Abstract
Spontaneous pain and function-associated pain are prevalent symptoms of multiple acute and chronic muscle pathologies. We established mouse models for evaluating spontaneous pain and bite-evoked pain from masseter muscle, and determined the roles of TRPV1 and the contribution of TRPV1- or NK1-dependent nociceptive pathways. Masseter muscle inflammation increased mouse grimace scale (MGS) scores and face wiping behavior which were attenuated by pharmacological or genetic inhibition of TRPV1. Masseter inflammation led to a significant reduction in bite force. Inhibition of TRPV1 only marginally relieved the inflammation-induced reduction of bite force. These results suggest differential extent of contribution of TRPV1 to the two types of muscle pain. However, chemical ablation of TRPV1-expressing nociceptors or chemogenetic silencing of TRPV1-lineage nerve terminals in masseter muscle attenuated inflammation-induced changes in both MGS scores and bite force. Furthermore, ablation of neurons expressing neurokinin 1 (NK1) receptor in trigeminal subnucleus caudalis also prevented both types of muscle pain. Our results suggest that TRPV1 differentially contribute to spontaneous pain and bite-evoked muscle pain, but TRPV1-expressing afferents and NK1-expressing second order neurons commonly mediate both types of muscle pain. Therefore, manipulation of the nociceptive circuit may provide a novel approach for management of acute or chronic craniofacial muscle pain.
Keywords: TRPV1, muscle pain, nociceptors, inflammation, dorsal horn, vanilloids, orofacial pain
INTRODUCTION
Musculoskeletal pain is a major medical problem19. Muscle pain to pressure (tenderness) and spontaneous pain are major symptoms of acute or chronic myopathies such as infection- or exercise-mediated myalgia, myofascial pain syndrome, temporomandibular joint disorders (TMJD), or low back pain. These conditions also interfere with normal muscle functions, e.g., limited range of motion in low back pain or decreased bite force in TMJD. Although mechanisms underlying muscle pain have been studied in multiple preclinical models21,37, molecular mechanisms and neural circuits underlying spontaneous muscle pain or muscle function-related pain, such as reduced bite force34,36, are not well understood. Scarcity of readily available rodent models for assessing spontaneous pain and muscle function-related pain is an impediment to progress.
TRPV1 is a receptor of capsaicin and contributes to thermal hyperalgesia in skin13. However, the data regarding the roles of TRPV1 in spontaneous pain produced by inflammation or injury of skin or joint are equivocal and seem to show different extent of contribution in different models20,25,43,44,57,60. TRPV1 is expressed in muscle nociceptors and contribute to mechanical hyperalgesia in response to external pressure following muscle inflammation, acid injection, occlusal interference, and eccentric or lengthening contraction9,11,18,45,59. It is not known, however, whether TRPV1 mediates spontaneous pain and muscle function-related pain during muscle inflammation.
Nociceptive primary afferents are mostly composed of peptidergic nociceptors, in which TRPV1 is enriched, and of non-peptidergic nociceptors, to which isolectin B4 (IB4) binds. In skin, TRPV1-expressing nociceptors are exclusively involved in thermal but not mechanical pain8,39. A high proportion of muscle nociceptors express TRPV1, whereas a relatively few muscle nociceptors are IB4-positive3,49. Despite the paucity, IB4-positive muscle afferents are known to contribute to muscle hyperalgesia and hyperalgesic priming in rats1,2. Since TRPV1 is involved in mechanical hyperalgesia following muscle inflammation11,45, it is reasonable to presume that TRPV1-expressing nociceptors are primary contributors to spontaneous pain and function-evoked pain following muscle inflammation. In contrast, central neural circuitry of muscle pain is less well understood. In spinal cord superficial laminae, the majority of projection neurons express neurokinin 1 (NK1) receptor54. Loss of NK1-expressing neurons in spinal cord reduces not only capsaicin-induced nocifensive behavior but also thermal hyperalgesia and mechanical allodynia under inflammation and neuropathy42. Chemical algogens or mechanical stimulation of muscle nociceptors also activates projection neurons of spinal cord15,17. However, it is not clear whether NK1-expressing neurons contribute to spontaneous pain and muscle function-related pain following muscle inflammation.
In this study, using newly established models to assess spontaneous pain and bite-evoked pain in mice with inflamed masseter muscle, we determined the roles of TRPV1 and TRPV1-expressing nociceptors as well as NK1-expressing second order neurons. This study provides one of the potential molecular mechanisms and neural circuits of muscle pain, which may lead to improved strategies to manage acute or chronic muscle pain conditions.
METHODS
Experimental animals
All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (Publication 85-23, Revised 1996) and under the University of Maryland approved Institutional Animal Care and Use Committee protocol. All animals were housed in a temperature-controlled room under a 12:12 light–dark cycle with access to food and water ad libitum.
Unless otherwise indicated, adult male C57BL/6 mice (https://www.jax.org/strain/000664) and TRPV1 knockout (KO) mice (https://www.jax.org/strain/003770) were used for behavioral assays. For chemogenetic silencing of TRPV1-expressing neurons, we used two mouse lines: First, TRPV1-Cre mice (https://www.jax.org/strain/017769)7 expresses Cre recombinase under the control of TRPV1 and labels afferents expressing TRPV1 in any stage of development (TRPV1-lineage). Consequently, TRPV1 is expressed in approximately half of TRPV1-lineage neurons in adult sensory ganglia7. Ablation of TRPV1-lineage neurons eliminates heat- and cold responses but mechanical sensitivity is not affected, which shows TRPV1-lineage neurons do not include mechano-sensitive neurons40. Second, we used a mouse line conditionally expressing an engineered human muscarinic 4 receptor (hM4Di; Gi-coupled Designer Receptors Exclusively Activated by Designer Drugs (DREADD)) from Rosa26 locus (R26-LSL-hM4Di; https://www.jax.org/strain/026219)61. Upon removal of loxP-stop-loxP cassette (LSL) by Cre recombination, the Gi-coupled hM4Di is expressed and the neuronal function is silenced by the administration of clozapine-N-oxide (CNO). In this line, mCitrine yellow fluorescent protein is also expressed to identify neurons expressing hM4Di.
For experiments in which facial grimace and wiping behaviors were assessed, 8 to 12 week-old mice were used. For all experiments involving bite force measurements, 12 to 16 week-old mice were used. In all behavioral assays, the animals were randomly allocated to experimental groups.
Drugs
Complete Freund adjuvant (CFA; Sigma) was emulsified in an equal volume of isotonic saline. Carrageenan (3%) was dissolved in PBS. AMG9810 (10 nmol/µl; Sigma), a TRPV1 antagonist, was dissolved in PBS containing 5% DMSO and 10% Tween-80. Resiniferatoxin (RTX; 100 ng/µl PBS; Sigma) was dissolved in PBS containing 1% DMSO and 10% Tween-80. Capsaicin was dissolved in 25% polyethylene glycol (0.5 mg/ml; a generous gift from Centrexion Therapeutics). The neurotoxin saporin conjugated to substance P (SP-Sap) or unconjugated saporin (Sap; 0.5 µg/µl; Advanced Targeting Systems, San Diego, CA, USA) were dissolved in isotonic saline. For intramuscular injection, mice were briefly anesthetized using 3% isoflurane. Intramuscular injection was performed using 1.0 ml syringe fitted with a 30 G needle.
Inflammation of masseter muscle
Unless otherwise indicated, inflammation was induced by injection of 20 µl CFA into mouse masseter muscle. For injection, mice were briefly anesthetized using 3% isoflurane. CFA was injected unilaterally or bilaterally as indicated in each experiment. Injection of CFA produced invariable swelling of the mouse masseter muscle. CFA-injected masseter muscle weighed greater by ~40% than contralateral saline-injected masseter muscle after 1 day (140.8±5.6 mg in CFA versus 105.5±7.7mg in saline; n=10, p<0.0001 in Student’s paired t-test). These data suggest that 20 µl of CFA in the masseter muscle produce a comparable level of inflammation, measured by the extent of edema, from mouse to mouse. In one experiment, we injected 3% carrageenan (20 µl in PBS) into masseter muscle.
Measurement of mouse grimace scale (MGS)
Standardized coding of facial expression provides a reliable, quantitative assay of spontaneous pain in mice35. We used a facial grimace scale to assess spontaneous pain from muscle inflammation and showed that inhibition of transient receptor potential ankyrin subtype 1 (TRPA1) attenuated spontaneous pain from masseter inflammation in rats5. Animals were acclimated to the testing environment for 3 days (30 minutes per day) prior to all behavioral assessments. For facial grimace scale, the mice were placed in a cubicle (9×5×5 cm high), with four transparent Plexiglas walls, a ventilated metal shelf bottom and an opaque middle wall that separated two cubicles, which allowed the recording of two mice at a time. Two digital video cameras (Sony HDR-CX230/B High Definition Handycam Camcorder) were placed at a fixed distance from the cubicle, with one on each side of the cubicle to maximize the probability of capturing the faces of the mice. The mice were videotaped for 30 min for each experimental time point. To capture facial images of mice in an unbiased manner, image extraction was performed by blinded experimenters. Images containing a clear view of the entire face were manually captured every 3 min during the video recording (10 images per 30 min session). In an experiment evaluating MGS scores following masseter injection of capsaicin, the mice were videotaped for 10 minutes in baseline and following capsaicin injection. Images were captured every two minutes (5 images per 10 minute session). For consistency in assessing MGS, one experimenter scored all the images in a blinded manner. Five facial action units (AUs) were scored: orbital tightening, nose bulge, cheek bulge, ear position, and whisker change. Each AU was scored 0, 1, or 2 based on criteria described previously35. A score of “0” represented absence of the AU, a score of “2” indicated obvious detection of the AU. A score of “1” was assigned when the scorer was not highly confident that the AU was present or absent, or when the AU exhibited a moderate appearance. An initial MGS score of each photograph was calculated by averaging the scores of the five AUs, and a mean MGS score was obtained from the 10 images, which was presumed to reflect the level of spontaneous pain. The mean MGS score of each mouse before CFA treatment was used as the baseline value. Time-dependent changes in MGS scores before and after CFA or vehicle were analyzed with two-way ANOVA with repeated measures. The effects of pharmacological or genetic manipulations were also analyzed with two-Way ANOVA with repeated measures. All multiple group comparisons were followed by Bonferroni post hoc test.
Measurement of face wiping behaviors
Facial injection of capsaicin induces wiping of face with forelimb, which has been used as an indicator of pain in mice53. To assess spontaneous pain, we measured face wiping behaviors following masseter injection of CFA. After facial grimace scale video recording, the same group of mice were used for measuring wiping behaviors as well. Each mouse was placed in plastic containers (9×9×13 cm high) with two mirrored back walls, affording the camera a four-sided view. A digital video camera (Sony HDR-CX230/B High Definition Handycam Camcorder) was placed at a fixed distance from the cubicle. Free behaviors of mice were recorded for 30 min. Wiping behaviors were analyzed as described previously53. We defined the wiping behavior as a motion of the ipsilateral forelimb that begins at the back of the masseter muscle in front of the ear and moves forward in one direction from caudal to rostral. We excluded similar behaviors such as face wiping using both forelimbs simultaneously or repeated wiping using both forelimbs alternatively, or hindlimb scratching directed to sites other than the cheek such as the nose, eye, or ear.
Bite force assay in mice
Reduced muscle force following muscle inflammation has been regarded as a model of muscle hyperalgesia27,48. Carregeenan-mediated reduction in grip strength was readily reversed by three different analgesics (opioid, NSAIDs, steroid)27, which argues against that the reduction of grip strength is entirely derived from impaired muscle contractility. We previously demonstrated that masseter injection of CFA resulted in reduced bite force in rats, and the reduction could be prevented by anti-inflammatory agents, suggesting that bite force measurement in rodents could be a useful method for studying muscle function-related pain from masseter muscle48. Recently, bite force measurement was also used to evaluate pain behaviors following inflammation in temporomandibular joint10. To assess bite-evoked pain associated with craniofacial muscle inflammation in mice, we measured changes in bite force. Mice were acclimated to the testing environment and handling for two days prior to behavioral testing. Bite force was measured using a bite force transducer consisting of two parallel aluminum plates connected to a force-displacement transducer (FT03; Grass instruments). Spike 2 software was used to measure the voltage changes from the transducer displacement. SigmaPlot 8.0 was used to convert the voltage change into force based on calibration using standard weights. Mice were placed in a modified 60-ml plastic syringe with a wide opening in one side to accommodate the head of the mouse. In order to prevent the mouse from escaping, the syringe plunger was inserted into the syringe to loosely restrain the mouse inside the syringe. The syringe containing the mouse was held manually and moved slowly at 0.5–1 cm/sec toward bite plates so that the mouse could bite the plates. Unlike rats that require a reward paradigm for producing continuous bites48, the induction of bite was instantaneous in mice once biting plates touched their lips. To minimize stress, the mouse was released immediately from syringe in case the mouse vigorously moved or tried to hide inside the syringe. Bite force was recorded for 120 sec per session and top five force measurements were averaged. We performed preliminary bite force measurement on day before the actual experiment. Mice showed biting less than 450 g in the preliminary measurement, they were not used for the actual assay afterward since mice showing weak bite force tended to show unreliable change in biting behaviors following inflammation. When cutoff was set at 450 g, masseter inflammation consistently produced averaged reduction of bite by approximately 30%. The baseline bite force measurements from all data from C57 mice included in the manuscript ranged from 484 to 870 (694±102 g, mean±SD, n=77), which indicates that the cutoff was more stringent than two SD below the average. The experimenter was blinded to the experimental groups.
Hargreaves assay for measuring thermal sensitivity in hind paw skin
The mice were acclimated to the testing environment for two days. The mice were placed on the glass platform of a Hargreaves device (PAW Thermal Stimulator, UC San Diego) under an acrylic box for at least 30 min for habituation. For testing, the mice were placed on the glass platform under an acrylic box for 10 minutes until they were settled down. Baseline latency of the radiant heat source was adjusted to a range of 10–12 s with a cutoff time of 20.5 s to prevent tissue damage. To record paw withdrawal latency in response to the heat stimulus, both hind paws were measured 3 times with a 10-min interval, and the average latency of three withdrawals was used for analysis. The experimenter performing the procedure was blinded to the experimental group status of the mouse.
Von Frey measurement in hind paw skin
Mice were placed under an acrylic box on elevated wire mesh platforms. All mice were habituated to the box in a behavioral room for at least 30 min per day for 3 days prior to the testing procedures. A series of von Frey filaments were applied perpendicularly to the plantar surface of hind paw. The von Frey filaments had bending forces ranging from 0.008 g to 4 g. Lifting of the hind paw or flinching immediately upon removal of the probing filament was defined as a response. Each von Frey filament was applied 5 times at intervals of a few seconds. The response frequencies [(number of responses/number of stimuli) ×100%] to a range of von Frey filament forces were determined and a stimulus-response frequency curve was plotted. The plot was fitted with a logistic function, from which EF50, the mechanical force that produced a 50% response frequency, was obtained.
Microinjection into trigeminal subnucleus caudalis (Vc)
Microinjection into Vc was performed as described previously28. Animals were anesthetized by intraperitoneal injection of Ketamine and Xylazine cocktail (100–150 mg/kg ketamine/10–16 mg/kg xylazine) and placed in a Kopf stereotaxic apparatus. After a midline incision (3–5 mm), an opening was made in the skull. A 0.5 µl Hamilton microsyringe was used for microinjection. The syringe needle was placed in the left or right Vc regions according to the stereotaxic coordinates of the mouse brain (7.80 mm anterior to bregma, 4.3 mm ventral to it, and ±1.60 mm lateral to the midline)46. To ablate central terminals of TRPV1-expressing afferents, RTX (50 ng in 0.5 µl PBS per side) was administered for 1 min (0.5 µl/min) in both the left and right side of Vc. PBS (0.5 µl per side) was used to treat control mice. To ablate NK1-expressing neurons, SP-Sap (0.25 µg in 0.5 µl PBS per side) or Sap (0.25 µg in 0.5 µl PBS per side) was administered in 1 min (0.5 µl/min) in both the left and right side of Vc. Following injection, the injection needle was held in the tissue for 2 min to allow diffusion before removal. After the surgery, the animal was returned to its home cage. MGS and bite force assay were performed 2–3 weeks after RTX or PBS injection or 4–5 weeks after SP-sap or Sap injection.
Immunohistochemistry
Immunohistochemical analysis was performed as described previously12. Mice were transcardially perfused with 3.7% paraformaldehyde. TG were dissected, cryoprotected, and sectioned at 12 µm intervals. The brainstem around the obex region was sectioned at 30 µm intervals. Conventional immunohistochemical procedures were performed with rabbit anti-NK1 (1:500; Novus Biologicals) and rabbit anti-TRPV1 (1:1000; a generous gift from Dr. Michael Caterina), and chicken anti-GFP (1:1000; Aves Labs, Inc; for detecting mCitrine) followed by appropriate secondary antibodies. Images were acquired by optical sectioning fluorescence microscopy (Zeiss Axiovert, Carl Zeiss MicroImaging).
Statistical analysis
Data are presented as mean±s.e.m. Comparisons between more than two groups with different time points were performed using two-way ANOVA with Bonferroni post tests. The criterion for statistical significance was P<0.05. All statistical analyses were performed using GraphPad Prism 6.0.
RESULTS
TRPV1 contributes to increased MGS and face wiping during muscle inflammation
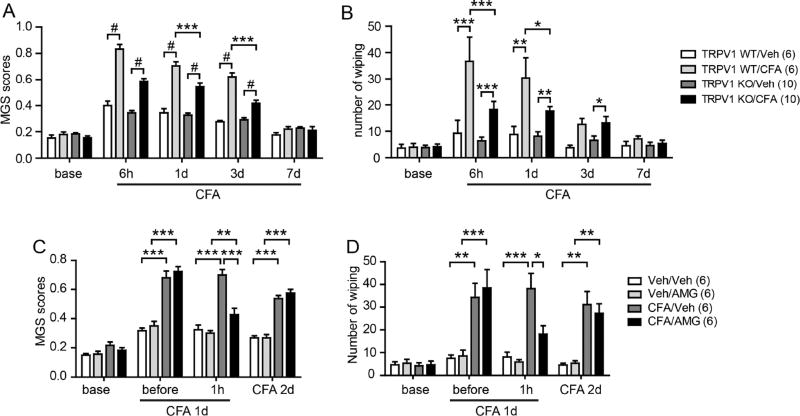
To assess the extent of spontaneous nocifensive behaviors during CFA-mediated muscle inflammation, we measured two outcomes: mouse grimace scale (MGS) scores and face wiping behaviors. When we compared these behaviors in vehicle- or CFA-injected mice, MGS scores were significantly greater in CFA-injected groups after 6 hours, 1 day, and 3 days than those in saline-injected groups. Face wiping behaviors were significantly greater in CFA-injected groups after 6 hours and 1 day than those in saline-injected groups. MGS and face wiping returned to control levels after 7 days (Fig. 1A, B). In contrast, MGS and face wiping were significantly reduced, but not completely ablated, in TRPV1 KO mice. To further determine the role of TRPV1 in spontaneous nocifensive behaviors during muscle inflammation, we tested the effects of AMG9810, a TRPV1 antagonist (Fig. 1C, D). Injection of AMG9810 into the masseter muscle 1 day after CFA injection significantly attenuated MGS and face wiping. These effects of the inhibitor were reversible, as inhibition was not observed 2 days after CFA injection. These results suggest that TRPV1 contributes to increased MGS and face wiping associated with muscle inflammation.
Figure 1. Pharmacological inhibition and knock-out of TRPV1 attenuates spontaneous pain during masseter inflammation in mice.
A–B. Averaged mouse grimace scale (MGS) scores (A) or number of face wiping behaviors (B) by mice following unilateral masseter injection of either CFA (20 µl) or vehicle (saline) in WT or TRPV1 KO mice.
C–D. Effects of AMG9810 (AMG, TRPV1 antagonist; 200 nmol in 20 µl) on MGS scores (C) and number of face wiping behaviors (D). Drugs or vehicle were injected on CFA day 1, and drug effects were evaluated 1 hour later (CFA day 1) and 1 day later (CFA day 2). *p<0.05, **p<0.01;***p<0.001, #p<0.0001; Bonferroni post-hoc test following two-way ANOVA with repeated measures. Numbers in parenthesis represent numbers of mice.
TRPV1 only marginally affects bite force reduction during muscle inflammation
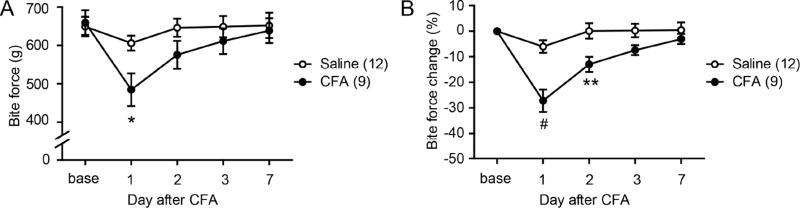
To assess muscle function-related nocifensive behaviors, we evaluated changes in bite force following inflammation of the masseter muscle (Fig. 2A). Bilateral injections of saline to the masseter muscle produced a slight decrease in bite force on 1 day, but the bite force recovered to baseline thereafter. In contrast, CFA injections to bilateral masseter muscles resulted in significantly reduced bite force (by approximately 30%), which gradually recovered toward baseline over 3 days (Fig. 2A). There was a large variation in individual bite force between animals. However, the baseline bite force measures between groups were not statistically different. Therefore, we normalized post-treatment bite force measures to the baseline value in each mouse. The plots of normalized data showed the same trend as those of the raw bite force data but with a lesser variation (Fig. 2B).
Figure 2. Measurement of bite-foce during masseter inflammation in mice.
Absolute bite force (A) or bite force change (B) measured before and after bilateral injection of vehicle (saline) or CFA (20 µl) into the masseter muscles; *, p<0.05; **p<0.01; #p<0.0001; Bonferroni post-hoc test following two-way ANOVA with repeated measurement. Numbers in parenthesis represent the numbers of mice.
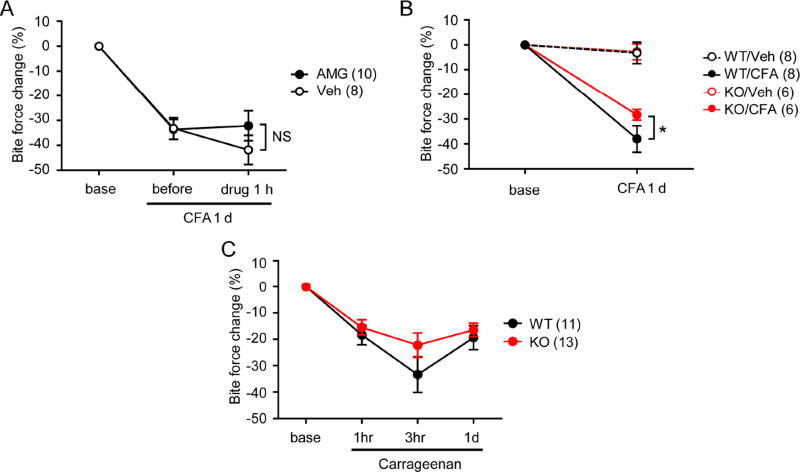
Since bite force reduction was maximal on CFA day 1, we tested the effects of the TRPV1 antagonist on that day (Fig. 3). Bilateral injection of vehicle to the masseter muscles on CFA day 1 slightly decreased bite force. Bilateral injection of AMG9810 (200 nmol/side, the dose that significantly inhibited MGS scores in Fig. 1) produced a slight, but non-significant, increase in bite force (Fig. 3A). TRPV1 knockout mice exhibited a small, but significantly less, reduction in bite force following CFA injection than WT mice (Fig. 3B). We further tested the contribution of TRPV1 to bite force changes induced by masseter injection of carrageenan, which also causes muscle inflammation47. Knockout of TRPV1 showed only modestly decreased bite force reduction 3 hours following carrageenan injection, which did not reach statistical significance (Fig. 3C). These results suggest that TRPV1 marginally contributes to reduction in bite force during muscle inflammation.
Figure 3. Limited contribution of TRPV1 to bite-evoked pain during masseter inflammation.
A. Effects of bilateral injection of AMG9810 (200 nmol/side) or vehicle into the masseter muscles 1 day following bilateral CFA injection. Bite force was measured before and 1hr after drug injection. NS, not significant.
B. Bite force of TRPV1 KO or WT mice measured before and 1 day after bilateral injections of saline or CFA (20 µl) into the masseter muscles. *, p<0.05; Bonferroni post-hoc test following two-way ANOVA with repeated measurement.
C. Bite force of TRPV1 KO or WT mice measured before and after bilateral injection of carrageenan (3%; 20 µl) into the masseter muscles.
TRPV1-expressing afferents mediate changes in MGS and bite force during masseter inflammation
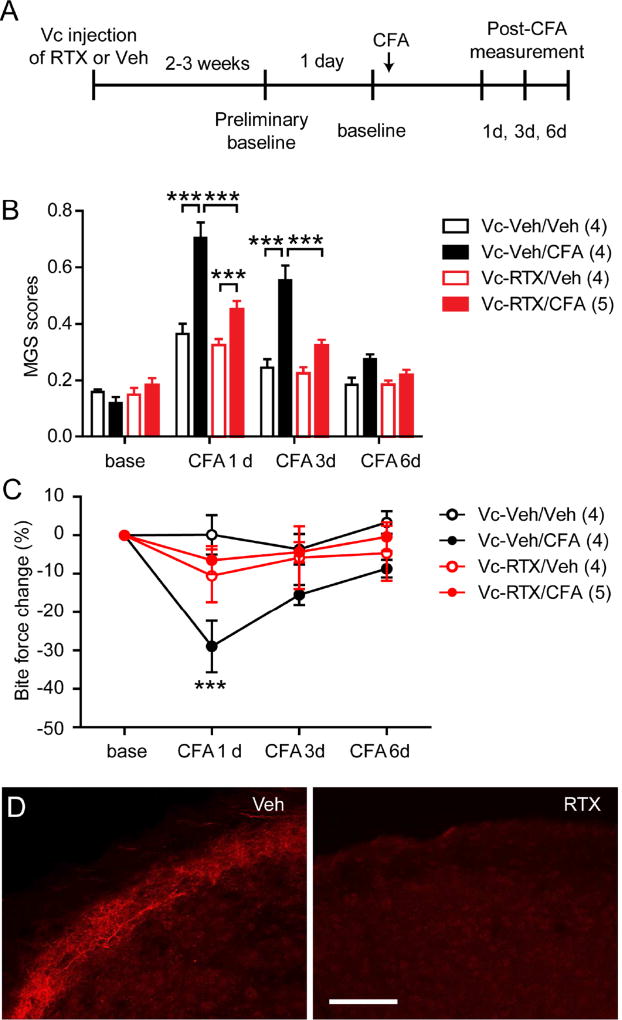
Next, we tested whether changes in MGS and bite force during muscle inflammation were dependent on TRPV1-expressing afferents (Fig. 4A). To prevent signal transmission through TRPV1-expressing afferents, we bilaterally injected RTX (50 ng/side) directly into Vc 2 weeks prior to behavioral measurements (Vc-RTX). Control animals received bilateral injections of vehicle (Vc-Veh). Intrathecal administration 200 ng RTX has been shown to ablate not only central terminals but also the majority of TRPV1-expressing primary afferents in TG39. Two weeks following Vc injection, we assessed MGS scores and bite force in CFA-inflamed and saline-treated control animals. We observed no differences in baseline MGS scores among the different treatment groups. In Vc-Veh animals with CFA-induced inflammation (Vc-Veh/CFA), MGS scores were significantly greater than those of Vc-Veh/Veh. In Vc-RTX/CFA animals, MGS scores were greater than those of Vc-RTX/Veh but significantly less than those in Vc-Veh/CFA animals (Fig. 4B). Bite force was also tested in the same animals. CFA injection but not vehicle (saline) control significantly decreased bite force of Vc-Veh mice. In contrast, neither CFA injection nor saline control decreased bite force of Vc-RTX mice. The reduction in bite force following CFA injection was significantly greater in Vc-Veh/CFA mice than in Vc-RTX/CFA mice (Fig. 4C). When TRPV1 expression was examined by immunohistochemical staining, the central terminals of TRPV1-expressing afferents in the Vc were totally ablated in Vc-RTX mice but not in Vc-Veh mice (Fig. 4D). These results suggest that changes in MGS and bite force during muscle inflammation are both primarily mediated by TRPV1-expressing nociceptors.
Figure 4. Ablation of central terminals of TRPV1-expressing afferents attenuates spontaneous and bite-evoked pain during masseter inflammation.
A. Time line of experiment. RTX (50 ng in 0.5 µl PBS per side) or vehicle (0.5 µl per side) was microinjected bilaterally into trigeminal nucleus caudalis (Vc) two weeks prior to the start of behavioral assessments. CFA (20 µl per side) was bilaterally injected into the masseter muscles. MGS and bite force were evaluated from same groups of animals 1, 3, and 6 days after CFA injections.
B–C. Averaged MGS scores (B) and normalized bite force (C) before and after masseter injection of Veh or CFA in Vc-Veh- or Vc-RTX-injected mice. ***p<0.001 (Vc-Veh/CFA vs Vc-RTX/CFA); post-hoc analysis following two-way ANOVA. Numbers in parenthesis represent the number of mice.
D. Representative immunohistochemical staining of TRPV1 in Vc from Vc-RTX mice or Vc-Veh mice. Scale bar, 100 µm.
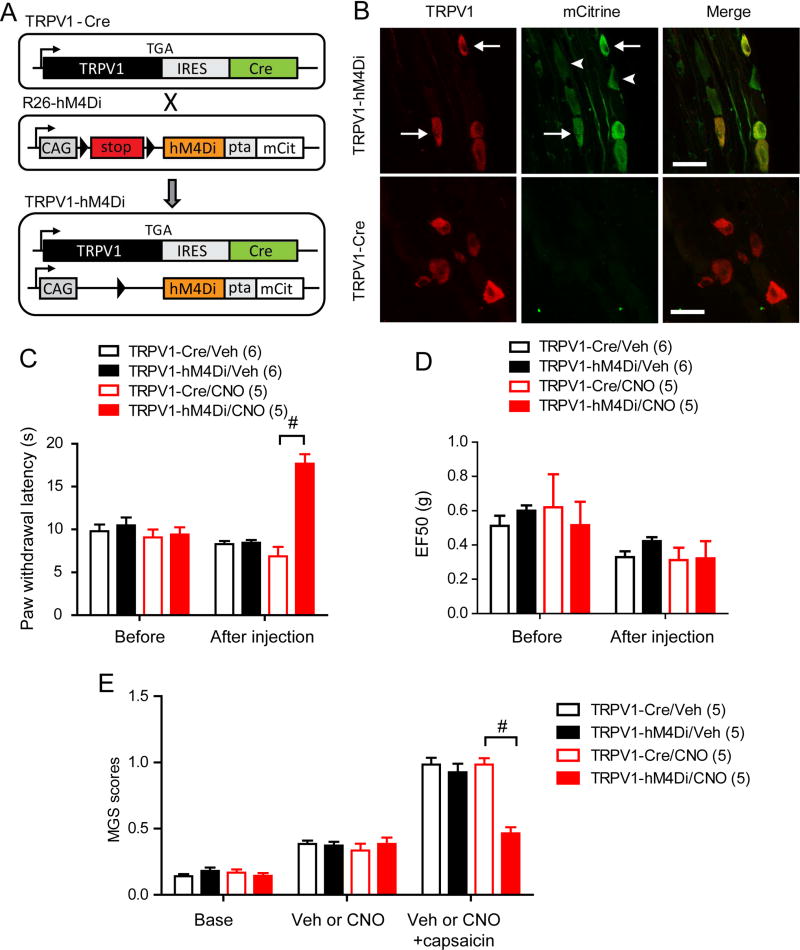
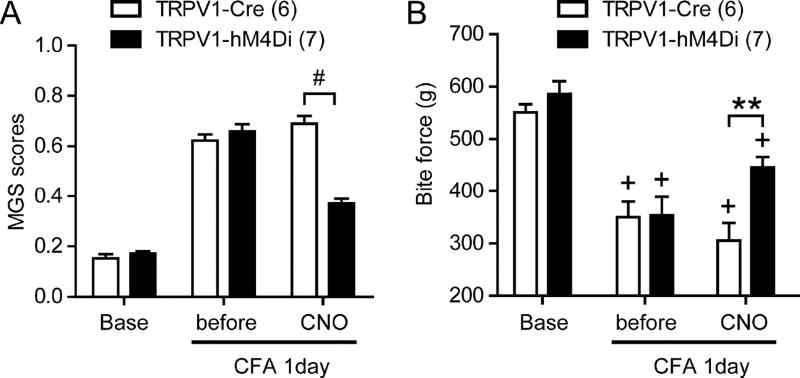
Although the results above indicates the importance of TRPV1-expressing afferents in transmission of muscle pain, we cannot exclude the possibility that structural or functional plasticity of pain pathways occur following initial excitation and ablation by RTX, which potentially compound the interpretation. To exclude such possibility, we further tested the contribution of TRPV1-expressing afferents by selectively silencing the activity of TRPV1-expressing afferents. Because these experiments require bilateral manipulation over large areas of muscle tissue, we chose a chemogenetic approach rather than an optogenetic approach. Designer Receptors Exclusively Activated by Designer Drugs (DREADD), engineered G-protein coupled receptors, are exclusively activated by biologically inert drugs such as clozapine-N-oxide (CNO)4. We took advantage of an inhibitory DREADD in which a modified human muscarinic M4 receptor is coupled to Gi (hM4Di). We employed a newly developed R26R-LSL-hM4Di/mCitrine mouse line that expresses floxed hM4Di and mCitrine from the R26 locus61 (Fig. 5A). We crossed this line with TRPV1-Cre mice, which express Cre recombinase only in TRPV1-lineage neurons. The offspring that were heterozygous for both R26R-hM4Di-mCitrine and TRPV1-Cre (TRPV1-hM4Di) expressed mCitrine in a subpopulation of TG neurons (Fig. 5B). All TRPV1-expressing neurons were mCitrine -positive, and approximately 45% of mCitrine-positive neurons expressed TRPV1, which is consistent with previous findings from TRPV1-reporter mice6. To assess the effects of hM4Di activation by CNO on TRPV1-expressing afferents, we assessed thermal sensitivity of hindpaw in TRPV1-hM4Di mice. Intraplantar injection of CNO, but not vehicle, significantly increased withdrawal latency to radiant heating in TRPV1-hM4Di mice compared to TRPV1-Cre control mice (Fig. 5C). In contrast, mechanical sensitivity of hindpaw in TRPV1-hM4Di mice was not significantly different from that of TRPV1-Cre mice following the injection of CNO or vehicle (Fig. 5D). Since TRPV1 lineage neurons contribute to thermal but not mechanical pain in skin8,39, these data validate specific inhibition of TRPV1-lineage neurons by CNO in TRPV1-hM4Di. Furthermore, masseter injection of CNO in TRPV1-hM4Di mice significantly attenuated MGS scores upon masseter injection of capsaicin compared to TRPV1-Cre (Fig. 5E). These results suggest that local injection of CNO effectively silenced TRPV1-lineage afferent terminals in TRPV1-hM4Di mice both in skin and muscle tissues. Next, we evaluated whether functional silencing of TRPV1-lineage muscle afferent terminals affected MGS and bite force during muscle inflammation (Fig. 6). TRPV1-hM4Di and TRPV1-Cre mice showed comparable MGS scores both at baseline and 1 day following CFA injection. When CNO was bilaterally injected to masseter muscles, MGS was significantly suppressed in TRPV1-hM4Di mice but not in TRPV1-Cre mice (Fig. 6A). We also evaluated bite force in the same animals. TRPV1-hM4Di and TRPV1-Cre mice showed comparable bite force at baseline and 1 day following CFA injection. Bilateral injection of CNO into masseter muscles significantly, but not completely, relived the decreased bite force in TRPV1-hM4Di mice but not in TRPV1-Cre mice (Fig. 6B). These results provide further evidence that muscle inflammation-induced changes in MGS and bite force depends on TRPV1-lineage neurons.
Figure 5. Chemogenetic silencing of TRPV1-lineage neurons attenuates heat- and capsaicin-evoked pain but not mechanical pain in mice.
A. Schematic of recombinant transgenes in mice used in the study. TRPV1-Cre mice were crossed with R26-hM4Di mice to generate TRPV1-hM4Di mice, in which an engineered inhibitory receptor is expressed in TRPV1-lineage neurons. IRES, internal ribosome entry sequence; Cre, Cre recombinase, CAG, CMV immediate enhancer/β-actin promoter; hM4Di, Gi-DREADD; pta, porcine Teschovirus cleavage site; mCit, mCitrine fluorescent protein.
B. Immunohistochemical labeling of TRPV1 (left; red) and mCitrine (middle; green) in trigeminal ganglia of TRPV1-hD4Mi (upper) and TRPV1-Cre mice (lower). Right panels show merged images. Examples of neurons expressing both mCitrine and TRPV1 (arrow) or mCitrine only (arrow head) are marked. Scale bar, 30 µm.
C. Thermal sensitivity of hindpaw evaluated by Hargreaves assay before and 15 min after intraplantar injection of vehicle or CNO (20 µg in 10 µl) in TRPV1-hM4Di or TRPV1-Cre.
D. Mechanical sensitity of hindpaw evaluated by Von Frey assay before and 1 hour after intraplantar injection of vehicle or CNO (20 µg in 10 µl) in TRPV1-hM4Di or TRPV1-Cre.
E. Changes in MGS scores before and after bilateral masseter injection of vehicle or CNO (20 µg in 10 µl per side) and following bilateral masseter injection of capsaicin (0.1 µg in 10 µl per side) in TRPV1-hM4Di or TRPV1-Cre mice. CNO was injected 30 min before capsaicin injection.
Figure 6. Chemogenetic silencing of TRPV1-lineage neurons attenuates spontaneous and bite-evoked pain during masseter inflammation.
Averaged MGS scores (A) and bite force (B) evaluated before or after bilateral masseter injection of CNO (20 µg in 20 µl per side) after 1day following bilateral masseter injection of CFA in TRPV1-hM4Di or TRPV1-Cre mice. MGS was evaluated after 30 min and bite force was evaluated after 1 hour following bilateral masseter CNO injection. **p<0.001, #p<0.0001, +p<0.0001 versus respective base; post-hoc test following two-way ANOVA.
NK1-expressing neurons in Vc mediate changes in MGS and bite force during muscle inflammation
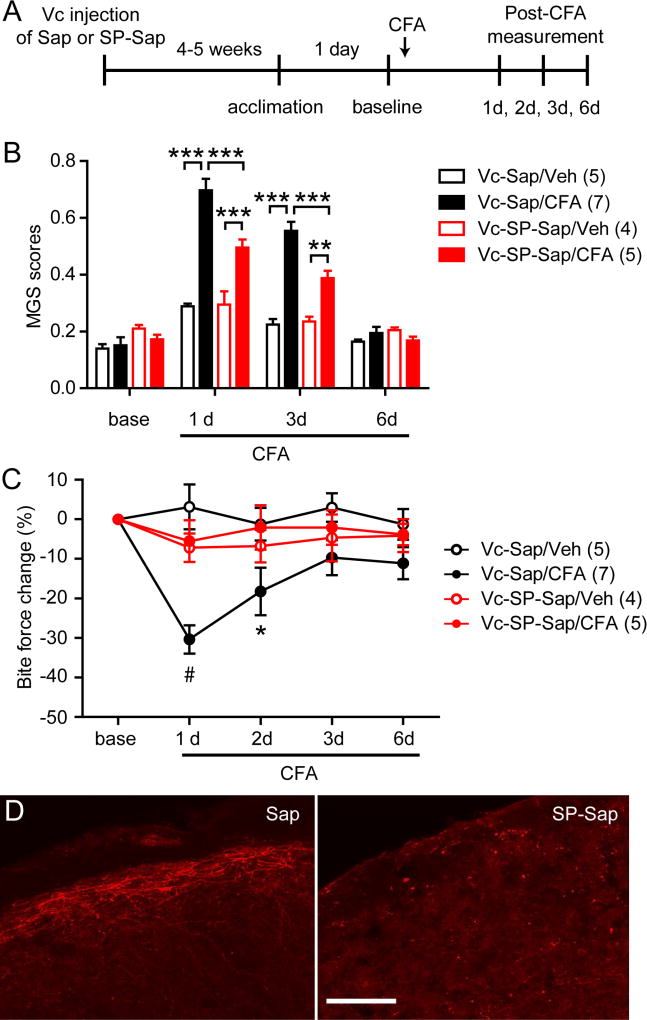
NK1-expressing second order neurons in spinal dorsal horn comprise a major ascending pathway for nociceptive signals54. Since TRPV1-expressing afferents are ultrastructurally and functionally connected with NK1-positive second order neurons14,26, we postulated that NK1-positive second order neurons were responsible for muscle inflammation-induced changes in MGS and bite force. To test this, we took advantage of selective chemical ablation of NK1-positive neurons using the neurotoxin saporin conjugated to substance P42. We microinjected SP-Sap or Sap control directly into Vc (bilaterally) 1 month prior to behavioral assays (Fig. 7A). Mice that received Sap control injection into Vc (Vc-Sap) showed comparable baseline MGS scores to mice that received saporin conjugated to substance P (SP-sap) injection into Vc (Vc-SP-Sap). Bilateral injections of CFA, but not saline, into the masseter muscles of Vc-Sap mice significantly increased MGS scores. MGS scores were also significantly increased by CFA injection in Vc-SP-Sap mice (Vc-SP-Sap/CFA), but the extent of increase was significantly less than that which occurred in Vc-Sap/CFA mice (Fig. 7B). We tested bite force in the same animals. Bilateral injections of CFA into the masseter muscles in Vc-Sap mice resulted in significantly decreased bite force compared to saline injection. In contrast, injections of CFA in Vc-SP-Sap mice did not result in decreased bite force compared to saline injection. The bite force of Vc-SP-Sap mice following CFA day 1 and day 2 were significantly greater than bite force of Vc-Sap mice following CFA injection (Fig. 7C). In immunohistochemical staining, NK1 receptor was substantially reduced in the superficial layer of Vc from mice injected with SP-Sap compared to mice injected with Sap control (Fig. 7D). These results indicate that myositis-induced changes in MGS and bite force largely depends on NK1-positive projection neurons.
Figure 7. Ablation of NK1-expressing second order neurons within Vc suppresses spontaneous and bite-evoked pain during masseter inflammation.
A. Time line of experiments. Saporin conjugated to substance P (SP-Sap; 0.25 µg in 0.5 µl PBS per side) or saporin (Sap; 0.25 µg in 0.5 µl PBS per side) was injected bilaterally into Vc one month prior to the start of assessments. CFA (20 µl) was injected bilaterally into the masseter muscles. MGS and bite force were measured from same animals on the indicated days.
B–C. Averaged MGS scores (B) and normalized bite force (C) before and after masseter injection of vehicle (saline) or CFA in mice injected with Vc-Sap or Vc-SP-Sap. #p<0.0001, ***p<0.001, *p<0.05 (Vc-Sap-CFA vs Vc-SP-Sap/CFA); post-hoc analysis following two-way ANOVA. Numbers in parenthesis represent the number of mice.
D. Representative immunohistochemical labeling of NK1 receptor in Vc from Vc-Sap-injected or Vc-SP-Sap-injected mice. Scale bar, 100 µm.
DISCUSSION
In this study, we adapted two different methods to assess non-evoked nocifensive behaviors associated with masseter inflammation in mice: MGS and face wiping35,53. Using these assays, we found that masseter inflammation commonly resulted in measurable effects, which were collectively interpreted as enhanced spontaneous pain from the masseter muscle. In addition, we assessed function-related pain by assessing bite force during masseter inflammation. Outcomes from MGS and bite force assay may be affected by factors not relevant with pain, e.g. stress or grooming35 and direct effects of inflammation on muscle contractility55. We cannot completely rule out potential contribution of these factors. We concluded, however, that the observed behaviors during masseter inflammation are primarily a consequence of nociception and likely reflect different types of pain based on our results that the manipulation of nociceptive pathway through inhibiting or ablating nociceptor terminals or second order neurons attenuated changes in MGS and bite force. TRPV1-positive afferents may mediate spontaneous pain due to inflammation and nerve injury: Deactivation of TRPV1-expressing fibers by capsaicin or RTX reduces conditioned place preference (CPP) following hindpaw inflammation and spinal nerve ligation injury or guarding behaviors following hindpaw incision22,29,41,43. In contrast, the contribution of TRPV1 molecule itself to spontaneous pain following tissue injury or inflammation is equivocal and seems to depend on pain models. TRPV1 antagonists do not affect guarding behaviors following skin plus deep tissue incision57 or intraplantar injection of CFA43. Inhibition of TRPV1 does not affect CPP in knee joint arthritis model either44. Inhibition of TRPV1, however, attenuates mouth rubbing in oral ulcerative mucositis or guarding in bone cancer20,25,60. Our data show that TRPV1 contribute to facial grimace and face wiping associated with masseter inflammation supporting its role in spontaneous pain. Constitutive activation of TRPV1 in peripheral terminals of muscle nociceptors might contribute to spontaneous pain. Low level, prolonged chemical activation of TRPV1 induces non-desensitizing firing of nociceptors similar to spontaneous firing58. Intraplantar injection of linoleic acid, an endogenous TRPV1 agonist, induces TRPV1-dependent spontaneous nocifensive behaviors during CFA-induced inflammation50. Therefore, it is possible that generation of putative endogenous ligands of TRPV1 during inflammation may cause constitutive activation of these channels leading to spontaneous pain. Constitutive activation of TRPV1-expressing nociceptive terminals not only induces spontaneous pain but can also drive central plasticity to produce referred hyperalgesia56.
Unlike spontaneous pain, the inhibition of TRPV1 only marginally contributed to bite force reduction following masseter inflammation. These results may appear contradictory to our previous findings that showed pressure-evoked pain from masseter inflammation depends on TRPV111. However, it is unclear whether the two types of muscle pain are mediated through similar mechanisms. Our data on time course and pharmacological sensitivity suggested that reduction of bite force and pressure-evoked withdrawal response may be generated through distinct neurobiological processes. Similar pharmacological disparity has been reported between weight bearing and tactile hypersensitivity following peripheral neuropathy, and was attributed to apparently different mechanisms30. Although we used the bite force reduction as a measurable index of muscle hyperalgesia, the mechanisms of associated hyperalgesia during masseter inflammation are unclear. Activation of fine muscle afferents inhibits alpha-motor neurons33, suggesting that masseter inflammation-mediated ongoing nociceptive inputs may produce inhibitory control of masseter activity. Alternatively, muscle contraction upon bite might cause mechanical irritation to muscle nociceptors under muscle inflammation and thus peripheral sensitization of muscle nociceptors leads to reduction of bite force as a nocifensive behavior as suggested in an ischemic contraction model38. Even so, it is possible that biting-evoked pain might involve indirect chemical transduction between non-neuronal cells within muscle and adjacent TRPV1-expressing nociceptive terminals, which is analogous to the stretch-evoked responses of bladder6. It is also possible that aversive ongoing inputs from muscle nociceptors might drive secondary mechanical hyperalgesia in the muscle. Major molecular contributors to bite-evoked muscle pain are not known either. Results from current study suggest that bite-evoked muscle pain may be attributable to unidentified molecules expressed in TRPV1-expressing afferents. The results from our recent transcriptome analysis suggested that a subset of genes enriched in TRPV1-lineage neurons were differentially regulated under masseter inflammation11. We are investigating the roles of these candidate genes to identify the primary molecular contributors to bite-evoked muscle pain.
Despite differential contributions of TRPV1 to spontaneous pain versus bite-evoked muscle pain, our data from chemical ablation or chemogenetic inhibition suggest that TRPV1-expressing primary afferents commonly contribute to the transmission of both types of pain from masseter muscle. Our findings on the roles of TRPV1-positive afferents in spontaneous muscle pain are consistent with reports that TRPV1-positive afferents contribute to CPP following hindpaw inflammation and spinal nerve ligation injury or to guarding behaviors following hindpaw incision22,29,41,43. Our finding of a role for TRPV1-expressing afferents in bite-evoked muscle pain is consistent with the effects of neonatal capsaicin treatment on grip strength change by carrageenan injection to triceps muscle27. Such effects of TRPV1-expressing afferents on muscle hyperalgesia would seem to conflict with findings made in regard to cutaneous pain, showing that TRPV1-expressing afferents are predominantly involved in thermal but not mechanical pain8,39. However, intra-articular injection of RTX attenuates weight-bearing responses in a knee arthritis model31, which is thought to represent mechanical hyperalgesia. These putatively conflicting results suggest that the contribution of TRPV1-expressing afferents to mechanical hyperalgesia may be tissue-dependent. Nonetheless, our data clearly show that TRPV1-expressing afferents contribute to spontaneous pain and bite-evoked pain from masseter muscle during inflammation. In rats, ablation of IB4-positive afferent terminals in spinal cord partially attenuates hyperalgesia from gastrocnemius muscle induced by carrageenan injection, eccentric exercise, or hindlimb vibration1,2. Along with our data, these reports indicate that pathological muscle pain is attributable to the two major nociceptive pathways, TRPV1-positive and IB4-positive afferents.
To further validate the roles of TRPV1-expressing afferents, we took advantage of the recently developed chemogenetic strategy by using a knock-in mouse line in which Cre-dependent inhibitory DREADD is expressed from Rosa26 locus. Recently, another independently generated inhibitory DREADD line was reported to show altered neuronal functions51. In our experiments by using TRPV1-hM4Di line, baseline mechanical or thermal in vivo phenotypes were not different from those in TRPV1-Cre control mice. Furthermore, spontaneous and bite-evoked pain during masseter inflammation was identical between two genotypes suggesting that any potential altered neuronal functions in the TRPV1-hM4Di line are dispensable in our measurements. Therefore, we interpreted the consequences of CNO as an inhibition of spontaneous and bite-evoked muscle pain by suppressing TRPV1-lineage neurons. Of note, chemogenetic silencing in this experiment was not confined to TRPV1-expressing neurons since TRPV1-lineage neurons include TRPV1-negative neurons in adult6. Therefore, this experiment argues for the involvement of TRPV1-expressing afferents but does not necessarily argue against the involvement of TRPV1-negative afferents.
Spontaneous pain and evoked pain are apparently mediated by different mechanisms (e.g. Eisenach et al.16). In rodents, spinal administration of an antagonist of the NK1 receptor attenuates myositis-induced hyperexcitability of spinal neurons in response to input from Aδ and c muscle afferents, but does not affect background activity24. In contrast, nitric oxide synthase inhibitor affects background activity but not excitability leading to activation of muscle afferents23. This neurophysiological evidence and our data support the notion that myositis-induced spontaneous pain and mechanically or functionally evoked pain are produced and regulated via differential molecular mechanisms at the level of both nociceptors and spinal cord. Our results, however, suggest that inflammation-induced changes in MGS scores and reductions of bite force depend on NK1-expressing neurons in Vc. Thus, NK1-expressing second order neurons likely comprise a primary common pathway for both spontaneous pain and bite-evoked pain from muscle. Our data also indicate that myositis-induced changes in MGS and bite force are consequences of transmission of nociceptive signals through TRPV1-expressing nociceptors and NK1-expressing nociceptive neurons. This provides clear evidence that MGS scores and bite force reduction represent nociception-related behaviors in inflamed mice. Masseter inflammation upregulates multiple genes from trigeminal ganglia implicated in synaptic function and structure11, which may produce central sensitization and lead to more widespread craniofacial pain such as secondary cutaneous hyperalgesia32. Further investigation of central mechanisms in craniofacial hyperalgesia following muscle inflammation is needed to address this issue.
In this study, we used a model of inflammation caused by injection of CFA into masseter muscles to assess spontaneous pain and bite-evoked pain. Inflammation is one of the major contributors to acute and chronic muscle pain. Acute trauma, injury, and infection are accompanied by inflammatory reactions in muscle, which cause peripheral and central sensitization leading to hyperalgesia37. Inflammatory mediators also appear to contribute to chronic muscle pain conditions, such as myofascial pain syndrome52. Therefore, rodent models of muscle inflammation have been widely used to study mechanisms of muscle pain21. However, multiple chronic muscle pain conditions, e.g., fibromyalgia, often do not involve overt muscle inflammation. It is possible that the mechanisms of spontaneous pain and bite-evoked pain associated with non-inflammatory muscle pathologies may be different from the mechanisms presented in this study.
We conclude that TRPV1 differentially contribute to spontaneous and bite-evoked pain during masseter inflammation, but that both types of muscle pain are mediated by TRPV1-expressing nociceptors as well as NK1-expressing second order neurons. Results of this study suggest that targeting TRPV1 might be used to treat spontaneous muscle pain; whereas, manipulation of muscle nociceptive terminals might be a potential therapeutic approach for simultaneously reducing multiple modes of muscle pain.
Perspective.
We report the profound contribution of TRPV1 to spontaneous muscle pain but not to bite-evoked muscle pain. These two types of muscle pain are transmitted through a common nociceptive pathway. These results may help to develop new strategies to manage multiple modes of muscle pain simultaneously by manipulating pain circuits.
Highlights.
We established models for spontaneous pain and bite-evoked pain from masseter muscle in mice.
TRPV1 is a major contributor to spontaneous pain but not bite-evoked pain from masseter muscle.
TRPV1-positive afferents and NK1-positive neurons commonly mediate both types of muscle pain.
Acknowledgments
The authors would like to thank Corinne O’Brien, Benjamin Brigoli, Mariama Magona, Yiwei Gao, Raime Shah, and Youping Zhang for technical help. Juhi Dwivedi, Alisha Karley, and Bri’Anna Horne for helping with video analysis.
This study was supported by National Institutes of Health grant R01 DE023846 (M.K.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures : The authors declare no conflict of interest.
References
- 1.Alvarez P, Gear RW, Green PG, Levine JD. IB4-saporin attenuates acute and eliminates chronic muscle pain in the rat. Exp Neurol. 2012;233:859–865. doi: 10.1016/j.expneurol.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez P, Green PG, Levine JD. Role for monocyte chemoattractant protein-1 in the induction of chronic muscle pain in the rat. Pain. 2014;155:1161–1167. doi: 10.1016/j.pain.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambalavanar R, Moritani M, Haines A, Hilton T, Dessem D. Chemical phenotypes of muscle and cutaneous afferent neurons in the rat trigeminal ganglion. J Comp Neurol. 2003;460:167–179. doi: 10.1002/cne.10655. [DOI] [PubMed] [Google Scholar]
- 4.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asgar J, Zhang Y, Saloman JL, Wang S, Chung MK, Ro JY. The role of TRPA1 in muscle pain and mechanical hypersensitivity under inflammatory conditions in rats. Neuroscience. 2015;310:206–215. doi: 10.1016/j.neuroscience.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavanaugh DJ, Chesler AT, Braz JM, Shah NM, Julius D, Basbaum AI. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci. 2011;31:10119–10127. doi: 10.1523/JNEUROSCI.1299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WN, Lee CH, Lin SH, Wong CW, Sun WH, Wood JN, Chen CC. Roles of ASIC3, TRPV1, and NaV1.8 in the transition from acute to chronic pain in a mouse model of fibromyalgia. Mol Pain. 2014;10:40. doi: 10.1186/1744-8069-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, Parekh PK, Moore C, Gereau RWt, Taylor AB, Wang F, Guilak F, Liedtke W. Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain. 2013;154:1295–1304. doi: 10.1016/j.pain.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung MK, Asgar J, Jennifer P, Ro JY. Transcriptome analysis of trigeminal ganglia following masseter muscle inflammation in rats. Mol pain. 2016 doi: 10.1177/1744806916668526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung MK, Jue SS, Dong X. Projection of non-peptidergic afferents to mouse tooth pulp. J Dent Res. 2012;91:777–782. doi: 10.1177/0022034512450298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung MK, Jung SJ, Oh SB. Role of TRP Channels in Pain Sensation. Adv Exp Med Biol. 2011;704:615–636. doi: 10.1007/978-94-007-0265-3_33. [DOI] [PubMed] [Google Scholar]
- 14.Clayton JS, Gaskin PJ, Beattie DT. Attenuation of Fos-like immunoreactivity in the trigeminal nucleus caudalis following trigeminovascular activation in the anaesthetised guinea-pig. Brain Res. 1997;775:74–80. doi: 10.1016/s0006-8993(97)00930-x. [DOI] [PubMed] [Google Scholar]
- 15.Craig AD, Kniffki KD. Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. J Physiol. 1985;365:197–221. doi: 10.1113/jphysiol.1985.sp015767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenach JC, Rauck RL, Curry R. Intrathecal, but not intravenous adenosine reduces allodynia in patients with neuropathic pain. Pain. 2003;105:65–70. doi: 10.1016/s0304-3959(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 17.Foreman RD, Schmidt RF, Willis WD. Effects of mechanical and chemical stimulation of fine muscle afferents upon primate spinothalamic tract cells. J Physiol. 1979;286:215–231. doi: 10.1113/jphysiol.1979.sp012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140:292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregory NS, Sluka KA. Anatomical and physiological factors contributing to chronic muscle pain. Curr Top Behav Neurosci. 2014;20:327–348. doi: 10.1007/7854_2014_294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamalainen MM, Subieta A, Arpey C, Brennan TJ. Differential effect of capsaicin treatment on pain-related behaviors after plantar incision. J Pain. 2009;10:637–645. doi: 10.1016/j.jpain.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoheisel U, Sander B, Mense S. Blockade of nitric oxide synthase differentially influences background activity and electrical excitability in rat dorsal horn neurones. Neurosci Lett. 1995;188:143–146. doi: 10.1016/0304-3940(95)11411-o. [DOI] [PubMed] [Google Scholar]
- 24.Hoheisel U, Sander B, Mense S. Myositis-induced functional reorganisation of the rat dorsal horn: effects of spinal superfusion with antagonists to neurokinin and glutamate receptors. Pain. 1997;69:219–230. doi: 10.1016/S0304-3959(96)03276-9. [DOI] [PubMed] [Google Scholar]
- 25.Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, Joshi SK, Ghilardi JR, Sevcik MA, Fryer RM, Segreti JA, Banfor PN, Marsh K, Neelands T, Bayburt E, Daanen JF, Gomtsyan A, Lee CH, Kort ME, Reilly RM, Surowy CS, Kym PR, Mantyh PW, Sullivan JP, Jarvis MF, Faltynek CR. Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermia. Pain. 2009;142:27–35. doi: 10.1016/j.pain.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Hwang SJ, Burette A, Rustioni A, Valtschanoff JG. Vanilloid receptor VR1-positive primary afferents are glutamatergic and contact spinal neurons that co-express neurokinin receptor NK1 and glutamate receptors. J Neurocytol. 2004;33:321–329. doi: 10.1023/B:NEUR.0000044193.31523.a1. [DOI] [PubMed] [Google Scholar]
- 27.Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–343. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- 28.Kim YS, Chu Y, Han L, Li M, Li Z, Lavinka PC, Sun S, Tang Z, Park K, Caterina MJ, Ren K, Dubner R, Wei F, Dong X. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81:873–887. doi: 10.1016/j.neuron.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152:1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King T, Rao S, Vanderah T, Chen Q, Vardanyan A, Porreca F. Differential blockade of nerve injury-induced shift in weight bearing and thermal and tactile hypersensitivity by milnacipran. J Pain. 2006;7:513–520. doi: 10.1016/j.jpain.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Kissin EY, Freitas CF, Kissin I. The effects of intraarticular resiniferatoxin in experimental knee-joint arthritis. Anesth Analg. 2005;101:1433–1439. doi: 10.1213/01.ANE.0000180998.29890.B0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiyomoto M, Shinoda M, Okada-Ogawa A, Noma N, Shibuta K, Tsuboi Y, Sessle BJ, Imamura Y, Iwata K. Fractalkine signaling in microglia contributes to ectopic orofacial pain following trapezius muscle inflammation. J Neurosci. 2013;33:7667–7680. doi: 10.1523/JNEUROSCI.4968-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kniffki KD, Schomburg ED, Steffens H. Synaptic effects from chemically activated fine muscle afferents upon alpha-motoneurones in decerebrate and spinal cats. Brain Res. 1981;206:361–370. doi: 10.1016/0006-8993(81)90537-0. [DOI] [PubMed] [Google Scholar]
- 34.Kroon GW, Naeije M. Electromyographic evidence of local muscle fatigue in a subgroup of patients with myogenous craniomandibular disorders. Arch Oral Biol. 1992;37:215–218. doi: 10.1016/0003-9969(92)90091-l. [DOI] [PubMed] [Google Scholar]
- 35.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 36.Marton K, Hermann P, Danko K, Fejerdy P, Madlena M, Nagy G. Evaluation of oral manifestations and masticatory force in patients with polymyositis and dermatomyositis. J Oral Pathol Med. 2005;34:164–169. doi: 10.1111/j.1600-0714.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- 37.Mense S, Gerwin RD. Muscle Pain Understanding the Mechanisms. Springer; 2010. [Google Scholar]
- 38.Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol. 1983;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci. 2010;43:157–163. doi: 10.1016/j.mcn.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell K, Lebovitz EE, Keller JM, Mannes AJ, Nemenov MI, Iadarola MJ. Nociception and inflammatory hyperalgesia evaluated in rodents using infrared laser stimulation after Trpv1 gene knockout or resiniferatoxin lesion. Pain. 2014;155:733–745. doi: 10.1016/j.pain.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- 43.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, Ossipov MH, Xie J, Dussor GO, King T, Porreca F. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153:924–933. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ota H, Katanosaka K, Murase S, Kashio M, Tominaga M, Mizumura K. TRPV1 and TRPV4 play pivotal roles in delayed onset muscle soreness. PLoS One. 2013;8:e65751. doi: 10.1371/journal.pone.0065751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 3. Elsevier Academic Press; Amsterdam ; Boston: 2008. [Google Scholar]
- 47.Pratt D, Fuchs PN, Sluka KA. Assessment of avoidance behaviors in mouse models of muscle pain. Neuroscience. 2013;248:54–60. doi: 10.1016/j.neuroscience.2013.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ro JY. Bite force measurement in awake rats: a behavioral model for persistent orofacial muscle pain and hyperalgesia. J Orofac Pain. 2005;19:159–167. [PubMed] [Google Scholar]
- 49.Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruparel S, Green D, Chen P, Hargreaves KM. The cytochrome P450 inhibitor, ketoconazole, inhibits oxidized linoleic acid metabolite-mediated peripheral inflammatory pain. Mol Pain. 2012;8:73. doi: 10.1186/1744-8069-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saloman JL, Scheff NN, Snyder LM, Ross SE, Davis BM, Gold MS. Gi-DREADD Expression in Peripheral Nerves Produces Ligand-Dependent Analgesia, as well as Ligand-Independent Functional Changes in Sensory Neurons. J Neurosci. 2016;36:10769–10781. doi: 10.1523/JNEUROSCI.3480-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah JP, Gilliams EA. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther. 2008;12:371–384. doi: 10.1016/j.jbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Todd AJ. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp Physiol. 2002;87:245–249. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- 55.Wagenmakers AJ. Muscle function in critically ill patients. Clin Nutr. 2001;20:451–454. doi: 10.1054/clnu.2001.0483. [DOI] [PubMed] [Google Scholar]
- 56.Walder RY, Radhakrishnan R, Loo L, Rasmussen LA, Mohapatra DP, Wilson SP, Sluka KA. TRPV1 is important for mechanical and heat sensitivity in uninjured animals and development of heat hypersensitivity after muscle inflammation. Pain. 2012;153:1664–1672. doi: 10.1016/j.pain.2012.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C, Gavva NR, Brennan TJ. Effect of AMG0347, a transient receptor potential type V1 receptor antagonist, and morphine on pain behavior after plantar incision. Anesthesiology. 2008;108:1100–1108. doi: 10.1097/ALN.0b013e31817302b3. [DOI] [PubMed] [Google Scholar]
- 58.Wu Z, Yang Q, Crook RJ, O'Neil RG, Walters ET. TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. Pain. 2013;154:2130–2141. doi: 10.1016/j.pain.2013.06.040. [DOI] [PubMed] [Google Scholar]
- 59.Xu XX, Cao Y, Ding TT, Fu KY, Li Y, Xie QF. Role of TRPV1 and ASIC3 channels in experimental occlusal interference-induced hyperalgesia in rat masseter muscle. Eur J Pain. 2016;20:552–563. doi: 10.1002/ejp.758. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi K, Ono K, Hitomi S, Ito M, Nodai T, Goto T, Harano N, Watanabe S, Inoue H, Miyano K, Uezono Y, Matoba M, Inenaga K. Distinct TRPV1- and TRPA1-based mechanisms underlying enhancement of oral ulcerative mucositis-induced pain by 5-fluorouracil. Pain. 2016;157:1004–1020. doi: 10.1097/j.pain.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 61.Zhu H, Aryal DK, Olsen RH, Urban DJ, Swearingen A, Forbes S, Roth BL, Hochgeschwender U. Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis. 2016;54:439–446. doi: 10.1002/dvg.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]