Abstract
Acute vascular injury occurs in a number of important clinical contexts, including spontaneous disease-related events (e.g. plaque rupture, thrombosis) and therapeutic interventions such as angioplasty, stenting, or bypass surgery. Endothelial cell (EC) disruption exposes the underlying matrix, leading to a rapid deposition of platelets, coagulation proteins, and leukocytes. A thrombo-inflammatory response ensues characterized by leukocyte recruitment, vascular smooth muscle cell (VSMC) activation, and the elaboration of cytokines, reactive oxygen species and growth factors within the vessel wall. A resolution phase of vascular injury may be described in which leukocyte efflux, clearance of debris, and re-endothelialization occurs. VSMC migration and proliferation leads to the development of a thickened neointima that may lead to lumen compromise. Subsequent remodeling involves matrix protein deposition, and return of EC and VSMC to quiescence. Recent studies suggest that specialized proresolving lipid mediators (SPM) modulate key aspects of this response, and may constitute an endogenous homeostatic pathway in the vasculature. SPM exert direct effects on vascular cells that counteract inflammatory signals, reduce leukocyte adhesion, and inhibit VSMC migration and proliferation. These effects appear to be largely G-protein coupled receptor-dependent. Across a range of animal models of vascular injury, including balloon angioplasty, bypass grafting, and experimental aneurysm formation, SPM accelerate repair and reduce lesion formation. With bioactivity in the pM-nM range, a lack of discernible cytotoxicity, and a spectrum of vasculo-protective properties, SPM represent a novel class of vascular therapeutics. This review summarizes current research in this field, including a consideration of critical next steps and challenges in translation.
Keywords: Resolution, specialized pro-resolving mediators, resolvins, omega-3 fatty acids, vascular injury, neointimal hyperplasia, restenosis
Vascular Injury: Clinical Relevance and Unmet Need
Atherosclerosis is a chronic inflammatory disease with potentially devastating consequences in the coronary, cerebrovascular and peripheral vascular systems (e.g. myocardial infarction, stroke, critical limb ischemia). Diet, exercise, lipid lowering and other vasculo-protective medications can help slow progression of disease, however interventions are frequently required for treatment of advanced symptoms. These interventions, both endovascular (angioplasty, stenting) and surgical (endarterectomy, bypass), repair or replace damaged blood vessels and improve end organ perfusion. All of these commonly performed procedures are associated with injury and acute inflammation in the vessel wall. It is now well established that this post-intervention inflammatory response induces a series of events that are central to vascular repair and remodeling. When excessive this response leads to aggressive neointimal hyperplasia (NIH), lumen narrowing (re-stenosis), and recurrent ischemia (Tanaka, Sukhova et al. 1993, Kornowski, Hong et al. 1998, Shah 2003, Muto, Model et al. 2010). Additional interventions to treat re-stenosis in the coronary and peripheral circulation are common, costly, and incur significant risk to affected patients. Accordingly efforts to ameliorate this response remain of major importance in the field of cardiovascular medicine and surgery.
Currently, therapeutic approaches to prevent re-stenosis are focused on anti-proliferative and anti-inflammatory agents, and are limited in efficacy. Importantly, these approaches universally delay rather than accelerate healing of the vessel wall. The “off target” effects of these agents include toxicity to the endothelium with associated risk for thrombosis, and immune cell suppression with associated risk for impaired healing or infection (Joner, Finn et al. 2006, Garg and Mauri 2007, Ostrovsky 2008). Presently these approaches are applied in the form of drug-eluting stents (DES) and drug-coated balloons (DCB) that have made positive impact in the field of coronary intervention and, to a much less degree, in the peripheral circulation. Peripheral artery interventions provide a stringent test of vascular patency with a large burden of disease that frequently extends into small caliber distal vessels, especially in the growing diabetes population presenting with advanced stages of disease. Despite ongoing technical improvements in endovascular technologies, surgical bypass grafting remains an important and commonly employed intervention for those with advanced atherosclerosis. Bypass grafting and other vascular procedures, such as creation of hemodialysis grafts and fistulas, incur a similar process of vessel injury, repair and remodeling that can ultimately lead to renarrowing and failure over time. Clinical trials testing anti-proliferative approaches to reduce NIH in these surgical scenarios have been uniformly disappointing (Alexander, Hafley et al. 2005, Conte, Bandyk et al. 2006, Ostrovsky 2008). Thus, there are no currently available therapies to reduce the incidence of re-stenosis following these common cardiovascular procedures. Risks of adverse effects in surgical patients, including wound complications or early thrombosis, highlight the importance of a safe, homeostatic approach that minimizes cytotoxicity while reducing vessel scarring.
The elucidation of biochemical pathways of resolution (Serhan 2007, Spite and Serhan 2010, Ortega-Gomez, Perretti et al. 2013, Serhan 2014) summarized in this compendium, suggest the hypothesis that excessive vessel scarring following intervention may represent an impairment of resolution. It follows that the local availability or activity of specialized pro-resolving mediators (SPM) may be an important modifier of vascular repair, representing a new potential therapeutic opportunity. This review provides a context for resolution pharmaco-biology in the setting of vascular injury, summarizes the mechanisms through which SPMs facilitate homeostasis within the vasculature, and evidence from animal studies supporting beneficial effects of SPM across a range of vascular injury models. Finally, we will comment on current gaps in knowledge and critical steps towards translation of “resolution pharmacology” in the arena of vascular intervention.
Vascular Injury: From Inflammation through Resolution to Repair
The clinical context of vascular injury takes many forms—e.g acute mechanical trauma from balloon angioplasty or surgery, ischemia-reperfusion of a harvested vein for bypass grafting, implantation of a metallic stent or prosthetic device, hemodynamic stress associated with venous arterialization. Endothelial (EC) injury, with variable damage to underlying vascular smooth muscle cells (VSMC), leads to a rapid and pronounced interaction with circulating blood elements and a thrombo-inflammatory response (Figure 1). This bears resemblance to other scenarios of sterile inflammation, but is compounded by amplification from platelets and elements of the coagulation cascade (e.g. tissue factor, fibrinogen) that are immediately activated once the thrombo-resistance of intact endothelium is lost. Apoptosis of EC and VSMC is an early event. Recruitment of leukocytes, particularly neutrophils (PMNs) and monocytes, to areas of denuded endothelium brings an influx of pro-inflammatory cytokines (e.g. IL-1β, IL-6, TNF-α), chemokines (e.g. MCP-1), and reactive oxygen species into the vessel wall. Together with platelet-derived products (e.g. PDGF) and other growth factors (e.g. bFGF) locally available within the vascular matrix, these mediators rapidly activate VSMCs from a basal contractile state to a synthetic, de-differentiated, neointimal phenotype. Expression of leukocyte adhesion molecules by activated VSMC and EC augments the inflammatory response. The phenotypic switch of VSMC to a synthetic state is a critical event in the vessel repair process that leads to NIH. Activated VSMC demonstrate enhanced migration, proliferation, resistance to apoptosis, expression of pro-inflammatory signals, and elaboration of matrix proteins. Spatial and temporal regulation of this phenotypic switch, and the subsequent conversion of VSMC back to a quiescent contractile state, correlates with the magnitude and distribution of the NIH lesion that results (Tanaka, Sukhova et al. 1993, Kornowski, Hong et al. 1998, Shah 2003, Muto, Model et al. 2010). The exact process by which return to quiescence occurs remains incompletely understood. A resolution phase in the vascular injury setting is characterized by: cessation of PMN recruitment, decline in vessel wall macrophage numbers and conversion from M1 to M2 phenotype, reduction in pro-inflammatory cytokine and growth factor expression, decline in the VSMC proliferative index, regeneration of an intact endothelium, and the initiation of matrix remodeling. Accelerating the transition to the resolution phase following acute vascular injury offers the potential to hasten vessel repair and thereby reduce downstream NIH.
Figure 1.
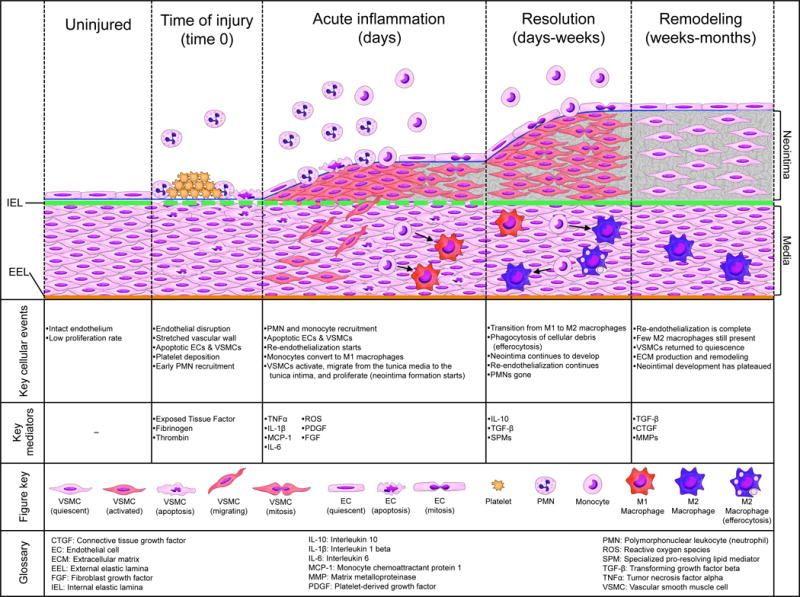
Schematic illustrating the temporal sequence of cellular and molecular events in the vessel wall following acute injury, characterizing the phases of injury, inflammation, resolution, and remodeling.
Clinical evidence linking the magnitude of the acute inflammatory response to the outcomes of vascular interventions comes from studies utilizing systemic biomarkers such as high sensitivity C-reactive protein (hsCRP), fibrinogen, serum amyloid A, interleukin (IL)-1, IL-6, and TNF-α (Buffon, Liuzzo et al. 1999, Schillinger, Exner et al. 2002). Patients with advanced atherosclerosis represent a substrate of chronic low-grade inflammation as a baseline state (Ridker 2003, Owens, Ridker et al. 2007). Initial clinical evidence for a relative “resolution deficit” in atherosclerosis was provided by Ho and colleagues, who demonstrated that circulating levels of the SPM aspirin-triggered lipoxin A4 (ATL; 15-epi-LXA4) were significantly lower in symptomatic peripheral arterial disease patients than in healthy controls, and correlated inversely with clinical severity (Ho, Spite et al. 2010). Similar findings have been reported in patients with coronary artery disease as well as cerebrovascular disease (Elajami, Colas et al. 2016, Thul, Labat et al. 2017). In these settings of advanced atherosclerosis, acute superimposed vascular injury (e.g. angioplasty or surgery) triggers a robust systemic inflammatory response reflected in circulating biomarkers. In a prospective cohort study of patients undergoing lower extremity vein bypass grafting for advanced leg ischemia, pre-operative levels of hsCRP and other inflammatory markers were strongly associated with postoperative events, largely downstream re-interventions for bypass graft stenosis (Owens, Ridker et al. 2007). Elevated plasma inflammatory markers were also associated with impaired early vein graft remodeling post-implantation, suggesting that hemodynamic adaptation of the vein graft to the arterial environment is influenced by the acute inflammatory response (Owens, Kim et al. 2012, Gasper, Owens et al. 2013). Biomarkers of resolution are currently in evolution, limited to date by the requirement of liquid chromatography-tandem mass spectrometry (LC-MS/MS) with validated standards to reliably identify and quantitate the bioactive lipid mediators (e.g. SPM) in blood and other tissues. Ongoing and future studies seek to identify a “resolution index” biomarker(s) that may potentially correlate with clinical disease progression or with responses to illness, injury, or intervention. Conceptually, the peri-procedural setting offers an opportunity for trials of resolution pharmacology to hasten recovery in the cardiovascular patient (Figure 2).
Figure 2.
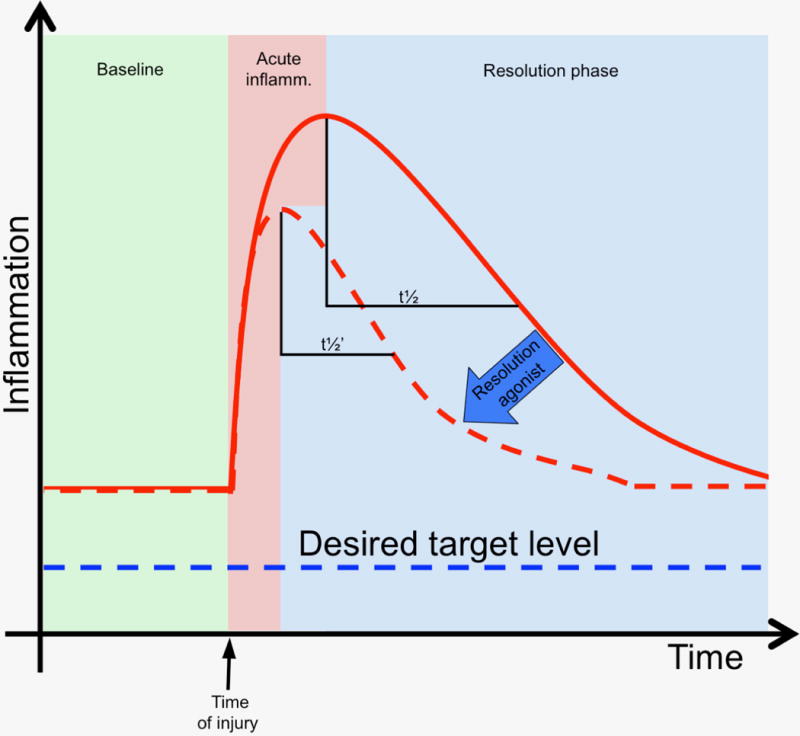
Systemic inflammation in clinical cardiovascular disease and intervention, and the opportunity for resolution pharmacology in the peri-procedural setting. Patients with clinical atherosclerosis manifest chronic low grade inflammation that can me measured by biomarkers such as C-reactive protein (CRP) and others. Acute vascular injury (e.g. angioplasty) is superimposed and generates a prototypic injury response. A resolution index can be measured from the temporal decline in the acute inflammatory response, from peak to 50% of peak values. The dashed curve represents a hypothetical response to a proresolving therapeutic.
Biosynthesis of SPM in the vasculature and evidence for receptor expression and activity
SPM are synthesized from their polyunsaturated fatty acid (PUFA) precursors via sequential actions of lipoxygenases (LOX) and hydrolases. Lipoxins, the first identified SPM, are downstream products of arachidonic acid (AA); the E-series resolvins (RvE) are derived from eicosapentaenoic acid (EPA), whereas the D-series resolvins (RvD), protectins and maresins are derived from docosahexaenoic acid (DHA). Similar to other autocoids, SPM are rapidly inactivated locally by enzymes, such as 15-prostaglandin dehydrogenase (PGDH). SPM biosynthesis has been demonstrated in isolated leukocytes (esp. PMNs and macrophages) as well as via leukocyte-epithelial and leukocyte-endothelial interactions. Sources of SPM within the vasculature remain incompletely defined but their presence has been detected in a number of studies. The presence of endogenous LXA4 after vascular intervention was initially described by Brazinski and colleagues, who identified intraluminal LXA4 after percutaneous transcoronary angioplasty in humans (25 ng/mL) (Brezinski, Nesto et al. 1992). Using LC-MS/MS analysis of whole vessel lysates, we identified various SPMs including RvD1, RvD5, Maresin-1 and LXB4 in rabbit femoral arteries both at baseline and after balloon angioplasty, with a trend towards increased SPM production after injury (Miyahara, Runge et al. 2013). SPM have been identified in mouse atherosclerotic lesions (Fredman, Hellmann et al. 2016, Viola, Lemnitzer et al. 2016) where they correlate with histologic signs of plaque stability. More recently it was demonstrated that isolated human artery segments and primary cultured human vascular cells generate D-series resolvins in the presence of precursors (DHA,17-HDHA) (Chatterjee, Komshian et al. 2017). Conditioned media from these DHA-supplemented vascular cells blunted leukocyte adhesion to activated EC, in a reaction that was attenuated by blocking the RvD1 receptors ALX and GPR32. These studies suggest that endogenous production of SPM within the vessel wall may represent a newly identified paracrine pathway to counter-regulate vascular inflammation and maintain homeostasis.
SPM exert their biologic actions on cells via G-protein coupled receptors (GPCR) such as ALX/FPR2, GPR32, ChemR23, BLT1, and GPR18 (Serhan 2014, Chiang, de la Rosa et al. 2017). Expression of ALX, GPR32, and ChemR23 has been demonstrated in vascular cells and tissues (Ho, Spite et al. 2010, Norling, Dalli et al. 2012, Miyahara, Runge et al. 2013, Petri, Laguna-Fernandez et al. 2015). Studies employing a mouse knockout of the ALX/FPR2 homologue demonstrated its functional role in mediating LxA4-induced effects on VSMC migration, proliferation, and on NIH following carotid ligation in-vivo (Petri, Laguna-Fernandez et al. 2015). However further studies are needed to fully characterize the regulation of SPM receptor expression and activity in vascular cells, and the relationships between SPM synthesis, degradation and downstream signaling events in vascular tissues, in both health and disease.
Direct effects of SPM on vascular cells
Elucidation of the cellular actions of SPM has largely focused on leukocytes, particularly PMN and macrophages (Serhan 2007, Serhan 2014). Effects on platelet aggregation, platelet-PMN interactions, and clot remodeling in-vivo have also demonstrated beneficial antithrombotic properties that are of clear relevance to vascular disease (Dona, Fredman et al. 2008, Chen, Fenet et al. 2009, Fredman and Serhan 2011, Gong, Lin et al. 2015, Elajami, Colas et al. 2016, Lannan, Spinelli et al. 2017). Evidence for direct effects of SPM on EC and VSMC demonstrates an anti-inflammatory, homeostatic profile of actions that may constitute an important vasculo-protective biochemical pathway (Table 1). SPM are bioactive in the pM-nM range, consistent with levels that have been measured in circulating blood (Colas, Shinohara et al. 2014) as well as in a growing range of human biological samples (Brezinski, Nesto et al. 1992, Ho, Spite et al. 2010, Colas, Shinohara et al. 2014, Elajami, Colas et al. 2016, Thul, Labat et al. 2017). Important for their consideration as candidate vascular therapeutics, we have observed no evidence of toxicity in EC or VSMC even when cells are exposed to micromolar ranges of resolvins and other SPM (Conte MS, unpublished data).
Table 1.
Summary of effects of SPM on vascular cells
| cell type | SPM* | Effects observed | Refs.+ |
|---|---|---|---|
| EC | RvD1, MaR1, PD1, LXA4, ATL |
|
Nascimento-Silva (2006), Paul-Clark (2004), Merched (2008), Tian (2009), Zhang (2013), Chatterjee (2014), Zhang (2016), Chattopadhyay (2017), Sok (2017) |
| VSMC | RvD1, RvD2, RvE1, MaR1, ALT |
|
Ho (2010), Miyahara (2013), Chatterjee (2014), Akagi (2015), Hiram (2015), Petri (2015), Mottola (2017), Wu (2017), |
| Fibroblasts | RvE1, LXA4, benzo-LXA4, ATL |
|
Martins (2009), Borgeson (2011), Qu (2012), Roach (2015) |
| Leukocytes | RvD1, RvD2, Mar1 |
|
Duffield (2006), Keyes (2010), Serhan(2014), Miyahara (2013), Akagi (2015), Pope (2016), Wu (2017) |
| Platelets | RvD1, RvE1, PD1 |
|
Dona (2008), Chen (2009), Fredman (2011), Lannan (2017) |
List of SPMs provided, however not every mechanism has been studied for each mediator.
Lists are not comprehensive.
EC = endothelial cell
VSMC = vascular smooth muscle cell
Endothelial cells (ECs) are located on the luminal surface and function to maintain the integrity of the vessel wall. Leukocyte-endothelial cell interactions are central in modulating vascular inflammation. Various reports have demonstrated that SPMs decrease leukocyte-endothelial cell interactions, potentially through up-regulation of endothelial cell nitric oxide (eNOS) and downregulation of adhesion molecules on both leukocytes and endothelial cells. For example. RvD1 and Mar1 were demonstrated to preserve endothelial cell function in various contexts through suppression of reactive oxygen species and regulation of adhesion molecules (Paul-Clark, Van Cao et al. 2004, Tian, Lu et al. 2009, Chattopadhyay, Raghavan et al. 2017). Merched and colleagues studied the actions of various SPMs (RvD1. PD1. LXA4) in vitro on endothelial cells harvested from human aortas and observed down-regulation of adhesion molecules (VCAM-1 and P-selectin) and pro-inflammatory cytokines (MCP-1 and IL-8) (Merched, Ko et al. 2008). Similar results has been observed in examining the effects of RvD1 and MaR1 in vitro on endothelial cells harvested from human greater saphenous veins. In these studies MaR1 attenuated TNF-α induced monocyte adhesion to ECs with associated down-regulation of E-selectin and attenuation of TNF-α induced production of proinflammatory cytokines (PDGF-BB. MCP-1. IL-8. IL-16. ICAM. Eotaxin-2. GM-CSF. TIMP2. MIP1-β. RANTES. IP10) (Chatterjee, Sharma et al. 2014). Endothelial regeneration is an important attribute in the setting of vascular injury, as EC migration and proliferation is required to re-establish a confluent monolayer. One study demonstrated enhancement of endothelial migration by RvD2 (Zhang, Sansbury et al. 2016). Studies from our laboratory have consistently demonstrated that SPM such as RvD1, RvD1, and MaR1 are non-toxic to endothelium and have no significant impact on human EC migration (Conte MS, unpublished data).
Mechanisms of SPM signaling in EC are under continued investigation, focused on modulation of prototypic inflammatory pathways. MaR1 attenuated TNF-α induced activation of the NF-kB pathway (IKK phosphorylation and nuclear translocation of the p65 subunit), as well as reactive oxygen species (ROS) production, with associated down-regulation of NADPH-oxidases (NOX1, NOX2, NOX4) (Chatterjee, Sharma et al. 2014). These effects appear related to a time-dependent increase in intracellular cyclic AMP (cAMP), suggesting a role for the cAMP/PKA pathway. Other investigators have demonstrated similar effects of SPM on intracellular signaling in ECs (Nascimento-Silva, Augusta Arruda et al. 2006, Zhang, Wang et al. 2013).
It is well established that VSMC activation, migration and proliferation are central to the pathobiology of NIH (Tanaka, Sukhova et al. 1993, Kornowski, Hong et al. 1998, Shah 2003, Muto, Model et al. 2010). Attenuation of VSMC migration by SPMs (ATL, RvD1, RvD2, RvE1, MaR1) has been a consistent finding in vitro, both with VSMC harvested from human saphenous veins (Ho, Spite et al. 2010) (Miyahara, Runge et al. 2013) and with arterial VSMC harvested from rodent aortas (Akagi, Chen et al. 2015) (Petri, Laguna-Fernandez et al. 2015, Wu, Mottola et al. 2017) as well as human pulmonary arteries (Hiram, Rizcallah et al. 2015). This effect has been demonstrated across several prototypic VSMC motogens including PDGF, thrombin, angiotensin II, TNF-α and IL-6 (Hiram, Rizcallah et al. 2015, Mottola, Wu et al. 2017, Wu, Mottola et al. 2017). Associated with this effect, resolvins induce rapid and reversible changes in VSMC cell-shape with a decreased length:width ratio corresponding to an anti-migratory phenotype (Ho, Spite et al. 2010, Miyahara, Runge et al. 2013, Mottola, Wu et al. 2017, Wu, Mottola et al. 2017). The anti-migratory effects of AT-RvD1 in human saphenous vein VSMC appear dependent on the cAMP/PKA pathway, with downstream involvement of Rac1, VASP, and paxillin ((Mottola, Wu et al. 2017)).
Several SPMs (RvD1, RvD2, MaR1) have demonstrated modest anti-proliferative effects on VSMC in vitro (Miyahara, Runge et al. 2013, Akagi, Chen et al. 2015, Petri, Laguna-Fernandez et al. 2015, Wu, Mottola et al. 2017). Unlike migration, proliferation can be easily observed in vivo and intravascular, perivascular and systemic delivery of SPMs (RvD2, RvD1 and RvD1/MaR1, respectively) attenuated VSMC proliferation in various models of vascular injury (Miyahara, Runge et al. 2013, Akagi, Chen et al. 2015, Wu, Mottola et al. 2017). Importantly, there has been no evidence of VSMC cytotoxicity related to SPMs within their therapeutic range either in vitro (Ho, Spite et al. 2010, Miyahara, Runge et al. 2013, Chatterjee, Sharma et al. 2014, Wu, Mottola et al. 2017) or in vivo (Wu, Mottola et al. 2017).
For vascular interventions that denude the endothelium (i.e. angioplasty and stenting), leukocyte-VSMC interactions are particularly important for early leukocyte recruitment. SPMs (RvD, RvD2, MaR1) decrease monocyte-VSMC adhesion with associated down-regulation of adhesion molecule expression (ICAM-1, VCAM-1) (Miyahara, Runge et al. 2013) (Chatterjee, Sharma et al. 2014). SPMs (RvD1, RvD2, MaR1) modulate inflammatory cytokine expression from TNF-α stimulated VSMC in vitro (decreased secretion of MCP-1, IL-1α, IL-1β, IL-6, IL-8 and GM-CSF) (Ho, Spite et al. 2010, Miyahara, Runge et al. 2013, Chatterjee, Sharma et al. 2014, Akagi, Chen et al. 2015). In vivo, RvD2 (10nM, intra-arterial for 20 minutes after injury) treatment decreased the expression of inflammatory cytokines (TNF-α, MCP-1, and IL-1α) in rabbit femoral arteries after angioplasty, which injures the vessel wall via stretch as well as endothelial denudation (Miyahara, Runge et al. 2013).
Many SPM-mediated intracellular signaling mechanisms demonstrated in leukocytes and endothelial cells appear relevant within VSMCs. Attenuation of the NF-kB signaling pathway has been observed in vitro after stimulation with TNF-α (RvD1, RvD2, MaR1) (Miyahara, Runge et al. 2013, Chatterjee, Sharma et al. 2014, Akagi, Chen et al. 2015, Wu, Mottola et al. 2017) and in vivo in rat carotid arteries after angioplasty (RvD1, 200 ng perivascular) (Wu, Mottola et al. 2017). Attenuation of VSMC-derived ROS by SPMs has been observed in vitro after stimulation with TNF-α (RvD1, RvD2, MaR1) (Miyahara, Runge et al. 2013, Chatterjee, Sharma et al. 2014, Akagi, Chen et al. 2015) and in vivo in rabbit femoral arteries and rat carotid arteries after angioplasty (Miyahara, Runge et al. 2013, Wu, Mottola et al. 2017). SPM (RvD1, MaR1) signaling in VSMC appears to involve the cAMP/PKA pathway (Chatterjee, Sharma et al. 2014, Mottola, Wu et al. 2017, Wu, Mottola et al. 2017).
To date, there is limited data relating to the effect of SPMs on vascular fibroblasts. Extrapolating from studies on vascular fibroblasts using precursors fatty acids, we find suggestions that SPMs may inhibit macrophage-mediated pro-inflammatory activation within vascular fibroblasts (Endo, Sano et al. 2014) and that SPMs may attenuate proliferation as well as conversion to pro-fibrotic myofibroblasts (Faggin, Puato et al. 2000). The myofibroblast is particularly important in the context of vein bypass grafting, as these cells not only can migrate to contribute to neointimal formation but can cause negative (inward) remodeling of the vessel wall (Garbey and Berceli 2013, Owens, Gasper et al. 2015). Direct evidence for anti-fibrotic effects of SPMs (RvE1, LXA4 and its synthetic analog benzo-LXA4) has been provided in a rodent model of renal fibrosis (unilateral ureteric obstruction), where these SPM attenuated myofibroblast activation and proliferation (Borgeson, Docherty et al. 2011, Qu, Zhang et al. 2012). Similarly, treatment with SPMs (ALX4, ATL) in models of pulmonary fibrosis attenuates myofibroblast activation and proliferation both in vitro and in vivo (Martins, Valenca et al. 2009, Roach, Feghali-Bostwick et al. 2015). These studies of renal and pulmonary myofibroblasts can be extrapolated to vascular fibroblasts in general and suggest that SPMs may provide homeostatic actions on all three layers of the vessel wall.
In-vivo effects of SPM in animal models of vascular injury
Neointimal hyperplasia (NIH)
There are various “proof-of-concept” animal models through which restenosis can be studied, with a common theme of inducing inflammation within the vessel wall. Ligation of the distal common carotid artery in mice produces profound alterations in flow dynamics proximally, leading to a extensive neointimal hyperplasia in the presence of an intact endothelium (Kumar and Lindner 1997, Holt and Tulis 2013). Petri and colleagues used this model to study the effects of aspirin-triggered lipoxin (ATL), with a focus on its signaling mechanism through the ALX receptor. Their investigations involved administration of ATL (250 ng) systemically through a continuous subcutaneous pump placed after carotid ligation and demonstrated a 50% decrease in neointimal formation by ATL in wild type mice, with no effect in ALX knockout mice (Petri, Laguna-Femandez et al. 2015). This murine model has also been used to study the effect of RvD2 and MaR1 on neointimal hyperplasia (Akagi, Chen et al. 2015). Serial intra-peritoneal injections of either RvD2 or MaR1 (100 ng injection at 0, 1, 3, 5, and 7 days after ligation) were found to result in a 62% decrease in neointimal formation by RvD2 and a 67% decrease in neointimal formation by MaR1. Of note, treatment with either SPM decreased neutrophil and macrophage recruitment to the vessel wall, with increased polarization of M2 macrophages, and reduced VSMC proliferation in this model (Akagi, Chen et al. 2015).
Although low flow-induced remodeling models provide useful information in the context of restenosis, models involving balloon angioplasty provide more fidelity to clinical vascular interventions. Balloon angioplasty of the rat carotid artery is a well-established model of stretch and endothelial denudation-induced injury and utilizes a similar catheter to that used for coronary and peripheral vascular interventions. Angioplasty produces a prototypic response to injury, involving VSMC migration to the intima, proliferation within the intima and subsequent formation of neointimal hyperplasia (Clowes, Reidy et al. 1983, Clowes, Reidy et al. 1983). Perivascular application of RvD1 (200 ng, delivered via either a biodegradable film or via a pluronic gel) significantly inhibited neointimal hyperplasia in this model. RvD1-loaded “wraps” reduced neointimal formation by 59% versus no-wrap controls and 45% versus vehicle-wrap controls, while RvD1-loaded gels reduced neointimal formation by 49% versus no-gel controls and 52% vehicle-gel controls. Of note, neither perivascular treatment was associated with infection, thrombosis or negative vessel remodeling. Proliferation, NF-kB activation and oxidative stress in the wall were all significantly lower in arteries treated with RvD1 (Wu, Mottola et al. 2017).
Balloon angioplasty of rabbit femoral arteries creates stretch and endothelial denudation-induced injury with ensuing neointimal hyperplasia similar to clinical restenosis (Simosa, Wang et al. 2005). Intraluminal incubation with RvD2 (10 nM) decreased neointimal hyperplasia in this model by 29% at 28 days post-injury, with associated reductions in leukocyte recruitment (41%), proliferation (51%), ROS and expression of inflammatory genes (TNF-α, MCP-1, and IL-1α) at 3 days post-injury. Of note, endogenous production of various SPMs was detected in both uninjured and injured rabbit arteries, with a trend towards increased SPM production after injury (Miyahara, Runge et al. 2013).
Vein bypass grafting offers the most durable long-term outcomes for peripheral and coronary occlusive disease, however injury related to vein harvest and hemodynamic stresses during arterialization cause inflammatory changes and subsequent restenosis. Many of the mechanisms of failure after vein bypass appear to be similar to those after arterial injury, as previously described (Muto, Model et al. 2010, Owens, Gasper et al. 2015, de Vries, Simons et al. 2016). A rabbit vein graft model can be used to investigate the neointimal response after vein bypass (Jiang, Wu et al. 2004, Wang, Sui et al. 2005, Owens, Gasper et al. 2015). We recently demonstrated that perivascular delivery of RvD1 (1 mg, via either a biodegradable film or gel) attenuated vein graft neointimal hyperplasia in this model. Specifically, RvD1-loaded gels reduced neointimal formation by ~60% while RvD1-loaded perivascular “wraps” reduced neointimal formation by 50% versus bypass-only controls. Perivascular RvD1 treatments did not influence rates of graft thrombosis, wound infection or death. Total leukocyte infiltration, macrophage infiltration as well as cellular proliferation were significantly lower in vein grafts treated with perivascular RvD1 (Wu, Werlin et al. 2017).
Ischemia and ischemia-reperfusion
Resolution biology is relevant for other acute vascular events, such as renal, mesenteric, cerebral and myocardial ischemia. Ischemia-reperfusion models involve end organ ischemia followed by a massive inflammatory insult during reperfusion, during which specific temporal spatial relationships are essential to prevent or minimize permanent damage. SPM administration in several of these models has suggested therapeutic potential. Duffield and colleagues demonstrated that systemic administration of either an RvD cocktail (3.5 – 35 μg total of a RvD1/RvD2/RvD3 cocktail prior to ischemia) or PD1 (3.5 – 35 μg prior to ischemia) decreased leukocyte infiltration, preserved renal function and inhibited renal fibrosis in a murine model of renal ischemia-reperfusion (Duffield, Hong et al. 2006). Brancaleone and colleagues demonstrated that intravenous administration of LXA4 (1 – 100 ng prior to reperfusion) decreased platelet-neutrophil aggregates in a murine model of mesenteric ischemia-reperfusion (Brancaleone, Gobbetti et al. 2013). Smith and colleagues reported that intravenous administration of ATL (0.5 – 4 μg prior to reperfusion) decreased leukocyte-endothelial cell interactions and provided a survival benefit in a murine model of cerebral ischemia-reperfusion (Smith, Gil et al. 2015). Keyes and colleagues employed intravenous administration of RvE1 (9 – 90 μg prior to reperfusion) in a rodent model of cardiac ischemia-reperfusion to decrease leukocyte infiltration as well as cardiomyocyte death and limit infarction size (Keyes, Ye et al. 2010).
Gilbert and colleagues demonstrated intraventricular injection of RvD1 (1 μg, at the time of coronary ligation) reduces infarct size and improves functional recovery in a rat model of myocardial infarction (Gilbert, Bernier et al. 2014). Similarly, subcutaneous delivery of RvD1 (3 μg/kg/day) decreases left ventricular scarring and improves left ventricular function in a mouse model of myocardial infarction (Kain, Ingle et al. 2015). Zhang and colleagues recently demonstrated improved skeletal muscle regeneration with administration of exogenous RvD2 in a mouse model of hindlimb ischemia (Zhang, Sansbury et al. 2016). All of these studies suggest deficient resolution may play a role in the downstream end organ pathogenesis after acute ischemia.
Aneurysm disease
Inflammation within arterial walls can lead to formation of life-threatening aneurysms, such as abdominal aortic aneurysms (AAA) (Shimizu, Mitchell et al. 2006). Various murine models of AAA formation have been developed to investigate therapeutics for treatment of AAA with most inhibitory strategies aimed at early inflammatory events. Treatment with n-3 PUFA has previously been implicated as a potential therapeutic strategy in this context (Wales, Kavazos et al. 2014). Pope and colleagues demonstrated that systemic administration of either RvD1 or RvD2 (100 ng/kg IP every 3 days) significantly decreased AAA formation in a surgical elastase-perfusion model, associated with preservation of elastin, reduced macrophage (but not T-cell) infiltration, a broad reduction in local inflammatory cytokine signals (TNF-α, IL-1α, IL-1β, MCP-1, CXCL-1, RANTES), increase anti-inflammatory cytokines (IL-10), and reduction in MMP activity by gelatin zymography (Pope, Salmon et al. 2016). When specifically examined, RvD2 treatment resulted in a shift in aortic wall macrophage phenotype favoring M2 polarization. They also examined the effects of RvD2 in a non-surgical model of angiotensin II infusion in the ApoE(−/−) mouse, with similar overall findings. Importantly, these investigators also tested the effects of RvD2 in a treatment model three days after aneurysms had been initiated, observing a 25% reduction in subsequent aortic dilation. Clinical relevance of inflammation-resolution has been demonstrated after surgical repair of AAA, with a distinct subset of patients having measurable early increases in SPM pathways postoperatively (Pillai, Leeson et al. 2012). These studies suggest that SPM may have important effects in both aneurysm pathogenesis and in postoperative repair.
Translation: resolution pharmacology in vascular injury
Although the optimal pharmacokinetics for therapeutic application of SPM after vascular injury remains unknown, local delivery at the time of injury might be ideal in surgical settings. Local approaches are also advantageous because enzymes that rapidly degrade SPM such as 15-PGDH are ubiquitous. Local vascular delivery can be achieved through direct injection into the vessel wall, drug coated balloons or drug eluting stents after endovascular interventions (angioplasty, stenting) or through perivascular approaches (gels, films) after open surgical procedures (endarterectomy, bypass). Recent studies have demonstrated efficacy in reducing NIH in animal models with both intraluminal and perivascular delivery of D-series resolvins (Miyahara, Runge et al. 2013, Wu, Mottola et al. 2017). For local vascular delivery, the small size and lipophilic nature of SPM as a class provide favorable tissue transfer properties similar to those of currently available therapies (Kotani, Awata et al. 2006, Tepe, Zeller et al. 2008, Hawkins and Hennebry 2011).
Perivascular delivery of SPM after open surgical procedures is facilitated as the target vessel is exposed. To maintain controlled and sustained delivery, biodegradable carrier gels or films can be utilized (Miyahara, Runge et al. 2013, Lance, Chatterjee et al. 2017, Wu, Mottola et al. 2017). We recently described a thin film poly(lactic-co-glycolic acid) [PLGA] device that allows for sustained and directed release of RvD1 that can be oriented towards the vessel wall as a “wrap” (Lance, Chatterjee et al. 2017). The device eluted biologically active RvD1 for more than 3 weeks in vitro. A similar approach was taken by Sok et al in loading AT-RvD1 into PLGA scaffolds implanted under mouse skin (Sok, Tria et al. 2017). Sustained release of SPM (e.g. weeks) is likely important for clinical vascular applications as the healing phase in human arteries is prolonged compared to the small animal models. Of note, stable synthetic benzo-analogs for various SPMs have recently been developed which resist degradation, but have not yet been investigated in the vascular arena (Petasis, Keledjian et al. 2008, Orr, Colas et al. 2015).
Systemic administration of SPM or augmentation of their biosynthetic pathways may also provide an approach to improve outcomes of clinical vascular interventions. The specific actions of SPMs could explain some of the cardio-protective benefit derived from dietary intake of their precursor omega-3 fatty acids (DHA and EPA) (Bang, Dyerberg et al. 1976, Dyerberg, Bang et al. 1978. Kagawa, Nishizawa et al. 1982, Investigators 1999, Investigators 2002, Yates, Calder et al. 2014). However, aggregate results of clinical trials involving omega-3 fatty acids in the cardiovascular setting are conflicting (Kris-Etherton 2002, Filion, Khoury et al. 2010, Mozaffarian and Wu 2011). These differences are likely related to the heterogeneity of these cohorts in addition to relatively low doses of omega-3 fatty acids that were administered (Rizos, Ntzani et al. 2012). Additionally, a fundamental challenge to these nutritional trials is the importance of the balance that exists between oral intake and metabolism of omega-3 and omega-6 fatty acids. This delicate balance may be dysregulated in aging and obesity, leading to impaired resolution (Lopez, Kabarowski et al. 2015, Halade, Kain et al. 2016). In both transgenic mice and rabbits overexpressing 15-LOX, one of the key enzymes involved in the biosynthesis of SPMs, atherogenesis is significantly reduced compared to wild type controls when these animals are fed a standard chow diet (Serhan, Jain et al. 2003, Merched, Ko et al. 2008). However, the same transgenic mice fed a high-cholesterol diet developed more significant atherosclerosis compared to wild type controls (Merched, Serhan et al. 2011). The reason for this is rooted in the fact that 15-LOX can also contribute to oxidation of low-density lipoprotein (Fredman and Spite 2017). In this scenario, diet composition determined the preferred pathway through which fatty acids were metabolized, leading to either SPM production or the alternative formation of pro-atherogenic lipids.
It has been well established that oral supplementation with omega-3 fatty acids or diets rich in their marine sources increase both plasma and cell-membrane levels of EPA and DHA (Grenon, Owens et al. 2015, Wang, Hjorth et al. 2015). Unfortunately, the relationship between blood levels of these n-3 fatty acids and the downstream biochemical pathways producing SPM is poorly understood. The physiological impact of oral supplementation of n-3 PUFA for each individual is variable depending on baseline characteristics such as prior dietary intake and hereditary metabolic factors (von Schacky 2014). Recently, the OMEGA-PAD-I Trial demonstrated the effects of short-term n-3 fatty acid supplementation on altering biochemical SPM pathways in PAD patients (Grenon, Owens et al. 2015). In this study, eighty subjects were randomized to either 4.4g of fish oil, corresponding to 2.6g of EPA and 1.8g of DHA daily, or placebo for one month. In the fish oil group there was a significant increase in the plasma levels of several downstream SPM pathway markers. Unfortunately not all lipid mediators that were investigated in the study were detected in subject plasma, so changes in most of the bioactive SPMs could not be assessed. This study provides a basis for continued investigation of oral supplementation of n-3 fatty acids in the context of vascular disease. Future efforts in this regard would benefit from greater consistency in formulations and dosing, and potential development of oral SPM cocktails.
In murine models of acute inflammation, such as peritonitis, periodontitis, and sepsis, various studies have demonstrated that oral administration of SPMs is effective (Spite, Norling et al. 2009, Recchiuti, Codagnone et al. 2014, Hasturk, Abdallah et al. 2015, Chacon, Phillips et al. 2016). The effect of omega-3 fatty acids in acute illness in humans has been studied frequently in the context of the intensive care unit. The most comprehensive meta-analysis on this topic has concluded that parenteral treatment with omega-3 containing lipid emulsions significantly reduces infections and trended towards a reduction in the number of days of mechanical ventilation as well as overall hospital length of stay (Manzanares, Langlois et al. 2015). Additionally, in subgroup analysis there was a trend toward decreased mortality in patients who received n-3 fatty acids via enteral nutrition. In a cohort of subjects undergoing major hepatobiliary operations, it was found that preoperative treatment with EPA for 5 days lead to significantly increased plasma RvE1 levels and fewer infectious complications, as well as overall lesser severity of complications, compared to a control group (Uno, Furukawa et al. 2016). Collectively, these studies begin to set the stage for future investigation of the effects of oral SPM or their precursors in the setting of an acute vascular intervention.
These studies also highlight a number of relevant issues and obstacles to systemic resolution therapies. First, there is a complex relationship between omega-3 and omega-6 fatty acids (Lands 1992, Yaqoob, Pala et al. 2000, Kris-Etherton 2002, Yates, Calder et al. 2014). Second, downstream bioactive mediators (SPMs) may be more potent and biologically relevant than their nutritional precursor fatty acids (Ho, Spite et al. 2010, Fredman and Serhan 2011) (Duffield, Hong et al. 2006). Third, biosynthesis pathways for both omega-3 and omega-6 fatty acids are complex and involve competition for enzymes to production various bioactive mediators (Levy, Clish et al. 2001, Serhan 2007, Spite and Serhan 2010, Merched, Serhan et al. 2011, Colas, Shinohara et al. 2014, Serhan 2014, Poorani, Bhatt et al. 2015) and this competition can produce isomers without proresolving actions (Dona, Fredman et al. 2008, Spite, Norling et al. 2009). Furthermore, similar pathways might play antagonistic roles in different cell types and/or different species (Wittwer and Hersberger 2007, Chatterjee, Komshian et al. 2017) and additional factors such as aging (common in the atherosclerosis population) may alter the biosynthetic pathways (Halade, Kain et al. 2016). An additional consideration is that oral administration of precursors might not produce adequate systemic or local amounts of specific bioactive mediators (Endo, Sano et al. 2014), especially in the setting of a high cholesterol diet (Faggin, Puato et al. 2000). Finally, and perhaps most importantly, tissue distribution and metabolism of orally administered SPM are not well understood and improved formulations will likely be needed to achieve consistent therapeutic levels in target tissues. A more complete understanding of cellular mechanisms of resolution within the vasculature (including SPM biosynthesis, degradation and receptor specificity and expression) is needed to accelerate the translation of resolution biology to vascular therapies.
Conclusions and next steps
In summary, the pharmaco-biology of resolution is a young and rapidly expanding field, with potential benefits across a wide range of disease states. The need for improved adjuvant therapies after cardiovascular interventions is clear, and restenosis remains one of the greatest challenges for cardiovascular biologists, interventionalists and surgeons alike (Hedman, Hartikainen et al. 2003, Conte, Bandyk et al. 2006). SPM exert homeostatic effects on vascular cells and their interactions with blood elements, reducing inflammation and improving healing in several preclinical models of vascular injury. Recent work highlights their potential as anti-restenosis agents. Current scientific challenges in the field include more readily available analytics, as well as optimal therapeutic formulations for early stage clinical studies. This review provides cautious optimism for the future therapeutic use of SPMs or their analogues in vascular injury, and highlights the need for ongoing basic and translational research.
Table 2.
Summary of effects of SPM in animal models of vascular injury
| Study | Animal Model | SPM and Delivery | Findings+ |
|---|---|---|---|
| Akagi et al (2015) | Carotid ligation (mouse) |
Systemic RvD2 or MaR1 (100 ng IP day 0, 1, 3, 5, 7) |
62–67% decrease in neointimal formation vs controls (14 days post-injury) |
| Petri et al (2015) | Carotid ligation (mouse) |
Systemic ATL (250 ng, cont SQ pump) |
50% decrease in neointimal formation by ATL in wild type mice, with no effect in ALX knockout mice (4 weeks post-injury) |
| Wu et at (2017) | Carotid angioplasty (rat) |
Perivascular RvD1 (200 ng, via wrap or gel) |
45–59% decrease in neointimal formation vs controls (14 days post-injury) |
| Miyahara et al (2013) | Femoral angioplasty (rabbit) |
Intraluminal RvD2 (10 nM ×20 min after angio) |
29% decrease in neointimal formation vs control (28 days post-injury) |
| Wu et al (2017) | Vein bypass (rabbit) |
Perivascular RvD1 (1 mg, via wrap or gel) |
38–63% decrease in neointimal formation vs controls (28 days post-injury) |
| Duffield et al (2006) | Renal I/R (mouse) |
Systemic RvD(1–3) or PD1 (3.5–35 μg prior to ischemia) |
Decrease leukocyte infiltration, preserve renal function and inhibit renal fibrosis |
| Brancaleone et al (2013) | Mesenteric I/R (mouse) |
Systemic LXA4 (1–100 ng prior to reperfuse) |
Decrease platelet-neutrophil aggregates |
| Smith et al (2015) | Cerebral I/R (mouse) |
Systemic ATL (0.5–4 μg prior to reperfuse) |
Decrease leukocyte-endothelial cell interactions and decrease mortality |
| Keyes et al (2010) | Cardiac I/R (rat) |
Systemic RvE1 (9–90 μg prior to reperfuse) |
Decrease leukocyte infiltration, decrease cardiomyocyte death and limit infarction size |
| Gilbert et al (2014) | Coronary ligation (rat) |
Systemic RvD1 (1 μg, at time of ischemia) |
Decrease infarct size and improve functional recovery |
| Kain et al (2015) | Coronary ligation (mouse) |
Systemic RvD1 (3 μg/kg/day SQ) |
Decrease left ventricular scarring and improves left ventricular function |
| Zhang et al (2016) | Hindlimb ischemia (mouse) |
Systemic RvD2 | Increased skeletal muscle regeneration |
| Pope et al (2016) | AAA formation (murine) |
Systemic RvD1 or RvD2 (100 ng/kg IP every 3 days) |
25–41% decrease in aortic diameter vs controls |
List of SPMs provided, however not every mechanism has been studied for each mediator.
Primary outcome listed only
I/R = ischemia reperfusion
AAA = abdominal aortic aneurysm
Acknowledgments
This work was supported by funding from National Heart, Lung and Blood Institute Grants HL119508 (MSC) and F32HL123318 (BW).
List of Acronyms Used
- AA
arachidonic acid
- AAA
abdominal aortic aneurysm
- ApoE
apolipoprotein-E
- ATL
aspirin-triggered lipoxin
- AT-RvD1
aspirin-triggered resolvin D1
- cAMP
cyclic adenosine monophosphate
- CRP
C-reactive protein
- DCB
drug coated balloon
- DES
drug eluting stent
- DHA
docosaheaenoic acid
- EC
endothelial cell
- EPA
eicosapentaenoic acid
- FGF
fibroblast growth factor
- GPCR
G-protein couple receptor
- 17-HDHA
17-hydroxy-docosahexaenoic acid
- hsCRP
high sensitivity C-reactive protein
- IL-1, 6, 8
interleukins (−1,6,8)
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LOX
lipoxygenase
- LXA4, LXB4
lipoxin-A4, B4
- M1/M2
macrophage phenotypes
- MaR1
maresin-1
- MCP-1
monocyte chemoattractant protein-1
- MMP
matrix metalloprotease
- NIH
neointimal hyperplasia
- PD1
protectin D1
- PDGF
platelet derived growth factor
- PGDH
prostaglandin dehydrogenase
- PKA
protein kinase A
- PLGA
poly (lactic-co-glycolic acid)
- PMN
polymorphonuclear leukocyte
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- Rv(D, E)
resolvin (D,E)
- SPM
specialized pro-resolving mediator
- TNF-α
tumor necrosis factor-α
- VSMC
vascular smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
MSC is an inventor on a patent assigned to Regents of the University of California and Brigham and Women’s Hospital.
References
- Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 2015;29(6):2504–2513. doi: 10.1096/fj.14-265363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J, Hafley G, Harrington R, Peterson E, Ferguson TJ, Lorenz T, Goyal A, Gibson M, Mack M, Gennevois D, Califf R, Kouchoukos N, P.I. Investigators Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294(19):2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- Bang HO, Dyerberg J, H N. The composition of food consumed by Greenland Eskimos. Acta Med Scand. 1976;200(1–2):69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- Borgeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O’Sullivan TP, Guiry PJ, Goldschmeding R, Higgins DF, Godson C. Lipoxin A(4) and benzo-lipoxin A(4) attenuate experimental renal fibrosis. FASEB J. 2011;25(9):2967–2979. doi: 10.1096/fj.11-185017. [DOI] [PubMed] [Google Scholar]
- Brancaleone V, Gobbetti T, Cenac N, le Faouder P, Colom B, Flower RJ, Vergnolle N, Nourshargh S, Perretti M. A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation. Blood. 2013;122(4):608–617. doi: 10.1182/blood-2013-04-496661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezinski DA, Nesto RW, Serhan CN. Angioplasty Triggers Intracoronary Leukotrienes and Lipoxin A4. Circulation. 1992;86:56–63. doi: 10.1161/01.cir.86.1.56. [DOI] [PubMed] [Google Scholar]
- Buffon A, Liuzzo G, Biasucci LM, Pasqualetti P, Ramazzotti V, Rebuzzi A, Crea F, Maseri A. Preprocedural Serum Levels of C-Reactive Protein Predict Early Complications and Late Restenosis After Coronary Angioplasty. J Am Coll Cardiol. 1999;34:1512–1521. doi: 10.1016/s0735-1097(99)00348-4. [DOI] [PubMed] [Google Scholar]
- Chacon AC, Phillips BE, Chacon MA, Brunke-Reese D, Kelleher SL, Soybel DI. Oral omega-3 fatty acids promote resolution in chemical peritonitis. J Surg Res. 2016;206(1):190–198. doi: 10.1016/j.jss.2016.06.036. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Komshian S, Sansbury BE, Wu B, Mottola G, Chen M, Spite M, Conte MS. Biosynthesis of pro-resolving lipid mediators by vascular cells and tissues. FASEB J. 2017 doi: 10.1096/fj.201700082R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Sharma A, Chen M, Toy R, Mottola G, Conte MS. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS One. 2014;9(11):e113480. doi: 10.1371/journal.pone.0113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay R, Raghavan S, Rao GN. Resolvin D1 via prevention of ROS-mediated SHP2 inactivation protects endothelial adherens junction integrity and barrier function. Redox Biol. 2017;12:438–455. doi: 10.1016/j.redox.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Fenet B, Michaud S, Tomczyk N, Vericel E, Lagarde M, Guichardant M. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett. 2009;583(21):3478–3484. doi: 10.1016/j.febslet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Chiang N, de la Rosa X, Libreros S, Serhan CN. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J Immunol. 2017;198(2):842–851. doi: 10.4049/jimmunol.1601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. Lab Invest. 1983;49(3):327–333. [PubMed] [Google Scholar]
- Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307(1):C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS, P.I. Investigators Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- de Vries MR, Simons KH, Jukema JW, Braun J, Quax PH. Vein graft failure: from pathophysiology to clinical outcomes. Nat Rev Cardiol. 2016;13(8):451–470. doi: 10.1038/nrcardio.2016.76. [DOI] [PubMed] [Google Scholar]
- Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112(3):848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D Series and Protectin D1 Mitigate Acute Kidney Injury. The Journal of Immunology. 2006;177(9):5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- Dyerberg J, Bang HO, Stoffersen E. Eicosapentaenoic Acid and Prevention of Thrombosis and Atherosclerosis. The Lancet. 1978;8081:117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016;30(8):2792–2801. doi: 10.1096/fj.201500155R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J Exp Med. 2014;211(8):1673–1687. doi: 10.1084/jem.20132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggin E, Puato M, C A, Franch R, Pauletto P, Sartore S. Fish Oil Supplementation Prevents Neointima Formation in Nonhypercholesterolemic Balloon-Injured Rabbit Carotid Artery by Reducing Medial and Adventitial Cell Activation. Arterioscler Thromb Vasc Biol. 2000;20:152–163. doi: 10.1161/01.atv.20.1.152. [DOI] [PubMed] [Google Scholar]
- Filion KB, Khoury FE, Schiller I, Dendukuri N, Brophy JM. Omega-3 fatty acids in high-risk cardiovascular patients: a meta-analysis of randomized controlled trials. BMC Cardiovascular Disorders. 2010;10(24):1–11. doi: 10.1186/1471-2261-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859. doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G, Serhan CN. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem J. 2011;437(2):185–197. doi: 10.1042/BJ20110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G, Spite M. Specialized pro-resolving mediators in cardiovascular diseases. Mol Aspects Med. 2017 doi: 10.1016/j.mam.2017.02.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbey M, Berceli SA. A dynamical system that describes vein graft adaptation and failure. J Theor Biol. 2013;336:209–220. doi: 10.1016/j.jtbi.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P, Mauri L. The conundrum of late and very late stent thrombosis following drug-eluting stent implantation. Curr Opin Cardiol. 2007;22:565–571. doi: 10.1097/HCO.0b013e3282f02100. [DOI] [PubMed] [Google Scholar]
- Gasper WJ, Owens CD, Kim JM, Hills N, Belkin M, Creager MA, Conte MS. Thirty-day vein remodeling is predictive of midterm graft patency after lower extremity bypass. J Vasc Surg. 2013;57(1):9–18. doi: 10.1016/j.jvs.2012.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert K, Bernier J, Godbout R, Rousseau G. Resolvin D1, a metabolite of omega-3 polyunsaturated fatty acid, decreases post-myocardial infarct depression. Mar Drugs. 2014;12(11):5396–5407. doi: 10.3390/md12115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Lin M, Piao L, Li X, Yang F, Zhang J, Xiao B, Zhang Q, Song WL, Yin H, Zhu L, Funk CD, Yu Y. Aspirin enhances protective effect of fish oil against thrombosis and injury-induced vascular remodelling. Br J Pharmacol. 2015;172(23):5647–5660. doi: 10.1111/bph.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenon SM, Owens CD, Nosova EV, Hughes-Fulford M, Alley HF, Chong K, Perez S, Yen PK, Boscardin J, Hellmann J, Spite M, Conte MS. Short-Term, High-Dose Fish Oil Supplementation Increases the Production of Omega-3 Fatty Acid-Derived Mediators in Patients With Peripheral Artery Disease (the OMEGA-PAD I Trial) J Am Heart Assoc. 2015;4(8):e002034. doi: 10.1161/JAHA.115.002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D - and E - series resolvins to modulate cardio - splenic and cardiorenal network following myocardial infarction. Aging. 2016;8(11):2611–2634. doi: 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Abdallah R, Kantarci A, Nguyen D, Giordano N, Hamilton J, Van Dyke TE. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35(5):1123–1133. doi: 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BM, Hennebry TA. Local paclitaxel delivery for treatment of peripheral arterial disease. Circ Cardiovasc Interv. 2011;4(3):297–302. doi: 10.1161/CIRCINTERVENTIONS.110.961052. [DOI] [PubMed] [Google Scholar]
- Hedman M, Hartikainen J, Syvanne M, Stjernvall J, Hedman A, Kivela A, Vanninen E, Mussalo H, Kauppila E, Simula S, Narvanen O, Rantala A, Peuhkurinen K, Nieminen MS, Laakso M, Yla-Herttuala S. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and instent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107(21):2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- Hiram R, Rizcallah E, Marouan S, Sirois C, Sirois M, Morin C, Fortin S, Rousseau E. Resolvin E1 normalizes contractility, Ca2+ sensitivity and smooth muscle cell migration rate in TNF-alpha- and IL-6-pretreated human pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2015;309(8):L776–788. doi: 10.1152/ajplung.00177.2015. [DOI] [PubMed] [Google Scholar]
- Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, Creager MA, Serhan CN, Conte MS. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177(4):2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AW, Tulis DA. Experimental Rat and Mouse Carotid Artery Surgery: Injury & Remodeling Studies. ISRN Minim Invasive Surg. 2013;2013 doi: 10.1155/2013/167407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators, G.-P. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. The Lancet. 1999;354(9177):447–455. [PubMed] [Google Scholar]
- Investigators, G.-P. Early Protection Against Sudden Death by n-3 Polyunsaturated Fatty Acids After Myocardial Infarction: Time-Course Analysis of the Results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105(16):1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Wu L, Miller BL, Goldman DR, Fernandez CMZ, Ozaki ZS, C K, Berceli SA. Novel vein graft model: adaptation to different flow environments. Am J Physiol Heart Circ Physiol. 2004;286:H240–H245. doi: 10.1152/ajpheart.00760.2003. [DOI] [PubMed] [Google Scholar]
- Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kagawa Y, Nishizawa M, Suzuki M, Miyatake T, Hamamoto T, Goto K, Motonaga E, Izumikawa H, Hirata H, A E. Eicosapolyenoic acids of serum lipids of Japanese islanders with low incidence of cardiovascular diseases. J Nutr Sci Vitaminol. 1982;28(4):441–453. doi: 10.3177/jnsv.28.441. [DOI] [PubMed] [Google Scholar]
- Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol. 2015;84:24–35. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;299(1):H153–164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- Kornowski R, Hong MK, Tio FO, Branwell O, Wu H, Leon MB. In-Stent Restenosis: Contributions of Inflammatory Responses and Arterial Injury to Neointimal Hyperplasia. J Am Coll Cardiol. 1998;31(1):224–230. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- Kotani J, Awata M, Nanto S, Uematsu M, Oshima F, Minamiguchi H, Mintz GS, Nagata S. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol. 2006;47(10):2108–2111. doi: 10.1016/j.jacc.2005.11.092. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM. Fish Consumption, Fish Oil, Omega-3 Fatty Acids and Cardiovascular Disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Kumar A, Lindner V. Remodeling With Neointima Formation in the Mouse Carotid Artery After Cessation of Blood Flow. Arteriosclerosis, Thrombosis and Vascular Biology. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- Lance KD, Chatterjee A, Wu B, Mottola G, Nuhn H, Lee PP, Sansbury BE, Spite M, Desai TA, Conte MS. Unidirectional and sustained delivery of the proresolving lipid mediator resolvin D1 from a biodegradable thin film device. J Biomed Mater Res A. 2017;105(1):31–41. doi: 10.1002/jbm.a.35861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands WEM. Biochemistry and physiology of n-3 fatty acid. FASEB J. 1992;6:2530–2536. doi: 10.1096/fasebj.6.8.1592205. [DOI] [PubMed] [Google Scholar]
- Lannan KL, Spinelli SL, Blumberg N, Phipps RP. Maresin 1 induces a novel pro-resolving phenotype in human platelets. J Thromb Haemost. 2017 doi: 10.1111/jth.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switch during acute inflammation: signals in resolution. Nature Immunology. 2001;2(7):612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Lopez EF, Kabarowski JH, Ingle KA, Kain V, Barnes S, Crossman DK, Lindsey ML, Halade GV. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am J Physiol Heart Circ Physiol. 2015;308(4):H269–280. doi: 10.1152/ajpheart.00604.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares W, Langlois PL, Dhaliwal R, Lemieux M, Heyland DK. Intravenous fish oil lipid emulsions in critically ill patients: an updated systematic review and meta-analysis. Crit Care. 2015;19:167. doi: 10.1186/s13054-015-0888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins V, Valenca SS, Farias-Filho FA, Molinaro R, Simoes RL, Ferreira TP, e Silva PM, Hogaboam CM, Kunkel SL, Fierro IM, Canetti C, Benjamim CF. ATLa, an aspirin-triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin-induced pulmonary fibrosis. J Immunol. 2009;182(9):5374–5381. doi: 10.4049/jimmunol.0802259. [DOI] [PubMed] [Google Scholar]
- Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22(10):3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched AJ, Serhan CN, Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. J Nutrigenet Nutrigenomics. 2011;4(1):12–24. doi: 10.1159/000326890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, Serhan CN, Conte MS. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27(6):2220–2232. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottola G, C A, Wu B, Chen M, Conte MS. Aspirin-triggered resolvin D1 attenuates PDGF-induced vascular smooth muscle cell migration via the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174936. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways and clinical events. J Am Coll Cardiol. 2011;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- Muto A, Model L, Ziegler K, Eghbalieh S, Dardik A. Mechanisms of vein graft adaptation to arterial circulation: Insights into the neointimal algorithm and management strategies. Circ J. 2010;74(8):1501–1512. doi: 10.1253/circj.cj-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento-Silva V, Augusta Arruda M, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: A novel antioxidative mechanism. Thrombosis and Haemostasis. 2006 [PubMed] [Google Scholar]
- Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol. 2012;32(8):1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SK, Colas RA, Dalli J, Chiang N, Serhan CN. Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am J Physiol Lung Cell Mol Physiol. 2015;308(9):L904–911. doi: 10.1152/ajplung.00370.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5(5):661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrovsky G. Angiotech suspends Vascular Wrap Trial Enrollment. Med Gadget 2008 [Google Scholar]
- Owens CD, Gasper WJ, Rahman AS, Conte MS. Vein graft failure. J Vasc Surg. 2015;61(1):203–216. doi: 10.1016/j.jvs.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens CD, Kim JM, Hevelone ND, Gasper WJ, Belkin M, Creager MA, Conte MS. An integrated biochemical prediction model of all-cause mortality in patients undergoing lower extremity bypass surgery for advanced peripheral artery disease. J Vasc Surg. 2012;56(3):686–695. doi: 10.1016/j.jvs.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens CD, Ridker PM, Belkin M, Hamdan AD, Pomposelli F, Logerfo F, Creager MA, Conte MS. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007;45(1):2–9. doi: 10.1016/j.jvs.2006.08.048. discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200(1):69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petasis NA, Keledjian R, Sun YP, Nagulapalli KC, Tjonahen E, Yang R, Serhan CN. Design and synthesis of benzo-lipoxin A4 analogs with enhanced stability and potent anti-inflammatory properties. Bioorg Med Chem Lett. 2008;18(4):1382–1387. doi: 10.1016/j.bmcl.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Petri MH, Laguna-Fernandez A, Gonzalez-Diez M, Paulsson-Berne G, Hansson GK, Back M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc Res. 2015;105(1):65–74. doi: 10.1093/cvr/cvu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri MH, Laguna-Fernandez A, Tseng CN, Hedin U, Perretti M, Back M. Aspirin-triggered 15-epi-lipoxin A(4) signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation. Int J Cardiol. 2015;179:370–372. doi: 10.1016/j.ijcard.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai PS, Leeson S, Porter TF, Owens CD, Kim JM, Conte MS, Serhan CN, Gelman S. Chemical mediators of inflammation and resolution in post-operative abdominal aortic aneurysm patients. Inflammation. 2012;35(1):98–113. doi: 10.1007/s10753-011-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorani R, Bhatt AN, Dwarakanath BS, Das UN. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.08.049. [DOI] [PubMed] [Google Scholar]
- Pope NH, Salmon M, Davis JP, Chatterjee A, Su G, Conte MS, Ailawadi G, Upchurch GR., Jr D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J. 2016;30(12):4192–4201. doi: 10.1096/fj.201600144RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Zhang X, Yao J, Song J, Nikolic-Paterson DJ, Li J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J Pathol. 2012;228(4):506–519. doi: 10.1002/path.4050. [DOI] [PubMed] [Google Scholar]
- Recchiuti A, Codagnone M, Pierdomenico AM, Rossi C, Mari VC, Cianci E, Simiele F, Gatta V, Romano M. Immunoresolving actions of oral resolvin D1 include selective regulation of the transcription machinery in resolution-phase mouse macrophages. FASEB J. 2014;28(7):3090–3102. doi: 10.1096/fj.13-248393. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Clinical Application of C-Reactive Protein for Cardiovascular Disease Detection and Prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association Between Omega-3 Fatty Acid Supplementation and Risk of Major Cardiovascular Disease Events. JAMA. 2012;308(10):1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- Roach KM, Feghali-Bostwick CA, Amrani Y, Bradding P. Lipoxin A4 Attenuates Constitutive and TGF-beta1-Dependent Profibrotic Activity in Human Lung Myofibroblasts. J Immunol. 2015;195(6):2852–2860. doi: 10.4049/jimmunol.1500936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillinger M, Exner M, M W, Rumpold H, A R, Sabeti S, Haumer M, Wagner O, Minar E. Vascular Inflammation and Percutaneous Transluminal Angioplasty of the Femoropopliteal Artery: Association with Restenosis. Vascular and Interventional Radiology. 2002;225(1):21–26. doi: 10.1148/radiol.2251011809. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced Inflammation and Tissue Damage in Transgenic Rabbits Overexpressing 15-Lipoxygenase and Endogenous Anti-inflammatory Lipid Mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- Shah PK. Inflammation, neointimal hyperplasia and restenosis: as the leukocytes roll, the arteries thicken. Circulation. 2003;107(17):2175–2177. doi: 10.1161/01.CIR.0000069943.41206.BD. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26(5):987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- Simosa HF, Wang G, Sui X, Peterson T, Narra V, Altieri DC, Conte MS. Survivin expression is up-regulated in vascular injury and identifies a distinct cellular phenotype. J Vasc Surg. 2005;41(4):682–690. doi: 10.1016/j.jvs.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Smith HK, Gil CD, Oliani SM, Gavins FN. Targeting formyl peptide receptor 2 reduces leukocyte-endothelial interactions in a murine model of stroke. FASEB J. 2015;29(5):2161–2171. doi: 10.1096/fj.14-263160. [DOI] [PubMed] [Google Scholar]
- Sok MC, Tria MC, Olingy CE, San Emeterio CL, Botchwey EA. Aspirin-Triggered Resolvin D1-modified materials promote the accumulation of pro-regenerative immune cell subsets and enhance vascular remodeling. Acta Biomater. 2017 doi: 10.1016/j.actbio.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107(10):1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Sukhova GK, Swanson SJ, Clinton SK, Ganz P, Cybulsky MI, Libby P. Sustained Activation of Vascular Cells and Leukocytes in the Rabbit Aorta After Balloon Injury. Circulation. 1993;88(1):1788–1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- Tepe G, Zeller T, Albrecht A, Heller S, Schwarzwälder U, Beregi JP, Claussen C, Oldencurh A, Scheller B, Speck U. Local Delivery of Paclitaxel to Inhibit Restenosis during Angioplasty of the Leg. NEJM. 2008;358(7):689–699. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- Thul S, Labat C, Temmar M, Benetos A, Back M. Low salivary resolvin D1 to leukotriene B4 ratio predicts carotid intima media thickness: A novel biomarker of non-resolving vascular inflammation. Eur J Prev Cardiol. 2017 doi: 10.1177/2047487317694464. 2047487317694464. [DOI] [PubMed] [Google Scholar]
- Tian H, Lu Y, Sherwood AM, Hongqian D, Hong S. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci. 2009;50(8):3613–3620. doi: 10.1167/iovs.08-3146. [DOI] [PubMed] [Google Scholar]
- Uno H, Furukawa K, Suzuki D, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, Miyazaki M. Immunonutrition suppresses acute inflammatory responses through modulation of resolvin E1 in patients undergoing major hepatobiliary resection. Surgery. 2016;160(1):228–236. doi: 10.1016/j.surg.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Viola J, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Döring Y, Drechsler M, Webe C, Zimmer R, Cenac N, Soehnlein O. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ Res. 2016;119(9):1030–1038. doi: 10.1161/CIRCRESAHA.116.309492. [DOI] [PubMed] [Google Scholar]
- von Schacky C. Omega-3 index and cardiovascular health. Nutrients. 2014;6(2):799–814. doi: 10.3390/nu6020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales KM, Kavazos K, Nataatmadja M, Brooks PR, Williams C, Russell FD. N-3 PUFAs protect against aortic inflammation and oxidative stress in angiotensin II-infused apolipoprotein E-/- mice. PLoS One. 2014;9(11):e112816. doi: 10.1371/journal.pone.0112816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Sui XX, Simosa HF, Jain MK, Altieri DC, Conte MS. Regulation of vein graft hyperplasia by survivin, an inhibitor of apoptosis protein. Arterioscler Thromb Vasc Biol. 2005;25(10):2081–2087. doi: 10.1161/01.ATV.0000183885.66153.8a. [DOI] [PubMed] [Google Scholar]
- Wang X, Hjorth E, Vedin I, Eriksdotter M, Freund-Levi Y, Wahlund LO, Cederholm T, Palmblad J, Schultzberg M. Effects of n-3 FA supplementation on the release of proresolving lipid mediators by blood mononuclear cells: the OmegAD study. J Lipid Res. 2015;56(3):674–681. doi: 10.1194/jlr.P055418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer J, Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot Essent Fatty Acids. 2007;77(2):67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Wu B, Mottola G, Chatterjee A, Lance KD, Chen M, Siguenza IO, Desai TA, Conte MS. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. J Vasc Surg. 2017;65(1):207–217 e203. doi: 10.1016/j.jvs.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Werlin E, Mottola G, Chatterjee A, Chen M, Lance KD, Bernards DA, Sansbury BE, M S, Desai TA, Conte MS. Perivascular Delivery of Resolvin D1 Inhibits Neointimal Hyperplasia in a Rabbit Vein Graft Model. Academic Surgical Congress Oral Presentation. 2017 doi: 10.1016/j.jvs.2018.05.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob P, Pala HS, C-B M, N EA, C PC. Encapsulated fish oil enriched in alpha-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur J Clin Invest. 2000;30(3):260–274. doi: 10.1046/j.1365-2362.2000.00623.x. [DOI] [PubMed] [Google Scholar]
- Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141(3):272–282. doi: 10.1016/j.pharmthera.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Zhang MJ, Sansbury BE, Hellmann J, Baker JF, Guo L, Parmer CM, Prenner JC, Conklin DJ, Bhatnagar A, Creager MA, Spite M. Resolvin D2 Enhances Postischemic Revascularization While Resolving Inflammation. Circulation. 2016;134(9):666–680. doi: 10.1161/CIRCULATIONAHA.116.021894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang T, Gui P, Yao C, Sun W, Wang L, Wang H, Xie W, Yao S, Lin Y, Wu Q. Resolvin D1 reverts lipopolysaccharide-induced TJ proteins disruption and the increase of cellular permeability by regulating IkappaBalpha signaling in human vascular endothelial cells. Oxid Med Cell Longev 2013. 2013:185715. doi: 10.1155/2013/185715. [DOI] [PMC free article] [PubMed] [Google Scholar]


