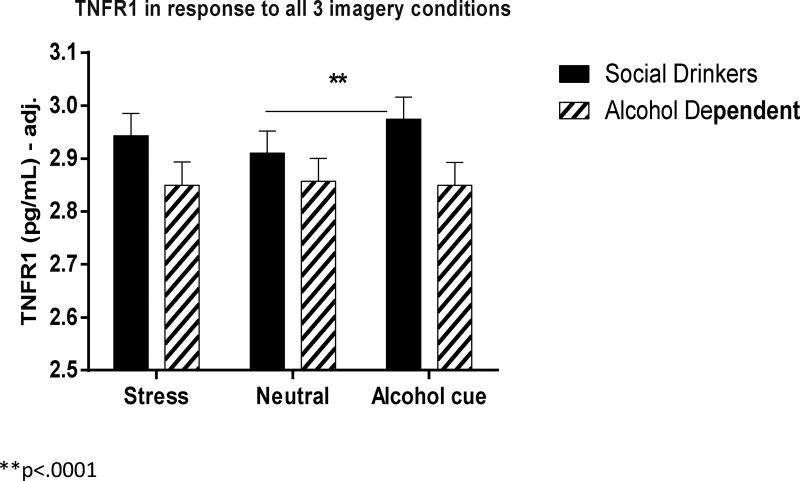
TNFR1
(Fig. 4) A significant Group × Imagery Condition interaction [F2, 1060 = 4.3, p=.01] indicated that a TNFR1 elevation was observed in the cue imagery condition compared with the neutral imagery condition in the control group (C>N, p<.0001). These increases were not observed in the AD group. After inclusion of covariates, only Age was seen to significantly affect the model (p=.02). However, a Group × Imagery Condition interaction [F2, 1038 = 4.8, p=.008] was still observed indicating an increased TNFR1 response to cue compared with neutral imagery in the control group (p<.0001), but not in the AD group.
Figure 4.
Correlation analyses also indicted that TNFR1 response was significantly negatively associated with alcohol craving immediately following exposure to cue-related imagery (r=−.32, p<.03). This indicates that the lower the TNFR1 response to cue, the higher the reported alcohol craving response.
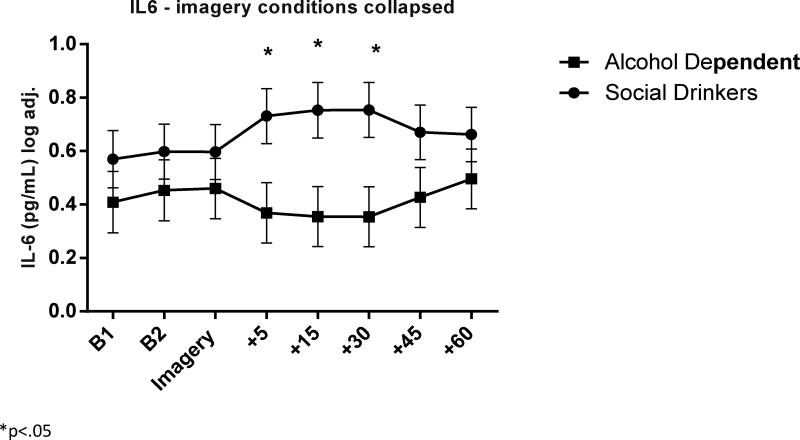
IL-6
(Fig. 5) A significant Group × Time-point interaction [F7, 940 = 3.3, p=.002] indicated that the AD group demonstrated a dampened IL-6 response compared with the control group following exposure to all three imagery conditions. Group variation in IL-6 was seen specifically 5 minutes (AD< SD, p=.08, trend), 15 minutes (AD< SD, p=.03) and 30 minutes (AD< SD, p<.03) following all three imageries.
Figure 5.
Following the addition of covariates into the model, only Age was seen to have a significant impact on the overall model. Although, a significant Group × Time-point interaction [F7, 899 = 3.3, p=.002] was still observed.
As no group variation in IL-6 was observed with regard to the imagery conditions, an exploratory analysis was conducted examining response to baseline, peak, and initial recovery time-point only, to see whether the peak or immediate IL-6 response better demonstrated any potential effects of imagery manipulation. Findings showed a Group × Imagery Condition [F2, 423 = 3.3, p<.04] where the control group demonstrated an elevation in IL-6 immediately following stress imagery exposure compared with the neutral condition. This was not observed in the alcohol dependent group.
DISCUSSION
In the current study we used a well-validated and reliable laboratory paradigm to model stress-induced alcohol craving in a group of early abstinent alcohol dependent individuals compared with a group of socially drinking controls. Broadly, findings indicated that during elevations in stress-induced and cue-induced craving, anxiety and negative mood, the alcohol group demonstrated a dampened concomitant response in peripheral cytokines compared with controls. While socially drinking controls demonstrated a relative activation of IL-10 and TNFR1 following exposure to alcohol cue as well as activation of IL-6 and a trend in TNFα and IL-10 elevations following stress, this was not observed in the alcohol dependent group.
Specifically, levels of anti-inflammatory cytokine IL-10 were shown to be lower overall in the alcohol group compared with the controls, both at baseline and following exposure to all three imagery conditions. Moreover, the attenuated response was significantly more robust following exposure to alcohol cue. While cue-related IL-10 attenuation was maintained after controlling for smoking status, the extent of overall dampening was not as robust. Interleukin-10 (IL-10) is typically classified as a T-Helper lymphocyte Type 2 cytokine responsible for humoral immunity, and a key communicator between immune and endocrine interactions (Asadullah et al., 2003). High secretion of IL-10 following partial activation of microglia following binge drinking has also been postulated to demonstrate the reparative role of microglia (Marshall et al., 2013). Interestingly, recent studies have also indicated attenuated basal levels of IL-10 in both early abstinent (Fox et al., 2012b) and actively using (Moreira et al., 2016) cocaine dependent individuals. Moreover, following three weeks of cocaine abstinence, a dampened IL-10 response was similarly observed following cocaine cue imagery compared with controls and concomitant to elevations in cocaine craving (Fox et al., 2012b).
A significant dampening in the soluble receptor TNFR1 (an indicator of pro-inflammation) and TNFα was also observed in the alcohol group compared with controls following exposure to alcohol-cue and stress cue, respectively. However it is important to note that these cue-related decreases may have been driven in part by adaptations in tonic levels due to a lack of significant time-point interactions. Findings corroborate studies that have shown acute ethanol to reduce the expression of TNFα receptors on human interferon gamma macrophages (Bermudez et al., 1991) and down regulate TNFα response to LPS and interferon (Verma et al., 1993). Dampened LPS-stimulated release of TNFα from alveolar microphages has also been observed in chronic drinkers both with (Wallaert et al., 1991) and without (Omidvari et al., 1998) liver disease compared with non-drinking controls.
A phasic suppression of the pro-inflammatory marker IL-6 was also shown directly in response to all three imagery conditions; no group variation was observed at baseline. Extended analysis indicated that IL-6 suppression was most robust immediately following exposure to the stress imagery. This suppression was again maintained after controlling for covariates. Additionally, our findings also corroborate studies that have assessed the effects of alcohol on stress-induced levels of IL-6. Injured trauma patients with a positive BAC have been shown to demonstrate lower levels of IL-6 than those with no BAC (Relja et al., 2016), and alcohol following heavy resistance exercise has also been shown to decrease the high levels of IL-6 induced by exercise (Levitt et al., 2015).
Despite some corroboration with prior alcohol research, the exact pattern of cytokine adaptations during early withdrawal remain highly ambiguous for several reasons. First, prior research shows considerable variation in experimental paradigms. For example, human studies assessing the pattern of TNFα and IL-6 produced following LPS/interferon stimulation from alcohol dependent individuals, have shown substantial variation as a function of alcohol consumption status and type of hepatic damage. Two studies by Laso and colleagues demonstrated that alcohol dependent individuals in withdrawal who also had liver cirrhosis showed no changes in TNFα and IL-6 production from peripheral blood monocytes (Laso et al., 2007b) or from decreased circulating dendritic cells (Laso et al., 2007a). Notably, attenuated levels of circulating cytokines were seen only in alcohol dependent individuals with cirrhosis who were still actively drinking (Laso et al., 2007a; Laso et al., 2007b). Second, the pleiotropic nature of cytokines also means that they are produced by many cells which display varied and often overlapping specificity (Dinarello et al., 2000). For example, in addition to pro-inflammatory properties, IL-6 also down-regulates TNFα and IL-1 synthesis and the production of TNFα and IL-1 antagonists (Tilg and Wedemeyer, 2014). Similarly, while endogenous soluble TNF-receptor-1 (sTNFR1) increases in response to TNF-mediated inflammation, its exact role remains unclear (Luna et al., 2013). IL-10 is also robustly produced by regulatory cells in addition to anti-inflammatory TH2 helper cells (Kidd, 2003; Moser and Murphy, 2000).
Due to these complexities, Pentol-Rol et al. (2009) suggest that ascertaining effector-regulator equilibrium may be a more meaningful assessment of immune function than Th1/Th2 balance. With this in mind, the current reduced levels of both IL-10 and TNFα measures in response to stressors, may therefore demonstrate an overall effector-regulator imbalance representing an extremely complex imbalance of cytokines and their feed forward loops and regulator mechanisms. One explanation may be that the attenuated levels of the specific pro- and anti-inflammatory cytokines in the current study are the result of chronic alcohol-related and stress-related pro-inflammatory, and hence anti-inflammatory, activation. Across time, chronic and repeated over-activity of stress and immune system mediators may produce immuno-suppressive effects (McEwan and Seeman, 1999) which reflect the magnitude of dysregulation. In terms of clinical utility, circulating cytokines may therefore reflect drinking severity and hence greater allostatic load typically associated with stress system sensitivity underlying the negative reinforcing effects of alcohol during early withdrawal.
Some support for this is shown in the current study where alcohol cue-related suppressions in both pro-inflammatory marker, TNFR1 and anti-inflammatory cytokine, IL-10 each showed modest significant negative correlations with corresponding elevations in alcohol craving. Findings from our recent study of problem drinkers have additionally shown immune-suppression to predict craving, severity of problem use and alcohol consumption (Milivojevic in press). Marcos et al., (2008) also demonstrated a significant association between the −592C>A polymorphism of IL-10 and alcoholism, with an excess of allele A carriers being observed in alcohol dependent individuals (Crawley et al., 1999). In addition, as outlined in the Introduction, disruption of immuno-regulatory mechanisms have been shown to underpin chronic deleterious mood and inhibitory function, similar to that associated with stress-induced motivation in many drugs of abuse. Taken together, peripheral immune system cytokines may play a potentially important diagnostic role in alcohol abuse (Achur et al., 2010).
Interpretation of the study findings should be viewed in light of certain methodological limitations. First, interpretation is restricted by the fact that the control group did not represent a population of regular smokers. Although efforts were made to account for nicotine withdrawal and smoking status, findings may still partially reflect nicotine-induced immune adaptations. With regard to this, attenuated tonic levels of IL-10 were less robust in the alcohol group following the addition of covariates to the statistical model. Second, it remains unclear whether the observed adaptations relate to aspects of alcohol consumption per se or to the affective and bio-physiological changes related to repeated withdrawals, or both. Third, although both groups were statistically matched for number of men and women in the present study, findings may still be diminished due to potentially incongruent sex-specific differences. Given sex-related differences in Hypothalamic-Pituitary-Gonadal (HPG) function observed during early abstinence from alcohol and drugs, there is a need to more thoroughly examine sex differences in a larger sample of male and female alcohol dependent individuals. Four, while dampening of both stress-induced TNFα levels and IL6 levels following exposure to all imagery conditions, occurred alongside increases in negative mood, anxiety and alcohol craving, no significant correlation was observed. One of the reasons for this may be related to the fact that the visual analog self-report measure of craving in this study did not fully capture biological adaptations to stress, which may also occur at slightly separate time-frames.
While further research is required to fully establish the precise immune mechanisms underlying stress-related craving, this study clearly highlights impairment of immune system effector-regulator mechanisms during a stress-induced craving state, compared with controls. Given the potential role of peripheral cytokines in affective and regulatory processes that underpin compulsive drinking, further research is encouraged to more fully explore the nature of immune system adaptations across a more protracted withdrawal period and in phenotypically varied sub-populations of drinkers.
Table 1.
Demographics and Alcohol Use
| N= 85 | Socially Drinking Controls (n=46) |
Alcohol Dependent (n=39) |
|---|---|---|
| Gender (% male) | 27 (58.7%) | 28 (71.8%) |
| Race | ||
| Caucasian | 26 (56.5%) | 27 (69.2%) |
| African American | 10 (21.7%) | 11 (28.2%) |
| Hispanic | 9 (19.6%) | 0 |
| Other | 1 (2.2%) | 1 (2.6%) |
| Age | 31.7 ± 10.0 | 37.6 ± 7.5 |
| IQ (Shipley) | 111.4 ± 10.9 | 108.4 ± 10.1 |
| No. of drinks consumed (last 30 days) | 13.7 ± 12.0 | 361.0 ± 283.5 |
| No. of days spent drinking (last 30 days) | 5.1 ± 6.2 | 18.5 ± 9.0 |
| No. of years drinking regularly | 8.0 ± 8.6 | 18.1 ± 8.5 |
| No. of Regular Smokers% | 26.1% | 92.3% |
| No. of cigarettes per day (last 30 days) | 8.4 ± 9.9 | 15.4 ± 8.0 |
| Lifetime mood disorder | 3 (6.5%) | 7 (17.9%) |
| Current mood disorder | 0 | 0 |
| Lifetime anxiety | 3 (6.5%) | 5 (12.8%) |
| Current anxiety | 1 (2.2%) | 3 (7.7%) |
Shaded area: p>.05
Acknowledgments
We would like to thank the staff at the Yale Stress Center, the Yale Center for Clinical Investigation, as well as the staff based at the Substance Abuse Center and the Clinical Neuroscience Research Unit of the Connecticut Mental Health Center for their assistance in completing this study.
FUNDING
This study was supported in part by grants R01: AA 20095 (Fox), R03:AA022500 (Fox), Peter F MacManus Charitable Trust (Fox) R01: AA 20504 (Sinha) R01: AA013892 (Sinha) U1DE019586 (Sinha).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no competing financial interests pertaining to the aims and results of this study
References
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12(6):305–20. doi: 10.1080/10673220490910844. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22(1):67–72. 1998 Review. [PMC free article] [PubMed] [Google Scholar]
- Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5(1):83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- Bermudez LE, Wu M, Martinelli J, et al. Ethanol affects release of TNF and GM-CSF and membrane expression of TNF receptors by human macrophages. Lymphokine Cytokine Res. 1991;10:413–419. [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, et al. Peroxisome proliferator-activated receptors alpha and gamma are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcohol Clin Exp Res. 2015;39:136–145. doi: 10.1111/acer.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, et al. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25(Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, et al. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011 Feb;25(2):221–9. doi: 10.1016/j.bbi.2010.10.008. 2011 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Maier SF, Meltzer HY, et al. Behavioral changes in rats after acute, chronic and repeated administration of interleukin-1beta: relevance for affective disorders. J Affect Disord. 2003;77:143–148. doi: 10.1016/s0165-0327(02)00118-0. [DOI] [PubMed] [Google Scholar]
- Brebner K, Hayley S, Zacharko R, et al. Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology. 2000;22:566–580. doi: 10.1016/S0893-133X(99)00166-9. [DOI] [PubMed] [Google Scholar]
- Brites D, Fernandes A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front Cell Neurosci. 17. 2015;9:476. doi: 10.3389/fncel.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behaviors and attitudes. Monographs of the Rutgers Center of Alcohol Studies. 1969;6 [Google Scholar]
- Cooney NL, Litt MD, Morse PA, et al. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Crawley E, Kay R, Sillibourne J, Patel P, Hutchinson I, Woo P. Polymorphic haplotypes of the interleukin-10 5' flanking region determine variable interleukin-10 transcription and are associated with particular phenotypes of juvenile rheumatoid arthritis. Arthritis Rheum. 1999;42(6):1101–8. doi: 10.1002/1529-0131(199906)42:6<1101::AID-ANR6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Proinflammatory cytokines. Chest. 2000 Aug;118(2):503–8. doi: 10.1378/chest.118.2.503. 2000 Review. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Thomas SE. Assessing craving for alcohol. Alcohol Res Health. 1999;23:179–186. [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52(1–2):40–51. doi: 10.1016/j.neuint.2007.06.037. Review. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress system--organization, physiology and immunoregulation. Neuroimmunomodulation. 2006;13(5–6):257–67. doi: 10.1159/000104853. Review. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for the DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Version 2.0, 4/97 revision) New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Fox HC, Anderson GM, Tuit K, et al. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012a;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Ansell EB, Simpson C, et al. Direct and indirect effects of immune system adaptations of problem drinking. Alcoholism: Clin Exp Res. 2013a doi: 10.1111/acer.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, et al. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, D'Sa C, Kimmerling A, et al. Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol. 2012b;27:156–166. doi: 10.1002/hup.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39:1527–1537. doi: 10.1038/npp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17(2):103–19. doi: 10.1080/10673220902899680. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, et al. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology. 2013b;38:1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, et al. Involvement of Purinergic P2X4 Receptors in Alcohol Intake of High-Alcohol-Drinking (HAD) Rats. Alcohol Clin Exp Res. 2015;39:2022–2031. doi: 10.1111/acer.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohier B, Goeb JL, Rannou-Dubas K, et al. Hepatitis C, alpha interferon, anxiety and depression disorders: a prospective study of 71 patients. World J Biol Psychiatry. 2003;4:115–118. doi: 10.1080/15622970310029904. [DOI] [PubMed] [Google Scholar]
- Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kühn M, Schuld A, Pollmächer T. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res. 1999;33(5):407–18. doi: 10.1016/s0022-3956(99)00021-7. [DOI] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Schafbauer T, et al. Endotoxin-induced systemic inflammation activates microglia: [(1)(1)C]PBR28 positron emission tomography in nonhuman primates. Neuroimage. 2012;63:232–239. doi: 10.1016/j.neuroimage.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007 May;21(4):374–83. doi: 10.1016/j.bbi.2007.01.010. Review. [DOI] [PubMed] [Google Scholar]
- Izard C. Patterns of emotions: A new analysis of anxiety and depression. New York: New York Academic Press; 1972. [Google Scholar]
- Jin LE. Reducing the harm of stress: medications to rescue the prefrontal cortex and overcome bad habits: the science of stress: focus on the brain, breaking bad habits, and chronic disease. Yale J Biol Med. 2011;84:479–482. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016 Aug;3(8):760–73. doi: 10.1016/S2215-0366(16)00104-8. 2016 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, et al. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin Exp Res. 2007a;31:846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, et al. Production of inflammatory cytokines by peripheral blood monocytes in chronic alcoholism: relationship with ethanol intake and liver disease. Cytometry B Clin Cytom. 2007b;72:408–415. doi: 10.1002/cyto.b.20169. [DOI] [PubMed] [Google Scholar]
- Levitt DE, Duplanty AA, Budnar RG., Jr The effect of post-resistance exercise alcohol ingestion on lipopolysaccharide-stimulated cytokines. Eur J Appl Physiol. 2015 doi: 10.1007/s00421-015-3278-6. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL. Inducing craving for alcohol in the laboratory. Alcohol Res Health. 1999;23:174–178. [PMC free article] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Marcos M, Pastor I, González-Sarmiento R, Laso FJ. Interleukin-10 gene polymorphism is associated with alcoholism but not with alcoholic liver disease. Alcohol Alcohol. 2008;43(5):523–8. doi: 10.1093/alcalc/agn026. [DOI] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol Dis. 2013;54:239–51. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Casachahua JD, Rinker JA, et al. IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav Immun. 2016;51:258–267. doi: 10.1016/j.bbi.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS1, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Potenza MN, Kober H, et al. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J Psychopharmacol. 2015;29:300–311. doi: 10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- Mercer D, Woody G. Addiction Counseling. University of Pennsylvania/ VAMC Center for Studies of Addiction; 1992. Unpublished manuscript. [Google Scholar]
- Milivojevic V, Fox HC, Sofuoglu M, et al. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology. 2016;65:44–53. doi: 10.1016/j.psyneuen.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V, Ansell EB, Simpson C, Siedlarz KM, Sinha R, Fox HC. Peripheral immune system adaptations and alcohol craving in problem non-dependent drinkers. ACER; (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FP, Medeiros JR, Lhullier AC, et al. Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug Alcohol Depend. 2016;158:181–185. doi: 10.1016/j.drugalcdep.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Moser M, Murphy KM. Dendritic cell regulation of TH1–TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidvari K, Casey R, Nelson S, et al. Alveolar macrophage release of tumor necrosis factor-alpha in chronic alcoholics without liver disease. Alcohol Clin Exp Res. 1998;22:567–572. doi: 10.1111/j.1530-0277.1998.tb04294.x. [DOI] [PubMed] [Google Scholar]
- Pentón-Rol G, Cervantes-Llanos M, Martínez-Sánchez G, Cabrera-Gómez JA, Valenzuela-Silva CM, Ramírez-Nuñez O, Casanova-Orta M, Robinson-Agramonte MA, Lopategui-Cabezas I, López-Saura PA. TNF-α and IL-10 downregulation and marked oxidative stress in Neuromyelitis Optica. Journal of Inflammation. 2009;6:18. doi: 10.1186/1476-9255-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlipný J1, Hess Z, Vrzalová J, Rosolová H, Beran J, Petrlová B. Lower serum levels of interleukin-6 in a population sample with symptoms of depression than in a population sample without symptoms of depression. Physiol Res. 2010;59(1):121–6. doi: 10.33549/physiolres.931695. Epub 2009 Feb 27. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relja B, Menke J, Wagner N, et al. Effects of positive blood alcohol concentration on outcome and systemic interleukin-6 in major trauma patients. Injury. 2016;47:640–645. doi: 10.1016/j.injury.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Salome N, Tasiemski A, Dutriez I, et al. Immune challenge induces differential corticosterone and interleukin-6 responsiveness in rats bred for extremes in anxiety-related behavior. Neuroscience. 2008;151:1112–1118. doi: 10.1016/j.neuroscience.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Macdougall MG, Hu F, et al. Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice. Mol Psychiatry. 2007;12:408–417. doi: 10.1038/sj.mp.4001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, et al. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, et al. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, et al. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Stowe RP, Peek MK, Cutchin MP, et al. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol A Biol Sci Med Sci. 2010;65:429–433. doi: 10.1093/gerona/glp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichner G, Horner MD, Harvey RT. Neuropsychological predictors of the attainment of treatment objectives in substance abuse patients. Int J Neurosci. 2001;106:253–263. doi: 10.3109/00207450109149753. [DOI] [PubMed] [Google Scholar]
- Tilg H, Wedemeyer H. UEG Week Vienna 2014 cutting edge symposium: Today's Science, Tomorrow's Medicine session features the immune system - a driving force in digestive health and disease. United European Gastroenterol J. 2014;2:149–150. doi: 10.1177/2050640614528146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine AD, Meyers CA, Kling MA, et al. Mood and cognitive side effects of interferon-alpha therapy. Semin Oncol. 1998;25:39–47. [PubMed] [Google Scholar]
- Verma BK, Fogarasi M, Szabo G. Down-regulation of tumor necrosis factor alpha activity by acute ethanol treatment in human peripheral blood monocytes. J Clin Immunol. 1993;13:8–22. doi: 10.1007/BF00920631. [DOI] [PubMed] [Google Scholar]
- Wallaert B, Aerts C, Colombel JF, et al. Human alveolar macrophage antibacterial activity in the alcoholic lung. Am Rev Respir Dis. 1991;144:278–283. doi: 10.1164/ajrccm/144.2.278. [DOI] [PubMed] [Google Scholar]
- Wen RT, Zhang M, Qin WJ, et al. The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol seeking and consumption in alcohol-preferring Fawn-Hooded rats. Alcohol Clin Exp Res. 2012;36:2157–2167. doi: 10.1111/j.1530-0277.2012.01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]