Abstract
Caffeine is a highly catabolic dietary stimulant. High caffeine concentrations (1–10 mM) have previously been shown to inhibit protein synthesis and increase protein degradation in various mammalian cell lines. The purpose of this study was to examine the effect of short-term caffeine exposure on cell signaling pathways that regulate protein metabolism in mammalian skeletal muscle cells. Fully differentiated C2C12 skeletal myotubes either received vehicle (DMSO) or 5 mM caffeine for 6 h. Our analysis revealed that caffeine promoted a 40% increase in autolysosome formation and a 25% increase in autophagic flux. In contrast, caffeine treatment did not significantly increase the expression of the skeletal muscle specific ubiquitin ligases MAFbx and MuRF1 or 20S proteasome activity. Caffeine treatment significantly reduced mTORC1 signaling, total protein synthesis and myotube diameter in a CaMKKβ/AMPK-dependent manner. Further, caffeine promoted a CaMKII-dependent increase in myostatin mRNA expression that did not significantly contribute to the caffeine-dependent reduction in protein synthesis. Our results indicate that short-term caffeine exposure significantly reduced skeletal myotube diameter by increasing autophagic flux and promoting a CaMKKβ/AMPK-dependent reduction in protein synthesis.
Keywords: Caffeine, Skeletal muscle, Protein synthesis, Autophagy, Myostatin
Introduction
Caffeine is a highly catabolic dietary stimulant. Previous investigations revealed that higher caffeine concentrations (1–10 mM) can significantly reduce and even inhibit protein synthesis in mammalian skeletal muscle cells (Lewis et al. 1982; Goodman 1987). The mechanistic target of rapamycin complex 1 (mTORC1) is a primary regulator of protein synthesis in eukaryotic cells (Zoncu et al. 2011). Activated mTORC1 increases protein synthesis by directly phosphorylating the ribosomal S6 kinase 1 (S6K1), which in turn directly phosphorylates ribosomal protein S6, resulting in increased ribosomal protein translation (Dennis et al. 2012). In addition, activated mTORC1 phosphorylates the eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1), which prevents 4EBP1 from associating with and thus repressing the eukaryotic translation initiation factor 4E (eIF4E) from recruiting the 40S ribosomal subunit to the 5′ end of mRNA (Csibi et al. 2010). The AMP-activated protein kinase (AMPK) directly inhibits the activation of mTORC1 in skeletal muscle cells (Mounier et al. 2009a, b), and caffeine has been shown to significantly increase AMPK activation in skeletal muscle cells (Egawa et al. 2011a, b; Mathew et al. 2014). Though caffeine has been shown to significantly reduce mTORC1 activation in numerous mammalian cell lines, it remains unclear if caffeine reduces protein synthesis in skeletal muscle cells in an AMPK-dependent manner.
Our lab recently demonstrated that treating C2C12 skeletal myotubes with 2.5–10 mM caffeine for 6 h promoted a calcium/calmodulin-activated protein kinase kinase β (CaMKKβ) mediated activation of AMPK and a subsequent AMPK-dependent increase in macroautophagy (hereafter referred simply as autophagy) (Mathew et al. 2014). However, there is much controversy over the analysis of autophagy in mammalian cells because the initiation of autophagy (autophagosome formation) does not always correlate with increased autolysosome formation and lysosomal degradation, a process referred to as autophagic flux (Klionsky et al. 2016). Previous investigations have revealed that AMPK can also promote ubiquitin proteasome-dependent protein degradation in skeletal muscle cells by increasing the expression of the skeletal muscle specific ubiquitin ligases muscle atrophy F box (MAFbx/Atrogin1) and muscle RING finger 1 (MuRF1) (Tong et al. 2009). It remains unclear, however, if high caffeine concentrations can significantly increase both autophagic flux and ubiquitin proteasome-dependent protein degradation in skeletal muscle cells.
Caffeine has also been shown to significantly increase the activation of calcium/calmodulin-activated protein kinase II (CaMKII) (Mathew et al. 2014). CaMKII is one of the primary upstream kinases of the transcription factor cyclic AMP/calcium response element binding protein (CREB) (Sun et al. 1994). CaMKII phosphorylates CREB at serine 133, which promotes CREB activation by increasing the binding of CREB to its DNA binding partner CREB-binding protein (CBP) (Sun et al. 1994). Previous research indicates that CaMKII can promote a CREB-dependent increase in the skeletal muscle specific growth inhibitor myostatin (Zuloaga et al. 2013). Myostatin reduces protein synthesis in skeletal muscle cells by inhibiting the mTORC1 pathway (Taylor et al. 2001; Whittemore et al. 2003). Though caffeine has been shown to increase CREB-dependent gene expression in mammalian cells (Connolly and Kingsbury 2010), it remains unclear if caffeine can promote a CREB-dependent increase in myostatin expression in skeletal muscle cells.
Our preliminary experiments revealed that exposing C2C12 skeletal myotubes to 5 mM caffeine for 6 h promoted a 38% reduction in mean myotube diameter when compared to control cells. Therefore, our objectives were to determine if short-term exposure of skeletal myotubes to a high-caffeine concentration could significantly increase autophagic flux and ubiquitin proteasome-dependent protein degradation. In addition, we sought to determine whether the caffeine-dependent reduction in protein synthesis in skeletal myotubes was dependent upon AMPK inhibition of mTORC1. A final objective was to determine if high caffeine could promote a CaM-KII-dependent increase in myostatin expression and to examine it’s potential role in regulating protein synthesis.
Materials and methods
Materials
Fetal bovine serum (FBS), horse serum (HS), penicillin/streptomycin (pen/strep), and Dulbecco’s modified Eagle’s medium (DMEM) were purchase from Life Technologies (Grand Island, NY, USA). Primary antibodies for tubulin, CaMKII and LC3B were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Primary antibodies for phospho-CaMKII (p-CaMKII, Ser286), and CaMKII were purchased from Cell Signaling Technology (Beverly, MA, USA). All secondary antibodies were purchased from Vector Laboratories (Burlingame, CA, USA). The pharmacological inhibitors STO609 (CaMKKβ inhibitor), KN93 (CaMKII inhibitor), KN92 (negative control for KN93), Dantrolene, and Bafilomycin A1 were purchased from EMD Millipore (Darmstadt, Germany).
Cell culture
C2C12 myoblasts were purchased from ATCC (Manassas, VA, USA). Myoblasts were grown to confluence in normal growth media (DMEM plus 10% FBS, 100 U/mL penicillin, 100 U/mL streptomycin) at 37 °C in a water-saturated atmosphere of 5% CO2. To promote differentiation of mono-nucleated myoblasts into multinucleated myotubes the media was switched to differentiation media (DMEM plus 2% HS, 100 U/mL penicillin, 100 U/mL streptomycin). Fully differentiated myotubes were normally present upon 5–6 days incubation in differentiation media.
Colocalization analysis
Myoblasts were seeded on 35 mm dishes that contained a 1.5 mm thick collagen coated coverslip (MatTek Corporation, Ashland, MA, USA) in normal growth media. Cells were stained with 1 μL/mL LysoTracker® Red DND-99 (Thermo Fisher Scientific, Carlsbad, CA, USA) and 1 μL/mL Hoechst 33342 in differentiation media for 30 min at 37 °C. All samples were washed in PBS and fixed with 4% paraformaldehyde for 20 min. Cells were permeated with 0.5% Triton® X-100 in PBS for 15 min and blocked in 0.5% BSA in PBS for 1 h. All samples were incubated in 1:400 LC3b primary antibody in PBS containing 0.25% BSA for 1 h at room temp, washed 3× in PBS and subsequently incubated with an AlexaFluor 488 conjugated secondary antibody at 1:4000 in PBS containing 0.25% BSA for 1 h at room temperature. Samples were analyzed using a Nikon Eclipse TS100-F microscope and NIS Elements Br software (Nikon Instruments, Melville, NY, USA). Briefly, images were captured in the FITC (LC3b) and TRITC (LysoTracker) channels using a 40× fluorite objective. Ten images were randomly captured on each coverslip (replicate sample). Colocalization analysis was conducted using Coloc2 plugin for Fiji (ImageJ) software (National Institutes of Health, Bethesda, Maryland, USA). A Pearson’s correlation coefficient was calculated for each image and all ten correlation coefficients were used to calculate the mean correlation coefficient for each sample replicate (n = 3).
Transmission electron microscopy (TEM)
Cells were grown in a 6-well plate as described above, where three of the wells were controls and three were treated with 5 mM caffeine for 6 h prior to harvesting. Cells were scraped from the wells and transferred to a microcentrifuge tube containing Karnovsky’s fixative (3.0% glutaraldehyde, 3.0% paraformaldehyde in 0.2 M sodium cacodylate buffer, pH 7.6) for 24 h at room temperature. Cells were then rinsed in 0.2 M sodium cacodylate, followed by secondary fixation in 0.5% osmium tetroxide in 0.2 M sodium cacodylate buffer. Samples were rinsed and dehydrated, after which they were embedded in Spurr epoxy resin (Electron Microscopy Sciences, Hatfield, PA, USA). Embedded samples were cut into a trapezoid with beveled edges. Samples were then sectioned at 90 nm on a Reichert Ultracut E ultramicrotome (Leica Microsystems, Inc., Bannockburn, IL, USA). A series of ten sections were cut for each of the six samples (wells) using a systematic random sampling method (Reed and Howard 1998), where different cells were represented in each section. Sections were collected and mounted on Formvar-coated (0.25 g Formvar in 100 ml ethylene dichloride), 200 μm mesh, high-transmission copper grids. Sections were stained with 2% uranyl acetate in 50% ethyl alcohol, and then stained with Reynolds lead citrate for 15 min each.
Sections were examined on a Technai G2 Spirit BioTwin TEM (FEI, Hillsboro, OR, USA). A total of 60 images were collected (30 each for controls and treatments), and images were analyzed in Image Pro Plus software (Media Cybernetics, Inc., Rockville, MD, USA). Point counting stereological analysis was used to determine volume density of autolysosomes.
Western blot analysis
Cell lysates were collected in lysis buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.004% sodium azide, 2 mM PMSF, 1 mM sodium orthovanadate, 10 μl protease inhibitor cocktail). An equal volume of supernatant was combined with 2× Laemmli buffer and boiled for 10 min. Twenty μg of each denatured sample was submitted to SDS–PAGE using either 7.5 or 12% polyacrylamide gels and subsequently transferred onto a PVDF membrane (EMD Millipore, Darmstadt, Germany). All membranes were blocked for 1 h in 5% bovine serum albumin (BSA) dissolved in Tris-buffered saline plus 0.1% Tween 20 (TBST), incubated with primary antibody (1/1000) over night at 4 °C and subsequently labeled with an appropriate HRP-labeled secondary antibody (1/10,000) for 1 h at room temperature. Once satisfactory images were obtained each membrane was stained with coomassie brilliant blue (R-250) for 5 min, washed in TBST and imaged for total protein assessment. Unless otherwise stated, all blots were normalized to total protein. Blots were developed using standard ECL detection and images were acquired on a FluorChem E System imager (Protein-Simple, Santa Clara, CA, USA). Digital images were analyzed using ImageJ 1.47v (National Institutes of Health, Bethesda, MD, USA).
Proteasome activity assay
Cell lysates were collected and homogenized in 250 μL of 0.5% NP-40 in PBS. The 20S proteasome activity was analyzed using a fluorometric assay to measure chymotrypsin-like activity in cell lysates according to the manufacturer’s instructions (BioVision Inc., Milpitas, CA, USA). Proteasome activity was measured by analyzing changes in fluorescence intensity at 350 nm of excitation and 440 nm of emission using an automatic multi-well plate reader (BioTek Instruments Inc., Winooski, VT, USA). The relative activity was standardized by sample protein concentration, which was determined using a Bradford assay.
Nascent protein synthesis assay
Myoblasts were seeded on 35 mm dishes that contained a 1.5 mm thick collagen coated coverslip (MatTek Corporation, Ashland, MA, USA) in normal growth media. Triplicate groups of cells were exposed to vehicle (DMSO, control) or a combination of 5 mM caffeine, 30 μM STO609, 10 μM KN93 or 10 μM KN92. All possible experimental scenarios were tested using these treatment groups. Protein synthesis analysis was conducted using a Molecular Probes Click-iT® Plus OPP Alexa Fluor® 488 Protein Synthesis Assay Kit (Thermo Fisher Scientific, Carlsbad, CA, USA) according to manufacturer’s protocol. Samples were analyzed using a Nikon Eclipse TS100-F microscope and NIS Elements Br software (Nikon Instruments, Melville, NY, USA). Ten images were randomly captured on each coverslip (replicate sample), which were subsequently used to calculate the mean FITC intensity of individual myotubes by creating a region of interest around myotubes in each image. The mean FITC intensity for each image calculated and used to calculate the average intensity for each experimental treatment (n = 3).
Myotube diameter
Myoblasts were seeded on 35 mm dishes that contained a 1.5 mm thick collagen coated coverslip (MatTek Corporation, Ashland, MA, USA) in normal growth media. Triplicate groups of cells were exposed to vehicle (DMSO, control) or a combination of 5 mM caffeine, 10 mM 3-methyladenine (3-MA), or 30 μM STO609. Samples were washed in PBS and fixed with 4% paraformaldehyde for 20 min. analyzed using a Nikon Eclipse TS100-F microscope and NIS Elements Br software (Nikon Instruments, Melville, NY, USA). Ten images were randomly captured on each coverslip (replicate sample). The diameter of every myotube (5–10) in each image was measured and used to calculate the mean myotube diameter for each sample replicate (n= 3).
RNA extraction and real time PCR
Cells were collected and washed twice with phosphate buffered saline (PBS) and then RNA was extracted using the RNeasy minikit from Qiagen (Valencia, CA). A total of 1 μg of RNA was used to create cDNA using a superscript first-strand synthesis reverse transcription kit (Invitrogen, Carlsbad, CA). Primers for murine myostatin (qMmuCED0045853) and GAPDH (qMmuCED0027497) were purchased from Bio-Rad (Hercules, CA). Real-time PCR was carried out in triplicate using the Bio-rad CFX Connect Real Time Detection system. The 20 μL reaction mix for real time PCR was made using cDNA, SYBR green (Bio-Rad, Hercules, CA), 1 μL each of forward and reverse primers, and H2O. The reaction cycle used was: 95 °C for 2 min for activation, 40 cycles of 95 °C for 5 s for denaturation and 60 °C for 30 s for annealing, 95 °C for 1 min, and 60 °C for 1 min followed by a melt curve analysis starting at 65 °C and increasing 0.5 °C each cycle through 95 °C for 5 s/step. Data was analyzed using the Biorad CFX manager software.
Statistical analysis
Each experiment was repeated at least three times. Statistical differences between groups were analyzed using a student’s t test or a one-way ANOVA with subsequent post hoc analysis, as appropriate. A P-value <0.05 was considered significant.
Results
Caffeine significantly increased autophagic flux in skeletal myotubes
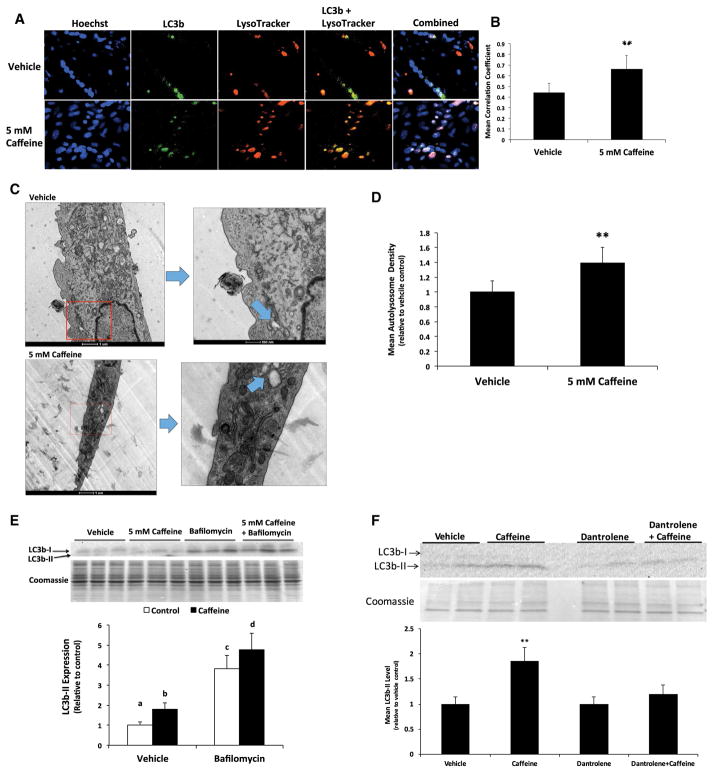
In the present study, we sought to confirm whether treating myotubes with 5 mM caffeine for 6 h could significantly increase autophagic flux. Caffeine significantly increased LC3b expression and LC3b and lysosome colocalization, as the mean Pearson’s coefficient for caffeine treated myotubes was 50% greater than that of vehicle treated cells (Fig. 1a, b). Initial transmission electron microscopy analysis revealed no difference in autolysosome number density between groups. However, the control cells were approximately 40% greater in volume than the caffeine treated cells, meaning a much larger cell volume was evaluated for the controls than the treatments. Further, about half of the images did not have autolysosomes present, but we noticed that autolysomes were present more frequently in the caffeine treated cells than in the control cells, despite the smaller cell size surveyed in the treatment group. To compensate for the relative rarity of the autolysosomes and the differences in cell volume, cells were evaluated based on presence/absence of autolysosomes per volume of cell analyzed. Cells were scored with a 1 if no lysosome was present, and a 2 if a lysosome was present, and the score for each cell was divided by the relative cell volume. When we evaluated the presence/absence score per cell volume, we found a 40% increase in autolysosome formation in caffeine vs. vehicle treated myotubes (Fig. 1c, d). A similar difference was observed if we evaluated autolysosome number using only the population of cells in the same size range for both groups. Additionally, we discovered that incubating myotubes with 4 μM bafilomycin, a well characterized inhibitor of autophagosome and lysosome fusion, in the presence of caffeine promoted a 25% increase in LC3b-II levels when compared to cells treated with bafilomycin alone (Fig. 1e). This result suggests that in vehicle treated cells, 5 mM caffeine increased the lysosomal degradation of LC3b-II by approximately 25%. Finally, to assess whether caffeine-dependent autophagy required ryanodine receptor-mediated calcium release, we treated skeletal myotubes with the specific ryanodine receptor inhibitor dantrolene. The addition 200 μM dantrolene completely inhibited the caffeine-dependent increase in LC3b-II protein levels, and thus autophagosome formation, in skeletal myotubes (Fig. 1f). These results strongly suggest that caffeine treatment promoted a calcium-dependent increase in autophagic flux in skeletal myotubes.
Fig. 1.
Caffeine significantly increased autophagic flux in skeletal myotubes. Fully differentiated C2C12 myotubes were treated with either vehicle (control) or 5 mM caffeine for 6 h (n = 3). a, b Caffeine promoted a 50% increase in LC3b/LysoTracker colocalization. c, d Transmission electron microscopy analysis revealed a 40% increase in autolysosome formation in caffeine vs. vehicle treated myotubes. e The addition 4 μM bafilomycin significantly increased the level of LC3b-II in caffeine treated myotubes when compared to cells treated with bafilomycin alone. f The addition of 200 μM dantrolene completely inhibited the caffeine-dependent increase in LC3b-II levels. a–dIndicate significant difference between treatments with out same letters (**P < 0.05)
Caffeine did not increase ubiquitin-dependent protein degradation in skeletal myotubes
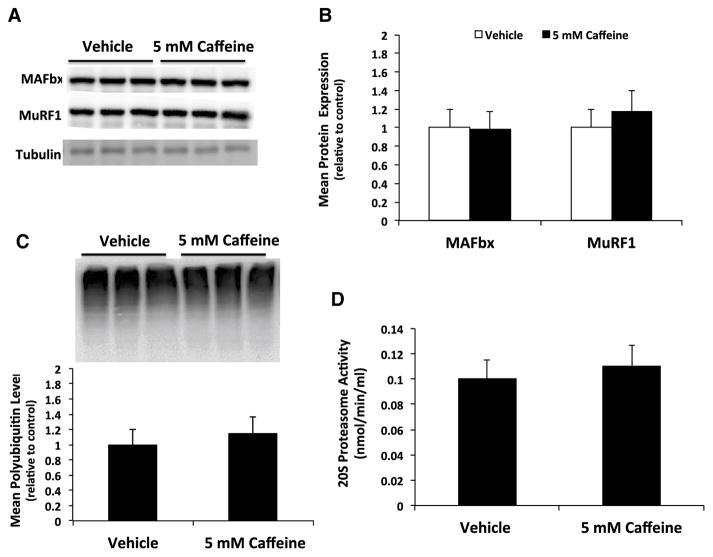
To determine if caffeine could promote a significant increase in ubiquitin-dependent protein degradation we first investigated the protein expression of the skeletal muscle specific ubiquitin ligases MAFbx and MuRF1. There was no significant difference in MAFbx or MuRF1 protein expression in caffeine vs. vehicle treated skeletal myotubes (Fig. 2a, b). In addition, we discovered that caffeine treatment did not significantly affect the level of total protein ubiquitination or 20S proteasome activity in skeletal myotubes (Fig. 2c, d). These results suggest that caffeine treatment did not significantly increase ubiquitin-dependent protein degradation in skeletal myotubes.
Fig. 2.
Caffeine does not significantly increase ubiquitin-dependent protein degradation in skeletal myotubes. Fully differentiated C2C12 myotubes were treated with either vehicle (control) or 5 mM caffeine for 6 h (n = 6). a, b Western blot analysis revealed that caffeine did not significantly increase the expression of the skeletal muscle specific ligases MAFbx and MuRF1. c Additional western blot analysis revealed that caffeine did not increase total protein ubiquitination. d An analysis of 20S proteasome enzyme activity revealed no significant difference in activity due to caffeine treatment
Inhibiting AMPK activation restored mTORC1 signaling and prevented the caffeine-dependent reduction in protein synthesis and myotube diameter
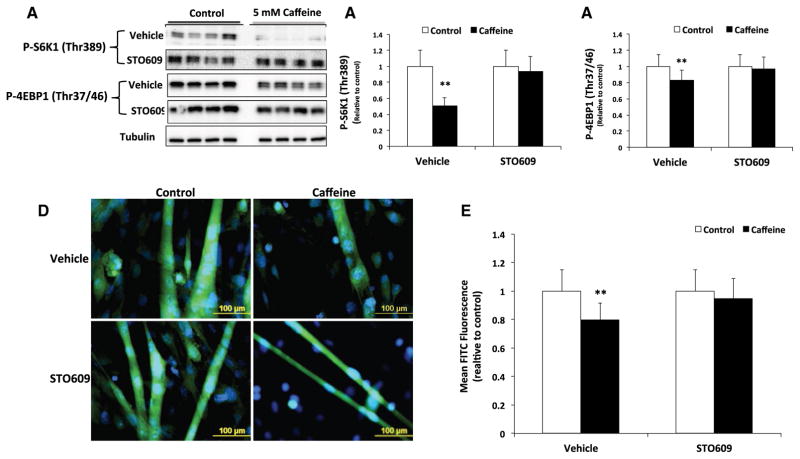
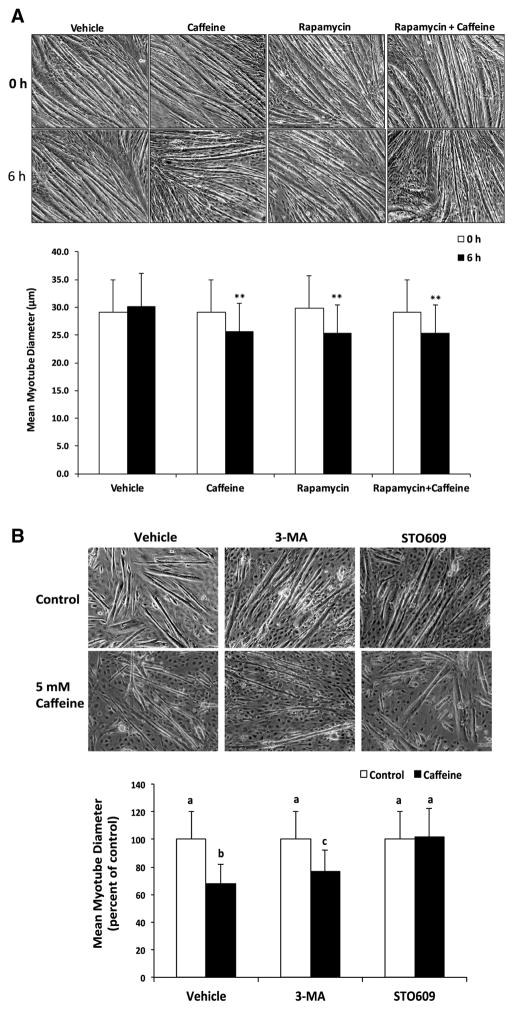
We previously demonstrated that caffeine primarily increases AMPK activation in a CaMKKβ-dependent manner in skeletal myotubes (Mathew et al. 2014). In the current study, we discovered that the addition of 30 μM STO609 prevented the significant (P < 0.05) reduction in P-S6K1 (Thr389) and P-4EBP1 (Thr37/46) in caffeine vs. vehicle treated skeletal myotubes (Fig. 3a–c). Fluorescence microscopy analysis revealed a 40% reduction (P < 0.05) in protein synthesis rate (measured as mean FITC fluorescence) in caffeine treated myotubes that was completely inhibited by the addition of STO609 (Fig. 3d, e). To further examine the effect of caffeine in promoting the AMPK-dependent inhibition of mTORC1, we conducted experiments to examine the effect of caffeine on myotube diameter. Treating myotubes with 5 mM caffeine or 1 μM rapamycin for 6 h promoted a 15% reduction in myotube diameter (Fig. 4a). Further, the addition of rapamycin and caffeine did not have an additive effect on myotube diameter (Fig. 4a), which likely indicates that caffeine reduced myotube diameter by directly inhibiting mTORC1 activity. In addition, 10 mM 3-methyladenine (3-MA), a specific autophagy inhibitor, partially attenuated the caffeine-dependent reduction in myotube diameter (Fig. 4b). However, the addition of 30 μM STO609 completely inhibited the caffeine-dependent reduction myotube diameter (Fig. 4b). Together, these results suggest that caffeine reduced cellular protein synthesis and skeletal myotube diameter via CaMKKβ/AMPK-dependent inhibition of mTORC1.
Fig. 3.
Caffeine significantly reduced protein synthesis in an AMPK-dependent manner in skeletal myotubes. Fully differentiated C2C12 myotubes were treated with either vehicle (control) or 5 mM caffeine for 6 h (n = 3–6). a–c Western blot analysis revealed that caffeine significantly reduced P-S6K1 (Thr 389) and P-4EBP1 (Thr 37/4), which was prevented by the addition 30 μM STO609 (CaMKKβ inhibitor). d, e Fluorescent microscopy analysis revealed that caffeine promoted a 25% reduction in protein synthesis in fully formed myotubes that was completely inhibited by the addition of STO609. (**P < 0.05)
Fig. 4.
Caffeine reduced myotube diameter in by promoting the CaMKKβ/AMPK-dependent inhibition of mTORC1. Fully differentiated C2C12 myotubes were treated with either vehicle (control) or 5 mM caffeine for 6 h (n = 3). a Microscopy analysis revealed that caffeine and 1 μM rapamycin promoted a 15% reduction in myotube diameter, though the addition of caffeine plus rapamycin did not have an additive affect. b The decrease in skeletal myotube diameter that was partially attenuated by 10 mM 3-methyladenine (3MA, autophagy inhibitor) and completely inhibited by 30 μM STO609 (CaMKKβ inhibitor). (**P < 0.05)
Inhibiting the CaMKII-dependent increase in myotstatin gene expression did not prevent the caffeine-induced reduction in protein synthesis in skeletal myotubes
To determine if caffeine could promote a CaMKII-dependent increase in myostatin gene expression we first examined the effect of caffeine on CaMKII and CREB phosphorylation. Caffeine treatment significantly (P < 0.05) increased both P-CaMKII and P-CREB (Ser133) levels when compared to vehicle treated myotubes (Fig. 5a, b). Further, we discovered that treating myotubes with 10 μM KN92 and caffeine increased myostatin mRNA expression by 65% (P < 0.05) when compared to cells treated with KN92 alone (Fig. 5c). The addition of 10 μM KN93 completely inhibited the caffeine-mediated increase in myostatin mRNA expression (Fig. 5c). In contrast, we discovered that caffeine did not significantly affect the expression of the inactive (52 kDa) or active (26 kDa) forms of myostatin protein in skeletal myotubes (Fig. 5d). Fluorescence microscopy analysis revealed that treating myotubes with KN93 did not prevent the caffeine-dependent reduction (P < 0.05) in protein synthesis when compared KN92 treated cells (Fig. 5e, f). Collectively, these results indicate that inhibiting the CaMKII-dependent increase in myostatin gene expression did not prevent the caffeine-induced reduction in cellular protein synthesis in skeletal myotubes.
Fig. 5.
Caffeine promoted a CaMKII-dependent increase in myostatin gene expression that did not significantly contribute to reduce protein synthesis. C2C12 myotubes were treated with either vehicle (control) or 5 mM caffeine for 6 h (n = 3–6). a, b Western blot analysis revealed that caffeine promoted a 40% increase in CaMKII and 5.5-fold increase in P-CREB (Ser 133). c Myotubes were either treated with 10 μM KN93, a specific CaMKII inhibitor, or KN92, a negative control, to examine the effect of caffeine on the CaMKII-dependent regulation of myostatin gene and protein expression. Caffeine promoted a 65% increase myostatin mRNA expression in KN92 treated myotubes. The addition of KN93 completely inhibited the caffeine dependent increase in myostatin mRNA expression. d Caffeine treatment had no significant effect on myostatin protein levels. e, f Fluorescence microscopy analysis revealed that caffeine promoted a 25% reduction in protein synthesis in myotubes treated with KN92 or KN93. (**P < 0.05)
Discussion
The purpose of this study was to further elucidate the pathways by which caffeine reduces protein synthesis and potentially increases protein degradation in skeletal muscle cells. Our results strongly suggest that treating skeletal myotubes with 5 mM caffeine for 6 h significantly increased autophagic flux (Fig. 1) without increasing ubiquitin proteasome-dependent degradation (Fig. 2). These results are in agreement with those of previous studies, which found that higher doses of caffeine (1.5–10 mM) increased autophagic flux in various mammalian cell lines (Saiki et al. 2011; Sinha et al. 2014). To the best of our knowledge the present study is the first to demonstrate that caffeine can significantly increase autophagic flux in mammalian skeletal myotubes. However, our results directly contrast the findings of Lewis et al. (1982) and Goodman (1987), which found that treating rat skeletal muscle cells with 4–10 mM caffeine did not significantly increase cellular protein degradation. This discrepancy is likely due to the fact that in the present study skeletal muscle cells were exposed to caffeine for 6 h. Lewis et al. (1982) and Goodman (1987) exposed skeletal muscles cells to caffeine for only 2 h. In addition, Lewis et al. (1982) and Goodman (1987) conducted ex vivo analysis using isolated rat extensor digitorum longus (EDL) muscles, whereas in the present study we utilized an in vitro approach using fully differentiated skeletal myotubes. These discrepancies in experimental design likely contributed to the contrasts in results. Regardless, the current study is the first to demonstrate that caffeine can significantly increase cellular protein degradation in mammalian skeletal myotubes.
Our next objective was to determine if caffeine reduces protein synthesis in an AMPK-dependent manner. We previously demonstrated that caffeine increases AMPK activation in skeletal muscle cells by promoting the calcium-dependent activation of CaMKKβ, which in turn directly phosphorylates and thus activates the kinase domain of AMPK (Mathew et al. 2014). Our results demonstrate that caffeine significantly reduced mTORC1 activity in a CaMKKβ/AMPK-dependent manner (Fig. 3). Further, the addition of STO609 prevented the significant reduction in cellular protein synthesis (Fig. 3) and myotube diameter (Fig. 4) in caffeine vs. vehicle treated myotubes. These results strongly suggest that caffeine reduces protein synthesis and myotube diameter by promoting a CaMKKβ/AMPK-dependent reduction in mTORC1 signaling. Previous studies have demonstrated that AMPK acts as a negative regulator of mTORC1-dependent protein in mammalian skeletal muscle cells during exercise (Dreyer et al. 2006) and mechanical overload (Mounier et al. 2009a, b). To the best of our knowledge, the current study is the first to demonstrate that caffeine reduces myotube diameter and protein synthesis in mammalian skeletal myotubes in a CaMKKβ/AMPK-dependent manner.
Our previous research demonstrated that acute caffeine treatment increases CaMKII activation in C2C12 skeletal myotubes (Mathew et al. 2014). A recent investigation revealed that CaMKII can promote a CREB-dependent increase in myostatin expression in C2C12 skeletal myotubes, as well (Whittemore et al. 2003). Therefore, we decided to investigate whether acute caffeine exposure could promote a CaMKII-dependent increase in myostatin expression in skeletal myotubes. Our analysis revealed that acute caffeine promoted a CaMKII-dependent increase in myostatin mRNA expression without significantly affecting myostatin protein levels (Fig. 4). In addition, we discovered that inhibiting CaMKII activation, and therefore myostatin gene expression, did not prevent the caffeine-dependent reduction in protein synthesis (Fig. 4). Therefore, we concluded that in the current study acute caffeine exposure promoted a CaMKII-dependent increase in myostatin gene expression that did not contribute significantly to the caffeine-dependent reduction in protein synthesis. To the best of our knowledge, however, the current study is the first to report that caffeine can significantly increase myostatin gene expression in skeletal myotubes. Future experiments are needed to determine whether chronic exposure to high doses of caffeine can negatively impact skeletal muscle growth in a myostatin-dependent manner.
According the model proposed by Reagan-Shaw et al. (2008), a 5 mM caffeine dose in an adult mouse translates to an equivalent dose of approximately 400 μM in an adult human. Given that >500 μM (100 mg/L) caffeine is considered to be potentially lethal for adult humans (Winek et al. 2001), the caffeine concentration used in the present study likely represents the upper physiological limit of mammalian skeletal muscle. However, the caffeine dose used in the present study is very similar to those of previous investigations (Lewis et al. 1982; Goodman 1987; Egawa et al. 2011a, b; Moore et al. 2017). As previously mentioned, Lewis et al. (1982) and Goodman (1987) discovered that treating rat EDL muscle with 4–10 mM caffeine significantly reduced protein synthesis. A recent investigation by Moore et al. (2017) revealed that supplementing drinking water with 1 g/L caffeine (5.0 mM) did not affect protein synthesis or growth of the plantaris muscle from rats 2-weeks after sham or synergist ablation surgery. A further consideration is the half-life of caffeine in rodents vs. humans. Previous research indicates that caffeine has a half-life of approximately 1 h in mice and rats (Arnaud 1987) and 5 h in humans (Bonati et al. 1985). Therefore, it is unclear if the results of the current study are truly indicative of the potential catabolic effects of high-caffeine concentrations on skeletal muscle in vivo. Regardless, the current study details the pathways by which high-caffeine concentrations promote protein degradation and reduce protein synthesis in murine skeletal myotubes.
Acknowledgments
Financial support was provided by Magellan Scholars Fellowships that were awarded by the Office of Undergraduate Research at the University of South Carolina to M. A. Hughes. Additional support was provided by a Magellan Mentors Award that was awarded by the University of South Carolina Upstate Office of Sponsored Awards and Research Support to B. L. Baumgarner. Research reported in this publication was also supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R15DK106688 to S. T. Kinsey.
References
- Arnaud MJ. The pharmacology of caffeine. Prog Drug Res. 1987;31:273–313. doi: 10.1007/978-3-0348-9289-6_9. [DOI] [PubMed] [Google Scholar]
- Bonati M, Latini R, Tognoni G, Young JF, Garattini S. Interspecies comparison of in vivo caffeine pharmacokinetics in man, monkey, rabbit, rat and mouse. Drug Metab Rev. 1985;15:1355–1383. doi: 10.3109/03602538409029964. [DOI] [PubMed] [Google Scholar]
- Connolly S, Kingsbury TJ. Caffeine modulates CREB-dependent gene expression in developing cortical neurons. Biochem Biophys Res Commun. 2010;397:152–156. doi: 10.1016/j.bbrc.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibi A, Cornille K, Leibovitch MP, Poupon A, Tintignac LA, Sanchez AM, Leibovitch SA. The translation regulatory subunit eIF3f controls the kinase-dependent mTOR signaling required for muscle differentiation and hypertrophy in mouse. PLoS One. 2010;5:e8994. doi: 10.1371/journal.pone.0008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MD, Jefferson LS, Kimball SR. Role of p70s6k1-mediated phosphorylation of eif4b and pdcd4 proteins in the regulation of protein synthesis. J Biol Chem. 2012;287:42890–42899. doi: 10.1074/jbc.M112.404822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volp E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa T, Hamada T, Ma X, Karaike K, Kameda N, Masuda S, Iwanaka N, Hayashi T. Caffeine activates preferentially α1-isoform of 5′ AMP-activated protein kinase in rat skeletal muscle. Acta Physiol. 2011a;2:227–238. doi: 10.1111/j.1748-1716.2010.02169.x. [DOI] [PubMed] [Google Scholar]
- Egawa T, Tsuda S, Ma X, Hamada T, Hayashi T. Caffeine modulates phosphorylation. FASEB J. 2011b;23:2264–2273. doi: 10.1152/japplphysiol.00249.2011. [DOI] [PubMed] [Google Scholar]
- Goodman MN. Differential effects of acute changes in cell Ca2+ concentration on myofibrillar and non-myofibrillar protein breakdown in the rat extensor digitorum longus muscle in vitro. Assessment by production of tyrosine and N tau-methylhistidine. Biochem J. 1987;24:121–127. doi: 10.1042/bj2410121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, Ait-Si-Ali S, Akematsu T, Akporiaye ET, Al-Rubeai M, Albaiceta GM, Albanese C, Albani D, Albert ML, Aldudo J, Algül H, Alirezaei M, Alloza I, Almasan A, Almonte-Beceril M, Alnemri ES, Alonso C, Altan-Bonnet N, Altieri DC, Alvarez S, Alvarez-Erviti L, Alves S, Amadoro G, Amano A, Amantini C, Ambrosio S, Amelio I, Amer AO, Amessou M, Amon A, An Z, Anania FA, Andersen SU, Andley UP, Andreadi CK, Andrieu-Abadie N, Anel A, Ann DK, Anoopkumar-Dukie S, Antonioli M, Aoki H, Apostolova N, Aquila S, Aquilano K, Araki K, Arama E, Aranda A, Araya J, Arcaro A, Arias E, Arimoto H, Ariosa AR, Armstrong JL, Arnould T, Arsov I, Asanuma K, Askanas V, Asselin E, Atarashi R, Atherton SS, Atkin JD, Attardi LD, Auberger P, Auburger G, Aurelian L, Autelli R, Avagliano L, Avantaggiati ML, Avrahami L, Awale S, Azad N, Bachetti T, Backer JM, Bae DH, Bae JS, Bae ON, Bae SH, Baehrecke EH, Baek SH, Baghdiguian S, Bagniewska-Zadworna A, Bai H, Bai J, Bai XY, Bailly Y, Balaji KN, Balduini W, Ballabio A, Balzan R, Banerjee R, Bánhegyi G, Bao H, Barbeau B, Barrachina MD, Barreiro E, Bartel B, Bartolomé A, Bassham DC, Bassi MT, Bast RC, Jr, Basu A, Batista MT, Batoko H, Battino M, Bauckman K, Baumgarner BL, Bayer KU, Beale R, Beaulieu JF, Beck GR, Jr, Becker C, Beckham JD, Bédard PA, Bednarski PJ, Begley TJ, Behl C, Behrends C, Behrens GM, Behrns KE, Bejarano E, Belaid A, Belleudi F, Bénard G, Berchem G, Bergamaschi D, Bergami M, Berkhout B, Berliocchi L, Bernard A, Bernard M, Bernassola F, Bertolotti A, Bess AS, Besteiro S, Bettuzzi S, Bhalla S, Bhattacharyya S, Bhutia SK, Biagosch C, Bianchi MW, Biard-Piechaczyk M, Billes V, Bincoletto C, Bingol B, Bird SW, Bitoun M, Bjedov I, Blackstone C, Blanc L, Blanco GA, Blomhoff HK, Boada-Romero E, Böckler S, Boes M, Boesze-Battaglia K, Boise LH, Bolino A, Boman A, Bonaldo P, Bordi M, Bosch J, Botana LM, Botti J, Bou G, Bouché M, Bouchecareilh M, Boucher MJ, Boulton ME, Bouret SG, Boya P, Boyer-Guittaut M, Bozhkov PV, Brady N, Braga VM, Brancolini C, Braus GH, Bravo-San Pedro JM, Brennan LA, Bresnick EH, Brest P, Bridges D, Bringer MA, Brini M, Brito GC, Brodin B, Brookes PS, Brown EJ, Brown K, Broxmeyer HE, Bruhat A, Brum PC, Brumell JH, Brunetti-Pierri N, Bryson-Richardson RJ, Buch S, Buchan AM, Budak H, Bulavin DV, Bultman SJ, Bultynck G, Bumbasirevic V, Burelle Y, Burke RE, Burmeister M, Bütikofer P, Caberlotto L, Cadwell K, Cahova M, Cai D, Cai J, Cai Q, Calatayud S, Camougrand N, Campanella M, Campbell GR, Campbell M, Campello S, Candau R, Caniggia I, Cantoni L, Cao L, Caplan AB, Caraglia M, Cardinali C, Cardoso SM, Carew JS, Carleton LA, Carlin CR, Carloni S, Carlsson SR, Carmona-Gutierrez D, Carneiro LA, Carnevali O, Carra S, Carrier A, Carroll B, Casas C, Casas J, Cassinelli G, Castets P, Castro-Obregon S, Cavallini G, Ceccherini I, Cecconi F, Cederbaum AI, Ceña V, Cenci S, Cerella C, Cervia D, Cetrullo S, Chaachouay H, Chae HJ, Chagin AS, Chai CY, Chakrabarti G, Chamilos G, Chan EY, Chan MT, Chandra D, Chandra P, Chang CP, Chang RC, Chang TY, Chatham JC, Chatterjee S, Chauhan S, Che Y, Cheetham ME, Cheluvappa R, Chen CJ, Chen G, Chen GC, Chen G, Chen H, Chen JW, Chen JK, Chen M, Chen M, Chen P, Chen Q, Chen Q, Chen SD, Chen S, Chen SS, Chen W, Chen WJ, Chen WQ, Chen W, Chen X, Chen YH, Chen YG, Chen Y, Chen Y, Chen Y, Chen YJ, Chen YQ, Chen Y, Chen Z, Chen Z, Cheng A, Cheng CH, Cheng H, Cheong H, Cherry S, Chesney J, Cheung CH, Chevet E, Chi HC, Chi SG, Chiacchiera F, Chiang HL, Chiarelli R, Chiariello M, Chieppa M, Chin LS, Chiong M, Chiu GN, Cho DH, Cho SG, Cho WC, Cho YY, Cho YS, Choi AM, Choi EJ, Choi EK, Choi J, Choi ME, Choi SI, Chou TF, Chouaib S, Choubey D, Choubey V, Chow KC, Chowdhury K, Chu CT, Chuang TH, Chun T, Chung H, Chung T, Chung YL, Chwae YJ, Cianfanelli V, Ciarcia R, Ciechomska IA, Ciriolo MR, Cirone M, Claerhout S, Clague MJ, Clària J, Clarke PG, Clarke R, Clementi E, Cleyrat C, Cnop M, Coccia EM, Cocco T, Codogno P, Coers J, Cohen EE, Colecchia D, Coletto L, Coll NS, Colucci-Guyon E, Comincini S, Condello M, Cook KL, Coombs GH, Cooper CD, Cooper JM, Coppens I, Corasaniti MT, Corazzari M, Corbalan R, Corcelle-Termeau E, Cordero MD, Corral-Ramos C, Corti O, Cossarizza A, Costelli P, Costes S, Cotman SL, Coto-Montes A, Cottet S, Couve E, Covey LR, Cowart LA, Cox JS, Coxon FP, Coyne CB, Cragg MS, Craven RJ, Crepaldi T, Crespo JL, Criollo A, Crippa V, Cruz MT, Cuervo AM, Cuezva JM, Cui T, Cutillas PR, Czaja MJ, Czyzyk-Krzeska MF, Dagda RK, Dahmen U, Dai C, Dai W, Dai Y, Dalby KN, Dalla Valle L, Dalmasso G, D’Amelio M, Damme M, Darfeuille-Michaud A, Dargemont C, Darley-Usmar VM, Dasarathy S, Dasgupta B, Dash S, Dass CR, Davey HM, Davids LM, Dávila D, Davis RJ, Dawson TM, Dawson VL, Daza P, de Belleroche J, de Figueiredo P, de Figueiredo RC, de la Fuente J, De Martino L, De Matteis A, De Meyer GR, De Milito A, De Santi M, de Souza W, De Tata V, De Zio D, Debnath J, Dechant R, Decuypere JP, Deegan S, Dehay B, Del Bello B, Del Re DP, Delage-Mourroux R, Delbridge LM, Deldicque L, Delorme-Axford E, Deng Y, Dengjel J, Denizot M, Dent P, Der CJ, Deretic V, Derrien B, Deutsch E, Devarenne TP, Devenish RJ, Di Bartolomeo S, Di Daniele N, Di Domenico F, Di Nardo A, Di Paola S, Di Pietro A, Di Renzo L, DiAntonio A, Díaz-Araya G, Díaz-Laviada I, Diaz-Meco MT, Diaz-Nido J, Dickey CA, Dickson RC, Diederich M, Digard P, Dikic I, Dinesh-Kumar SP, Ding C, Ding WX, Ding Z, Dini L, Distler JH, Diwan A, Djavaheri-Mergny M, Dmytruk K, Dobson RC, Doetsch V, Dokladny K, Dokudovskaya S, Donadelli M, Dong XC, Dong X, Dong Z, Donohue TM, Jr, Doran KS, D’Orazi G, Dorn GW, 2nd, Dosenko V, Dridi S, Drucker L, Du J, Du LL, Du L, du Toit A, Dua P, Duan L, Duann P, Dubey VK, Duchen MR, Duchosal MA, Duez H, Dugail I, Dumit VI, Duncan MC, Dunlop EA, Dunn WA, Jr, Dupont N, Dupuis L, Durán RV, Durcan TM, Duvezin-Caubet S, Duvvuri U, Eapen V, Ebrahimi-Fakhari D, Echard A, Eckhart L, Edelstein CL, Edinger AL, Eichinger L, Eisenberg T, Eisenberg-Lerner A, Eissa NT, El-Deiry WS, El-Khoury V, Elazar Z, Eldar-Finkelman H, Elliott CJ, Emanuele E, Emmenegger U, Engedal N, Engelbrecht AM, Engelender S, Enserink JM, Erdmann R, Erenpreisa J, Eri R, Eriksen JL, Erman A, Escalante R, Eskelinen EL, Espert L, Esteban-Martínez L, Evans TJ, Fabri M, Fabrias G, Fabrizi C, Facchiano A, Færgeman NJ, Faggioni A, Fairlie WD, Fan C, Fan D, Fan J, Fang S, Fanto M, Fanzani A, Farkas T, Faure M, Favier FB, Fearnhead H, Federici M, Fei E, Felizardo TC, Feng H, Feng Y, Feng Y, Ferguson TA, Fernández ÁF, Fernandez-Barrena MG, Fernandez-Checa JC, Fernández-López A, Fernandez-Zapico ME, Feron O, Ferraro E, Ferreira-Halder CV, Fesus L, Feuer R, Fiesel FC, Filippi-Chiela EC, Filomeni G, Fimia GM, Fingert JH, Finkbeiner S, Finkel T, Fiorito F, Fisher PB, Flajolet M, Flamigni F, Florey O, Florio S, Floto RA, Folini M, Follo C, Fon EA, Fornai F, Fortunato F, Fraldi A, Franco R, Francois A, François A, Frankel LB, Fraser ID, Frey N, Freyssenet DG, Frezza C, Friedman SL, Frigo DE, Fu D, Fuentes JM, Fueyo J, Fujitani Y, Fujiwara Y, Fujiya M, Fukuda M, Fulda S, Fusco C, Gabryel B, Gaestel M, Gailly P, Gajewska M, Galadari S, Galili G, Galindo I, Galindo MF, Galliciotti G, Galluzzi L, Galluzzi L, Galy V, Gammoh N, Gandy S, Ganesan AK, Ganesan S, Ganley IG, Gannagé M, Gao FB, Gao F, Gao JX, García Nannig L, García Véscovi E, Garcia-Macía M, Garcia-Ruiz C, Garg AD, Garg PK, Gargini R, Gassen NC, Gatica D, Gatti E, Gavard J, Gavathiotis E, Ge L, Ge P, Ge S, Gean PW, Gelmetti V, Genazzani AA, Geng J, Genschik P, Gerner L, Gestwicki JE, Gewirtz DA, Ghavami S, Ghigo E, Ghosh D, Giammarioli AM, Giampieri F, Giampietri C, Giatromanolaki A, Gibbings DJ, Gibellini L, Gibson SB, Ginet V, Giordano A, Giorgini F, Giovannetti E, Girardin SE, Gispert S, Giuliano S, Gladson CL, Glavic A, Gleave M, Godefroy N, Gogal RM, Jr, Gokulan K, Goldman GH, Goletti D, Goligorsky MS, Gomes AV, Gomes LC, Gomez H, Gomez-Manzano C, Gómez-Sánchez R, Gonçalves DA, Goncu E, Gong Q, Gongora C, Gonzalez CB, Gonzalez-Alegre P, Gonzalez-Cabo P, González-Polo RA, Goping IS, Gorbea C, Gorbunov NV, Goring DR, Gorman AM, Gorski SM, Goruppi S, Goto-Yamada S, Gotor C, Gottlieb RA, Gozes I, Gozuacik D, Graba Y, Graef M, Granato GE, Grant GD, Grant S, Gravina GL, Green DR, Greenhough A, Greenwood MT, Grimaldi B, Gros F, Grose C, Groulx JF, Gruber F, Grumati P, Grune T, Guan JL, Guan KL, Guerra B, Guillen C, Gulshan K, Gunst J, Guo C, Guo L, Guo M, Guo W, Guo XG, Gust AA, Gustafsson ÅB, Gutierrez E, Gutierrez MG, Gwak HS, Haas A, Haber JE, Hadano S, Hagedorn M, Hahn DR, Halayko AJ, Hamacher-Brady A, Hamada K, Hamai A, Hamann A, Hamasaki M, Hamer I, Hamid Q, Hammond EM, Han F, Han W, Handa JT, Hanover JA, Hansen M, Harada M, Harhaji-Trajkovic L, Harper JW, Harrath AH, Harris AL, Harris J, Hasler U, Hasselblatt P, Hasui K, Hawley RG, Hawley TS, He C, He CY, He F, He G, He RR, He XH, He YW, He YY, Heath JK, Hébert MJ, Heinzen RA, Helgason GV, Hensel M, Henske EP, Her C, Herman PK, Hernández A, Hernandez C, Hernández-Tiedra S, Hetz C, Hiesinger PR, Higaki K, Hilfiker S, Hill BG, Hill JA, Hill WD, Hino K, Hofius D, Hofman P, Höglinger GU, Höhfeld J, Holz MK, Hong Y, Hood DA, Hoozemans JJ, Hoppe T, Hsu C, Hsu CY, Hsu LC, Hu D, Hu G, Hu HM, Hu H, Hu MC, Hu YC, Hu ZW, Hua F, Hua Y, Huang C, Huang HL, Huang KH, Huang KY, Huang S, Huang S, Huang WP, Huang YR, Huang Y, Huang Y, Huber TB, Huebbe P, Huh WK, Hulmi JJ, Hur GM, Hurley JH, Husak Z, Hussain SN, Hussain S, Hwang JJ, Hwang S, Hwang TI, Ichihara A, Imai Y, Imbriano C, Inomata M, Into T, Iovane V, Iovanna JL, Iozzo RV, Ip NY, Irazoqui JE, Iribarren P, Isaka Y, Isakovic AJ, Ischiropoulos H, Isenberg JS, Ishaq M, Ishida H, Ishii I, Ishmael JE, Isidoro C, Isobe K, Isono E, Issazadeh-Navikas S, Itahana K, Itakura E, Ivanov AI, Iyer AK, Izquierdo JM, Izumi Y, Izzo V, Jäättelä M, Jaber N, Jackson DJ, Jackson WT, Jacob TG, Jacques TS, Jagannath C, Jain A, Jana NR, Jang BK, Jani A, Janji B, Jannig PR, Jansson PJ, Jean S, Jendrach M, Jeon JH, Jessen N, Jeung EB, Jia K, Jia L, Jiang H, Jiang H, Jiang L, Jiang T, Jiang X, Jiang X, Jiang X, Jiang Y, Jiang Y, Jiménez A, Jin C, Jin H, Jin L, Jin M, Jin S, Jinwal UK, Jo EK, Johansen T, Johnson DE, Johnson GV, Johnson JD, Jonasch E, Jones C, Joosten LA, Jordan J, Joseph AM, Joseph B, Joubert AM, Ju D, Ju J, Juan HF, Juenemann K, Juhász G, Jung HS, Jung JU, Jung YK, Jungbluth H, Justice MJ, Jutten B, Kaakoush NO, Kaarniranta K, Kaasik A, Kabuta T, Kaeffer B, Kågedal K, Kahana A, Kajimura S, Kakhlon O, Kalia M, Kalvakolanu DV, Kamada Y, Kambas K, Kaminskyy VO, Kampinga HH, Kandouz M, Kang C, Kang R, Kang TC, Kanki T, Kanneganti TD, Kanno H, Kanthasamy AG, Kantorow M, Kaparakis-Liaskos M, Kapuy O, Karantza V, Karim MR, Karmakar P, Kaser A, Kaushik S, Kawula T, Kaynar AM, Ke PY, Ke ZJ, Kehrl JH, Keller KE, Kemper JK, Kenworthy AK, Kepp O, Kern A, Kesari S, Kessel D, Ketteler R, Kettelhut Ido C, Khambu B, Khan MM, Khandelwal VK, Khare S, Kiang JG, Kiger AA, Kihara A, Kim AL, Kim CH, Kim DR, Kim DH, Kim EK, Kim HY, Kim HR, Kim JS, Kim JH, Kim JC, Kim JH, Kim KW, Kim MD, Kim MM, Kim PK, Kim SW, Kim SY, Kim YS, Kim Y, Kimchi A, Kimmelman AC, Kimura T, King JS, Kirkegaard K, Kirkin V, Kirshenbaum LA, Kishi S, Kitajima Y, Kitamoto K, Kitaoka Y, Kitazato K, Kley RA, Klimecki WT, Klinkenberg M, Klucken J, Knævelsrud H, Knecht E, Knuppertz L, Ko JL, Kobayashi S, Koch JC, Koechlin-Ramonatxo C, Koenig U, Koh YH, Köhler K, Kohlwein SD, Koike M, Komatsu M, Kominami E, Kong D, Kong HJ, Konstantakou EG, Kopp BT, Korcsmaros T, Korhonen L, Korolchuk VI, Koshkina NV, Kou Y, Koukourakis MI, Koumenis C, Kovács AL, Kovács T, Kovacs WJ, Koya D, Kraft C, Krainc D, Kramer H, Kravic-Stevovic T, Krek W, Kretz-Remy C, Krick R, Krishnamurthy M, Kriston-Vizi J, Kroemer G, Kruer MC, Kruger R, Ktistakis NT, Kuchitsu K, Kuhn C, Kumar AP, Kumar A, Kumar A, Kumar D, Kumar D, Kumar R, Kumar S, Kundu M, Kung HJ, Kuno A, Kuo SH, Kuret J, Kurz T, Kwok T, Kwon TK, Kwon YT, Kyrmizi I, La Spada AR, Lafont F, Lahm T, Lakkaraju A, Lam T, Lamark T, Lancel S, Landowski TH, Lane DJ, Lane JD, Lanzi C, Lapaquette P, Lapierre LR, Laporte J, Laukkarinen J, Laurie GW, Lavandero S, Lavie L, LaVoie MJ, Law BY, Law HK, Law KB, Layfield R, Lazo PA, Le Cam L, Le Roch KG, Le Stunff H, Leardkamolkarn V, Lecuit M, Lee BH, Lee CH, Lee EF, Lee GM, Lee HJ, Lee H, Lee JK, Lee J, Lee JH, Lee JH, Lee M, Lee MS, Lee PJ, Lee SW, Lee SJ, Lee SJ, Lee SY, Lee SH, Lee SS, Lee SJ, Lee S, Lee YR, Lee YJ, Lee YH, Leeuwenburgh C, Lefort S, Legouis R, Lei J, Lei QY, Leib DA, Leibowitz G, Lekli I, Lemaire SD, Lemasters JJ, Lemberg MK, Lemoine A, Leng S, Lenz G, Lenzi P, Lerman LO, Lettieri Barbato D, Leu JI, Leung HY, Levine B, Lewis PA, Lezoualc’h F, Li C, Li F, Li FJ, Li J, Li K, Li L, Li M, Li M, Li Q, Li R, Li S, Li W, Li W, Li X, Li Y, Lian J, Liang C, Liang Q, Liao Y, Liberal J, Liberski PP, Lie P, Lieberman AP, Lim HJ, Lim KL, Lim K, Lima RT, Lin CS, Lin CF, Lin F, Lin F, Lin FC, Lin K, Lin KH, Lin PH, Lin T, Lin WW, Lin YS, Lin Y, Linden R, Lindholm D, Lindqvist LM, Lingor P, Linkermann A, Liotta LA, Lipinski MM, Lira VA, Lisanti MP, Liton PB, Liu B, Liu C, Liu CF, Liu F, Liu HJ, Liu J, Liu JJ, Liu JL, Liu K, Liu L, Liu L, Liu Q, Liu RY, Liu S, Liu S, Liu W, Liu XD, Liu X, Liu XH, Liu X, Liu X, Liu X, Liu Y, Liu Y, Liu Z, Liu Z, Liuzzi JP, Lizard G, Ljujic M, Lodhi IJ, Logue SE, Lokeshwar BL, Long YC, Lonial S, Loos B, López-Otín C, López-Vicario C, Lorente M, Lorenzi PL, Lõrincz P, Los M, Lotze MT, Lovat PE, Lu B, Lu B, Lu J, Lu Q, Lu SM, Lu S, Lu Y, Luciano F, Luckhart S, Lucocq JM, Ludovico P, Lugea A, Lukacs NW, Lum JJ, Lund AH, Luo H, Luo J, Luo S, Luparello C, Lyons T, Ma J, Ma Y, Ma Y, Ma Z, Machado J, Machado-Santelli GM, Macian F, MacIntosh GC, MacKeigan JP, Macleod KF, MacMicking JD, MacMillan-Crow LA, Madeo F, Madesh M, Madrigal-Matute J, Maeda A, Maeda T, Maegawa G, Maellaro E, Maes H, Magariños M, Maiese K, Maiti TK, Maiuri L, Maiuri MC, Maki CG, Malli R, Malorni W, Maloyan A, Mami-Chouaib F, Man N, Mancias JD, Mandelkow EM, Mandell MA, Manfredi AA, Manié SN, Manzoni C, Mao K, Mao Z, Mao ZW, Marambaud P, Marconi AM, Marelja Z, Marfe G, Margeta M, Margittai E, Mari M, Mariani FV, Marin C, Marinelli S, Mariño G, Markovic I, Marquez R, Martelli AM, Martens S, Martin KR, Martin SJ, Martin S, Martin-Acebes MA, Martín-Sanz P, Martinand-Mari C, Martinet W, Martinez J, Martinez-Lopez N, Martinez-Outschoorn U, Martínez-Velázquez M, Martinez-Vicente M, Martins WK, Mashima H, Mastrianni JA, Matarese G, Matarrese P, Mateo R, Matoba S, Matsumoto N, Matsushita T, Matsuura A, Matsuzawa T, Mattson MP, Matus S, Maugeri N, Mauvezin C, Mayer A, Maysinger D, Mazzolini GD, McBrayer MK, McCall K, McCormick C, McInerney GM, McIver SC, McKenna S, McMahon JJ, McNeish IA, Mechta-Grigoriou F, Medema JP, Medina DL, Megyeri K, Mehrpour M, Mehta JL, Mei Y, Meier UC, Meijer AJ, Meléndez A, Melino G, Melino S, de Melo EJ, Mena MA, Meneghini MD, Menendez JA, Menezes R, Meng L, Meng LH, Meng S, Menghini R, Menko AS, Menna-Barreto RF, Menon MB, Meraz-Ríos MA, Merla G, Merlini L, Merlot AM, Meryk A, Meschini S, Meyer JN, Mi MT, Miao CY, Micale L, Michaeli S, Michiels C, Migliaccio AR, Mihailidou AS, Mijaljica D, Mikoshiba K, Milan E, Miller-Fleming L, Mills GB, Mills IG, Minakaki G, Minassian BA, Ming XF, Minibayeva F, Minina EA, Mintern JD, Minucci S, Miranda-Vizuete A, Mitchell CH, Miyamoto S, Miyazawa K, Mizushima N, Mnich K, Mograbi B, Mohseni S, Moita LF, Molinari M, Molinari M, M⊘ller AB, Mollereau B, Mollinedo F, Mongillo M, Monick MM, Montagnaro S, Montell C, Moore DJ, Moore MN, Mora-Rodriguez R, Moreira PI, Morel E, Morelli MB, Moreno S, Morgan MJ, Moris A, Moriyasu Y, Morrison JL, Morrison LA, Morselli E, Moscat J, Moseley PL, Mostowy S, Motori E, Mottet D, Mottram JC, Moussa CE, Mpakou VE, Mukhtar H, Mulcahy Levy JM, Muller S, Muñoz-Moreno R, Muñoz-Pinedo C, Münz C, Murphy ME, Murray JT, Murthy A, Mysorekar IU, Nabi IR, Nabissi M, Nader GA, Nagahara Y, Nagai Y, Nagata K, Nagelkerke A, Nagy P, Naidu SR, Nair S, Nakano H, Nakatogawa H, Nanjundan M, Napolitano G, Naqvi NI, Nardacci R, Narendra DP, Narita M, Nascimbeni AC, Natarajan R, Navegantes LC, Nawrocki ST, Nazarko TY, Nazarko VY, Neill T, Neri LM, Netea MG, Netea-Maier RT, Neves BM, Ney PA, Nezis IP, Nguyen HT, Nguyen HP, Nicot AS, Nilsen H, Nilsson P, Nishimura M, Nishino I, Niso-Santano M, Niu H, Nixon RA, Njar VC, Noda T, Noegel AA, Nolte EM, Norberg E, Norga KK, Noureini SK, Notomi S, Notterpek L, Nowikovsky K, Nukina N, Nürnberger T, O’Donnell VB, O’Donovan T, O’Dwyer PJ, Oehme I, Oeste CL, Ogawa M, Ogretmen B, Ogura Y, Oh YJ, Ohmuraya M, Ohshima T, Ojha R, Okamoto K, Okazaki T, Oliver FJ, Ollinger K, Olsson S, Orban DP, Ordonez P, Orhon I, Orosz L, O’Rourke EJ, Orozco H, Ortega AL, Ortona E, Osellame LD, Oshima J, Oshima S, Osiewacz HD, Otomo T, Otsu K, Ou JH, Outeiro TF, Ouyang DY, Ouyang H, Overholtzer M, Ozbun MA, Ozdinler PH, Ozpolat B, Pacelli C, Paganetti P, Page G, Pages G, Pagnini U, Pajak B, Pak SC, Pakos-Zebrucka K, Pakpour N, Palková Z, Palladino F, Pallauf K, Pallet N, Palmieri M, Paludan SR, Palumbo C, Palumbo S, Pampliega O, Pan H, Pan W, Panaretakis T, Pandey A, Pantazopoulou A, Papackova Z, Papademetrio DL, Papassideri I, Papini A, Parajuli N, Pardo J, Parekh VV, Parenti G, Park JI, Park J, Park OK, Parker R, Parlato R, Parys JB, Parzych KR, Pasquet JM, Pasquier B, Pasumarthi KB, Patschan D, Patterson C, Pattingre S, Pattison S, Pause A, Pavenstädt H, Pavone F, Pedrozo Z, Peña FJ, Peñalva MA, Pende M, Peng J, Penna F, Penninger JM, Pensalfini A, Pepe S, Pereira GJ, Pereira PC, Pérez-de la Cruz V, Pérez-Pérez ME, Pérez-Rodríguez D, Pérez-Sala D, Perier C, Perl A, Perlmutter DH, Perrotta I, Pervaiz S, Pesonen M, Pessin JE, Peters GJ, Petersen M, Petrache I, Petrof BJ, Petrovski G, Phang JM, Piacentini M, Pierdominici M, Pierre P, Pierrefite-Carle V, Pietrocola F, Pimentel-Muiños FX, Pinar M, Pineda B, Pinkas-Kramarski R, Pinti M, Pinton P, Piperdi B, Piret JM, Platanias LC, Platta HW, Plowey ED, Pöggeler S, Poirot M, Polčic P, Poletti A, Poon AH, Popelka H, Popova B, Poprawa I, Poulose SM, Poulton J, Powers SK, Powers T, Pozuelo-Rubio M, Prak K, Prange R, Prescott M, Priault M, Prince S, Proia RL, Proikas-Cezanne T, Prokisch H, Promponas VJ, Przyklenk K, Puertollano R, Pugazhenthi S, Puglielli L, Pujol A, Puyal J, Pyeon D, Qi X, Qian WB, Qin ZH, Qiu Y, Qu Z, Quadrilatero J, Quinn F, Raben N, Rabinowich H, Radogna F, Ragusa MJ, Rahmani M, Raina K, Ramanadham S, Ramesh R, Rami A, Randall-Demllo S, Randow F, Rao H, Rao VA, Rasmussen BB, Rasse TM, Ratovitski EA, Rautou PE, Ray SK, Razani B, Reed BH, Reggiori F, Rehm M, Reichert AS, Rein T, Reiner DJ, Reits E, Ren J, Ren X, Renna M, Reusch JE, Revuelta JL, Reyes L, Rezaie AR, Richards RI, Richardson DR, Richetta C, Riehle MA, Rihn BH, Rikihisa Y, Riley BE, Rimbach G, Rippo MR, Ritis K, Rizzi F, Rizzo E, Roach PJ, Robbins J, Roberge M, Roca G, Roccheri MC, Rocha S, Rodrigues CM, Rodríguez CI, de Cordoba SR, Rodriguez-Muela N, Roelofs J, Rogov VV, Rohn TT, Rohrer B, Romanelli D, Romani L, Romano PS, Roncero MI, Rosa JL, Rosello A, Rosen KV, Rosenstiel P, Rost-Roszkowska M, Roth KA, Roué G, Rouis M, Rouschop KM, Ruan DT, Ruano D, Rubinsztein DC, Rucker EB, 3rd, Rudich A, Rudolf E, Rudolf R, Ruegg MA, Ruiz-Roldan C, Ruparelia AA, Rusmini P, Russ DW, Russo GL, Russo G, Russo R, Rusten TE, Ryabovol V, Ryan KM, Ryter SW, Sabatini DM, Sacher M, Sachse C, Sack MN, Sadoshima J, Saftig P, Sagi-Eisenberg R, Sahni S, Saikumar P, Saito T, Saitoh T, Sakakura K, Sakoh-Nakatogawa M, Sakuraba Y, Salazar-Roa M, Salomoni P, Saluja AK, Salvaterra PM, Salvioli R, Samali A, Sanchez AM, Sánchez-Alcázar JA, Sanchez-Prieto R, Sandri M, Sanjuan MA, Santaguida S, Santambrogio L, Santoni G, Dos Santos CN, Saran S, Sardiello M, Sargent G, Sarkar P, Sarkar S, Sarrias MR, Sarwal MM, Sasakawa C, Sasaki M, Sass M, Sato K, Sato M, Satriano J, Savaraj N, Saveljeva S, Schaefer L, Schaible UE, Scharl M, Schatzl HM, Schekman R, Scheper W, Schiavi A, Schipper HM, Schmeisser H, Schmidt J, Schmitz I, Schneider BE, Schneider EM, Schneider JL, Schon EA, Schönenberger MJ, Schönthal AH, Schorderet DF, Schröder B, Schuck S, Schulze RJ, Schwarten M, Schwarz TL, Sciarretta S, Scotto K, Scovassi AI, Screaton RA, Screen M, Seca H, Sedej S, Segatori L, Segev N, Seglen PO, Seguí-Simarro JM, Segura-Aguilar J, Seki E, Sell C, Seiliez I, Semenkovich CF, Semenza GL, Sen U, Serra AL, Serrano-Puebla A, Sesaki H, Setoguchi T, Settembre C, Shacka JJ, Shajahan-Haq AN, Shapiro IM, Sharma S, She H, Shen CK, Shen CC, Shen HM, Shen S, Shen W, Sheng R, Sheng X, Sheng ZH, Shepherd TG, Shi J, Shi Q, Shi Q, Shi Y, Shibutani S, Shibuya K, Shidoji Y, Shieh JJ, Shih CM, Shimada Y, Shimizu S, Shin DW, Shinohara ML, Shintani M, Shintani T, Shioi T, Shirabe K, Shiri-Sverdlov R, Shirihai O, Shore GC, Shu CW, Shukla D, Sibirny AA, Sica V, Sigurdson CJ, Sigurdsson EM, Sijwali PS, Sikorska B, Silveira WA, Silvente-Poirot S, Silverman GA, Simak J, Simmet T, Simon AK, Simon HU, Simone C, Simons M, Simonsen A, Singh R, Singh SV, Singh SK, Sinha D, Sinha S, Sinicrope FA, Sirko A, Sirohi K, Sishi BJ, Sittler A, Siu PM, Sivridis E, Skwarska A, Slack R, Slaninová I, Slavov N, Smaili SS, Smalley KS, Smith DR, Soenen SJ, Soleimanpour SA, Solhaug A, Somasundaram K, Son JH, Sonawane A, Song C, Song F, Song HK, Song JX, Song W, Soo KY, Sood AK, Soong TW, Soontornniyomkij V, Sorice M, Sotgia F, Soto-Pantoja DR, Sotthibundhu A, Sousa MJ, Spaink HP, Span PN, Spang A, Sparks JD, Speck PG, Spector SA, Spies CD, Springer W, Clair DS, Stacchiotti A, Staels B, Stang MT, Starczynowski DT, Starokadomskyy P, Steegborn C, Steele JW, Stefanis L, Steffan J, Stellrecht CM, Stenmark H, Stepkowski TM, Stern ST, Stevens C, Stockwell BR, Stoka V, Storchova Z, Stork B, Stratoulias V, Stravopodis DJ, Strnad P, Strohecker AM, Ström AL, Stromhaug P, Stulik J, Su YX, Su Z, Subauste CS, Subramaniam S, Sue CM, Suh SW, Sui X, Sukseree S, Sulzer D, Sun FL, Sun J, Sun J, Sun SY, Sun Y, Sun Y, Sun Y, Sundaramoorthy V, Sung J, Suzuki H, Suzuki K, Suzuki N, Suzuki T, Suzuki YJ, Swanson MS, Swanton C, Swärd K, Swarup G, Sweeney ST, Sylvester PW, Szatmari Z, Szegezdi E, Szlosarek PW, Taegtmeyer H, Tafani M, Taillebourg E, Tait SW, Takacs-Vellai K, Takahashi Y, Takáts S, Takemura G, Takigawa N, Talbot NJ, Tamagno E, Tamburini J, Tan CP, Tan L, Tan ML, Tan M, Tan YJ, Tanaka K, Tanaka M, Tang D, Tang D, Tang G, Tanida I, Tanji K, Tannous BA, Tapia JA, Tasset-Cuevas I, Tatar M, Tavassoly I, Tavernarakis N, Taylor A, Taylor GS, Taylor GA, Taylor JP, Taylor MJ, Tchetina EV, Tee AR, Teixeira-Clerc F, Telang S, Tencomnao T, Teng BB, Teng RJ, Terro F, Tettamanti G, Theiss AL, Theron AE, Thomas KJ, Thomé MP, Thomes PG, Thorburn A, Thorner J, Thum T, Thumm M, Thurston TL, Tian L, Till A, Ting JP, Titorenko VI, Toker L, Toldo S, Tooze SA, Topisirovic I, Torgersen ML, Torosantucci L, Torriglia A, Torrisi MR, Tournier C, Towns R, Trajkovic V, Travassos LH, Triola G, Tripathi DN, Trisciuoglio D, Troncoso R, Trougakos IP, Truttmann AC, Tsai KJ, Tschan MP, Tseng YH, Tsukuba T, Tsung A, Tsvetkov AS, Tu S, Tuan HY, Tucci M, Tumbarello DA, Turk B, Turk V, Turner RF, Tveita AA, Tyagi SC, Ubukata M, Uchiyama Y, Udelnow A, Ueno T, Umekawa M, Umemiya-Shirafuji R, Underwood BR, Ungermann C, Ureshino RP, Ushioda R, Uversky VN, Uzcátegui NL, Vaccari T, Vaccaro MI, Váchová L, Vakifahmetoglu-Norberg H, Valdor R, Valente EM, Vallette F, Valverde AM, Van den Berghe G, Van Den Bosch L, van den Brink GR, van der Goot FG, van der Klei IJ, van der Laan LJ, van Doorn WG, van Egmond M, van Golen KL, Van Kaer L, van Lookeren Campagne M, Vandenabeele P, Vandenberghe W, Vanhorebeek I, Varela-Nieto I, Vasconcelos MH, Vasko R, Vavvas DG, Vega-Naredo I, Velasco G, Velentzas AD, Velentzas PD, Vellai T, Vellenga E, Vendelbo MH, Venkatachalam K, Ventura N, Ventura S, Veras PS, Verdier M, Vertessy BG, Viale A, Vidal M, Vieira HL, Vierstra RD, Vigneswaran N, Vij N, Vila M, Villar M, Villar VH, Villarroya J, Vindis C, Viola G, Viscomi MT, Vitale G, Vogl DT, Voitsekhovskaja OV, von Haefen C, von Schwarzenberg K, Voth DE, Vouret-Craviari V, Vuori K, Vyas JM, Waeber C, Walker CL, Walker MJ, Walter J, Wan L, Wan X, Wang B, Wang C, Wang CY, Wang C, Wang C, Wang C, Wang D, Wang F, Wang F, Wang G, Wang HJ, Wang H, Wang HG, Wang H, Wang HD, Wang J, Wang J, Wang M, Wang MQ, Wang PY, Wang P, Wang RC, Wang S, Wang TF, Wang X, Wang XJ, Wang XW, Wang X, Wang X, Wang Y, Wang Y, Wang Y, Wang YJ, Wang Y, Wang Y, Wang YT, Wang Y, Wang ZN, Wappner P, Ward C, Ward DM, Warnes G, Watada H, Watanabe Y, Watase K, Weaver TE, Weekes CD, Wei J, Weide T, Weihl CC, Weindl G, Weis SN, Wen L, Wen X, Wen Y, Westermann B, Weyand CM, White AR, White E, Whitton JL, Whitworth AJ, Wiels J, Wild F, Wildenberg ME, Wileman T, Wilkinson DS, Wilkinson S, Willbold D, Williams C, Williams K, Williamson PR, Winklhofer KF, Witkin SS, Wohlgemuth SE, Wollert T, Wolvetang EJ, Wong E, Wong GW, Wong RW, Wong VK, Woodcock EA, Wright KL, Wu C, Wu D, Wu GS, Wu J, Wu J, Wu M, Wu M, Wu S, Wu WK, Wu Y, Wu Z, Xavier CP, Xavier RJ, Xia GX, Xia T, Xia W, Xia Y, Xiao H, Xiao J, Xiao S, Xiao W, Xie CM, Xie Z, Xie Z, Xilouri M, Xiong Y, Xu C, Xu C, Xu F, Xu H, Xu H, Xu J, Xu J, Xu J, Xu L, Xu X, Xu Y, Xu Y, Xu ZX, Xu Z, Xue Y, Yamada T, Yamamoto A, Yamanaka K, Yamashina S, Yamashiro S, Yan B, Yan B, Yan X, Yan Z, Yanagi Y, Yang DS, Yang JM, Yang L, Yang M, Yang PM, Yang P, Yang Q, Yang W, Yang WY, Yang X, Yang Y, Yang Y, Yang Z, Yang Z, Yao MC, Yao PJ, Yao X, Yao Z, Yao Z, Yasui LS, Ye M, Yedvobnick B, Yeganeh B, Yeh ES, Yeyati PL, Yi F, Yi L, Yin XM, Yip CK, Yoo YM, Yoo YH, Yoon SY, Yoshida K, Yoshimori T, Young KH, Yu H, Yu JJ, Yu JT, Yu J, Yu L, Yu WH, Yu XF, Yu Z, Yuan J, Yuan ZM, Yue BY, Yue J, Yue Z, Zacks DN, Zacksenhaus E, Zaffaroni N, Zaglia T, Zakeri Z, Zecchini V, Zeng J, Zeng M, Zeng Q, Zervos AS, Zhang DD, Zhang F, Zhang G, Zhang GC, Zhang H, Zhang H, Zhang H, Zhang H, Zhang J, Zhang J, Zhang J, Zhang J, Zhang JP, Zhang L, Zhang L, Zhang L, Zhang L, Zhang MY, Zhang X, Zhang XD, Zhang Y, Zhang Y, Zhang Y, Zhang Y, Zhang Y, Zhao M, Zhao WL, Zhao X, Zhao YG, Zhao Y, Zhao Y, Zhao YX, Zhao Z, Zhao ZJ, Zheng D, Zheng XL, Zheng X, Zhivotovsky B, Zhong Q, Zhou GZ, Zhou G, Zhou H, Zhou SF, Zhou XJ, Zhu H, Zhu H, Zhu WG, Zhu W, Zhu XF, Zhu Y, Zhuang SM, Zhuang X, Ziparo E, Zois CE, Zoladek T, Zong WX, Zorzano A, Zughaier SM. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SE, Anderson P, Goldspink DF. The effects of calcium on protein turnover in skeletal muscles of the rat. Biochem J. 1982;204:257–264. doi: 10.1042/bj2040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew TS, Ferris RK, Downs RM, Kinsey ST, Baumgarner BL. Caffeine promotes autophagy in skeletal muscle cells by increasing the calcium-dependent activation of AMP-activated protein kinase. Biochem Biophys Res Commun. 2014;453:411–418. doi: 10.1016/j.bbrc.2014.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TM, Mortensen XM, Ashby CK, Harris AM, Kump KJ, Laird DW, Adams AJ, Bray JK, Chen T, Thomson DM. The effect of caffeine on skeletal muscle anabolic signaling and hypertrophy. Appl Physiol Nutr Metab. 2017 doi: 10.1139/apnm-2016-0547. [DOI] [PubMed] [Google Scholar]
- Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Pende M, Daegelen D, Sakamoto K, Foretz M, Viollet B. Important role for AMPKα1 in limiting skeletal muscle cell hypertrophy. FASEB J. 2009a;7:2264–2273. doi: 10.1096/fj.08-119057. [DOI] [PubMed] [Google Scholar]
- Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Pende M, Daegelen D, Sakamoto K, Foretz M, Viollet B. Important role for AMPKα1 in limiting skeletal muscle cell hypertrophy of insulin receptor substrate-1 and impairs insulin signal transduction in rat skeletal muscle. J Appl Physiol. 2009b;111:1629–1636. [Google Scholar]
- Reed MG, Howard CV. Surface-weighted star volume: concept and estimation. J Microsc. 1998;190:350–356. doi: 10.1046/j.1365-2818.1998.00331.x. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K-I, Imoto M, Hattori N. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 2011;7:176–187. doi: 10.4161/auto.7.2.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha RA, Farah BL, Singh BK, Siddique MM, Li Y, Wu Y, Yen PM. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology. 2014;4:1366–1380. doi: 10.1002/hep.26667. [DOI] [PubMed] [Google Scholar]
- Sun P, Enslen H, Myung P, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH, Jr, Kull FC, Jr, Gonzalez-Cadavid N. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab. 2001;280:E221–E228. doi: 10.1152/ajpendo.2001.280.2.E221. [DOI] [PubMed] [Google Scholar]
- Tong JF, Yan X, Zhu MJ, Du M. AMP-activated protein kinase enhances the expression of muscle-specific ubiquitin ligases despite its activation of IGF-1/Akt signaling in C2C12 myotubes. J Cell Biochem. 2009;108:458–468. doi: 10.1002/jcb.22272. [DOI] [PubMed] [Google Scholar]
- Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O’Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965–971. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- Winek CL, Wahba WW, Winek CL, Balzer TW. Drug and chemical blood-level data 2001. Forensic Sci Int. 2001;122:107–123. doi: 10.1016/s0379-0738(01)00483-2. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Sabatini DM, Efeyan A. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga R, Fuentes EN, Molina A, Valdés JA. The cAMP response element binding protein (CREB) is activated by insulin-like growth factor-1 (IGF-1) and regulates myostatin gene expression in skeletal myoblast. Biochem Biophys Res Commun. 2013;440:258–264. doi: 10.1016/j.bbrc.2013.09.067. [DOI] [PubMed] [Google Scholar]